Uterine Artery Embolization as a Gateway to Conservative Fibroid Surgery
Abstract
1. Introduction
2. Case Presentation
2.1. Patient Information
2.2. Clinical Course
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCT | Controlled Clinical Trial |
| MRI | Magnetic Resonance Imaging |
| PUAE | Preoperative Uterine Artery Embolization |
| RCS | Retrospective Cohort Study |
| RCTs | Randomized Controlled Trials |
| RR | Retrospective Review |
| RS | Retrospective Study |
| SR/MA | Systematic Review and Meta-analysis |
| UA | Uterine Artery |
| UAE | Uterine Artery Embolization |
| ER | Endometrial receptivity |
| IUAs | Intrauterine adhesions |
References
- Stewart, E.A.; Cookson, C.L.; Gandolfo, R.A.; Schulze-Rath, R. Epidemiology of uterine fibroids: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1501–1512. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Uterine fibroid management: From the present to the future. Hum. Reprod. Update 2016, 22, 665–686. [Google Scholar] [CrossRef]
- De La Cruz, M.S.; Buchanan, E.M. Uterine Fibroids: Diagnosis and Treatment. Am. Fam. Physician 2017, 95, 100–107. [Google Scholar] [PubMed]
- Micić, J.; Macura, M.; Andjić, M.; Ivanović, K.; Dotlić, J.; Micić, D.D.; Arsenijević, V.; Stojnić, J.; Bila, J.; Babić, S.; et al. Currently Available Treatment Modalities for Uterine Fibroids. Medicina 2024, 60, 868. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.; Pereira, L.; Berghella, V. Pregnancy After Uterine Artery Embolization. Obstet. Gynecol. 2002, 100, 869–872. [Google Scholar] [PubMed]
- Lee, S.J.; Ko, H.S.; Na, S.; Bae, J.Y.; Seong, W.J.; Kim, J.W.; Shin, J.; Cho, H.J.; Choi, G.Y.; Kim, J.; et al. Nationwide population-based cohort study of adverse obstetric outcomes in pregnancies with myoma or following myomectomy: Retrospective cohort study. BMC Pregnancy Childbirth 2020, 20, 716. [Google Scholar] [CrossRef]
- Xu, F.; Deng, L.; Zhang, L.; Hu, H.; Shi, Q. The comparison of myomectomy, UAE and MRgFUS in the treatment of uterine fibroids: A meta analysis. Int. J. Hyperth. 2021, 38, 24–29. [Google Scholar] [CrossRef]
- Manyonda, I.T.; Bratby, M.; Horst, J.S.; Banu, N.; Gorti, M.; Belli, A.M. Uterine artery embolization versus myomectomy: Impact on quality of life—Results of the FUME (Fibroids of the Uterus: Myomectomy versus Embolization) Trial. Cardiovasc. Interv. Radiol. 2012, 35, 530–536. [Google Scholar] [CrossRef]
- Daniels, J.; Middleton, L.J.; Cheed, V.; McKinnon, W.; Rana, D.; Sirkeci, F.; Manyonda, I.; Belli, A.M.; Lumsden, M.A.; Moss, J.; et al. Uterine artery embolisation versus myomectomy for premenopausal women with uterine fibroids wishing to avoid hysterectomy: The FEMME RCT. Health Technol. Assess. 2022, 26, 1–74. [Google Scholar] [CrossRef]
- Russ, M.; Hees, K.A.; Kemmer, M.; Richter, R.; Kroncke, T.; Schnapauff, D.; Heimann, U.; David, M. Preoperative Uterine Artery Embolization in Women Undergoing Uterus-Preserving Myomectomy for Extensive Fibroid Disease: A Retrospective Analysis. Gynecol. Obstet. Investig. 2022, 87, 38–45. [Google Scholar] [CrossRef]
- Bula Ibula, D.; Balestra, A.; Tanos, P.; Nisolle, M.; Karampelas, S. Uterine artery embolization before myomectomy: Is it worth the trouble? J. Minim. Invasive Gynecol. 2025, 32, 386–394. [Google Scholar] [CrossRef]
- Butori, N.; Tixier, H.; Filipuzzi, L.; Mutamba, W.; Guiu, B.; Cercueil, J.P.; Douvier, S.; Sagot, P.; Krausé, D.; Loffroy, R. Interest of uterine artery embolization with gelatin sponge particles prior to myomectomy for large and/or multiple fibroids. Eur. J. Radiol. 2011, 79, 1–6. [Google Scholar] [CrossRef]
- Dumousset, E.; Chabrot, P.; Rabischong, B.; Mazet, N.; Nasser, S.; Darcha, C.; Garcier, J.M.; Mage, G.; Boyer, L. Preoperative uterine artery embolization (PUAE) before uterine fibroid myomectomy. Cardiovasc. Interv. Radiol. 2008, 31, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Ustünsöz, B.; Uğurel, M.S.; Bozlar, U.; Duru, N.K.; Ustünsöz, A. Is Uterine Artery Embolization Prior to Myomectomy for Giant Fibroids Helpful? Diagn. Interv. Radiol. 2007, 13, 210–212. [Google Scholar] [PubMed]
- Tixier, H.; Grevoul, J.; Loffroy, R.; Lauferon, J.; Guiu, B.; Mutamba, W.; Filipuzzi, L.; Cercueil, J.P.; Douvier, S.; Krause, D.; et al. Preoperative embolization or ligature of the uterine arteries in preparation for conservative uterine fibroma surgery. Acta Obstet. Gynecol. Scand. 2010, 89, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Ghiaroni, J.; Lopez, G.E.; Coutinho Junior, A.C.; Schanaider, A. Uterine artery embolization with spherical PVA-PVAc particles as preparation for surgical resection of myomas. Rev. Col. Bras. Cir. 2013, 40, 386–391. [Google Scholar] [CrossRef]
- Malartic, C.; Morel, O.; Fargeadou, Y.; Le Dref, O.; Fazel, A.; Barranger, E.; Soyer, P. Conservative two-step procedure including uterin artery embolization with embosphere and surgical myomectomy for the treatment of multiple fibroids: Preliminary experience. Eur. J. Radiol. 2012, 81, 1–5. [Google Scholar] [CrossRef]
- Goldman, K.N.; Hirshfeld-Cytron, J.E.; Pavone, M.E.; Thomas, A.P.; Vogelzang, R.L.; Milad, M.P. Uterine artery embolization immediately preceding laparoscopic myomectomy. Int. J. Gynaecol. 2012, 116, 105–108. [Google Scholar] [CrossRef]
- McLucas, B.; Voorhees, W.D., 3rd. The effectiveness of combined abdominal myomectomy and uterine artery embolization. Int. J. Gynecol. 2015, 130, 241–243. [Google Scholar] [CrossRef]
- Ouyang, Y.; Peng, Y.; Zheng, M.; Mao, Y.; Gong, F.; Li, Y.; Chen, H.; Li, X. The impact of intrauterine adhesions on endometrial receptivity in patients undergoing in vitro fertilization-embryo transfer. Front. Endocrinol. 2025, 15, 1489839. [Google Scholar] [CrossRef]
- Hiraga, H.; Hoshino, K.; Yokoyama, E.; Takahashi, Y.; Sato, T.; Takahashi, Y.; Toratani, J.; Kuga, A.; Takahashi, A.; Watanabe, Z.; et al. Fertility Complications After Uterine Artery Embolization for Postpartum Hemorrhage: A Case of Infertility and Miscarriage Following Stillbirth. Cureus 2025, 17, e82609. [Google Scholar] [CrossRef]
- Komatsu, H.; Taniguchi, F.; Harada, T. Impact of myomectomy on the obstetric complications: A large cohort study in Japan. Int. J. Gynaecol. 2023, 162, 977–982. [Google Scholar] [CrossRef]
- Van der Kooij, S.M.; Bipat, S.; Hehenkamp, W.J.K.; Ankum, W.M.; Reekers, J.A. Uterine artery embolization vs surgery in the treatment of symptomatic fibroids: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2011, 205, 513–517. [Google Scholar] [CrossRef]
- Kinariwala, D.J.; Khaja, M.S.; McCann, S.; Sheeran, D.; Park, A.W.; Stone, J.; Wilkins, L.R.; Matsumoto, A.H.; Redick, D.L. Preoperative uterine artery embolization before hysterectomy or myomectomy: A single-center review of 53 patients. Clin. Imaging 2023, 101, 121–125. [Google Scholar] [CrossRef]
- Liu, W.M.; Tzeng, C.R.; Yi-Jen, C.; Wang, P.H. Combining the uterine depletion procedure and myomectomy may be useful for treating symptomatic fibroids. Fertil. Steril. 2004, 82, 205–210. [Google Scholar] [CrossRef]
- Leo, L.; Thomasset, R.; Massaro, A.; Tinelli, R.; Masturzo, B.; Remorgida, V.; Libretti, A.; Natrella, M. A 40-year-old woman with inoperable uterine fibroids treated with combined uterine artery embolization and relugolix. Am. J. Case Rep. 2025, 26, e946334. [Google Scholar] [CrossRef]
| Variable | Details |
|---|---|
| Age | 34 years |
| Country of origin | Madagascar |
| Family history | Father: prostate carcinoma; sister: uterine fibromatosis; sister: ovarian cysts |
| Past medical history | Left breast fibroadenoma, chronic iron-deficiency anemia |
| Surgical history | None |
| Allergies | None known |
| BMI | 21.7 kg/m2 |
| Lifestyle | Non-smoker, non-drinker |
| Gynecologic history | Menarche at 14; regular 28-day cycles; virgo; G0P0 |
| Infections | No history of PID or HSV |
| Latest Pap test | Negative (2025) |
| Blood pressure | Normal values |
| Date | Event |
|---|---|
| 19 June 2024 | Initial laboratory tests showing iron-deficiency anemia. |
| November 2024 | First consultation with GP for abdominal mass and fatigue. Ultrasound and blood tests ordered; oral iron continued. |
| December 2024 | Worsening pelvic pain, metrorrhagia, mass enlargement, constipation. Labs confirm worsening iron deficiency. |
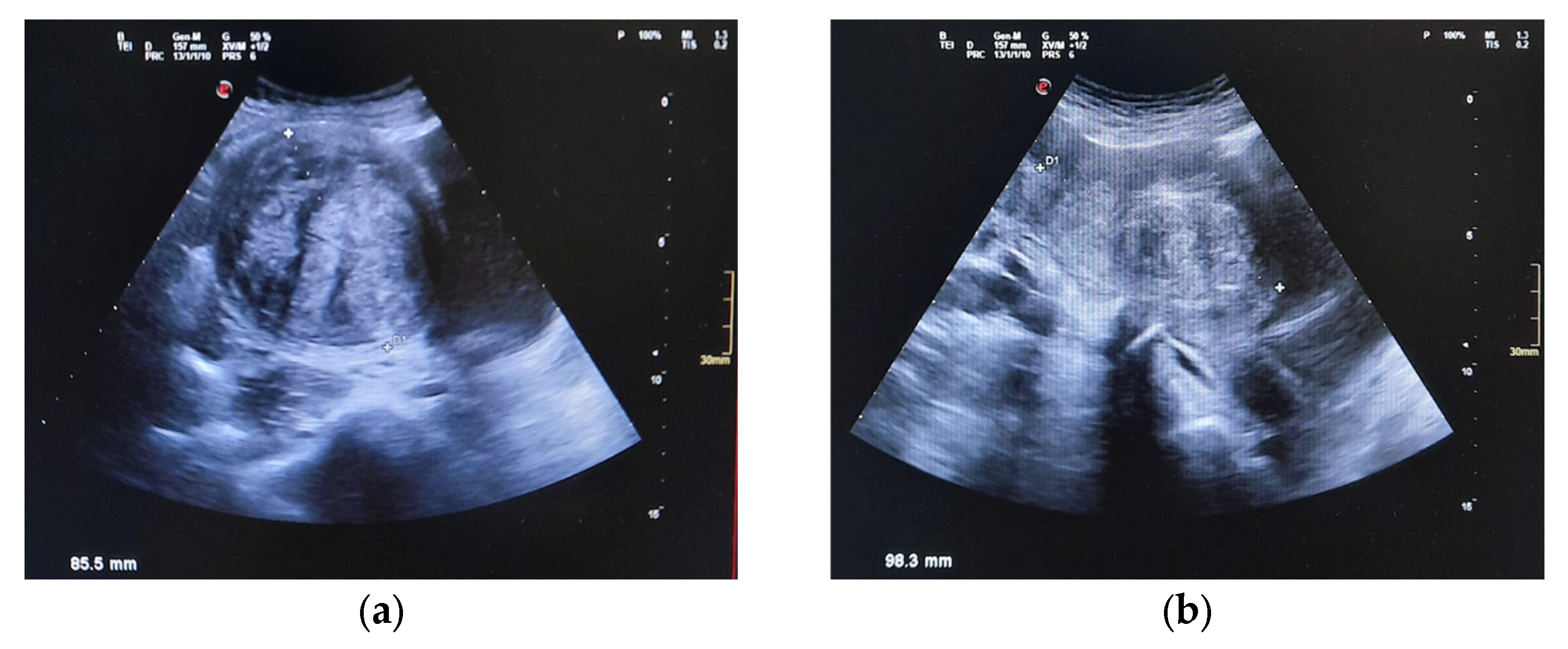
| 27 December 2024 | Abdominal US reveals 9 cm pelvic mass suggestive of fibroids; urgent gynecologic evaluation recommended. |
| Jan–Feb 2025 | Hematologic stabilization under iron therapy; tumor markers normal. |
| 28 February 2025 | First gynecologic exam at Beauregard Hospital: large fibroid uterus confirmed; plan for conservative management. Relugolix/estradiol/norethisterone (Ryeqo®) initiated. |
| April 2025 | Partial symptom improvement under medical therapy. |
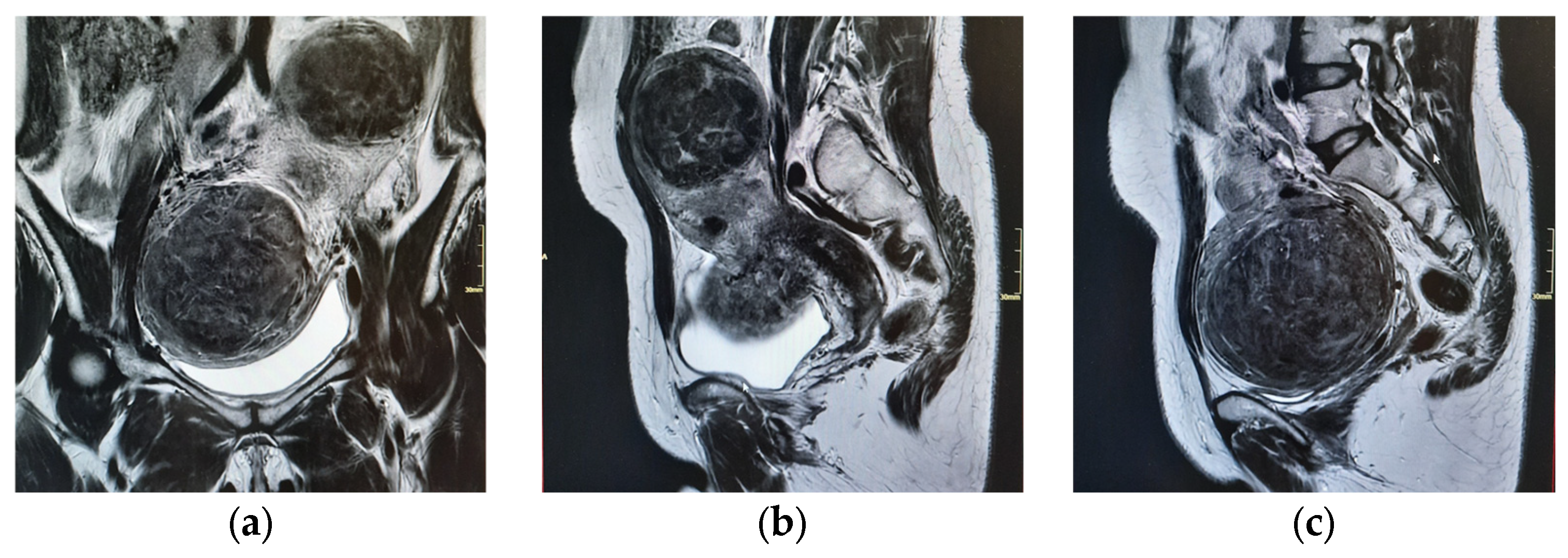
| 15 April 2025 | Pelvic MRI confirms multiple intramural and submucosal fibroids; significant uterine deformation. |
| 9 May 2025 | Informed consent for open multiple myomectomy; multidisciplinary decision to perform PUAE preoperatively. |
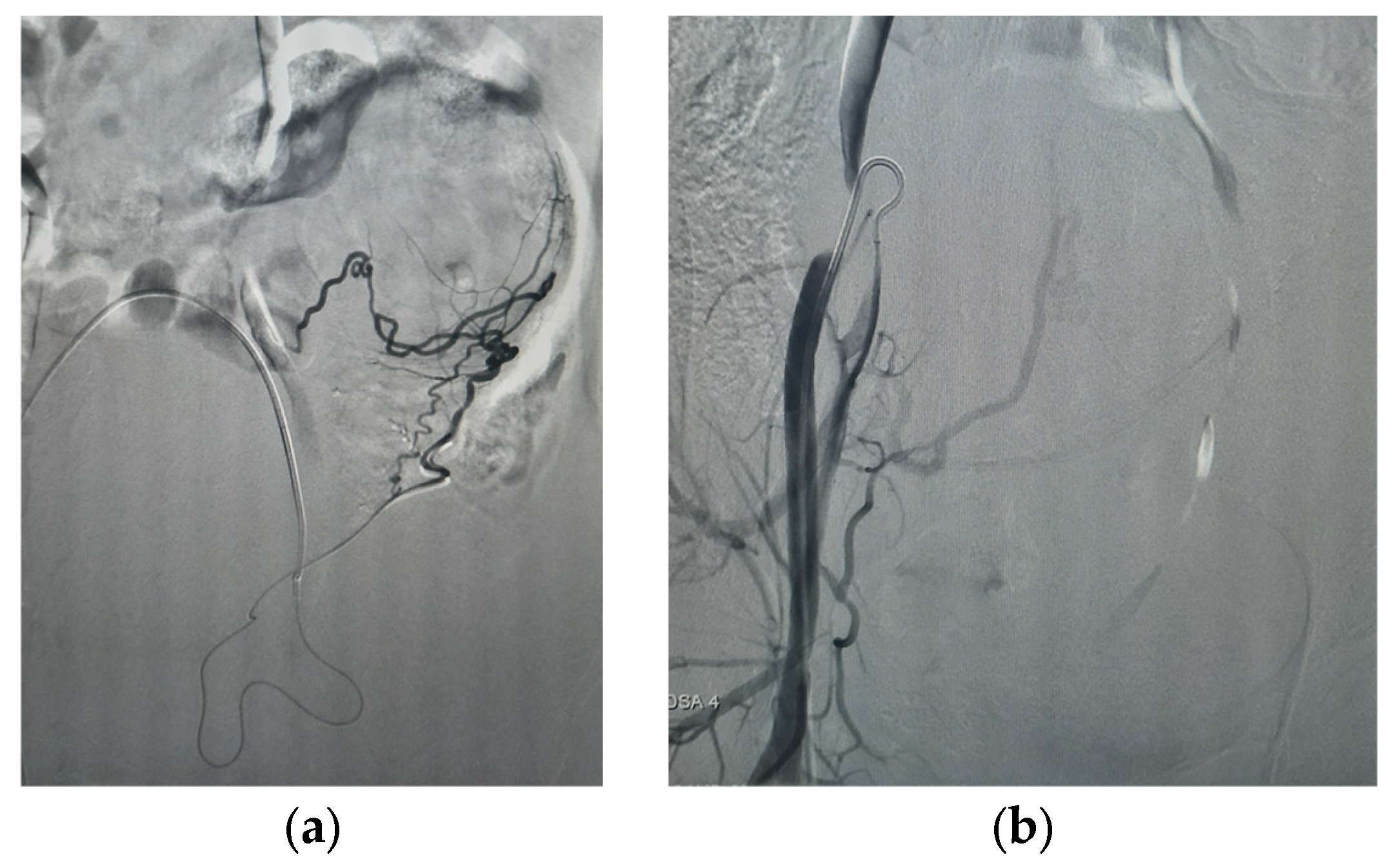
| 5 June 2025 (morning) | Selective bilateral PUAE performed using 800 μm particles + gelfoam; adequate devascularization achieved. |
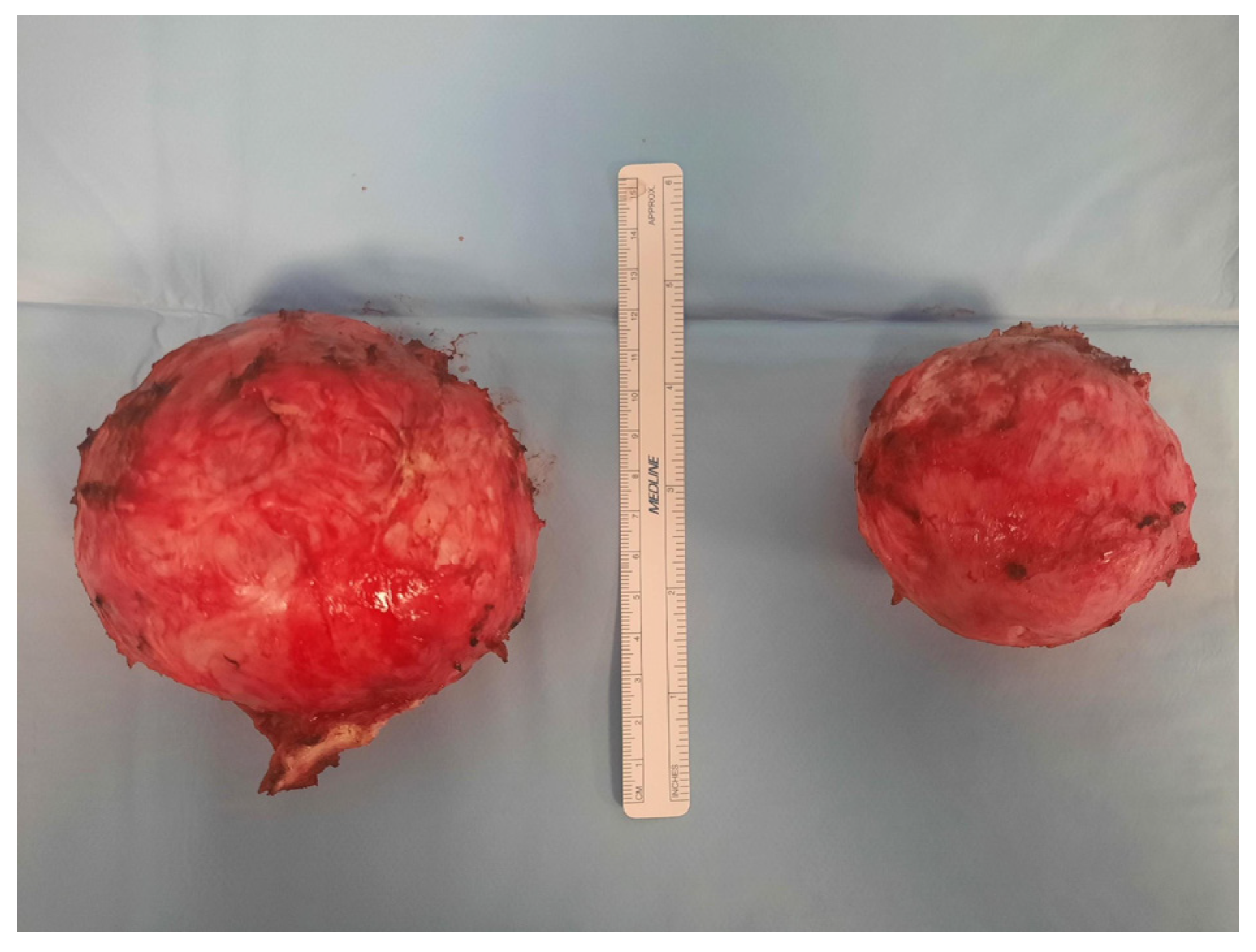
| 5 June 2025 (same day) | Longitudinal laparotomic multiple myomectomy performed; minimal blood loss (~100 mL), no transfusion needed. |
| 8 June 2025 | Patient discharged in stable condition; advised to continue Ryeqo® for 1 month; avoid conception for 12 months. |
| ~July 2025 | Postoperative follow-up at 40 days: symptoms resolved; normal ultrasound; no pelvic pain or urinary issues. |
| Pathology | Histology confirms leiomyomas. |
| Author (Year) | Study Type | Sample Size | Topic | Main Outcomes |
|---|---|---|---|---|
| Bula et al. (2025) [11] | RCS | 192 | Presence vs. absence of preoperative UAE | Reduced blood loss with UAE, no significant difference in postoperative Hb levels, no need of transfusion nor hysterectomy conversions; UAE associated with complications |
| Butori et al. (2012) [12] | RS | 33 | Use of UAE before myomectomy | Reduced intraoperative blood loss, increased chances of conservative surgery |
| Dumousset et al. (2008) [13] | RS | 22 | Use of UAE before laparoscopic myomectomy | Improved surgical visibility, reduced bleeding |
| Kinariwala et al. (2023) [24] | RR | 53 | UAE before surgery | Usefulness in selected patients should be evaluated in further studies |
| Liu et al. (2020) [25] | CCT | 486 | Ligation of UA before myomectomy | With UA ligation, blood loss and recurrence were reduced, useful in symptomatic fibroids |
| Malartic et al. (2012) [17] | RS | 12 | UAE before myomectomy | Safe in premenopausal women, reduced need of blood transfusion, decreased uterine volume |
| McLucas et al. (2015) [19] | RS | 20 | UAE before myomectomy | Helpful in premenopausal women with fibroids >4 cm, reduced recurrence, increased chances of uterus preservation |
| Russ et al. (2022) [10] | RS | 78 | Long- vs. short-term PUAE | Useful in women with enlarged uterus, reduced blood loss, postoperative transfusion and complications |
| Tixier et al. (2010) [15] | RS | 100 | UAE before myomectomy vs. surgery | Useful in case of high-risk hemorrhage |
| Van der Kooij et al. (2011) [23] | SR/MA | 11 RCTs | UAE vs. surgery | UAE is effective but associated with higher risk of long-term reintervention |
| Goldman et al. (2012) [18] | RS | 26 | UAE immediately before surgery | UAE facilitated minimally invasive surgery for larger uteri and larger myomas, with no differences in operative time or blood loss |
| Ghiaroni et al. (2013) [16] | RS | 12 | UAE with PVA before myomectomy | Reduction in uterine volume, decreased intraoperative bleeding, use of smaller incisions |
| Ustünsöz et al. (2007) [14] | RS | 30 | UAE before surgery vs. surgery | UAE before surgery is more effective |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brazzelli, B.; Libretti, A.; Thomasset, R.; Natrella, M.; Messina, A.; Leo, L. Uterine Artery Embolization as a Gateway to Conservative Fibroid Surgery. Reprod. Med. 2025, 6, 40. https://doi.org/10.3390/reprodmed6040040
Brazzelli B, Libretti A, Thomasset R, Natrella M, Messina A, Leo L. Uterine Artery Embolization as a Gateway to Conservative Fibroid Surgery. Reproductive Medicine. 2025; 6(4):40. https://doi.org/10.3390/reprodmed6040040
Chicago/Turabian StyleBrazzelli, Bianca, Alessandro Libretti, Raphael Thomasset, Massimiliano Natrella, Alessandro Messina, and Livio Leo. 2025. "Uterine Artery Embolization as a Gateway to Conservative Fibroid Surgery" Reproductive Medicine 6, no. 4: 40. https://doi.org/10.3390/reprodmed6040040
APA StyleBrazzelli, B., Libretti, A., Thomasset, R., Natrella, M., Messina, A., & Leo, L. (2025). Uterine Artery Embolization as a Gateway to Conservative Fibroid Surgery. Reproductive Medicine, 6(4), 40. https://doi.org/10.3390/reprodmed6040040









