Abstract
Background: Myocardial scar and fibrosis predict adverse cardiac outcomes. Late gadolinium enhancement (LGE) cardiac magnetic resonance (CMR) is the reference standard for detection. However, it requires gadolinium-based contrast agents (GBCAs), which may be unsuitable for some patients. Cine balanced steady-state free precession (bSSFP) sequences are universally acquired in routine CMR. They may enable contrast-free scar detection via radiomics analysis. Aim: To systematically review the diagnostic accuracy of cine CMR radiomics for myocardial scar or fibrosis detection. The reference standard is visual or threshold-based LGE. Methods: This review followed PRISMA guidelines and was registered in PROSPERO (CRD420251121699). We searched MEDLINE, Embase, and Cochrane Library up to 8 August 2025. Eligible studies compared cine CMR radiomics with LGE-based assessment in patients with suspected or known scar/fibrosis. Quality was assessed using QUADAS-2 and Radiomics Quality Score (RQS). Results: Five retrospective studies (n = 1484) were included. Two focused on myocardial infarction, two on hypertrophic cardiomyopathy, and one on ischaemic versus dilated cardiomyopathy. Diagnostic performance was good to excellent (AUC 0.74–0.96). Methodological heterogeneity was substantial in reference standards, segmentation, preprocessing, feature selection, and modelling. Only one study used external validation. QUADAS-2 showed high bias risk in patient selection and index test domains. RQS scores were low (30–42%), indicating limited reproducibility and validation. Conclusions: Cine CMR radiomics shows promise as a non-contrast alternative for detecting myocardial scar and fibrosis. However, methodological standardisation, multicentre validation, and prospective studies are needed before clinical adoption.
1. Introduction
Accurate detection of myocardial scar and fibrosis is clinically essential, as both replacement and interstitial fibrosis are key drivers of adverse cardiac remodelling and occur across a spectrum of conditions, including ischaemic injury, myocarditis, and cardiomyopathies. These pathologies exhibit distinct morphologic patterns and underlying mechanisms that influence prognosis and management [1,2,3]. Imaging-detected myocardial scar or fibrosis consistently predicts poorer outcomes in both ischaemic and non-ischaemic cardiomyopathies, with greater scar burden and specific distribution patterns associated with higher risks of mortality, heart failure events, and ventricular arrhythmias [4,5,6]. Therefore, imaging methods capable of reliably detecting and quantifying myocardial scar are of major clinical importance.
Cardiac magnetic resonance (CMR) is widely regarded as the reference standard for non-invasive assessment of myocardial structure, function, and scar, owing to its high spatial and temporal resolution and the lack of ionising radiation [7,8,9]. Late gadolinium enhancement (LGE) is the clinical gold standard for detecting and quantifying focal myocardial scar or fibrosis, providing high-contrast visualisation of fibrotic tissue through differential gadolinium distribution. It offers excellent spatial resolution and reproducibility, with robust evidence supporting its application across a wide range of cardiac pathologies [9,10]. However, LGE performance may vary across diseases: in ischaemic heart disease, it clearly delineates transmural scar in the presence of contrast administration, while in hypertrophic cardiomyopathy, diffuse interstitial fibrosis may be underestimated due to absent or subtle enhancement [11,12].
Despite its strengths, LGE requires intravenous gadolinium-based contrast agents (GBCAs), which, although generally well tolerated, are contraindicated in severe renal impairment because of the risk of nephrogenic systemic fibrosis and may also be unsuitable in patients with hypersensitivity [13]. LGE also prolongs scan time, depicts scar at a single time point, and requires additional imaging beyond routine cine sequences. Concerns over the environmental impact of unmetabolised gadolinium excretion have further prompted interest in non-contrast alternatives [14,15]. Studies report anthropogenic gadolinium concentrations in surface waters up to 0.1–1 μg/L in urban areas, reflecting widespread contamination from medical use. Cine balanced Steady-State Free Precession (bSSFP) imaging, the backbone of routine CMR protocols, is universally acquired and provides high-resolution, high-contrast visualisation of ventricular anatomy and wall motion without the need for contrast agents, offering a potential platform for scar and fibrosis assessment [9,10]. Recent studies have explored non-contrast methods like T1ρ mapping for fibrosis detection [16,17].
Radiomics converts medical images into quantitative features. These include shape, intensity, and texture metrics. It characterises tissue heterogeneity beyond visual perception [18,19]. In CMR, radiomics captures sub-visual changes. It improves diagnosis, prognosis, and classification with proper feature selection, machine learning, and validation [18,19,20]. Although established in oncology, its use in cardiac MRI for scar detection via cine imaging remains in its early stages [18,19]. Multiple studies have demonstrated that cine CMR radiomics can differentiate between scarred and non-scarred myocardium when benchmarked against visual or threshold-based LGE assessment [21,22,23,24,25]. However, marked methodological heterogeneity, including variations in segmentation protocols, feature extraction methods, modelling strategies, and validation approaches, limits direct comparison and generalisability. Given the universal acquisition of cine sequences in routine CMR, integrating radiomics to non-invasively and efficiently identify scar or fibrosis offers the potential to substantially expand the diagnostic utility of CMR without increasing scan time or requiring contrast administration.
This systematic review aims to critically appraise and synthesise the available evidence on the diagnostic accuracy of cine CMR radiomics for myocardial scar and fibrosis detection. We also appraise methodological quality using both the QUADAS-2 framework and the Radiomics Quality Score, in order to highlight areas for improvement and promote reproducibility in future research.
2. Materials and Methods
2.1. Protocol and Registration
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [26]. The protocol was prospectively registered in PROSPERO (registration number: CRD420251121699).
2.2. Eligibility Criteria
A PICO framework was used to define eligibility criteria. Eligible studies included
- Population: Patients undergoing cardiac magnetic resonance (CMR) for suspected or known myocardial scar or fibrosis.
- Intervention/index test: Radiomics analysis applied to cine balanced steady-state free precession (bSSFP).
- Comparator/Reference standard: Visual or quantitative threshold-based assessment of scar or fibrosis on CMR.
- Outcome: Diagnostic accuracy studies directly comparing radiomics-derived metrics to the reference standard.
Only studies assessing myocardial scar or fibrosis detection or quantification using radiomics (cine or LGE CMR) compared against a visual or threshold-based reference standard for scar/fibrosis were included. Studies focusing on acute myocardial infarction, microvascular obstruction, or other pathologies without scar/fibrosis quantification as the reference standard were excluded.
2.3. Information Sources and Search Strategy
A comprehensive literature search was conducted in MEDLINE, Embase, and the Cochrane Library from database inception to 8 August 2025, without language restrictions. The search combined terms related to radiomics, cardiac MRI, cine imaging, and myocardial scar/fibrosis, including: “Radiomics”, “Texture Analysis”, “Radiomic”, “Cardiac MRI”, “CMR”, “Magnetic Resonance Imaging”, “bSSFP”, “Cine MRI”, “Myocardial Fibrosis”, “Myocardial Scar”, “Myocardial Infarction”, “Hypertrophic Cardiomyopathy”, and “Ischemic Cardiomyopathy”. The complete search strategies for each database are provided in Supplementary Materials Text S1. To ensure completeness, reference lists of all included studies and relevant reviews were manually screened, and grey literature sources were searched for additional eligible studies.
2.4. Study Selection
All search results were imported into the reference management software EndNote version 21, and duplicates were removed. Two reviewers (CM and RD) independently screened titles and abstracts, followed by a full-text assessment of potentially eligible studies against the pre-determined inclusion criteria. Disagreements were resolved by consensus or adjudication by a third reviewer (HT).
2.5. Quality Assessment
Methodological quality and risk of bias for each study were assessed using the QUADAS-2 tool, which evaluates four domains: patient selection, index test, reference standard, and flow/timing, with applicability concerns assessed in parallel. In addition, radiomics-specific methodological quality was appraised using the Radiomics Quality Score (RQS), a 16-item framework assessing aspects such as image protocol reporting, feature robustness testing, model validation, biological/clinical validation, and data/code sharing. Scoring details are provided in Figure 1.
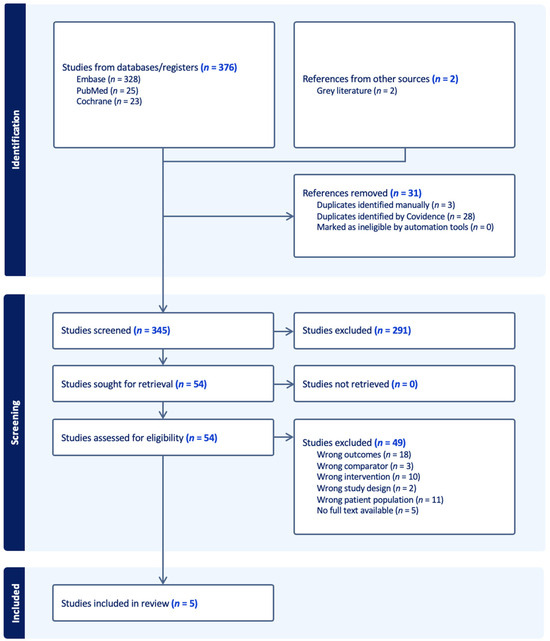
Figure 1.
PRISMA flow chart.
2.6. Data Extraction
Two reviewers (CM and RD) independently extracted data using a pre-piloted form capturing study characteristics (year, country, patient population), MRI acquisition parameters, segmentation and feature extraction methods, modelling approaches, performance metrics, comparator performance, and validation strategies. Discrepancies were resolved by consensus or adjudication by a third reviewer (HCT). Data extraction and management were performed using Covidence, the Cochrane Collaboration’s screening and data extraction platform.
2.7. Data Synthesis
Due to substantial heterogeneity in populations, reference standards, radiomics workflows, validation strategies, and reporting of performance metrics, a quantitative meta-analysis was not feasible. Instead, a narrative synthesis with structured tabulation was performed, grouping studies by reference standard and population where possible to facilitate qualitative comparison and identify methodological trends. Only a subset of studies reported operating-point metrics such as sensitivity, specificity, or accuracy. Most results were limited to Area Under the receiver operating characteristic Curve (AUCs), which, while comparable across studies, constrain assessment of clinically applicable thresholds
3. Results
3.1. Study Selection and Characteristics
The systematic search identified five studies meeting the predefined inclusion criteria, yielding a total of 1484 participants. All were retrospective observational studies, conducted between 2018 and 2025, with sample sizes ranging from 42 to 718 participants. Two studies focused exclusively on patients with prior myocardial infarction, two on hypertrophic cardiomyopathy, and one on the differentiation between ischaemic and dilated cardiomyopathy. In all cases, cine CMR radiomics was compared against LGE-based assessment of scar or fibrosis, with the reference standard defined either visually or via quantitative thresholding. While three studies included only internal cross-validation or train/test splits, one incorporated a fully independent external test cohort. The methodological characteristics of each study are summarised in Table 1, highlighting the variation in study populations and design across included research.

Table 1.
Methodological characteristics.
3.2. MRI Acquisition
All studies used cine balanced steady-state free precession (bSSFP) imaging as the index sequence, with the majority performed at 1.5 T; one study included both 1.5 T and 3.0 T scanners from multiple vendors. Where reported, cine coverage was a short-axis stack (±long-axis views) with typical temporal resolution of ~30–50 ms. LGE was performed in all studies to establish the reference standard; contrast type/dose and breath-hold/gating details were variably reported. MRI acquisition parameters are presented in Table 2, demonstrating considerable technical heterogeneity that may influence feature robustness and generalisability.

Table 2.
MRI acquisition parameters.
3.3. Segmentation, Feature Processing, and Modelling
Segmentation approaches varied: three studies used fully manual Left Ventricle (LV) border delineation (3D Slicer or ITK-SNAP), and two applied semi-automated methods (e.g., cvi42) with manual correction. Most analyses targeted the whole LV myocardium in 2D or 3D, with scar/fibrosis regions defined from LGE. End-diastolic and/or end-systolic phases were analysed, and some combined features across slices or phases. Feature extraction, most often via PyRadiomics with Image Biomarker Standardisation Initiative (IBSI) compliance, followed pre-processing steps such as isotropic voxel resampling, grey-level discretisation (30–120 bins), intensity normalisation, and—in one study—Z-score normalisation. The reasons for specific preprocessing choices, such as bin numbers, were not explained in the studies. Extracted features included shape, first-order, and higher-order texture metrics (e.g., GLCM, GLRLM, GLSZM, NGTDM, wavelets). Feature selection methods included ICC filtering, RFECV, Boruta, and LASSO. Models ranged from logistic regression, SVM, random forest, and XGBoost to a hybrid deep learning–radiomics approach in one study. Table 3 summarises these methodological components in detail, underscoring the variability in radiomics workflows and its potential impact on reproducibility and cross-study comparison.

Table 3.
Segmentation, feature processing & modelling.
3.4. Diagnostic Performance
Cine CMR radiomics demonstrated good to excellent discrimination for LGE-defined scar or fibrosis across all included studies.
In myocardial infarction cohorts, Avard et al. [21] and Baessler et al. [22] reported AUCs of 0.92–0.93 in internal validation, with sensitivities and specificities around 86% and 82%, respectively.
In hypertrophic cardiomyopathy, Fahmy et al. [23] achieved an AUC of 0.81 using a hybrid deep learning + radiomics model, while Pu et al. [25] reported an AUC of 0.906 for a purely radiomics-based approach. Pu et al. also performed external validation, which yielded a reduced but still clinically relevant AUC of 0.74 in an independent cohort.
In the ischaemic vs. dilated cardiomyopathy setting, Lasode et al. [24] achieved AUCs of 0.915–0.956 for classification tasks including scar/no-scar detection, using repeated cross-validation. Radiologist cine reads, reported for context, had true-positive and false-positive rates of ~0.87 and 0.32 for ICM vs. DCM discrimination, although no formal statistical comparisons (e.g., DeLong test) were provided.
Full performance metrics are provided in Table 4.

Table 4.
Feature assessment & diagnostic performance.
3.5. Risk of Bias and Applicability (QUADAS-2)
The QUADAS-2 assessment revealed several recurrent sources of bias. Patient selection was frequently high risk, particularly in Avard et al. [21] and Baessler et al. [22], which used case–control designs (e.g., MI vs. healthy controls), potentially inflating diagnostic accuracy (Supplementary Materials Table S1). The index test domain was high risk in Lasode et al. [24], where feature selection occurred before cross-validation, raising optimism bias concerns; in others, including Fahmy et al. [23] and Pu et al. [25], reporting was insufficient to determine whether modelling was fully nested within validation folds. Reference standard and flow/timing domains were generally low risk across all studies, as cine and LGE were acquired in the same session. Applicability concerns mainly reflected narrow patient populations or highly selective inclusion criteria.
3.6. Radiomics Quality Score (RQS)
RQS totals ranged from 11 to 15 out of 36 (30.6–41.7%) (Supplementary Materials Table S2). Strengths across studies included use of feature reduction, multiple testing correction, and some discussion of clinical utility. Common weaknesses were the absence of phantom or test–retest analyses, limited external validation (Pu et al. [25] being the only example), lack of model calibration or decision curve analysis, and minimal open science practices—only Pu et al. [25] shared code, and only Fahmy et al. [23] reported model calibration. No study performed cost-effectiveness analysis or prospective validation.
3.7. Synthesis and Sources of Heterogeneity
Marked methodological heterogeneity was observed. Variations included reference standard type (visual vs. threshold-based LGE), segmentation scope (single slice vs. whole LV; 2D vs. 3D), pre-processing protocols, feature extraction parameters, and modelling strategies. Validation approaches ranged from internal cross-validation (Avard et al. [21], Baessler et al. [22], Fahmy et al. [23], Lasode et al. [24]) to independent external datasets (Pu et al. [25]). Inconsistent reporting of operating-point metrics and 2 × 2 contingency data prevented pooled meta-analysis, so findings are presented narratively with structured tabulation to facilitate methodological and performance comparisons.
4. Discussion
This systematic review synthesises evidence on the diagnostic accuracy of cine CMR radiomics for myocardial scar and fibrosis detection. It uses visual or threshold-based LGE as the reference standard. Across five retrospective studies with 1484 participants, cine CMR radiomics achieved good-to-excellent performance (AUC 0.74–0.96) in ischaemic and non-ischaemic cardiomyopathies [21,22,23,24,25]. Our findings suggest cine radiomics detects subtle myocardial changes not visible on standard assessment. This could enable scar and fibrosis evaluation without GBCAs.
The clinical impact of contrast-free scar detection is substantial. LGE CMR is the gold standard for focal scar. However, it faces limitations from GBCA contraindications, environmental concerns, and extra scan time [13,14]. Cine bSSFP sequences are routine and can be processed retrospectively for radiomics without workflow changes [8,10]. In patients with renal impairment or gadolinium hypersensitivity, cine radiomics offers a safer alternative. It may also help in resource-limited settings, where contrast access is restricted by cost or supply issues [27,28].
LGE limitations vary by disease. In ischaemic heart disease, it visualises transmural scar well but requires contrast [11]. In hypertrophic cardiomyopathy, it often underestimates diffuse fibrosis [12]. Cine radiomics quantifies textural heterogeneity in non-contrast images. It reveals sub-visual changes. Thus, it complements LGE as a contrast-free screening tool. It may improve sensitivity to diffuse fibrosis in HCM and support scar detection in MI without gadolinium. Overall, cine radiomics expands detectable myocardial abnormalities.
However, our review highlights significant methodological heterogeneity across published studies. Key sources of variation included the type of reference standard (visual versus threshold-based LGE), segmentation scope (whole LV versus single-slice; 2D versus 3D), pre-processing pipelines (voxel resampling, grey-level discretisation, intensity normalisation), and feature extraction and selection strategies. The variation in preprocessing choices, such as grey-level discretisation bin numbers (30–120), was not justified in the included studies. This potentially impacts feature robustness. Additionally, MRI acquisition parameters were variably reported, with some missing details that could affect reproducibility. Modelling approaches also differed widely, ranging from traditional classifiers (logistic regression, SVM) to hybrid deep learning–radiomics pipelines. This diversity hampers direct comparison, prevents meaningful meta-analysis, and may contribute to the variability in reported performance. Clinical heterogeneity was also evident, with study populations spanning myocardial infarction, hypertrophic cardiomyopathy, and dilated cardiomyopathy. Such differences in disease substrate complicate interpretation and restrict the generalisability of findings to any single clinical context.
Validation strategies represent another important limitation. Only one study (Pu et al.) [25] incorporated an independent external test set. Most relied solely on internal cross-validation, which is more prone to optimism bias, especially when feature selection is not nested within validation folds. The relatively modest Radiomics Quality Score (RQS) ratings (30–42% of the maximum) underscore persistent methodological shortcomings, such as the absence of phantom or test–retest analyses, inadequate reporting of model calibration, limited use of decision curve analysis, lack of cost-effectiveness evaluation, and minimal adoption of open science practices. Furthermore, the evidence base remains very narrow, with only five studies available, which constrains the robustness of conclusions and limits confidence in their broader generalisability. We recommend developing consensus guidelines for radiomics standardisation in cardiac imaging to address these issues.
These limitations highlight the need for caution when interpreting the present diagnostic performance of cine CMR radiomics in a clinical context. While the diagnostic accuracy is encouraging, robust prospective studies with standardised pipelines and multicentre validation are required to confirm generalisability and reproducibility. Future research should assess whether cine radiomics offers added value beyond conventional functional parameters, and explore its prognostic significance and potential influence on clinical decision-making. Compared to other contrast-free techniques like native T1 and T2 mapping, which provide parametric tissue information, radiomics offers complementary textural analysis from routine cine sequences without extra scans. Incorporating head-to-head comparisons with emerging tissue characterisation techniques, such as native T1/T2 mapping and extracellular volume (ECV) quantification, will also be important to determine the relative and incremental value of radiomics in this setting.
5. Conclusions
Cine CMR radiomics shows promising potential as a non-contrast, time-efficient method for myocardial scar and fibrosis detection, achieving high diagnostic accuracy when compared with LGE. Nevertheless, clinical adoption will require stronger evidence from larger, well-designed, and externally validated studies to mitigate concerns around methodological variability and generalisability. Further work should also explore the integration of radiomics into clinical workflows, its applicability across different disease substrates, and its potential to improve patient selection and outcomes. If validated, cine radiomics could ultimately complement or, in selected cases, replace LGE, expanding the diagnostic capabilities of routine CMR while reducing reliance on gadolinium-based contrast.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/hearts6040027/s1, Text S1: Systematic Search Strategy; Table S1: QUADAS-2 score; Table S2: Radiomics Quality Score (RQS) details.
Author Contributions
Conceptualisation, C.P.M., R.S.D. and H.C.T.; methodology, C.P.M., R.S.D. and H.C.T.; formal analysis, C.P.M., R.S.D. and H.C.T.; investigation, C.P.M., R.S.D. and H.C.T.; writing—original draft preparation, C.P.M., A.M.K. and P.D.; writing—review and editing, C.P.M., A.M.K., P.D., A.J. and S.M.; supervision, A.J. and S.M.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data underlying this systematic review are derived from previously published studies, which are cited in the manuscript. The extracted datasets used and analysed during the current review (including study characteristics and outcome data) are available from the corresponding author on reasonable request. No new patient-level data were generated for this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Giordano, C.; Francone, M.; Cundari, G.; Pisano, A.; d’Amati, G. Myocardial fibrosis: Morphologic patterns and role of imaging in diagnosis and prognostication. Cardiovasc. Pathol. 2022, 56, 107391. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Arvanitaki, A.; Karvounis, H.; Neubauer, S.; Ferreira, V.M. Myocardial Tissue Characterization and Fibrosis by Imaging. JACC Cardiovasc. Imaging 2020, 13, 1221–1234. [Google Scholar] [CrossRef]
- Gupta, S.; Ge, Y.; Singh, A.; Gräni, C.; Kwong, R.Y. Multimodality Imaging Assessment of Myocardial Fibrosis. JACC Cardiovasc. Imaging 2021, 14, 2457–2469. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.M.; Greve, A.M.; Aspelund, T.; Schelbert, E.B.; Cao, J.J.; Danielsen, R.; Þorgeirsson, G.; Sigurðsson, S.; Eiríksdóttir, G.; Harris, T.B.; et al. Prevalence and prognosis of ischaemic and non-ischaemic myocardial fibrosis in older adults. Eur. Heart J. 2019, 40, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Ambale-Venkatesh, B.; Lima, J.A.C. Cardiac MRI: A central prognostic tool in myocardial fibrosis. Nat. Rev. Cardiol. 2015, 12, 18–29. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. JACC 2022, 79, e263–e421. [Google Scholar] [CrossRef]
- Rajiah, P.S.; François, C.J.; Leiner, T. Cardiac MRI: State of the Art. Radiology 2023, 307, e223008. [Google Scholar] [CrossRef]
- Beijnink, C.W.H.; van der Hoeven, N.W.; Konijnenberg, L.S.F.; Kim, R.J.; Bekkers, S.C.A.M.; Kloner, R.A.; Everaars, H.; El Messaoudi, S.; van Rossum, A.C.; van Royen, N.; et al. Cardiac MRI to Visualize Myocardial Damage after ST-Segment Elevation Myocardial Infarction: A Review of Its Histologic Validation. Radiology 2021, 301, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Edvardsen, T.; Asch, F.M.; Davidson, B.; Delgado, V.; DeMaria, A.; Dilsizian, V.; Gaemperli, O.; Garcia, M.J.; Kamp, O.; Lee, D.C.; et al. Non-invasive imaging in coronary syndromes: Recommendations of the European Association of Cardiovascular Imaging and the American Society of Echocardiography, in collaboration with the American Society of Nuclear Cardiology, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e6–e33. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction. Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Jada, L.; Holtackers, R.J.; Martens, B.; Nies, H.M.J.M.; Van De Heyning, C.M.; Botnar, R.M.; Wildberger, J.E.; Ismail, T.F.; Razavi, R.; Chiribiri, A. Quantification of myocardial scar of different etiology using dark- and bright-blood late gadolinium enhancement cardiovascular magnetic resonance. Sci. Rep. 2024, 14, 5395. [Google Scholar] [CrossRef]
- Schieda, N.; Blaichman, J.I.; Costa, A.F.; Glikstein, R.; Hurrell, C.; James, M.; Maralani, P.J.; Shabana, W.; Tang, A.; Tsampalieros, A.; et al. Gadolinium-Based Contrast Agents in Kidney Disease: A Comprehensive Review and Clinical Practice Guideline Issued by the Canadian Association of Radiologists. Can. J. Kidney Health Dis. 2018, 5, 1–17. [Google Scholar] [CrossRef]
- Polacin, M.; Karolyi, M.; Eberhard, M.; Gotschy, A.; Baessler, B.; Alkadhi, H.; Kozerke, S.; Manka, R. Segmental strain analysis for the detection of chronic ischemic scars in non-contrast cardiac MRI cine images. Sci. Rep. 2021, 11, 12376. [Google Scholar] [CrossRef] [PubMed]
- Lenkinski, R.E.; Rofsky, N.M. Contrast Media–driven Anthropogenic Gadolinium: Knowns and Unknowns. Radiology 2024, 312, e240020. [Google Scholar] [CrossRef]
- Shu, H.; Xu, H.; Pan, Z.; Liu, Y.; Deng, W.; Zhao, R.; Sun, Y.; Wang, Z.; Yang, J.; Gao, H.; et al. Early detection of myocardial involvement by non-contrast T1ρ mapping of cardiac magnetic resonance in type 2 diabetes mellitus. Front. Endocrinol. 2024, 15, 1335899. [Google Scholar] [CrossRef]
- Zhu, H.; Xie, K.; Qian, Y.; Zou, Z.; Jiang, M.; Pu, J. Recent Progresses in the Multimodality Imaging Assessment of Myocardial Fibrosis. Rev. Cardiovasc. Med. 2024, 25, 5. [Google Scholar] [CrossRef] [PubMed]
- Raisi-Estabragh, Z.; Izquierdo, C.; Campello, V.M.; Martin-Isla, C.; Jaggi, A.; Harvey, N.C.; Lekadir, K.; Petersen, S.E. Cardiac magnetic resonance radiomics: Basic principles and clinical perspectives. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 349–356. [Google Scholar] [CrossRef]
- Baeßler, B.; Engelhardt, S.; Hekalo, A.; Hennemuth, A.; Hüllebrand, M.; Laube, A.; Scherer, C.; Tölle, M.; Wech, T. Perfect Match: Radiomics and Artificial Intelligence in Cardiac Imaging. Circ. Cardiovasc. Imaging 2024, 17, e015490. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, C.; Zhao, S.; Xie, L.; Tian, Y. Cardiac magnetic resonance radiomics for disease classification. Eur. Radiol. 2023, 33, 2312–2323. [Google Scholar] [CrossRef]
- Avard, E.; Shiri, I.; Hajianfar, G.; Abdollahi, H.; Kalantari, K.R.; Houshmand, G.; Kasani, K.; Bitarafan-Rajabi, A.; Deevband, M.R.; Oveisi, M.; et al. Non-contrast Cine Cardiac Magnetic Resonance image radiomics features and machine learning algorithms for myocardial infarction detection. Comput. Biol. Med. 2022, 141, 105145. [Google Scholar] [CrossRef] [PubMed]
- Baessler, B.; Mannil, M.; Oebel, S.; Maintz, D.; Alkadhi, H.; Manka, R. Subacute and Chronic Left Ventricular Myocardial Scar: Accuracy of Texture Analysis on Nonenhanced Cine MR Images. Radiology 2018, 286, 103–112. [Google Scholar] [CrossRef]
- Fahmy, A.S.; Rowin, E.J.; Arafati, A.; Al-Otaibi, T.; Maron, M.S.; Nezafat, R. Radiomics and deep learning for myocardial scar screening in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2022, 24, 40. [Google Scholar] [CrossRef]
- Lasode, J.; Chantaksinopas, W.; Khongwirotphan, S.; Chattranukulchai, P.; Vorasettakarnkij, Y.; Sriswasdi, S.; Tumkosit, M.; Rakvongthai, Y. Radiomics for differential diagnosis of ischemic and dilated cardiomyopathy using non-contrast-enhanced cine cardiac magnetic resonance imaging. Radiol. Med. 2025, 130, 650–661. [Google Scholar] [CrossRef]
- Pu, C.; Hu, X.; Lv, S.; Wu, Y.; Yu, F.; Zhu, W.; Zhang, L.; Fei, J.; He, C.; Ling, X.; et al. Identification of fibrosis in hypertrophic cardiomyopathy: A radiomic study on cardiac magnetic resonance cine imaging. Eur. Radiol. 2023, 33, 2301–2311. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W65–W94. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.; Ding, H.; Adedeji, F.; Qin, C.; Obungoloch, J.; Asllani, I.; Anazodo, U.; Ntusi, N.A.B.; Mammen, R.; Niendorf, T.; et al. Bringing MRI to low- and middle-income countries: Directions, challenges and potential solutions. NMR Biomed. 2024, 37, e4992. [Google Scholar] [CrossRef] [PubMed]
- Bendszus, M.; Laghi, A.; Munuera, J.; Tanenbaum, L.N.; Taouli, B.; Thoeny, H.C. MRI Gadolinium-Based Contrast Media: Meeting Radiological, Clinical, and Environmental Needs. J. Magn. Reson. Imaging 2024, 60, 1774–1785. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).