Achilles and the Tortoise: Rethinking Evidence Generation in Cardiovascular Surgery and Interventional Cardiology
Abstract
1. Introduction
2. Materials and Methods
3. Results
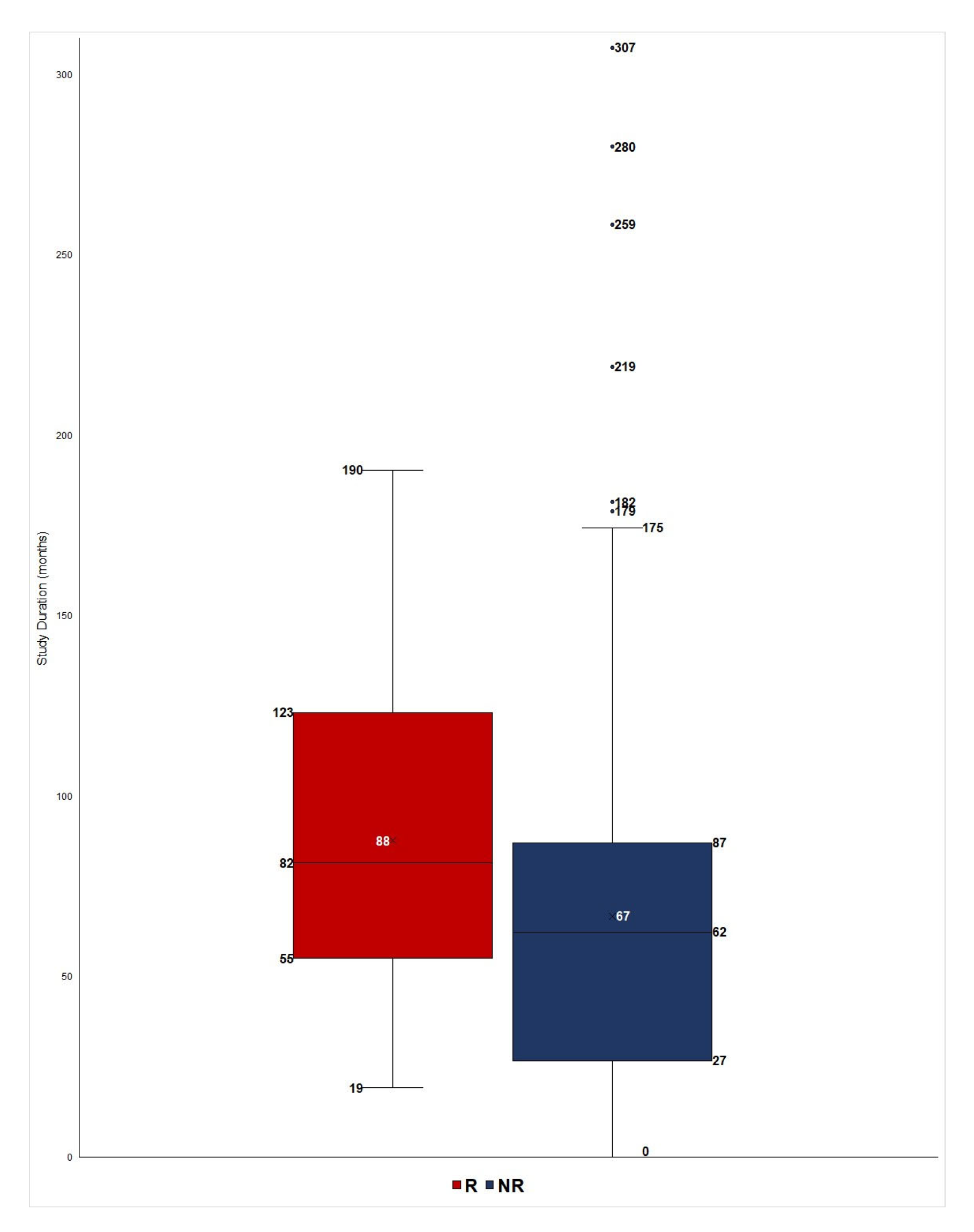
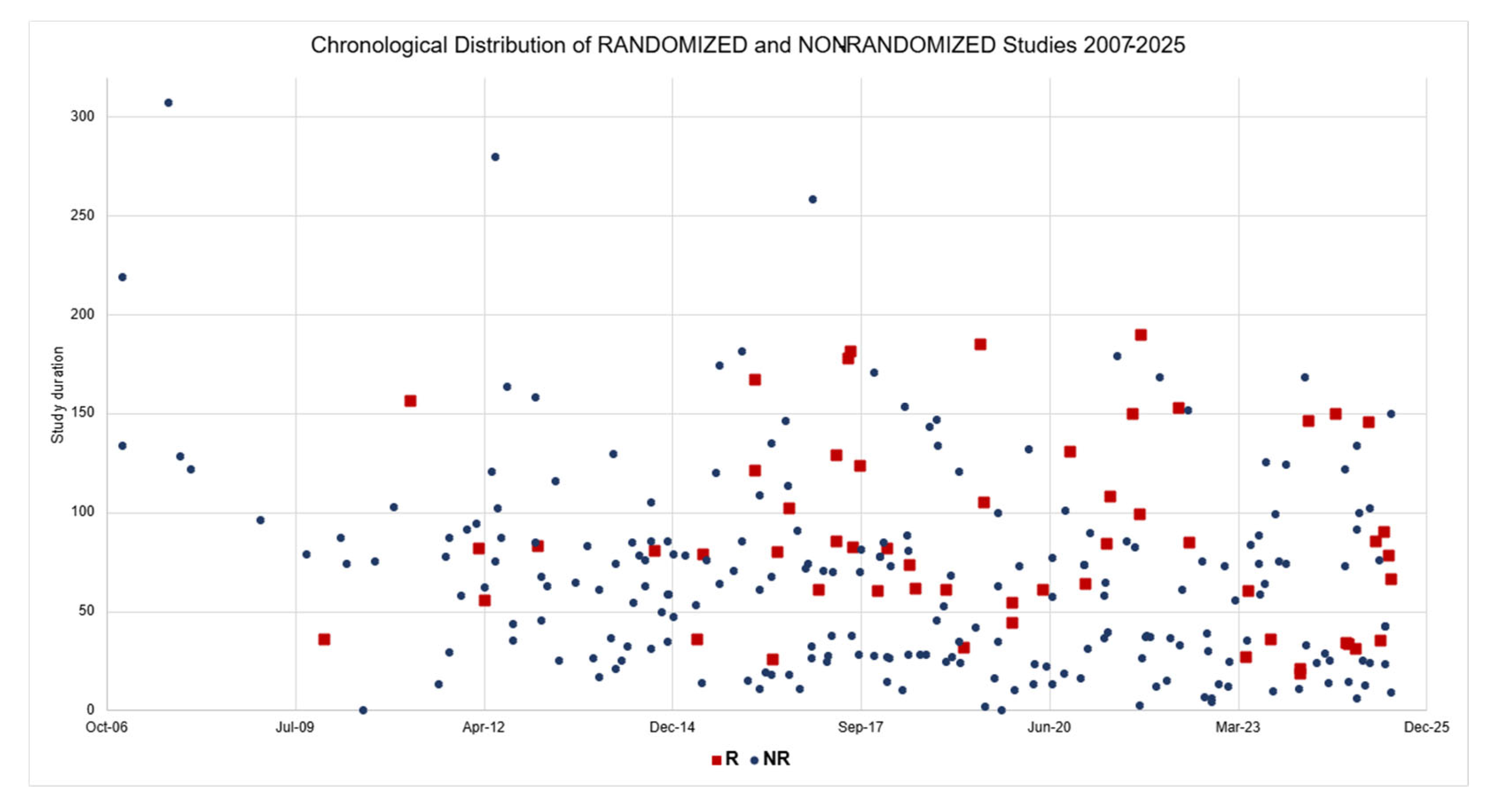
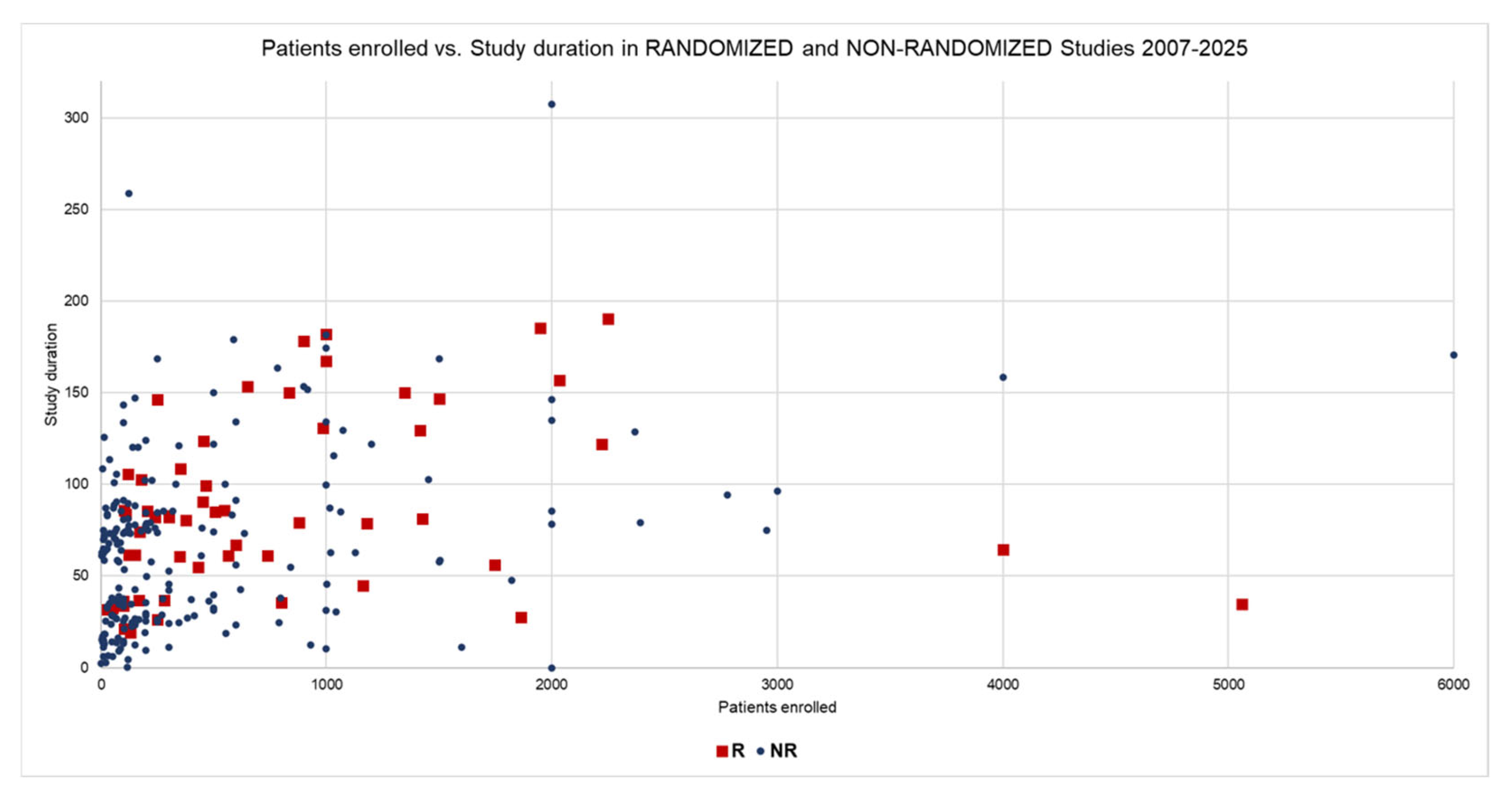
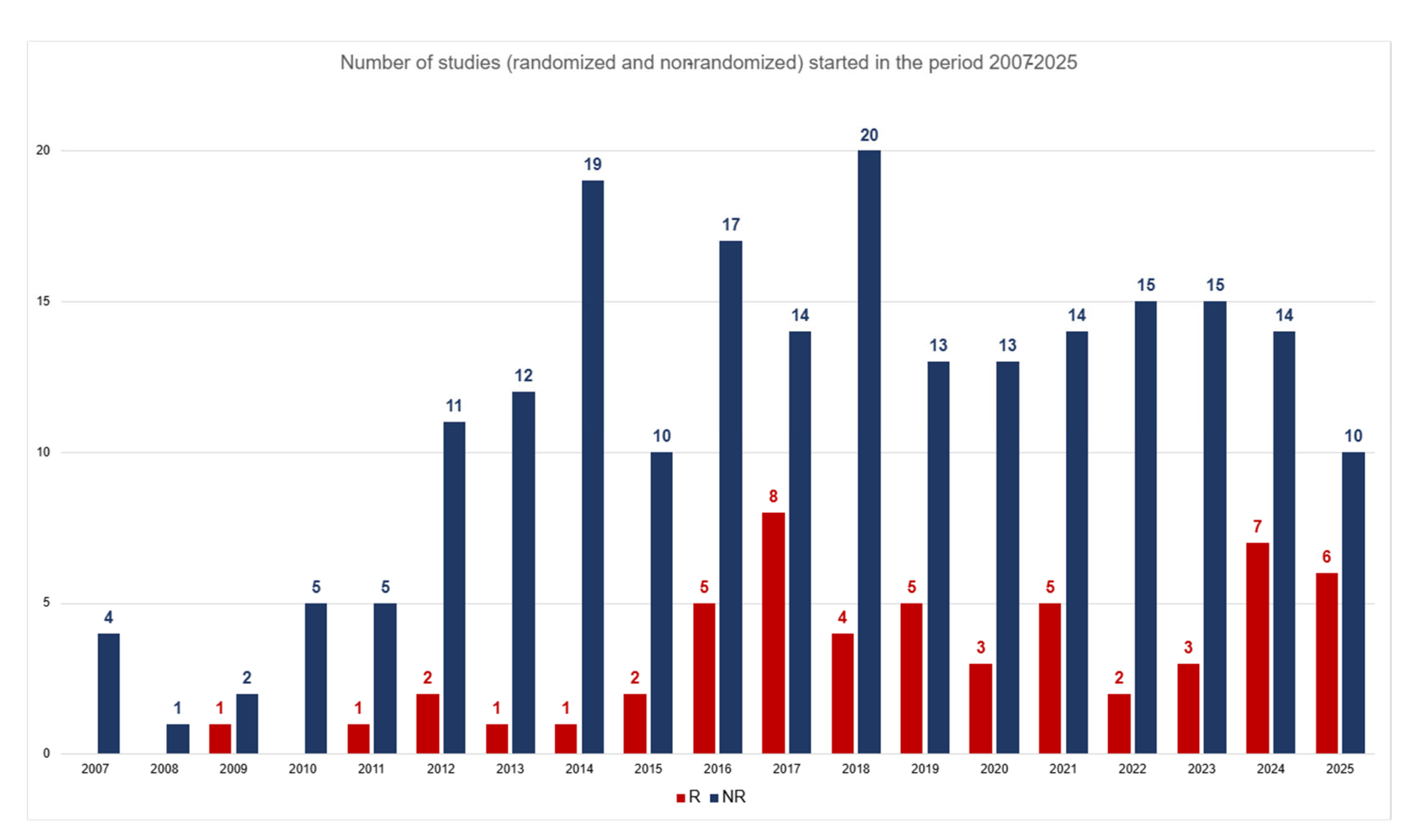
3.1. Analysis 1
3.2. Analysis 2
4. Discussion
4.1. The Structural Drivers of the Evidence-Validation Gap
- (a)
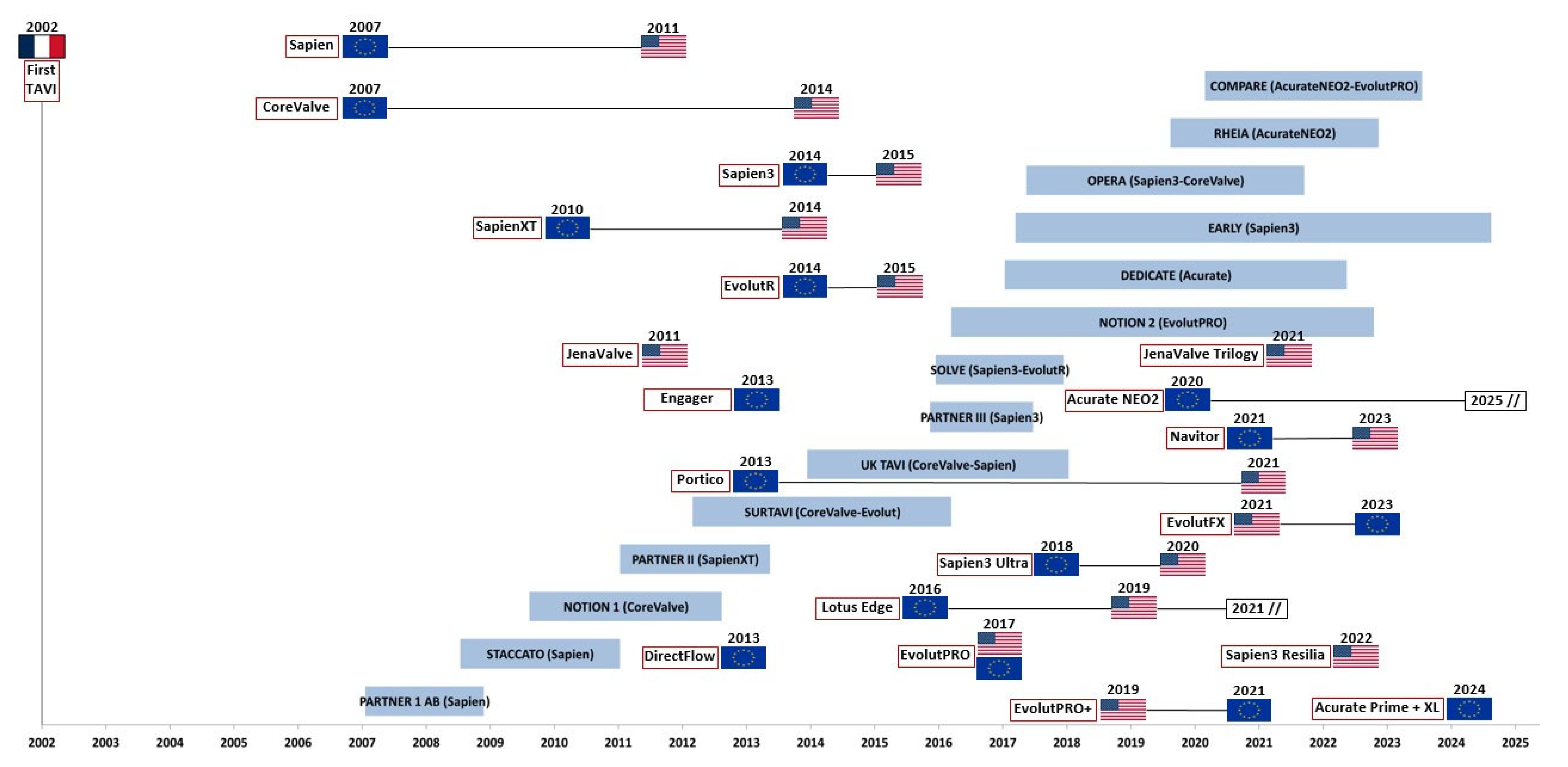
- The Regulatory and Commercial Timeline: The current regulatory pathways for medical devices, such as the FDA’s 510(k) and the CE marking process, allow new valve iterations to be approved based on substantial equivalence to a predicate device and limited new clinical data. This system permits—and commercial incentives encourage—the market release of new models long before the long-term data for their predecessors has matured. Our timeline (Figure 1) visually captures this phenomenon. Figure 1 is primarily descriptive and historical and serves to illustrate this structural “leapfrogging” problem, not to prove clinical harm which, if present, is already contemplated and reported in the intermediate results of the studies themselves.
- (b)
- The Methodological Lag of Traditional RCTs: Conventional RCTs, the gold standard for establishing efficacy, are inherently slow, costly, and ill-suited for a field with rapid technological iteration. By the time a definitive RCT reaches its primary endpoint, the device it studied may no longer be the state-of-the-art.
- (c)
- The Fundamental Constraint of Long-Term Assessment: The topic is complex also because the validation of a new prosthesis depends not only on immediate data (mortality, failure, complications) but above all on the long-term durability of the bioprostheses. First, in terms of immediate results, TAVI must compete with the mortality rate of surgical series, which, in centers of excellence, is very close to zero. Then, regarding durability, this figure is obtained with a follow-up of at least 10 years. Therefore, a follow-up period of at least this order of magnitude must be added to the duration of a study’s enrollment phase. Consequently, making a conclusive judgment on a prosthesis’ performance requires an extremely long time compared to the patient’s care needs. In this respect too, TAVI must compete with the durability of surgically implanted bioprostheses, which already have a well-established follow-up. This requirement “seizes” a significant number of patients for a long time from the real world where subsequent models are introduced. Even comparison of these patients with those enrolled in subsequent studies is limited by the poor reliability of matching.
4.2. The Evolving Role of Randomized and Non-Randomized Evidence
4.3. Existing and Proposed Frameworks for Adaptive Evidence Generation
4.4. A Comprehensive Analysis of the Variables—Evidence Analytics and Precision Medicine
5. Limitations
6. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RCTs | Randomized clinical trials |
| OS | Observational Studies |
| TAVI | Transcatheter aortic valve implantation |
| SVR | Surgical (aortic) valve replacement |
| AI | Artificial intelligence |
| R | Randomized studies |
| NR | Non-randomized studies |
References
- Internet Encyclopedia of Philosophy. Zeno’s Paradoxes. Available online: https://iep.utm.edu/zenos-paradoxes/ (accessed on 25 March 2024).
- Sattler, B.M. VI—Paradoxes as Philosophical Method and Their Zenonian Origins. Proc. Aristot. Soc. 2021, 121, 153–181. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.H.; Klaaborg, K.E.; Nissen, H.; Terp, K.; Mortensen, P.E.; Kjeldsen, B.J.; Jakobsen, C.J.; Andersen, H.R.; Egeblad, H.; Krusell, L.R.; et al. A prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: The STACCATO trial. EuroIntervention 2012, 8, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Hørsted Thyregod, H.G.; Højsgaard Jørgensen, T.; Ihlemann, N.; Steinbrüchel, D.A.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; De Backer, O.; Olsen, P.S.; Søndergaard, L. Transcatheter or surgical aortic valve implantation: 10-year outcomes of the NOTION trial. Eur. Heart J. 2024, 45, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Vergallo, R.; Volpe, M. The UK TAVI trial: An independent, pragmatic study extending the evidence for the treatment of symptomatic severe aortic stenosis. Eur. Heart J. 2022, 43, 2919–2920. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Thiele, H.; Kurz, T.; Feistritzer, H.J.; Stachel, G.; Hartung, P.; Eitel, I.; Marquetand, C.; Nef, H.; Doerr, O.; Lauten, A.; et al. Comparison of newer generation self-expandable vs. balloon-expandable valves in transcatheter aortic valve implantation: The randomized SOLVE-TAVI trial. Eur. Heart J. 2020, 41, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, T.H.; Thyregod, H.G.H.; Savontaus, M.; Willemen, Y.; Bleie, Ø.; Tang, M.; Niemela, M.; Angerås, O.; Gudmundsdóttir, I.J.; Sartipy, U.; et al. Transcatheter aortic valve implantation in low-risk tricuspid or bicuspid aortic stenosis: The NOTION-2 trial. Eur. Heart J. 2024, 45, 3804–3814. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, M.; Walther, T.; Hamm, C.; Falk, V.; Frey, N.; Thiele, H.; Hagl, C.; Landmesser, U.; Borger, M.; Massberg, S.; et al. The DEDICATE Trial: An independent all-comers trial of transcatheter aortic valve implantation vs. surgical aortic valve replacement in patients at low to intermediate operative risk is recruiting patients. Eur. Heart J. 2019, 40, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Schwartz, A.; Oldemeyer, B.; Cohen, D.J.; Redfors, B.; Prince, H.; Zhao, Y.; Lindman, B.R.; Pibarot, P.; Leon, M.B. Design and rationale of the evaluation of transcatheter aortic valve replacement compared to surveillance for patients with asymptomatic severe aortic stenosis: The EARLY TAVR trial. Am. Heart J. 2024, 268, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Saia, F.; Pilgrim, T.; Abdel-Wahab, M.; Garot, P.; Valvo, R.; Gandolfo, C.; Branca, L.; Latib, A.; Santos, I.A.; et al. Transcatheter Aortic Valve Replacement with the Latest-Iteration Self-Expanding or Balloon-Expandable Valves: The Multicenter OPERA-TAVI Registry. JACC Cardiovasc. Interv. 2022, 15, 2398–2407. [Google Scholar] [CrossRef] [PubMed]
- Tchetche, D.; Pibarot, P.; Bax, J.J.; Bonaros, N.; Windecker, S.; Dumonteil, N.; Nietlispach, F.; Messika-Zeitoun, D.; Pocock, S.J.; Berthoumieu, P.; et al. Transcatheter vs. surgical aortic valve replacement in women: The RHEIA trial. Eur. Heart J. 2025, 46, 2079–2088. [Google Scholar] [CrossRef]
- Terkelsen, C.J.; Thim, T.; Freeman, P.; Dahl, J.S.; Nørgaard, B.L.; Kim, W.Y.; Tang, M.; Sørensen, H.T.; Christiansen, E.H.; Nissen, H. Randomized comparison of TAVI valves: The Compare-TAVI trial. Am. Heart J. 2024, 274, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Writing Group for the Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Five-year clinical functional outcome comparing bypass surgery angioplasty in patients with multivessel coronary disease Amulticenter randomized trial. JAMA 1997, 277, 715–721, PMID: 9042843. [Google Scholar] [CrossRef]
- Hannan, E.L.; Racz, M.J.; Walford, G.; Jones, R.H.; Ryan, T.J.; Bennett, E.V.; Culliford, A.T.; Isom, O.W.; Gold, J.P.; Rose, E.A. Long-Term Outcomes of Coronary-Artery Bypass Grafting versus Stent Implantation. N. Engl. J. Med. 2005, 352, 2174–2183. [Google Scholar] [CrossRef]
- Serruys, P.W.; Morice, M.C.; Kappetein, A.P.; Colombo, A.; Holmes, D.R.; Mack, M.J.; Ståhle, E.; Feldman, T.E.; van den Brand, M.; Bass, E.J.; et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 2009, 360, 961–972. [Google Scholar] [CrossRef]
- Stone, G.W.; Sabik, J.F.; Serruys, P.W.; Simonton, C.A.; Généreux, P.; Puskas, J.; Kandzari, D.E.; Morice, M.C.; Lembo, N.; Brown, W.M., III; et al. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N. Engl. J. Med. 2016, 375, 2223–2235. [Google Scholar] [CrossRef]
- Stone, G.W.; Sabik, J.F.; Serruys, P.W.; Simonton, C.A.; Généreux, P.; Puskas, J.; Kandzari, D.E.; Morice, M.C.; Lembo, N.; Brown, W.M., 3rd; et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE). Lancet 2016, 388, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Farkouh, M.E.; Domanski, M.; Sleeper, L.A.; Siami, F.S.; Dangas, G.; Mack, M.; Yang, M.; Cohen, D.J.; Rosenberg, Y.; Solomon, S.D.; et al. Strategies for multivessel revascularization in patients with diabetes. N. Engl. J. Med. 2012, 367, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Kappetein, A.P.; Sabik, J.F.; Pocock, S.J.; Morice, M.C.; Puskas, J.; Kandzari, D.E.; Karmpaliotis, D.; Brown WM3rd Lembo, N.J.; Banning, A.; et al. Five-Year Outcomes after PCI or CABG for Left Main Coronary Disease. N. Engl. J. Med. 2019, 381, 1820–1830. [Google Scholar] [CrossRef]
- Feldman, T.; Kar, S.; Elmariah, S.; Smart, S.C.; Trento, A.; Siegel, R.J.; Apruzzese, P.; Fail, P.; Rinaldi, M.J.; Smalling, R.W.; et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J. Am. Coll. Cardiol. 2015, 66, 2844–2854. [Google Scholar] [CrossRef]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter mitral-valve repair in patients with heart failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef]
- Stone, G.W.; Abraham, W.T.; Lindenfeld, J.; Kar, S.; Grayburn, P.A.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Rinaldi, M.; Kapadia, S.R.; et al. Five-year follow-up after transcatheter repair of secondary mitral regurgitation. N. Engl. J. Med. 2023, 388, 1509–1519. [Google Scholar] [CrossRef]
- Lim, D.S.; Smith, R.L.; Gillam, L.D.; Zahr, F.; Chadderdon, S.; Makkar, R.; von Bardeleben, R.S.; Kipperman, R.M.; Rassi, A.N.; Szerlip, M.; et al. Randomized Comparison of Transcatheter Edge-to-Edge Repair for Degenerative Mitral Regurgitation in Prohibitive Surgical Risk Patients. JACC Cardiovasc. Interv. 2022, 15, 2523–2536. [Google Scholar] [CrossRef] [PubMed]
- Obadia, J.F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef]
- Deharo, P.; Obadia, J.F.; Guerin, P.; Cuisset, T.; Avierinos, J.F.; Habib, G.; Torras, O.; Bisson, A.; Vigny, P.; Etienne, C.S.; et al. Mitral transcatheter edge to edge repair versus isolated mitral surgery for severe mitral regurgitation: A French nationwide study. Eur. Heart J. 2024, 45, 940–949. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H. Transcatheter Aortic Valve Implantation: Two Decades of a Revolutionary and Ongoing Odyssey. Circulation 2024, 150, 821–822. [Google Scholar] [CrossRef] [PubMed]
- Figulla, H.R.; Franz, M.; Lauten, A. The History of Transcatheter Aortic Valve Implantation (TAVI)—A Personal View over 25 Years of Development. Cardiovasc. Revasc Med. 2020, 21, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Naser, N. Transcatheter Aortic Valve Implantation Two Decades of Evolution—TAVI From Current Perspective. Acta Inform. Med. 2023, 31, 312–321. [Google Scholar] [CrossRef]
- Bourantas, C.V.; Serruys, P.W. Evolution of Transcatheter Aortic Valve Replacement. Circ. Res. 2014, 114, 1037–1051. [Google Scholar] [CrossRef]
- Angioletti, C.; Moretti, G.; Manetti, S.; Pastormerlo, L.; Vainieri, M.; Passino, C. The evolution of TAVI performance overtime: An overview of systematic reviews. BMC Cardiovasc. Disord. 2024, 24, 314. [Google Scholar] [CrossRef]
- Andersen, H.R. How Transcatheter Aortic Valve Implantation (TAVI) Was Born: The Struggle for a New Invention. Front. Cardiovasc. Med. 2021, 8, 722693. [Google Scholar] [CrossRef] [PubMed]
- Bourantas, C.V.; Modolo, R.; Baumbach, A.; Søndergaard, L.; Prendergast, B.; Ozkor, M.; Kennon, S.; Mathur, A.; Mullen, M.J.; Serruys, P.W. The evolution of device technology in transcatheter aortic valve implantation. EuroIntervention 2019, 14, e1826–e1833. [Google Scholar] [CrossRef]
- Juarez-Casso, F.M.; Crestanello, J.A. The Evolving Role of Surgical Aortic Valve Replacement in the Era of Transcatheter Valvular Procedures. J. Clin. Med. 2023, 12, 5299. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S. Randomized Clinical Trials and Observational Studies Are More Often Alike Than Unlike. JAMA Intern. Med. 2014, 174, 1557. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.; Tannock, I. Randomised controlled trials and population-based observational research: Partners in the evolution of medical evidence. Br. J. Cancer 2014, 110, 551–555. [Google Scholar] [CrossRef]
- Fernainy, P.; Cohen, A.A.; Murray, E.; Losina, E.; Lamontagne, F.; Sourial, N. Rethinking the pros and cons of randomized controlled trials and observational studies in the era of big data and advanced methods: A panel discussion. BMC Proc 2024, 18 (Suppl. S2), 1. [Google Scholar] [CrossRef]
- Kjell Benson, B.A.; Arthur, J.; Hartz, M.D. A Comparison of Observational Studies and Randomized, Controlled Trials. N. Engl. J. Med. 2000, 342, 1878–1886. [Google Scholar] [CrossRef]
- Ceelen, W.; Soreide, K. Randomized controlled trials and alternative study designs in surgical oncology. Eur. J. Surg. Oncol. 2023, 49, 1331–1340. [Google Scholar] [CrossRef]
- Hannan, E.L. Randomized Clinical Trials and Observational Studies: Guidelines for Assessing Respective Strengths and Limitations. JACC Cardiovasc. Interv. 2008, 1, 211–217. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Concato, J.; Shah, N.; Horwitz, R.I. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N. Engl. J. Med. 2000, 342, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-world evidence—What is it and what can it tell us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Sa, Y.K.; Hwang, B.H.; Chung, W.B.; Lee, K.Y.; Lee, J.; Kang, D.; Ko, Y.G.; Yu, C.W.; Kim, J.; Choi, S.H.; et al. Real-World Comparison of Transcatheter Versus Surgical Aortic Valve Replacement in the Era of Current-Generation Devices. J. Clin. Med. 2023, 12, 571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hannan, E.L.; Samadashvili, Z.; Jordan, D.; Sundt, T.M., 3rd; Stamato, N.J.; Lahey, S.J.; Gold, J.P.; Wechsler, A.; Ashraf, M.H.; Ruiz, C.; et al. Thirty-Day Readmissions After Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis in New York State. Circ. Cardiovasc. Interv. 2015, 8, e002744. [Google Scholar] [CrossRef] [PubMed]
- Leha, A.; Huber, C.; Friede, T.; Bauer, T.; Beckmann, A.; Bekeredjian, R.; Bleiziffer, S.; Herrmann, E.; Möllmann, H.; Thomas Walther, T.; et al. Development and validation of explainable machine learning models for risk of mortality in transcatheter aortic valve implantation: TAVI risk machine scores. Eur. Heart J. Digit. Health 2023, 4, 225–235. [Google Scholar] [CrossRef]
- Ludman, P.F. The UK transcatheter aortic valve implantation registry; one of the suite of registries hosted by the National Institute for Cardiovascular Outcomes Research (NICOR). Heart 2012, 98, 1787–1789. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef] [PubMed]
- Hamm, C.W.; Möllmann, H.; Holzhey, D.; Beckmann, A.; Veit, C.; Figulla, H.R.; Cremer, J.; Kuck, K.H.; Lange, R.; Zahn, R.; et al. The German Aortic Valve Registry (GARY): In-hospital outcome. Eur. Heart J. 2014, 35, 1588–1598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kambhampati, N.T.; Ahamed, H.; Velayudhan, K.K.; David, S.; Chandra Hakeem, S.; Pillai, G.; Kartha, N. Cytochrome P450 2C19 Polymorphisms and Its Association with Major Adverse Cardiac Events in Post-coronary Intervention Patients on Clopidogrel in the Tertiary Care Center. Cureus 2023, 15, e34737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Veradigm, LLC. Veradigm Real-World Evidence (RWE) Analytics Platform. Veradigm. 2023. Available online: https://www.veradigm.com/real-world-evidence (accessed on 26 October 2023).
- Birnbaum, B.; Nussbaum, N.; Seidl-Rathkopf, K.; Agrawal, M.; Estevez, M.; Estola, E.; Haimson, J.; He, L.; Larson, B.; Richardson, M.; et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv 2021, arXiv:2102.07271. [Google Scholar]
- Liu, F.; Panagiotakos, D. Real-world data: A brief review of the methods, applications, challenges and opportunities. BMC Med. Res. Methodol. 2022, 22, 287. [Google Scholar] [CrossRef]
- Aikawa, M.; Sonawane, A.R.; Chelvanambi, S.; Asano, T.; Halu, A.; Matamalas, J.T.; Singh, S.A.; Uchida, S.; Aikawa, E.; Arenas, A.; et al. Precision cardiovascular medicine: Shifting the innovation paradigm. Front. Sci. 2025, 3, 1474469. [Google Scholar] [CrossRef]
| Data | Group R | Group NR | t-Test | Mann–Whitney |
|---|---|---|---|---|
| Duration (Mean) 1 | 87.7 | 66.1 | 0.0006 | 0.0001 |
| Duration (Median) | 81.6 | 62.1 | ||
| Enrolled Patients (Mean) | 814.9 | 689.4 | 0.83 | 0.0002 |
| Enrolled Patients (Median) | 460.0 | 150.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirillo, M. Achilles and the Tortoise: Rethinking Evidence Generation in Cardiovascular Surgery and Interventional Cardiology. Hearts 2025, 6, 28. https://doi.org/10.3390/hearts6040028
Cirillo M. Achilles and the Tortoise: Rethinking Evidence Generation in Cardiovascular Surgery and Interventional Cardiology. Hearts. 2025; 6(4):28. https://doi.org/10.3390/hearts6040028
Chicago/Turabian StyleCirillo, Marco. 2025. "Achilles and the Tortoise: Rethinking Evidence Generation in Cardiovascular Surgery and Interventional Cardiology" Hearts 6, no. 4: 28. https://doi.org/10.3390/hearts6040028
APA StyleCirillo, M. (2025). Achilles and the Tortoise: Rethinking Evidence Generation in Cardiovascular Surgery and Interventional Cardiology. Hearts, 6(4), 28. https://doi.org/10.3390/hearts6040028







