Abstract
Amino acids are not just monomers of proteins, but they can also carry biological functions. L-cysteine (Cys), L-proline (Pro), L-asparagine (Asn), and L-glutamic acid (Glu) were used to evaluate how different amino acid chemistries alter the morphology and size of the silver nanoparticles (AgNPs) synthesized in the presence of two carbohydrate ligands, which were lactose methoxyaniline (LMA) and galactose 5-aminosalicylic acid (G5AS). UV–vis, infrared (IR), High-Resolution Transmission Electron Microscopy (HR-TEM) and X-ray diffraction (XRD) characterizations revealed that the effect of amino acids on the characteristics of the AgNPs showed dependence on the carbohydrate ligand chemistry. In the case of LMA, AgNPs shifted from aggregates to anisotropic nanoparticles, larger aggregates, and a mixture of anisotropic and 1D nanoparticles in the presence of Cys, Glu, Asn and Pro amino acids, respectively. In contrast to this, the introduction of Cys and Asn caused the formation of cluster-like AgNPs and larger rounded nanoparticles, while G5AS-synthesized AgNPs were multigonal 0D particles. Moreover, Glu and Pro contributed the resistance of silver oxide formation on the particles. Antibacterial characterization showed that LMA_Glu_AgNPs were the most effective ones, while LMA_Cys_AgNPs and G5AS_Cys_AgNPs, which were the smallest AgNPs, did not show any significant antibacterial activity.
1. Introduction
Amino acids functionally contain a primary amino group (-NH2), a carboxyl group (COOH), and some of them contain thiol (-SH), hydroxyl (-OH), and some other special groups [1]. Due to their carboxyl and amino groups, they have been used in the synthesis of amphiphilic compounds for biomedical applications. These applications include antimicrobial, anticancer, and pH-dependent release of drugs [2]. Since the direct use of amino acids in AgNP synthesis is difficult, external reducing agents are included in the reaction medium. Potassium hydroxide [1] and light [3,4] are widely used as co-reducing agents. Unlike AgNP synthesis, gold nanoparticles can be produced by heat treatment (e.g., 80 °C) as an alternative to external reducing [5]. In a study, 21 amino acids were tested; only histidine, cystine, methionine, tyrosine, and tryptophan allowed AgNP synthesis when light was used as the power source [4]. In addition to natural amino acids, N-cholyl amino acid derivatives (e.g., N-cholyl L-tryptophan, N-cholyl L-valine, etc.) were also used in AgNPs synthesis [3]. Furthermore, amino acids have also been reported to be used in the modification of AgNP surface chemistry to enhance antibacterial properties [6] and improve the antifouling capacity of nanocomposites [7]. Similarly, amino acid modification enhanced the release of Ag+ ions from citrate-synthesized AgNPs, thereby increasing the antibacterial capacity [8]. Similarly, amino acids have been used to improve the surface chemistry of gold nanoparticles in order to increase gene release and the interaction with DNA [9,10]. In addition to the gold and silver nanoparticles, they have also been reported to be used in the synthesis of selenium particles [5,11,12]. In addition to the metallic nanoparticles, the synthesis of oxide nanoparticles (inorganic nanoparticles) such as titanium oxide (TiO2) [13] and iron oxide (IONP) [14,15] can be achieved using amino acids.
Silver nanoparticles are of interest for biological and biomedical applications. In particular, they have the potential to combat bacterial strains possessing multidrug resistance [16]. Therefore, AgNPs have been widely studied for their reproducible synthesis, biocompatibility, and ability to be synthesized without the use of toxic agents (e.g., even cow urine was reported [17]), and high bioactivity (e.g., high bactericidal activity at low concentrations) [18]. In this study, we used sugar ligands as the reducing agent in the synthesis of AgNPs, where amino acids played the morphology and crystal structure directing agents. The findings showed that amino acids can alter AgNPs’ morphology and the presence of silver oxide (Ag2O) abundance. Antibacterial studies showed that the altered morphology and crystallography in response to the used amino acids and sugar ligands affected the antibacterial activity. Further optimizations are needed to fine-tune the morphology and crystallography of the AgNPs to enhance possible antibacterial activity.
2. Materials and Methods
2.1. Materials
L-Glutamic acid (≥99.5%), L-proline (≥99.5%), L-cysteine (≥99.5%), L-asparagine (≥99.5%), 5-amino salicylic acid (95%), 4-methoxyaniline (99%), acetic acid (glacial, ReagentPlus®, ≥99%), ethanol (≥99.5%, ACS reagent, 200 proof), acetone (ACS reagent, ≥99.5%), D-galactose (BioXtra, ≥99% (HPLC)), β-d-lactose monohydrate (≥99% (HPLC), BioUltra), α-D-glucose (anhydrous, 96%), crystal violet (≥90% purity anhydrous basis, ACS reagent, powder), agar (powder, suitable for microbiology), 96-well plate (P6991), and meat extract (quality level 200) were purchased from Sigma-Aldrich. Pure water was produced in the lab using a Humana Zeneer pure water instrument. The TEM grids were 300M Lacey Carbon (Cat. Nu 01895-F, TedPella, Redding, CA, USA). Lactose methoxyaniline (LMA) and galactose 5-aminosaliyclic acid (G5AS) were previously synthesized as described in the literature [19,20].
2.2. Synthesis and Characterization of the Amino Acid-Conjugated Silver Nanoparticles
The stock solutions were 9 mM, 50 mg/mL, and 100 mg/mL for amino acids, sugar ligands, and silver nitrate (AgNO3), respectively. In the case of the sugar ligand-synthesized AgNPs, 200 μL of the stock sugar ligand and AgNO3 stocks were added into 4.6 mL of deionized water in polypropylene sterile tubes. Amino acid-supported AgNPs were prepared using 4.6 mL, 200 μL, and 200 μL from the stocks of amino acids, sugar ligands, and AgNO3, respectively.
UV–vis spectroscopy was used for surface plasmon resonance (SPR) characterization [21] in the range of 350–900 nm (MutiskunTM Go, Thermo Fischer Scientific, Waltham, MA, USA). Morphological characterizations were performed using High-Resolution Transmission Electron Microscopy (TEM, FEI TALOS F200S TEM 200 kV, Hillsboro, OR, USA), and X-ray diffraction (XRD, Bruker D8 Advance, Berlin, Germany) studies were performed for crystallography characterization. The protein corona formation test was performed to reveal the stability of the AgNPs under rich protein environments [22]. A total of 10 μL AgNPs from the prepared medium was placed in a 96-well plate, and 90 μL of 10 g/L meat extract containing 2 g/L D-glucose (pH~7.4) was added on top of the AgNPs in the wells. After six hours incubation at 37 °C, SPR patterns were characterized using UV–vis spectroscopy.
2.3. Antibacterial Activity Performance of the Synthesized Nanoparticles
Antibacterial characterizations of the AgNPs were performed by minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and total biofilm formation tests as explained in the literature [20]. In the study, multidrug-resistant gram (+) E. casseliflavus, S. epidermidis, E. faecialis, and S. aureus, and gram (−) E. coli and P. aeruginosa species were used. The nanoparticles and silver nitrate were mixed with the growth media for two hours prior to bacteria inoculation in order to provide enough time for media and agent interaction.
3. Results and Discussion
3.1. UV–Vis Characterizations
Sugar ligands were shown to be effective in metallic nanoparticle synthesis, including AgNPs [23]. Hereby, we chose to use cysteine, glutamic acid, proline, and asparagine amino acids to delve into the effect of amino acid chemistry on the AgNPs morphology. The amino acids were selected randomly since none of them allowed for the synthesis of stable AgNPs at a decent level in the pre-tests. In the first step, 50 μL of AgNO3 (stock 100 mg/mL) was added into the reaction medium containing 200 μL sugar ligand, 4.6 mL amino acid, and 200 μL deionized water. G5AS and LMA sugar ligands promoted the synthesis of AgNPs instantly. Proline and asparagine did not show any inhibiting effect on the AgNPs initiated by G5AS and LMA, where glutamic acid also did not prevent AgNPs formation in the presence of G5AS ligand as well. However, cysteine impeded AgNP formation for at least 16 h.
The λmax of the SPR bands were 525 nm for G5AS_AgNPs, 510 and 600 nm for G5AS_Cys_AgNPs, while 535 nm for G5AS_Pro_AgNPs, G5AS_Glu_AgNPs, and G5AS_Asn_AgNPs (Figure 1A). Interestingly, only G5AS_Cys_AgNPs gave two distinct SPR bands. In the case of LMA-synthesized AgNPs, the three peaks were observed at 465 nm, 510 nm, 585 nm for LMA_Pro_AgNPs, the three peaks were also obtained at 470, 510 and 585 for LMA_Asn_AgNPs, and 510 nm and 565 nm were there for LMA_Glu_AgNPs while there were no clear SPR bands for LMA_AgNP and LMA_Cys_AgNPs (Figure 1B).
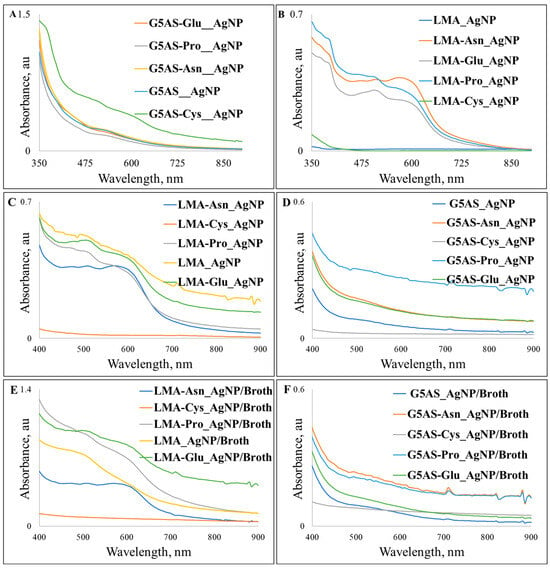
Figure 1.
UV–vis spectra of the silver nanoparticles synthesized in the presence of G5AS (A) and LMA (B) along with their storage stability (C,D) and protein corona characteristics (E,F).
The samples kept at room temperature for six months were tested for their stability (Figure 1C,D) while protein corona formation tests were performed for the two-day-old samples (Figure 1E,F). Agglomeration of the G5AS-synthesized AgNPs started within two months of the incubation, and the presence of larger aggregates was clear within 6 months, so sonication in a sonication bath for one minute was applied to all the samples (Heidolph™ Tuttnauer Clean and Simple™ Ultrasonic Bath, Wood Dale, IL, USA). The most dramatic change was observed for G5AS_Cys_AgNPs, which lost the inherent clear SPR bands, while G5AS_AgNPs, G5AS_Asn_AgNPs, and G5AS_Glu_AgNPs gave blue shifts to the 520, 515, and 515 nm wavelengths, respectively. In contrast to this, G5AS_Pro_AgNPs gave an extended SPR peak starting from 515 nm up to 600 nm in response to six months of storage. Protein corona formation tests revealed a 10 nm red shift was observed for G5AS_AgNPs (535 nm SPR peak), while G5AS_Pro_AgNPs, G5AS_Asn_AgNPs, and G5AS_Glu_AgNPs gave blue shifts to 520, 530, and 530 nm. G5AS_Cys_AgNPs gave only one peak at 530 nm upon protein corona formation. Among the LMA-synthesized AgNPs, only LMA_AgNPs gave a difference, as a clear λmax was observed at 600 nm for the incubated AgNPs, which was protected during protein corona formation. In the protein corona formation tests, λmax 470 nm in LMA_Pro_AgNPs became lost and λmax 590 and 585 nm shifted to 610 and 605 nm in LMA_Pro_AgNPs and LMA_Asn_AgNPs spectra, respectively. The literature reveals that proteins were shown to play critical roles in the stabilization of AgNPs [24], and can form multiple soft and hard layers on nanoparticles [25], which can easily cause red shifts in SPR bands of AgNPs [26,27]. In contrast to this, blue shifts upon protein corona formation were reported rarely in the literature [28], and it is possible that the observed blue shifts are related to the stabilization of the AgNPs, where proteins prevented further growth in the reaction tubes. Observation of the blue shifts could also refer to the alteration in size and/or morphology of the nanoparticles [29]. However, it should be noted that amino acid functionalization could also take part in protein–AgNPs interaction since different amino acid introductions caused distinct outcomes.
The formation of LMA_AgNPs aggregates may explain the absence of the SPR characteristic bands. This is because the individual LMA_AgNPs aggregate to form nanoclusters and have 310 × 375 nm cross-sections. However, LMA-Cys_AgNPs do not give characteristic SPR peaks, and their UV–vis spectra were very weak, maybe due to their very low concentrations during the measurements because the spherical and anisotropic particles below 50 nm are expected to give strong SPR peaks below 450 nm [30]. G5AS-Cys_AgNPs, on the other hand, showed strong SPR peaks, probably due to their higher density and G5AS sugar ligands. Morphologically, in addition to the spherical structures, the presence of anisotropic particles also contributed to this observation [30,31]. Although the surface plasmon resonances of silver nanoparticles are thought to be below 500 nm [32], it has been shown in the literature that anisotropic AgNPs can give SPR peaks even above 700 nm [30]. When the HR-TEM results of silver nanoparticles are combined with UV–vis results, a situation emerges where changes in the shape of AgNP particles contribute to changes in the SPR peaks and UV–vis spectra. The fact that LMA-Asn_AgNPs gave three different SPR peaks is due to the nanoplate-like structures [30]. The nanoclusters of LMA-Glu_AgNPs formed by the combination of different morphological particles gave more than one SPR peak, which was due to the morphological difference. When we look at LMA-Pro_AgNPs and G5AS-Pro_AgNPs, it can be mentioned that there is a similarity in their morphology given by the LMA-Glu_AgNP and G5AS-Asn_AgNP SPR peaks, respectively.
It is known that the optical behavior of the sugar ligands used in our study (especially G5AS) creates plasmonic interactions that may also cause differences in UV–vis spectra. Another situation is that when the studies with plant extracts are examined, the formation of more than one SPR peak is due to the fact that flavonoids from the extract chemistry not only change the morphology but also affect the plasmonic character [33]. This strengthens the thesis that the plasmonic characters we measured are affected by the sugar ligands and the presence of the amino acids.
3.2. HR-TEM and XRD Characterizations
LMA_AgNPs are mostly rounded with a ~25 × ~50 nm cross-section, which form aggregates that have 310 × 375 nm edges (Figure 2). The XRD 2θ peaks at 38.11° (111), 44.36° (200), 64.39° (220) and 77.53° (311) refer to the presence of face centric cubic (fcc) crystal structure while 2θ 31.44° (111) and 68.98° (220) might refer to the presence of centric cubic crystals of Ag2O particles (Figure 2C). The tiny peak at 35.14° can be assigned to (004) 4H hcp AgNPs. The d spacing (Figure 2B) with 2.03 Å refers to the (220) reflection of the fcc AgNP crystal structure. Figure 2B reveals the presence of (110) fcc Ag2O, (111) and (200) fcc AgNP, and (1–12) 4H hcp AgNPs. So, the LMA_AgNPs are composed of Ag2O and AgNP nanoparticles, which have been reported in the literature [34,35]. Moreover, Figure 2B clearly shows that there are two distinct layers for the AgNPs, where the outer layer is most probably an Ag2O layer. Additionally, the Fast Fourier Transform (FFT) insets show that the particles have a polycrystalline nature.
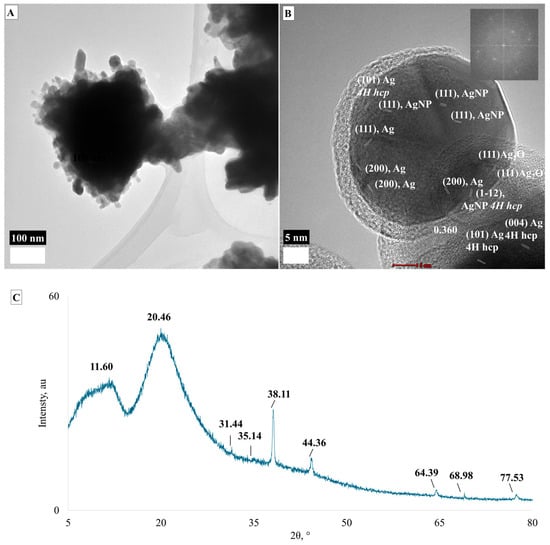
Figure 2.
TEM images (A,B) and XRD spectrum (C) of LMA_AgNPs.
Figure 3 shows the morphology and crystal structure of LMA-Cys-AgNPs. LMA-Cys-AgNPs do not have a homogeneous size and shape (Figure 3A). Approximately 10% of LMA-Cys-AgNPs have a size between 20 and 50 nm, while the majority (˃80%) have a rounded morphology between 5 and 15 nm. Very few AgNPs with anisotropic 15 × 30 nm cross-sections were observed as well. Interplanar spacings (d spacings, dsp) obtained from SAED analysis (Figure 3D) were 2.27, 1.8, 1.49, 1.37, 1.21, 1.15, and 1.01 Å assigned to (1–12), (104), (110), (112), (202), (203) and (0010) hkl miller indices, so LMA_Cys_AgNPs have hexagonal 4H crystal faces [36] while 2.08 Å can be assigned to (200) fcc AgNPs. Additionally, 1.47 Å can also be assigned to (220) fcc AgNPs. The inner bright reflection revealing 0.341 nm d spacing could be (110) fcc Ag2O [37]. The d spacings from HR-TEM images (Figure 3B,C) 2.52, 2.01, 1.8 Å could refer to (004), (103), (104) 4H hcp AgNPs, while 2.07 Å refers to (200) fcc AgNPs. The d spacings 3.36, 2.74, and 1.94 Å refer to (110), (111), and (211) fcc Ag2O.
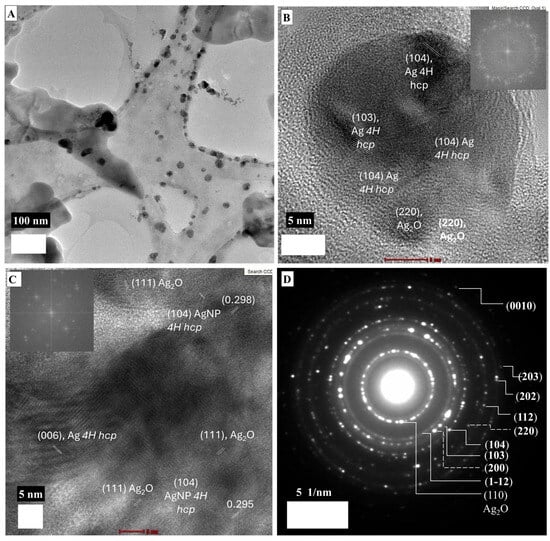
Figure 3.
TEM images (A–C) and SAED (D) micrograph of LMA-Cys_AgNPs.
LMA_Glu_AgNPs showed an aggregate-like structure with 6.34 μm disorganized form that contains protruding edges (Figure 4A). However, Figure 4B shows that the aggregate was composed of anisotropic nanoparticles with round cross sections of 38 × 106 nm, and it can be seen from these parts that the nanoparticles are not far from each other and bound together by a strong interaction, rather than loose interactions. HR-TEM images from different edges gave dsp 2.24 Å, which was assigned to (1–12) 4H hcp AgNPs (Figure 4C). Figure 4D shows that the nanoparticles are metallic Ag particles with a mixture of 4H hcp and fcc phases.
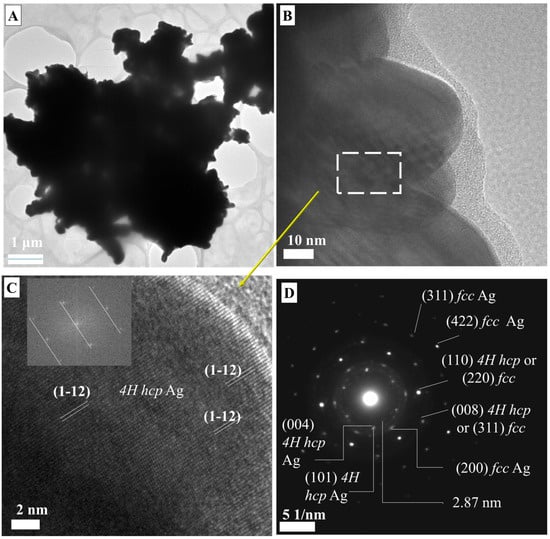
Figure 4.
TEM micrographs (A–C) and SAED spectrum (D) of LMA-Glu_AgNPs.
Figure 5 shows that LMA-Asn_AgNPs have a very different morphology compared to other AgNPs synthesized with LMA. The different edge lengths of the nanoplate-like morphological structures in Figure 5 mean that the energies that stabilize the surfaces are different. Non-nanoplate hexagonal structures were also found, and their edge lengths were determined as 920 × 987 × 620 × 863 × 863 × 582 × 726 nm. Figure 5C also shows the formation of rounded and anisotropic nanoparticles with dimensions between 8 and 17 nm. The dsp 2.39 and 2.06 Å were assigned to (111) and (200) fcc AgNPs. It is possible that the growth of LMA_Asn_AgNPs nanoplates proceeded through a self-seeding route as described in the literature [38]; the initially formed AgNPs served as a surface for further growth of the particles to give large nanoplates. However, it is not possible to come up with a concluding remark for this observation because of the study limitations that can be simply explained by the experimental setup. Further studies dealing with different asparagine/sugar ligand ratios are needed to explain how asparagine plays a role in obtaining the nanoplates.
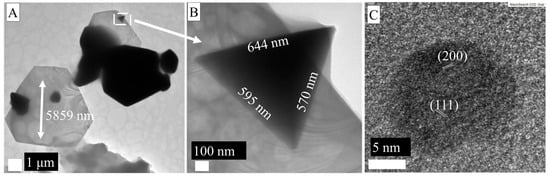
Figure 5.
(A–C) TEM micrographs of LMA_Asn_AgNPs.
LMA_Pro_AgNPs are a mixture of anisotropic 0D particles with a 48 ± 24 nm size range and 1D particles with 25–255 nm cross-sections. HR-TEM images gave dsp 2.78, 1.94, 2.36 Å that were assigned to (111), (211), and (200) of fcc Ag2O [37], while 2.36 Å dsp could also be assigned to (111) fcc AgNPs as well (Figure 6).
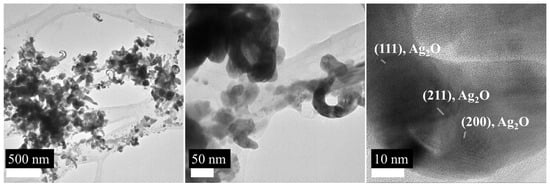
Figure 6.
TEM images of the LMA_Pro_AgNPs.
G5AS_AgNPs (Figure 7) also formed nanoclusters similar to LMA_AgNPs. These nanoclusters consist of anisotropic particles with a size distribution of 45–100 nm (Figure 7A). In Figure 7B, the weak interplanar characters at 5 nm resolution indicate that the crystalline character is not very strong. SAED characterization shows that the nanoparticles have hexagonal-closed crystal character with Miller indices of (112) and (1–12) (Figure 7C) while dsp 2.03 and 2.44 Å can be assigned to (200) fcc and (101) 4H hcp AgNP.
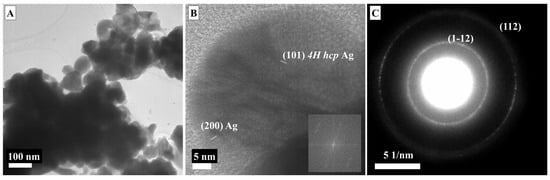
Figure 7.
TEM micrographs (A,B) and SAED spectrum (C) of G5AS_AgNPs.
Unlike G5AS_AgNPs, G5AS-Cys_AgNPs did not form nanoclusters (Figure 8). Figure 8A shows the overall image of the nanoparticles. Nanoparticles are commonly concentrated between 21 and 27 nm (~80%) and ~15% around 40 nm. The presence of <5nm nanoparticles is also observed at around 5%. The dsp 2.52, 1.98, and 1.54 Å are assigned to (004), (103), and (105) 4H hcp AgNPs, while dsp 3.30 Å reveals the presence of (110) fcc Ag2O. It is possible that SAED patterns of fcc and 4H hcp phases of AgNPs overlap with fcc Ag2O. The dsp 2.35 Å can result from (111) fcc AgNP and (200) Ag2O, while dsp 1.66 and 1.37 Å can be a combination of (006) 4H hcp AgNP + (220) Ag2O and (112) 4H hcp AgNP + (222) Ag2O, respectively (Figure 8B). XRD analysis (Figure 8C) shows that the nanoparticle is a mixture of AgNP and Ag2O nanoparticles. AgNP was characterized with 31.78°, 38.20°, 64.54°, and 77.88° 2Ɵ angles, which correspond to (113), (111), (220), and (311) Miller indices [39]. The presence of Ag2O nanoparticles was characterized with 27.43° (110) [39], 33.15° (111) [40], and 69.30° (222) face-centric cubic crystalline [41,42]. The peaks at 2Ɵ 30.05°, 33.15°, and 43.48° can be assigned to AgO [43]. The peaks at 22.11° and 25.51° could not be assigned to AgNPs (can be related to forbidden XRD reflection) or Ag2O nanoparticles.
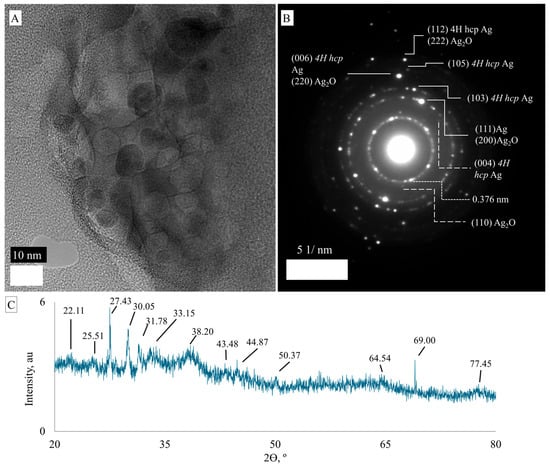
Figure 8.
TEM micrograph (A), SAED spectrum (B) and XRD spectrum (C) of G5AS-Cys_AgNPs.
Figure 9A shows G5AS_Asn_AgNPs are in the range of 48–320 nm with a heterogeneous morphology. In contrast, Figure 9B shows a heterogeneous surface with (110) fcc Ag2O and (101) 4H hcp metallic AgNPs, while Figure 9C reveals single-crystalline fcc Ag2O (110) Miller indices. The SAED pattern gave dsp 0.94 and 0.83 Å that can be assigned to (331) and (422) fcc AgNPs, while dsp 2.44, 1.76, and 2.58 Å were assigned to (101), (104), and (105) 4H hcp Miller indices of AgNPs (Figure 9D). The presence of Ag2O crystals was revealed by dsp 1.34 Å, which is assigned to (222) fcc Ag2O [37]. Moreover, the dsp 2.37 and 1.007 Å can be assigned to a combination of (111) fcc AgNP and (200) fcc Ag2O and (0010) 4H hcp AgNP and (400) fcc AgNP, respectively.
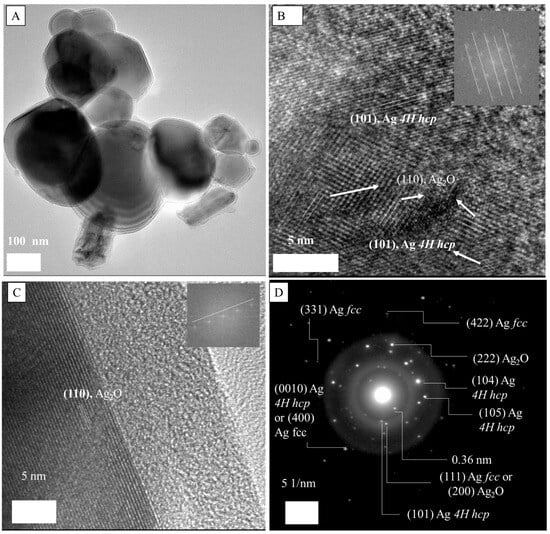
Figure 9.
TEM (A–C) and SAED spectrum (D) of G5AS_Asn_AgNPs.
The co-existence of fcc and 4H hcp crystal structures of metallic nanoparticles has been reported [44,45,46]. The hexagonal close-packed structure of AgNPs is very rare and accepted as metastable, and interestingly, the formation of silver oxides on co-existing fcc/hcp silver can happen as well [44]. It is noteworthy to mention that silver oxide nanoparticles can grow on AgNPs [47]. The presence of hcp crystal pattern was reported as interlayers grown within fcc silver ore, and also hcp formation was reported for different AgNPs structures as well. Transformation of hcp to fcc is possible, and recrystallization was a possible way to do so [48]. In our case, XRD samples were prepared through lyophilization for 24 h at −80 °C, which can be one of the possibilities for the discrepancy between the TEM-SAED and XRD results. The other possibility could be that only a few layers between the fcc core and Ag2O layer can contain 4H hcp silver, which is visible by TEM-SAED, while XRD can penetrate a deeper region of the AgNPs that provides information about the major crystalline structure. The latter claim seems more realistic since Grouchko et al. (2016) reported the co-existence of 4H hcp and fcc through the TEM-SAED technique, for whose XRD results only revealed the presence of fcc AgNPs [49]. The authors also claimed that the coalescence of fcc and hcp particles resulted in larger hcp AgNPs. Additionally, Carlos et al. (2020) reported the co-existence of 4H hcp and fcc AgNPs along with a silver oxide layer [50]. HR-TEM-SAED-based characterization of AgNPs synthesized using plant extracts was reported [51].
The d spacing at 2.15 Å could not be identified, but based on the literature, the d spacing (2.12 Å) can be assigned to Ag2O or AgNPs [37]. However, some papers claim the d spacing of 0.21 nm (they did not include the third digit, so we cannot know) belongs to (200) fcc AgNPs [52] while some claim it belongs to (111) fcc AgNPs [53]. It is possible that because of the lack of a long enough lattice fringe, the assignment cannot be conducted precisely in our case. The d spacings 3.76 ± 0.21 Å and 2.97 ± 0.02 Å can be assigned to AgO or Ag2O3 nanoparticles [35]. Additionally, the d spacing 2.97 ± 0.02 Å can also be related to a tetragonal or orthorhombic crystal structure [54] grown on silver oxide nanoparticles.
3.3. Antibacterial Characteristics of the Nanoparticles
Screening tests with 1 mg/mL of AgNP formulations synthesized in the study, LMA_Cys_AgNPs, and G5AS_Cys_AgNPs did not show antibacterial activity, even though they gave the smallest sizes. This could be related to the extreme stability of the silver nanoparticles, resulting from thiolation by cysteine since ionization must take place for AgNPs to reveal their toxicities [29]. The other formulations were tested at 104, 52, 13, 6.5, 3.25, and 1.625 µg/mL (left to right). None of the formulations gave an MIC value ≤ 6.5 µg/mL, so the results shown in Figure 10 are related to 13 µg/mL applied concentration except LMA_Cys_AgNPs and G5AS_Cys_AgNPs, which were applied at 1 mg/mL.
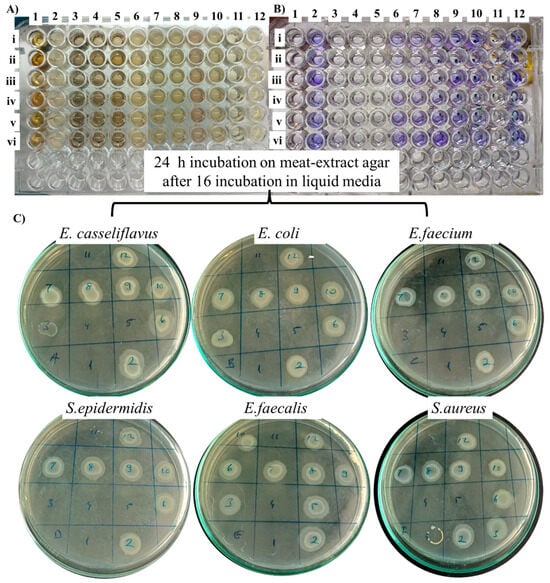
Figure 10.
Antibacterial activity of the nanoparticles based on MIC (A), biofilm formation (B) and MBC (C) characteristics. 1: LMA_AgNP, 2: LMA_Cys_AgNP, 3: LMA_Asn_AgNP, 4: LMA_Glu_AgNP, 5: LMA_Pro_AgNP, 6: G5AS_Asn_AgNP, 7: G5AS_Pro_AgNP, 8: G5AS_Glu_AgNP, 9: G5AS_Cys, 10: G5AS_AgNP, 11: AgNO3 (100 µg/mL), and 12: Control. Bacterial species were E. casseliflavus (i), E. coli (ii), E. faecialis (iii), S. epidermidis (iv), P. aeruginosa (v), and S. aureus (vi).
LMA_Glu_AgNP gave MIC/MBC at 13 µg/mL for all the tested bacterial species. LMA_AgNPs showed MIC/MBC value at 13 µg/mL for E. casseliflavus, E. coli, E. faecialis, S. epidermidis, and P. aeruginosa species, while at the same concentration, LMA_Pro_AgNPs gave MIC/MBC values for E. casseliflavus, E. coli, E. faecialis, S. epidermidis and S. aureus. In contrast to these, LMA_Asn_AgNPs only gave MIC/MBC values at 13 µg/mL for S. epidermidis (only MIC) and P. aeruginosa. Silver nitrate was at 100 µg/mL MIC/MBC for E. casseliflavus and E. coli, while S. epidermidis and S. aureus still showed some growth at this concentration. The elimination of biofilm development at 100% for all the tested bacterial species was obtained at this concentration by LMA_AgNP, LMA_Asn_AgNP, LMA_Glu_AgNP, LMA_Pro_AgNP and AgNO3. Even though the antibacterial activity contribution of amino acids for the AgNPs is limited, some of the literature is given in Table 1, which includes their contribution to the nanoparticle as well.

Table 1.
Comparison of the findings with the reported amino acid supported AgNPs and silver composite nanoparticles.
Silver ions can interact with the proteins within the growth media, so it is not expected to show very strong antibacterial activity in comparison to the corresponding nanoparticles. The results were unexpected since LMA_Glu_AgNPs were in the form of aggregates but still showed the most diverse antibacterial activity. In the light of the literature, smaller nanoparticles undergo faster dissolution which turns into an antibacterial activity [59]. Based on the UV–vis spectra, LMA_Glu_AgNPs underwent size and morphology changes (Figure 1), and as seen in Figure 4 LMA_Glu_AgNP, the aggregate is composed of smaller particles, so it is possible that proteins within the growth media could detach the individual particles from each other. The literature shows that glutamic acid can be used in silver nanoparticle complexes in order to enhance the antibacterial activity [60]. Further studies are needed to understand how exactly these particles possess their antibacterial activities.
4. Conclusions
The amino acids L-cysteine, L-proline, L-asparagine and L-glutamic acid showed a dramatic effect on the morphology and size of the AgNPs synthesized in the presence of lactose methoxyaniline and galactose 5-aminosalicylic acid. The effects were not universal; rather, they showed dependence on the sugar ligand chemistry. The antibacterial activities showed an unexpected result, with the largest nanoparticle system showing the best antibacterial activity for different bacterial species. This screening study can call for further research on the development of amino acid functionalized silver nanoparticles synthesized in the presence of sugar ligands by altering the pH of the reaction media, the amino acid/sugar ligand mole ratio, and the time-dependent addition of amino acids in the reaction media.
Author Contributions
Conceptualization, I.Y. and Z.S.; methodology, I.Y.; software, Ş.B.; validation, Ş.B., A.G. and I.Y.; formal analysis, Ş.B.; investigation, Ş.B.; resources, Z.S.; data curation, Ş.B.; writing—original draft preparation, Ş.B. and I.Y.; writing—review and editing, I.Y. and Ş.B.; visualization, Ş.B.; supervision, I.Y.; project administration, Z.S.; funding acquisition, I.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Kastamonu University Internal Funds, grant number KÜ-BAP01/2021-25.
Data Availability Statement
All the raw data will be available upon reasonable request.
Acknowledgments
We thank Aslı Uğurlu Bayarslan of Kastamonu University for providing the amino acids used in the study. We also thank BUMER Bayburt University of Türkiye for their support with the HR-TEM studies.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AgNP | Silver Nanoparticle |
| Ag2O | Silver oxide |
| LMA | Lactose metoxyaniline |
| G5AS | Galactose 5-aminosaliyclic acid |
| HR-TEM: | High-Resolution Transmission Electron Microscopy |
| SAED | Selected Area Diffraction |
| XRD | X-ray Diffraction |
| dsp | Interplanar spacing |
| MIC | Minimum Inhibitory Concentration |
| MBC | Minimum Bactericidal Concentration |
References
- Shankar, S.; Rhim, J.W. Amino acid mediated synthesis of silver nanoparticles and preparation of antimicrobial agar/silver nanoparticles composite films. Carbohydr. Polym. 2015, 130, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ménard-Moyon, C.; Bianco, A. Self-assembly of amphiphilic amino acid derivatives for biomedical applications. Chem. Soc. Rev. 2022, 51, 3535–3560. [Google Scholar] [CrossRef]
- Annadhasan, M.; SankarBabu, V.R.; Naresh, R.; Umamaheswari, K.; Rajendiran, N. A sunlight-induced rapid synthesis of silver nanoparticles using sodium salt of N-cholyl amino acids and its antimicrobial applications. Colloids Surf. B Biointerfaces 2012, 96, 14–21. [Google Scholar] [CrossRef]
- de Matos, R.A.; Courrol, L.C. Biocompatible silver nanoparticles prepared with amino acids and a green method. Amino Acids 2017, 49, 379–388. [Google Scholar] [CrossRef]
- Maruyama, T.; Fujimoto, Y.; Maekawa, T. Synthesis of gold nanoparticles using various amino acids. J. Colloid Interface Sci. 2015, 447, 254–257. [Google Scholar] [CrossRef]
- Chandraker, K.; Nagwanshi, R.; Jadhav, S.K.; Ghosh, K.K.; Satnami, M.L. Antibacterial properties of amino acid functionalized silver nanoparticles decorated on graphene oxide sheets. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 181, 47–54. [Google Scholar] [CrossRef]
- Tharmavaram, M.; Pandey, G.; Khatri, N.; Rawtani, D. L-arginine-grafted halloysite nanotubes as a sustainable excipient for antifouling composite coating. Mater. Chem. Phys. 2023, 293, 126937. [Google Scholar] [CrossRef]
- Shi, J.; Sun, X.; Zou, X.; Zhang, H. Amino acid-dependent transformations of citrate-coated silver nanoparticles: Impact on morphology, stability and toxicity. Toxicol. Lett. 2014, 229, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.S.; Kim, C.K.; Han, G.; Forbes, N.S.; Rotello, V.M. Efficient gene delivery vectors by tuning the surface charge density of amino acid-functionalized gold nanoparticles. ACS Nano 2008, 2, 2213–2218. [Google Scholar] [CrossRef]
- Ghosh, P.S.; Han, G.; Erdogan, B.; Rosado, O.; Krovi, S.A.; Rotello, V.M. Nanoparticles featuring amino acid-functionalized side chains as DNA receptors. Chem. Biol. Drug Des. 2007, 70, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Li, X.; Zheng, W.; Feng, Y.; Wong, Y.S.; Chen, T. PH-responsive cancer-targeted selenium nanoparticles: A transformable drug carrier with enhanced theranostic effects. J. Mater. Chem. B 2014, 2, 5409–5418. [Google Scholar] [CrossRef]
- Wangoo, N.; Bhasin, K.K.; Mehta, S.K.; Suri, C.R. Synthesis and capping of water-dispersed gold nanoparticles by an amino acid: Bioconjugation and binding studies. J. Colloid Interface Sci. 2008, 323, 247–254. [Google Scholar] [CrossRef]
- Durupthy, O.; Bill, J.; Aldinger, F. Bioinspired synthesis of crystalline TiO2: Effect of amino acids on nanoparticles structure and shape. Cryst. Growth Des. 2007, 7, 2696–2704. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Ghasemi, Y.; Rasoul-Amini, S.; Barar, J.; Davaran, S. Impact of amino-acid coating on the synthesis and characteristics of iron-oxide nanoparticles (IONs). Bull. Korean Chem. Soc. 2012, 33, 3957–3962. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Ghasemi, Y.; Rasoul-Amini, S.; Barar, J.; Davaran, S. Preparation of novel magnetic fluorescent nanoparticles using amino acids. Colloids Surf. B Biointerfaces 2013, 102, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Chahar, V.; Sharma, B.; Shukla, G.; Srivastava, A.; Bhatnagar, A. Study of antimicrobial activity of silver nanoparticles synthesized using green and chemical approach. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 554, 149–155. [Google Scholar] [CrossRef]
- Santhosh, A.S.; Sandeep, S.; Manukumar, H.M.; Mahesh, B.; Kumara Swamy, N. Green synthesis of silver nanoparticles using cow urine: Antimicrobial and blood biocompatibility studies. JCIS Open 2021, 3, 100023. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, applications, toxicity and toxicity mechanisms of silver nanoparticles: A review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef]
- Yazgan, İ.; Osonga, F.J.; Miller, R.M.; Kariuki, V.M.; Zhang, J.; Feng, J.; Skeete, Z.; Crapo, H.; Schulte, J.; Panetier, J.; et al. Green one-pot sugar-ligands synthesized property-controlled gold-nanoparticles. ACS Agric. Sci. Technol. 2021, 1, 379–389. [Google Scholar] [CrossRef]
- Sancak, S.; Yazgan, İ.; Bayarslan, A.U.; Ayna, A.; Evecen, S.; Taşdelen, Z.; Gümüş, A.; Sönmez, H.A.; Demir, M.A.; Demir, S.; et al. Surface chemistry dependent toxicity of inorganic nanostructure glycoconjugates on bacterial cells and cancer cell lines. J. Drug Deliv. Sci. Technol. 2023, 79, 104054. [Google Scholar] [CrossRef]
- Kariuki, V.M.; Hoffmeier, J.C.; Yazgan, I.; Sadik, O.A. Seedless synthesis and SERS characterization of multi-branched gold nanoflowers using water soluble polymers. Nanoscale 2017, 9, 8330–8340. [Google Scholar] [CrossRef]
- Mytych, J.; Zebrowski, J.; Lewinska, A.; Wnuk, M. Prolonged Effects of Silver Nanoparticles on p53/p21 Pathway-Mediated Proliferation, DNA Damage Response, and Methylation Parameters in HT22 Hippocampal Neuronal Cells. Mol. Neurobiol. 2017, 54, 1285–1300. [Google Scholar] [CrossRef]
- Yazgan, I.; Gümüş, A.; Gökkuş, K.; Demir, M.A.; Evecen, S.; Sönmez, H.A.; Miller, R.M.; Bakar, F.; Oral, A.; Popov, S.; et al. On the effect of modified carbohydrates on the size and shape of gold and silver nanostructures. Nanomaterials 2020, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Ballottin, D.; Fulaz, S.; Souza, M.L.; Corio, P.; Rodrigues, A.G.; Souza, A.O.; Gaspari, P.M.; Gomes, A.F.; Gozzo, F.; Tasic, L. Elucidating Protein Involvement in the Stabilization of the Biogenic Silver Nanoparticles. Nanoscale Res. Lett. 2016, 11, 313. [Google Scholar] [CrossRef]
- Brahmkhatri, V.P.; Singh, A.; Chakraborty, A.; Sharma, R.S.; Chandra, K.; Atreya, H.S. Multilayer protein corona on gold nanorod surface: First evidence of soft corona protein-protein interactions using solution NMR spectroscopy. Appl. Surf. Sci. Adv. 2022, 11, 100272. [Google Scholar] [CrossRef]
- Banerjee, V.; Das, K.P. Interaction of silver nanoparticles with proteins: A characteristic protein concentration dependent profile of SPR signal. Colloids Surf. B Biointerfaces 2013, 111, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Tomak, A.; Yilancioglu, B.; Winkler, D.; Karakus, C.O. Protein corona formation on silver nanoparticles under different conditions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129666. [Google Scholar] [CrossRef]
- Shannahan, J.H.; Podila, R.; Aldossari, A.A.; Emerson, H.; Powell, B.A.; Ke, P.C.; Rao, A.M.; Brown, J.M. Formation of a protein corona on silver nanoparticles mediates cellular toxicity via scavenger receptors. Toxicol. Sci. 2015, 143, 136–146. [Google Scholar] [CrossRef]
- MiclǍuş, T.; Beer, C.; Chevallier, J.; Scavenius, C.; Bochenkov, V.E.; Enghild, J.J.; Sutherland, D.S. Dynamic protein coronas revealed as a modulator of silver nanoparticle sulphidation in vitro. Nat. Commun. 2016, 7, 11770. [Google Scholar] [CrossRef]
- Amendola, V.; Bakr, O.M.; Stellacci, F. A study of the surface plasmon resonance of silver nanoparticles by the discrete dipole approximation method: Effect of shape, size, structure, and assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- Bastús, N.G.; Piella, J.; Puntes, V. Quantifying the Sensitivity of Multipolar (Dipolar, Quadrupolar, and Octapolar) Surface Plasmon Resonances in Silver Nanoparticles: The Effect of Size, Composition, and Surface Coating. Langmuir 2016, 32, 290–300. [Google Scholar] [CrossRef]
- Yeshchenko, O.A.; Dmitruk, I.M.; Alexeenko, A.A.; Kotko, A.V.; Verdal, J.; Pinchuk, A.O. Size and Temperature Effects on the Surface Plasmon Resonance in Silver Nanoparticles. Plasmonics 2012, 7, 685–694. [Google Scholar] [CrossRef]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Elyamny, S.; Eltarahony, M.; Abu-Serie, M.; Nabil, M.M.; Kashyout, A.E.H.B. One-pot fabrication of Ag @Ag2O core–shell nanostructures for biosafe antimicrobial and antibiofilm applications. Sci. Rep. 2021, 11, 22543. [Google Scholar] [CrossRef]
- Korkmaz, N.; Karadağ, A. Microwave assisted green synthesis of Ag, Ag2O, and Ag2O3 nanoparticles. J. Turkish Chem. Soc. Sect. A Chem. 2021, 8, 585–592. [Google Scholar] [CrossRef]
- Rodríguez-León, E.; Iñiguez-Palomares, R.; Navarro, R.E.; Herrera-Urbina, R.; Tánori, J.; Iñiguez-Palomares, C.; Maldonado, A. Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res. Lett. 2013, 8, 318. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Mao, X.; Ortiz, L.A.; Sadoway, D.R. Oriented silver oxide nanostructures synthesized through a template-free electrochemical route. J. Mater. Chem. 2011, 21, 432–438. [Google Scholar] [CrossRef]
- Jiang, X.; Zeng, Q.; Yu, A. A self-seeding coreduction method for shape control of silver nanoplates. Nanotechnology 2006, 17, 4929–4935. [Google Scholar] [CrossRef]
- Shinde, B.H.; Shinde, P.B.; Inamdar, A.K.; Inamdar, S.N.; Chaudhari, S.B. Green synthesis of silver/silver oxide composite nanoparticles using Cuscuta Reflexa plant for the insecticidal applications. Mater. Today Proc. 2023, 92, 549–553. [Google Scholar] [CrossRef]
- Jahan Tamanna, N.; Sahadat Hossain, M.; Mohammed Bahadur, N.; Ahmed, S. Green synthesis of Ag2O & facile synthesis of ZnO and characterization using FTIR, bandgap energy & XRD (Scherrer equation, Williamson-Hall, size-train plot, Monshi-Scherrer model). Results Chem. 2024, 7, 101313. [Google Scholar] [CrossRef]
- Laouini, S.E.; Bouafia, A.; Soldatov, A.V.; Algarni, H.; Tedjani, M.L.; Ali, G.A.M.; Barhoum, A. Green synthesized of Ag/Ag2O nanoparticles using aqueous leaves extracts of phoenix dactylifera l. And their azo dye photodegradation. Membranes 2021, 11, 468. [Google Scholar] [CrossRef]
- Dharmaraj, D.; Krishnamoorthy, M.; Rajendran, K.; Karuppiah, K. Antibacterial and cytotoxicity activities of biosynthesized silver oxide (Ag2O) nanoparticles using Bacillus paramycoides. J. Drug Deliv. Sci. Technol. 2021, 61, 102111. [Google Scholar] [CrossRef]
- Sabzi, M.; Mersagh Dezfuli, S. A study on the effect of compositing silver oxide nanoparticles by carbon on the electrochemical behavior and electronic properties of zinc-silver oxide batteries. Int. J. Appl. Ceram. Technol. 2018, 15, 1446–1458. [Google Scholar] [CrossRef]
- Bastl, Z.; Bezdička, P.; Pola, J. IR laser-induced ablation of Ag in dielectric breakdown of gaseous hydrocarbons: Simultaneous occurrence of metastable hcp and stable fcc Ag nanostructures in C:H shell. J. Photochem. Photobiol. A Chem. 2010, 213, 114–122. [Google Scholar] [CrossRef]
- Zhuang, J.; He, F.; Liu, X.; Si, P.; Gu, F.; Xu, J.; Wang, Y.; Xu, G. In-situ growth of heterophase Ni nanocrystals on graphene for enhanced catalytic reduction of 4-nitrophenol. Nano Res. 2022, 15, 1230–1237. [Google Scholar] [CrossRef]
- Orhan, O.K.; Ponga, M. Surface-plasmon properties of noble metals with exotic phases. J. Phys. Chem. C 2021, 125, 21521–21532. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.R.; Cao, W.; Zhen, J.; Wu, J.H. Screw-Dislocation-Driven Hierarchical Superstructures of Ag-Ag2O-AgO Nanoparticles. Crystals 2020, 10, 1084. [Google Scholar] [CrossRef]
- Thevamaran, R.; Griesbach, C.; Yazdi, S.; Ponga, M.; Alimadadi, H.; Lawal, O.; Jeon, S.; Thomas, E.L. Dynamic martensitic phase transformation in single-crystal silver microcubes. Acta Mater. 2020, 182, 131–143. [Google Scholar] [CrossRef]
- Grouchko, M.; Popov, I.; Uvarov, V.; Magdassi, S.; Kamyshny, A. Coalescence of Silver Nanoparticles at Room Temperature: Unusual Crystal Structure Transformation and Dendrite Formation Induced by Self-Assembly. Langmuir 2016, 25, 2501–2503. [Google Scholar] [CrossRef]
- Carlos, J.; Antonieta, M.; Paola, K.; Alejandra, R.; Contreras, S.; Mac, J.H. Characterization of Silver Nanoparticles Obtained by a Green Route and Their Evaluation in the Bacterium of Pseudomonas aeruginosa. Crystals 2020, 10, 395. [Google Scholar] [CrossRef]
- Tepe, M.; Zeybek, M.S. Chemistry of plant extracts directs the silver nanostructures’ crystal structure into hexagonally close-packed: A comparative study using elecampane and blueberry extracts. Anadian J. Chem. 2024, 102, 431–447. [Google Scholar] [CrossRef]
- Tang, S.; Vongehr, S.; Meng, X. Carbon spheres with controllable silver nanoparticle doping. J. Phys. Chem. C 2010, 114, 977–982. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, X.; Cai, H.; Cao, C. Facile and one-step synthesis of monodisperse silver nanoparticles using gum acacia in aqueous solution. J. Mol. Liq. 2014, 196, 135–141. [Google Scholar] [CrossRef]
- Ram, S.; Gautam, A.; Fecht, H.J.; Cai, J.; Bansmann, J.; Behm, R.J. A new allotropic structure of silver nanocrystals nucleated and grown over planar polymer molecules. Philos. Mag. Lett. 2007, 87, 361–372. [Google Scholar] [CrossRef]
- Perni, S.; Hakala, V.; Prokopovich, P. Biogenic synthesis of antimicrobial silver nanoparticles capped with L-cysteine. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 460, 219–224. [Google Scholar] [CrossRef]
- Almufarij, R.S.; Ali, A.E.; Elbah, M.E.; Elmaghraby, N.S.; Khashaba, M.A.; Abdel-Hamid, H.; Fetouh, H.A. Preparation, Characterization of New Antimicrobial Antitumor Hybrid Semi-Organic Single Crystals of Proline Amino Acid Doped by Silver Nanoparticles. Biomedicines 2023, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Gibała, A.; Żeliszewska, P.; Gosiewski, T.; Krawczyk, A.; Duraczyńska, D.; Szaleniec, J.; Szaleniec, M.; Oćwieja, M. Antibacterial and antifungal properties of silver nanoparticles—Effect of a surface-stabilizing agent. Biomolecules 2021, 11, 1481. [Google Scholar] [CrossRef]
- Chandra, A.; Singh, M. Biosynthesis of amino acid functionalized silver nanoparticles for potential catalytic and oxygen sensing applications. Inorg. Chem. Front. 2018, 5, 233–257. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1–10. [Google Scholar] [CrossRef]
- Stevanović, M.; Bračko, I.; Milenković, M.; Filipović, N.; Nunić, J.; Filipič, M.; Uskoković, D.P. Multifunctional PLGA particles containing poly(l-glutamic acid)-capped silver nanoparticles and ascorbic acid with simultaneous antioxidative and prolonged antimicrobial activity. Acta Biomater. 2014, 10, 151–162. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).