Abstract
Super-resolution microscopy (SRM) has revolutionized our understanding of subcellular structures, including cell organelles and viruses. For human immunodeficiency virus (HIV), SRM has significantly advanced knowledge of viral structural biology and assembly dynamics. This review analyzes how SRM techniques (particularly PALM, STORM, STED, and SIM) have been applied over the past decade to study HIV structural components and assembly. By categorizing and comparing studies based on SRM methods, HIV components, and labeling strategies, we assess the strengths and limitations of each approach. Our analysis shows that PALM is most commonly used for live-cell imaging of HIV Gag, while STED is primarily used to study the viral envelope (Env). STORM and SIM have been applied to visualize various components, including Env, capsid, and matrix. Antibody labeling is prevalent in PALM and STORM studies, targeting Env and capsid, whereas fluorescent protein labeling is mainly associated with PALM and focused on Gag. A recent emphasis on Gag and Env points to deeper investigation into HIV assembly and viral membrane dynamics. Insights from SRM studies of HIV not only enhance virological understanding but also inform future research in therapeutic strategies and delivery systems, including extracellular vesicles.
1. Introduction
Super-resolution microscopy (SRM) encompasses an array of techniques that surpass the diffraction limit of light, enabling imaging of subcellular-sized constructs at high resolution [1,2]. Achieving this improved resolution depends on specific SRM methods, including structured illumination microscopy (SIM), stimulated emission depletion (STED) microscopy, and single-molecule localization microscopy (SMLM) techniques such as PALM (PhotoActivatable Localization Microscopy) and STORM (STochastic Optical Reconstruction Microscopy) [1]. Collectively, these approaches resolve objects down to tens of nanometers, offering unprecedented insight into nanoscale features that cannot be clearly visualized by standard optical fluorescence microscopy [3,4,5].
SRM is particularly valuable for studying entities such as viruses, extracellular vesicles (EVs), large protein complexes, and lipid nanoparticles. One such entity is the human immunodeficiency virus (HIV), a retrovirus approximately 100–120 nm in diameter [6,7]. HIV (Figure 1) is composed of a viral membrane, internal protein matrix (MA), cone-shaped capsid (CA), nucleocapsid (NC), viral RNA genome (vRNA), and the envelope glycoprotein (Env), none of which can be resolved by conventional light microscopy methods [6]. Since the initial discovery of HIV, a wide range of biochemical, genomic, and virologic assays have been applied to advance our understanding of HIV biology, from insight into its replication and maturation to the regulation and structure of the HIV genome [8]. For instance, DNase I footprinting has been used to identify cellular factors involved in the transcriptional regulation of HIV, shedding light on how host proteins interact with the viral genome to modulate gene expression [9]. Selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE) has provided detailed insights into the higher-order structural organization of the HIV genome by distinguishing reactive and stable regions along the RNA strand [10]. These methodologies have provided the backbone of our current understanding of HIV biology and presently continue to have a significant impact on HIV research.
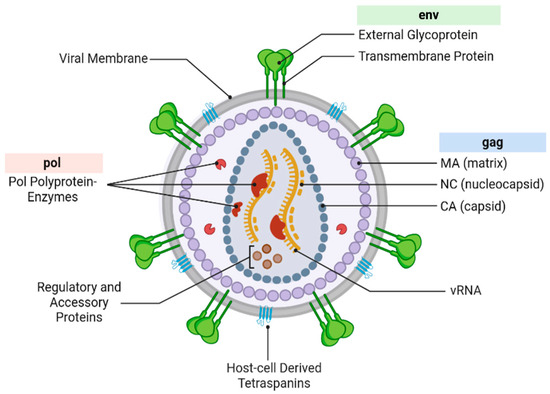
Figure 1.
The general structure of the HIV virion with key components highlighted and labeled. Components unique to a particular type or strain, such as Vpx, which is unique to HIV-2, are not included or specifically highlighted in the figure. Figure developed with BioRender [11].
Building on these insights, imaging techniques have been relied upon as a way to visualize the structure and spatial arrangement of HIV more tangibly. Specifically, electron microscopy (EM) methods have been instrumental in revealing key structural features of HIV and its interactions with host cells at nanoscale resolution [12]. Cryo-electron tomography (Cryo-ET) studies resolved the organization of the Gag structural protein within the HIV capsid while also revealing the arrangement of trimeric Env on native virions, both alone and in complex with CD4 and various antibodies [13,14,15]. More recently, single-particle EM has shed light on the mechanisms of broadly neutralizing antibodies, such as 10E8, which targets the HIV fusion protein by wedging into the membrane proximal external region (MPER) and bending the spike before lifting it up from the HIV lipid membrane [16].
While these approaches have greatly advanced HIV research, SRM offers a unique opportunity to further our understanding of HIV by bridging molecular specificity with high-resolution imaging. For instance, SRM methods are relatively easy to prepare and can provide dynamic studies of HIV under live-cell conditions [1,2,3]. SRM is also capable of multi-color labeling at nanoscale resolution, which enhances the information obtained from visualizing molecular interactions or tracking viral components in living cells, in turn making SRM a powerful complement to traditional techniques [2,3]. SRM, therefore, positions itself as a critical tool for advancing our knowledge of HIV structure and assembly mechanisms. By leveraging techniques like SIM, STED, PALM, and STORM, researchers have been able to visualize the localization of HIV components, investigate the dynamics of Gag assembly, and probe interactions between the Env glycoprotein and lipid components from the host cell [1,17,18,19]. Figure 2 highlights some of these SRM visualizations of various HIV components. Moreover, SRM imaging strategies used for HIV can be extended to other enveloped viruses or related structures, such as extracellular vesicles (EVs), enabling the evaluation of their composition, protein interactions, and nucleic acid payloads [6,20,21].
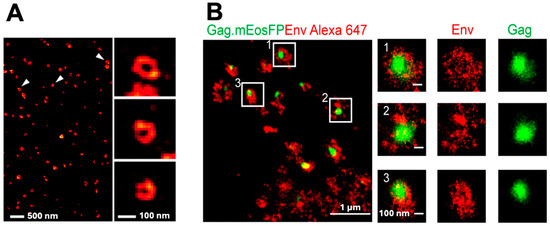
Figure 2.
Published examples of SRM images of HIV and its components. STED microscopy image of silicon SiR-CLIP-stained Gag in HIV particles (A) [22]. White arrows indicate the particles shown enlarged in the right column. Superposition of a PALM image of mEos fluorescent protein-stained Gag (green) overlayed on the corresponding dSTORM image of AF647-stained Env at HIV assembly sites on infected HeLa cells (B) [23]. The regions boxed in white indicate assembly sites enlarged in the second column to the right. The third and fourth columns show the separated Gag and Env overlays. (A) adapted with permission from Hanne J, Göttfert F, Schimer J, Anders-Össwein M, Konvalinka J, Engelhardt J, Müller B, Hell SW, Kräusslich H-G.: Stimulated Emission Depletion Nanoscopy Reveals Time-Course of Human Immunodeficiency Virus Proteolytic Maturation. ACS Nano. 2016;10(9):8215–8222. https://doi.org/10.1021/acsnano.6b03850. Copyright 2016 American Chemical Society. (B) adapted with permission under the Creative Commons Attribution License from Muranyi W, Malkusch S, Müller B, Heilemann M, Kräusslich H-G.: Super-Resolution Microscopy Reveals Specific Recruitment of HIV-1 Envelope Proteins to Viral Assembly Sites Dependent on the Envelope C-Terminal Tail. PLoS Pathogens. 2013;9(2):e1003198. https://doi.org/10.1371/journal.ppat.1003198. Copyright 2013 Muranyi et al. on PLoS Pathogens.
In this review, we examined how SRM has been applied over the past decade to study the structure and assembly of HIV virions and their native components. We further investigated how SRM methods and HIV components of focus have evolved over time, asked which SRM techniques were most commonly used, and considered how labeling strategies influenced imaging outcomes. These findings serve as a reference for guiding future SRM-based studies, not only for HIV but also for other particles of interest in virology and medicine.
2. Methods
A literature search was performed using PubMed. Keywords for the search were defined after reviewing the literature as to the names and variations of super-resolution microscopy currently available for use. Subsequently, each super-resolution method was searched alongside the term for HIV on PubMed to evaluate if they were ever used in the context of HIV research. Terms that did not appear were excluded from the final search criteria. Table 1 highlights the initial list of proposed terms and excluded terms alongside the final search query. Terms that did not yield any publications with HIV in initial searches were excluded from the final search criteria (i.e., RIM, LLS, ISM, etc.). The selected keywords in the final search were chosen to encompass as many relevant papers as possible while avoiding redundancy and reducing the appearance of unrelated publications. For example, if a search term was found to have yielded the same publications as another (i.e., papers found with the “STORM” search term all appeared within searches using less generalized terms such as “super-resolution microscopy” and “single molecule localization”), it was removed from the final search query. Additionally, certain abbreviations for SRM methods were avoided if they were found to share an abbreviation with another common scientific term, such as SIM, which could refer to structured illumination microscopy, single-ion monitoring, or single-minded genes.

Table 1.
The table of proposed search terms, excluded terms, and final search query.
Within this search, only studies published in English with a publication date from January 2011 until mid-June 2023 were included. Overall, 121 publications met the basic search criteria (Figure 3). A total of 18 papers were identified as being review publications and were removed. For the remaining 103 papers, a list of criteria was developed to determine which papers best matched the goals for this review, as shown in Table 2. We focused on the structural and assembly dynamics of HIV, as driven by HIV native components. With this scope in mind, we excluded papers that focused mainly on the pathology of HIV in human cells, hijacked host cell proteins, HIV drugs, extracellular vesicles, and virus-like particles (VLPs). VLP studies that used HIV VLPs to study HIV-related assembly dynamics (i.e., Gag-driven processes of assembly) remained in the study. Finally, if a publication relied on previously published data, rather than having obtained new data, only the original source study for those data was included to reduce redundancy. Ultimately, we identified 32 papers eligible for the final review. Any paper that met all defined criteria was also checked manually on PubPeer to avoid including papers suspected of research misconduct or poor research practice in our review [24,25]. From our assessment on PubPeer, none of the 32 publications met this final exclusion condition.
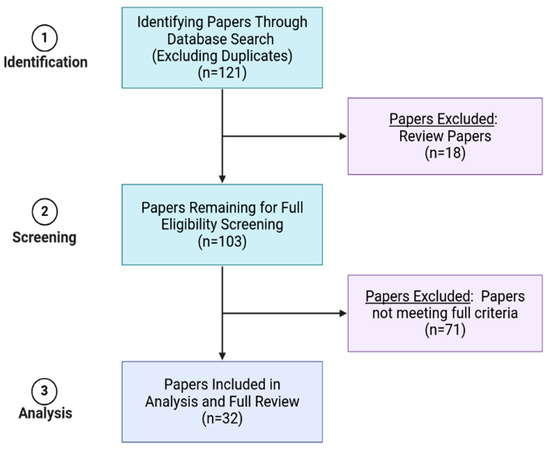
Figure 3.
Diagram outlining the paper identification and screening process. Figure developed with Biorender [11].

Table 2.
Paper criteria for inclusion in the final review.
For the final group of papers, three categorizations were performed, specifically by (1) the component of HIV studied, (2) the method of SRM performed, and (3) the type of labeling method used for SRM imaging. “Component of HIV studied” was restricted to those components studied by SRM. Thus, a paper on Gag and Env that detected only Gag by SRM was categorized as “Gag,” as was a study of full-length Gag (i.e., not matrix or capsid specifically). If the paper focused on matrix and/or capsid, respectively, then it was categorized by these proteins. Furthermore, studies that tagged Gag or another HIV protein (i.e., Vpr) with a fluorescent signal to localize SRM imaging of another HIV component were not considered SRM studies of the fluorescently labeled Gag/HIV protein. Lastly, when compiling the data for graphical analysis, components with the fewest appearances (studied in 4 or fewer papers) were combined into an “other” category.
For the SRM method category, only those methods used to study the HIV component(s) were considered. Frequently used subtypes were split from a broader category (i.e., PALM and STORM were split from general SMLM due to high occurrence, but not iPALM from PALM or dSTORM from STORM).
Finally, for the labeling category, only the labeling strategy used for SRM imaging was considered. Again, frequently used subtypes of labels were split from the general category: e.g., Fab fragments were differentiated from general antibody-based labeling, but nanobody and chromobody-based labeling (less than 4 total uses) were combined with the Fab fragment category. Similarly, SNAP, CLIP, and click chemistry occurred infrequently and were combined into the “other” category for graphical visualization and analysis. Genomic labeling, albeit with only two use cases, was not added to the “other” category due to its distinct approach. This resulted in five labeling categories: “protein-based” (fluorescent proteins such as GFP, mEOS2, etc.), “antibody,” “Fab fragment/nanobody/chromobody,” “genomic labeling,” and “other.”
3. Results
Among the 32 papers included for analysis, nine used STED, six SIM, six STORM alone, eight PALM alone, and three utilized both PALM and STORM. In terms of the HIV components studied, it was much more common for a publication to examine more than one component than to use multiple SRM methods. More than one HIV component was imaged in ten papers, with the most frequent pairing being Env and Gag (four out of these ten). For single-component SRM analyses, eight studies investigated Env, six focused on Gag, four on capsid, one on gRNA, one on protease, and two on Vpx. A tabulated summary of each publication’s SRM method, HIV component, and primary findings appears below, while Figure 4, Figure 5 and Figure 6 highlight key aspects of the temporal and correlative analyses discussed later.
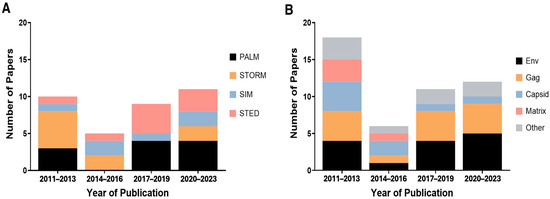
Figure 4.
Compilation of SRM method usage to study HIV over time (A) and the HIV components studied over time from each SRM HIV publication (B) between the years 2011 to late June 2023. Years were stratified into groups of 3 to enhance visibility relating to changes in use over time. In B, less commonly observed components were grouped under the category “other,” which ultimately included vRNA, Vpx, Vpr, and IN based on the papers in use for this review. Note that since some research studies have used more than one SRM method to study a component of HIV or may have used one SRM method to study multiple HIV components, the total number of papers in a year bracket may not match between (A,B).
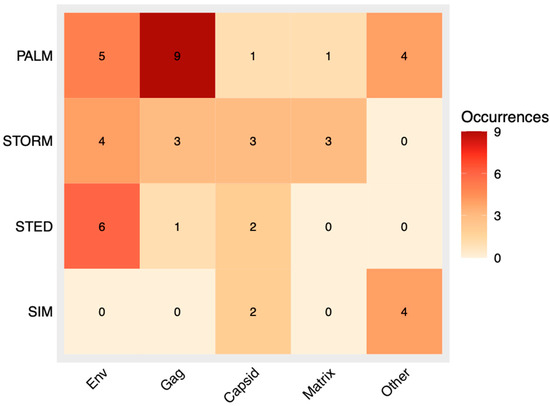
Figure 5.
Heatmap displaying associations between each SRM method and the component of HIV studied with said method. The more common a particular combination, the darker the corresponding square in the heatmap will become. As a baseline, combinations not found within the selection of papers used for this review are indicated by an off-white color.
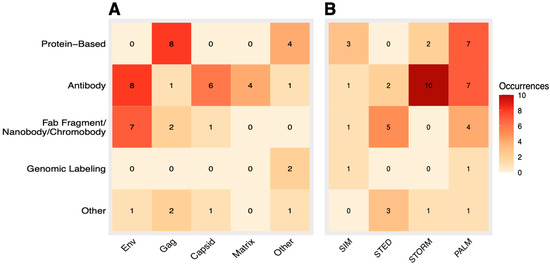
Figure 6.
Heatmaps displaying associations between each labeling method and the corresponding component of HIV studied (A) and the associations between labeling method and the SRM imaging method used in the study (B). Both heatmaps share the same vertical axis and color legend. Fab fragments, nanobodies, and chromobodies were categorized under one group for clearer visualization. Labeling methods included under the “other” category were those that appeared rather infrequently, specifically tag-based labeling (SNAP, CLIP, and FlAsH) along with click-chemistry labeling.
3.1. Env
Of the eight publications centered on Env, the primary themes included Env clustering, mobility, and distribution on the virion. In one of the earliest Env-focused papers, Chojnacki et al. (2012) used STED to show how virus maturation drives Env clustering, mediated by interior Gag interactions [26]. Later STED-based efforts (2017, 2021) employed scanning fluorescence spectroscopy (sSTED-FCS) to reveal how HIV-1 maturation regulates Env mobility, with a distinct reduction in Env motility observed at the site of assembly [27,28]. Chen et al. (2020) used both iPALM and STORM to quantify how SERINC2 or SERINC5 insertion affects Env clustering, finding that Env clusters persisted under SERINC2 but were disrupted under SERINC5 [29]. Nieto-Garai et al., 2020, leveraged STED to elucidate the role of gp41–cholesterol interactions in stabilizing Env clusters, noting that either truncation of gp41 or cholesterol depletion dispersed those clusters and diminished viral entry [10]. Carravilla et al. (2019), also using STED, discovered that the gp41 membrane proximal external region (MPER) is accessible in the native Env state, thus allowing anti-MPER antibodies to engage [30]. In another labeling approach, Sakin et al. (2017) integrated non-canonical amino acids into Env for click labeling and subsequent STED imaging, observing results consistent with classical immunostaining [31]. Finally, Mengistu et al. (2015) leveraged dSTORM to examine the exposure of diverse gp120 epitopes upon target cell binding, determining that epitopes—both neutralizing and non-neutralizing—rapidly became accessible [32]. Collectively, these SRM studies have provided further insight into how Env clustering, mobility, and spatial organization are tightly regulated by factors such as virus maturation, lipid composition, and host restriction factors. By resolving how these nanoscale features influence Env accessibility, antibody targeting, and fusion competency, SRM has provided a functional framework for understanding how Env structure and positioning govern HIV-1 infectivity and immune evasion.
3.2. Gag
Six publications examined Gag as their sole SRM target, consistently addressing assembly and maturation. In the earliest example, Malkusch and Muranyi et al. (2012) mapped Gag assembly clusters in fixed cells by dSTORM, classifying three distinct distribution patterns that aligned with EM findings [33]. Later, Hanne et al. (2016) used STED to highlight the structural differences between immature (ring-like) and mature (densely packed) Gag lattices [22]. Pedersen et al. (2018) combined iPALM with scanning EM to uncover lattice imperfections in considerable detail [34], and Saha and Saffarian (2020) used iPALM-based time-lapse imaging to reveal that Gag lattice organization exhibits dynamic behavior rather than remaining static [35]. Floderer et al. (2018) combined PALM with other analyses to show that vRNA acts as a key spatiotemporal organizer of Gag assembly at the plasma membrane [17]. Finally, Mao et al. (2021) used dSTORM alongside bimolecular fluorescence complementation (BiFC), enabling them to isolate specific Gag–Gag interactions and evaluate nonspecific overlaps within Gag clusters [36]. Together, these SRM-based investigations have revealed that Gag assembly is not a uniform or static process, but one shaped by spatial heterogeneity, dynamic remodeling, and molecular-level regulation. High-resolution nanoscale imaging by SRM revealed features such as lattice defects, maturation-dependent architecture, and the organizing role of viral RNA, further clarifying how Gag drives virion formation and structural transitions critical for HIV infectivity.
3.3. Env + Gag
Of the four studies that simultaneously visualized Env and Gag, most focused on how Gag assembly affects Env localization and dynamics. Roy et al. (2013) used STORM to observe that, as Gag accumulates at the plasma membrane, Env aggregates into larger, less mobile clusters [37]. A 2018 study by Buttler et al. leveraged 3D iPALM to assess Env neck-biased distribution on assembling virions and found that Env encounters the Gag lattice in later assembly stages, with variations depending on cell type and the Env cytoplasmic tail [38]. Groves et al. (2020) used PALM imaging to reconstruct viral assembly sites and showed that Gag lattice formation correlates with reduced Env mobility [39]. Snetkov et al. (2022), using PALM, uncovered the critical function of a conserved tryptophan in the Env cytoplasmic tail, governing Env–Gag recruitment, Env diffusion, and fusion capability [40]. Muranyi et al. (2013) added insights on Env interaction with the matrix domain, using dSTORM to detect Env clusters at Gag assembly sites, then comparing these observations with results from matrix–domain mutations [23]. Together, these studies have shown that the spatial and temporal coordination between Gag and Env is more dynamic and regulated than previously understood, with Env recruitment, clustering, and mobility tightly linked to Gag lattice formation and maturation. High-resolution SRM imaging has exposed key mechanistic details, including the timing of Env incorporation, the influence of the Env cytoplasmic tail, and the functional consequences of Gag–Env interactions, advancing our understanding of how these proteins interact and work in concert for efficient virion assembly.
3.4. Matrix, Capsid, and Integrase
Matrix (MA) was commonly studied alongside capsid (CA). In 2012, Pereira et al. used dSTORM to track how MA and CA reorganize immediately upon viral fusion with lymphoid cells [41]. Pham and Tabarin et al. (2015) also used dSTORM, reporting that CD4 receptor engagement triggers structural expansion of the virion, provided the capsid core is stable and mature [42]. Four papers evaluated capsid alone. Helma et al. (2012) introduced a fluorescent “chromobody” for 3D-SIM imaging of the capsid, enabling real-time visualization of HIV assembly sites [43]. Hulme et al. (2015) applied SIM to establish that capsid proteins persist in nuclear complexes after uncoating [44], whereas Zila et al. (2019) used STED to see that intact capsids associate with nuclear pores before genome release [45]. Schifferdecker et al. (2022) implemented minimal click labeling to confirm that a complete HIV capsid can pass through nuclear pores [46]. Another study by Lelek et al. (2012) performed PALM imaging of integrase (IN) in combination with capsid immunostaining, distinguishing immature from mature virions based on IN clustering [47]. Meanwhile, Lehmann et al. (2011) provided a comprehensive view of MA, CA, IN, Env, and Vpr, all labeled for PALM, charting how these components form clusters at assembly sites [48]. Collectively, these studies highlight how SRM has been instrumental in dissecting the spatial organization and remodeling of internal viral components across the HIV lifecycle. From tracking MA and CA rearrangements during fusion and entry to confirming the passage of intact capsids through nuclear pores, these approaches have provided further insight into long-standing questions about viral uncoating and nuclear import. SRM also enabled the direct visualization of integrase clustering patterns and the coordinated assembly of multiple viral proteins at budding sites, offering an integrated view of virion formation. Rather than treating these components as static or isolated, high-resolution imaging (especially in live-cell contexts) has clarified how their localization, maturation states, and interactions evolve dynamically to support infection and replication.
3.5. Protease, Vpx, and vRNA
One paper (Tabler et al., 2022) focused on HIV-1 protease, using SIM to visualize Vpr as a protease activity reporter. They found that protease is activated both in budding virions and within infected cells [49]. Two studies analyzing Vpx in HIV-2/SIV contexts [Singh et al. (2019, 2020)] applied SIM to show that Vpx localizes near the nuclear membrane, disrupts nuclear envelope arrangements, and interacts with Nup153 to facilitate nuclear import, contingent on Vpx phosphorylation by MAPK/ERK-2 [50,51]. Lastly, two publications delved into HIV vRNA using SRM. Ferrer et al. (2016) employed 3D-SIM to image the spatial arrangement of vRNA heterodimers, revealing that vRNA dimerization occurs largely in the cytosol of infected cells, guided by Gag and SL1/DIS sequences [52]. Yang et al. (2018) combined PALM and single-particle tracking to demonstrate that vRNA helps orchestrate Gag multimer formation and ensures continued stability of the Gag assembly process [19]. While diverse in focus, these studies underscore the flexibility of SRM in resolving underexplored facets of HIV biology at the nanoscale. By visualizing protease activity in situ, mapping the nuclear trafficking role of Vpx, and detailing the organization and function of vRNA during assembly, SRM has exposed dynamic processes that are otherwise difficult to capture. These findings broaden the scope of viral components accessible to direct imaging, revealing how transient interactions and spatial regulation contribute to HIV replication. Together, they demonstrate that even vRNA and non-structural proteins can yield pivotal insights when viewed through the lens of super-resolution microscopy.
Across studies targeting Env, Gag, MA, CA, integrase, vRNA, and accessory proteins, SRM has enabled researchers to visualize HIV biology with a level of spatial precision that conventional fluorescence microscopy cannot achieve [2,3]. As noted in Section 3.1, Section 3.2, Section 3.3, Section 3.4 and Section 3.5, this expanded resolution has revealed dynamic viral processes such as protein clustering, lattice remodeling, and RNA–protein coordination that would be difficult to discern by conventional imaging. Thus, by aligning SRM strategies with carefully designed labeling strategies and experimental goals, researchers can extract mechanistic insights from the sub-viral architecture of HIV, making SRM not just a technical advance but a transformative tool for molecular virology. As a result, this warrants a deeper analysis between the trends and associations that may exist between the SRM method, the HIV component(s) of interest, and the labeling method of choice across these 32 studies, as explored in Section 3.6, Section 3.7, Section 3.8 and Section 3.9.
3.6. Time Analysis of SRM and the HIV Components
The use of SRM to image HIV evolved over time (Figure 4A). From 2011 to 2013, the majority of studies used single-molecule localization microscopy (SMLM)—particularly PALM and STORM—with only one STED and one SIM publication, despite earlier availability of STED and SIM commercialization coinciding with PALM/STORM [1]. STED usage increased post-2017, and overall SRM choices became more varied, wherein one or two SRM methods did not overwhelmingly predominate usage during the most recent timeframe (2020–2023) compared with prior years (e.g., STORM in the 2011–2013 timeframe). Notably, SIM remained comparatively less common, except in 2014–2016 when its usage matched STORM. Such discrepancies could reflect SIM’s more restrictive resolution limits, limited access, or random variation, given the small number of total studies. Gaps are also evident, with no PALM studies published from 2014 to 2016 and none with STORM from 2017 to 2019.
Next, we tracked which HIV components were imaged over time (Figure 4B). Early SRM publications (2011–2013) shared focus among Gag, Env, capsid, and matrix, with two also including integrase (IN) and one examining Vpr [47,48]. A similar pattern persisted from 2014 to 2016, although fewer studies emerged overall. More recently, research into full-length Gag and Env increased, whereas matrix-focused studies ceased after 2017, and only two publications examined capsid in that time [45,46]. Meanwhile, two studies addressed Vpx and one covered vRNA (alongside Gag) [19,50,51]. Thus, recent SRM work has shifted toward probing Gag and Env, likely reflecting the biological and experimental relevance of these targets in HIV assembly, function, and interactions. Gag is the central structural protein driving viral assembly, making it important for imaging studies focused on particle formation [6]. As derivatives of full-length Gag, capsid (CA) and matrix (MA) are less central to the primary virion assembly process. Similarly, Env plays a critical role in viral entry and is a key determinant of infectivity [6]. Its position on the virion surface not only makes it highly accessible for various imaging labeling strategies but also marks it as a major target for therapeutic antibody development. Given their essential roles in virion structure, assembly, and host cell entry, it is unsurprising that SRM studies have increasingly prioritized Gag and Env to further insight into HIV biology.
3.7. Correlation Analysis of SRM and HIV Component
Further analysis also investigated how the choice of SRM method related to the HIV components imaged (Figure 5). PALM was used most with Gag, then Env, and sparingly with other proteins. STORM appeared across Gag, Env, capsid, and matrix, while STED was most frequent for Env but was also found in Gag and two capsid studies [22,45,46]. SIM did not dominate any single component but was uniquely linked to Vpx, driven by two papers from the same group [50,51]. This distribution suggests, unsurprisingly, that researchers may tend to use the SRM platform to which they have access unless a project’s specific requirements favor another technique.
A strong association emerged between Gag and PALM, likely due to PALM’s capacity for live-cell imaging (unlike STORM, due to toxic imaging buffers), which is advantageous in assembly-related studies [4,53]. By contrast, Env research showed diverse SRM usage, reflecting varied contexts (e.g., immature vs. mature virions, assembly sites). Capsid and matrix studies displayed more method heterogeneity, indicating less reliance on PALM and live-cell contexts compared with Gag. For other components (Vpx, vRNA, IN, and Vpr), the limited number of papers constrains clear conclusions. Of note, the single vRNA study that used PALM did so specifically to visualize Gag–vRNA interactions under live-cell conditions [19].
3.8. Correlation Analysis of Labeling Method and HIV Component
We next examined how different labeling strategies correlated with each HIV component under SRM (Figure 6A). Overall, antibody-, protein-, and Fab fragment-based methods were most common. Gag was predominantly imaged via protein-based labeling (e.g., the photoactivatable fluorescent protein mEOS2 [17]), whereas Env was typically labeled by either antibody or Fab fragment approaches at similar rates. This difference partly reflects their distinct locations: Env is surface-exposed, facilitating antibody binding, while Gag resides within the virion or cytosol, favoring protein tagging for live-cell studies [6]. With surface-exposed epitopes, Env is more immediately accessible to antibody-based probes. For targets inside the virion or cell, such as Gag, a permeabilization step may be needed for antibody exposure and binding, which may be incompatible with live-cell imaging (i.e., live-cell PALM) [54,55]. Hence, an internal protein component such as Gag, Vpr, or Vpx would favor a labeling strategy that incorporates a fluorescent protein label into its structure, especially if live-cell imaging is important for the context of the study. In contrast with Gag, however, capsid and matrix were often visualized by antibody labeling, a trend driven by the frequent use of fixed-cell/virion STORM, which allows permeabilization-based antibody access. Less-studied components (e.g., integrase, Vpr, Vpx) exhibited varied strategies: integrase was FlAsH- or antibody-tagged, whereas Vpr and Vpx were tagged exclusively via protein-based methods. As expected, genomic labeling (FISH or smFISH) was used solely for vRNA analysis.
3.9. Correlation Analysis of Labeling Method and SRM Technique
Finally, we mapped each SRM method to its corresponding labeling strategy (Figure 6B). SR SIM showed no strong pattern due to fewer overall data points, splitting relatively evenly among protein, antibody, nanobody/chromobody, and genomic labels. SR STED similarly used several labeling techniques, though Fab fragment-based approaches were most frequent. This was primarily attributable to the prominence of Env in STED studies, given that Env was the only target labeled with Fab fragments (Figure 6A). For PALM and STORM, clearer associations emerged. PALM favored protein-based and antibody-based labels, reflecting that Env and Gag were its primary targets, with Env commonly being antibody-labeled and Gag typically being protein-labeled (Figure 6A). PALM also exhibited more labeling diversity than STORM, which was limited to three labeling methods, favoring antibody-based approaches consistent with the high prevalence of Env, matrix, and capsid in STORM studies. Antibody-based or Fab fragment labeling is relatively simple and cost-effective [56,57], although for internal components requiring live-cell imaging, such as Gag, protein-based tags may be preferred.
4. Conclusions
This review underscores the significant impact of SRM in advancing our understanding of HIV structure and assembly. Over the past decade, usage of SRM methods such as PALM, STORM, SIM, and STED has evolved, with a trend towards more variability in the choice of microscopy methodology. PALM is frequently noted for usage in live-cell imaging contexts of HIV Gag, while STORM and STED are more evenly applied across various components. Additionally, the earliest studies rather equally favored imaging various HIV components, but recent research has increasingly focused on Gag and Env, reflecting a deeper exploration into HIV assembly and component interactions. The choice of SRM method and labeling technique varies, with protein-based labeling being prevalent for internal generalized Gag and antibody-based methods being used for surface components. Further key findings from this review, as well as some strengths and limitations for each SRM found in this review, are summarized in Table 3.

Table 3.
Summary of the SRM methods in HIV analysis: associations, strengths, and limitations.
Each of the four SRM methods covered here has advantages and disadvantages that influence the choice of method and experimental design (Table 3). STED offers improved spatial resolution and fast imaging, but it requires intense laser power that can lead to phototoxicity, limiting its utility in sensitive live-cell contexts [2,3]. PALM allows live-cell imaging with genetically encoded photoactivatable proteins but suffers from low photon output, which can reduce signal quality [2,4]. STORM provides exceptional resolution (~20 nm) in fixed samples but depends on specialized, often cytotoxic imaging buffers. SIM offers the broadest compatibility with standard dyes and live-cell imaging but achieves more modest resolution gains (~100 nm) [2,3,4,5]. Researchers should ultimately consider the specific advantages and limitations of each SRM method and labeling strategy in relation to their research goals, with some contexts offering more flexibility than others.
Additionally, with newer SRM methods continuing to push the boundaries of optical resolution, techniques like MINFLUX, expansion microscopy (ExM), and lattice light sheet (LLS) microscopy may provide new options for high-resolution studies in HIV and other applications [2]. For instance, MINFLUX enables near-molecular (~2 nm) precision at low excitation power, pushing the resolution boundary to lower sizes, while ExM physically enlarges samples for nanoscale imaging on standard microscopes, widening the accessibility of SRM [58,59]. LLS combines gentle illumination with high speed to capture dynamic processes with reduced phototoxicity [60]. Together, these and future innovations in SRM have the potential to enable access to even deeper insight into the nano-sized world of viruses and other subcellular constructs.
Overall, the application of SRM to HIV research has significantly advanced our understanding of the structural biology of HIV and virus–host interactions. By compiling and analyzing the methods and findings from past studies, this review aims to bring to light current SRM applications as an aid for future research efforts directed at unraveling the complexities of HIV and related particles through SRM. While this review has centered on HIV structure and assembly, SRM techniques have also contributed, and will continue to contribute, to studies of HIV in broader contexts, including virus–host interactions, immune evasion, and antiretroviral drug targeting. Ultimately, the continued innovation and application of SRM techniques will be instrumental in furthering our knowledge to assist in the fight against HIV and other viral diseases. It may also drive future developments of virus-like biological nanoparticles as novel therapeutics and drug delivery vehicles, ranging from artificial lipid nanoparticles to extracellular vesicles.
Author Contributions
Conceptualization, A.J., O.G. and K.W.W.; methodology, A.J. and O.G.; data Curation, A.J.; formal Analysis, A.J.; investigation, A.J.; resources, K.W.W.; writing—original draft preparation, A.J.; writing—review and editing, A.J., O.G. and K.W.W.; visualization, A.J. and O.G.; supervision, O.G. and K.W.W.; project administration, O.G.; funding acquisition, O.G. and K.W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This report was supported by the JHU CFAR NIH/NIAID fund P30AI094189 (to O.G.). The Witwer lab is supported in part by the NIH through AI144997, NCI/Common Fund CA241694, NIMH MH118164, The Paul G. Allen Frontiers Foundation, and the Richman Family Precision Medicine Center of Excellence in Alzheimer’s Disease at Johns Hopkins University.
Data Availability Statement
The original contributions presented in this study are included in the article. The total counts for each super-resolution microscopy method, HIV component, and labeling method are derived from the 32 papers discussed and cited in Section 3. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
K.W.W. is or has been an advisory board member of ShiftBio, Exopharm, NeuroDex, NovaDip, and ReNeuron; holds stock options with NeuroDex; and privately consults as Kenneth Witwer Consulting. Ionis Pharmaceuticals, Yuvan Research, AgriSciX, and the Michael J. Fox Foundation have sponsored or are sponsoring research in the Witwer laboratory.
References
- Prakash, K.; Diederich, B.; Heintzmann, R.; Schermelleh, L. Super-resolution microscopy: A brief history and new avenues. Philos. Transact. A Math. Phys. Eng. Sci. 2022, 380, 20210110. [Google Scholar] [CrossRef]
- Schermelleh, L.; Ferrand, A.; Huser, T.; Eggeling, C.; Sauer, M.; Biehlmaier, O.; Drummen, G.P.C. Super-resolution microscopy demystified. Nat. Cell Biol. 2019, 21, 72–84. [Google Scholar] [CrossRef]
- Huang, B.; Babcock, H.; Zhuang, X. Breaking the Diffraction Barrier: Super-Resolution Imaging of Cells. Cell 2010, 143, 1047–1058. [Google Scholar] [CrossRef]
- Khater, I.M.; Nabi, I.R.; Hamarneh, G. A Review of Super-Resolution Single-Molecule Localization Microscopy Cluster Analysis and Quantification Methods. Patterns 2020, 1, 100038. [Google Scholar] [CrossRef]
- Galbraith, C.G.; Galbraith, J.A. Super-resolution microscopy at a glance. J. Cell Sci. 2011, 124, 1607–1611. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Human Immunodeficiency Virus-1. Biological Agents; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- HIV Structure and Organisation. HIV Management Guidelines. Available online: https://hiv.guidelines.org.au/management/basic-hiv-virology/hiv-structure-and-organisation/ (accessed on 6 August 2024).
- Wainberg, M.A.; Jeang, K.-T. 25 years of HIV-1 research-progress and perspectives. BMC Med. 2008, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.A.; Wu, F.K.; Mitsuyasu, R.; Gaynor, R.B. Interactions of cellular proteins involved in the transcriptional regulation of the human immunodeficiency virus. EMBO J. 1987, 6, 3761–3770. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.M.; Dang, K.K.; Gorelick, R.J.; Leonard, C.W.; Bess, J.W.; Swanstrom, R.; Burch, C.L.; Weeks, K.M. Architecture and Secondary Structure of an Entire HIV-1 RNA Genome. Nature 2009, 460, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Scientific Image and Illustration Software|BioRender. Available online: https://www.biorender.com/ (accessed on 25 September 2024).
- Cyrklaff, M.; Frischknecht, F.; Kudryashev, M. Functional insights into pathogen biology from 3D electron microscopy. FEMS Microbiol. Rev. 2017, 41, 828–853. [Google Scholar] [CrossRef]
- Briggs, J.A.G.; Riches, J.D.; Glass, B.; Bartonova, V.; Zanetti, G.; Kräusslich, H.-G. Structure and assembly of immature HIV. Proc. Natl. Acad. Sci. USA 2009, 106, 11090–11095. [Google Scholar] [CrossRef]
- Liu, J.; Bartesaghi, A.; Borgnia, M.J.; Sapiro, G.; Subramaniam, S. Molecular architecture of native HIV-1 gp120 trimers. Nature 2008, 455, 109–113. [Google Scholar] [CrossRef]
- White, T.A.; Bartesaghi, A.; Borgnia, M.J.; de la Cruz, M.J.V.; Nandwani, R.; Hoxie, J.A.; Bess, J.W.; Lifson, J.D.; Milne, J.L.S.; Subramaniam, S. Three-Dimensional Structures of Soluble CD4-Bound States of Trimeric Simian Immunodeficiency Virus Envelope Glycoproteins Determined by Using Cryo-Electron Tomography. J. Virol. 2011, 85, 12114–12123. [Google Scholar] [CrossRef]
- Rantalainen, K.; Berndsen, Z.T.; Antanasijevic, A.; Schiffner, T.; Zhang, X.; Lee, W.-H.; Torres, J.L.; Zhang, L.; Irimia, A.; Copps, J.; et al. HIV-1 Envelope and MPER Antibody Structures in Lipid Assemblies. Cell Rep. 2020, 31, 107583. [Google Scholar] [CrossRef]
- Floderer, C.; Masson, J.-B.; Boilley, E.; Georgeault, S.; Merida, P.; El Beheiry, M.; Dahan, M.; Roingeard, P.; Sibarita, J.-B.; Favard, C.; et al. Single molecule localisation microscopy reveals how HIV-1 Gag proteins sense membrane virus assembly sites in living host CD4 T cells. Sci. Rep. 2018, 8, 16283. [Google Scholar] [CrossRef]
- Nieto-Garai, J.A.; Arboleya, A.; Otaegi, S.; Chojnacki, J.; Casas, J.; Fabriàs, G.; Contreras, F.; Kräusslich, H.; Lorizate, M. Cholesterol in the Viral Membrane is a Molecular Switch Governing HIV-1 Env Clustering. Adv. Sci. 2020, 8, 2003468. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qu, N.; Tan, J.; Rushdi, M.N.; Krueger, C.J.; Chen, A.K. Roles of Gag-RNA interactions in HIV-1 virus assembly deciphered by single-molecule localization microscopy. Proc. Natl. Acad. Sci. USA 2018, 115, 6721–6726. [Google Scholar] [CrossRef]
- Nolte-‘t Hoen, E.; Cremer, T.; Gallo, R.C.; Margolis, L.B. Extracellular vesicles and viruses: Are they close relatives? Proc. Natl. Acad. Sci. USA 2016, 113, 9155–9161. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Hanne, J.; Göttfert, F.; Schimer, J.; Anders-Össwein, M.; Konvalinka, J.; Engelhardt, J.; Müller, B.; Hell, S.W.; Kräusslich, H.-G. Stimulated Emission Depletion Nanoscopy Reveals Time-Course of Human Immunodeficiency Virus Proteolytic Maturation. ACS Nano 2016, 10, 8215–8222. [Google Scholar] [CrossRef] [PubMed]
- Muranyi, W.; Malkusch, S.; Müller, B.; Heilemann, M.; Kräusslich, H.-G. Super-Resolution Microscopy Reveals Specific Recruitment of HIV-1 Envelope Proteins to Viral Assembly Sites Dependent on the Envelope C-Terminal Tail. PLoS Pathog. 2013, 9, e1003198. [Google Scholar] [CrossRef]
- PubPeer-The Online Journal Club. Available online: https://www.pubpeer.com/ (accessed on 28 September 2023).
- Byrne, J.A.; Barnett, A.G. The research literature is an unsafe workplace. Account. Res. 2024, 1–8. [Google Scholar] [CrossRef]
- Chojnacki, J.; Staudt, T.; Glass, B.; Bingen, P.; Engelhardt, J.; Anders, M.; Schneider, J.; Müller, B.; Hell, S.W.; Kräusslich, H.-G. Maturation-Dependent HIV-1 Surface Protein Redistribution Revealed by Fluorescence Nanoscopy. Science 2012, 338, 524–528. [Google Scholar] [CrossRef]
- Chojnacki, J.; Waithe, D.; Carravilla, P.; Huarte, N.; Galiani, S.; Enderlein, J.; Eggeling, C. Envelope glycoprotein mobility on HIV-1 particles depends on the virus maturation state. Nat. Commun. 2017, 8, 545. [Google Scholar] [CrossRef]
- Chojnacki, J.; Eggeling, C. Super-Resolution STED Microscopy-Based Mobility Studies of the Viral Env Protein at HIV-1 Assembly Sites of Fully Infected T-Cells. Viruses 2021, 13, 608. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Sood, C.; Marin, M.; Aaron, J.; Gratton, E.; Salaita, K.; Melikyan, G.B. Super-Resolution Fluorescence Imaging Reveals That Serine Incorporator Protein 5 Inhibits Human Immunodeficiency Virus Fusion by Disrupting Envelope Glycoprotein Clusters. ACS Nano 2020, 14, 10929–10943. [Google Scholar] [CrossRef]
- Carravilla, P.; Chojnacki, J.; Rujas, E.; Insausti, S.; Largo, E.; Waithe, D.; Apellaniz, B.; Sicard, T.; Julien, J.-P.; Eggeling, C.; et al. Molecular recognition of the native HIV-1 MPER revealed by STED microscopy of single virions. Nat. Commun. 2019, 10, 78. [Google Scholar] [CrossRef]
- Sakin, V.; Hanne, J.; Dunder, J.; Anders-Össwein, M.; Laketa, V.; Nikić, I.; Kräusslich, H.-G.; Lemke, E.A.; Müller, B. A Versatile Tool for Live-Cell Imaging and Super-Resolution Nanoscopy Studies of HIV-1 Env Distribution and Mobility. Cell Chem. Biol. 2017, 24, 635–645.e5. [Google Scholar] [CrossRef]
- Mengistu, M.; Ray, K.; Lewis, G.K.; DeVico, A.L. Antigenic Properties of the Human Immunodeficiency Virus Envelope Glycoprotein Gp120 on Virions Bound to Target Cells. PLoS Pathog. 2015, 11, e1004772. [Google Scholar] [CrossRef]
- Malkusch, S.; Muranyi, W.; Müller, B.; Kräusslich, H.-G.; Heilemann, M. Single-molecule coordinate-based analysis of the morphology of HIV-1 assembly sites with near-molecular spatial resolution. Histochem. Cell Biol. 2012, 139, 173–179. [Google Scholar] [CrossRef]
- Pedersen, M.; Jamali, S.; Saha, I.; Daum, R.; Bendjennat, M.; Saffarian, S. Correlative iPALM and SEM resolves virus cavity and Gag lattice defects in HIV virions. Eur. Biophys. J. 2018, 48, 15–23. [Google Scholar] [CrossRef]
- Saha, I.; Saffarian, S. Dynamics of the HIV Gag Lattice Detected by Localization Correlation Analysis and Time-Lapse iPALM. Biophys. J. 2020, 119, 581–592. [Google Scholar] [CrossRef]
- Mao, S.; Ying, Y.; Ma, Z.; Yang, Y.; Chen, A.K. A Background Assessable and Correctable Bimolecular Fluorescence Complementation System for Nanoscopic Single-Molecule Imaging of Intracellular Protein-Protein Interactions. ACS Nano 2021, 15, 14338–14346. [Google Scholar] [CrossRef]
- Roy, N.H.; Chan, J.; Lambelé, M.; Thali, M. Clustering and Mobility of HIV-1 Env at Viral Assembly Sites Predict Its Propensity To Induce Cell-Cell Fusion. J. Virol. 2013, 87, 7516–7525. [Google Scholar] [CrossRef]
- Buttler, C.A.; Pezeshkian, N.; Fernandez, M.V.; Aaron, J.; Norman, S.; Freed, E.O.; van Engelenburg, S.B. Single molecule fate of HIV-1 envelope reveals late-stage viral lattice incorporation. Nat. Commun. 2018, 9, 1861. [Google Scholar] [CrossRef]
- Groves, N.S.; Bruns, M.M.; van Engelenburg, S.B. A Quantitative Live-Cell Superresolution Imaging Framework for Measuring the Mobility of Single Molecules at Sites of Virus Assembly. Pathogens 2020, 9, 972. [Google Scholar] [CrossRef]
- Snetkov, X.; Haider, T.; Mesner, D.; Groves, N.; van Engelenburg, S.B.; Jolly, C. A Conserved Tryptophan in the Envelope Cytoplasmic Tail Regulates HIV-1 Assembly and Spread. Viruses 2022, 14, 129. [Google Scholar] [CrossRef]
- Pereira, C.F.; Rossy, J.; Owen, D.M.; Mak, J.; Gaus, K. HIV taken by STORM: Super-resolution fluorescence microscopy of a viral infection. Virol. J. 2012, 9, 84. [Google Scholar] [CrossRef]
- Pham, S.; Tabarin, T.; Garvey, M.; Pade, C.; Rossy, J.; Monaghan, P.; Hyatt, A.; Böcking, T.; Leis, A.; Gaus, K.; et al. Cryo-electron microscopy and single molecule fluorescent microscopy detect CD4 receptor induced HIV size expansion prior to cell entry. Virology 2015, 486, 121–133. [Google Scholar] [CrossRef]
- Helma, J.; Schmidthals, K.; Lux, V.; Nüske, S.; Scholz, A.M.; Kräusslich, H.-G.; Rothbauer, U.; Leonhardt, H. Direct and Dynamic Detection of HIV-1 in Living Cells. PLoS ONE 2012, 7, e50026. [Google Scholar] [CrossRef]
- Hulme, A.E.; Kelley, Z.; Foley, D.; Hope, T.J. Complementary Assays Reveal a Low Level of CA Associated with Viral Complexes in the Nuclei of HIV-1-Infected Cells. J. Virol. 2015, 89, 5350–5361. [Google Scholar] [CrossRef]
- Zila, V.; Müller, T.G.; Laketa, V.; Müller, B.; Kräusslich, H.-G. Analysis of CA Content and CPSF6 Dependence of Early HIV-1 Replication Complexes in SupT1-R5 Cells. mBio 2019, 10, e02501-19. [Google Scholar] [CrossRef]
- Schifferdecker, S.; Zila, V.; Müller, T.G.; Sakin, V.; Anders-Össwein, M.; Laketa, V.; Kräusslich, H.-G.; Müller, B. Direct Capsid Labeling of Infectious HIV-1 by Genetic Code Expansion Allows Detection of Largely Complete Nuclear Capsids and Suggests Nuclear Entry of HIV-1 Complexes via Common Routes. mBio 2022, 13, e0195922. [Google Scholar] [CrossRef]
- Lelek, M.; Di Nunzio, F.; Henriques, R.; Charneau, P.; Arhel, N.; Zimmer, C. Superresolution imaging of HIV in infected cells with FlAsH-PALM. Proc. Natl. Acad. Sci. USA 2012, 109, 8564–8569. [Google Scholar] [CrossRef]
- Lehmann, M.; Rocha, S.; Mangeat, B.; Blanchet, F.; Uji-i, H.; Hofkens, J.; Piguet, V. Quantitative Multicolor Super-Resolution Microscopy Reveals Tetherin HIV-1 Interaction. PLoS Pathog. 2011, 7, e1002456. [Google Scholar] [CrossRef]
- Tabler, C.O.; Wegman, S.J.; Chen, J.; Shroff, H.; Alhusaini, N.; Tilton, J.C. The HIV-1 Viral Protease Is Activated during Assembly and Budding Prior to Particle Release. J. Virol. 2022, 96, e0219821. [Google Scholar] [CrossRef]
- Singh, S.P.; Raja, S.; Mahalingam, S. Lentiviral Vpx induces alteration of mammalian cell nuclear envelope integrity. Biochem. Biophys. Res. Commun. 2019, 511, 192–198. [Google Scholar] [CrossRef]
- Singh, S.P.; Raja, S.; Mahalingam, S. Viral protein X unlocks the nuclear pore complex through a human Nup153-dependent pathway to promote nuclear translocation of the lentiviral genome. Mol. Biol. Cell 2020, 31, 304–317. [Google Scholar] [CrossRef]
- Ferrer, M.; Clerté, C.; Chamontin, C.; Basyuk, E.; Lainé, S.; Hottin, J.; Bertrand, E.; Margeat, E.; Mougel, M. Imaging HIV-1 RNA dimerization in cells by multicolor super-resolution and fluctuation microscopies. Nucleic Acids Res. 2016, 44, 7922–7934. [Google Scholar] [CrossRef]
- Henriques, R.; Griffiths, C.; Hesper Rego, E.; Mhlanga, M.M. PALM and STORM: Unlocking live-cell super-resolution. Biopolymers 2011, 95, 322–331. [Google Scholar] [CrossRef]
- Slastnikova, T.A.; Ulasov, A.V.; Rosenkranz, A.A.; Sobolev, A.S. Targeted Intracellular Delivery of Antibodies: The State of the Art. Front. Pharmacol. 2018, 9, 1208. [Google Scholar] [CrossRef]
- Amidzadeh, Z.; Behbahani, A.B.; Erfani, N.; Sharifzadeh, S.; Ranjbaran, R.; Moezi, L.; Aboualizadeh, F.; Okhovat, M.A.; Alavi, P.; Azarpira, N. Assessment of Different Permeabilization Methods of Minimizing Damage to the Adherent Cells for Detection of Intracellular RNA by Flow Cytometry. Avicenna J. Med. Biotechnol. 2014, 6, 38–46. [Google Scholar]
- Allaire, A.; Picard-Jean, F.; Bisaillon, M. Immunofluorescence to Monitor the Cellular Uptake of Human Lactoferrin and its Associated Antiviral Activity Against the Hepatitis C Virus. J. Vis. Exp. JoVE 2015, 53053. [Google Scholar] [CrossRef]
- Gunasekara, H.; Perera, T.; Anderson, J.; Saed, B.; Ramseier, N.; Keshta, N.; Hu, Y.S. Superresolution Imaging with Single-Antibody Labeling. Bioconjug. Chem. 2023, 34, 825. [Google Scholar] [CrossRef]
- Chen, F.; Tillberg, P.W.; Boyden, E.S. Expansion microscopy. Science 2015, 347, 543–548. [Google Scholar] [CrossRef]
- Balzarotti, F.; Eilers, Y.; Gwosch, K.C.; Gynnå, A.H.; Westphal, V.; Stefani, F.D.; Elf, J.; Hell, S.W. Nanometer resolution imaging and tracking of fluorescent molecules with minimal photon fluxes. Science 2017, 355, 606–612. [Google Scholar] [CrossRef]
- Chen, B.-C.; Legant, W.R.; Wang, K.; Shao, L.; Milkie, D.E.; Davidson, M.W.; Janetopoulos, C.; Wu, X.S.; Hammer, J.A.; Liu, Z.; et al. Lattice light-sheet microscopy: Imaging molecules to embryos at high spatiotemporal resolution. Science 2014, 346, 1257998. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).