Abstract
Two new species of free-living marine nematodes were collected in the Yellow Sea, China, and they are described herein as Actinonema sinica sp. nov. and Comesoma zhangi sp. nov. Actinonema sinica sp. nov. is characterized by short cephalic setae; lateral differentiation consisting of a row of longitudinal sclerotized bars and beginning at the level of anterior third of the pharyngeal region; horn-shaped telamons; a curved rod-shaped gubernaculum; and an elongate conical tail with a smooth, pointed tip. Comesoma zhangi sp. nov. is characterized by long, thick cephalic setae, reaching up to 28 µm in length; a cup-shaped buccal cavity lacking a tooth and narrowing posteriorly with small projections; an amphidial fovea with two turns; slender, arcuate spicules 2.6 times the cloacal body diameter in length, lacking a proximal capitulum; a plate-like gubernaculum without apophysis; and the absence of precloacal supplements. Updated keys to the valid species of the genus Actinonema and the genus Comesoma are provided. A comparative morphological table of all currently accepted species of Comesoma is also provided.
1. Introduction
Free-living marine nematodes are the most dominant and diverse meiofaunal group in marine benthic habitats. However, a large number of nematode species are still undescribed. The nematode diversity in the marine regions of China remains largely unknown due to insufficient sampling efforts. Currently, approximately 600 species of free-living marine nematodes have been recorded from Chinese seas, which is estimated to represent less than half of the actual species diversity [1,2].
The Yellow Sea, located on the western edge of the Pacific Ocean, between China and the Korean Peninsula, is characterized by a high abundance of meiofauna, with free-living marine nematodes being the dominant group. They typically account for up to 90% of the total meiofaunal abundance in benthic habitats. The mean abundance of free-living nematodes has been estimated at 1663 ± 502 individuals 10 cm2 in the intertidal zone and 1184 ± 488 individuals 10 cm2 in the subtidal zone [3]. To evaluate nematode diversity in the Yellow Sea, large-scale biodiversity surveys and taxonomic investigations were initiated at the beginning of this century. These efforts have resulted so far in the identification of more than 400 nematode species, including 126 species that are new to science, representing approximately 30% of the total recorded fauna [1,4]. Nevertheless, the overall species richness of nematodes in this region remains uncertain, and new, additional species continue to be discovered [5,6]. This paper describes two new species of free-living marine nematodes discovered in the Yellow Sea, China.
The genus Actinonema Cobb, 1920 [7] belongs to the family Chromadoridae Filipjev, 1917, which includes five subfamilies, and the genus Actinonema belongs to the subfamily Euchromadorinae Gerlach & Riemann, 1973, all of which are marine species. Based on the literature and the database at Nemys [8], the genus comprises thirteen valid species [8,9,10]. In 1998, Muthumbi and Vincx reviewed two similar genera, Actinonema and Rhips Cobb, 1920, and assessed the taxonomic importance of the generic features of the two genera, noting that some species of Actinonema completely lack spicules [11]. Currently, three species, Actinonema diplobulba Chu, Hao & Huang, 2023 [9], A. pachydermatum Cobb, 1920 [7], and A. falciforme Shi, Yu & Xu, 2018 [12], have been recorded in Chinese seas. These species were found to inhabit the fine sand of intertidal beaches or subtidal silt–clay sediments. Regarding feeding modes among marine nematodes, Actinonema species are epistrate feeders, and they feed on diatoms. They have a buccal cavity with teeth, which they use for scraping food, piercing algal cell walls, and extracting cell content.
The genus Comesoma Bastian, 1865 [13] belongs to the family Comesomatidae Filipjev, 1918. Currently, thirteen valid species of Comesoma have been recorded worldwide [8]. Two species, C. sinica Fu, Cai, Leduc & Lin, 2022 [14] and C. quattuordecimsupplementata Xiao & Guo, 2023 [15], were discovered in Chinese seas. Both species are distributed in the fine sand of intertidal sand beaches. In terms of feeding, Comesoma species are non-selective deposit feeders. They have a cup-shaped buccal cavity without teeth, obtaining food by relying on the suction of the esophagus and the movements of the lips and the anterior part of the mouth. They mainly feed on fragments of decomposed organic matter.
The present work describes two new species of free-living marine nematodes from the Yellow Sea. As a result, the total number of nematode species recorded from the Yellow Sea has increased from 404 to 406, while the number of new species described from this region has risen from 126 to 128. In addition, this study contributes to improving the baseline data on the species diversity of free-living marine nematodes in Chinese marine waters.
2. Materials and Methods
In July 2018, undisturbed sediments were taken from a grid of 31 sampling stations between 32° N and 38°50′ N, and between 120°20′ E and 124°10′ E in the Yellow Sea, China. Underwater sediments were collected using a 0.1 m2 modified Gray-O’Hara box corer (Instrument and Equipment Factory of Ocean University of China, Qingdao, China). Meiofaunal samples were obtained from the top 0–8 cm layer of sediment using a 2.9 cm diameter cut-off syringe. The samples were fixed in an equal volume of 10% formalin solution. In the laboratory, samples were stained with 0.1% Rose Bengal solution for about six hours. The stained material was then passed through a set of two sieves with mesh sizes of 500 μm and 42 μm and rinsed with tap water to remove fine silt and to separate macrofauna from meiofauna. The residue retained on the 42 µm mesh was centrifuged in Ludox-TM (50% colloidal silica suspension in water; Sigma Aldrich Co., St. Louis, MO, USA) with a specific gravity of 1.15 g/mL [16] to isolate the meiofauna from the heavier sediment particles. The supernatant was collected after three rounds of centrifugation and filtered again through the 42 μm sieve to remove residual Ludox-TM. The retained sample was transferred into a Petri dish using distilled water, and the meiofauna were sorted under a stereoscopic microscope (Olympus SZX12, Olympus Corporation, Tokyo, Japan).
Specimens were transferred to a cavity block containing a solution composed of 5% glycerol, 5% absolute ethanol, and 90% distilled water by volume [17]. After gradual evaporation of the ethanol, the specimens were mounted in glycerin on permanent slides. The descriptions were made using a differential interference contrast microscope (Leica DM 2500, Leica Microsystems, CMS GmbH, Wetzlar, Germany. Assembled by Leica Microsystems, Shanghai, China), and photographs were taken with a Leica DMC 5400 digital camera (Leica Microsystems (Switzerland) Ltd., Heerbrugg, made in Germany). Line drawings were prepared with the aid of a camera lucida. All measurements were made using Leica LAS X software (version 3.3.3, Leica Microsystems, CMS GmbH, Wetzlar, Germany), with all curved structures measured along the midline of the curve. Type specimens were deposited at the Marine Biological Museum of the Chinese Academy of Sciences, Qingdao.
Abbreviations used in this article are as follows: a: ratio of body length to maximum body diameter; a.b.d.: body diameter at the cloaca or anus; At: number of turns of amphidial fovea; b: ratio of body length to pharynx length; c: ratio of body length to tail length; c′: ratio of tail length to cloacal or anal body diameter; c.b.d.: corresponding diameter of body; Cs: cephalic setae length; hd: head diameter; L: body length; Ps: number of precloacal supplements; Spic: spicule length measured as an arc; V%: position of vulva from anterior end expressed as a percentage of total body length.
3. Results and Discussion
3.1. Description of Actinonema sinica sp. nov. (Figure 1 and Figure 2)
Class Chromadorea Inglis, 1983
Order Chromadorida Chitwood, 1933
Family Chromadoridae Filipjev, 1917
Genus Actinonema Cobb, 1920
Diagnosis (emended from Tchesunov [18] and Muthumbi1 & Vincx [11]). Cuticle heterogeneous with lateral differentiation as a ridge beginning at the level of the pharynx. Anterior sensilla arranged in a 6 + 10 pattern with six outer labial and four cephalic setae arranged in a single circle. Amphidial fovea conspicuous transversally oval, and with a double contour. Buccal cavity with a large dorsal tooth. Male monorchic, with anterior testis. Spicules simple, maybe absent; telamon curved, usually L-shaped; gubernaculum simple, without apophysis. Precloacal supplements absent. Female with two opposed and reflexed ovaries, the anterior gonad positioned to the right of the intestine and the posterior gonad to the left. Tail conical.

Figure 1.
Line drawings of Actinonema sinica sp. nov. (a) Lateral view of the holotype anterior end, showing the cephalic setae, amphid, and buccal cavity tooth; (b) Lateral view of the holotype cloacal region, showing the telamon and gubernaculum; (c) Entire view of a female; (d) Entire view of the holotype. Scale bars: (a,b) = 10 μm; (c,d) = 30 μm.
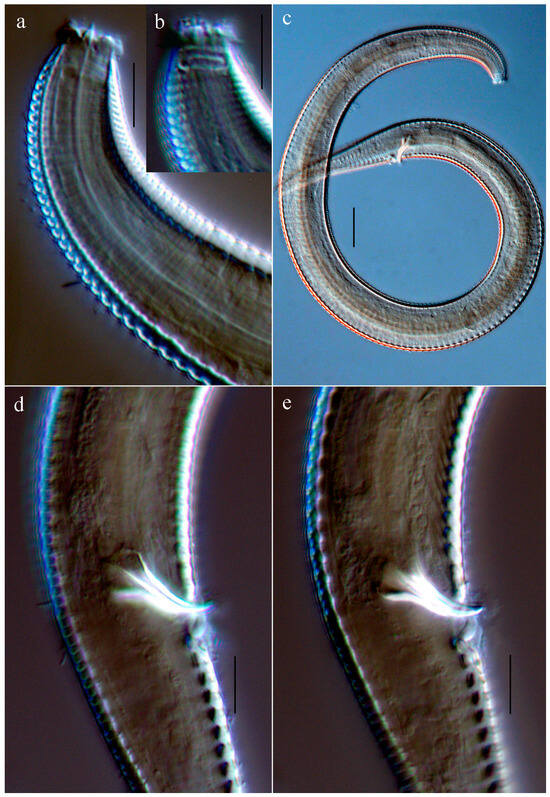
Figure 2.
Microphotographs of Actinonema sinica sp. nov. (a,b) Lateral view of the holotype anterior end, showing the buccal cavity tooth, cephalic setae, and amphid; (c) Entire view of a male; (d,e) Lateral view of the holotype cloacal region, showing the telamon and gubernaculum. Scale bars: (a,b,d,e) = 10 μm; (c) = 30 μm.
3.1.1. Type Material
Three males and three females were measured. Holotype ♂1 and paratype ♀1 are mounted on slide YS3400-3-0-3. Paratype ♂2 and ♀2 are on slide YS3400-4-0-14, ♂3 on slide YS3400-3-2-10, and ♀3 on slide YS3400-4-0-13.
3.1.2. Type Locality and Habitat
Holotypes and paratypes were all collected from silt–clay sediments on the seafloor at Station 3400-3 (34° N, 121°30′ E, water depth 17 m) and 3300-4 (33° N, 123° E, water depth 30 m) in the Yellow Sea.
3.1.3. Etymology
The species epithet sinica is derived from the Latin adjective referring to their country of origin, China.
3.1.4. Measurements
All measurement data are presented in Table 1.

Table 1.
Individual measurements of Actinonema sinica sp. nov. and Comesoma zhangi sp. nov. (in µm except for ratios).
3.1.5. Description
Males. Body slender and tapering towards the ends. Cuticle thick, annulated and heterogeneously punctated. Lateral differentiation consists of a row of longitudinal sclerotized bars, beginning at the level of anterior third of the pharynx and terminating near the middle of the tail. Anterior end blunt. Inner labial sensilla inconspicuous; outer labial and cephalic setae short (2–3 μm), positioned at the same level. Amphidial fovea transversely oval with a double contour, the anterior border situated 4–5 µm behind the head end; 6–7 µm in width, corresponding to 55–64% of the body diameter. Few somatic setae present in pharyngeal region and on tail. Mouth opening surrounded by sclerotized rugae; buccal cavity with a prominent dorsal tooth and two small subventral teeth. Pharynx cylindrical, with a posterior oval bulb. Nerve ring encircling the pharynx at approximately 58% of its length from the anterior end. Cardia small and conical. Reproductive system has an outstretched testis located to the right of the intestine. Spicules absent, while telamon present, sclerotized, 24–31 µm long, horn-shaped, slightly curved, with a broad proximal end that gradually tapers to a pointed distal end. Gubernaculum as a sclerotized rod, anterior half curved dorsally, 15–23 µm long. Tail elongate conical with a smooth pointed end.
Females. Body slender, tapering at both ends; tail relatively long and more slender. Cuticle thick, annulated, and heterogeneously punctated. Lateral differentiation represented by a longitudinal row of sclerotized bars, commencing at the anterior third of the pharynx and terminating near the mid-tail region. Anterior end blunt. Inner labial sensilla indistinct. Outer labial and cephalic setae short (2–3 μm), inserted at same level. Amphidial fovea transversely oval with a double contour, 7–8 µm wide, accounting for 64–73% of the body diameter. Mouth opening surrounded by sclerotized rugae. Buccal cavity with a prominent dorsal tooth and two small subventral teeth. Pharynx cylindrical with an oval bulb. Nerve ring encircling the pharynx at approximately 57% of its length from the anterior end. Reproductive system with two reflexed ovaries: the anterior ovary to the right and the posterior ovary to the left of the intestine. Oviduct a narrow tube. Vagina straight, 0.35 times vulval body diameter in the depth. Vulva non-protruding, located at 47–51% of the body length from the anterior end. Spermatheca not observed.
3.1.6. Differential Diagnosis and Discussion
Actinonema sinica sp. nov. is characterized by short cephalic setae; lateral differentiation consists of a row of longitudinal sclerotized bars, beginning at the level of anterior third of the pharynx; a horn-shaped telamon and a curved rod-shaped gubernaculum; and an elongate conical tail with a smooth, pointed tip. These characters agree well with the generic diagnosis of the genus.
The genus Actinonema was established by Cobb in 1920, and, to date, thirteen valid species, including recombined species such as A. megamphida (Boucher, 1971), have been identified [8,10]. The new species is similar to A. megamphida and A. pachydermatum Cobb, 1920 [7] in body size and shape, telamon, and gubernaculum, but differs from the former species by its narrower head (9 µm vs. 14–18 µm in width), smaller amphidial fovea (6–8 µm vs. 10–13 µm in width), and shorter cephalic setae (3 µm vs. 4–5 µm in length). The new species differs from A. pachydermatum by its narrower head (9 µm vs. 11.5 µm in width), lateral differentiation with longitudinal sclerotized bars beginning at the level of the anterior third of the pharynx (vs. irregular plates and bodies, beginning at the end of the pharynx), and curved, rod-shaped gubernaculum (vs. a thin, straight, plate-shaped gubernaculum). The new species is also distinguished from the other two species discovered in the Chinese seas, A. diplobulba Chu, Hao & Huang, 2023 [9], and A. falciforme Shi, Yu & Xu, 2018 [12]. The new species differs from A. diplobulba by having a single pharyngeal bulb (vs. double posterior bulbs), absence of spicules (vs. spicules present, slender, and bent), and a different telamon (horn-shaped vs. L-shaped). The new species differs from A. falciforme by the latter species having a smaller body (428–560 µm in length and 19–26 µm in maximum width), a falciform telamon, and lacking a gubernaculum. Actinonema sinica sp. nov. is distinguished from the other known species of the genus by its unique combination of characteristics: a longitudinal row of bar-shaped lateral differentiations beginning at the level of the anterior third of the pharynx, absence of spicules, a horn-shaped telamon, and a curved rod-shaped gubernaculum.
Updated identification key to valid Actinonema species, including the new species (modified after Chu et al. [9])
- 1 Head end with six sclerotic cones .......................................................................................... 2
- – Head end without sclerotic cones ........................................................................................... 5
- 2. Cephalic setae 1 μm long; tail elongate, posterior flagellum exceeding 70% of total tail length................................................................................ A. dolichurum Gagarin & Long, 2017
- – Cephalic setae > 2 μm long; tail short, lacking distinct posterior flagellum...................... 3
- 3. Dorsal tooth large; cuticle lacking V-shaped lateral differentiation........................... A. pachydermatum Cobb, 1920
- – Dorsal tooth small; cuticle with V-shaped lateral differentiation ..................................... 4
- 4. Body slender (a = 32–44); spicules absent .............. A. smolae Muthumbi and Vincx, 1998
- – Body thick (a = 22–23); spicules present ....................................... A. parvum Gagarin, 2015
- 5. Tail long with posterior flagellum exceeding 70% of total tail length ............................. 6
- – Tail short; posterior flagellum absent or indistinct.............................................................. 7
- 6. Males with cuticular extension anterior to cloaca ........ A. longicaudatoides Gagarin, 2015
- – Males without cuticular extension anterior to cloaca ……A. longicaudatum (Steiner 1918) Wieser 1954
- 7. Spicules present in males........................................................................................................ 8
- – Spicules absent in males......................................................................................................... 12
- 8. Telamon absent ....................................................................................................................... 9
- – Telamon present ...................................................................................................................... 11
- 9. Dorsal tooth small; males lacking distinct gubernaculum……...A. fidatum Vitiello, 1970
- – Dorsal tooth large; males with distinct gubernaculum……………………………..…….10
- 10. Spicules slender (24–31 μm), with narrow proximal end ………….A. grafi Jensen, 1991
- – Spicules conical (18–19 μm), with broad proximal end ……. megamphida (Boucher, 1971)
- 11. Pharynx with double posterior bulbs ................. A. diplobulba Chu, Hao & Huang, 2023
- – Pharynx with a single posterior bulb ............................................... A. celtica Boucher, 1976
- 12. Telamon present, falciform; gubernaculum absent…….A. falciforme Shi, Yu & Xu, 2018
- – Both telamon and gubernaculum present…………………………………………………..13
- 13. Telamon L-shaped, broad at mid-length.........A. paraceltica Muthumbi and Vincx, 1998
- – Telamon horn-shaped, broad proximally, tapering posteriorly………. A. sinica sp. nov.
3.2. Comesoma zhangi sp. nov. (Figure 3 and Figure 4)
Class Chromadorea Inglis, 1983
Order Araeolaimida De Coninck and Schuurmans Stekhoven, 1933
Family Comesomatidae Filipjev, 1918
Genus Comesoma Bastian, 1865
Diagnosis (emended from Fonseca & Bezerra [19]). Cuticle with transverse rows of very fine punctations, without lateral differentiation. Anterior sensilla arranged in a 6 + 6 + 4 pattern with two lateral outer labial setae usually longer than the other four outer labial setae. Subcephalic setae arranged in one or more circles of four setae each. Amphids mutispiral. Anterior portion of the buccal cavity cup-shaped, while the posterior portion is weakly sclerotized, and bears small blunt projections at the border to anterior portion. Spicules long and slender. Gubernaculum plate-like, usually without apophysis. Precloacal supplements may be present or absent.

Figure 3.
Line drawings of Comesoma zhangi sp. nov. (a) Entire view of the holotype; (b) entire view of a female; (c) lateral view of the holotype anterior end, showing the cephalic and subcephalic setae, amphid, pharynx, and excretory system; (d) lateral view of the holotype posterior end, showing the spicules, gubernaculum, and caudal glands; (e) lateral view of a female anterior end, showing the cephalic and subcephalic setae, amphid, pharynx, and excretory system. Scale bars: (a,b) = 100 μm; (c–e) = 20 μm.
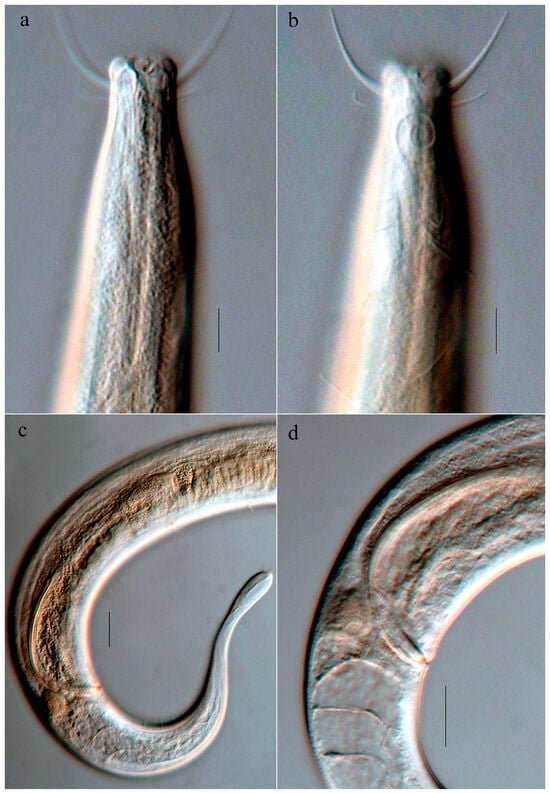
Figure 4.
Microphotographs of Comesoma zhangi sp. nov. (a,b) Lateral view of the holotype anterior end, showing the buccal cavity, cephalic, subcephalic and cervical setae, and amphid; (c) lateral view of the holotype posterior end, showing the spicules and tail; (d) lateral view of the holotype cloacal region, showing the spicules, gubernaculum, and caudal glands. Scale bars: (a,b) = 10 μm; (c,d) = 20 μm.
3.2.1. Type Material
One male and one female were measured. Holotype ♂1 and paratype ♀1 are mounted on slide WHSD5-1-4.
3.2.2. Type Locality and Habitat
Holotype and paratype female 1 were collected from intertidal fine sand in Weihai City, the Yellow Sea (37° 8.6′ N 122°45.9′ E).
3.2.3. Etymology
The specific epithet zhangi is dedicated to Professor Zhinan Zhang, a nematologist at the Ocean University of China.
3.2.4. Measurements
All measurement data are presented in Table 1.
3.2.5. Description
Male. Body cylindrical, gradually tapering towards both ends. Cuticle bearing transverse rows of fine punctations starting from the posterior half of the amphid and extending nearly to the tail tip. Lateral differentiation absent. Head blunt, slightly set off by a contraction at the level of the cephalic setae. Head diameter 15 μm. Anterior sensilla arranged in three distinct circles; six small inner labial papillae, six outer labial papillae, and four very long, thick cephalic setae, 27 μm long, equivalent to 1.8–1.9 times the head diameter, located about half a head diameter from the anterior end; a circle of four short subcephalic setae (12 μm long) follows immediately behind. Cervical setae (10–14 µm long) numerous and scattered. Amphidial fovea multispiral with two turns, 12 µm wide, occupying approximately 75% of the corresponding body diameter, and located immediately posterior to the circle of subcephalic setae. Buccal cavity small, cup-shaped without a tooth; its posterior portion narrowed and bearing small projections. Pharynx cylindrical, gradually enlarging posteriorly but not forming a true posterior bulb. Nerve ring located at about mid-length of the pharynx. Secretory-excretory system composed of a large unicellular gland cell located at the level of the anterior intestine; the secretory-excretory duct with a distinct ampulla opens immediately posterior to the nerve ring, approximately 145 µm from the anterior end. Tail conico-cylindrical, with a conical anterior half and a cylindrical posterior half; tail tip slightly swollen, with two terminal setae about 5 μm long. Subventral caudal setae measure 6–8 µm long. Three caudal glands and a spinneret present.
Reproductive system has two opposed, outstretched testes. The anterior testis lies to the left of the intestine, and the posterior testis to the right. Spicules slender, arcuate, approximately 2.6 times the cloacal body diameter, with a slightly pointed distal end and lacking a proximal capitulum. Gubernaculum plate-like, 26 µm long, without apophysis. Precloacal supplements absent. A single short precloacal setae, 4 µm long, is located immediately anterior to the cloaca.
Female. Body cylindrical, gradually tapering towards both ends. Cuticle with transverse rows of fine punctation; lateral differentiation absent. Head blunt, slightly set off. Anterior sensilla arranged in three distinct circles: six inner labial small papillae, six outer labial papillae, and four long, thick cephalic setae (28 μm, 1.9 head diameter long), located approximately half a head diameter from the anterior end; followed by a circle of four short subcephalic setae (12 μm long). Cervical setae numerous, scattered, 6–10 μm long. Amphidial fovea multispiral with two turns, 8 µm wide, occupying approximately 53% of the corresponding body diameter, located just posterior to the circle of subcephalic setae. Buccal cavity small, cup-shaped, without tooth; its posterior portion narrowed and bearing small projections. Pharynx cylindrical, gradually widening posteriorly but not forming true posterior bulb. Nerve ring located at about mid-length of the pharynx. Secretory-excretory system well-developed; the excretory duct with distinct ampulla opens immediately posterior to the nerve ring. Tail conico-cylindrical, tail tip slightly swollen, without caudal setae. Three caudal glands and a spinneret present.
Reproductive system has two opposed, outstretched ovaries. The anterior ovary lies to the left of the intestine and the posterior ovary to the right. Vagina short, with thick walls. Spermatheca present. Vulva outwardly protruding, situated at mid-body.
3.2.6. Diagnosis and Discussion
Comesoma zhangi sp. nov. is characterized by very long, thick cephalic setae (up to 28 µm in length); a cup-shaped buccal cavity without a tooth; a multispiral amphidial fovea with two turns; slender, arcuate spicules measuring approximately 2.6 times the cloacal body diameter and lacking a proximal capitulum; a plate-like gubernaculum without an apophysis; and an absence of precloacal supplements.
The genus Comesoma was established by Bastian in 1865 [13]. Currently, thirteen valid species of Comesoma have been recorded worldwide [8]. However, C. heterosetosum Gerlach, 1955 [20] and C. simile Cobb, 1898 [21] were described solely based on female specimens, lacking descriptions of male characters, and should therefore be considered species inquirendae. Comesoma zhangi sp. nov. is most similar to C. sinica Fu, Cai, Leduc & Lin, 2022 [14] in body shape and size. However, the new species differs from C. sinica by having longer and thicker cephalic setae (1.8–1.9 vs. 1.3–1.5 times the corresponding body diameter), fewer turns of the amphidial fovea (2 vs. 2.5 turns), shorter spicules (2.6 vs. 3.5–4.3 times the cloacal body diameter), and by the absence of precloacal supplements in males (vs. approximately 20 pore-like precloacal supplements in C. sinica). The new species differs from another species found in China, C. quattuordecimsupplementata Xiao & Guo, 2023 [15], by its longer body length (2182–2500 µm vs. 1293–1498 µm long), longer and thicker cephalic setae (27–28 µm vs. 9–10 µm), fewer turns of the amphidial fovea (2 vs. 2.5–2.75 turns), longer tail (5–5.4 vs. 4.2–4.4 times the cloacal body diameter), and absence of precloacal supplements (vs. 14 fibriform precloacal supplements in C. quattuordecimsupplementata). Comesoma zhangi sp. nov. can be identified among its congeners by its very long, thick cephalic setae, relatively shorter spicules (2.6 times the cloacal body diameter), and absence of precloacal supplements. The difference between Comesoma zhangi sp. nov. and other known species in the genus can be inferred from the key below.
Regrettably, only one male and one female were found in the samples, suggesting that this is a rare species. Although the number of specimens is limited, the morphological characteristics of the present species are distinct and allow it to be clearly distinguished from other known species of the genus. The morphological data of all known valid Comesoma species, together with those of the new species, are presented in Table 2.

Table 2.
Morphometric data of all valid Comesoma species and the new species (after Xiao and Guo [15]).
Key to valid Comesoma species with males, including the new species
- 1. Body thick; de Man ratio a equal or less than 30................................................................. 2
- – Body slender; de Man ratio a higher than 30........................................................................ 4
- 2. Cephalic setae longer than 1 hd; spicules 6.3 a.b.d…..…… C. minimum Chitwood, 1937
- – Cephalic setae 0.7–0.9 hd; spicules shorter than 4 a.b.d. …...……………………………..3
- 3. Body length shorter than 2 mm; amphidial fovea with 2.5 to 2.75 turns……………….C. quattuordecimsupplementata Xiao & Guo, 2023
- – Body length longer than 3 mm; amphidial fovea with 1.75 turns……………….. C. solum Pastor de Ward, 1984
- 4. Spicules longer than 270 μm; amphidial fovea with 2 turns…… C. arenae Gerlach, 1956
- – Spicules shorter than 200 μm; amphidial fovea with 2 to 2.75 turns……………………. 5
- 5. Body length 3.6–4.6 mm; spicules 165–198 μm…………………………………………… 6
- – Body length less than 3.5 mm……………………………………………………………….. 7
- 6. Body length 4.6 mm long; spicules 165 μm …………….. C. stenocephalum Filipjev, 1918
- – Body length 3.6 mm long; spicules 198 μm ……………………... C. vulgare Bastian, 1865
- 7. Spicules longer than 150 μm……………………………………………………………….. 8
- – Spicules shorter than 120 μm……………………………………………………………….11
- 8. Cuticle with longitudinal rows of markings; transverse markings absent………………
- .............................................................................................................. . C. profundi Bastian, 1865
- – Cuticle lacking longitudinal markings; transverse punctations present………………... 9
- 9. Cephalic setae 0.6–0.8 hd; de Man ratio b = 7.6–9.1……… C. hermani Chen & Vincx, 1998
- – Cephalic setae 1.3–1.5 hd; de Man ratio b = 8.2–12……………………………………… 10
- 10. Cuticle with lateral differentiation; de Man c = 8.5–11.3 ………….. C. bermudense Jensen & Gerlach, 1977
- – Cuticle lacking lateral differentiation; de Man c = 12–17………. C. sinica Fu, Cai, Leduc & Lin, 2022
- 11. Body length 1.6 mm; amphidial fovea with 2.5 turns; de Man ratio c = 9………………...
- ………………………………………………………………. C. tenuispiculum (Ditlevsen, 1921)
- – Body length 2.1–2.5 mm; amphidial fovea with 2 turns; de Man ratio c = 11.5–12.8……..
- …………………………………………………………………………………C. zhangi sp. nov.
Author Contributions
Preparation of specimens, data measurement, X.S.; writing—review and editing, Y.H.; funding acquisition, Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant number 41676146.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are very thankful to all crew members of R/V Kexue 3 for their kind help in sample collection and to Ruobing Bai for her help in preparing the original draft of the manuscript. We are sincerely grateful to two anonymous reviewers for their patient reviewing and improvement of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sun, X.Y.; Bai, R.B.; Zhai, H.X.; Huang, Y. Species list of free-living marine nematodes in the Yellow Sea, China. J. Liaocheng Univ. 2024, 37, 34–52. [Google Scholar] [CrossRef]
- Huang, M.; Zhai, H.X. Two new species of the genus Halichoanolaimus (Nematoda, Selachinematidae) from the intertidal zone of the Yellow Sea, China. ZooKeys 2024, 1208, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.D.; Chu, M.D.; Huang, Y. Trefusia brevicauda sp. nov. and Conilia parasinensis sp. nov. (Nematoda: Enoplida) from the Yellow Sea, China. Cah. Biol. Mar. 2022, 63, 257–267. [Google Scholar] [CrossRef]
- Sun, L.Y.; Zhai, H.X.; Huang, Y. Three new species of the order Enoplida (Nematoda) from the Yellow Sea China. J. Oce. Lim. 2025, 43, 984–995. [Google Scholar] [CrossRef]
- Huang, M.; Sun, X.Y.; Bai, R.B. Two new species of Eleutherolaimus Filipjev, 1922 (Nematoda: Linhomoeidae) from the Yellow Sea, China. J. Nat. Hist. 2025, 59, 1127–1140. [Google Scholar] [CrossRef]
- Zhai, H.X.; Liu, L.; Huang, M. Two new nematode species of the family Sphaerolaimidae (Nematoda, Monhysterida) from the Yellow Sea, China. Zootaxa 2025, 5631, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Cobb, N.A. One hundred new nemas (type species of 100 new genera). Contributions to a science of nematology. Contrib. A Sci. Nematol. 1920, 9, 217–343. [Google Scholar]
- Nemys: World Database of Nematodes. Available online: https://www.marinespecies.org/imis.php?dasid=66&doiid=366 (accessed on 1 August 2025).
- Chu, M.D.; Hao, Y.D.; Huang, M. Two new species of Chromadorida (Nematoda) from the inter-tidal zone of the Yellow Sea, China. J. Nat. Hist. 2023, 57, 1364–1376. [Google Scholar] [CrossRef]
- Boucher, G.; de Bovée, F. Trochamus carinatus gen. et sp. n. et Adeuchromadora megamphida gen. et sp. n. Chromadoridae Nematoda) à dix soies céphaliques de la vase terrigène côtière de Banyuls-sur-Mer. Vie Milieu. 1971, 22, 231–241. [Google Scholar]
- Muthumbi, A.W.; Vincx, M. Chromadoridae (Chromadorida: Nematoda) from the Indian Ocean: Difficulties in morphological identification of Actinonema Cobb, 1920 and Rhips, Cobb, 1920. Hydrobiologia 1998, 364, 155–167. [Google Scholar] [CrossRef]
- Shi, B.Z.; Yu, T.T.; Xu, K.D. A new free-living nematode, Actinonema falciforme sp. nov. (Nematoda: Chromadoridae), from the continental shelf of the East China Sea. Acta Oceanol. Sin. 2018, 37, 152–156. [Google Scholar] [CrossRef]
- Bastian, H.C. Monograph of the Anguillulidae, or Free Nematoids, Marine, Land, and Freshwater; with Descriptions of 100 New Species. Tran. Lin. Soc. Lond. 1865, 25, 73–184. [Google Scholar] [CrossRef]
- Fu, S.J.; Cai, L.Z.; Leduc, D.; Lin, H.S. One new species of Comesoma Bastian, 1865 (Nematoda: Araeolaimida: Comesomatidae) and redescription with molecular characterisation of Sphaerolaimus callisto Zograf, Pavlyuk, Trebukhova & Nguyen, 2020 (Nematoda: Monhysterida: Sphaerolaimidae) from the South China Sea. Nematology 2022, 24, 1139–1155. [Google Scholar]
- Xiao, Y.P.; Guo, Y.Q. Three new free-living marine nematode species of the family Comesomatidae from Mangrove wetlands in Jinmen County, Taiwan. Zootaxa 2023, 5369, 513–532. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, V.N.; Bouwman, L.A. A simple density separation technique for quantitative isolation of meiobenthos using the colloidal silica Ludox-TM. Mar. Biol. 1977, 42, 143–148. [Google Scholar] [CrossRef]
- McIntyre, A.D.; Warwick, R.M. Meiofauna techniques. In Methods for the Study of Marine Benthos; Holme, N.A., McIntyre, A.D., Eds.; Blackwell Scientific Publications: Oxford, UK, 1984; pp. 217–244. [Google Scholar]
- Tchesunov, A.V. Order Chromadorida. In Handbook of Zoology; Schmidt-Rhaesa, A., Ed.; De Gruyter: Berlin, Germany, 2014; Volume 2, pp. 373–398. [Google Scholar]
- Fonseca, G.; Bezerra, T.N. Order Araeolaimida. In Handbook of Zoology; Schmidt-Rhaesa, A., Ed.; De Gruyter: Berlin, Germany, 2014; Volume 2, pp. 435–465. [Google Scholar]
- Gerlach, S.A. Zur Kenntnis der freilebenden marinen Nematoden von San Salvador. Z. Für Wiss. Zool. 1955, 158, 249–303. [Google Scholar]
- Cobb, N.A. Australian free-living marine nematodes. Proc. Linn. Soc. New South Wales 1898, 23, 383–407. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).