Abstract
Direct Z-scheme heterojunctions are known for their unique carrier mobility mechanism, which significantly improves photocatalytic water splitting efficiency. In this study, we use first-principles simulations to determine the stability, electrical, and photocatalytic properties of a SnC/SnS2 heterojunction. Analyses of the projected energy band and state density demonstrate that the SnC/SnS2 heterojunction exhibits an indirect band gap of 0.80 eV and a type-II band alignment. Analysis of its work function shows that the SnC/SnS2 heterojunction has a built-in electric field pointing from the SnC monolayer to the SnS2 monolayer. The band edge position and the differential charge density indicate that the SnC/SnS2 heterostructure exhibits a Z-scheme photocatalytic mechanism. Furthermore, the SnC/SnS2 heterojunction exhibits excellent visible-light absorption and high solar-to-hydrogen efficiency of 32.8%. It is found that the band gap and light absorption of the heterojunction can be effectively tuned by biaxial strain. These results demonstrate that the fabricated SnC/SnS2 heterojunction has significant photocatalysis potential.
1. Introduction
The current global situation is facing two key challenges: energy scarcity [1,2] and environmental pollution [3]. The depletion of old fossil energy sources [4] and air pollution [5] resulting from their use have a considerable negative influence on our quality of life and future possibilities for sustainable development. The search for new clean energy sources is urgent. Hydrogen energy is the best energy source for future sustainable development [6,7], and the semiconductor photocatalytic hydrolysis technique is regarded as the most efficient method of hydrogen production [8]. Two-dimensional (2D) materials exhibit superior photocatalytic properties compared with those of semiconductor materials [9,10]. However, in some 2D materials, rapid photogenerated electron–hole recombination results in diminished photocatalytic activity [11,12]. In this regard, heterojunctions [13,14] have been proposed as an effective strategy to overcome the deficiency of electron–hole recombination and diminished photocatalytic activity.
By utilizing the energy band difference between two semiconductors, photogenerated electrons and holes at the interface of heterojunctions can be separated, thereby improving carrier separation efficiency [15,16]. Photocatalytic heterostructures include type-II and Z-scheme heterostructures. However, conventional type-II heterojunctions face challenges in effectively separating photogenerated electrons and holes at the heterojunction interface due to limitations in their band structure, which consequently reduces the efficiency of photocatalytic reactions. To overcome this difficulty, Yu et al. [17] introduced the concept of direct Z-type heterostructure photocatalysts. Z-type heterojunctions generate photogenerated electrons and holes in different semiconductor materials, thereby separating them at the interface and improving carrier separation efficiency. Numerous studies on Z-type heterojunctions have been reported, including GeC/HfS2 [18], g-C3N4/MoS2 [19] and GaN/BS [20].
The graphene-like structure SnC exhibits structural stability and an indirect bandgap [21,22]. It also exhibits excellent electron conductivity and superior light absorption [22]. In addition, SnC can efficiently convert light energy into chemical energy, making it a promising photocatalytic material [23,24]. SnC has high theoretical capacity and conductivity, making it suitable as an anode material for potassium- and sodium-ion batteries [25,26]. In addition, SnC has a suitable band edge position and can be used as a photocatalytic reduction reaction material [25,26]. Currently, several studies on SnC heterojunction photocatalysis have been reported. Because of the suitable band gap of SnC, a heterojunction composed of SnC and HfSSe exhibits high interlayer carrier recombination [27]. In addition, a high light absorption capacity [28] was reported in a heterojunction formed by SnC and BAs. A high energy conversion rate [29] was found in a heterojunction composed of SnC and PtSe2.
Due to its suitable band gap, good photovoltaic performance, and unique optical properties, SnS2 exhibits potential for use in a wide variety of fields [30,31,32]. In the field of optoelectronic devices, Huang et al. [33] employed chemical vapor deposition (CVD) to prepare SnS2 nanosheets with excellent optoelectronic properties. In the field of battery materials, SnS2 exhibits good conductivity and chemical stability and has been a popular electrode material for lithium batteries [34,35,36]. Chen et al. [37] synthesized SnS2 thin films using CVD to optimize preparation conditions and achieve efficient photoelectrochemical water decomposition under visible light. In addition, a SnSe2/SnS2 heterojunction with an exceptionally high switching ratio and high responsivity in response to electric fields has been fabricated [38]. A SnO2/SnS2 heterojunction with remarkable activity and exceptional stability in electrocatalytic hydrogen evolution reactions (HERs) has also been reported [39]. A recent study indicated that heterojunction constructed from SnC and CdS exhibit excellent photocatalytic performance [40,41].
In this work, by using first-principles calculations, we constructed a SnC/SnS2 heterojunction and investigated its electronic and photocatalytic properties. We found that the SnC/SnS2 is a z-scheme heterojunction that effectively separates photogenerated electrons and holes. Furthermore, the SnC/SnS2 heterojunctions exhibit excellent light absorption performance and high solar-to-hydrogen (STH) efficiency, making it a promising photocatalytic material.
2. Computation Details
All calculations were carried out using the Vienna Ab initio Simulation Package (VASP), which is based on density functional theory (DFT) [42]. To describe the exchange–correlation interaction, the Perdew–Burke–Ernzerhof (PBE) function was used, which is based on the generalized gradient approximation (GGA) [43]. The cut-off energy was set at 500 eV and a k-point grid of was sampled in the Brillouin zone. All atoms were relaxed sufficiently to reach the convergence criteria of eV for total energy and for maximum force. Furthermore, because the PBE functional typically results in an underestimation of the band gap and optical absorption, we used a hybrid Heyd–Scuseria–Ernzerhof functional (HSE06) method to calculate the band structure [44]. In particular, the DFT-D3 method was applied to correct the interlayer van deer Waals (vdW) interaction [45]. The vacuum thickness along the z-axis was set to 20 to prevent interactions between adjacent layers. In addition, dipole corrections were used to obtain more accurate potential energy and forces [46].
The thermal stability of the SnC/SnS2 heterojunction was verified via using ab initio molecular dynamics (AIMD) [47]. A 5 × 5 × 1 supercell was used to calculate the energy over 5000 fs at 300 K. In addition, Phonopy 2.12.0 software was used to analyze the phonon dispersion spectrum to investigate the dynamic stability of the SnC/SnS2 heterojunction [48].
3. Results and Discussion
3.1. Geometric Structure and Stability
To construct the heterojunction, we calculated the structural and electronic properties of each component in the SnC/SnS2 heterojunction. The top and side views of the optimized crystal structure of each material are shown in Figure 1. The optimized lattice constants of the SnC and SnS2 monolayers are 3.59 and 3.70 Å, respectively, which are consistent with the values reported before [29,49]. The band structure calculated using the HSE06 method indicates that the SnC and SnS2 monolayers possess indirect bandgaps of 1.77 eV and 2.46 eV, respectively, which agree well with those reported in previous reports [49,50]. The valence band maximum (VBM) and conduction band minimum (CBM) of SnC are −4.87 and −3.10 eV, respectively. In contrast, the VBM and CBM of SnS2 are −7.17 and −4.71 eV, respectively.
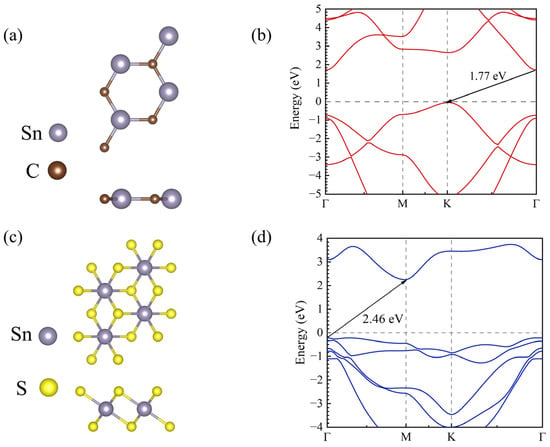
Figure 1.
Top and side views of the optimized structures of (a) SnC monolayer and (c) SnS2 monolayer; (b,d) their corresponding band structures, respectively.
The lattice constants are close; thus, the constructed heterostructure composed of SnC and SnS2 only exhibits a lattice mismatch rate of 2.96%. By shifting and rotating the SnC layer, we constructed six configurations for the SnC/SnS2 heterojunction: AA, AB, AC, BA, BB, and BC (Figure 2). The three configurations of BA, BB and BC were constructed by rotating the SnC layer by 180°. A and B on the left of the name represent the Sn and C atoms in the upper SnC layer of the heterojunction, respectively. A, B, and C on the right side of the name represent the alignment mode, where the upper atoms align with the lower S atom, the upper S atom, and the Sn atom in the lower SnS2 layer, respectively.
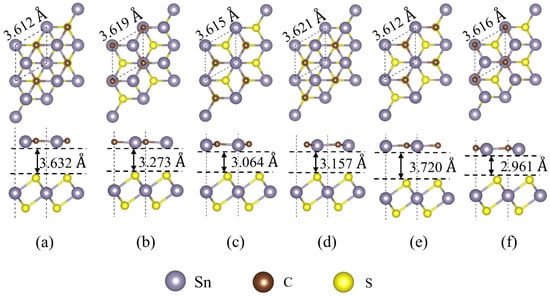
Figure 2.
Top and side views of the optimized structures of SnC/SnS2 heterostructure with six different configurations: (a) AA, (b) AB, (c) AC, (d) BA, (e) BB and (f) BC configurations. The BA, BB and BC configurations are obtained by rotating the SnC layer by 180°.
To identify the most stable configuration, the binding energies of the six heterostructures were calculated. The following formula was used to calculate the binding energy Eb [51]:
where , , and denote the total energies of SnC/SnS2 vdWHs, SnC, and SnS2 layers, respectively.
Table 1 shows that the binding energy of each configuration is negative, confirming their energy stability. The BC configuration exhibits the lowest binding energy (−3.983 eV). Table 1 also lists the optimized lattice constants and equilibrium interlayer distances for the six heterostructures. The interlayer distances for the AA, AB, AC, BA, BB, and BC configurations are 3.632, 3.273, 3.064, 3.157, 3.720, and 2.961 Å, respectively. This suggests that the SnC and SnS2 monolayers are connected and bound together by interlayer vdW forces.

Table 1.
Optimized lattice constants, equilibrium interlayer distance d, and binding energy Eb for the stacking of SnC/SnS2 heterostructure.
To further verify the stability of the BC configuration, we applied AIMD. Figure 3a shows that during a 5000-fs AIMD simulation at 300 K, no deformation was observed in the structural integrity of the SnC/SnS2 heterojunction structure and that the energy remains within a certain range, demonstrating its stability. Meanwhile, we monitored the lattice constants and the average bond distance values before and after the simulation. It was found that the lattice constants remain almost unchanged during the simulation, while the average bond distance is within 5%. It further demonstrates the stability of SnC/SnS2 heterojunction. Furthermore, we calculated the phonon spectrum of the SnC/SnS2 heterojunction with BC configuration. The results presented in Figure 3b indicate the absence of any negative frequency within the Brillouin zone. This implies that the SnC/SnS2 vdW heterostructure with BC configuration is stable [52]. Therefore, in the following discussion, we use the BC structure as the typical configuration for the SnC/SnS2 heterojunction.
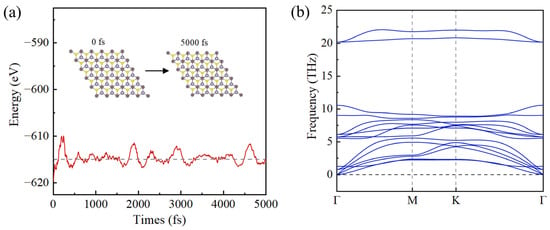
Figure 3.
(a) The variation in total energy of SnC/SnS2 heterostructure at 300 K under continuous 5000 fs AIMD simulation. The insets show the initial and final structures in the AIMD simulation. (b) Phonon dispersion of SnC/SnS2 heterojunction with BC configuration.
3.2. Electronic Properties
To investigate the electronic properties of the SnC/SnS2 heterostructure, we calculate its band structure and density of states (DOS) using the HSE06 method. As shown in Figure 4a, the SnC/SnS2 heterostructure exhibits an indirect bandgap of 0.80 eV. The CBM of the heterostructure is positioned at the M point, which is contributed by SnS2. Its VBM is situated at the K point, which is contributed by SnC. This suggests that the heterostructure exhibits a type-II band alignment, which effectively inhibits the recombination of photogenerated electrons and holes, thereby enhancing photocatalytic efficiency.
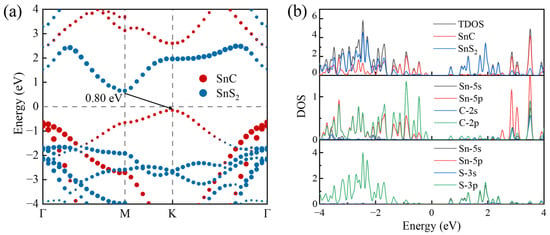
Figure 4.
(a) The projected band structures and (b) the total and partial DOS of the SnC/SnS2 heterostructure obtained by HSE06 function.
Figure 4b shows the DOS of the SnC/SnS2 vdW heterostructure. Based on this figure, the VBM of the heterojunction mostly originates from SnC, whereas its CBM originates from SnS2. The partial DOS indicates that the CBM of the heterostructure is primarily derived from the Sn-p orbital. In contrast, its VBM is primarily derived from the S-p and C-p orbitals. These findings are consistent with the analysis of the band structures, indicating that the contact between SnC and SnS2 exhibits a type-II band alignment. However, polar solutions such as water may have an effect on the stability and band gap of the heterojunction. Therefore, these factors should be taken into account in practical application [53].
To evaluate the interlayer charge transfer, we further explore the work function. The work functions of the SnC and SnS2 monolayers and heterojunction were calculated as follows:
where , and represent the work function, vacuum level and Fermi level, respectively. The work functions of the SnC monolayer and SnS2 monolayer are 4.729 eV [54] and 6.586 eV [55], respectively. The disparity in work function between SnC and SnS2 monolayers leads to the redistribution of charges. When the two monolayers come into contact, electrons move from the SnC monolayer to the SnS2 monolayer and the holes are moved from the SnS2 monolayer to the SnC monolayer until their Fermi levels are aligned. In addition, a built-in electric field is generated, and its direction changes from the SnC layer to the SnS2 layer. The built-in electric field causes the bending of the band edges in SnC and similar bending for SnS2 has been reported before in the literature [56]. In addition, as shown in Figure 5a, after the formation of the heterostructure, an electrostatic potential difference of 0.426 eV exists between the SnC layer and SnS2 layers. Electrostatic potential differences affect the photocatalytic performance of photocatalytic materials [18].
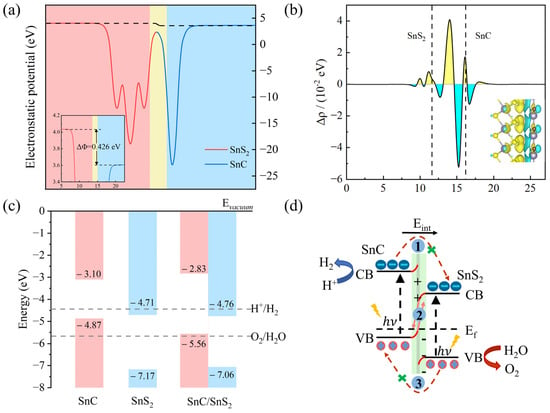
Figure 5.
(a) The electrostatic potential of the SnC/SnS2 heterostructure along the Z axis. (b) The mean charge density difference along Z-axis of the heterostructure, where yellow represents electron accumulation and blue represents electron consumption. (c) The band alignment of the SnC/SnS2 heterostructure. (d) Photo-generated carrier migration path for Z-scheme system.
To further evaluate the charge transfer, we also calculated the average charge density difference along the Z-axis and the differential charge density. The average charge density difference along the Z-axis is defined as follows:
where , and denote the charge densities of the SnC/SnS2 heterostructure, SnC, and SnS2 along the Z-axis, respectively. Figure 5b shows that charge density redistribution is primarily observed at the interface between SnC and SnS2. Here, the positive charges are concentrated at SnC, whereas the negative charges accumulate at SnS2. This creates a built-in electric field from the SnC to SnS2 layer.
A suitable band edge position is of paramount importance in the construction of a photocatalytic heterostructure. The band arrangement of the SnC and SnS2 monolayer in a heterojunction is shown in Figure 5c. According to Equations (4) and (5), at = 0, the SnC layer only crosses the reduction potential of water (−4.44 eV) and the SnS2 only crosses the oxidation potential of water (−5.67 eV). The energy bands exhibit a staggered arrangement.
As presented in Figure 5d, when sunlight shines on the SnC/SnS2 heterojunction, electrons in the valence band (VB) of each monolayer are stimulated to move to the conduction band (CB), resulting in an equal number of holes in the VB, thereby forming photogenerated electrons and holes. Furthermore, the built-in electric field can accelerate the recombination of photogenerated electrons at the CBM of SnS2 and holes at the VBM of SnC. However, it can also hinder holes from the VBM of SnS2 to the VBM of SnC. This analysis shows that the constructed SnC/SnS2 heterojunction exhibits the character of a direct Z-scheme heterojunction photocatalyst.
3.3. Photocatalytic Performance
The light absorption coefficient is an important index of heterostructure photocatalytic performance. Therefore, we computed the light absorption coefficients of the SnC and SnS2 monolayers and SnC/SnS2 heterostructure. For a more precise calculation of light absorption, all calculations were performed using the HSE06 function. The light absorption coefficient can be calculated as follows [57]:
where and denote the light speed and angular frequency in vacuum, respectively, and and denote the real and imaginary parts of the dielectric constant, respectively.
Figure 6 shows that the two monolayers exhibit low visible-light absorption. However, the SnC/SnS2 heterostructure exhibits a substantial increase in its absorption coefficient within the visible-light spectrum. This phenomenon can be attributed to the decrease in the band gap following the combination of the SnC and SnS2 monolayers.
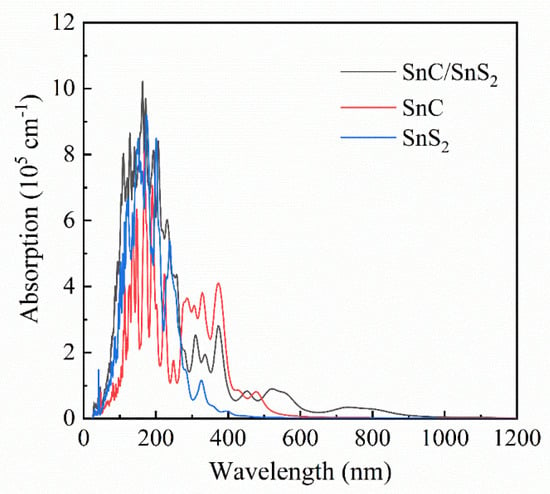
Figure 6.
The optical absorption spectra of SnC, SnS2 and SnC/SnS2 heterostructure.
In addition, we calculated the STH efficiency to further assess the photocatalytic capacity of the SnC/SnS2 heterostructure. The recombination of interlayer charges in direct Z-scheme heterostructures results in the consumption of electrons and holes. The STH efficiency can be expressed as follows [58]:
where denotes a physical quantity that represents the ability of materials to absorb light and denotes the proportion of electrons and holes involved in the photoelectric conversion process, also known as the carrier utilization efficiency. can be calculated as follows:
where denotes the photon flux density of solar radiation under standard test conditions (AM1.5G) at a photon energy of ; denotes the band gap of the SnC/SnS2 heterostructure. ηcu can be expressed as follows:
where denotes the minimum electrochemical potential difference required for a water decomposition reaction in a standard state and represents the minimum photon energy required fo the water splitting reaction to occur, which is calculated as follows:
where and denote the overpotentials of the HER and oxygen evolution reaction (OER), respectively.
As shown in Figure 5c, is equal to , and is equal to . Using Equations (5)–(8), the STH efficiency of the SnC/SnS2 heterostructure is 32.8%. Typically, STH efficiency above 10% is proposed for commercial use. The STH efficiency of the SnC/SnS2 heterostructure is significantly higher than that reported in the literature, e.g., GaTe/AsP (14.1%) [59], PtTe2/Sb2S3 (28.8%) [60] and β-SnSe/HfS2 (14.29%) [61]. This implies that the SnC/SnS2 heterojunction has broad prospects in the industry for clean energy.
To further evaluate the photocatalytic capacity of the SnC/SnS2 heterojunction, we calculated the Gibbs free energy redox reaction at pH = 0. The Gibbs free energy of redox reactions can be calculated as follows [62,63]:
where ΔE, and denote the differences in the total energy, zero-point energy, and entropy between products and reactants of the reactions, respectively. The temperature (T) in the calculation is set to 298.15 K. And represents the potential with respect to the standard water reduction potential of the standard hydrogen electrode (SHE).
The Gibbs free energy change curves of HER and OER for the SnC/SnS2 heterojunction are shown in Figure 7. The HER process comprises the following two steps:
where ∗ represents the adsorption site of heterostructure and denotes the heterojunction after the adsorption of H atoms. We find that the energy of is the lowest at the top position of the C atom. Therefore, we chose this structure to calculate the free energy. As shown in Figure 7a, in the absence of an external potential, the free energy of the heterojunction HER reaction is −0.72 eV. The OER process can be concluded as follows:
where ∗ denotes the active site of heterostructure and , and denote the configuration of the heterojunction after adsorption of OH, O, and OOH, respectively. We found that the lowest energy points of , , are the top position of the S atom, the top position of the Sn atom, and the top position of the S atom, respectively. And we used the corresponding structure with the lowest energy to calculate the free energy. For OER, the photogenerated hole potential (Uh) represents the energy difference from SHE to VBM of SnS2 [63]. When the external potential is 0 V, the overpotential of heterojunction is 2.5 V, and the overall OER curve shows an upward trend. When the external potential is 1.23 V, the heterojunction overpotential decreases; however, the OER still cannot proceed spontaneously. When U = Uh = 2.62V, the overall OER curve shows a downward trend, indicating that the OER is exothermic and is spontaneous under light.
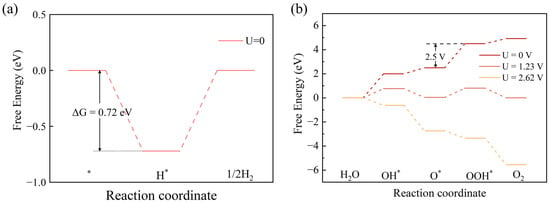
Figure 7.
(a) The free energy of SnC/SnS2 heterostructure (a) HER and (b) OER.
3.4. Strain Engineering
According to previous research reports, strain engineering can effectively regulate the properties of 2D materials [64,65]. The above discussion demonstrates that the SnC/SnS2 heterojunction exhibits excellent photocatalytic properties and is a candidate 2D vdW heterojunction material with significant application potential. Therefore, we investigated the effect of strain on the electronic and optical properties of the SnC/SnS2 heterojunction.
In particular, the effect of biaxial strain on the SnC/SnS2 heterojunction was investigated by applying a plane biaxial strain between −6% and 6%. Here, negative values represent compressive strains, while positive values denote tensile strains, respectively. The degree of strain was calculated as follows:
where and a denote the lattice constants of the initial and the post-strain heterojunction, respectively. In addition, we calculated the strain energy of the SnC/SnS2 heterostructure to ensure that the strain degree was within the elastic range, as follows:
where and denote the energies of the SnC/SnS2 heterostructure with and without strain, respectively.
As shown in Figure 8a, with an increase in tensile (compression) strain, the total energy of the SnC/SnS2 heterojunction gradually increases, and a perfect quadratic function relationship is formed between them, indicating that SnC/SnS2 heterojunction is a flexible material and exhibits reversibility when the external strain is within its elastic limit. As the degree of strain changes, the band gap of the SnC/SnS2 heterojunction changes. At −2% strain, the heterojunction band gap reaches its maximum (0.81 eV). At 6% strain, the heterojunction band gap reaches the minimum (0.36 eV).
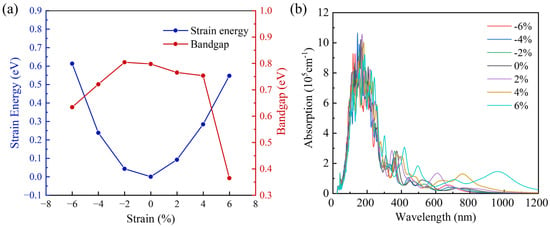
Figure 8.
(a) Bandgap calculated by HSE06 and strain energy under biaxial strain. (b) The optical absorption spectra calculated by HSE06 under biaxial strain.
In addition, by applying biaxial strain on the SnC/SnS2 heterojunctions, we investigated the optical properties of the studied heterojunctions. As shown in Figure 8b, varies with strain. Under compressive strain, the light absorption of the heterojunction exhibits no obvious change. Under tensile strain, the heterojunction absorption curve changes. Under 2% tensile strain, a high absorption peak at 500 mm~700 mm appears in the heterojunction. Under 4% tensile strain, a high absorption peak at 600–850 mm can be found in the heterojunction. Under 6% tensile strain, the heterojunction exhibits multiple high absorption peaks at 400–1100 mm. These results demonstrate that strain is an effective way to tune the optical properties of SnC/SnS2 heterojunctions.
4. Conclusions
In this study, first-principles calculations were applied to explore the structure, electronic and photocatalytic properties of a SnC/SnS2 heterojunction. The BC configuration exhibited the lowest binding energy; thus, its stability was verified. Our results demonstrate that the calculated band gap of the SnC/SnS2 heterostructure exhibits a type-II band alignment. The predicted energy gap of our proposed SnC/SnS2 heterostructure is 0.80 eV. In addition, the SnC/SnS2 vdW heterostructure is identified as a typical Z-scheme heterostructure based on analyses of the work function, band alignment, and mean charge density difference. In particular, the STH efficiency we predicted in the SnC/SnS2 heterojunction reaches 32.8%. Both the electronic structure as well as the optical properties of the heterojunction can be effectively modulated by biaxial strain. These results imply that the fabricated SnC/SnS2 heterojunction is a promising material with excellent photocatalytic performance, which can be realized by applying biaxial strain in experiments. Thus, our calculations have a guiding significance to the experiment. In our previous work, we firstly investigated the physical properties of a GeSe/SnSe heterostructure in theory and found that it exhibits high carrier mobility, strong light absorption, and superior PCE properties [66]. Subsequently, we successfully prepared a GeSe/SnSe heterojunction and found that it exhibits excellent photoelectric properties and can be used as a near-infrared photodetector [67]. From a viewpoint of outlooking, the calculation expectation in this study can be used as a guide for subsequent experiments.
Author Contributions
S.Z.: Data curation; Writing—original draft. Y.M.: Conceptualization, Funding acquisition, Supervision; Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by Natural Science Foundation of Hunan Province, China (Grant No. 2023JJ30567).
Data Availability Statement
The data that support the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Armaroli, N.; Balzani, V. The Future of Energy Supply: Challenges and Opportunities. Angew. Chem. Int. Ed. 2006, 46, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Dorian, J.P.; Franssen, H.T.; Simbeck, D.R. Global challenges in energy. Energy Policy 2006, 34, 1984–1991. [Google Scholar] [CrossRef]
- Ukaogo, P.O.; Ewuzie, U.; Onwuka, C.V. 21—Environmental pollution: Causes, effects, and the remedies. In Microorganisms for Sustainable Environment and Health; Chowdhary, P., Raj, A., Verma, D., Akhter, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 419–429. [Google Scholar]
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Vohra, K.; Vodonos, A.; Schwartz, J.; Marais, E.A.; Sulprizio, M.P.; Mickley, L.J. Global mortality from outdoor fine particle pollution generated by fossil fuel combustion: Results from GEOS-Chem. Environ. Res. 2021, 195, 110754. [Google Scholar] [CrossRef]
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F. Hydrogen energy. Philos. Trans. R. Soc. A. 2007, 365, 1043–1056. [Google Scholar] [CrossRef]
- Veziroğlu, T.N.; Şahin, S. 21st Century’s energy: Hydrogen energy system. Energy Convers. Manage 2008, 49, 1820–1831. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Low, J.; Cao, S.; Yu, J.; Wageh, S. Two-dimensional layered composite photocatalysts. Chem. Commun. 2014, 50, 10768–10777. [Google Scholar] [CrossRef]
- Di, J.; Xiong, J.; Li, H.; Liu, Z. Ultrathin 2D Photocatalysts: Electronic-Structure Tailoring, Hybridization, and Applications. Adv Mater 2017, 30, 1704548. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.; Jaroniec, M. All-Solid-State Z-Scheme Photocatalytic Systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. A review on TiO2-based Z-scheme photocatalysts. Chin. J. Catal. 2017, 38, 1936–1955. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Robert, D.; Weber, J.V. Bi2S3/TiO2 and CdS/TiO2 heterojunctions as an available configuration for photocatalytic degradation of organic pollutant. J. Photochem. Photobiol. A Chem. 2004, 163, 569–580. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Robert, D.; Weber, J.V. Photocatalytic activity of Cu2O/TiO2, Bi2O3/TiO2 and ZnMn2O4/TiO2 heterojunctions. Catal. Today 2005, 101, 315–321. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4–TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, Y.; Liu, J.; Lv, L.; Zhou, M.; Yang, X.; Meng, X.; Zhou, Z. Internal electric field enhanced photoelectrochemical water splitting in direct Z-scheme GeC/HfS2 heterostructure: A first-principles study. Appl Phys Lett 2023, 122, 043902. [Google Scholar] [CrossRef]
- Xing, F.; Wang, C.; Liu, S.; Jin, S.; Jin, H.; Li, J. Interfacial Chemical Bond Engineering in a Direct Z-Scheme g-C3N4/MoS2 Heterojunction. ACS Appl. Mater. Interfaces 2023, 15, 11731–11740. [Google Scholar] [CrossRef]
- Luo, Q.; Yin, S.; Sun, X.; Tang, Y.; Feng, Z.; Dai, X. GaN/BS van der Waals heterostructure: A direct Z-scheme photocatalyst for overall water splitting. Appl. Surf. Sci. 2023, 609, 155400. [Google Scholar] [CrossRef]
- Şahin, H.; Cahangirov, S.; Topsakal, M.; Bekaroglu, E.; Akturk, E.; Senger, R.T.; Ciraci, S. Monolayer honeycomb structures of group-IV elements and III-V binary compounds: First-principles calculations. Phys Rev B. 2009, 80, 155453. [Google Scholar] [CrossRef]
- Hoat, D.M.; Naseri, M.; Ponce-Péreze, R.; Hieu, N.N.; Rivas-Silva, J.F.; Vu, T.V.; Tong, H.D.; Cocoletzi, G.H. Structural and electronic properties of chemically functionalized SnC monolayer: A first principles study. Mater. Res. Express 2019, 7, 015013. [Google Scholar] [CrossRef]
- Jin, H.; Dai, Y.; Huang, B.-B. Design of Advanced Photocatalysis System by Adatom Decoration in 2D Nanosheets of Group-IV and III–V Binary Compounds. Sci. Rep. 2016, 6, 23104. [Google Scholar] [CrossRef] [PubMed]
- Arellano, L.G.; Marcos-Viquez, A.L.; DESantiago, F.; Miranda, Á.; Pérez, L.A.; Nakamura, J.; Cruz-Irisson, M. Hydrogen storage on tin carbide monolayers with transition metal adatoms. Int. J. Hydrogen Energy 2023, 48, 37500–37509. [Google Scholar] [CrossRef]
- Rehman, J.; Fan, X.; Laref, A.; Zheng, W.T. Adsorption and Diffusion of Potassium on 2D SnC Sheets for Potential High-Performance Anodic Applications of Potassium-Ion Batteries. ChemElectroChem 2020, 7, 3832–3838. [Google Scholar] [CrossRef]
- Khalid Butt, M.; Muhammad Zeeshan, H.; An Dinh, V.; Zhao, Y.; Wang, S.; Jin, K. Monolayer SnC as anode material for Na ion batteries. Comput. Mater. Sci. 2021, 197, 110617. [Google Scholar] [CrossRef]
- Tang, W.; Wang, G.; Fu, C.; Wang, B.; Yuan, H.; Chen, H. Engineering two-dimensional SnC/HfSSe heterojunction as a direct Z-scheme photocatalyst for water splitting hydrogen evolution. Appl. Surf. Sci. 2023, 626, 157247. [Google Scholar] [CrossRef]
- Sheng, R.Q.; Deng, X.Q.; Zhang, Z.H.; Fan, Z.Q. Tunable electronic and optical properties of SnC/BAs heterostructure by external electric field and vertical strain. Phys. Lett. A 2020, 384, 126150. [Google Scholar] [CrossRef]
- Liang, K.; Wang, J.; Wei, X.; Zhang, Y.; Yang, Y.; Liu, J.; Tian, Y.; Duan, L. Theoretical design of direct Z-scheme SnC/PtSe2 heterostructure with enhanced photocatalytic performance and tunable optoelectronic properties. Phys. E 2024, 155, 115825. [Google Scholar] [CrossRef]
- Setayeshmehr, M.; Haghighi, M.; Mirabbaszadeh, K. A review of tin disulfide (SnS2) composite electrode materials for supercapacitors. Energy Storage 2021, 4, e295. [Google Scholar] [CrossRef]
- Mishra, R.K.; Choi, G.J.; Choi, H.J.; Singh, J.; Lee, S.H.; Gwag, J.S. Potentialities of nanostructured SnS2 for electrocatalytic water splitting: A review. J. Alloys Compd. 2022, 921, 166018. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, F.; Zhang, Y.; Hu, J. Review on the advancement of SnS2 in photocatalysis. J. Mater. Chem. A 2023, 11, 7331–7343. [Google Scholar] [CrossRef]
- Huang, Y.; Deng, H.-X.; Xu, K.; Wang, Z.-X.; Wang, Q.-S.; Wang, F.-M.; Wang, F.; Zhan, X.-Y.; Li, S.-S.; Luo, J.-W.; et al. Highly sensitive and fast phototransistor based on large size CVD-grown SnS2 nanosheets. Nanoscale 2015, 7, 14093–14099. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Qiu, W.; Jian, J.; Huang, Y.; Luo, Y.; Yang, H.; Liang, C.; Lu, X.; Tong, Y. Vanadium Nitride Nanowire Supported SnS2 Nanosheets with High Reversible Capacity as Anode Material for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 23205–23215. [Google Scholar] [CrossRef]
- Wang, G.; Peng, J.; Zhang, L.; Zhang, J.; Dai, B.; Zhu, M.; Xia, L.; Yu, F. Two-dimensional SnS2@PANI nanoplates with high capacity and excellent stability for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 3659–3666. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, D.; Wu, J.; Wang, Z.; Huang, S.; Xu, Y.; Chen, Z.; Zhao, B.; Zhang, J. Sandwich-like SnS2/Graphene/SnS2 with Expanded Interlayer Distance as High-Rate Lithium/Sodium-Ion Battery Anode Materials. ACS Nano 2019, 13, 9100–9111. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wang, S.; Cao, F.; Tian, W.; Long, R.; Li, L. Doping-Induced Amorphization, Vacancy, and Gradient Energy Band in SnS2 Nanosheet Arrays for Improved Photoelectrochemical Water Splitting. Angew. Chem. Int. Ed. 2019, 58, 6761–6765. [Google Scholar] [CrossRef]
- Cong, X.; Shah, M.N.U.; He, W. Straddling SnSe2/SnS2 van der Waals tunneling heterostructures for high performance broadband photodetectors. J. Mater. Chem. C 2024, 12, 5411–5419. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, R.; Xiang, H.; Yang, C.; Zhong, W.; Zhang, C.; Zhang, Q.; Li, X.; Yang, N. Exposing highly active (100) facet on a SnS2/SnO2 electrocatalyst to boost efficient hydrogen evolution. Appl. Catal. B 2021, 292, 120200. [Google Scholar] [CrossRef]
- Huang, Y.; Song, S.; Lian, J.; Cao, J.; Zheng, Y.; Wang, J.; Zhu, M.; Pan, J.; Li, C. Hollow cubic SnS2/CdS nanoheterojunction for enhanced photocatalytic hydrogen evolution and degradation via MOFs in situ sulfuration. Int. J. Hydrogen Energy 2024, 64, 1030–1039. [Google Scholar] [CrossRef]
- Reddy, P.A.K.; Han, H.; Kim, K.C.; Bae, S. Rational Synthesis of S-Scheme CdS/SnS2 Photocatalysts with Isolated Redox Cocatalysts for Enhanced H2 Production. ACS Sustain. Chem. Eng. 2024, 12, 4979–4992. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurateab initioparametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Bengtsson, L. Dipole correction for surface supercell calculations. Phys. Rev. B 1999, 59, 12301–12304. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Baroni, S.; de Gironcoli, S.; Dal Corso, A.; Giannozzi, P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 2001, 73, 515–562. [Google Scholar] [CrossRef]
- Wang, J.; Luan, L.; Chen, J.; Zhang, Y.; Wei, X.; Fan, J.; Ni, L.; Liu, C.; Yang, Y.; Liu, J.; et al. PtSe2/SnS2 heterostructure as a direct Z-scheme photocatalyst for water decomposition. Mater. Sci. Semicond. Process 2023, 155, 107225. [Google Scholar] [CrossRef]
- Luo, Y.-F.; Zhang, Y.; Li, J.-H.; Yang, Y.-S.; Sun, S.-Z.; Duan, L. The SnC/WS2 vdW heterojunction: A prospective direct Z-scheme photocatalyst for overall water decomposition with high STH efficiency and catalytic activity. Mol. Catal. 2024, 557, 113983. [Google Scholar] [CrossRef]
- Singh, A.; Jain, M.; Bhattacharya, S. MoS2 and Janus (MoSSe) based 2D van der Waals heterostructures: Emerging direct Z-scheme photocatalysts. Nanoscale Adv. 2021, 3, 2837–2845. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Choi, J.; Zhang, H.; Du, H.; Choi, J.H. Understanding Solvent Effects on the Properties of Two-Dimensional Transition Metal Dichalcogenides. ACS Appl. Mater. Interfaces 2016, 8, 8864–8869. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, X.; Nie, Y.; Zhu, H.; Xiang, G. Achieving highly efficient 2D SnC monolayer-based photocatalyst for water splitting via a synergistic strategy of S-scheme heterostructure construction and silicon doping. Nanoscale 2024, 16, 4866–4871. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, X.; Zhao, S.; Wang, S.; Cui, J. Mechanism of photocatalytic water splitting of 2D WSeTe/XS2 (X = Hf, Sn, Zr) van der Waals heterojunctions under the interaction of vertical intrinsic electric and built-in electric fiel. Appl. Surf. Sci. 2022, 599, 154012. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T., Jr. Band Bending in Semiconductors: Chemical and Physical Consequences at Surfaces and Interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef]
- Sun, M.; Chou, J.-P.; Gao, J.; Cheng, Y.; Hu, A.; Tang, W.; Zhang, G. Exceptional Optical Absorption of Buckled Arsenene Covering a Broad Spectral Range by Molecular Doping. ACS Omega 2018, 3, 8514–8520. [Google Scholar] [CrossRef]
- Fu, C.-F.; Sun, J.; Luo, Q.; Li, X.; Hu, W.; Yang, J. Intrinsic Electric Fields in Two-dimensional Materials Boost the Solar-to-Hydrogen Efficiency for Photocatalytic Water Splitting. Nano Lett. 2018, 18, 6312–6317. [Google Scholar] [CrossRef]
- Sun, S.-Z.; Zhang, Y.; Luo, Y.-F.; Yang, Y.-S.; Li, J.-H.; Duan, L.; Xie, J.; Guo, T.-T. A direct Z-scheme GaTe/AsP van der Waals heterostructure: A promising high efficiency photocatalyst for overall water splitting with strong optical absorption and superior catalytic activity. Surf. Sci. 2024, 749, 122553. [Google Scholar] [CrossRef]
- Wang, F.; Yang, C.-L.; Wang, M.-S.; Ma, X.-G. PtTe2/Sb2S3 Nanoscale Heterostructures for the Photocatalytic Direct Z-Scheme with High Solar-to-Hydrogen Efficiency: A Theoretical Investigation. ACS Appl. Nano Mater. 2023, 6, 5591–5601. [Google Scholar] [CrossRef]
- He, L.; Long, X.; Zhang, C.; Ma, K.; She, L.; Mi, C.; Yu, M.; Xie, Z.; Wang, L. Direct Z-scheme β-SnSe/HfS2 heterostructure for photocatalytic water splitting: High solar-to-hydrogen efficiency and excellent carrier mobility. Mater. Today Commun. 2024, 38, 108127. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef] [PubMed]
- Valdés, Á.; Qu, Z.W.; Kroes, G.J.; Rossmeisl, J.; Nørskov, J.K. Oxidation and Photo-Oxidation of Water on TiO2 Surface. J. Phys. Chem. C 2008, 112, 9872–9879. [Google Scholar] [CrossRef]
- Liang, K.; Wang, J.; Wei, X.; Zhang, Y.; Yang, Y.; Liu, J.; Tian, Y.; Duan, L. SnC/PtS2 heterostructure: A promising direct Z-scheme photocatalyst with tunable electronic optical properties and high solar-to-hydrogen efficiency. Int. J. Hydrogen Energy 2023, 48, 38296–38308. [Google Scholar] [CrossRef]
- Vn, D.; Sen, S.; Chattopadhyaya, M. A density functional study of type I to type II crossover in g-C3N4/CoN4 heterostructure in presence of external perturbation. Int. J. Hydrogen Energy 2024, 91, 1136–1148. [Google Scholar]
- Mao, Y.; Xu, C.; Yuan, J.; Zhao, H. A two-dimensional GeSe/SnSe heterostructure for high performance thin-film solar cells. J. Mater. Chem. A 2019, 7, 11265–11271. [Google Scholar] [CrossRef]
- Mao, Y.; Deng, T.; Li, Y.; He, F. The GeSe/SnSe heterojunction photodetector with self-powered characteristics and high infrared response performance. Appl. Phys. Lett. 2024, 124, 181106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).