Crystal Engineering of Conglomerates: Dilution of Racemate-Forming Fe(II) and Ni(II) Congeners into Conglomerate-Forming [Zn(bpy)3](PF6)2
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedure for [M(bpy)3](PF6)2
2.2. General Procedure for [MxZn(1−x)(bpy)3](PF6)2
2.3. X-ray Diffraction
2.4. IR Spectroscopy
2.5. EDX
2.6. XNCD
3. Results and Discussion
3.1. Phase Determination of Pure [M(bpy)3](PF6)2 in Powders and Crystals
3.2. Molecular Alloys of [NixZn(1−x)(bpy)3](PF6)2
3.3. Molecular Alloys of [FexZn(1−x)(bpy)3](PF6)2
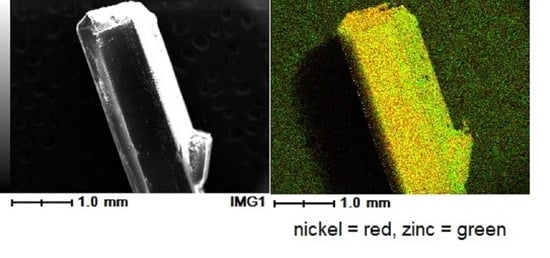
3.4. EDX
3.5. XNCD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srisanga, S.; Ter Horst, J.H. Racemic Compound, Conglomerate, or Solid Solution: Phase Diagram Screening of Chiral Compounds. Cryst. Growth Des. 2010, 10, 1808–1812. [Google Scholar] [CrossRef]
- Jacques, J.; Collet, A.; Wilen, S.H. Enantiomers, Racemates, and Resolutions; J. Wiley & Sons, Inc.: New York, NY, USA, 1981. [Google Scholar]
- Pasteur, L. Mémoire Sur La Relation Qui Peut Exister Entre La Forme Cristalline et La Composition Chimique, et Sur La Cause de La Polarisation Rotatoire. C. R. Acad. Sci. Paris 1848, 26, 535–538. [Google Scholar]
- Levilain, G.; Coquerel, G. Pitfalls and Rewards of Preferential Crystallization. CrystEngComm 2010, 12, 1983–1992. [Google Scholar] [CrossRef]
- Sögütoglu, L.-C.; Steendam, R.R.E.; Meekes, H.; Vlieg, E.; Rutjes, F.P.J.T. Viedma Ripening: A Reliable Crystallisation Method to Reach Single Chirality. Chem. Soc. Rev. 2015, 44, 6723–6732. [Google Scholar] [CrossRef] [PubMed]
- Levilain, G.; Eicke, M.J.; Seidel-Morgenstern, A. Efficient Resolution of Enantiomers by Coupling Preferential Crystallization and Dissolution. Part 1: Experimental Proof of Principle. Cryst. Growth Des. 2012, 12, 5396–5401. [Google Scholar] [CrossRef]
- Coquerel, G. Preferential Crystallization. In Novel Optical Resolution Technologies; Sakai, K., Hirayama, N., Tamura, R., Eds.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–51. ISBN 978-3-540-46320-7. [Google Scholar]
- Binev, D.; Seidel-Morgenstern, A.; Lorenz, H. Continuous Separation of Isomers in Fluidized Bed Crystallizers. Cryst. Growth Des. 2016, 16, 1409–1419. [Google Scholar] [CrossRef]
- Dunn, A.S.; Szilagyi, B.; ter Horst, J.H.; Nagy, Z.K. Enabling Mechanical Separation of Enantiomers through Controlled Batchwise Concomitant Crystallization: Digital Design and Experimental Validation. Cryst. Growth Des. 2020, 20, 7726–7741. [Google Scholar] [CrossRef]
- Eicke, M.J.; Levilain, G.; Seidel-Morgenstern, A. Efficient Resolution of Enantiomers by Coupling Preferential Crystallization and Dissolution. Part 2: A Parametric Simulation Study to Identify Suitable Process Conditions. Cryst. Growth Des. 2013, 13, 1638–1648. [Google Scholar] [CrossRef]
- Galan, K.; Eicke, M.J.; Elsner, M.P.; Lorenz, H.; Seidel-Morgenstern, A. Continuous Preferential Crystallization of Chiral Molecules in Single and Coupled Mixed-Suspension Mixed-Product-Removal Crystallizers. Cryst. Growth Des. 2015, 15, 1808–1818. [Google Scholar] [CrossRef]
- Galland, A.; Dupray, V.; Berton, B.; Morin-Grognet, S.; Sanselme, M.; Atmani, H.; Coquerel, G. Spotting Conglomerates by Second Harmonic Generation. Cryst. Growth Des. 2009, 9, 2713–2718. [Google Scholar] [CrossRef]
- Newman, A.C.D.; Powell, H.M. The Spontaneous Optical Resolution of Solvated Tri-o-Thymotide. J. Chem. Soc. 1952, 718, 3747–3751. [Google Scholar] [CrossRef]
- Harfouche, L.C.; Brandel, C.; Cartigny, Y.; Ter Horst, J.H.; Coquerel, G.; Petit, S. Enabling Direct Preferential Crystallization in a Stable Racemic Compound System. Mol. Pharm. 2019, 16, 4670–4676. [Google Scholar] [CrossRef] [PubMed]
- Mbodji, A.; Gbabode, G.; Sanselme, M.; Cartigny, Y.; Couvrat, N.; Leeman, M.; Dupray, V.; Kellogg, R.M.; Coquerel, G. Evidence of Conglomerate with Partial Solid Solutions in Ethylammonium Chlocyphos. Cryst. Growth Des. 2020, 20, 2562–2569. [Google Scholar] [CrossRef]
- Mbodji, A.; Gbabode, G.; Sanselme, M.; Couvrat, N.; Leeman, M.; Dupray, V.; Kellogg, R.M.; Coquerel, G. Family of Conglomerate-Forming Systems Composed of Chlocyphos and Alkyl-Amine. Assessment of Their Resolution Performances by Using Various Modes of Preferential Crystallization. Cryst. Growth Des. 2019, 19, 5173–5183. [Google Scholar] [CrossRef]
- Cortijo, M.; Valentín-Pérez, Á.; Rogalev, A.; Wilhelm, F.; Sainctavit, P.; Rosa, P.; Hillard, E.A. Rapid Discrimination of Crystal Handedness by X-Ray Natural Circular Dichroism (XNCD) Mapping. Chem. Eur. J. 2020, 26, 13363–13366. [Google Scholar] [CrossRef]
- Atzori, M.; Ludowieg, H.D.; Valentín-Pérez, Á.; Cortijo, M.; Breslavetz, I.; Paillot, K.; Rosa, P.; Train, C.; Autschbach, J.; Hillard, E.A.; et al. Validation of Microscopic Magnetochiral Dichroism Theory. Sci. Adv. 2021, 7, eabg2859. [Google Scholar] [CrossRef]
- Sessoli, R.; Boulon, M.-E.; Caneschi, A.; Mannini, M.; Poggini, L.; Wilhelm, F.; Rogalev, A. Strong Magneto-Chiral Dichroism in a Paramagnetic Molecular Helix Observed by Hard X-rays. Nat. Phys. 2015, 11, 69–74. [Google Scholar] [CrossRef]
- Train, C.; Nuida, T.; Gheorghe, R.; Gruselle, M.; Ohkoshi, S. Large Magnetization-Induced Second Harmonic Generation in an Enantiopure Chiral Magnet. J. Am. Chem. Soc. 2009, 131, 16838–16843. [Google Scholar] [CrossRef]
- Atzori, M.; Breslavetz, I.; Paillot, K.; Inoue, K.; Rikken, G.L.J.A.; Train, C. A Chiral Prussian Blue Analogue Pushes Magneto-Chiral Dichroism Limits. J. Am. Chem. Soc. 2019, 141, 20022–20025. [Google Scholar] [CrossRef]
- Bernal, I.; Kauffman, G.B. The Spontaneous Resolution of Cis-Bis(Ethylenediamine)Dinitrocobalt(III) Salts: Alfred Werner’s Overlooked Opportunity. J. Chem. Educ. 1987, 64, 604–610. [Google Scholar] [CrossRef]
- Björemark, P.M.; Jönsson, J.; Håkansson, M. Absolute Asymmetric Synthesis: Viedma Ripening of [Co(Bpy)3]2+ and Solvent-Free Oxidation to [Co(Bpy)3]3+. Chem. Eur. J. 2015, 21, 10630–10633. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.K.; Ramanujam, R.; Bernal, I.; Fronczek, F.R. The Phenomenon of Conglomerate Crystallization. Part 57. Control of the Crystallization Behavior by the Choice of the Counter Ion. Part 9. The Stereochemistry and Crystallization Architecture of [(3,2,3-Tet)Co(N3)2]X (X = Cl(I), Br(II), I(III), NO3 (IV), and PF6 (V)). Cryst. Growth Des. 2002, 2, 205–212. [Google Scholar]
- Neill, D.; Riley, M.J.; Kennard, C.H.L. Tris(Ethylenediamine-N,N′)Zinc(II) Dinitrate. Acta Cryst. C 1997, 53, 701–703. [Google Scholar] [CrossRef]
- Caughlan, C.N.; Emerson, K. Crystal and Molecular Structure of Tris(Ethylenediamine)Nickel(II) Sulfate, Ni(NH2CH2CH2NH2)3SO4. Inorg. Chem. 1970, 9, 2421–2424. [Google Scholar] [CrossRef]
- Deisenroth, S. CSD Structure Code YEGLUR, Untersuchung der Folgeprozesse des 57Co-Kernzerfalls in der Komplexverbindung [57Co/Mn(bipy)3](PF6)2. Ph.D. Thesis, University of Mainz Institut fur Anorganische Chemie und Analytische Chemie, Mainz, Germany, 1996. [Google Scholar]
- Archer, C.M.; Dilworth, J.R.; Thompson, R.M.; McPartlin, M.; Povey, D.C.; Kelly, J.D. The Synthesis and Reactivity of a New Technetium(III) Precursor. The Crystal Structures of [TcCl3(MeCN){P(C6H4Me-3)3}2] and [Tc(Bipy)3]2+(Bipy = 2,2′-Bipyridine). J. Chem. Soc. Dalton Trans. 1993, 3, 461–466. [Google Scholar] [CrossRef]
- Menteş, A.; Singh, K. Tris(2,2′-Bipyridine-Κ2N,N′)Cobalt(II) Bis (Hexa fluoridophosphate). Acta Cryst. E 2013, 69, m58. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Huang, W. Tris(2,2′-Bipyridyl-Κ2N,N′)Copper(II) Hexa fluoridophosphate. Acta Cryst. E 2007, 63, m835–m836. [Google Scholar] [CrossRef]
- Breu, J.; Domel, H.; Stoll, A. Racemic Compound Formation versus Conglomerate Formation with [M(Bpy)3](PF6)2 (M = Ni, Zn, Ru); Molecular and Crystal Structures. Eur. J. Inorg. Chem. 2000, 2000, 2401–2408. [Google Scholar] [CrossRef]
- Dick, S. Crystal structure of tris(2,2′-bipyridine)iron(II) bis(hexafluorophosphate), (C10H8N2)3Fe(PF6)2. Z. Für Krist. New Cryst. Struct. 1998, 213, 370. [Google Scholar] [CrossRef]
- Rillema, D.P.; Jones, D.S.; Woods, C.; Levy, H.A. Comparison of the Crystal Structures of Tris Heterocyclic Ligand Complexes of Ruthenium(II). Inorg. Chem. 1992, 31, 2935–2938. [Google Scholar] [CrossRef]
- Rillema, D.P.; Jones, D.S. Structure of Tris(2,2′-Bipyridyl)Ruthenium(II) Hexafluorophosphate, [Ru(Bipy)3][PF6]2; X-Ray Crystallographic Determination. J. Chem. Soc., Chem. Commun. 1979, 19, 849–851. [Google Scholar] [CrossRef]
- Bouzaid, J.; Schultz, M.; Lao, Z.; Bartley, J.; Bostrom, T.; McMurtrie, J. Supramolecular Selection in Molecular Alloys. Cryst. Growth Des. 2012, 12, 3906–3916. [Google Scholar] [CrossRef]
- Constable, E.C.; Raithby, P.R.; Smit, D.N. The X-Ray Crystal Structure of Tris (2,2′-Bipyridine)Osmium(II) Hexafluorophosphate. Polyhedron 1989, 8, 367–369. [Google Scholar] [CrossRef]
- Richter, M.M.; Scott, B.; Brewer, K.J.; Willett, R.D. Crystal and Molecular Structure of Tris(2,2′-Bipyridyl)Osmium(II) Bis(Hexafluorophosphate). Acta Cryst. C 1991, 47, 2443–2444. [Google Scholar] [CrossRef]
- Biner, M.; Buergi, H.B.; Ludi, A.; Roehr, C. Crystal and Molecular Structures of [Ru(Bpy)3](PF6)3 and [Ru(Bpy)3](PF6)2 at 105 K. J. Am. Chem. Soc. 1992, 114, 5197–5203. [Google Scholar] [CrossRef]
- Hadadzadeh, H.; Mansouri, G.; Rezvani, A.; Khavasi, H.R.; Skelton, B.W.; Makha, M.; Charati, F.R. Mononuclear Nickel(II) Complexes Coordinated by Polypyridyl Ligands. Polyhedron 2011, 30, 2535–2543. [Google Scholar] [CrossRef]
- Brewer, B.; Brooks, N.R.; Abdul-Halim, S.; Sykes, A.G. Differential metathesis reactions of 2,2’-bipyridine and 1,10-phenanthroline complexes of cobalt(II) and nickel(II): Cocrystallization of ionization isomers {[cis-Ni(phen)2(H2O)2][cis-Ni(phen)2(H2O)Cl]} (PF6)3·4.5H2O, and a Synthetic Route to Asymmetric Tris-Substituted Complexes. J. Chem. Crystallogr. 2003, 12, 651–662. [Google Scholar]
- Breu, J.; Domel, H.; Norrby, P.-O. Racemic Compound Formation versus Conglomerate Formation with [M(Bpy)3](PF6)2 (M = Ni, Zn, Ru); Lattice Energy Minimisations and Implications for Structure Prediction. Eur. J. Inorg. Chem. 2000, 2000, 2409–2419. [Google Scholar] [CrossRef]
- Habib, F.; Lin, P.-H.; Long, J.; Korobkov, I.; Wernsdorfer, W.; Murugesu, M. The Use of Magnetic Dilution to Elucidate the Slow Magnetic Relaxation Effects of a Dy 2 Single-Molecule Magnet. J. Am. Chem. Soc. 2011, 133, 8830–8833. [Google Scholar] [CrossRef]
- Flores Gonzalez, J.; Douib, H.; Le Guennic, B.; Pointillart, F.; Cador, O. Ytterbium-Centered Isotopic Enrichment Leading to a Zero-Field Single-Molecule Magnet. Inorg. Chem. 2021, 60, 540–544. [Google Scholar] [CrossRef]
- Balogh, M.C. New Luminescent Materials, Bio-Inspired and Recyclable, Based on Lanthanide Complexes. Ph.D. Thesis, Université de Lyon, Lyon, France, 2016. [Google Scholar]
- Bruker. SAINT+; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. SHELXS 97, Program for the Solution of Crystal Structure. University of Göttingen: Göttingen, Germany, 1990. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.a.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General Features. Zeitschrift für Krist. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kundu, N.; Mandal, D.; Chaudhury, M.; Tiekink, E.R.T. Luminescence Characteristics and X-ray Crystal Structure of [Cd(Bipy)3][PF6]2 (Bipy = 2,2′-Bipyridine). Appl. Organomet. Chem. 2005, 19, 1268–1270. [Google Scholar] [CrossRef]
- Stewart, B.; Peacock, R.D.; Alagna, L.; Prosperi, T.; Turchini, S.; Goulon, J.; Rogalev, A.; Goulon-Ginet, C. Circular Dichroism at the Edge: Large X-Ray Natural CD in the 1s → 3d Pre-Edge Feature of 2[Co(En)3Cl3]·NaCl·6H2O. J. Am. Chem. Soc. 1999, 121, 10233–10234. [Google Scholar] [CrossRef]
- Srinivasan, A.; Cortijo, M.; Bulicanu, V.; Naim, A.; Clerac, R.; Sainctavit, P.; Rogalev, A.; Wilhelm, F.; Rosa, P.; Hillard, E.A. Enantiomeric Resolution and X-ray Optical Activity of a Tricobalt Extended Metal Atom Chain. Chem. Sci. 2018, 9, 1136–1143. [Google Scholar] [CrossRef]





| Compound | MSO4·nH2O | Mass MSO4·nH2O |
|---|---|---|
| [Mn(bpy)3](PF6)2 | MnSO4·H2O | 0.169 g |
| [Fe(bpy)3](PF6)2 | FeSO4·7H2O | 0.278 g |
| [Co(bpy)3](PF6)2 | CoSO4·7H2O | 0.281 g |
| [Cu(bpy)3](PF6)2 | CuSO4·5H2O | 0.250 g |
| [Ni(bpy)3](PF6)2 | NiSO4·7H2O | 0.281 g |
| [Zn(bpy)3](PF6)2 | ZnSO4·7H2O | 0.288 g |
| mmol NiSO4·7H2O | mmol ZnSO4·7H2O | Mass ZnSO4·7H2O | Mass NiSO4·7H2O |
| 0.1 | 0.9 | 0.259 g | 0.028 g |
| 0.2 | 0.8 | 0.230 g | 0.056 g |
| 0.3 | 0.7 | 0.201 g | 0.084 g |
| 0.4 | 0.6 | 0.173 g | 0.112 g |
| 0.5 | 0.5 | 0.144 g | 0.140 g |
| 0.6 | 0.4 | 0.115 g | 0.169 g |
| 0.7 | 0.3 | 0.086 g | 0.197 g |
| 0.8 | 0.2 | 0.058 g | 0.225 g |
| 0.9 | 0.1 | 0.029 g | 0.253 g |
| mmol FeSO4·7H2O | mmol ZnSO4·7H2O | Mass ZnSO4·7H2O | Mass FeSO4·7H2O |
| 0.1 | 0.9 | 0.259 g | 0.028 g |
| 0.2 | 0.8 | 0.230 g | 0.056 g |
| 0.3 | 0.7 | 0.201 g | 0.083 g |
| 0.4 | 0.6 | 0.173 g | 0.111 g |
| 0.5 | 0.5 | 0.144 g | 0.139 g |
| Metal | Phase | a (Å) | c (Å) | V (Å3) | GOF | V (A3) SC a |
|---|---|---|---|---|---|---|
| Fe | β | 10.6392(11) | 16.588(3) | 1626.1(4) | 1.46 | 1561.3 |
| Co | γ | 10.4818(9) | 26.525(3) | 2523.9(5) | 1.24 | 2432.0 |
| Ni | γ | 10.5058(11) | 26.367(4) | 2520.3(5) | 1.48 | 2415.8 |
| Cu | γ | 10.4921(13) | 26.428(4) | 2519.6(7) | 1.72 | 2482.1 |
| Zn | γ | 10.5046(9) | 26.455(3) | 2528.1(4) | 1.52 | 2507.9 |
| M(II) | T | [M−N]avg (Å) | Ref. | |
|---|---|---|---|---|
| Racemic β-phase | Fe | rt | 1.967 | [32] |
| Ru | rt | 2.055 | [33] | |
| Os | rt | 2.056 | [36] | |
| Ni | rt | 2.078 2.076(2) | [40] This work a | |
| Tc | rt | 2.076 | [28] | |
| Chiral γ-phase | Ni | rt | 2.090 | [39] |
| Cu | rt | 2.119 | [30] | |
| Co | rt | 2.142 | [23] | |
| Zn | rt | 2.159(5) | This work a | |
| Mn | rt | 2.249 | [27] | |
| Cd | 223 K | 2.335 | [51] |
| Ni (mol %) | a (Å) | c (Å) | V (Å3) | GOF |
|---|---|---|---|---|
| 10 | 10.5022(5) | 26.453(1) | 2526.8(2) | 1.37 |
| 20 | 10.4978(5) | 26.457(2) | 2525.0(3) | 1.37 |
| 30 | 10.4953(5) | 26.447(1) | 2522.9(2) | 1.34 |
| 40 | 10.4969(5) | 26.443(2) | 2523.3(2) | 1.41 |
| 60 | 10.4964(5) | 26.440(2) | 2522.8(3) | 1.41 |
| 70 | 10.4965(5) | 26.430(2) | 2521.9(2) | 1.54 |
| 80 | 10.4932(5) | 26.419(2) | 2519.2(2) | 1.54 |
| Ni(II) (mol %) | β-Phase (%) | aγ (Å) | cγ (Å) | vγ (Å3) | aβ (Å) | cβ (Å) | vβ (Å3) |
|---|---|---|---|---|---|---|---|
| 10 | 0 | 10.479(1) | 26.402(6) | 2510.6(5) | – | – | – |
| 20 | 0 a | 10.475(1) | 26.393(6) | 2508.3(5) | – | – | – |
| 30 | 45 | 10.478(2) | 26.400(12) | 2510(1) | 10.796(3) | 16.570(8) | 1673(1) |
| 40 | 57 | 10.477(4) | 26.395(17) | 2509(1) | 10.787(3) | 16.562(8) | 1669(2) |
| 50 | 56 | 10.477(3) | 26.402(12) | 2510(1) | 10.780(2) | 16.564(6) | 1667(1) |
| 60 | 100 b | – | – | – | 10.777(2) | 16.571(6) | 1666.7(5) |
| 70 | 100 | – | – | – | 10.770(2) | 16.562(6) | 1663.8(5) |
| 80 | 100 | – | – | – | 10.769(2) | 16.569(6) | 1664.2(5) |
| 90 | 100 | – | – | – | 10.759(2) | 16.564(6) | 1660.6(5) |
| Fe(II) (mol %) | β-Phase (%) | aγ (Å) | cγ (Å) | vγ (Å3) | aβ (Å) | cβ (Å) | vβ (Å3) |
|---|---|---|---|---|---|---|---|
| 10 | 1 | 10.500(4) | 26.426(12) | 2523(2) | 10.74 | 16.59 | 1657 |
| 20 | 4 | 10.498(4) | 26.417(13) | 2521(2) | 10.741(21) | 16.587(49) | 1657(2) |
| 30 | 12 | 10.497(4) | 26.385(14) | 2518(2) | 10.752(4) | 16.582(12) | 1660(2) |
| 40 | 27 | 10.497(4) | 26.373(13) | 2517(2) | 10.754(5) | 16.582(13) | 1660(2) |
| 50 | 65 | 10.504(5) | 26.346(17) | 2517(2) | 10.753(4) | 16.584(9) | 1661(2) |
| Fe(II) (mol %) | β-Phase (%) | aγ (Å) | cγ (Å) | vγ (Å3) | aβ (Å) | cβ (Å) | vβ (Å3) |
|---|---|---|---|---|---|---|---|
| 10 | 7 | 10.476(2) | 26.393(8) | 2509(1) | 10.706(11) | 16.517(28) | 1639(1) |
| 20 | 29 | 10.476(3) | 26.395(10) | 2509(2) | 10.666(4) | 16.539(11) | 1629(1) |
| 30 | 59 | 10.478(6) | 26.380(20) | 2508(3) | 10.644(4) | 16.533(11) | 1622(2) |
| 40 | 90 | 10.476(10) | 26.310(43) | 2500(4) | 10.632(3) | 16.533(7) | 1619(4) |
| 50 | 54 | 10.477(4) | 26.384(13) | 2508(2) | 10.630(3) | 16.536(8) | 1618(2) |
| Theoretical | Found | Phase | Theoretical | Found | Phase | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ni (%) | Zn (%) | Ni (%) | Zn(%) | Fe (%) | Zn (%) | Fe (%) | Zn (%) | ||
| 10 | 90 | 10.6 | 89.4 | γ | 10 | 90 | 2.4 | 97.6 | γ |
| 30 | 70 | 24.4 | 75.6 | γ | 20 | 80 | 2.5 | 97.5 | γ |
| 50 | 50 | 9.1 | 90.9 | γ | 30 | 70 | 1.4 | 98.6 | γ |
| 50 | 50 | 54.3 | 45.7 | β | 30 | 70 | 1.4 | 98.6 | γ |
| 60 | 40 | 66.6 | 33.4 | β | 30 | 70 | 86.2 | 13.8 | β |
| 90 | 10 | 95.5 | 4.5 | β | 40 | 60 | 61.3 | 38.7 | β |
| 90 | 10 | 94.9 | 5.1 | β | 40 | 60 | 2.2 | 97.8 | γ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serdan, U.; Robin, L.; Marchivie, M.; Gonidec, M.; Rosa, P.; Duverger-Nédellec, E.; Pouget, E.; Sainctavit, P.; Arrio, M.-A.; Juhin, A.; et al. Crystal Engineering of Conglomerates: Dilution of Racemate-Forming Fe(II) and Ni(II) Congeners into Conglomerate-Forming [Zn(bpy)3](PF6)2. Chemistry 2023, 5, 255-268. https://doi.org/10.3390/chemistry5010020
Serdan U, Robin L, Marchivie M, Gonidec M, Rosa P, Duverger-Nédellec E, Pouget E, Sainctavit P, Arrio M-A, Juhin A, et al. Crystal Engineering of Conglomerates: Dilution of Racemate-Forming Fe(II) and Ni(II) Congeners into Conglomerate-Forming [Zn(bpy)3](PF6)2. Chemistry. 2023; 5(1):255-268. https://doi.org/10.3390/chemistry5010020
Chicago/Turabian StyleSerdan, Ugo, Lucas Robin, Mathieu Marchivie, Mathieu Gonidec, Patrick Rosa, Elen Duverger-Nédellec, Emilie Pouget, Philippe Sainctavit, Marie-Anne Arrio, Amélie Juhin, and et al. 2023. "Crystal Engineering of Conglomerates: Dilution of Racemate-Forming Fe(II) and Ni(II) Congeners into Conglomerate-Forming [Zn(bpy)3](PF6)2" Chemistry 5, no. 1: 255-268. https://doi.org/10.3390/chemistry5010020
APA StyleSerdan, U., Robin, L., Marchivie, M., Gonidec, M., Rosa, P., Duverger-Nédellec, E., Pouget, E., Sainctavit, P., Arrio, M.-A., Juhin, A., Rogalev, A., Wilhelm, F., & Hillard, E. A. (2023). Crystal Engineering of Conglomerates: Dilution of Racemate-Forming Fe(II) and Ni(II) Congeners into Conglomerate-Forming [Zn(bpy)3](PF6)2. Chemistry, 5(1), 255-268. https://doi.org/10.3390/chemistry5010020