Hydrotrifluoromethylation of Styrene and Phenylacetylene Derivatives under Visible-Light Photoredox Conditions
Abstract
:1. Introduction
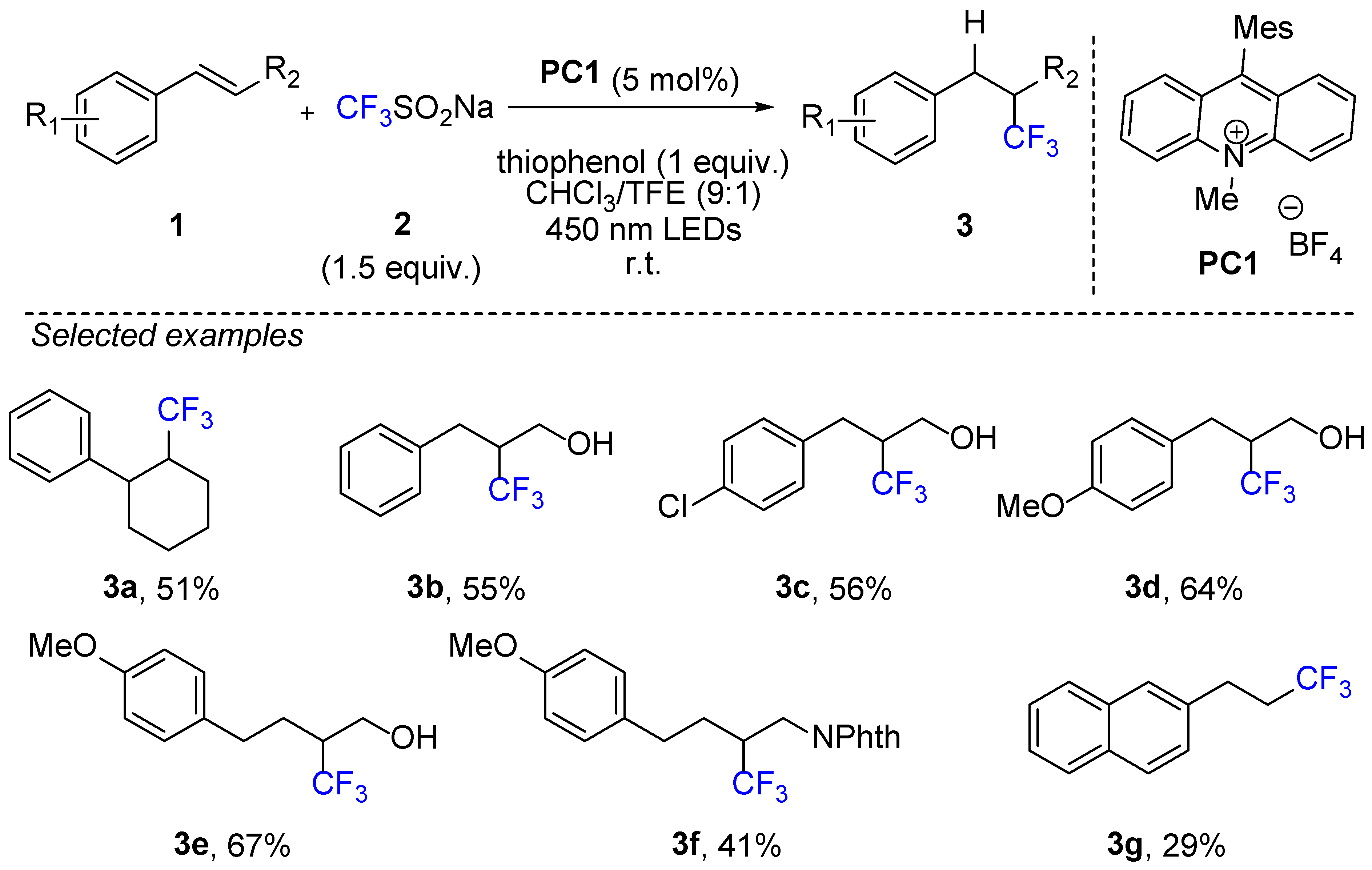
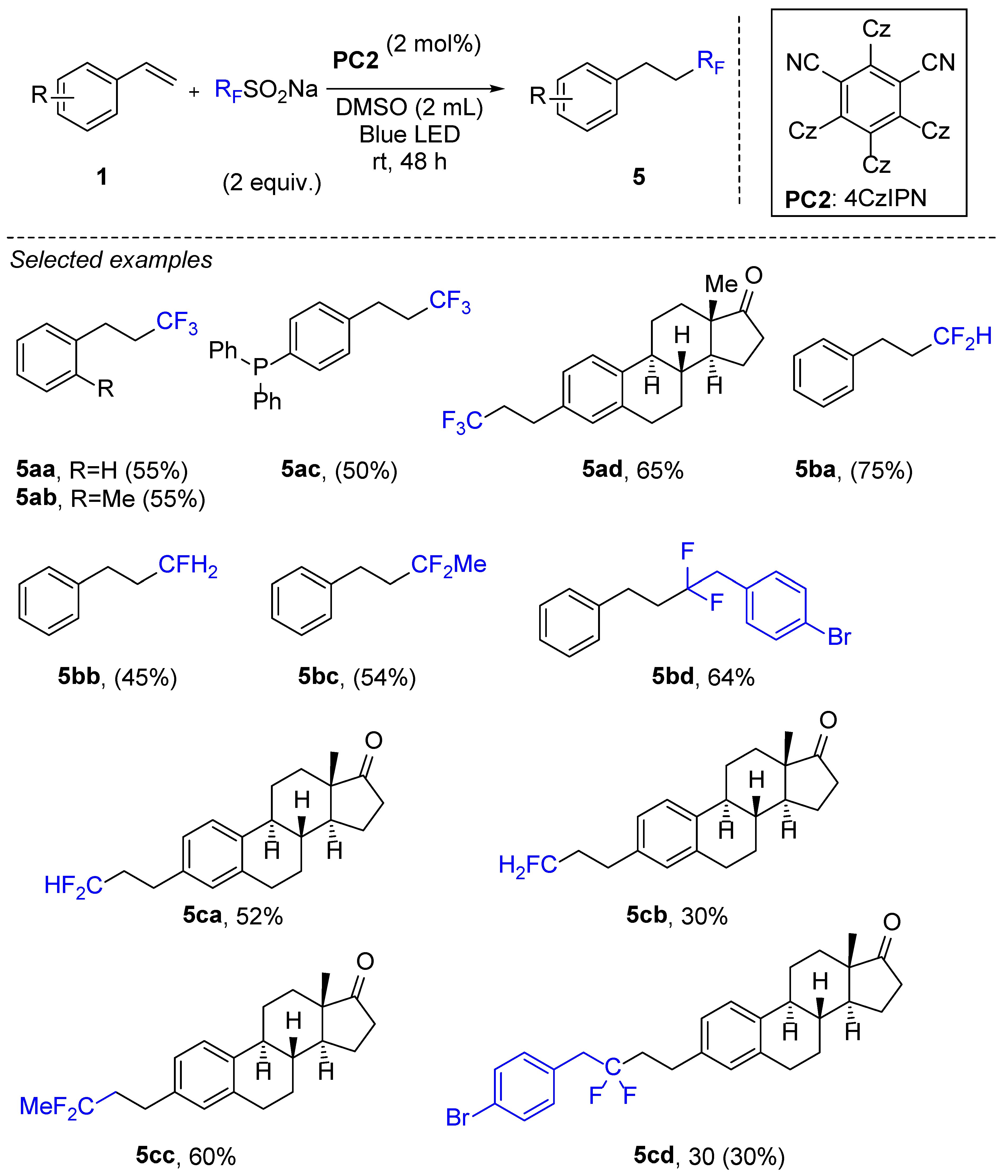
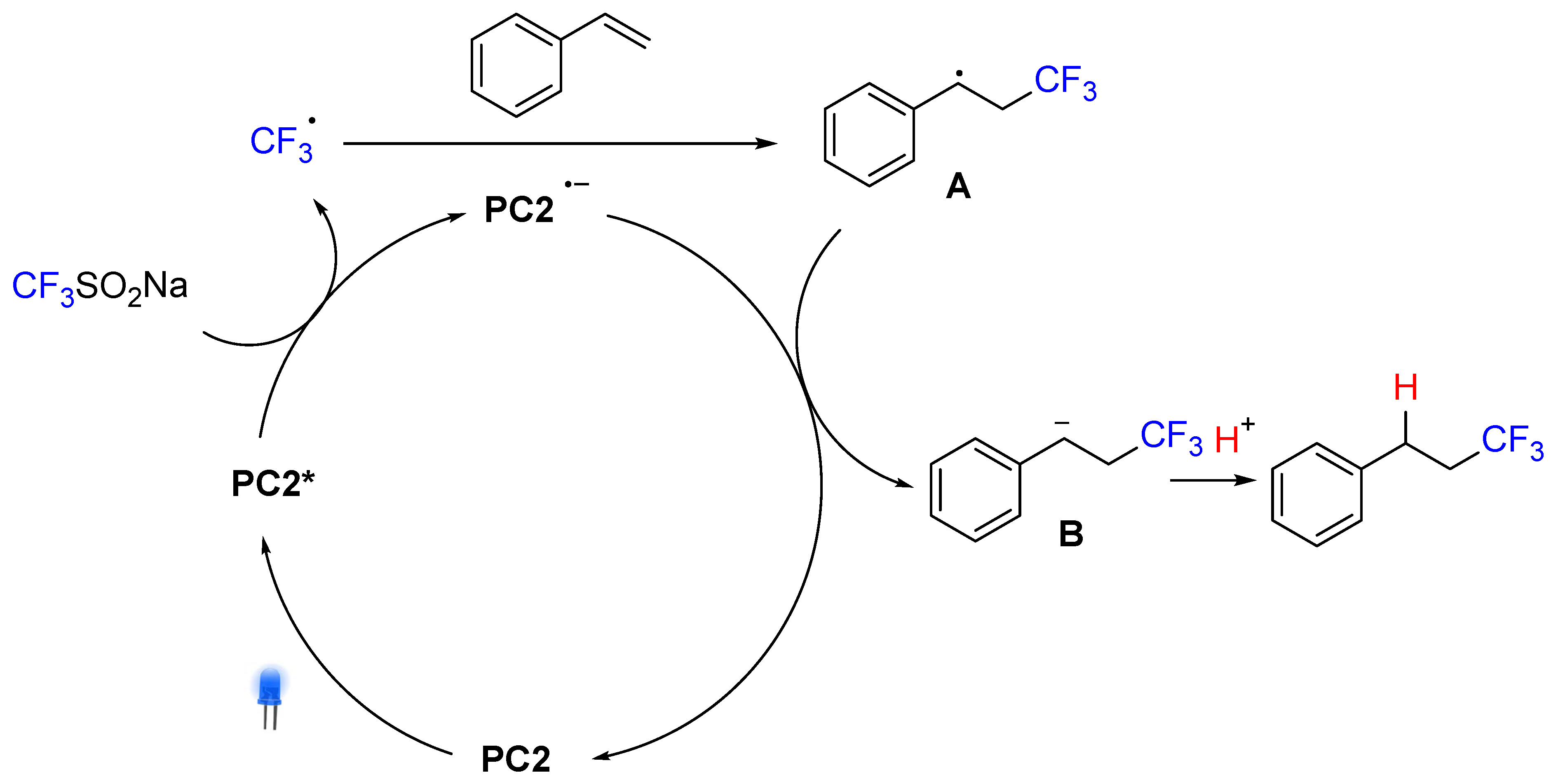
2. Hydrotrifluoromethylation of Styrene Derivatives
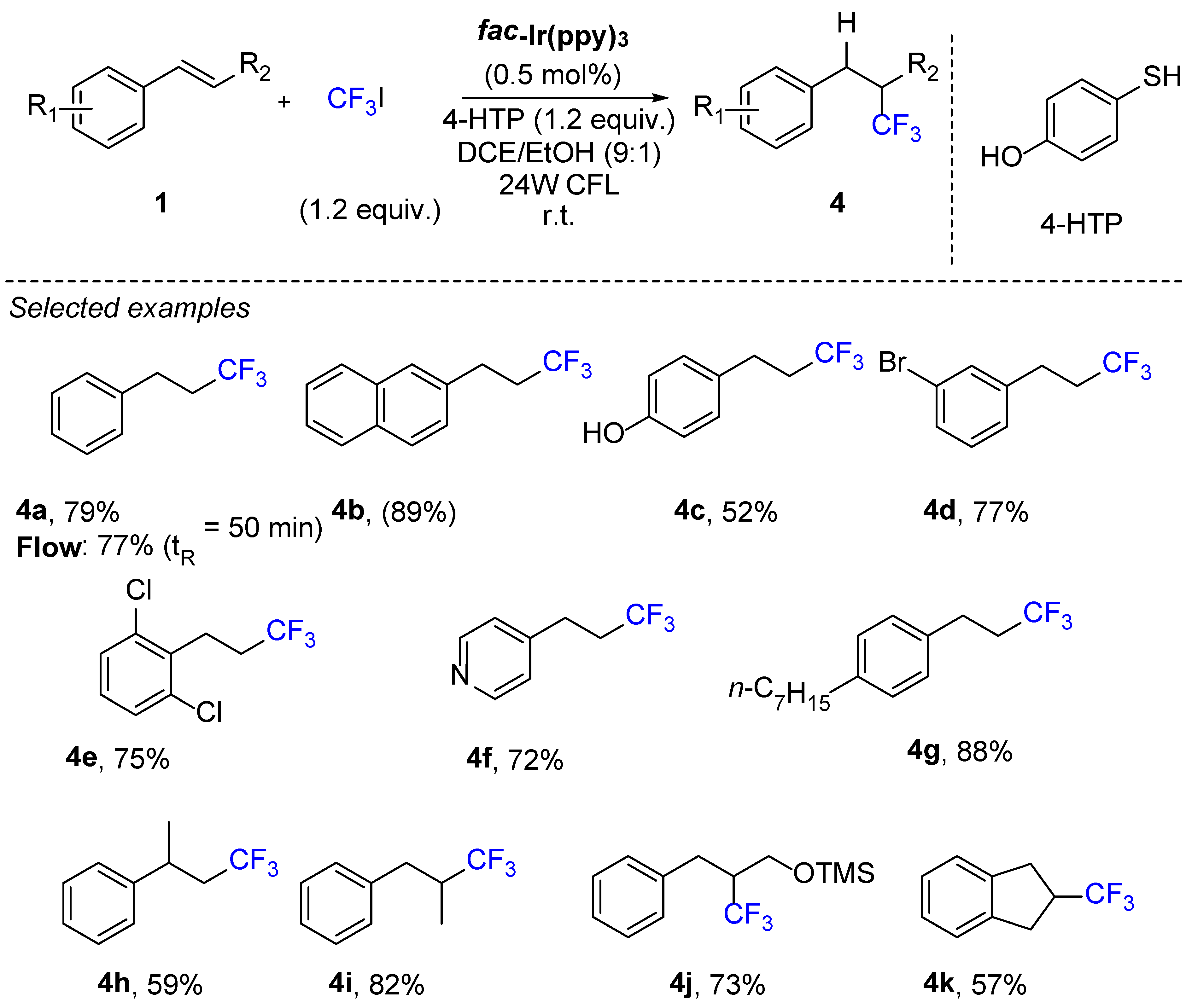
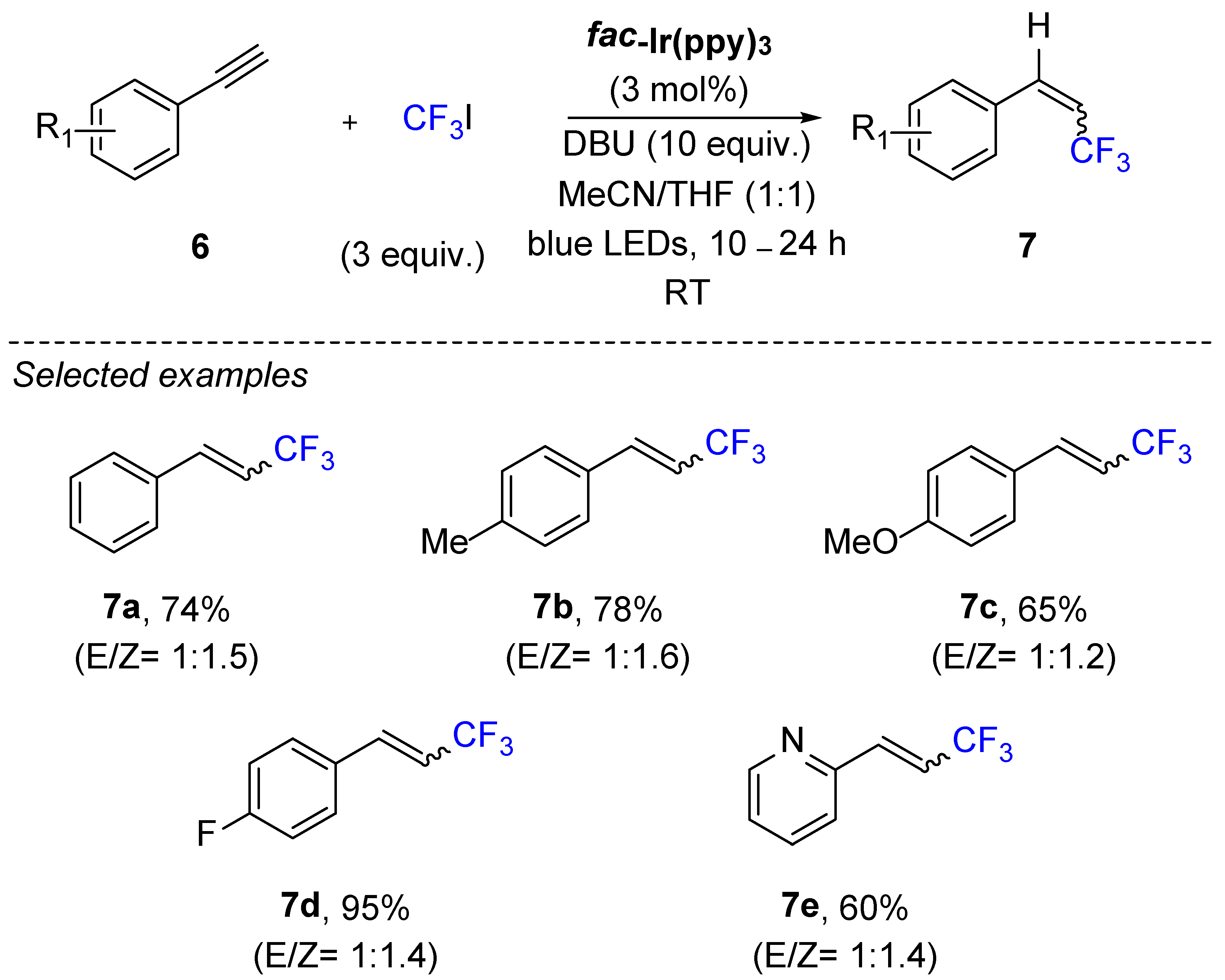
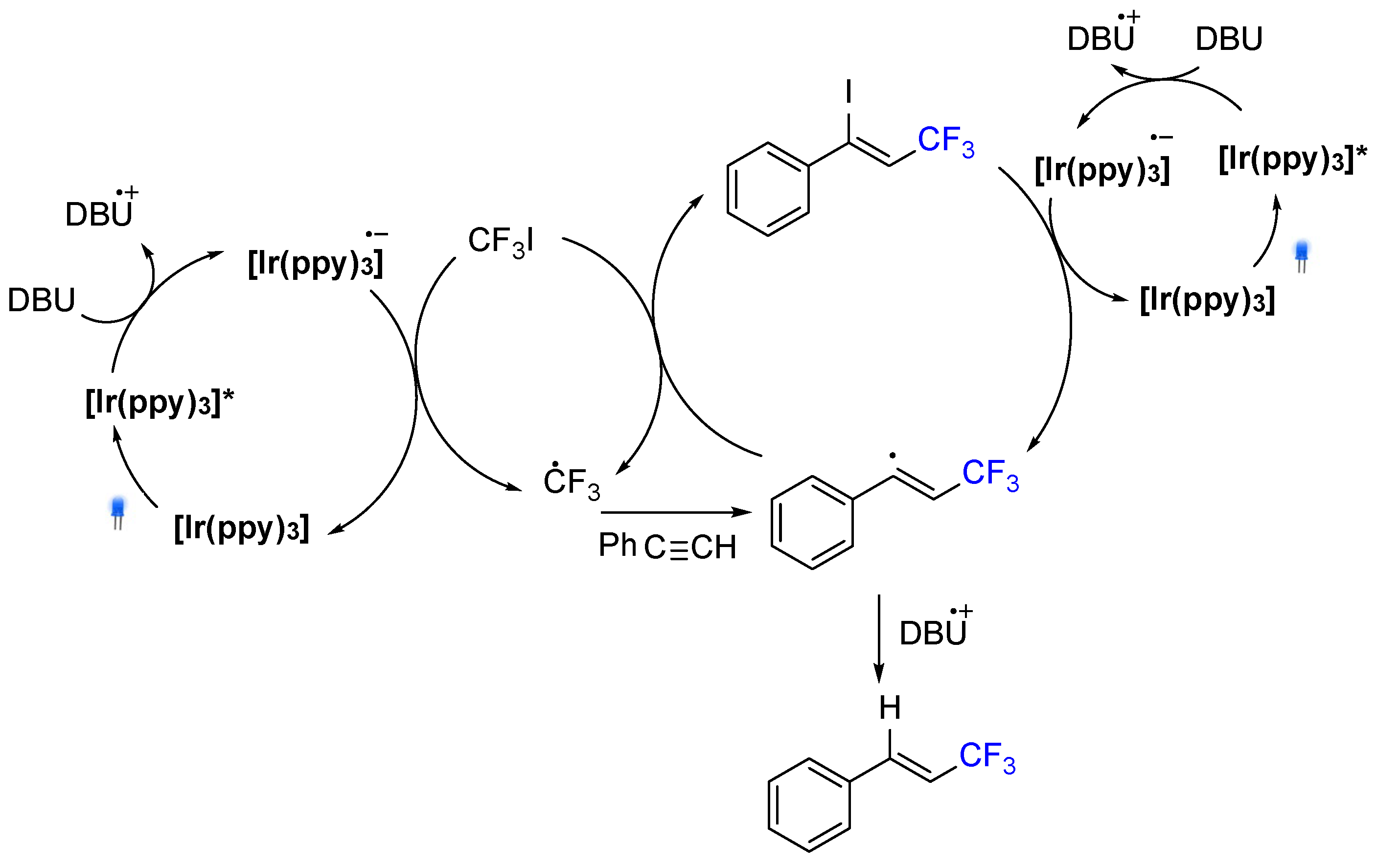
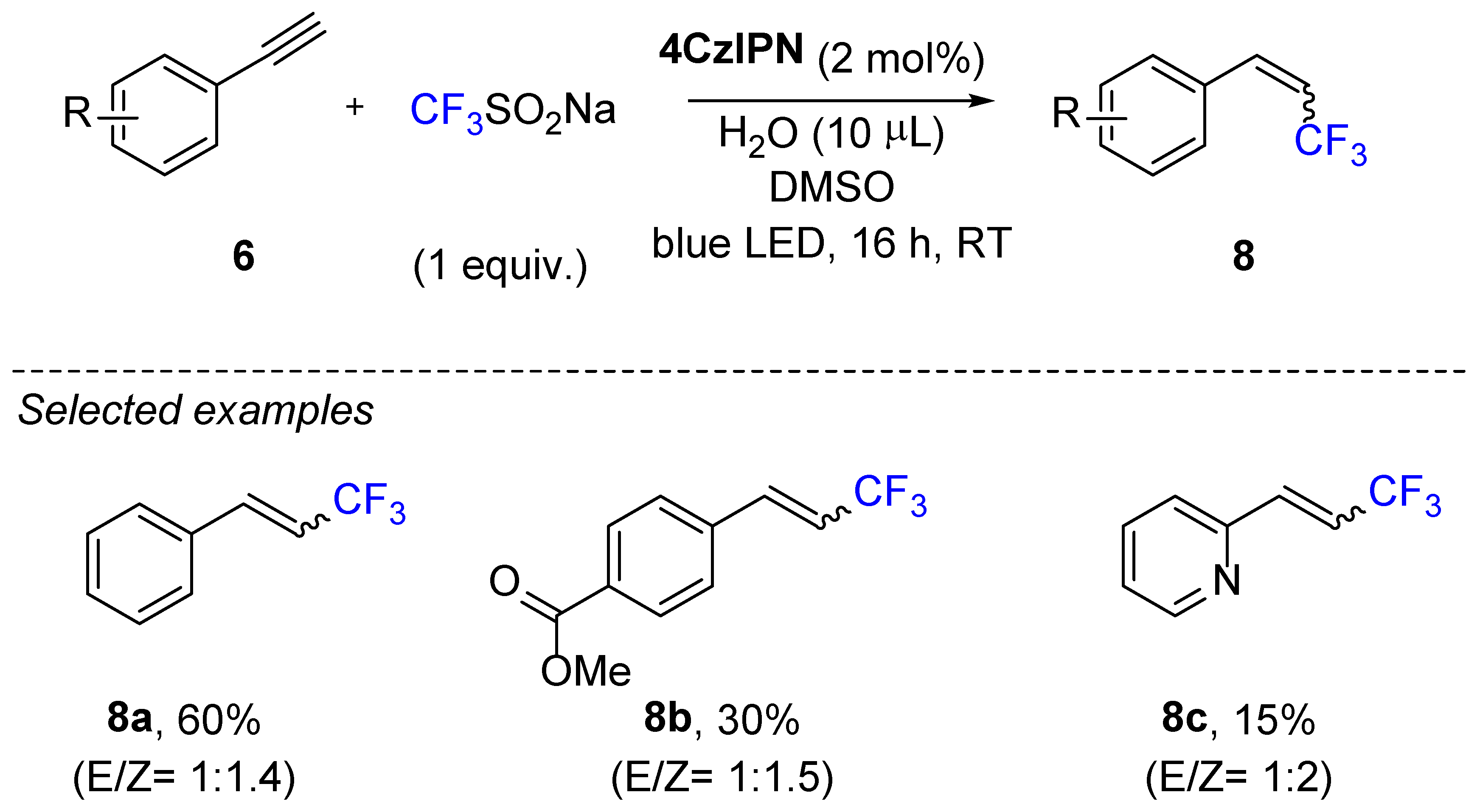
3. Hydrotrifluromethylation of Phenylacetylenes Derivatives
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Fluorine in Life Sciences: Pharmaceuticals, Medicinal Diag-nostics, and Agrochemicals; Haufe, G.; Leroux, F. (Eds.) Elsevier Science: London, UK, 2018; pp. 459–518. [Google Scholar]
- Leo, A.; Hansch, C.; Elkins, D. Partition coefficients and their uses. Chem. Rev. 1971, 71, 525–616. [Google Scholar] [CrossRef]
- Hagmann, W.K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef] [PubMed]
- Tlili, A.; Lakhdar, S. Acridinium Salts and Cyanoarenes as Powerful Photocatalysts: Opportunities in Organic Synthesis. Angew. Chem. Int. Ed. 2021, 60, 19526–19549. [Google Scholar] [CrossRef] [PubMed]
- Vega-PeÇaloza, A.; Mateos, J.; Companyó, X.; Escudero-Casao, M.; Dell’Amico, L. A Rational Approach to Organo-Photocatalysis: Novel Designs and Structure-Property Relationships. Angew. Chem. Int. Ed. 2021, 60, 1082–1097. [Google Scholar] [CrossRef] [PubMed]
- Marzo, L.; Pagire, S.K.; Reiser, O.; König, B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef] [PubMed]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Poliakoff, M.; George, M.W. Manufacturing chemicals with light: Anyrole in the circular economy? Phil. Trans. R. Soc. 2020, 378, 20190260. [Google Scholar] [CrossRef] [PubMed]
- Yungyeong, L.; Min, S.K. Emerging Organic Photoredox Catalysts for Organic Transformations. Eur. J. Org. Chem. 2020, 2020, 6028–6043. [Google Scholar]
- Courant, T.; Masson, G. Recent Progress in Visible-Light Photoredox-Catalyzed Intermolecular 1,2-Difunctionalization of Double Bonds via an ATRA-Type Mechanism. J. Org. Chem. 2016, 81, 6945–6952. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Akita, M. Fine Design of Photoredox Systems for Catalytic Fluoromethylation of Carbon–Carbon Multiple Bonds. Acc. Chem. Res. 2016, 49, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, Y.J.; Kim, S.M.; Yang, J.W.; Kim, S.W.; Cho, E.J. Hydrotrifluoromethylation and iodotrifluoromethylation of alkenes and alkynes using an inorganic electride as a radical generator. Nat. Commun. 2014, 5, 4881. [Google Scholar] [CrossRef] [PubMed]
- Wilger, D.J.; Gesmundo, N.J.; Nicewicz, D.A. Catalytic hydrotrifluoromethylation of styrenes and unactivated aliphatic alkenes via an organic photoredox system. Chem. Sci. 2013, 4, 3160. [Google Scholar] [CrossRef]
- Straathof, S.N.J.W.; Cramer, E.; Hessel, V.; Noel, T. Practical Photocatalytic Trifluoromethylation and Hydrotrifluoromethylation of Styrenes in Batch and Flow. Angew. Chem. Int. Ed. 2016, 55, 15549–15553. [Google Scholar] [CrossRef] [PubMed]
- Louvel, D.; Souibgui, A.; Taponard, A.; Rouillon, J.; ben Mosbah, M.; Moussaoui, Y.; Pilet, G.; Khrouz, L.; Monnereau, C.; Vantourout, J.C.; et al. Tailoring the Reactivity of the Langlois Reagent and Styrenes with Cyanoarenes Organophotocatalysts under Visible-Light. Adv. Synth. Catal. 2022, 364, 139–148. [Google Scholar] [CrossRef]
- Iqbal, N.; Jung, J.; Park, S.; Cho, E.J. Controlled Trifluoromethylation Reactions of Alkynes through Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2014, 53, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Souibgui, A.; ben Mosbah, M.; Moussaoui, Y.; Tlili, A. unpublished results.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souibgui, A.; ben Mosbah, M.; ben Salem, R.; Moussaoui, Y.; Tlili, A. Hydrotrifluoromethylation of Styrene and Phenylacetylene Derivatives under Visible-Light Photoredox Conditions. Chemistry 2022, 4, 1010-1015. https://doi.org/10.3390/chemistry4030068
Souibgui A, ben Mosbah M, ben Salem R, Moussaoui Y, Tlili A. Hydrotrifluoromethylation of Styrene and Phenylacetylene Derivatives under Visible-Light Photoredox Conditions. Chemistry. 2022; 4(3):1010-1015. https://doi.org/10.3390/chemistry4030068
Chicago/Turabian StyleSouibgui, Amel, Mongi ben Mosbah, Ridha ben Salem, Younes Moussaoui, and Anis Tlili. 2022. "Hydrotrifluoromethylation of Styrene and Phenylacetylene Derivatives under Visible-Light Photoredox Conditions" Chemistry 4, no. 3: 1010-1015. https://doi.org/10.3390/chemistry4030068
APA StyleSouibgui, A., ben Mosbah, M., ben Salem, R., Moussaoui, Y., & Tlili, A. (2022). Hydrotrifluoromethylation of Styrene and Phenylacetylene Derivatives under Visible-Light Photoredox Conditions. Chemistry, 4(3), 1010-1015. https://doi.org/10.3390/chemistry4030068








