Abstract
Structurally divergent molecules containing bulky substituents tend to produce porous materials via frustrated packing. Two rigid tetrahedral cores, tetraphenylmethane and 1,3,5,7-tetraphenyladamantane, grafted peripherally with four (trimethylsilyl)ethynyl moieties, were found to have only isolated voids in their crystal structures. Hence, they were modified into tecton-like entities, tetrakis(4-(iodoethynyl)phenyl)methane [I4TEPM] and 1,3,5,7-tetrakis(4-(iodoethynyl)phenyl)adamantane [I4TEPA], in order to deliberately use the motif-forming characteristics of iodoethynyl units to enhance crystal porosity. I4TEPM not only holds increased free volume compared to its precursor, but also forms one-dimensional channels. Furthermore, it readily co-crystallizes with Lewis basic solvents to afford two-component porous crystals.
1. Introduction
According to Kitaigorodskii’s principle of close packing [1,2,3,4,5], molecules in crystals tend to dovetail and pack as efficiently as possible in order to maximize attractive dispersion forces and to minimize free energy. In other words, void space in crystals is always unfavorable. Thus, the construction of porous materials from discrete organic molecules (i.e., molecular porous materials (MPMs)) demands some special tactics [6,7,8,9,10,11]. For example, the packing of molecules specifically designed to bear sufficiently large and dimensionally fixed inner cavities or clefts (e.g., molecular cages and bowl-shaped compounds) can lead to porous structures [12,13,14].
Another viable synthetic strategy towards MPMs is to employ molecules with bulky, divergent and/or awkward shapes so that they no longer have the ability to pack tightly. Molecules such as 4-p-Hydroxyphenyl-2,2,4-trimethylchroman (Dianin’s compound) [15,16], tris(o-phenylenedioxy)cyclotriphosphazene (TPP) [17,18,19] and 3,3′,4,4′-tetrakis(trimethylsilylethynyl)biphenyl (TTEB) [20] are well-known for producing MPMs merely as a consequence of frustrated packing, even though they do not have pre-fabricated molecular free volumes.
We have now expanded this idea to a family of tetrahedral molecules substituted at the four vertices with bulky groups. Here, we report the synthesis and structural investigation of tetrakis(4-((trimethylsilyl)ethynyl)phenyl)methane (TMS4TEPM) and 1,3,5,7-tetrakis(4-((trimethylsilyl)ethynyl)phenyl)adamantane (TMS4TEPA) (Scheme 1). By affixing large trimethylsilylethynyl (TMS-acetylenyl) moieties to the parent tetraphenylmethane (TPM) and 1,3,5,7-tetraphenyladamantane (TPA) core units, our aim was to disturb close-packing and to realize more open crystalline solids.
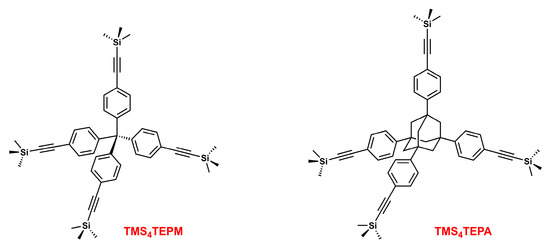
Scheme 1.
Structural formulas of tetrakis(4-((trimethylsilyl)ethynyl)phenyl)methane (TMS4TEPM) and 1,3,5,7-tetrakis(4-((trimethylsilyl)ethynyl)phenyl)adamantane (TMS4TEPA).
Even though molecular shape is of primary importance in crystal packing, it is not the only structure-directing factor. The presence of functional units that can partake in directional and energetically significant non-covalent interactions has a major influence on molecular arrangement. With tectons (i.e., molecules featuring multiple peripheral binding sites) [21,22,23,24], the structure is built up so as to saturate the maximum amount of interactions, which is usually accompanied by compromises regarding dense-packing. Their association induces the assembly of networks where each molecule is positioned, through directional molecular recognition events, in a definite way with respect to its neighbors. Moreover, unlike van der Waals contacts, intermolecular point contacts consume only a limited amount of molecular surface, thereby leaving more usable surface. In this context, a great body of work has been done with hydrogen-bonding tectons to build so-called hydrogen-bonded organic frameworks (HOFs) [25,26,27,28]. Some notable examples include triptycenetrisbenzimidazolone (TTBI) [29], triaminotriazine-functionalized spirobifluorene [30,31] and polyfluorinated triphenylbenzene equipped with pyrazole [32].
Molecular tectonics based on halogen bonding (XB) is still in its infancy [33,34]. We therefore decided to modify the TPM and TPA scaffolds and transform them into new tecton-like entities, tetrakis(4-(iodoethynyl)phenyl)methane (I4TEPM) and 1,3,5,7-tetrakis(4-(iodoethynyl)phenyl)adamantane (I4TEPM) (Scheme 2). When iodine is directly bonded to an sp-hybridized carbon, it is strongly polarized, resulting in a more pronounced electron-deficient region (i.e., σ-hole) at the tip along the C–I bond axis [35,36,37,38]. The iodoethynyl functionality is, therefore, a perfect candidate for σ-hole/XB interactions. Alhough largely overlooked in molecular tectonics and crystal engineering, it can direct the assembly of network structures through C≡C–I···(C≡C) interactions (wherein the ethynyl π system acts as the XB acceptor) [39,40,41]. These T-shaped contacts frequently lead to zigzag chain motifs and are topologically parallel to those formed by C≡C–H···(C≡C) and C≡C–Br···(C≡C) contacts [37,42,43,44,45,46,47,48,49,50,51,52], but preferably serve as a stronger counterpart. Additional features that make the iodoethynyl unit well-suited for devising molecular building blocks include its structural rigidity, steric openness and core expanding ability.
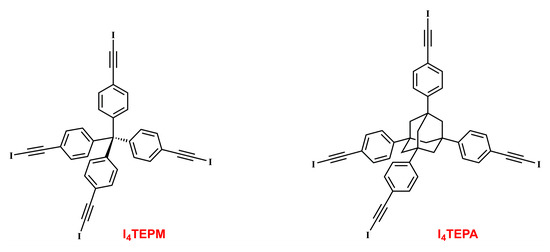
Scheme 2.
Structural formulas of tetrakis(4-(iodoethynyl)phenyl)methane (I4TEPM) and 1,3,5,7-tetrakis(4-(iodoethynyl)phenyl)adamantane (I4TEPM).
2. Results and Discussion
The four molecules of interest were obtained according to the synthetic pathways shown in Scheme 3 and Scheme 4. Starting with commercially available tetraphenylmethane, TMS4TEPM was prepared in two steps (tetra-para-bromination followed by coupling with trimethylsilylacetylene) with an overall yield of 78%. The synthesis of TMS4TEPA required three steps (Friedel-Crafts reaction of 1-bromoadamantane and benzene, tetra-para-iodination followed by coupling with trimethylsilylacetylene), and the yield over these three steps was 50% (with respect to 1-bromoadamantane).
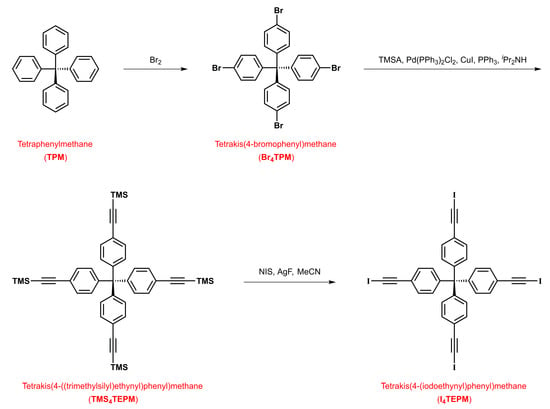
Scheme 3.
Synthetic route to TMS4TEPM and I4TEPM.

Scheme 4.
Synthetic route to TMS4TEPA and I4TEPA.
Both I4TEPM and I4TEPA were accessible from the corresponding TMS derivatives, TMS4TEPM and TMS4TEPA, via one-pot/in situ desilylative iodination using silver(I) fluoride and N-iodosuccinimide. This direct trimethylsilyl-to-iodo transformation allowed us to avoid potentially unstable ethynyl intermediates and to achieve the target compounds in moderate yields (56% and 63%, respectively). Even though the 1H and proton-decoupled 13C-NMR spectra of these four-fold symmetric tetraiodoethynyl species are quite simple, the signals display considerable solvent dependency due to their XB-based complexation ability, with the alkynyl carbon bonded to iodine being most strongly affected (I4TEPM: 7.0 ppm in CDCl3 versus 18.4 ppm in DMSO-d6, I4TEPA: 6.2 ppm in CDCl3 versus 17.0 ppm in DMSO-d6). It is also worth mentioning that the 1H-NMR spectrum of I4TEPA exhibits conspicuous second order (leaning/roofing) effects.
Crystals of TMS4TEPM suitable for single-crystal X-ray analysis were obtained by slow evaporation of either tetrahydrofuran/ethanol or chloroform/ethanol solution. For TMS4TEPA, X-ray quality crystals could be harvested from hexane, heptane, heptane/dichloromethane or chloroform/ethanol. As anticipated, structural determination revealed that both are somewhat porous in nature (14.9% and 14.5% free volume, respectively). They, however, do not form empty-channel structures; instead, they have disconnected spatial voids or “porosity without pores”, as described by Barbour (Figure 1) [53]. The overall packing is mainly mediated by extensive phenyl embraces.
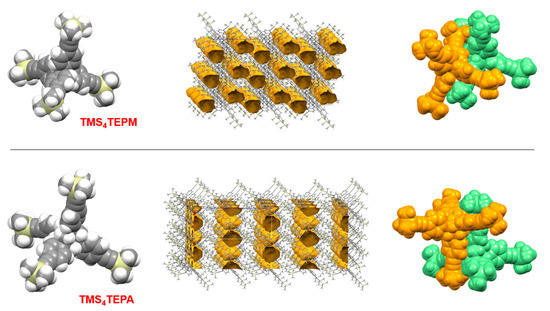
Figure 1.
Crystal structures of TMS4TEPM and TMS4TEPA. (from left) Single molecules, overall packing and phenyl embraces (representative structures are shown from disordered structures).
In order to get some insight about the electron density/charge distribution over the free tetraiodoethynyl tectons and the degree of activation of XB donor sites (i.e., iodine atoms) delivered by sp-hybridized carbons [35,36,37,38], their molecular electrostatic potential (MEP) maps were generated (Figure 2). As expected, both I4TEPM and I4TEPA were found to have well-built σ-holes (+172.4 and +170.7 kJ/mol, respectively) on each iodine atom. Indeed, these σ-hole potential values are significantly higher than those of other closely-related tetra-halogenated molecules (see Supplementary Materials, Figure S33).
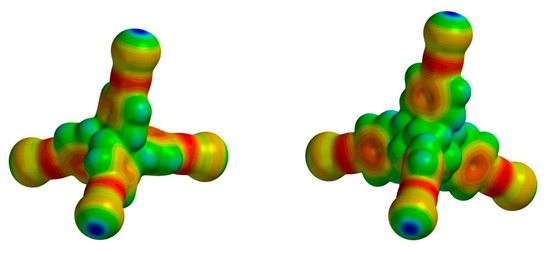
Figure 2.
Molecular electrostatic potential (MEP) surfaces of the free tetraiodoethynyl tectons, I4TEPM and I4TEPA. Both plots have been set to the same color scale for visual comparison. Range: from −80 kJ/mol (red) to +175 kJ/mol (blue).
We then tried to grow crystals of I4TEPM and I4TEPA but were successful only with the former. The structural analysis of I4TEPM crystals (harvested from hexanes) showed that the molecules are arranged in stacks which, in turn, are linked together by C≡C–I···(C≡C) halogen bonds, with near orthogonal approach of C–I donors towards C≡C triple bonds (detailed geometrical data are given in Table 1). In each I4TEPM molecule, only two iodoethynyl arms participate in these T-shaped contacts, and the remaining two form weak C≡C–I···π(phenyl) interactions. The extended (and possibly cooperative) zigzag arrays of the C≡C–I···π(ethynyl) interactions ultimately make ladder-like motifs between individual molecular rows, leading to an infinite two-dimensional network (Figure 3 left). I4TEPM shares these packing features with its bromo analog, tetrakis(4-(bromoethynyl)phenyl)methane (Br4TEPM) [42], but not with tetrakis(4-ethynylphenyl)methane (TEPM), which forms an interwoven diamondoid net [44].

Table 1.
XB interaction parameters in the studied crystal structures.
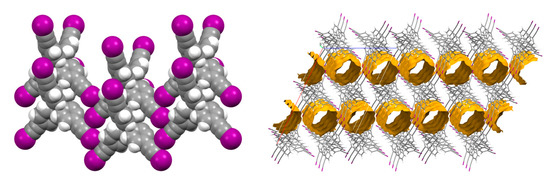
Figure 3.
Crystal structure of I4TEPM, showing halogen bonding (XB)-driven network formation (left) and void space in overall packing (right).
In contrast to the structure of TMS4TEPM with isolated voids, I4TEPM possesses one-dimensional channels along the crystallographic b axis (Figure 3 right). These channels account for 26.5% of the crystal volume, which is roughly twice as high as that of TMS4TEPM. Another point worth emphasizing is that the precursor molecules, tetraphenylmethane (TPM), tetrakis(4-bromophenyl)methane (Br4TPM) and tetrakis(4-iodophenyl)methane (I4TPM), all form non-porous structures (see Supplementary Materials, Figure S34), highlighting the effectiveness of our strategy.
Since MPMs are usually held together by relatively weak interactions, they are not as rigid and robust as zeolites, metal-organic frameworks (MOFs) or covalent-organic frameworks (COFs). In most cases, attempts at activation (i.e., removal of entrapped guest molecules) cause structural disintegration. Hence, the real challenge lies in attaining permanently porous molecular materials that can behave analogously to framework-type solids. Most importantly, I4TEPM, sustained primarily by the iodoethynyl catemer motif (i.e., the infinite C≡C–I···C≡C–I··· synthon), can maintain its structural integrity upon guest solvent loss, indicating its potential to exhibit permanent porosity.
In addition to tectonic construction, we also wanted to test the suitability of I4TEPM in modular construction by co-crystallizing it with appropriate Lewis basic (i.e., XB-accepting) co-formers, in order to realize multicomponent architectures. With tetraphenylphosphonium halide salts (Ph4P+X−; X− = Cl−, Br−, I−), it readily afforded diamondoid (dia) frameworks, but interpenetration and the inclusion of bulky Ph4P+ cations gave rise to highly compact arrangements within those solids [54]. As a charge-neutral co-crystallizing partner, our first choice was pyridine, one of the simplest XB acceptors, even though it cannot lead I4TEPM to a polymeric assembly. We managed to get a binary crystalline material (confirmed by IR, NMR and TGA) but the structural characterization was not successful, as those crystals were quite fragile and rapidly deteriorated during data collection. This intrigued us to try out other Lewis basic/coordinating solvents with multiple bond forming ability. In three cases, with tetrahydrofuran (THF), dimethyl sulfoxide (DMSO) and 1,4-dioxane, I4TEPM afforded crystalline binary solids.
Crystallization of I4TEPM in THF/methanol afforded crystals of I4TEPM·2THF where each THF molecule forms two halogen bonds in a bifurcated manner and connect adjacent I4TEPM molecules together, thereby forming a one-dimensional twisted ribbon-like architecture (Figure 4a left). The resulting lattice comprises isolated voids that account for 14.4% of unit cell volume (Figure 4a right).
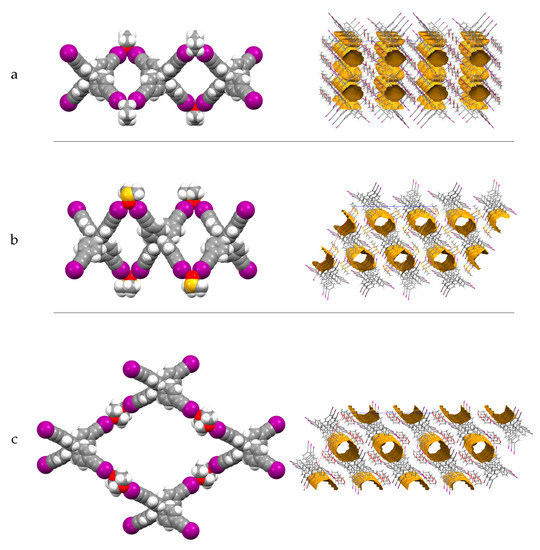
Figure 4.
Crystal structures of (a) I4TEPM·2THF, (b) I4TEPM·2DMSO and (c) I4TEPM·2Dioxane, showing XB-directed chain/net formation (left) and void space in overall packing (right).
Crystallization of I4TEPM from neat DMSO or DMSO/methanol yielded crystals of I4TEPM·2DMSO which has XB interactions analogous to those observed in I4TEPM·2THF. Once again, the coordinating solvent acts as a bridging ligand and gives rise to a twisted-ribbon supramolecular chain (Figure 4b left), with one-dimensional channels of 21.0% free volume in the overall packing (Figure 4b right).
By using 1,4-dioxane/dichloromethane as the solvent system, crystals of I4TEPM·2Dioxane could be obtained. As expected, dioxane serves as a linear ditopic ligand, so the structure propagates into two dimensions (Figure 4c left). As in I4TEPM·2DMSO, the structure creates one-dimensional channels parallel to the crystallographic c axis, holding 21.0% free volume (Figure 4c right).
Unfortunately, as is the case with many other crystalline solvates, all these binary crystals are unstable at room temperature. Once removed from the mother liquor, they gradually become opaque because of the partial loss of halogen-bonded and freely-occupying solvent molecules. The DSC and TGA thermograms (Figure 5), however, show that the solvents are somewhat strongly attached to the crystal lattice. In particular, for I4TEPM·2THF and I4TEPM·2Dioxane, the removal temperatures are noticeably higher than their respective boiling points.
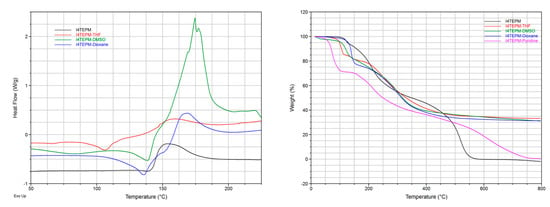
Figure 5.
(left) DSC traces (Tzero aluminum pan, 1–2 mg sample size, 5 °C·min−1 heating rate, nitrogen atmosphere) and (right) TGA traces (platinum pan, 5–10 mg sample size, 10 °C·min−1 heating rate, nitrogen atmosphere).
Table 1 presents XB distances and angles of I4TEPM and its binary crystals/solvates, along with the normalized distance (ND) and the percent radii reduction (%RR) values, which are two common indicators used as rough measures of the XB strength. In I4TEPM, C≡C–I···(C≡C) interactions are not symmetric and the C–I donors reach more toward terminal acetylenic carbons. Consequently, one I···C separation is significantly longer (with a low %RR value) and deviates from linearity. The %RR values calculated for XBs observed in the three solvates are greater than 15% (except in one case), reflecting the moderate strength of those interactions. Moreover, all bonds have near-linear (> 170° angles, again one exception) arrangements, reflecting their high directionality.
3. Conclusions
The solid-state packing behavior of tetrakis(4-((trimethylsilyl)ethynyl)phenyl)methane [TMS4TEPM] and 1,3,5,7-tetrakis(4-((trimethylsilyl)ethynyl)phenyl)adamantane [TMS4TEPA] showed some degree of extrinsic porosity. These two molecules were converted into tecton-like derivatives with XB capability, I4TEPM and I4TEPA, in order to investigate the power of iodoethynyl recognition sites in the context of solid-state packing and extrinsic porosity. Our results demonstrate that, even though I4TEPA tends not to form crystalline unary or binary solids, I4TEPM crystallizes into porous solids in its neat form as well as with suitable co-formers. The binary systems formed with coordinating solvents (i.e., I4TEPM·4Pyridine, I4TEPM·2THF, I4TEPM·2DMSO and I4TEPM·2Dioxane) are prone to collapse upon solvent removal. It is therefore rational to think that I4TEPM would offer more stable crystals if the co-formers employed are solids at ambient conditions. Efforts to explore these new possibilities, especially utilizing molecules with tetrahedrally-disposed XB accepting sites (e.g., tetraazaadamantane, tetrakis(4-pyridyl)cyclobutane, tetrakis(4-pyridyloxymethyl)methane) are currently being undertaken in our lab.
4. Materials and Methods
Unless otherwise noted, all reagents, solvents and precursors (tetraphenylmethane and 1-bromoadamantane) were purchased from commercial sources and used as received, without further purification. Nuclear magnetic resonance (NMR) spectra were recorded at room temperature on a Varian Unity Plus (400 MHz) spectrometer (Agilent Technologies, Inc., Santa Clara, CA, USA). Chemical shifts for 1H-NMR spectra were referenced to the residual protio impurity peaks in the deuterated solvents, while 13C{1H} NMR spectra were referenced against the solvent 13C resonances. A Nicolet 380 FT-IR system (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used for the infrared (IR) spectroscopic analysis. Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) were performed on TA Q20 and TA Q50 (TA Instruments, New Castle, DE, USA), respectively. In order to calculate the molecular surface electrostatic potentials of tetra-halogenated TPM and TPA species, their geometries were optimized (using Spartan ‘14 software [55]) at hybrid functional B3LYP/6-311+G** and B3LYP/6-311++G** levels of theory, respectively, and potential values were subsequently mapped onto 0.002 au isosurface. Detailed crystallographic information about data collections, solutions, and refinements can be found in the Supplementary Materials. Structural visualizations and void mapping were done using Mercury software [56]. For free volume calculations, the voids function in Mercury (with contact surface, 1.2 Å probe radius and 0.2 Å approximate grid spacing) and/or the solvent-masking tool in Olex2 (with its default parameters) were employed [56,57].
4.1. Synthesis of Tetrakis(4-bromophenyl)methane (Br4TPM)
The bromination of tetraphenylmethane was performed neat using an excess of molecular bromine. To a 100-mL round-bottom flask containing tetraphenylmethane (2.00 g, 6.24 mmol, 1 equiv.), bromine liquid (6.4 mL, 124.8 mmol, 20 equiv.) was added carefully at 0 °C. After attaching a water-cooled reflux condenser, the resultant dark reddish slurry was stirred vigorously at room temperature for one hour, and then cooled to −78 °C by using a dry ice/acetone bath. Ethanol (25 mL) was added slowly and the reaction mixture was allowed to warm to room temperature overnight. Then, to destroy excess/unreacted bromine, it was treated with 40% aqueous solution of sodium bisulfite (approximately 75 mL) and stirred for an additional 30 min until the orange color disappeared. The tan colored solid was collected by filtration, washed well with distilled water (100 mL) and oven-dried at 60 °C for five hours. This solid was further purified by re-crystallization from chloroform/ethanol (2:1), affording tetrakis(4-bromophenyl)methane, Br4TPM, as an off-white crystalline material. Yield: 3.65 g (5.74 mmol, 92%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.39 (d, 8H); 7.01 (d, 8H). 13C-NMR (100 MHz, CDCl3) δ (ppm): 144.64, 132.57, 131.30, 121.02, 63.84. ATR-FTIR (cm−1): 3059, 1919, 1569, 1478, 1395, 1185, 1077, 1007, 948, 808, 753.
4.2. Synthesis of Tetrakis(4-((trimethylsilyl)ethynyl)phenyl)methane (TMS4TEPM)
This step involved a Sonogashira cross-coupling reaction of tetrakis(4-bromophenyl)methane with trimethylsilylacetylene. Tetrakis(4-bromophenyl)methane (3.50 g, 5.50 mmol, 1 equiv.) and triphenylphosphine (462 mg, 1.76 mmol, 32 mol%) were placed in a 250-mL round-bottomed flask. Diisopropyl amine (100 mL) was added and the resulting solution was purged with dinitrogen gas for 30 min. Then, bis(triphenylphosphine)palladium(II) dichloride (618 mg, 0.88 mmol, 16 mol%), copper(I) iodide (168 mg, 0.88 mmol, 16 mol%) and trimethylsilylacetylene (6.2 mL, 44.0 mmol, 8 equiv.) were added. The reaction flask was fitted to a water-jacketed condenser, cooled to −78 °C, subjected to a brief vacuum/backfill cycle and refluxed for 24 h under nitrogen atmosphere. After removing volatile materials in vacuo, the residue was re-dissolved in chloroform (100 mL) and filtered through a pad of Celite, using an extra 50 mL portion of chloroform to wash the filter pad. The combined filtrate was then washed with distilled water (2 × 25 mL) and brine (25 mL), dried over anhydrous magnesium sulfate, and evaporated to dryness under vacuum. The crude product was flash-column-chromatographed on silica gel using pure hexanes followed by hexanes/ethyl acetate (4:1) as eluents to obtain the title compound, TMS4TEPM, as a pale yellowish solid. Yield: 3.30 g (4.68 mmol, 85%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.33 (d, 8H), 7.05 (d, 8H), 0.24 (s, 36H). 13C-NMR (100 MHz, CDCl3) δ (ppm): 146.21, 131.59, 130.95, 121.42, 104.82, 95.00, 64.98, 0.18. ATR-FTIR (cm−1): 2957, 2157, 1496, 1405, 1247, 1187, 1019, 835, 758.
4.3. Synthesis of Tetrakis(4-(iodoethynyl)phenyl)methane (I4TEPM)
The one-pot/in situ desilylative iodination (i.e., direct trimethylsilyl-to-iodo conversion) method was employed. Acetonitrile (150 mL) was transferred into a 250-mL round-bottom flask that contained tetrakis(4-((trimethylsilyl)ethynyl)phenyl)methane (2.50 g, 3.54 mmol, 1 equiv.). The flask was wrapped in aluminium foil, and then silver(I) fluoride (2.70 g, 21.3 mmol, 6 equiv.) and N-iodosuccinimide (4.78 g, 21.3 mmol, 6 equiv.) were added. It was then evacuated (while stirring), refilled with nitrogen and stirred at room temperature for 24 h. Distilled water (200 mL) was added and the resulting mixture was extracted with diethyl ether (4 × 50 mL). The combined organic layers were washed with saturated sodium bisulfite (40 mL), distilled water (40 mL) and brine (40 mL), and dried over anhydrous magnesium sulfate. The evaporation of the solvent under reduced pressure resulted in an orange colored residue. Additional cleanup by column chromatography (silica gel, hexanes/ethyl acetate = 9:1) gave the desired compound, I4TEPM, as a yellow solid. Crystals suitable for single-crystal X-ray diffraction were grown from hexanes. Yield: 1.83 g (1.98 mmol, 56%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.32 (d, 8H), 7.06 (d, 8H). 13C-NMR (100 MHz, CDCl3) δ (ppm): 146.34, 132.04, 130.87, 121.81, 93.87, 65.02, 7.03. 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 7.37 (d, 8H), 7.04 (d, 8H). 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 145.68, 131.66, 130.36, 121.08, 92.11, 64.26, 18.41. ATR-FTIR (cm−1): 2944, 2167, 1490, 1400, 1186, 1112, 1016, 955, 898, 827, 722.
4.4. Synthesis of 1,3,5,7-Tetraphenyladamantane (TPA)
In a 250-mL round-bottom flask, tert-butyl bromide (3.9 mL, 34.9 mmol, 2.5 equiv.) was added to a solution of 1-bromoadamantane (3.00 g, 13.9 mmol, 1 equiv.) in anhydrous benzene (30 mL). The flask was placed in an ice bath and aluminium chloride (186 mg, 1.39 mmol, 10 mol%) was carefully charged to the chilled stirring solution. The mixture was then heated under reflux until the evolution of hydrogen bromide ceased (the top of the condenser was connected to a gas absorption trap containing 30% aqueous sodium hydroxide). The resultant heterogeneous mixture was allowed to cool to room temperature and filtered, and the residue was washed sequentially with chloroform (30 mL), water (50 mL) and chloroform (30 mL). The off-white solid was further purified by washing overnight with refluxing chloroform in a Soxhlet apparatus, which gave 1,3,5,7-tetraphenyladamantane, TPA, as a fine white powder. Yield: 5.04 g (11.4 mmol, 82%). Mp: > 300 °C. ATR-FTIR (cm−1): 3055, 3020, 2918, 2849, 1597, 1493, 1442, 1355, 1263, 1078, 1030, 918, 889, 844, 788, 760, 746, 699.
4.5. Synthesis of 1,3,5,7-Tetrakis(4-iodophenyl)adamantane (I4TPA)
To a 250-mL round-bottom flask containing a suspension of 1,3,5,7-tetraphenyladamantane (4.00 g, 9.08 mmol, 1 equiv.) in chloroform (100 mL) was added iodine (5.76 g, 22.7 mmol, 2.5 equiv.). This mixture was stirred vigorously at room temperature until the iodine fully dissolved. The flask was flushed with nitrogen gas and bis(trifluoroacetoxy)iodo)benzene (9.76 g, 22.7 mmol, 2.5 equiv.) was added. The resulting mixture was stirred at room temperature for 12 h. It was then filtered off, and the collected solid was washed with an excess amount of chloroform (200 mL). The combined dark purple filtrate was washed with 5% sodium bisulfite solution twice (2 × 50 mL), followed by distilled water (100 mL) and saturated sodium chloride solution (100 mL). It was dried with anhydrous magnesium sulfate and the solvent was removed under reduced pressure, which resulted in a pale-yellow solid. After refluxing in methanol (200 mL) for 12 h, the pure compound, I4TPA, was isolated as a white solid by filtration and air-drying. Yield: 5.91 g (6.26 mmol, 69%). 1H-NMR (400 MHz, CDCl3) δ (ppm): 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.67 (d, 8H), 7.18 (d, 8H), 2.06 (s, 12H). 13C-NMR (100 MHz, CDCl3) δ (ppm): 148.63, 137.75, 127.34, 91.96, 46.92, 39.29. ATR-FTIR (cm−1): 3046, 2928, 2898, 2851, 1900, 1782, 1647, 1579, 1483, 1441, 1390, 1355, 1180, 1120, 1064, 1001, 888, 819, 775, 701, 659.
4.6. Synthesis of 1,3,5,7-Tetrakis(4-((trimethylsilyl)ethynyl)phenyl)adamantane (TMS4TEPA)
As in the synthesis of TMS4TEPM, this step involved a four-fold Sonogashira cross-coupling reaction of 1,3,5,7-tetrakis(4-iodophenyl)adamantane (I4TPA) with trimethylsilylacetylene. Yield: 88%. 1H-NMR (400 MHz, CDCl3) δ (ppm): 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.45 (d, 8H), 7.38 (d, 8H), 2.09 (s, 12H), 0.24 (s, 36H). 13C-NMR (100 MHz, CDCl3) δ (ppm): 149.63, 132.29, 125.13, 121.32, 105.19, 94.20, 46.97, 39.53, 0.25. ATR-FTIR (cm−1): 3033, 2958, 2897, 2852, 2155, 1604, 1502, 1445, 1398, 1355, 1248, 1115, 1016, 859, 835, 758.
4.7. Synthesis of 1,3,5,7-Tetrakis(4-(iodoethynyl)phenyl)adamantane (I4TEPA)
The same one-pot desilylative iodination method described above for the synthesis of I4TEPM (i.e., the direct trimethylsilyl-to-iodo transformation using silver(I) fluoride and N-iodosuccinimide) was employed. Yield: 63%. 1H-NMR (400 MHz, CDCl3) δ (ppm): 7.42 (d, 8H), 7.39 (d, 8H), 2.09 (s, 12H). 13C-NMR (100 MHz, CDCl3) δ (ppm): 149.82, 132.64, 125.16, 121.57, 94.16, 46.88, 39.50, 6.18. 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 7.51 (d, 8H), 7.37 (d, 8H), 2.00 (s, 12H). 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 150.14, 131.74, 125.48, 120.50, 92.59, 45.48, 38.95, 17.02. ATR-FTIR (cm−1): 3033, 2919, 2896, 2849, 2165, 1908, 1701, 1603, 1501, 1439, 1355, 1241, 1176, 1115, 1016, 837, 822, 769, 693.
4.8. Synthesis of I4TEPM·4pyridine
In a 2-dram glass vial, I4TEPM (10 mg, 0.011 mmol) was dissolved in 0.5 mL of pyridine. This open vial was placed in a second larger container (50-mL glass jar) containing 10 mL of pyridine/methanol (1:4) mixture. The outer container was then closed/sealed, and the apparatus was kept at ambient conditions to allow the vapor from methanol (anti-solvent) to diffuse into the sample solution. When the total volume of the inner vial became ~3 mL, it was taken out and, after partially tightening the lid, left undisturbed at ambient conditions to allow the solvents to evaporate slowly. Colorless/pale-yellow crystals were observed after few days. ATR-FTIR (cm−1): 3032, 2923, 2851, 2158, 1909, 1587, 1493, 1438, 1405, 1210, 1185, 1147, 1066, 1017, 997, 955, 827, 745, 699.
4.9. Synthesis of I4TEPM·2THF
In a 2-dram glass vial, I4TEPM (10 mg, 0.011 mmol) was dissolved in 1 mL of tetrahydrofuran. After adding 1 mL of methanol, the vial (with a partially-tightened screw cap) was left undisturbed at ambient conditions to allow the solvents to evaporate slowly. Colorless/pale-yellow crystals suitable for single-crystal X-ray diffraction were observed after few days. ATR-FTIR (cm−1): 2974, 2865, 2165, 1684, 1588, 1494, 1423, 1404, 1365, 1190, 1115, 1044, 1018, 884, 830, 809.
4.10. Synthesis of I4TEPM·2DMSO
In a 2-dram glass vial, I4TEPM (10 mg, 0.011 mmol) was dissolved in 0.5 mL of dimethyl sulfoxide. The vial (with a partially-tightened screw cap) was then allowed to stand at room temperature for one week, during which time colorless/pale-yellow crystals suitable for single-crystal X-ray diffraction were appeared. ATR-FTIR (cm−1): 3032, 2986, 2908, 2160, 1494, 1429, 1398, 1308, 1186, 1113, 1039, 1014, 945, 826, 697.
4.11. Synthesis of I4TEPM·2dioxane
In a 2-dram glass vial, I4TEPM (10 mg, 0.011 mmol) was suspended in 0.5 mL 1,4-dioxane. After adding a few drops of methylene chloride, the vial was sealed and heated to obtain a clear solution. Colorless/pale-yellow crystals suitable for single-crystal X-ray diffraction were harvested by slow evaporation. ATR-FTIR (cm−1): 2958, 2906, 2851, 2171, 1490, 1448, 1401, 1369, 1288, 1252, 1186, 1113, 1077, 1016, 976, 866, 829, 735.
Supplementary Materials
NMR and IR spectra, and crystallographic data are available online at https://www.mdpi.com/2624-8549/2/1/11/s1. The crystallographic data for this paper (CCDC 1971906–1971911) can also be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Author Contributions
C.A.G. and C.B.A. conceived and designed the experiments; C.A.G. performed the experiments; A.S.S. and E.W.R. performed the single-crystal X-ray crystallography; C.A.G. and C.B.A. analyzed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the U. S. Army Research Laboratory and the U. S. Army Research Office, grant number W911NF-13-1-0387.
Acknowledgments
We are grateful to Victor W. Day at the University of Kansas for collecting some single-crystal X-ray data. He, in turn, acknowledges the NSF-MRI grant CHE-0923449 which was used to purchase an X-ray diffractometer and software used in this study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Kitaigorodskii, A.I. Molecular Crystals and Molecules; Academic Press: New York, NY, USA, 1973. [Google Scholar]
- Kitaigorodskii, A.I. Organic Chemical Crystallography; Consultants Bureau: New York, NY, USA, 1961. [Google Scholar]
- Kitaigorodskii, A.I. Non-bonded interactions of atoms in organic crystals and molecules. Chem. Soc. Rev. 1978, 7, 133–163. [Google Scholar] [CrossRef]
- Kitaigorodskii, A.I. The principle of close packing and the condition of thermodynamic stability of organic crystals. Acta Crystallogr. 1965, 18, 585–590. [Google Scholar] [CrossRef]
- Kitaigorodskii, A.I. The close-packing of molecules in crystals of organic compounds. J. Phys. (USSR) 1945, 9, 351–352. [Google Scholar]
- Lü, J.; Cao, R. Porous organic molecular frameworks with extrinsic porosity: A platform for carbon storage and separation. Angew. Chem. Int. Ed. 2016, 55, 9474–9480. [Google Scholar] [CrossRef] [PubMed]
- Hashim, M.I.; Hsu, C.-W.; Le, H.T.M.; Miljanić, O.Š. Organic molecules with porous crystal structures. Synlett 2016, 27, 1907–1918. [Google Scholar]
- Tian, J.; Thallapally, P.K.; McGrail, B.P. Porous organic molecular materials. CrystEngComm 2012, 14, 1909–1919. [Google Scholar] [CrossRef]
- Mastalerz, M. Permanent porous materials from discrete organic molecules—towards ultra-high surface areas. Chem. Eur. J. 2012, 18, 10082–10091. [Google Scholar] [CrossRef]
- McKeown, N.B. Nanoporous molecular crystals. J. Mater. Chem. 2010, 20, 10588–10597. [Google Scholar] [CrossRef]
- Holst, J.R.; Trewin, A.; Cooper, A.I. Porous organic molecules. Nat. Chem. 2010, 2, 915–920. [Google Scholar] [CrossRef]
- Hasell, T.; Cooper, A.I. Porous organic cages: Soluble, modular and molecular pores. Nat. Rev. Mater. 2016, 1, 16053. [Google Scholar] [CrossRef]
- Evans, J.D.; Sumby, C.J.; Doonan, C.J. Synthesis and applications of porous organic cages. Chem. Lett. 2015, 44, 582–588. [Google Scholar] [CrossRef]
- Zhang, G.; Mastalerz, M. Organic cage compounds—from shape-persistency to function. Chem. Soc. Rev. 2014, 43, 1934–1947. [Google Scholar] [CrossRef] [PubMed]
- Imashiro, F.; Yoshimura, M.; Fujiwara, T. ‘Guest-free’ Dianin’s compound. Acta Crystallogr. C 1998, 54, 1357–1360. [Google Scholar] [CrossRef]
- Barrer, R.M.; Shanson, V.H. Dianin’s compound as a zeolitic sorbent. J. Chem. Soc. Chem. Commun. 1976, 333–334. [Google Scholar] [CrossRef]
- Kaleta, J.; Bastien, G.; Wen, J.; Dračínský, M.; Tortorici, E.; Císařová, I.; Beale, P.D.; Rogers, C.T.; Michl, J. Bulk inclusions of double pyridazine molecular rotors in hexagonal tris(o-phenylene)cyclotriphosphazene. J. Org. Chem. 2019, 84, 8449–8467. [Google Scholar] [CrossRef]
- Sozzani, P.; Bracco, S.; Comotti, A.; Ferretti, L.; Simonutti, R. Methane and carbon dioxide storage in a porous van der Waals crystal. Angew. Chem. Int. Ed. 2005, 44, 1816–1820. [Google Scholar] [CrossRef]
- Allcock, H.R.; Siegel, L.A. Phosphonitrilic compounds. III. Molecular inclusion compounds of tris(o-phenylenedioxy)phosphonitrile trimer. J. Am. Chem. Soc. 1964, 86, 5140–5144. [Google Scholar] [CrossRef]
- Msayib, K.J.; Book, D.; Budd, P.M.; Chaukura, N.; Harris, K.D.M.; Helliwell, M.; Tedds, S.; Walton, A.; Warren, J.E.; Xu, M.C.; et al. Nitrogen and hydrogen adsorption by an organic microporous crystal. Angew. Chem. Int. Ed. 2009, 48, 3273–3277. [Google Scholar] [CrossRef]
- Wuest, J.D. Engineering crystals by the strategy of molecular tectonics. Chem. Commun. 2005, 5830–5837. [Google Scholar] [CrossRef]
- Hosseini, M.W. Molecular tectonics: From simple tectons to complex molecular networks. Acc. Chem. Res. 2005, 38, 313–323. [Google Scholar] [CrossRef]
- Hosseini, M.W. Reflexion on molecular tectonics. CrystEngComm 2004, 6, 318–322. [Google Scholar] [CrossRef]
- Su, D.; Wang, X.; Simard, M.; Wuest, J.D. Molecular tectonics. Supramol. Chem. 1995, 6, 171–178. [Google Scholar] [CrossRef]
- Lin, R.-B.; He, Y.; Li, P.; Wang, H.; Zhou, W.; Chen, B. Multifunctional porous hydrogen-bonded organic framework materials. Chem. Soc. Rev. 2019, 48, 1362–1389. [Google Scholar] [CrossRef] [PubMed]
- Hisaki, I.; Xin, C.; Takahashi, K.; Nakamura, T. Designing hydrogen-bonded organic frameworks (HOFs) with permanent porosity. Angew. Chem. Int. Ed. 2019, 58, 11160–11170. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, J.-W.; Zhang, J.-H.; Lai, S.; Zhong, D.-C. Hydrogen-bonded organic frameworks: Design, structures and potential applications. CrystEngComm 2018, 20, 5884–5898. [Google Scholar] [CrossRef]
- Han, Y.-F.; Yuan, Y.-X.; Wang, H.-B. Porous hydrogen-bonded organic frameworks. Molecules 2017, 22, 266. [Google Scholar] [CrossRef]
- Mastalerz, M.; Oppel, I.M. Rational construction of an extrinsic porous molecular crystal with an extraordinary high specific surface area. Angew. Chem. Int. Ed. 2012, 51, 5252–5255. [Google Scholar] [CrossRef]
- Fournier, J.H.; Maris, T.; Wuest, J.D. Molecular tectonics. Porous hydrogen-bonded networks built from derivatives of 9,9’-spirobifluorene. J. Org. Chem. 2004, 69, 1762–1775. [Google Scholar] [CrossRef]
- Demers, E.; Maris, T.; Wuest, J.D. Molecular tectonics. Porous hydrogen-bonded networks built from derivatives of 2,2’,7,7’-tetraphenyl-9,9’-spirobi[9 H-fluorene]. Cryst. Growth Des. 2005, 5, 1227–1235. [Google Scholar] [CrossRef]
- Chen, T.H.; Popov, I.; Kaveevivitchai, W.; Chuang, Y.C.; Chen, Y.S.; Daugulis, O.; Jacobson, A.J.; Miljanic, O.S. Thermally robust and porous noncovalent organic framework with high affinity for fluorocarbons and CFCs. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Shankar, S.; Chovnik, O.; Shimon, L.J.W.; Lahav, M.; van der Boom, M.E. Directed molecular structure variations of three-dimensional halogen-bonded organic frameworks (XBOFs). Cryst. Growth Des. 2018, 18, 1967–1977. [Google Scholar] [CrossRef]
- Nikolayenko, V.I.; Castell, D.C.; van Heerden, D.P.; Barbour, L.J. Guest-induced structural transformations in a porous halogen-bonded framework. Angew. Chem. Int. Ed. 2018, 57, 12086–12091. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.T.; Ellington, T.L.; Allen, K.E.; Gorden, J.D.; Rheingold, A.L.; Tschumper, G.S.; Hammer, N.I.; Watkins, D.L. Systematic experimental and computational studies of substitution and hybridization effects in solid-state halogen bonded assemblies. Cryst. Growth Des. 2018, 18, 3244–3254. [Google Scholar] [CrossRef]
- González, L.; Gimeno, N.; Tejedor, R.M.; Polo, V.; Ros, M.B.; Uriel, S.; Serrano, J.L. Halogen-bonding complexes based on bis(iodoethynyl)benzene units: A new versatile route to supramolecular materials. Chem. Mater. 2013, 25, 4503–4510. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Baldrighi, M.; Desper, J.; Metrangolo, P.; Resnati, G. Supramolecular hierarchy among halogen-bond donors. Chem. Eur. J. 2013, 19, 16240–16247. [Google Scholar] [CrossRef]
- Sarwar, M.G.; Dragisic, B.; Salsberg, L.J.; Gouliaras, C.; Taylor, M.S. Thermodynamics of halogen bonding in solution: Substituent, structural, and solvent effects. J. Am. Chem. Soc. 2010, 132, 1646–1653. [Google Scholar] [CrossRef]
- Baldrighi, M.; Bartesaghi, D.; Cavallo, G.; Chierotti, M.R.; Gobetto, R.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Polymorphs and co-crystals of haloprogin: An antifungal agent. CrystEngComm. 2014, 16, 5897–5904. [Google Scholar] [CrossRef]
- Lemouchi, C.; Vogelsberg, C.S.; Zorina, L.; Simonov, S.; Batail, P.; Brown, S.; Garcia-Garibay, M.A. Ultra-fast rotors for molecular machines and functional materials via halogen bonding: Crystals of 1,4-bis(iodoethynyl)bicyclo[2.2.2]octane with distinct gigahertz rotation at two sites. J. Am. Chem. Soc. 2011, 133, 6371–6379. [Google Scholar] [CrossRef][Green Version]
- Dunitz, J.D.; Gehrer, H.; Britton, D. The crystal structure of diiodacetylene: An example of pseudosymmetry. Acta Crystallogr. B 1972, 28, 1989–1994. [Google Scholar] [CrossRef]
- Guo, W.Z.; Galoppini, E.; Gilardi, R.; Rydja, G.I.; Chen, Y.H. Weak intermolecular interactions in the crystal structures of molecules with tetrahedral symmetry: Diamondoid nets and other motifs. Cryst. Growth Des. 2001, 1, 231–237. [Google Scholar] [CrossRef]
- Kaleta, J.; Bastien, G.; Císařová, I.; Batail, P.; Michl, J. Molecular rods: Facile desymmetrization of 1,4-diethynylbicyclo[2.2.2]octane. Eur. J. Org. Chem. 2018, 2018, 5137–5142. [Google Scholar] [CrossRef]
- Galoppini, E.; Gilardi, R. Weak hydrogen bonding between acetylenic groups: The formation of diamondoid nets in the crystal structure of tetrakis(4-ethynylphenyl)methane. Chem. Commun. 1999, 173–174. [Google Scholar] [CrossRef]
- Dikundwar, A.G.; Sathishkumar, R.; Guru Row, T.N.; Desiraju, G.R. Structural variability in the monofluoroethynylbenzenes mediated through interactions involving “organic” fluorine. Cryst. Growth Des. 2011, 11, 3954–3963. [Google Scholar] [CrossRef]
- Thakur, T.S.; Sathishkumar, R.; Dikundwar, A.G.; Guru Row, T.N.; Desiraju, G.R. Third polymorph of phenylacetylene. Cryst. Growth Des. 2010, 10, 4246–4249. [Google Scholar] [CrossRef]
- Dziubek, K.; Podsiadło, M.; Katrusiak, A. Nearly isostructural polymorphs of ethynylbenzene: Resolution of ≡CH···π(arene) and cooperative ≡CH···π(C≡C) interactions by pressure freezing. J. Am. Chem. Soc. 2007, 129, 12620–12621. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.K.; Nangia, A. Ethynyl group as a supramolecular halogen and C≡C–H···C≡C trimer synthon in 2,4,6-tris(4-ethynylphenoxy)-1,3,5-triazine. Cryst. Growth Des. 2007, 7, 393–401. [Google Scholar] [CrossRef]
- Ohkita, M.; Suzuki, T.; Nakatani, K.; Tsuji, T. Polar assembly of 2,6-diethynylpyridine through C(sp2)–H···N, C(sp)–H···π and π–π stacking interactions: Crystal structure and nonlinear optical properties. Chem. Lett. 2001, 30, 988–989. [Google Scholar] [CrossRef]
- Robinson, J.M.; Kariuki, B.M.; Harris, K.D.; Philp, D. Interchangeability of halogen and ethynyl substituents in the solid state structures of di- and tri-substituted benzenes. J. Chem. Soc. Perkin Trans. 1998, 2, 2459–2470. [Google Scholar] [CrossRef]
- Weiss, H.-C.; Bläser, D.; Boese, R.; Doughan, B.; Haley, M. C–H···π interactions in ethynylbenzenes: The crystal structures of ethynylbenzene and 1,3,5-triethynylbenzene, and a redetermination of the structure of 1,4-diethynylbenzene. Chem. Commun. 1997, 1703–1704. [Google Scholar] [CrossRef]
- Steiner, T.; Starikov, E.B.; Amado, A.M.; Teixeira-Dias, J.J.C. Weak hydrogen bonding. Part 2. The hydrogen bonding nature of short C–H···π contacts: Crystallographic, spectroscopic and quantum mechanical studies of some terminal alkynes. J. Chem. Soc. Perkin Trans. 1995, 2, 1321–1326. [Google Scholar] [CrossRef]
- Barbour, L.J. Crystal porosity and the burden of proof. Chem. Commun. 2006, 11, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, C.A.; Đaković, M.; Aakeröy, C.B. Diamondoid architectures from halogen-bonded halides. Chem. Commun. 2018, 54, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Wavefunction, Inc. Irvine, CA 92612, USA. Available online: https://www.wavefun.com/spartan (accessed on 8 March 2020).
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).