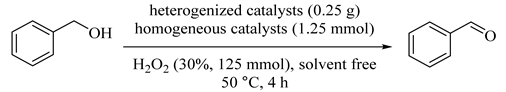
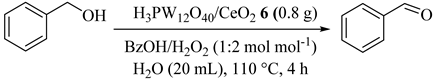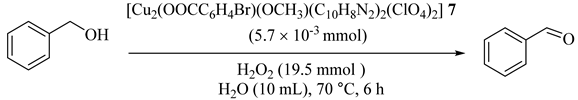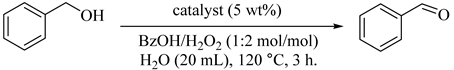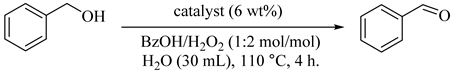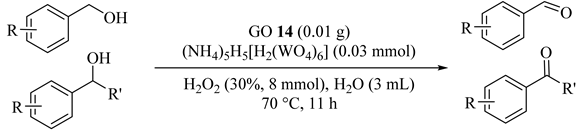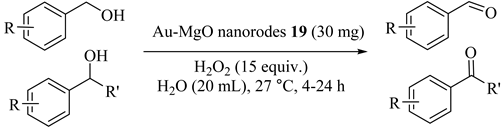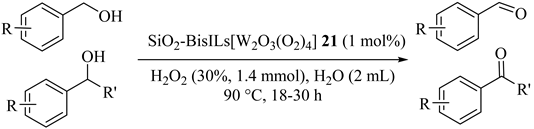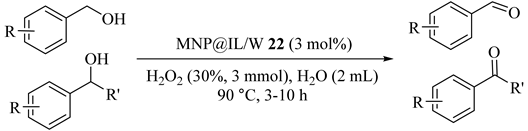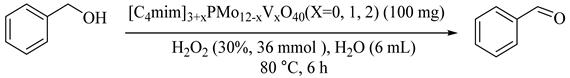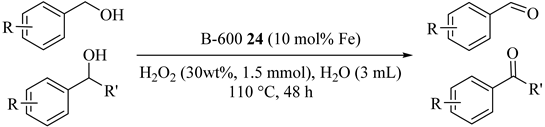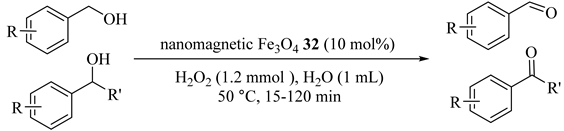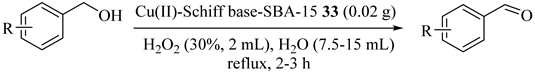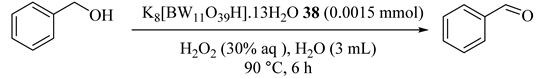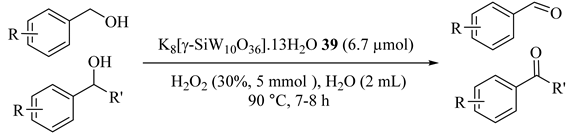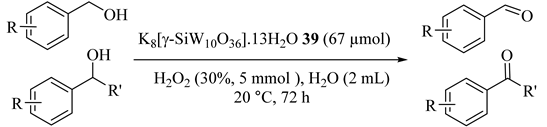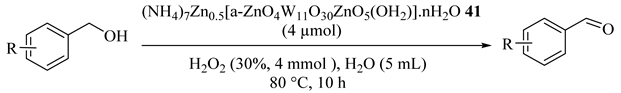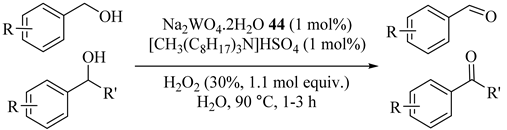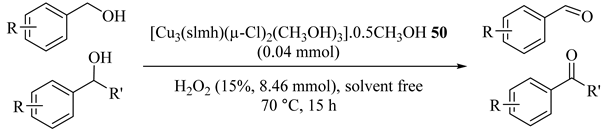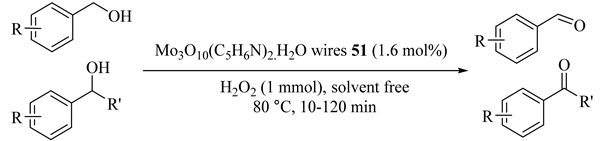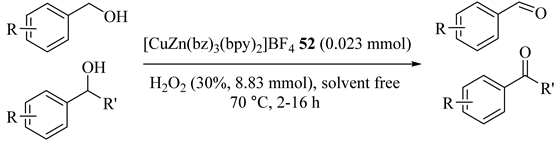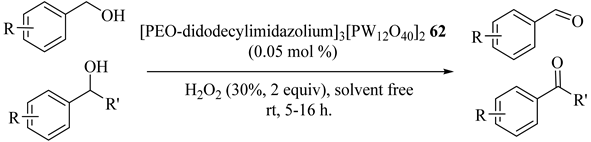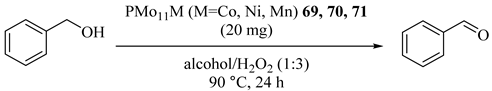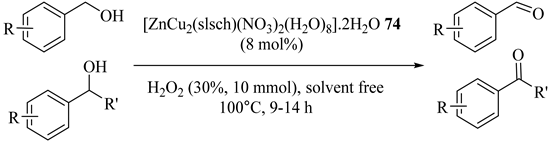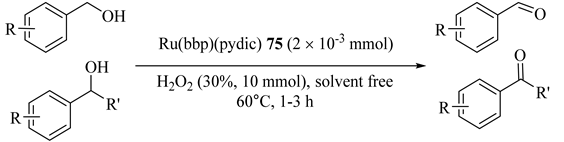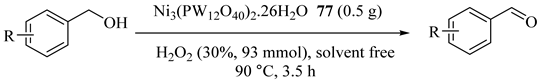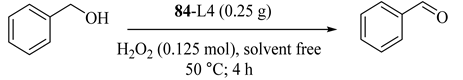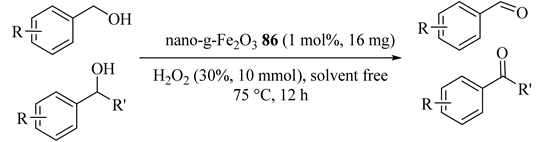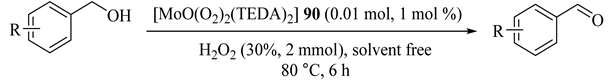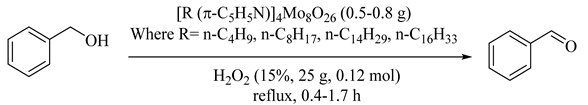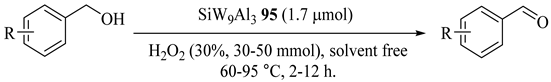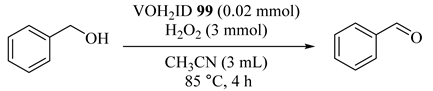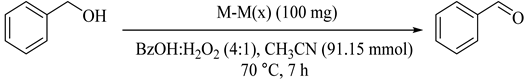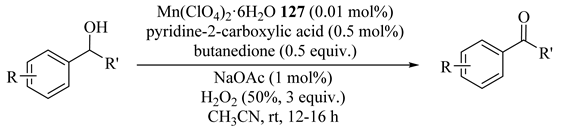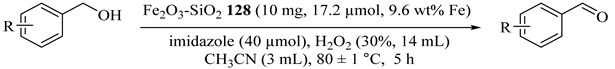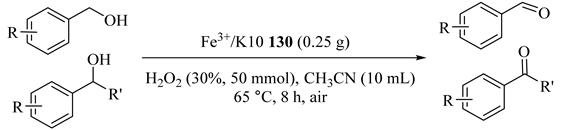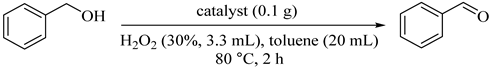Abstract
Among a plethora of known and established oxidant in organic chemistry, hydrogen peroxide stands in a special position. It is commercially and inexpensively available, highly effective, selective, and more importantly it is compatible with current environmental concerns, dictated by principles of green chemistry. Several chemicals or their intermediates that are important in our daily life such as pharmaceuticals, flavors, fragrances, etc. are products of oxidation of alcohols. In this review, we introduce hydrogen peroxide as an effective, selective, green and privileged oxidant for the catalyzed oxidation of primary and secondary benzylic and heterocyclic alcohols to corresponding carbonyl compounds in different media such as aqueous media, under solvent-free conditions, various organic solvent, and dual-phase system.
1. Introduction
Nowadays, energy and environmental crises have resulted in the discovery of green technologies for progressive use in the chemical industry. Exploration of green chemical reaction approaches have especially shaped our daily lives. The use of alternative non-toxic and green solvents, benign reagents and substrates, and the application of new catalytic systems with the same efficiency or even superior efficiency have also reduced the risk of chemical reactions and resulted in safety [1,2].
Oxidation of primary and secondary alcohols to their corresponding aldehydes and ketones, respectively, are considered as a pivotal reaction in organic transformations due to the wide applications of these products as precursors or intermediates for construction of several drugs, fragrances, and vitamins. Because of the low selectivity of some reactions such as oxidation, the resulting byproducts generate waste and pollution. Therefore, selective oxidation of organic compounds as a critical chemical transformation has wide-ranging applications in the chemical, industrial, and biological processes which needs more attention. Traditionally, selective oxidation of alcohols has needed toxic, corrosive, and expensive stoichiometric inorganic oxidants such as Cr(VI), Mn(VII), heptavalent iodine, and DMSO-coupled reagents which create both environmental and economic concerns [3,4]. Due to an urgent and important demand for greener approaches, the use of toxic solvents and reagents such as organic peroxides must be avoided. Thus, green solvents such as water or solvent-free systems, clean oxidants such as O2 or H2O2, and also recyclable catalysts should be considered in order to comply with the principles of green chemistry proposed by Anastas and Warner [2]. According to the atom economy principle, established by Trost in 1991, in oxidation reactions, with respect to the total mass of the oxidant, the mass amount of oxygen transferred to the substrate should be optimal [5]. Although molecular oxygen is considered to be an ideal oxidant, its use sometimes is problematic since it needs harsh reaction conditions such as high temperature or pressure and it also shows poor selectivity.
Nowadays, among the common oxidants, hydrogen peroxide (H2O2) with 47% oxygen content has been found to be a more suitable and practical oxidant. Moreover, with the transfer of one oxygen atom to the substrate in the oxidation reaction, one equivalent of H2O is practically formed as an ideal and green expected byproduct [6,7,8,9,10]. In addition, due to its good solubility in water and many organic solvents, H2O2 is a very operative oxidant in liquid phase reactions [9,10]. Furthermore, its safe storage, operation, and transportation of aqueous hydrogen peroxide, along with its commercially availability, justify its wide applications [11,12].
Selective oxidation of aliphatic or aromatic alcohols to the corresponding carbonyl compounds using hydrogen peroxide either in the absence or presence of catalysts in different solvents under neutral, alkaline, or acid conditions have been extensively studied [13,14,15,16,17,18]. Our research group was also interested in oxidation [19,20,21,22], using hydrogen peroxide as an oxidant of choice [23,24] and especially, selective oxidation of alcohols to the corresponding carbonyl compounds [25,26,27]. We recently reviewed the applications of pyridinium chlorochromate (PCC) as an important selective oxidant for oxidation of primarily and secondary alcohols to their corresponding carbonyl compounds [28]. As a continuation of these interests, herein, we try to highlight the importance and efficacy of hydrogen peroxide as an effective and green oxidant in the catalyzed selective oxidation of primary and secondary benzylic alcohols and primary heterocyclic alcohols to their corresponding carbonyl compounds. This review is also intended to cover recent literature, focusing on new methods of selective catalytic oxidation of benzylic and heterocyclic alcohols using hydrogen peroxide in aqueous phase, a solvent-free system, in various solvents, and dual-phase systems. In addition, we present an overview on the reasonable reaction mechanism and promising catalytic effects of the selective oxidation of alcohols which actually can be considered as dehydrogenation of alcohols.
2. Selective Catalytic Oxidation of Benzylic and Heterocyclic Alcohols
2.1. Oxidation of Benzylic and Heterocyclic Alcohols in Aqueous Media
Epichlorohydrin (ECH)-modified Fe3O4 microspheres (1) for the selective oxidation of benzyl alcohol to benzaldehyde with H2O2 has been reported [29,30]. The ECH-derived hydroxyl groups have been shown to be milder and beneficial for the structural stability of Fe3O4 as compare with corrosive organic acid and iron-chelating additives. With ECH modification, a few surface active sites have been occupied, so the activation energy for H2O2 on Fe3O4 microspheres decomposition increased from 50.1 to 116.3 kJ mol−1. Therefore, the promotional effect of short-chain saturated alcohols as additives for Fe3O4-catalyzed decomposition in H2O2 was established for the first time, and exploited to improve the catalytic performance of Fe3O4 microspheres for the selective oxidation of benzyl alcohol to corresponding benzaldehyde with H2O2 in water (Table 1) [31]. The results showed that applications of only Fe3O4 microspheres converted only 5.7% of PhCH2OH (Table 1, Entry 1). Although, the catalytic activity of Fe3O4 microspheres were suggestively improved by the addition of small amounts of short-chain saturated alcohols (0.4 mol% with regards to H2O2) (Table 1, Entries 2–7), the selectivity for benzaldehyde reduced to 83 to 87%, due to over-oxidation to benzoic acid as a byproduct. The better promotional effect (same dosage, 0.4 mol%) of secondary and tertiary alcohols (41.8% to 48.9% in Table 1, Entries 2–4) on the conversion of benzyl alcohol (13.5% to 22.8% in Table 1, Entries 5–7) are observed as compared with primary alcohols. As shown in Table 1, for each alcohol, the optimal dosage could be different additives to accomplish the highest conversion of benzyl alcohol. Although the dosage of all alcohol additives was fixed (0.4 mol% with regard to hydrogen peroxide), the conversion of PhCH2OH may not be the optimal value for each alcohol additive.

Table 1.
Catalytic oxidation of benzyl alcohol to benzaldehyde with H2O2 in water on Fe3O4 microspheres 1 with 0.4 mol% of alcohol additives a.
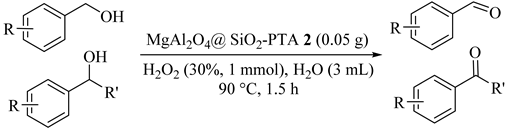
Recyclable and reusable nanocatalyst (MgAl2O4@SiO2–PTA) (2) was applied for the selective oxidation of primary and secondary alcohols (Table 2) to the corresponding aldehydes (for example, benzyl alcohol Entry 1) and ketones (for example, 1-phenyl ethanol Entry 7) with hydrogen peroxide as oxidant and water [32]. The oxidation reaction of 1-phenyl ethanol to acetophenone was more efficient than other reactions (98%). The catalyst was successfully used five times without any loss of its high catalytic activity. In benzylic alcohol oxidation, since the replacement was different from electron donating to electron withdrawing (Table 2, Entries 2–8), the yield of products decreased from 94% to 81% [33].

Table 2.
Synthesis of diversified benzylic alcohol in the presence of MgAl2O4@ SiO2–PTA 2 a.
A heterogeneous organocatalyst “glycoluril” (3) was applied for the oxidation of alcohols to the corresponding carbonyls with excellent conversion and selectivity (more than 90%) in an aqueous medium using hydrogen peroxide (Table 3) [34]. The catalyst was successfully recycled for >10 times. Depending on the functional groups substituted on the aromatic rings of benzylic alcohols (Table 3, Entries 1–5), good conversions and selectivities were obtained with different reaction times. In addition, heteroaromatic substrates were successfully converted into the corresponding aldehydes in a short reaction time.

Table 3.
Substrate scope for the oxidation reaction a.
Green, chemoselective, facile, and efficient oxidation of primary and secondary benzylic alcohols to corresponding aldehydes or ketones in aqueous H2O2 was reported by Kamal Amani and co-workers [35] in the presence of GO/Fe3O4/HPW (4) nanocomposite catalyst with approximately 75% to 99% conversions, without any overoxidation to acid (100% selectivity), as shown in Table 4.

Table 4.
Catalytic performance of the GO/Fe3O4/HPW 4 nanocatalyst for the selective oxidation of alcohols with H2O2 under optimal reaction conditions a.
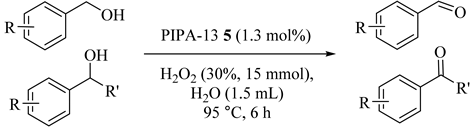
In water, exchanging the protons of HPW with PEG-bridged di-imidazolium cations produced double catalytic sites in a single molecule of Keggin-type phosphotungstic acid (H3PW12O40, HPW)-based di-imidazolium ionic liquid (IL) hybrid (5) catalyst which enhanced the reaction rate and resulted in higher selectivity of benzyl alcohol oxidation with H2O2 (30 wt%) in water with excellent catalytic efficiency, convenient recovery, and steady reusability. Higher conversions (71%) and selectivity (82%) were obtained by the di-cation IL-based PIPA-0 due to the di-cationic structure of imidazolium IL (Table 5, Entry 4). In addition, the built-in phase transfer capability of PIPA-n that arose from PEG modifier provided the higher activity of PIPA-n (n = 4, 8, and 13) [36].

Table 5.
Results of the selective oxidation of alcohols to aldehydes with H2O2 in water over various PW-based catalysts a.
Incipient wetness impregnation method was applied for the preparation of ceria (CeO2) supported tungstophosphoric acid (H3PW12O40; HPW) catalysts. As illustrated in Table 6, H3PW12O40/CeO2 (6) catalyst among the various catalysts showed the best results for selective oxidation of benzyl alcohol (BzOH) with hydrogen peroxide (20 wt% H2O2) [37]. In addition, response surface methodology (RSM) based on the Box–Behnken design model showed 95.2% conversion of benzyl alcohol and a 94.2% yield of benzaldehyde with 98.9% selectivity which was in good agreement with the experimental results [37]. Reusability of the catalyst were further successfully tested for six consecutive runs.

Table 6.
Comparison of catalytic performances over various catalyst during oxidation of benzyl alcohol a.
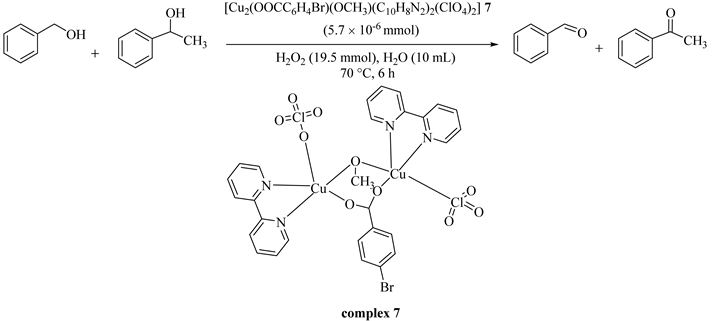
Unver [38] reported the synthesis of a water-soluble dinuclear Cu(II) complex, [Cu2(OOCC6H4Br)(OCH3)(C10H8N2)2(ClO4)2] (7) (4-bromobenzoic acid = HOOCC6H4Br; 2-2′-bipyridyl = C10H8N2) which was successfully used without any additives under mild conditions in the oxidation of primary and secondary alcohols in water (Table 7). The catalyst showed high TON values (up to 100), good to moderate yields, and high selectivity with no traces of carboxylic acid producing during or after the reactions. In addition, the competition between the pure substrates as compared with the mixtures of alcohols, under the same conditions, resulted in higher product yields (Table 8) [38].

Table 7.
Oxidation of various alcohols with copper (II) complex 7 in water a.

Table 8.
Selective oxidation of selected alcohols with copper (II) complex 7 in water a.
Cobalt zeolitic imidazolate framework (ZIF-9@Zeolite) (8) was prepared and under a simple and clean protocol, aldehydes, as oxidative products of alcohols, were efficiently obtained in high yields (Table 9), generating water as the only byproduct and with no decrease in catalyst activity even after four catalytic cycles. Benzyl alcohols with electron-withdrawing substituent also yielded the corresponding aldehydes high percent (Table 9, Entries 4, 7, 11, and 12) [39].

Table 9.
Oxidation of alcohols to aldehydes using ZIF-9@Zeolite 8 a.
The influence of polyetheramine (Jeffamine®) as a di-block copolymer with ethylene oxide and propylene oxide moieties along with terminal amine on phosphotungstic acid (PTA) (9) which is a polyoxometalate catalyst was determined, in detail, in H2O2-mediated oxidation of BzOH in water. Recyclable PTA-Jffamine® catalyst not only enhanced the conversion of BzOH as compared with pristine PTA, but also facilitated the easy separation of catalyst and benzaldehyde (BzH) from the reaction mixture. In addition, with and without pure-PTA as the catalyst, the BzOH oxidation reaction was investigated (Table 10). Very low conversions of BzOH were obtained for reactions with and without pure-PTA (Entries 1 and 2). In pH = 3.5, the conversion was only 25% after 1.5 h at low Jeffamine® concentration, but dramatically increased to 100% at higher Jeffamine® contents at (pH = 4.5, 6.5, 7.5, and 8.5) which had not been reported earlier (Table 10) [40].

Table 10.
Summary of GC-MS results obtained from reactions performed at various conditions a.
Water-soluble heteropolyacid-based ionic liquids were prepared by modifying tungstophosphoric acid (H3PW12O40) and propyl sulfonic acid-functionalized ionic complex. Among various organic TPA salts, the [DMBPSH]H2PW12O40 (10) catalyst, due to strong acidity and excellent surface activity played as an effective and reusable catalyst, exhibited the best oxidative activity with a desirable BzH selectivity of 97.0% and an excellent BzOH conversion of 98.5% under optimum conditions (Table 11) [41]. Additionally, after six consecutive experimental cycles, the catalyst showed no decreasing in conversion and selectivity.

Table 11.
Catalytic performance of various catalysts during oxidation of benzyl alcohol with H2O2 a.
A series of diverse amino acids such as phenylalanine, alanine, and glycine functionalized tungstophosphoric acid (TPA;H3PW12O40) composite and were efficiently applied as recyclable thermally stable, eco-friendly, and cost-effective heterogeneous catalysts in the selective oxidation of benzyl alcohol (Table 12) [42]. Although [GlyH]H2PW12O40 (11) had more Brønsted acidity than [PheH]H2PW12O40 (12), [PheH]H2PW12O40 (12) exhibited the best catalytic activity including conversion 97.9%, selectivity 97.4%, and yield of 95.4% [42,43]. This phenomenon indicated that catalytic oxidation required only a modest acidity.

Table 12.
Catalytic performances of various catalysts during oxidation of benzyl alcohol with hydrogen peroxide a.
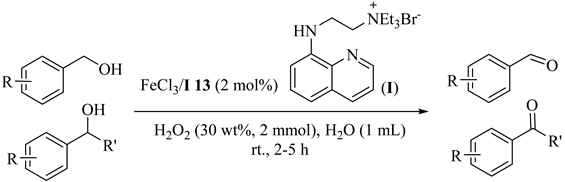
Oxidation of primary alcohols to aldehydes catalyzed by H2O2 as an oxidant and a reusable and water-soluble iron (III) catalyst in water (Table 13) [44]. This novel and reusable FeCl3 complex (13) in situ formed with quaternary ammonium salt-functionalized 8-aminoquinoline. The reaction showed not only unique chemoselectivity similar to the oxidation a benzylic primary alcohol even in the presence of an aliphatic one, but also exhibited broad functional-group tolerance.

Table 13.
The oxidation of benzylic primary alcohols to aldehydes a.
Various alcohols were oxidized with hydrogen peroxide over ammonium tungstate promoted by GO (14) as heterogeneous acid catalyst (Table 14) [45]. The aromatic primary alcohols and secondary alcohols were converted to the corresponding aldehydes and ketones in excellent to satisfactory yields. In addition, the catalytic system could be efficiently reused in at least seven cycles.

Table 14.
Substrate scope of the alcohol oxidation reaction a.
Cu(II) nanoparticles immobilized on nanocage-like mesoporous KIT-5 as a support and a 3-aminopropyltriethoxysilane (APTES) group as a coordinating agent for Cu(II) provided an active catalyst in the selective oxidation of primary and secondary alcohols in water. The computational investigation also confirmed the catalytic role of APTES-KIT-5 silica-supported copper(II) nanocatalyst (15). The results in Table 15 showed that the oxidation of various benzylic alcohols were considerably dependent on substituents (–Cl, –OCH3, –OH, –NO2, and –NH2) and on their positions [46]. In addition, aldehydes obtained with excellent selectivity without overoxidation into carboxylic acids from the oxidation of primary alcohols and secondary benzylic alcohols oxidation provided the corresponding ketone in satisfactory yield (Entry 12). The mentioned heterogeneous nanocatalyst could be recovered and reused six times.

Table 15.
Cu(OAc)2 supported on AK 15 as catalyst in oxidation of alcohols to corresponding aldehydes a.
Tungstate salt with imidazolium (((1,3,5-triazine-2,4,6-triyl)tris(1-octyl-1H-imidazol-3-ium))2(WO4=)3 (16) framework provided a catalytic system, under neutral aqueous reaction conditions, for the highly selective oxidation of primary benzylic alcohols (bearing both electron-releasing and electron-withdrawing groups) using H2O2 as a green oxidant (Table 16) [47]. The catalyst could be reused for at least seven subsequent reaction cycles.

Table 16.
Oxidation of substituted benzylic alcohols to aldehydes using imidazolium WO4= salt 16 as catalyst a.
Via simple method, Na7PW11O39 (PW11) was immobilized on quanternary ammonium functionalized chloromethylated polystyrene (DMA16/CMPS) (PW11-DMA16/CMPS (17)) and used as high active, stable, recoverable, and recyclable catalyst in the oxidation of aliphatic and aromatic alcohol with H2O2 (Table 17) [48]. Although, benzhydrol oxidation is difficult because of the deficiency of interaction between the alcohol moiety and the catalyst [49], in this catalytic system, benzhydrol attained 100% conversion and 97.5% selectivity (Entry 1).

Table 17.
Oxidation of various alcohols catalyzed by PW11-DMA16/CMPS 17 with 30% H2O2 a.
As reported in Table 18, Ramazani et al. [50] introduced nanomagnetic MgFe2O4 (18) as an active, and reusable (seven runs) catalyst for the oxidation of various primary and secondary alcohols in good yields in water as a solvent, and either oxone (at room temperature) or H2O2 (at 60 °C) as an oxidant. Overoxidation of aldehydes to the corresponding carboxylic acids was not observed which emphasized that the aldehyde selectivity, in most cases, was quite high (>99%).

Table 18.
Oxidation of various alcohols using MgFe2O4 MNPs 18 as catalyst in the present of H2O2 in water a.
A solution based chemical reduction method was applied for the production of gold nanoparticles supported on magnesium oxide nanorods (Au-MgO) (19) which were found to be an efficient heterogeneous catalyst with hydrogen peroxide for the base free oxidation of alcohols in aqueous medium at room temperature, Table 19 [51]. It is worthwhile to mention that the catalyst was reused for five cycles.

Table 19.
Oxidation of alcohols using Au-MgO nanorodes 19 a.
Perez [52] reported the application of copper(II) complexes, copper, and copper oxide nanoparticles supported on SBA-15 applied in the benzyl alcohol oxidation in aqueous phase as catalyst and in the presence of H2O2 (Table 20). Immobilization of ionic liquid containing copper followed by chemical reduction method provided the catalyst which showed the highest benzyl alcohol oxidation activity with a 73% conversion and 54% selectivity with 30 min reaction time. Different copper species of the catalyst explored different conversion and selectivity. The highest activity, with 73% conversion and 54% selectivity for benzaldehyde, was obtained in metallic copper nanoparticle catalyst such as Cu/IMILeSBA-15-G1 (20) (Entry 9), in 30 min. The 20 had also the higher activity as compare with similar previously reported catalyst such as copper nanoparticle-polyacrylamide/SBA-15 and polymer-supported copper(II)-L-valine complexes [53,54].

Table 20.
Benzyl alcohol oxidation catalyzed by the different copper supported catalysts a.
The efficient and selective oxidation of primary and secondary alcohols were reported by Shi [55] who immobilized ionic liquids/peroxotungstates/SiO2 (21) catalyzed reactions in the presence of H2O2 as an oxidant in neat water (Table 21). Hydrophilic imidazoliums and hydrophobic hydrocarbon chains in the ILs caused to diffusion of both hydrophobic alcohols and hydrophilic H2O2 oxidant into the micro reactor and provided carbonyls catalyzed by the peroxotungstates. Substituted-benzylic alcohols were oxidized to selective carbonyl products in satisfied yields but electronic effect of substituted groups on the activity was significantly observed. In addition, aromatic secondary alcohols were also selectively oxidized to ketones in good yields. Moreover, the catalyst was easily recovered by filtration and reused at least for six times.

Table 21.
The selective oxidation of different alcohols catalyzed by SiO2-BisILs[W2O3(O2)4] 21 with H2O2 a.
Bis-imidazolium tungstate ionic liquid produced magnetically recoverable catalyst, at least 5 times, which were extremely dispersible in water and could selectively oxidized a wide variety of alcohols and sulfides using H2O2 oxidant. The results in Table 22 demonstrated the key role of MNP@IL/W (22) as both oxidant and phase transfer catalyst in various alcohol oxidations [56]. Good to excellent conversion and selectivity was also obtained in oxidation of secondary alcohols.

Table 22.
Oxidation of alcohols to aldehydes catalyzed by MNP@IL/W 22 a.
Using the anion-exchange method produced some hybrid materials ([C4mim]3+xPMo12−xVxO40, x = 0, 1, 2) based on V-substituted phosphomolybdic acid H3+xPMo12−xVxO40 (x = 0, 1, 2) and ionic liquid 1-butyl-3-methyl imidazolium bromide ([C4mim]Br) and applied in the benzyl alcohol oxidation reaction Table 23 [57]. The hybrids [C4mim]3+xPMo12−xVxO40 demonstrated much higher catalytic activities than both corresponding moieties. Particularly, [C4mim]4PMo11VO40 (23) provided 34% benzyl alcohol conversion and 99% selectivity for benzaldehyde under the optimized conditions (Entry 7) and was reused for five runs without much decrease in selectivity and conversion. In this case, the reaction mixture turned out to be L-L-S triphase system because of the insolubility of the solid hybrid and immiscibility between water and benzyl alcohol [57]. Although high selectivities for benzaldehyde were obtained, in all cases, conversions of the benzyl alcohol were below 50% which justified more efforts to improve the catalytic performances by modifiying morphologies and hydrophilic and hydrophobic properties of the hybrid catalysts in upcoming work.

Table 23.
Catalytic performances of various POM catalyst for oxidation of benzyl alcohol with H2O2 a.
Catalytic activities of Fe3O4@C materials in neat water were examined in the selective oxidation of alcohols using H2O2 under base-free conditions. Both aryl and alkyl alcohols as a comprehensive substrate scope were oxidized with high activity and selectivity over the B-600 materials (24) (Table 24) [58]. In addition, the magnetic catalyst could be easily removed using an external magnetic field and reused at least four times. Different primary and secondary benzylic alcohols with electron-donating or -withdrawing functional groups converted their corresponding substituted aldehyde or ketones into good to excellent yields (Entries 1–14) but difference in activities were seen which were attributed to their compositions and structures. Secondary benzylic alcohols such as 1-phenylethanol and derivatives were converted to the corresponding ketones in relatively lower yields as compared with primary benzylic alcohols due to the steric effect of the α-CH position in secondary alcohols (Entries 11–15).

Table 24.
Oxidation of various alcohols by B-600 24 in water a.
Recyclable heterogeneous catalyst β-CD grafted on lignin cross linked by epichlorohydrin (EPI) as crosslinking agent (l-β-CD) (25) were prepared and under mild reaction were used in the oxidation of BzOH to BzH conditions. The catalyst provided the solution selective oxidation of BzOH in high selectivity (>99%) and catalytic activity was not significantly decreased after five cycles. As illustrated in Table 25, (25) was applied for different benzyl alcohol oxidations to the corresponding BzHs. The results not only emphasized the catalytic power of the 25/H2O2/NaHCO3 system for the oxidation of various substituted benzyl alcohols in good yields (79% to 99%) and high selectivity (>99%), but also obviously showed the electronic and steric hindrance effect of substituent groups on the catalytic oxidation of substrates [59].

Table 25.
Scope oxidation of alcohols catalyzed by l-β-CD 25 in water a.
Yadollahi [60] reported the application of sodium and potassium salts of a sandwich-type tetracobalt tungstophosphate, [Co4(H2O)2(PW9O34)2]10− catalysts for the selective oxidation of alcohols with H2O2 in water. In general, the Na10[Co4(H2O)2(PW9O34)2]·27H2O complex (26) presented better activity and was recycled for five times. Results in Table 26 showed that alcohols with electron-donating substituent converted to their corresponding aldehydes even faster than benzyl alcohol [60].

Table 26.
Selective oxidation of different alcohols to the corresponding aldehyde using Na-Co-POM 26 a.
Direct solvothermal synthesis or post-synthetic modification were applied for the preparation of ECH-modified Fe3O4 microspheres in ethylene glycol (MEG). As summarized in Table 27, the catalyst was successfully performed for the selective oxidation of BzOH to BzH in water with H2O2 [29]. The reaction was sensitive to both reaction temperature and the molar ratio of H2O2 to BzOH. Magnetic Fe3O4 microspheres (27) were recoverable and reusable at least five times without loss of catalytic selectivity and activity after NaBH4 reduction and ECH modification. In light of the above, surface modification with organic groups increased the catalytic performance of Fe3O4 and exhibited suitable interactions with H2O2.

Table 27.
Catalytic oxidation of benzyl alcohol to benzaldehyde with H2O2 a.
Benzyl alcohol oxidation catalyzed by gold nanoparticles supported on gamma alumina (γ-Al2O3) were prepared using a deposition-precipitation method. The Nano Au/c-Al2O3 (28) selectivity provided benzaldehyde (over 98%) under environment-friendly conditions and could be recovered after three times. More investigation showed that changes in support material, even the same type of alumina (γ-Al2O3), had a significant effect on both catalytic activity and physical–chemical of the catalysts. Gold particles were responsible for catalytic efficiency since in the tests conducted in like conditions using no catalyst or γ-alumina catalyst caused a very low conversion of benzyl alcohol and yield of benzaldehyde (<1%) ()(Table 28) [61]. For all the supports, both the conversion of benzyl alcohol and the selectivity of benzaldehyde also increased. As a result, for the 2% Au/γ-Al2O3 catalysts, higher yields of benzaldehyde were achieved8)(Table 29) [61]. While the benzaldehyde selectivities were high in the S723-supported catalysts, the conversion of the benzyl alcohol was comparatively low.

Table 28.
Effect of Au catalyst concentration on the oxidation of benzyl alcohol a.

Table 29.
Effect of nanogold on the oxidation of benzyl alcohol over various support materials a.
Platinum nanoparticles supported on Ca(Mg)-ZSM-5 (29) were a highly stable and selective catalyst for the room-temperature oxidation of alcohols in water. In situ EPR measurement and the radical trapping technique demonstrated that •OH radicals generated by O–O cleavage bond of H2O2 intermediate as the rate determining step, contributed to the H abstraction of the α-C–H bond of alcohols to provide aldehydes/ketones. PtNPs on different supports applied for the oxidation reactions of alcohols in aqueous solution at room temperature and the results are summarized in Table 30 [62]. From the results, the basic zeolites, such as Mg-ZSM-5 and Ca-ZSM-5, exhibited the optimal performance during benzyl alcohol oxidation, with 95% conversion and 99% of selectivity at a carbon balance of 98%, (Entries 1 and 2). The PtNPs catalytic power extremely depended on the size of Pt which was sized similar to the PtNPs/Ca-ZSM-5 catalyst with a Pt mean size of ~5.8 nm, and when prepared by the impregnation method, could only convert 89% of the benzyl alcohol in 20 h of reaction (Entry 2). In contrast, high basicity supports, such as CaO and MgO, significantly suppressed the oxidation process (Entries 6 and 7). The PtNPs on inert supports, such as silica gel, exhibited some activity but did not exceed the conversion upper of 70%, even with a prolonged reaction time (Entry 5). Moreover, the application of AuNPs or PdNPs, instead of PtNPs, yielded no benzaldehyde product under identical experimental conditions which could be attributed to the unique catalytic property of PtNPs (Entries 8 and 9). In addition, by replacing water with other solvents, only a slight conversion of benzyl alcohol was observed (Entries 13–15) which indicated the important role of water in the PtNP catalyzed alcohol oxidation procedure. The catalyst could be steady and reused even after four reaction cycles with no Pt leaching and less than 1% Ca leaching.

Table 30.
The catalytic property of PtNPs on different supports a.
An acid-base reaction using a Keggin-type phosphotungstic acid and TEA was applied for the synthesis of several triethylamine (TEA) salts of phosphotungstic acid ((TEAH)nH3-nPW12O40 (n = 1, 2, 3)). These catalysts used for the alcohol oxidation reactions, and their catalytic activity, selectivity, and recovery rate are listed in Table 31 [63]. The (TEAH)H2PW12O40 (30) catalyst showed the best results, with 99.6% conversion of benzyl alcohol and 100% of selectivity to benzaldehyde under optimized reaction conditions. The activity and selectivity were also essentially unchanged even in the third and the fifth cycles.

Table 31.
Activity and selectivity in the oxidation of benzyl alcohol with the catalysts a.
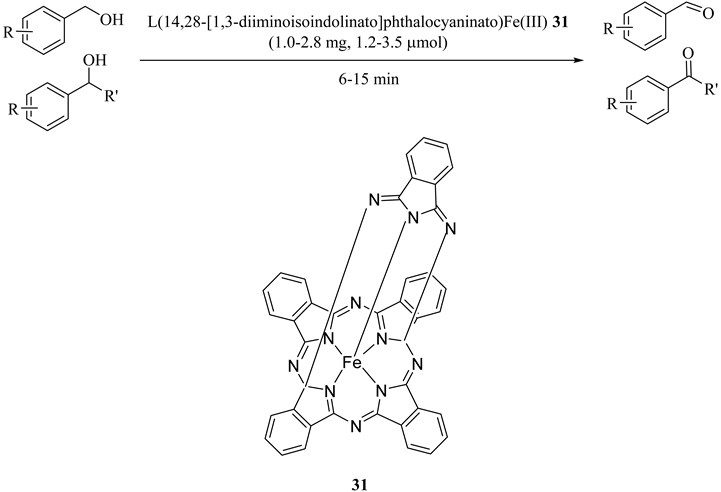
An iron(III) complex formulated as L(14,28-[1,3-diiminoisoindolinato]phthalocyaninato)Fe(III) (31) in which L was a labile axial ligand was synthesized. The efficiency of the prepared catalyst was investigated by the oxidations of primary and secondary benzylic alcohols with both hydrogen peroxide and tert-butyl hydroperoxide (TBHP). Benzyl alcohol, 4-chlorobenzyl alcohol, 1-phenylethanol, and diphenylmethanol (benzhydrol), as illustrated in Table 32, were effectively oxidized without needing highly problematic oxidants or adding organic solvent. In primary alcohol oxidation (benzyl alcohol and 4-chlorobenzyl alcohol) no significant carboxylic acid as overoxidation products were observed. Moreover, secondary alcohols produced ketones with excellent selectivity [64].

Table 32.
Summary of results from catalysis and control experiments a.
A straightforward method was suggested for the oxidation of alcohols to their corresponding carbonyl compounds using nanomagnetic Fe3O4 (32) catalyst in water by H2O2 and the results are summarized in Table 33 [65]. High yields and the best selectivity (>99%) were obtained by producing aldehyde without observing overoxidation of aldehydes to parallel carboxylic acids [66].

Table 33.
Oxidation of various alcohols by using H2O2 in the presence of nanomagnetic Fe3O4 32 catalyst in water at 50 °C a.
Grafting the Cu(II) Schiff base complex onto the channels of mesoporous silica material SBA-15 (Cu(II)-Shiff base-SBA-15 (33)) was conducted by Ma et al. [67], and provided an effective catalyst for the selective oxidation of alcohols in water phase with hydrogen peroxide. Benzyl alcohol converted 98.5% with 100% of the selectivity to benzyl aldehyde. Other substituted benzyl alcohol was also successfully oxidized under the optimal conditions in which the substituted groups impacted the catalytic activity (Table 34).

Table 34.
Selective oxidation of other primary alcohols a.
Malakooti et al. [68] reported the catalytic activity of the Fe2O3/SBA-15 (34) catalyst in oxidation of alcohols. As compared with different heterogeneously catalyzed procedures [7,69] the catalyst showed good activity, and therefore the catalyst was successfully examined for the oxidation of a variety of primary and secondary alcohols using H2O2 (Table 35). Benzyl alcohols were converted to their corresponding aldehydes with excellent yields and also alcohols containing heteroatom such as furfuryl alcohol, hindered substituted alcohols, and secondary benzylic alcohols were oxidized. No further oxidation of aldehyde products to carboxylic acid were seen and the selectivity of the catalyst was also confirmed. The recovered catalyst was reused for at least six successive runs with only a few decreasing in conversion.

Table 35.
Oxidation of alcohols to aldehyde using Fe2O3/SBA-15 34 catalyst in water in the presence of H2O2 a.
Simple and inexpensive iron(III)-benzenetricarboxylate (Fe-BTC) (35) metal-organic gel catalyst, which was previously synthesized [70], was applied in a catalytic system for the oxidation of alcohols with H2O2 oxidant in water with no proof of other side products (Table 36) [71].

Table 36.
Fe-BTC gel 35 catalyzed oxidation of various alcohols a.
Two cobalt (II) and cobalt (III) complexes of a terpyridine based ligand, (40-(2-thienyl)-2,2′,6′,2′′-terpyridine (L)), were prepared in which each complex had two units of the tridentate ligand. The cobaltous complex and the cobaltic complex showed the formula [Co(L)2](NO3)2.2CH3OHH2O (36) and [Co(L)2](NO3)3.2CH3OH (37), respectively. The aromatic alcohol oxidation reactions in the presence of catalysts was performed and the results are summarized in Table 37 [72]. As it was clear, in oxidation by hydrogen peroxide, the alcohols with more solubility in an aqueous media provided more reactivity towards and needed less reaction times. It is worthwhile mentioning that the cobaltous species was more effective than the cobalt (III) catalyst for alcohol oxidation reactions.

Table 37.
Oxidation of a wide range of alcohols using the Co(II) and Co(III) complexes (36, 37) as catalyst in water at room temperature a,b.
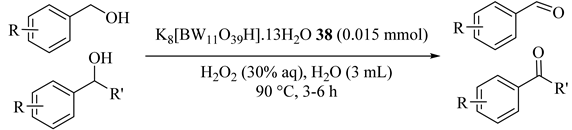
Efficient usage of Lacunary Keggin-tungstoborate of K8[BW11O39H]·13H2O (38) catalyst was reported for the first time in an aqueous/oil system for the oxidation of alcohols [73]. Benzyl alcohol oxidation provided benzaldehyde in high conversion and selectivity and secondary alcohols delivered high yields of ketones. The molar ratio of H2O2/benzyl alcohol was optimized (1:1) because a greater content of oxidant decreased the activity and selectivity of the oxidation product which benzoic acid produced (Table 38 and Table 39). The catalyst could be reused after four consecutive cycles of the reaction.

Table 38.
Oxidation of benzyl alcohol catalyzed by K8[BW11O39H].13H2O 38 with H2O2 a.

Table 39.
Oxidation of various alcohols catalyzed by K8[BW11O39H].13H2O 38 with H2O2 a.
Using easily prepared water-soluble POMs, K8[γ-SiW10O36]·13H2O (39) precatalyst for the selective oxidation of alcohols were reported for the first time. Benzyl alcohol, 1-phenylethanol, and benzhydrol, etc. [74] as activated benzylic alcohols with 30% H2O2 at 90 °C were selectively oxidized to the corresponding ketone in high yields (Table 40) and the catalyst was recycled five times. With the molar ratio of H2O2 to benzyl alcohol 5:1, benzyl alcohol was absolutely converted to benzoic acid after 7 h at 90 °C (Entry 1). By decreasing the temperature of the reaction from 90 to 70 °C and the molar ratio of H2O2 to benzyl alcohol 1:1, benzaldehyde was obtained as the only oxidation product (Entry 2).

Table 40.
Selective oxidation of alcohol catalyzed by K8[γ-SiW10O36]·13H2O 39 with H2O2 at 90 °C a.
To gain more understanding of the catalytic system, the reactions were performed at 20 °C and the results are shown in Table 41. Dropping the reaction temperature from 90 to 20 °C was needed to achieve good yield, longer time (even some days), and more amount of catalyst (Entries 1 and 2). These data indicated that the high reaction temperature and the good water-solubility of alcohols provided high yields of products.

Table 41.
Selective oxidation of alcohol catalyzed by K8[γ-SiW10O36].13H2O 39 with H2O2 at 20 °C a.
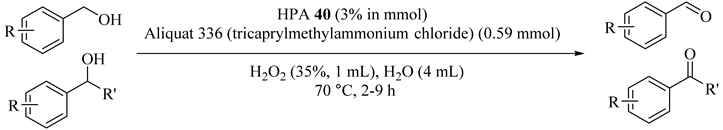
Keggin-type heteropolyacids (40) and hydrogen peroxide as a multiphase system were applied for the selective oxidation of alcohols to ketones or aldehydes (Table 42) with no appreciable detection of higher oxidation state by-product in most cases (Table 42). In the case of benzyl alcohols and 4-chloro benzyl alcohol, the formation of small amounts of benzoic and 4-chlorobenzoic acid due to the addition of hydrogen peroxide twice was decomposed rapidly under homogeneous conditions (Table 42, Entries 6 to 10) [75].

Table 42.
Oxidation of alcohols with hydrogen peroxide catalyzed by different heteropolyacids at 70 °C in multiphase conditions a.
Easy product isolation, good turnovers, and high selectivities were achieved using the Zn substituted polyoxoanion (NH4)7Zn0.5[α-ZnO4W11O30ZnO5(OH2)]·nH2O (41) as the catalyst for the oxidation of organic functionalities. As illustrated in Table 43, only minor (≤5%) amounts of benzoic acids as overoxidation byproduct was provided and the catalyst could be reused three times with little loss in its efficacy [76]. In addition, in contrast to a previously reported system [77,78] using Na2WO4, a phase transfer catalyst and H2O2 in water, in this system, no phase transfer co-catalyst was required.

Table 43.
Oxidation of benzyl alcohol by (NH4)7Zn0⋅5[α-ZnO4W11O30ZnO5(OH2)]⋅nH2O 41 in water with aqueous hydrogen peroxide as an oxidant a.
Alkali-treated ZSM-5 zeolite (42) was obtained by the alkali-treatment modification of the commercially available ZSM-5 zeolite with high concentration NaOH solution at low temperature. The oxidation reaction in the presence of the catalyst (Table 44) showed 53% conversion of BzOH and 86% selectivity to benzaldehyde (BzH). Additionally, the catalyst could be reused for more than six times and was very stable [79]. The results also showed that with an increase of the SiO2/Al2O3 ratio in as-received zeolites, the conversion of BzOH decreased seriously which could be attributed to decreasing the concentration of Lewis acids on the surface of treated zeolite.

Table 44.
Selective oxidation of benzyl alcohol to benzaldehyde by hydrogen peroxide over different catalyst a.
Among different W- and Mo-based heteropolyoxometalate catalysts which were used in the oxidation of aromatic alcohols in the presence of hydrogen peroxide in water, dodecatungstophosphoric acid, H3PW12O40 (43), had the most efficiency. This catalytic system provided a highly selective, efficient, fast, environmentally friendly, and inexpensive approach for the conversion of alcoholic functions to carbonyl groups with H2O2 in water, and even after fifteen runs, the efficiency of the oxygenation system was ~10% decreased (Table 45) [80].

Table 45.
Oxidation of some aromatic alcohols with 34% H2O2 in water catalyzed by H2PW12O40 43 a.
Table 46 [81] summarizes the application of cheap, effective, organic-solvent-free and phase-transfer WO42− catalyst (Na2WO4·2H2O (44)) and aqueous hydrogen peroxide for six primary or secondary alcohol oxidations which are liquids or melt below 90 °C [77,82]. Primary alcohols, such as benzyl alcohol, were easily oxidized to aldehydes with no overoxidation.

Table 46.
Phase-transfer catalyzed oxidation of alcohols a.
2.2. Oxidation of Benzylic and Heterocyclic Alcohols in a Solvent-Free System
Green oxidation of primary and secondary alcohols in the presence of nano-MoO3/copper Schiff base complex (45), using H2O2, was investigated under solvent-free conditions with high conversion and excellent selectivity. The benefits of the reaction also included the ease of isolating products from green media, and the reusability of the catalyst for six times without loss of activity and selectivity (Table 47) [83]. Further oxidation such as the preparation of acid or ester were not investigating (Table 47, Entries 1–4 and 6–12) and, additionally, benzyl phenyl sulfide oxidation provided no sulfoxide or sulfone byproducts (Entry 5).

Table 47.
The oxidation of different alcohols catalyzed by NMCS bio-nanocomposite 45 using H2O2 within 3 h at 80 °C under ultrasonic irradiation a.
Dioxo-molybdenum(VI) complex supported functionalized Merrifield resin (MR-SB-Mo) (46). The catalyst efficiently and selectively oxidized a wide variety of alcohols to aldehydes or ketones using H2O2 as an oxidant with reasonably good TOF (660 h−1 in case of benzyl alcohol) under solvent-free reaction conditions and did not lead to overoxidized products under optimized conditions (Table 48) [84]. The catalyst afforded regeneration and could be reused for at least five reaction cycles without loss of efficiency and product selectivity. In the case of substituted benzyl alcohols, different types of substituents such as –F, –Cl, –Br, –OMe, –OH, and –NO2 were well-tolerated during the oxidation process (Table 48, Entries 2–7), some of which could be utilized for further derivation. One of the notable aspects of the developed catalytic system was its ability to oxidize benzyl alcohol to benzaldehyde at a relatively higher scale (10 g scale) without losing the catalytic efficiency and product selectivity (Table 48, Entry 1d), which provides its potential application in commercial processes.

Table 48.
Oxidation of alcohols to aldehydes or ketones catalyzed by MR-SB-Mo 46 using 30% aqueous H2O2 as oxidant a.
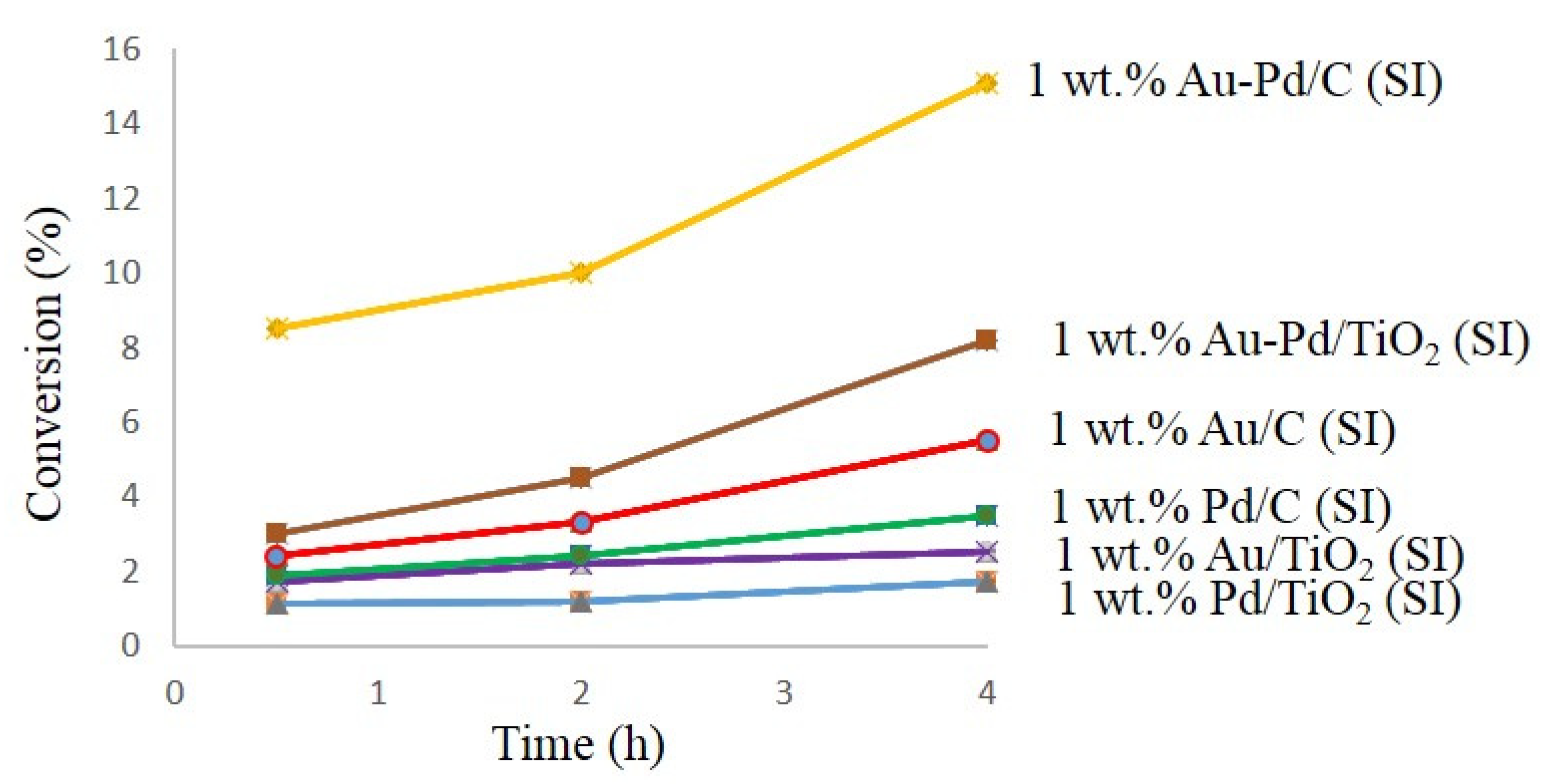
Active Au-Pd catalysts on carbon and titanium oxide (Au-Pd/C (47) and Au-Pd/TiO2 (48)) were applied for the benzyl alcohol oxidation without adding solvents. The results showed that carbon-supported bimetallic catalysts provided a higher conversion as compared with Au-Pd/TiO2 48 catalyst, due to the vast superficial area of carbon supported catalyst as compared with the titanium supported catalyst (Figure 1) [85,86]. These results demonstrated the critical importance of correct selection of support for the metal nanoparticles immobilization in the catalyst preparation approaches. Additionally, some dissimilarities in the selectivity and catalytic activities of catalysts could be the result of both the interaction of catalyst support and the influence of the shape of metal particles.
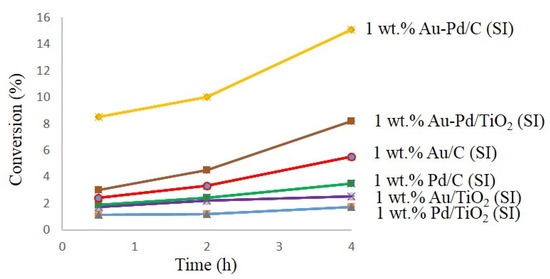
Figure 1.
Curves conversion versus time for carbon-supported catalysts and titanium supported catalysts.
Fe3O4/GrOSi(CH2)3–NH2/HPMo nanocomposite (49) as a magnetically recyclable (at least four times) heterogeneous separable oxidation catalyst was applied for selective oxidation of alcohols to corresponding aldehydes (Table 49, Entries 1–10) and ketones (Table 49, Entries 11–13) with very high selectivity (≥99%) in moderate to excellent yields (60% to 96%) with H2O2 under solvent-free conditions (Table 49) [87].

Table 49.
Results of various alcohol oxidations with H2O2 catalyzed by the Fe3O4/GrOSi(CH2)3-NH2/HPMo 49 catalyst a.
Homotrinuclear copper catalyst [Cu3(slmh)(µ-Cl)2(CH3OH)3]·0.5CH3OH (50), where H4slmh stands for disalicylaldehyde malonoyldihydrazone was prepared by Lal et al., in 2017 [88]. This heterogeneous catalyst oxidized several aromatic ring substituted benzylic alcohols by aqueous hydrogen peroxide and the results are summarized in Table 50 [88]. No benzoic acid production was observed in benzylic alcohol oxidations. Moreover, recyclability of the catalyst showed the five consecutive runs ability with no loss of activity.

Table 50.
Oxidation of alcohols catalyzed by [Cu3(slmh)(µ-Cl)2(CH3OH)3]·0.5CH3OH 50 under solvent-free conditions in the absence of base and co-catalyst a.
The selective oxidation of alcohols using green oxidants, H2O2, in the presence of Mo3O10(C5H6N)2.H2O (51) showed the efficiency of the catalyst for the oxidations of alcohols to the corresponding ketones or aldehydes under solvent-free conditions over reaction-controlled phase-transfer catalysis (Table 51) [89]. The method was so selective with no overoxidation product preparation even with longer reaction time. In addition, MoOx-pyridine could efficiently oxidize secondary benzylic alcohols to their corresponding ketones with excellent conversions and high selectivity (Table 51, Entries 8–11) [89]. It is worthwhile to mention that the catalyst could be reused in H2O2 systems for at least four runs.

Table 51.
Oxidation of alcohols to corresponding aldehydes or ketones using Mo3O10(C5H6N)2·H2O wires (MoOx-pyridine) 51 catalyst a.
Water-soluble heterobimetallic complex [CuZn(bz)3(bpy)2]BF4 (52) was applied for selective oxidations of primary alcohols to the corresponding aldehydes with no base or co-catalyst. In addition, the catalyst could be recyclable and keep its activity intact over five cycles (Table 52) [90].

Table 52.
Oxidation of alcohols catalyzed by [CuZn(bz)3(bpy)2]BF4 52 under solvent-free condition in the absent of base a.
Table 53 presents the application of Cu(II) Schiff base complex [Cu(HL)(H2O)NO3] (53) using 2-[(2-hydroxy-1,1-dimethyl-ethylimino)methylphenol (H2L) in the oxidation of different alcohols [91]. Primary benzylic alcohols either with electron donating or electron withdrawing groups underwent selective and efficient oxidation to the corresponding aldehydes with no overoxidation (Table 53, Entries 1–8). The oxidation of secondary alcohols provided ketones in good to high yield (Table 53, Entries 10–12). The catalyst was also reused five times without losing of its activity.

Table 53.
Oxidation of alcohols using H2O2 catalyzed by [Cu(HL)(H2O)NO3] 53 a.
The treatment of mordenite acid using hydrochloric acid (MOR-HC) (54), nitric acid (MOR-HN) (55), and oxalic acid (MOR-Ox) improved its textural properties such as increasing the BET surface area, total pore volume, mesopore volume, and external surface area (Table 54) [92]. These improvements influenced the catalytic activity of the mordenite in oxidation of benzyl alcohol not only in benzyl alcohol conversion both also in benzaldehyde selectivities. In terms of catalytic properties, the highest improvement was observed in the case of the nitric acid treated sample as follows: benzyl alcohol conversion >99% and benzaldehyde selectivities >99%. In addition, the fittingness of the MOR-HN for various acid catalyzed bulky molecular transformations was observed. Decreasing acid site density of mordenite by dealumination and the increasing porosity, resulted in controlling overoxidation of benzaldehyde to undesired benzoic acid in the MOR-HN catalyst [92].

Table 54.
Performance of various catalysts in benzyl alcohol oxidation a.
Tin-containing 1,3-din-butylimidazolium bromide ([BBIM]Br)-SnCl2 (56) was prepared by Xing et al. [93] and successfully applied for the simple and efficient oxidation of benzyl alcohol using hydrogen peroxide as the oxidant (Table 55). In this catalyst, coordination of Sn species with the imidazole ring resulted in the easy recovery and reusability of the catalyst for six reaction runs without loss of catalytic activity.

Table 55.
Catalytic properties of [BBIM]Br-SnCl2 56 in the oxidation of various alcohols with H2O2 a.
The CoFe2O4 nanoparticles (NPs) (57) catalyzed the solvent-free oxidation of benzyl alcohol (BzOH) to benzaldehyde (BzH) with hydrogen peroxide (Table 56) [94]. Excellent results, >99% conversation of BzOH and 100% selectivity, were obtained with no obvious loss of catalyst activity after three consecutive runs.

Table 56.
Selective oxidation of benzyl alcohol to benzaldehyde catalyzed by CoFe2O4 NPs 57 a.
The Mn- and Co-substituted polyoxotungstates [MPW11O39]5− (M = Mn or Co) was immobilized on MCM-41 or into the interlayer of Mg3Al-layered double hydroxide. The catalysts showed catalytic activity in the presence of H2O2 for the solvent-free oxidation of benzyl alcohol for at least four times with no loss of activity and selectivity. As illustrated in Table 57 [95], POM loaded-LDH (58) and POM loaded-LDH-adipate (59) catalysts were better catalysts than POM-free catalysts due to easier reduction of metal ion via interaction of POM anion with the clay surface [96]. In addition, the basic sites of LDH-supported catalysts resulted in higher selectivity for benzaldehyde as compared with the unsupported catalysts [97].

Table 57.
Oxidation of benzyl alcohol with various catalyst a.
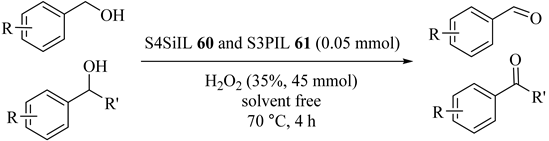
Two long chain multi-SO3H functionalized heteropolyanion-based ionic liquids S4SiIL (60) and S3PIL (61) as homogeneous catalysts provided aldehydes and ketones with 63% to 100% yields through the oxidation of alcohols with no phase transfer catalyst and could be reused five times. The corresponding benzoic acids of benzyl alcohols were obtained with 64% to 94% yields (Table 58) [99]. For 60, benzaldehyde was obtained in 94% yield, whereas for 61, the benzoic acid provided 83% yield.

Table 58.
Results of selective oxidation of alcohols catalyzed by S4SiIL 60 and S3PIL 61 a.
A polyethylene oxide-supported long-chain imidazolium polyoxometalate hybrid catalyst ([PEO-didodecylimidazolium]3[PW12O40]2) (62) was employed as the recyclable catalyst (could be reused six times) for the selective oxidation reactions of alcohols using H2O2 at room temperature, without significant loss of its activity, as shown in Table 59 [100]. Ketones or aldehydes were the only produced products. Primary benzylic alcohols had the best activities with no significant effects on the electronic properties, electron-withdrawing, or electron-donating substituents, or steric hindrance of the groups on the benzene ring [100].

Table 59.
Oxidation of various alcohols a.
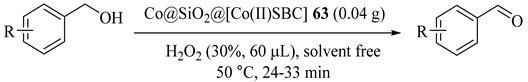
Schiff base complexes of transition metal ions supported on silica coated magnetic metallic cobalt nanoparticles (Co@SiO2@[M(II)SBC], M = Co, Cu, Ni, Zn) (63, 64, 65, 66) as heterogeneous catalysts were prepared and explored for the oxidations of the alcohols to corresponding aldehydes in high yields. The catalysts could be recyclable five times. The obtained results, as shown in Table 60 [101], indicated that alcohol conversions occurred during approximately half an hour with 100% selectivity. Although all of these catalysts selectively converted different alcohols to corresponding aldehyde, the Ni(II) catalysts were more active than three others and converted alcohols to aldehydes in shorter time.

Table 60.
Investigation of catalytic activity of Co@SiO2@[Co(II)SBC] 63 catalysts on the oxidation reaction a.
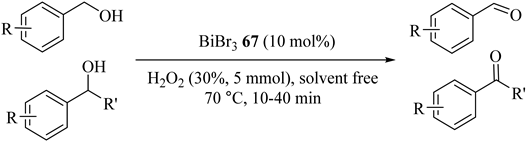
Bismuth tribromide (BiBr3) (67) catalyzed oxidations of both primary and secondary benzylic alcohols with aqueous H2O2 to corresponding carbonyl compounds in high yields, as summarized in Table 61 [102]. Without overoxidation, primary benzylic alcohols resulted in exclusive formation of aldehydes. Generally, the oxidation of primary benzylic alcohols proceeded slower than primary secondary alcohols.

Table 61.
Oxidation of alcohols with H2O2 and BiBr3 67 a.
Three amphiphilic sulphonato-salen-chromium(III) complexes immobilized on MCM-41 (Cr(SO3-salphen)-MCM-41) (68) were applied for the selective oxidation of BzOH to BzH with H2O2, and the results are listed in Table 62 [103]. The introduction of hydrophilic sulfonic acid groups to the catalyst structure enhanced accessibility of the catalyst with oxidant which increased the catalytic act in oxidation reactions as compare with their corresponding lipophilic complexes. The best results were also obtained, i.e., 60.3% benzyl alcohol conversion with 100% benzaldehyde selectivity. In addition, Cr(SO3-salphen)-MCM-41 complex could be reused for five runs.

Table 62.
Catalytic performance of samples in benzyl alcohol oxidation a.
Cesium salt of transition metal (M = Co, Mn, Ni) substituted phosphomolybdates PMo11M as catalysts for the oxidation of alcohol were applied and the catalyst showed the activity in the order of activity PMo11Co (69) ≥ PMo11Ni (70) > PMo11Mn (71) as illustrated in Table 63 [104]. In addition, the catalysts could be reused up to 2 cycles.

Table 63.
Oxidation of various alcohols catalyzed by PMo11M (M = Co, Mn, Ni), under optimized conditions a.
Using cationic surfactants with different carbon-chain lengths for the functionalization of the Vcontaining Keggin POM H4PMo11VO40 provided a series of polyoxometalate (POM)-based amphiphilic catalysts. The catalysts were for selective benzyl alcohol oxidation by H2O2 (Table 64) [105] and the best catalytic efficiency was obtained by (ODA)4PMo11VO40 (72) (ODA: octadecylmethylammonium) due to its amphiphilic property (60.6% of benzyl alcohol conversion with 99% of selectivity for benzaldehyde). Furthermore, the catalyst could be recycled over four run reactions without losing activity.

Table 64.
Selective oxidation of benzyl alcohol to benzaldehyde with H2O2 in the presence of the catalysts investigated a.
A simple adsorption method provided heterogeneous copper(II)-cysteine/SiO2-Al2O3 (73) catalyst which represented good activity and selectivity in aromatic alcohol oxidation reactions, as shown in Table 65 [106]. All reactants were completely converted to selective carbonyl compounds. In addition, shifting the substituent from electron withdrawing (Entries 2–5) to electron donating (Entries 6–9) remarkably increased the yield of corresponding aldehydes from 45% to 98%. The catalyst could also be recycled over five times.

Table 65.
Solvent-free oxidation of various aromatic alcohols over Cu(II)-cysteine/SiO2-Al2O3 73 a.
Lal et al. [107] reported the solvent-free oxidation of primary (Entries 2–5, 9) and secondary (Entries 6–8) benzylic alcohols using a heterotrinuclear complex containing a dicopper(II)–monozinc(II) centre, [ZnCu2(slsch)(NO3)2(H2O)8]·2H2O (74), with hydrogen peroxide, as summarized in Table 66, with good yield and excellent selectivity and four consecutive run reusability.

Table 66.
Hydrogen peroxide mediated oxidation of alcohols to aldehydes and ketones a.
Under mild conditions, primary and secondary alcohols were oxidized by applying rutheniumbis(benzimidazole)pyridinedicarboxylate complex [Ru(bbp)(pydic)] (75) as catalyst and H2O2 as oxidant. Aldehydes and ketones were prepared with good yield and excellent selectivity (Table 67) [108].

Table 67.
Oxidation of various alcohols with H2O2 catalyzed by Ru(bbp)(pydic) 75 a.
Keggintype polyoxometallates, such as M3(PW12O40)2 [M = Ni, Zn, Co, Mn, Cu; denoted as MPW12], FePW12O40 [FePW12] (76), Ni3(PMo12O40)2 [NiPMo12] (77), and Ni2SiW12O40 [NiSiW12] (78) were synthesized as catalysts, under solvent-free condition, for the selective oxidation of BzOH into BzH with hydrogen peroxide (Table 68) [109]. Among them, Ni3(PW12O40)2·26H2O (77) provided high catalytic activity, TON of 550.6 mol/(mol cat.), and 87.3% selectivity. The above catalyst was also recovered and reused for three times.

Table 68.
Oxidation of alcohols a.
Appling L-aspartic acid coupled with imidazolium based ionic liquid [L-AAIL] (79), in the presence of hydrogen peroxide as an ideal oxidant, provided a green protocol for the selective oxidation of alcohols which could be recycled and reused for seven runs. The substituted primary benzylic alcohols were selectively oxidized to aldehydes with 88% to 96% yield (Table 69, Entries 1–6, 9) and the secondary alcohols also produced ketones with 76% to 84% yield (Table 69, Entries 7, 8, 10) [110].

Table 69.
L-AAIL 79 catalyzed oxidation of alcohols by hydrogen peroxide at 25 °C a.
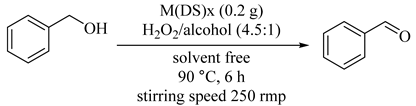
Under solvent-free conditions, hydrogen peroxide as oxidant and metal dodecanesulfonate salts, M(DS)x [M = Fe3+ (80), Cu2+ (81), Ni2+ (82) and Sn2+ (83), DS = dodecanesulfonate, x = 3 or 2] as catalysts were conducted by biphasic catalysis for benzyl alcohol oxidation to benzaldehyde. The results are summarized in Table 70 [111] and indicate that Fe(DS)3 (80) catalyst represented a surprisingly high activity, exhibiting nearly 100% conversion of BzOH and TON of 194.4 mol/(mol cat) as compare with other catalytic systems, due to its difference in Lewis acidity (Entry 1) [112]. In addition, this catalyst could be recovered and reused three times. Overoxidation of BzH into benzylic acid was minimized in biphasic operation systems due to reducing the contact opportunity between BzH in bulk organic phase and the catalyst on the interphase or H2O2 that was soluble in the aqueous phase.

Table 70.
Catalytic activity of various dodecanesulfonate salts a.
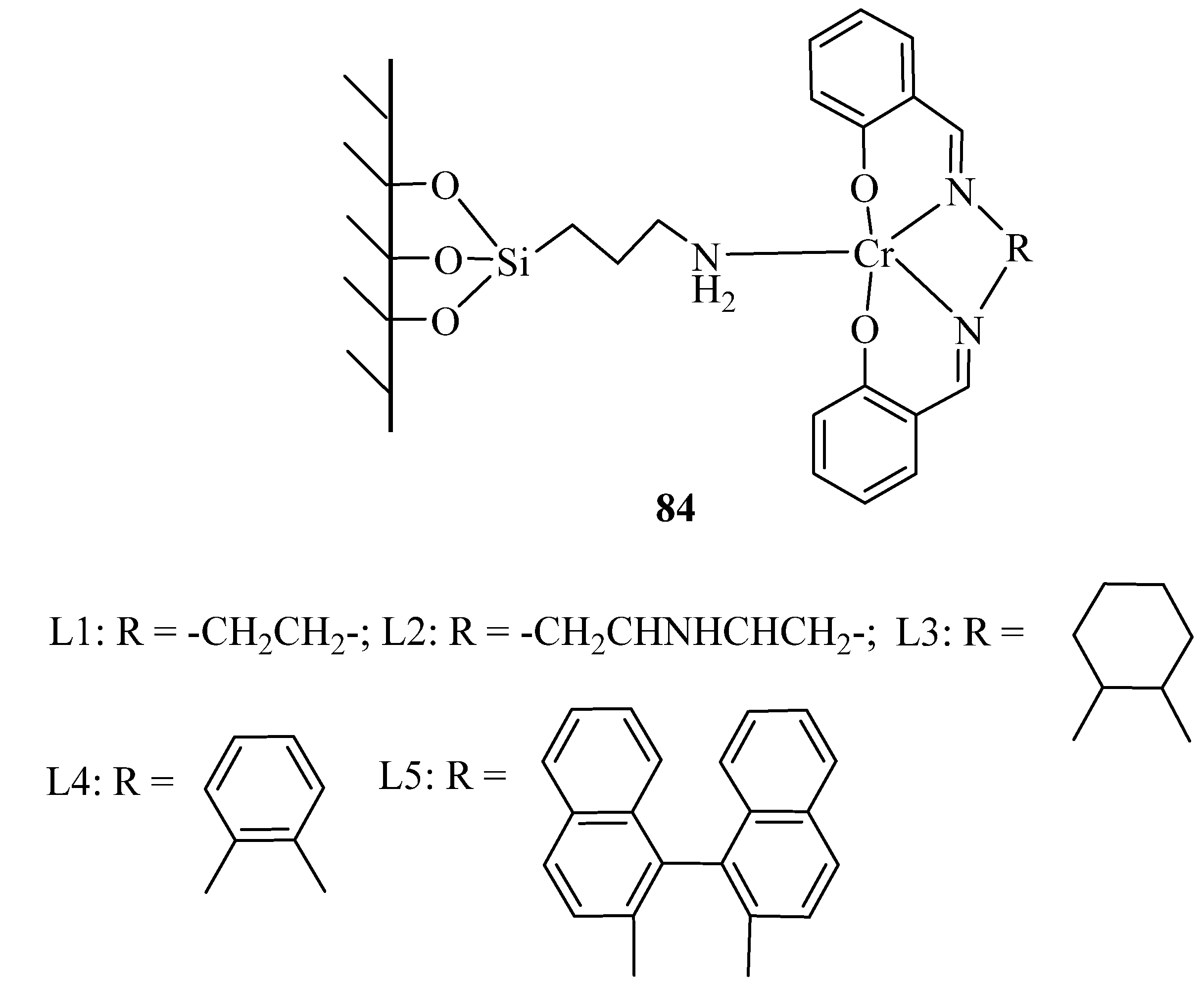
A series of conventional chromium(III) Schiff base complexes were immobilized on MCM-41. Without any organic solvent, phase transfer catalyst, or additive, the mentioned complexes showed much higher catalytic performance in benzyl alcohol conversion to benzaldehyde, due to their corresponding homogeneous analogs. The catalyst represented difference activity by varying ligand in the following order: 84-L4 > 84-L2 > 84-L1 > 84-L5 > 84-L3 (Figure 2) [113]. The difference could be attributed to ligand structures which 84-L4 with π-extended coordination structure exhibiting the best catalytic performance.
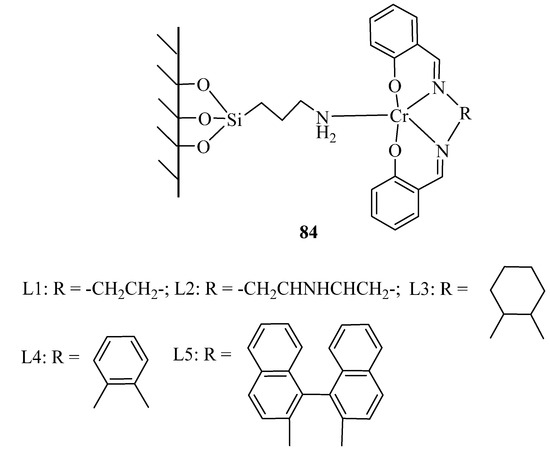
Figure 2.
Homogeneous chromiun (III) Schiff base complexes and their immobilized analouge were prepared as described in “experimental” section.
Different alcohol oxidations were also applied over the representative catalyst 84-L4 (Table 71) [113]. All benzylic alcohols were oxidized with good yields, and the major products were the corresponding ketones or aldehydes. Overoxidation of aldehydes into their corresponding acids were also observed. The catalyst could be reusedfor four catalytic runs, with only a little change in the catalytic performance.

Table 71.
Catalytic performance of the representative immobilized complex in the oxidation of alcohols a.
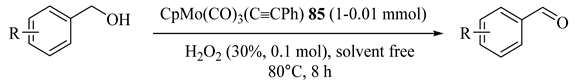
As illustrated in Table 72 [114], green oxidant hydrogen peroxide and cyclopentadienyl molybdenum acetylide catalyst, CpMo(CO)3(C≡CPh) (85), were used to explore selective oxidations of different aromatic alcohols to aldehydes with very high conversion (90%) and aldehyde selectivity (90%). The catalytic system could be reused even after five recycles with no decrease in alcohol conversion and aldehyde selectivity.

Table 72.
Oxidation of different alcohols a.
Nano-γ-Fe2O3 86 as a selective and active catalyst for alcohol and olefin oxidation yielded the corresponding aldehyde, with good to excellent selectivity, without losing catalyst activity even after reusing 5 times. As illustrated in Table 73 [115], significant impact on the catalyst activity was provided by substitutions on the aromatic ring of the benzyl alcohol. Moreover, as compared with benzyl alcohol, 1-phenylethanol, diphenylmethanol, and 1-phenyl-1-propanol gave good conversions with lower selectivity (Table 73, Entries 4–6).

Table 73.
Selective oxidation of alcohols to aldehydes and ketones with nano-γ-Fe2O3 86 a.
Some methyl-containing Cr(salen) complexes which immobilized on MCM-41 were prepared as catalysts and exhibited for the selective oxidation of benzyl alcohol (BzOH) with H2O2. All organic solvent-free systems, with no phase transfer catalyst or additive, showed much higher catalytic activity than their homogeneous analogue. Benzaldehyde (BzH), benzoic acid, and benzyl benzoate were only detected as products (Table 74) [116]. By increasing methyl content of catalysts, from Cr(salen)-MCM-41(CH3)1 (87) to Cr(salen)-MCM-41(CH3)3 (89), BzOH conversion and H2O2 efficiency decreased slowly, whereas BzH selectivity increased, as can be seen in the presence of Cr(salen)-MCM-41(CH3)2 (88) and 89, BzOH was completely oxidized to BzH with no the formation of byproducts. The best BzOH conversion of 65.0% with 100% selectivity to benzaldehyde (BzH) was reached using catalytic system 88 [116].

Table 74.
Catalytic performance of the obtained complexes in the selective oxidation of benzyl alcohol a.
Wang et al. reported the preparation of MoVI oxo-diperoxo complex [MoO(O2)2(TEDA)2] (90) (TEDA = 1,4-diazabicyclo [2.2.2]octane) [117], which successfully catalyzed the high yield oxidations of alcohols to their corresponding carbonyl groups by H2O2. The catalyst was active even for three recycling experiments with no overoxidation (Table 75).

Table 75.
[MoO(O2)2(TEDA)2]-catalyzed oxidation of selected alcohols using H2O2 as oxidant a.
Some tetra-alkylpyridinium octamolybdate (91–94) were used as catalysts for selective oxidations of benzyl alcohol. Among them, as illustrated in Table 76, [118], tetraalkylpyridinium octamolybdate exhibited high activity and selectivity due to oxygen transfer ability from hydrogen peroxide to the substrate. This matter is clear by 82.3% to 94.8% benzyl alcohol conversion and 87.9% to 96.7% benzaldehyde selectivity. The simple and easy catalyst preparations and utilization, high recovery, and short reaction time made the catalytic system an ideal choice for future investigations.

Table 76.
Oxidation of benzyl alcohol to benzaldehyde by hydrogen peroxide over tetra-alkylpyridinium octamolybdate catalysts a.
Na4H3[SiW9Al3(H2O)3O37]·12H2O(SiW9Al3) (95) was synthesized and applied as the catalyst for organic-solvent-free selective oxidations of alcohol to ketones using H2O2 without any phase-transfer catalyst under mild, safe, and simple reaction conditions (Table 77) [119]. Benzaldehydes was prepared with selective benzylic alcohol oxidations in moderate to good yields without overoxidation. Additionally, N-atom of 2-pyridinemethanol and S-atom of 2-thiophenemethanol showed no oxidation.

Table 77.
The selective oxidation of alcohols with H2O2 catalyzed by SiW9Al3 95 without solvent a.
2.3. Oxidation of Benzylic and Heterocyclic Alcohols in the Presence of Various Solvents
2.3.1. Acetonitrile Solvent
Keggin-type polyoxometalate [n-C4H9)4N]x[PW11ZnO39]·nH2O was successfully immobilized on imidazole functionalized ionic liquid-modified mesoporous MCM-41 by physical adsorption (PW11Zn@MCM-41-Im) (96) [120]. The supported ionic liquid catalyst was easily recovered by simple filtration and reused in four reaction runs. Different alcohols were efficiently oxidized in reflux condition, as shown in Table 78, with high yields and good selectivity. A slight influence on the reaction of benzylic alcohols and electronic nature (electron-withdrawing and electron-donating groups) of the substituent were observed (Table 78, Entries 2–6).

Table 78.
Oxidation of alcohols with H2O2 catalyzed by PW11Zn@MCM-41-Im 96 under reflux conditions a.
Three paramagnetic metal complexes of 3-hydroxy-3,3′-biindoline-2,2′-dione (dihydroindolone, H4ID) (MH2ID) with Ni2+, Cu2+, and VO2+ ions with were synthesized and applied in oxidation reactions using aqueous H2O2 in acetonitrile. Although all the NiH2ID (97), CuH2ID (98), and VOH2ID (99) catalysts showed good catalytic activity, chemo-, and regioselectivity, VOH2ID had the highest potential due to more Lewis acid character and the high oxidation number of the central V4+ ion in 99 as compared with both Ni2+- and Cu2+-species in the same homogenous aerobic atmosphere [121]. As illustrated in Table 79, 99 afforded the best amount of the selective product such as benzaldehyde (85%) [122].

Table 79.
Oxidation process catalyzed by VOH2ID 99 using an aqueous H2O2 in acetonitrile a.
Iron catalysts supported on porous furfuryl alcohol derived resins were synthesized and applied for the selective comparison of benzyl alcohol to benzaldehyde. As shown in Table 80, FeCl2 (0.5 mol%) as a reference reaction was tested for the oxidation of 0.77 M benzyl alcohol with 2.3 eq. H2O2 in acetonitrile [123]. After microwave irradiation (5 min), 53% conversion with 78% selectivity to benzaldehyde was detected. Loading iron catalysts supported on P420 (Fe/P420 (100)) or P500resin (Fe/P500 (101)) with 10 times lower Fe content (0.05 mol%), similar selectivity with lower conversion in the product mixture was obtained. (Table 80 and Table 81) [123]. In addition, the selectivity in the final reaction mixture by preventing further oxidation to benzoic acid was optimized by continuous addition of H2O2 between 1.5 and 2.3 eq.

Table 80.
Benzyl alcohol conversion and selectivity using Fe nanoparticles supported on P420 resin 100 as compared with free dissolved FeCl2, using 1.5 and 2.3 equiv. H2O2 a.

Table 81.
Benzyl alcohol conversion and selectivity using Fe nanoparticles supported on P500 101 as compared with dissolved FeCl2, using 1.5 and 2.3 equiv. H2O2 a.
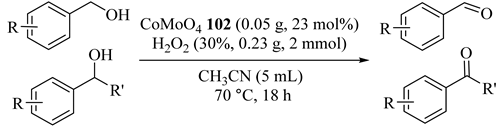
Metal molybdates were coupled with Co, Ni, and Cu with Mo, respectively, for the preparation of bimetallic complexes which was used successfully in alcohol oxidation reactions by Xinhua et al., in 2018 [124]. The 100% selectivity in conversion of the alcohols to corresponding carbonyl compounds showed noticeable performance of theses catalysts which could be reused three times. The results represented that CoMoO4 (102) catalyst provided lower conversion and much higher selectivity (approximately 100%). In addition, non-terminal alcohols could be oxidized to ketones sufficiently (Table 82, Entries 2 and 3). Although NiMoO4 (103) had near 100% selectivity, Cu4Mo3O12 (104) provided no product (Table 82, Entry 1) due to the existence of a bimetallic combination effect in the oxidation reactions.

Table 82.
Selective oxidation of different alcohols a.
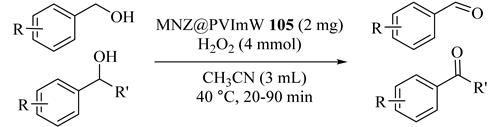
Baghbanian et al. [125] immobilized 12-tungstophosphoric acid onto poly(N-vinylimidazole) which was modified by magnetic nanozeolite (MNZ@PVImW) (105). The results of successful catalytic oxidations of benzylic alcohols in the presence of synthesized catalyst under optimized reaction conditions are summarized in Table 83. In addition, without loss of catalytic activity, the catalyst could be reused eight times.

Table 83.
Oxidation of various alcohols catalyzed by MNZ@PVImW 105 a.
A simple and efficient catalytic oxidation of benzylic and hetero-aryl alcohols to their corresponding carbonyl compound using recoverable heterobimetallic sodium-dioxidovanadium (V) complexes (106) was reported (Table 84) [126].

Table 84.
Catalytic oxidation of alcohols by heterobimetallic vanadium(V) complexes 106 a.
The hydrothermal method was applied for the preparation of MCM-41 nanostructure-modified with vanadium, iron, and cobalt. Under optimized conditions, a molar ratio substrate/oxidant of 4/1 and 7 h of reaction, as summarized in Table 85, BzH was the main product with 7% and 12% yield for V-M(60) (107) and Fe-M(60) (108), respectively [127]. High TON (1100 mol/mol V), 95% selectivity to BzH, and 31.7% yield made V-M(60) performance better than 108 and Co-M(60) (109) due to effective dispersion of vanadium species in the framework that could be considered as the active sites for the oxidation reaction. Moreover, the catalyst could be effectively reused and recovered for at least three cycles without loss of its activity and selectivity.

Table 85.
Benzyl alcohol oxidation with H2O2 on M-M(x) catalysts under standards conditions a.
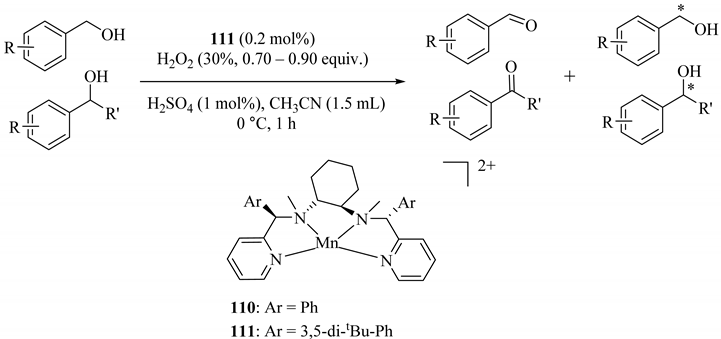
Using a synthetic manganese catalyst for catalytic oxidation and oxidative kinetic resolution (OKR) of secondary alcohols in the presence of an environmentally benign oxidant hydrogen peroxide and a small amount of additive sulfuric acid provided the high yields of products (up to 93%) with excellent enantioselectivity (>90% ee in the OKR of secondary alcohols). Moreover, 111 provided a higher enantiomeric excess (90% ee) than 110 (65%) in 1-phenylethanol oxidation (Table 86, Entry 1 and footnote c) [128].

Table 86.
Substrate scope of oxidative kinetic resolution of secondary alcohols using the 111/H2O2/H2SO4 catalytic system. a,b
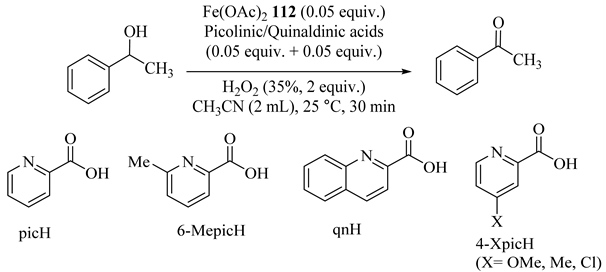
A variety of picolinic/quinaldinic acids as ligands mixed in situ with Fe(OAc)2 (112) catalyzed the H2O2 oxidation of 1-phenylethanol especially with 6-methylpicolinic acid (6-MepicH) and 4-chloropicolinic acid (4-ClpicH) (Table 87) [129]. The oxidation possesses proceeded using 35% aq. H2O2 in CH3CN solution with isolated Fe complexes (113) as the catalyst (Table 88) [129]. In addition, the results showed that the redox potential of Fe III and the lability of picolinate or quinaldinate ligand were important factors for the catalytic reaction.

Table 87.
Iron-catalyzed oxidation of 1-phenylethanol by various combinations of picolinic/quinaldinic acids a.

Table 88.
Catalytic oxidation of 1-phenylethanol by isolated Fe complexes 113 a.
The coprecipitation approach was applied for hybrid chromium (VI)-based magnetic nanocomposite catalyst (Fe3O4@SiO2@PPh3@Cr2O72− (114)) preparation by Maleki et al., in 2016 [130]. This catalyst was used for the first report of using magnetic nanocomposites with ultrasonic irradiation for the oxidation of benzyl alcohol to benzaldehyde with hydrogen peroxide at room temperature (Table 89). Total conversion of benzyl alcohol to benzaldehyde and the reusability of the catalyst at least five times were the benefit of this system.

Table 89.
The oxidations of benzyl alcohol to benzaldehyde in the presence of Fe3O4@SiO2@PPh3@Cr2O72− nanocomposite 114 a.
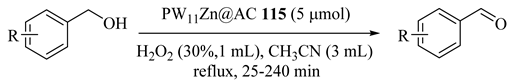
Yadollahi et al. [131] first reported using transition metal-substituted polyoxometalate [PW11ZnO39]5− (PW11Zn) (POMs) supported on activated carbon (AC) for oxidation of alcohols in the presence of H2O2 and CH3CN as solvent (Table 90). In addition, with high selectivity, the oxidation of p-hydroxybenzyl alcohol without oxidation of the hydroxyl group was carried out (Table 90, Entry 13) and showed no change in the catalytic activity/selectivity of PW11Zn@AC (115) for at least five catalytic cycles of sequence loading.

Table 90.
Oxidation of various alcohols with hydrogen peroxide catalyzed by PW11Zn@AC 115 a.
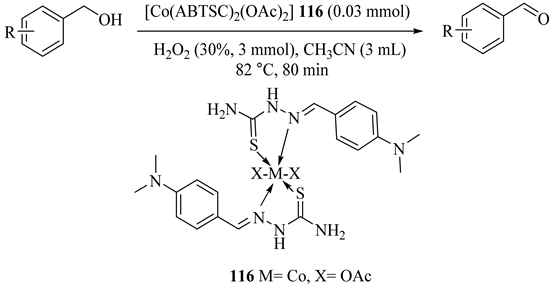
Two thiosemicarbazide Schiff bases, 1-(4-dimethylaminobenzylidene) thiosemicarbazide (ABTSC) and 1-(2-pyridincarboxyl-idene) thiosemicarbazide (TCTS), were applied for the coordination with Co(II), Ni(II), Zn(II), Cd(II), and Ag(I) transition metal salts, chloride, and acetate, and then under optimized conditions proceeded successfully for the oxidation of benzylic alcohols, Table 91 [132]. The environmentally friendly oxidation catalytic systems exhibited great activities in successive runs without loss in activity. The best results under the optimum conditions were obtained with Co(ABTSC)2(OAc)2 (116) catalyst as in 4-methoxybenzyl alcohol oxidation to aldehyde with 95% conversion and 100% benzaldehyde selectivity.

Table 91.
Oxidation benzyl alcohol derivatives for complex [Co(ABTSC)2(OAc)2] 116 a.
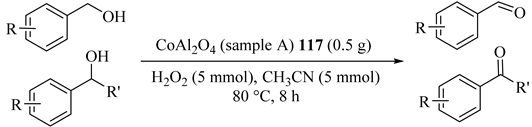
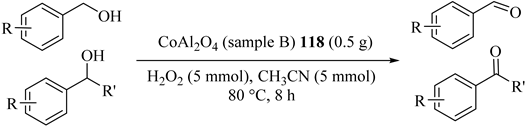
Nanocrystalline cobalt aluminate (CoAl2O4), using both conventional (sample A: CoAl2O4-CCM) (117) and microwave combustion method (sample B: CoAl2O4-MCM) (118), was synthesized for comparative investigation in various alcohol oxidations to corresponding carbonyl compounds, as shown in Table 92 and Table 93 [133]. 117 showed weaker ferromagnetic in nature as compared with CoAl2O4-MCM and both also showed a lower conversion rate in the oxidation of benzyl alcohol [134]. This result was confirmed more powerful for 118 and similar results were achieved within a shorter time. In addition, both catalytic systems catalytic were reusable activity/selectivity during at least five catalytic cycles.

Table 92.
Oxidation of substituted alcohols to aldehydes using cobalt aluminate (CoAl2O4) (sample A) 117 under the optimum conditions a.

Table 93.
Oxidation of substituted alcohols to aldehydes using cobalt aluminate (CoAl2O4) (sample B) 118 under optimum conditions a.
The sol-gel technique via hydrolysis of tetraethyl orthosilicate (TEOS) was applied for the preparation of mono substituted Keggin type POMs, [n-C4H9)4N]x[PW11MO39]·nH2O (PWM) (M = Cr, Mn, Fe, Co, Ni, and Cu) in silica matrix (PWM/SiO2) and were examined in the oxidation reactions of different aromatic alcohols with aqueous 30% H2O2. Because for the preparation of benzaldehyde, PWFe/SiO2 (119) composite showed the highest activity (80% yields) among all of catalytic systems, further oxidation was performed by this catalyst (Table 94) [33]. Benzyl alcohol its derivatives with electron donating and withdrawing groups in para-, orto- and meta-positions, provided yields as excellent as primarily aromatic alcohols to the corresponding aldehydes. The 119 catalyst could also be recovered for five oxidation runs.

Table 94.
Oxidation of alcohols with hydrogen peroxide catalyzed by PWFe/SiO2 119 a.
Oxidation of para-substituted phenyl methyl sulfides and benzyl alcohols with H2O2 in acetonitrile solution were examined by a series of diiron (III) complexes of 1,3-bis(2′-arylimino)isoindoline, [(Fe(L)Cl)2O], and 1,4-di-(2′-aryl)aminophthalazine, [Fe2(µ-OMe)2(H2L)Cl4], 1,4-di-(4′-methyl-2′-thiazolyl)aminophthalazine, and 1,4-di-(2′-benzthiazolyl)-aminophthalazine (Table 95) [135]. The results of the [Fe2(µ-O)(L3,5,7)2Cl2] (120–122) catalysts confirmed that complex 120 provided the highest conversion because of catalyzing both oxygen-atom transfer and hydrogen-atom abstraction. In addition, among phthalazine-based complexes, the best oxidative activity was found with 124, since benzyl alcohol gave the benzaldehyde with 100% selectivity by catalysts in all these reactions, and no other products were provided.

Table 95.
Results of the oxidation of benzyl alcohols catalyzed by isoindoline- ([Fe2(µ-O)(L3,5,7)2Cl2]) (120–122) and phthalazine-based ([Fe2(µ-OMe)2(H24,6,8)Cl4]) (123–125) diiron complexes a.
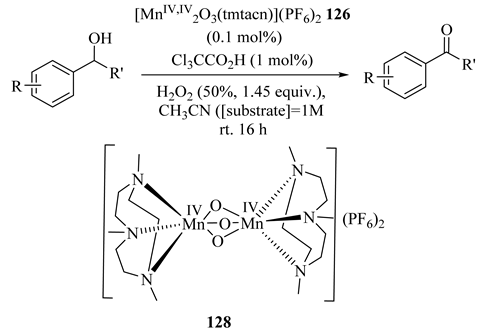
The oxidation of alcohols employing H2O2 as oxidant by manganese-containing catalytic system [MnIV,IV2O3(tmtacn)2]2+ (126)/carboxylic acid (where tmtacn = N,N’,N’’-trimethyl-1,4,7-triazacyclononane was reported (Table 96) [136]. Co-catalyst trichloroacetic acid provided the most active catalyst system and side reactions due to side products were not observed [137,138].

Table 96.
Oxidation of secondary alcohols to ketones using H2O2 catalyzed by [MnIV,IV2O3(tmtacn)](PF6)2 (126) a.
As illustrated in Table 97, an in situ prepared catalyst based on manganese(II) salts (Mn(ClO4)2·6H2O (127)), pyridine-2-carboxylic acid, and butanedione with H2O2 as oxidant at ambient temperatures as catalyst system provided good-to-excellent yields and conversions with high TONs (up to 10,000) [139]. In addition, secondary alcohols were converted to ketones selectively in substrates bearing multiple alcohol groups which reduced the need to introduction protecting groups prior to the oxidation and subsequent removal. In general, the results confirmed that benzyl CH oxidation proceeded in preference to aliphatic CH oxidation.

Table 97.
Oxidation of secondary alcohols a.
Chemoselective oxidation of alcohols to their corresponding carbonyl compounds in short reaction times and high yields were reported by an efficient protocol including H2O2 as oxidant at 80 °C in acetonitrile in the presence of nanoparticles of Fe2O3-SiO2 (128) (Table 98) [140]. The catalyst was successfully recycled at least five times with no substantial loss in the activity of the reused catalyst.

Table 98.
Oxidation of various alcohols catalyzed by (9.6 wt% Fe) Fe2O3-SiO2/Imidazole/H2O2 a.
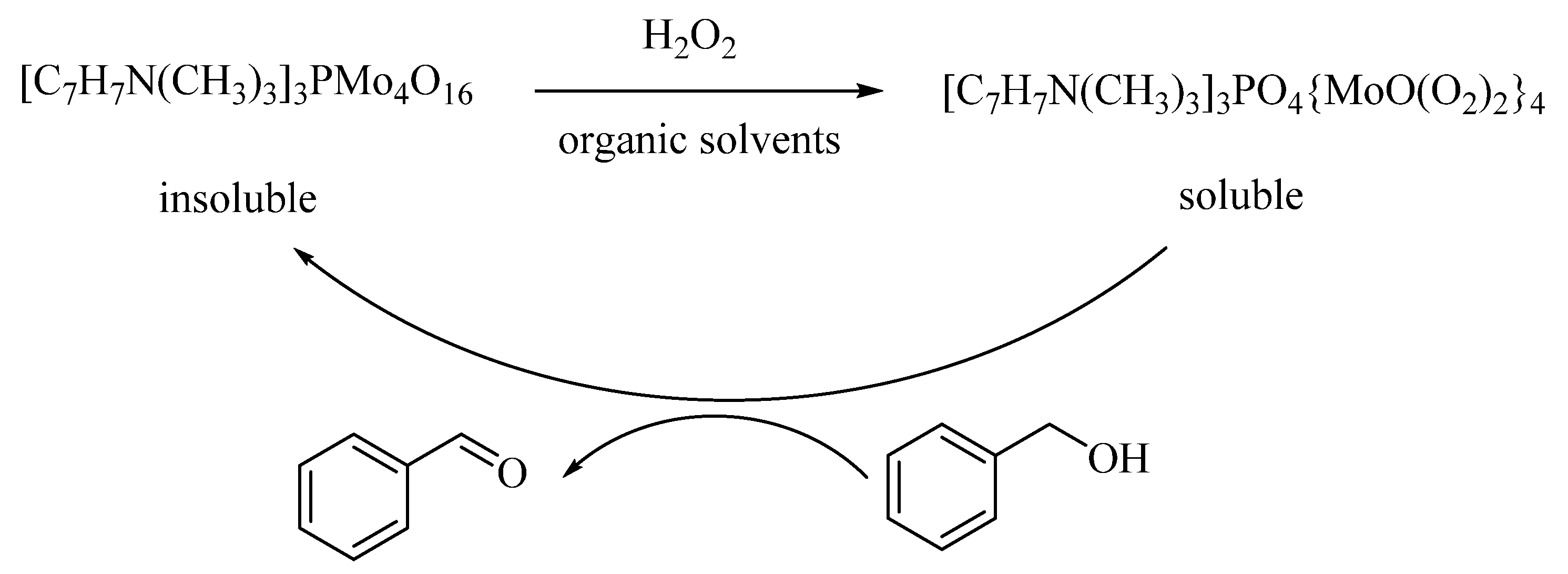
In the presence of H2O2, a reusable (three times) and high stable heteropolyoxometalates [C7H7N(CH3)3]3PMo4O16 (BTPM) (140) catalyst was catalyzed for selective oxidation of BzH with selectivity more than 99% and 92.8% of BzOH conversion (Scheme 1) [141].
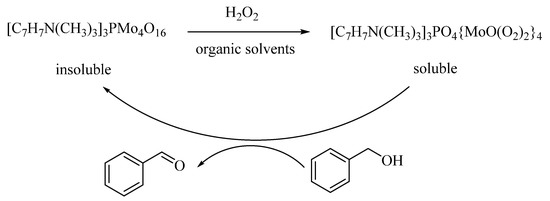
Scheme 1.
Process of the catalytic oxidation of BzOH.
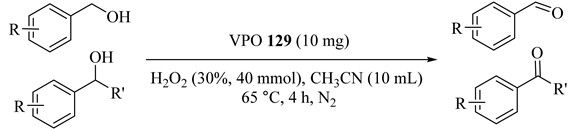
Vanadium phosphorus oxide (VPO) (129) was introduced as an operational catalyst in the presence of hydrogen peroxide for the preparation of aldehydes and ketones from alcohol oxidations without employing any sacrificial oxidants or base at 65 to 70 °C in acetonitrile solvent (Table 99) [142]. Although both activated and non-activated alcohols were selectively and efficiently converted to the corresponding carbonyls with no producing byproducts, activated alcohols such as benzhydrol, benzyl alcohol, and 1-phenylethanol (Entries 1, 2, and 3, respectively) afforded a much higher conversion. The catalyst could also be easily reused for five successive cycle runs with a slight decrease in its efficiency.

Table 99.
Oxidation of various alcohols over VPO 129 catalyst using H2O2 and acetonitrile a.
An environmentally friendly protocol including hydrogen peroxide, liquid phase at atmospheric pressure over Fe3+/montmorillonite-K10 (130) catalyst synthesized by the ion-exchange method at pH = 4 in acetonitrile solvent was proposed for the oxidation of various primary and secondary aromatic, as illustrated in Table 100 [143]. Oxidation of the -OH group adjacent to the benzene ring (activated alcohols) was easier such as in 1-phenylethanol (Entry 1) and benzyl alcohols (Entries 2–3) and also it showed higher conversions (86% to 95%). The catalyst was also reused for five times.

Table 100.
Oxidation of various alcohols over Fe3+/K10 130 catalyst using H2O2 and acetonitrile a.
2.3.2. Toluene Solvent
Oxo-vanadium (V=O) was immobilized on the surface of Fe3O4@PDA [VO(PDA)@Fe3O4] as a magnetic adsorbent and stabilizing agent. VO(PDA)@ Fe3O4 (131) nanoparticles demonstrated high catalytic activity as a recyclable catalyst in the chemoselective oxidation of benzylic alcohols to aldehydes using hydrogen peroxide in high yield (Table 101) [144]. The synthesized catalyst could be reused seven times and could be easily separated.

Table 101.
Oxidation of benzylic alcohols catalyzed by VO(PDA)@Fe3O4 131 a.
The deposition-precipitation method was applied for the preparation of a series of bimetallic copper-nickel (CuNix, x = 0.1, 0.2, 0.5, and 1) nanoparticles supported on activated carbon (AC) for assessing the effect of different ratios of Ni added into the Cu catalyst. A comparison between bimetallic Cu-Ni nanoparticles and monometallic Cu and Ni nanoparticles showed that the catalytic activity increased in the presence of the CuNi1/AC (132) catalyst for the oxidation of benzyl alcohols to the corresponding aldehyde in a short reaction time at 80 °C (Table 102) [145].

Table 102.
Catalytic performance of the catalysts for the oxidation of benzyl alcohol to benzaldehyde a.
Redox reaction between in situ generated zero-valent cobalt nanomaterial and KMnO4 produced gram-scale synthesis of recoverable heterobimetallic Co-Mn oxide (Co2Mn3O8) (133) in water was successfully applied for oxidation of benzyl, aliphatic, cinamyl, pyridine, and thiophene moiety alcohols to aldehydes/ketones, both in the presence of hydrogen peroxide, with excellent selectivity (>99%) and yield (Table 103) [146].

Table 103.
Substrate scope for Co2Mn3O8-promoted oxidation of a variety of alcohols in the presence of hydrogen peroxide a.
The selective oxidation of alcohols catalyzed successfully by Fe3O4@mSiO2/NH-PV2W (134) as a novel magnetically recyclable catalyst was prepared by immobilization of divanadium-substituted Keggin phosphotungstic acid H5PV2W10O40 on mesoporous silica-coated Fe3O4 core-shell nanoparticles (Table 104) [147]. With an external magnetic field, the catalyst was easily separated after the completion of the reactions and without any loss of its catalytic activity, the recovered catalyst could be reused at least five times. Although the catalyst showed high catalytic activities with 98% and 99% selectivity for conversion of benzyl alcohol to benzaldehyde, the secondary benzyl alcohols were reluctantly oxidated to corresponding ketones such as 40% and 36% conversion oxidation for 1-phenylethanol, as shown in Entry 4.

Table 104.
Selective oxidation of different alcohols catalyzed by Fe3O4@SiO2/NH-V2PW (20%) 134 a.
2.3.3. Acetic Acid Solvent
A simple combination included NaBr (135), H2O2, and AcOH was overlooked among many reported catalytic systems for catalytic oxidations of alcohols [148,149,150]. Oxidation of secondary benzylic alcohols bearing substituents such as Cl, Br, NO2, and diarylmethanol derivatives in aqueous hydrogen peroxide as terminal oxidant were explored by this system with excellent yields (Table 105) [151].

Table 105.
Substrate scope under optimized conditions a.
Under optimized conditions, substrate having p-methoxypenyl group provided the oxidation product c′ with poor yield due to formation of major product 4-methoxy-α-methylbenzyl acetate (d) and 4-methoxy-α-methylbenzyl hydroperoxide (e). Without NaBr catalyst, d and e products were largely prepared which indicated that the formation of these products occurred independently from the oxidation reaction pathway. With more experience, the best optimized condition was performed in AcOH-EtOAc (3:7, 0.25 M), giving the desired ketone c′ in 86% isolated yield (Table 106, Entries 1–5).

Table 106.
Optimization of conditions for substrate c a.
As illustrated in Table 107, this protocol was successfully applied for the preparation of corresponding oxidative products f′–j′ a variety of substrates such as 1-o-methoxyphenyl-1-ethanol (f), cyclopropylphenylmethanol (g), and various cyclic substrates h–j with good yields (Table 107) [150,151].

Table 107.
The preparation of corresponding oxidative products f′–j′ a.
Heterogeneous catalytic oxidation of preyssler type heteropolyacid supported onto silica gel, H14[NaP5W30O110]/SiO2 (136) was reported for the converting of substituted benzyl alcohols converted to the corresponding benzaldehyde derivatives, as shown in Table 108 [26]. Using 0.03 g H14-P5 supported onto silica gel in refluxing acetic acid represented the best results with excellent yields. After extended reaction times, overoxidation products such as carboxylic acids were not observed and the catalytic activity remained after three runs.

Table 108.
Oxidation of alcohols using H14-P5 supported onto silica gel 136 a.
2.3.4. Dimethylacetamide Solvent
As shown in Table 109, a series of heteropolytungstates catalysts, Q3PW12O40 (137), Q3PMo12O40 (138), and Q3PMo4O24 (139) (Q+ represents [C7H7N(CH3)3]+), were applied for the BzOH oxidation reactions by Jian [152]. Using 137 and 138 as catalyst needed no feature of the reaction-controlled phasetransfer catalysis, within the determined reaction time, the catalysts were still soluble in the solvent, and the reaction conversions were poor (38.5% and 24.6%, respectively). Applying [C7H7N(CH3)3]9PW9O34 (140) as catalyst under optimal conditions, DMAc, H2O2/BzOH:1, 95.0% conversion of benzyl alcohol (based on H2O2) and over 99% selectivity of benzaldehyde was achieved. In addition, the catalyst could be reused and recycled three times.

Table 109.
Catalytic oxidation of BzOH by various heteropolyoxometalates with H2O2 a.
2.3.5. Methanol Solvent
As presented in the Table 110, Dabiri et al. [153] reported oxidation of alcohols to the related carbonyl compounds using a vanadium Schiff base complex on nano silica catalyst (VSBC@NS) (141). Nano silica NH2-functionalized, 2,4-dihydroxy benzaldehyde, and VO(acac)2 were reacted and provided heterogeneous 141 catalysts which showed good efficiency, chemical stability, and at least five times reusability in selective oxidation of alcohols to aldehydes.

Table 110.
Oxidation of alcohols a.
2.3.6. tert-Buthanol Solvent
Tandon and co-workers [154] reported a quasi-homogenous reaction system included using 1:1.5 t-butanol-H2O2, not expensive PTC tetrabutyl ammonium hydrogen sulfate (TBAHSO4) as the phase transfer and heteropolyanion Na2WO4 (142) as the catalyst for simple secondary alcohol oxidations to ketones at 90 °C. As shown in Table 111, 100% yield of products in 30 min confirmed the power of this catalytic system.

Table 111.
Oxidation of secondary alcohols with hydrogen peroxide in tert-butanol a.
Oxidation of benzylic alcohols also provided corresponding aldehydes or acids with good yields (Table 112) [154]. Substitution of the aromatic ring influenced the state of oxidation like p-methoxybenzaldehyde was produced with 87% yield from p-methoxybenzyl alcohol in four hours and further oxidation did not occur; whereas p-nitrobenzaldehyde (62%) and p-nitrobenzoic acid (30%) were produced from p-nitrobenzyl alcohol oxidation. In addition, non-benzylic primary alcohols were directly converted to their corresponding carboxylic acids (Table 112, Entry 3). The results showed that, generally, secondary alcohols were more reactive towards oxidation than primary alcohols.

Table 112.
Oxidation of primary alcohols with hydrogen peroxide in tert-butanol.
2.3.7. Xylene Solvent
Hayashi, in 2012 [155], reported using 30% H2O2 in the presence of activated carbon (143) for the oxidation of secondary benzylic alcohols to ketones (Table 113). At first, 9-fluorenol was selected as a substrate (Table 114) and 9-fluorenone was obtained with 77% yield (100 weight% of activated carbon, m-xylene, 95 °C, 18 h). When ten equivalents of 30% H2O2 were used, less (4 equiv.) and more amounts (15 equiv.) of 30% H2O2 caused a decrease of yield (Table 114, Entries 1 (68%) and 3 (35%), respectively). After drying, the activated carbon was recovered and reused without deactivation.

Table 113.
Oxidation of various alcohols to the corresponding carbonyl compounds a.

Table 114.
Oxidation of 9-fluorenol a.
2.3.8. Chloroform Solvent
A solvent-controlled selective oxidation of benzyl alcohol (BnOH) by 30 wt% H2O2 with iron(III) tosylate (Fe(OTs)3·6H2O) (144) was reported (Table 115) [156]. The results confirmed that using different solvents provided dissimilar products such as in chloroform, quantitative conversion to benzaldehyde (BzH) was shown, while in acetonitrile, benzoic acid was detected with high yield.

Table 115.
Oxidation of benzyl alcohol catalyzed by Fe(OTs)3.6H2O 144 with H2O2 a.
2.4. Oxidation of Benzylic and Heterocyclic Alcohols in Dual-Phase System
2.4.1. Acetonitrile-Water
Tungstate ions immobilized on the homemade periodic mesoporous organosilica with imidazolium ionic liquid framework which was denoted as WO4=@PMO-IL (145) were prepared and applied with hydrogen peroxide in the oxidation of primary and secondary alcohols to the corresponding carbonyl compounds (Table 116) [157]. Various primary even pretty hindered substrate 2-nitro-benzyl alcohol were also converted to the corresponding aldehyde with excellent selectivities and good yields without any overoxidation (Table 116, Entry 5). With acceptable to excellent yield, under the same reaction conditions, secondary aromatic alcohols were also converted to the corresponding ketones (Table 116, Entries 7–9). In addition, the catalyst could be also efficiently recovered and reused in seven subsequent reaction cycles without deactivation [157].

Table 116.
Oxidation of various alcohols with WO=4@PMO-IL 145 catalyst a.
The mild alcohol oxidations with hydrogen peroxide were catalyzed by heterogeneous catalyst iron(III) tetrakis(p-sulfonatophenylporphyrinato)acetate supported on polyvinylpyridine and Amberlite IRA-400 (146). As illustrated in Table 117, benzyl alcohol and substituted benzyl alcohols were efficiently converted to their corresponding aldehydes with no significant effect of substituents on the oxidation process [158]. In addition, secondary alcohols were converted to their corresponding ketones with good yields. The catalysts could also be applied in four regeneration cycles without significant loss of their activity.

Table 117.
Performance of catalysts in the oxidation of alcohols with H2O2 a.
Tetrabutylammonium decatungstate (VI) (TBADT) (147) as catalyst was applied in the oxidation of selected alcohols with hydrogen peroxide as a green oxidant using acetonitrile/water 1,2- or dichloroethane/water as a solvent system. Microwave irradiation combined with elevated pressure in two phase system acetonitrile/water resulted in the highest conversions of substrates in the range of 80% to 100% (Table 118) [159]. TBADT also conserved its catalytic activity for two regeneration cycles.

Table 118.
Oxidation of selected alcohols in the acetonitrile/water system a.
2.4.2. Water-Toluene
H2O2 in the presence of catalytic amounts of titanium oxide loaded SiO2-based phase-boundary (148) was used to investigate oxidation of various hydrophobic alcohols, especially relatively bulky alcohols with their oxidation limited by well-known titanium-containing microporous materials due to their limitations of pore size (Table 119) [160,161]. Primary benzylic alcohols were efficiently converted into corresponding aldehydes. The rate of their oxidation depended not only on substituents (–Cl, –OCH3, and –CH3) but also on their positions (p-, m-, and o-) (Entries 1–6). Correspondingly, in relatively high selectivity, secondary benzylic alcohols were oxidized to corresponding ketones (Entries 7 and 8).

Table 119.
Oxidation of various alcohols catalyzed by w/o-Ti-SiO2 148 using H2O2 as an oxidant a.
2.4.3. Dichloromethane-Water
A tetrameric DABCO–bromine complex (149) as a novel active bromine complex was introduced for the fast oxidation of allylic, benzylic, primary, and secondary alcohols to carbonyl compounds with excellent yields and without overoxidation (Table 120) [27].

Table 120.
Oxidation of alcohols to aldehydes and ketones with the DABCO-bromine complex 149 a.
3. Conclusions
In this review, an attempt has been made to summarize some efficient catalytic systems for the selective oxidation of primary and secondary alcoholic OH groups to carbonyl or carboxyl functional groups, using H2O2 as the green oxidant. Different chemical environments such as an aqueous media, a solvent-free system, and various organic solvent and dual-phase systems in the present of hydrogen peroxide oxidant indicated good selectivity and in most cases no overoxidation to acid were observed. Moreover, in many articles, electron-donating were compared with electron-withdrawing functional groups in benzyl alcohol derivative oxidations. In most literature, it was found that there were different yields and conversions of primary and secondary benzylic alcohols with electron-donating and -withdrawing functional groups. It was also shown that electron-donating or -withdrawing functional groups had the same effect on the oxidation results of many researches. In light of the above, it is hoped that this review can assist as a valuable critical overview of the area and the contributions help in further research in this field.
Author Contributions
This work has been completed in continuation of M.M.H., N.G. and E.H. activities in the field of oxidation of alcohols. All authors have read and agreed to the published version of the manuscript.
Funding
This work supported by five year granted research chair by INSF as well as Alzahra Universoty Research Council.
Acknowledgments
The authors are thankful to Alzahra University research Council for partial financial assistance. MMH also appreciates financial support from Iran National Science Foundation (INSF), granted via an individual granted research chair.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| PCC | pyridinium chlorochromate |
| BzOH | benzyl alcohol |
| BzH | benzaldehyde |
| RSM | response surface methodology |
| PTA | phosphotungstic acid |
| APTES | 3-aminopropyltriethoxysilane |
| TLC | thin layer chromatography |
| MNPs | magnetic nanoparticles |
| EPI | epichlorohydrin |
| MEG | ethylene glycol |
| EPR | electron paramagnetic resonance spectroscopy |
| TEA | trimethylamine |
| TBHP | tert-butyl hydroperoxide |
| Fe-BTC | iron(III)-benzenetricarboxylate |
| BET | Brunauer–Emmett–Teller |
| TEDA | 1,4-diazabicyclo[2.2.2]octane |
| OKR | oxidative kinetic resolution |
| POM | Polyoxometalate |
| AC | activated carbon |
| ABTSC | 1-(4-dimethylaminobenzylidene) thiosemicarbazide |
| TCTS | 1-(2-pyridincarboxyl-idene) thiosemicarbazide |
| TEOS | tetraethyl orthosilicate |
| TBA | tetra-n-butylammonium |
| PVP | polyvinylpolypyrrolidone |
| VPO | vanadium phosphorus oxide |
| DMAc | dimethylacetamide |
| UHP | urea-hydrogen peroxide |
| PEGDME250 | polyethylene glycol dimethyl ether 250 |
| TBAHSO4 | tetrabutyl ammonium hydrogen sulfate |
| PTC | phase transfer catalyst |
| TBADT | tetrabutylammonium decatungstate |
| DABCO | 1,4-diazabicyclo[2.2.2] octane |
References
- Chen, G.; Zhou, Y.; Long, Z.; Wang, X.; Li, J.; Wang, J. Mesoporous polyoxometalate-based ionic hybrid as a triphasic catalyst for oxidation of benzyl alcohol with H2O2 on water. ACS Appl. Mater. Interfaces 2014, 6, 4438–4446. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Principles of green chemistry. In Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Sheldon, R.A.; Arends, I.; Dijksman, A. New developments in catalytic alcohol oxidations for fine chemicals synthesis. Catal. Today 2000, 57, 157–166. [Google Scholar] [CrossRef]
- Lee, D.G.; Spitzer, U.A. Aqueous dichromate oxidation of primary alcohols. J. Org. Chem. 1970, 35, 3589–3590. [Google Scholar] [CrossRef]
- Trost, B.M. The atom economy--a search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]
- Carey, J.S.; Laffan, D.; Thomson, C.; Williams, M.T. Analysis of the reactions used for the preparation of drug candidate molecules. Org. Biomol. Chem. 2006, 4, 2337–2347. [Google Scholar] [CrossRef]
- Parmeggiani, C.; Cardona, F. Transition metal based catalysts in the aerobic oxidation of alcohols. Green Chem. 2012, 14, 547–564. [Google Scholar] [CrossRef]
- Goti, A.; Cardona, F. Hydrogen peroxide in green oxidation reactions: Recent catalytic processes. In Green Chemical Reactions; Springer: Berlin/Heidelberg, Germany, 2008; pp. 191–212. [Google Scholar]
- Jones, C. Applications of Hydrogen Peroxide and Derivatives; Royal Society of Chemistry: Cambridge, UK, 1999; Volume 5, pp. 65–69. [Google Scholar]
- Strukul, G. Catalytic Oxidations with Hydrogen Peroxide as Oxidant; Kluwer Academic: Dordrecht, The Netherlands, 1992. [Google Scholar]
- Sato, K.; Hyodo, M.; Aoki, M.; Zheng, X.-Q.; Noyori, R. Oxidation of sulfides to sulfoxides and sulfones with 30% hydrogen peroxide under organic solvent-and halogen-free conditions. Tetrahedron 2001, 57, 2469–2476. [Google Scholar] [CrossRef]
- Dumitriu, E.; Guimon, C.; Cordoneanu, A.; Casenave, S.; Hulea, T.; Chelaru, C.; Martinez, H.; Hulea, V. Heterogeneous sulfoxidation of thioethers by hydrogen peroxide over layered double hydroxides as catalysts. Catal. Today 2001, 66, 529–534. [Google Scholar] [CrossRef]
- Noyori, R.; Aoki, M.; Sato, K. Green oxidation with aqueous hydrogen peroxide. Chem. Commun. 2003, 16, 1977–1986. [Google Scholar] [CrossRef]
- Dixneuf, P.; Cadierno, V. Metal-Catalyzed Reactions in Water; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Guajardo, N.; Carlesi, C.; Schrebler, R.; Morales, J. Applications of liquid/liquid biphasic oxidations by hydrogen peroxide with ionic liquids or deep eutectic solvents. ChemPlusChem 2017, 82, 165–176. [Google Scholar] [CrossRef]
- Ahmad, J.U.; Räisänen, M.T.; Leskelä, M.; Repo, T. Copper catalyzed oxidation of benzylic alcohols in water with H2O2. Appl. Catal A. Gen. 2012, 411, 180–187. [Google Scholar] [CrossRef]
- Ahmad, J.U.; Figiel, P.J.; Räisänen, M.T.; Leskelä, M.; Repo, T. Aerobic oxidation of benzylic alcohols with bis (3, 5-di-tert-butylsalicylaldimine) copper (II) complexes. Appl. Catal A. Gen. 2009, 371, 17–21. [Google Scholar] [CrossRef]
- Figiel, P.J.; Leskelä, M.; Repo, T. TEMPO-Copper (II) Diimine-Catalysed Oxidation of Benzylic Alcohols in Aqueous Media. Adv. Synth. Catal 2007, 349, 1173–1179. [Google Scholar] [CrossRef]
- Heravi, M.M.; Ajami, D.; Ghassemzadeh, M. Wet alumina supported chromium (VI) oxide: A mild, efficient and inexpensive reagent for oxidative deprotection of trimethylsilyl and tetrahydropyranyl ethers in a solventless system. Synthesis 1999, 1999, 393–394. [Google Scholar] [CrossRef]
- Heravi, M.M.; Behbahani, F.K.; Oskooie, H.A.; Shoar, R.H. Catalytic aromatization of Hantzsch 1,4-dihydropyridines by ferric perchlorate in acetic acid. Tetrahedron Lett. 2005, 46, 2775–2777. [Google Scholar] [CrossRef]
- Heravi, M.M.; Abdolhosseini, N.; Oskooie, H.A. Regioselective and high-yielding bromination of aromatic compounds using hexamethylenetetramine–bromine. Tetrahedron Lett. 2005, 46, 8959–8963. [Google Scholar] [CrossRef]
- Heravi, M.M.; Behbahani, F.K.; Oskooie, H.A.; Shoar, R.H. Mild and efficient tetrahydropyranylation of alcohols and dehydropyranylation of THP ethers catalyzed by ferric perchlorate. Tetrahedron Lett. 2005, 46, 2543–2545. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Tavakoli, N. N-oxidation of pyridine carboxylic acids using hydrogen peroxide catalyzed by a green heteropolyacid catalyst: Preyssler’s anion,[NaP5W30O110] 14−. J. Mol. Catal A Chem. 2006, 252, 219–225. [Google Scholar] [CrossRef]
- Bamoharram, F.F.; Heravi, M.M.; Roshani, M.; Akbarpour, M. Catalytic performance of Preyssler heteropolyacid as a green and recyclable catalyst in oxidation of primary aromatic amines. J. Mol. Catal A Chem. 2006, 255, 193–198. [Google Scholar] [CrossRef]
- Heravi, M. ‘Zeofen’, a user-friendly oxidizing reagent. Chem. Commun. 1999, 9, 833–834. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V.; Bakhtiari, K.; Oskooie, H.A.; Bamoharram, F.F. Green and reusable heteropolyacid catalyzed oxidation of benzylic, allylic and aliphatic alcohols to carbonyl compounds. Catal. Commun. 2007, 8, 315–318. [Google Scholar] [CrossRef]
- Heravi, M.M.; Derikvand, F.; Ghassemzadeh, M.; Neumüller, B. Synthesis, characterization and structure of a tetrameric DABCO–bromine complex: A novel oxidizing agent for oxidation of alcohols to carbonyl compounds. Tetrahedron Lett. 2005, 46, 6243–6245. [Google Scholar] [CrossRef]
- M Heravi, M.; Fazeli, A.; Faghihi, Z. Recent Advances in Application of Pyridinium Chlorochromate (PCC) in Organic Synthesis. Curr. Org. Synth. 2016, 13, 220–254. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, C.; Chen, R.; Chen, F. Selective oxidation of benzyl alcohol to benzaldehyde with H2O2 in water on epichlorohydrin-modified Fe 3 O 4 microspheres. New J. Chem. 2015, 39, 4924–4932. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, J.; Huang, X.; Chen, F. One-step nonaqueous synthesis of modified magnetic Fe3O4 microspheres by using epichlorohydrin as functional solvent. Chem. Lett. 2018, 48, 22–25. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, S.; Chen, R.; Chen, F. Promotional effect of short-chain saturated alcohols on Fe3O4-catalyzed decomposition of H2O2 and its application in selective oxidation of benzyl alcohol. J. Chem. Technol. Biot. 2019, 94, 1613–1621. [Google Scholar] [CrossRef]
- Hajavazzade, R.; Kargarrazi, M.; Mahjoub, A.R. Phosphotungstic Acid Supported on MgAl2O4 Nanoparticles as an Efficient and Reusable Nanocatalyst for Benzylic Alcohols Oxidation with Hydrogen Peroxide. J. Inorg. Organomet. P 2019, 29, 1–10. [Google Scholar] [CrossRef]
- Farsani, M.R.; Yadollahi, B. Synthesis, characterization and catalytic performance of a Fe polyoxometalate/silica composite in the oxidation of alcohols with hydrogen peroxide. J. Mol. Catal A Chem. 2014, 392, 8–15. [Google Scholar] [CrossRef]
- Patel, P.; Nandi, S.; Menapara, T.; Biradar, A.V.; Nagarale, R.K.; Khan, N.H.; Kureshy, R.I. Glycoluril: A heterogeneous organocatalyst for oxidation of alcohols and benzylic sp3 carbons. App. Catal. A. Gen. 2018, 565, 127–134. [Google Scholar] [CrossRef]
- Darvishi, K.; Amani, K.; Rezaei, M. Preparation, characterization and heterogeneous catalytic applications of GO/Fe3O4/HPW nanocomposite in chemoselective and green oxidation of alcohols with aqueous H2O2. Appl. Organomet. Chem. 2018, 32, e4323. [Google Scholar] [CrossRef]
- Hao, P.; Zhang, M.; Zhang, W.; Tang, Z.; Luo, N.; Tan, R.; Yin, D. Polyoxometalate-based Gemini ionic catalysts for selective oxidation of benzyl alcohol with hydrogen peroxide in water. Catal. Sci. Technol. 2018, 8, 4463–4473. [Google Scholar] [CrossRef]
- Xiaoxiang, H.; Yingying, K.; Chunhua, X.; Xiujuan, T.; Qing, C.; Kuiwu, W.; Chin-Te, H.; Li-Li, L.; Shang-Bin, L. Heteropoly Tungstate Supported on Metal Oxide Catalysts for Liquid Phase Oxidation of Benzyl Alcohol with Hydrogen Peroxide. J. Braz. Chem. Soc. 2018, 29, 88–98. [Google Scholar] [CrossRef]
- Ünver, H. Crystal structure of an alkoxide bridged dinuclear copper (II) complex: Mild and selective oxidation of primary and secondary alcohols in water. Transit. Met. Chem. 2018, 43, 641–646. [Google Scholar] [CrossRef]
- Hashemian, S.; Sedrpoushan, A.; Eshbala, F.H. Co-Zeolite Imidazolate Frameworks (ZIF-9@ Zeolite) as Heterogen Catalyst for Alcohols Oxidation. Catal. Lett. 2017, 147, 196–203. [Google Scholar] [CrossRef]
- Bhattacharjee, R.R.; Mal, S.S. A Liquid Derivative of Phosphotungstic Acid as Catalyst for Benzyl Alcohol Oxidation in Water: Facile Separation and Stability of Benzaldehyde at Room Temperature. ChemistrySelect 2017, 2, 4368–4375. [Google Scholar] [CrossRef]
- Kai, O.; Xiaoxiang, H.; Chunhua, X.; Xiujuan, T.; Qing, C. Water-soluble heteropolyacid-based ionic liquids as effective catalysts for oxidation of benzyl alcohol in water with hydrogen peroxide. Green Process. Synth. 2017, 6, 385–395. [Google Scholar] [CrossRef]
- Han, X.; Kuang, Y.; Xiong, C.; Tang, X.; Chen, Q.; Hung, C.-T.; Liu, L.-L.; Liu, S.-B. Heterogeneous amino acid-based tungstophosphoric acids as efficient and recyclable catalysts for selective oxidation of benzyl alcohol. Korean J. Chem. Eng. 2017, 34, 1914–1923. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Li, X.; Zhao, J.; Bai, S.; Liu, J.; Yang, Q. A yolk–shell nanoreactor with a basic core and an acidic shell for cascade reactions. Angew. Chem. Int. Ed. 2012, 51, 9164–9168. [Google Scholar] [CrossRef]
- Yan, Q.; Fang, Y.C.; Jia, Y.X.; Duan, X.H. Chemoselective hydrogen peroxide oxidation of primary alcohols to aldehydes by a water-soluble and reusable iron (III) catalyst in pure water at room temperature. New J. Chem. 2017, 41, 2372–2377. [Google Scholar] [CrossRef]
- Fu, H.; Hu, C.; Huang, Z.; Zhou, J.; Peng, X. Ammonium Tungstate as an Effective Catalyst for Selective Oxidation of Alcohols to Aldehydes or Ketones with Hydrogen Peroxide under Water–A Synergy of Graphene Oxide. Synlett 2018, 29, 447–451. [Google Scholar]
- Mirsafaei, R.; Heravi, M.M.; Hosseinnejad, T.; Ahmadi, S. Copper (II) nanoparticles: An efficient and reusable catalyst in green oxidation of benzyl alcohols to benzaldehydes in water. Appl. Organomet. Chem 2016, 30, 823–830. [Google Scholar] [CrossRef]
- Hosseini Eshbala, F.; Mohanazadeh, F.; Sedrpoushan, A. Tungstate ions (WO4=) supported on imidazolium framework as novel and recyclable catalyst for rapid and selective oxidation of benzyl alcohols in the presence of hydrogen peroxide. Appl. Organomet. Chem 2017, 31, e3597. [Google Scholar] [CrossRef]
- Wang, H.; Fang, L.; Yang, Y.; Hu, R.; Wang, Y. Immobilization Na7PW11O39 on quanternary ammonium functionalized chloromethylated polystyrene by electrostatic interactions: An efficient recyclable catalyst for alcohol oxidation. App. Catal A Gen 2016, 520, 35–43. [Google Scholar] [CrossRef]
- Sloboda-Rozner, D.; Witte, P.; Alsters, P.L.; Neumann, R. Aqueous Biphasic Oxidation: A Water-Soluble Polyoxometalate Catalyst for Selective Oxidation of Various Functional Groups with Hydrogen Peroxide. Adv. Synth. Catal. 2004, 346, 339–345. [Google Scholar] [CrossRef]
- Sadri, F.; Ramazani, A.; Ahankar, H.; Taghavi Fardood, S.; Azimzadeh Asiabi, P.; Khoobi, M.; Woo Joo, S.; Dayyani, N. Aqueous-phase oxidation of alcohols with green oxidants (Oxone and hydrogen peroxide) in the presence of MgFe2O4 magnetic nanoparticles as an efficient and reusable catalyst. J. Nanostruct. 2016, 6, 264–272. [Google Scholar]
- Emayavaramban, P.; Babu, S.G.; Karvembu, R.; Kadirvelu, K.; Dharmaraj, N. Gold Nanoparticles Supported on Magnesium Oxide Nanorods for Oxidation of Alcohols. J. Nanosci. Nanotechno. 2016, 16, 2517–2526. [Google Scholar] [CrossRef]
- Cruz, P.; Pérez, Y.; del Hierro, I.; Fajardo, M. Copper, copper oxide nanoparticles and copper complexes supported on mesoporous SBA-15 as catalysts in the selective oxidation of benzyl alcohol in aqueous phase. Microporous Mesoporous Mater. 2016, 220, 136–147. [Google Scholar] [CrossRef]
- Kalbasi, R.J.; Nourbakhsh, A.A.; Zia, M. Aerobic Oxidation of Alcohols Catalyzed by Copper Nanoparticle-Polyacrylamide/SBA-15 as Novel Polymer-Inorganic Hybrid. J. Inorg. Organomet. P 2012, 22, 536–542. [Google Scholar] [CrossRef]
- Valodkar, V.; Tembe, G.; Ravindranathan, M.; Ram, R.; Rama, H. Catalytic oxidation by polymer-supported copper (II)–l-valine complexes. J. Mol. Catal. A Chem. 2004, 208, 21–32. [Google Scholar] [CrossRef]
- Fan, J.; Pu, F.; Sun, M.; Liu, Z.-W.; Han, X.-Y.; Wei, J.-F.; Shi, X.-Y. Immobilized bis-layered ionic liquids/peroxotungstates as an efficient catalyst for selective oxidation of alcohols in neat water. New J. Chem. 2016, 40, 10498–10503. [Google Scholar] [CrossRef]
- Zohreh, N.; Tavakolizadeh, M.; Hosseini, S.H.; Jahani, M.; Pourjavadi, A.; Bennett, C. Highly dispersible bis-imidazolium/WO 4 2− modified magnetic nanoparticles: A heterogeneous phase transfer catalyst for green and selective oxidations. New J. Chem. 2016, 40, 10325–10332. [Google Scholar] [CrossRef]
- Tong, J.; Su, L.; Li, W.; Wang, W.; Ma, H.; Wang, Q. Hybrids of [C4mim] 3+ xPMo12− xVxO40: A new catalyst for oxidation of benzyl alcohol to benzaldehyde in water with greatly improved performances. Polyhedron 2016, 115, 282–287. [Google Scholar] [CrossRef]
- Yao, X.; Bai, C.; Chen, J.; Li, Y. Efficient and selective green oxidation of alcohols by MOF-derived magnetic nanoparticles as a recoverable catalyst. RSC Adv. 2016, 6, 26921–26928. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X.; Yao, X.; Fang, Y.; Chen, H.; Ji, H. β-cyclodextrin grafted on lignin as inverse phase transfer catalyst for the oxidation of benzyl alcohol in H2O. Tetrahedron 2016, 72, 1773–1781. [Google Scholar] [CrossRef]
- Farsani, M.R.; Assady, E.; Jalilian, F.; Yadollahi, B.; Rudbari, H.A. Green oxidation of alcohols with hydrogen peroxide catalyzed by a tetra-cobalt polyoxometalate in water. J. Iran. Chem. Soc. 2015, 12, 1207–1212. [Google Scholar] [CrossRef]
- Long, N.Q.; Quan, N.A. Highly selective oxidation of benzyl alcohol to benzaldehyde catalyzed by nano Au/γ-Al2O3 under environment-friendly conditions. React. Kinet. Mech. Catal. 2015, 114, 147–155. [Google Scholar] [CrossRef]
- Hong, Y.; Yan, X.; Liao, X.; Li, R.; Xu, S.; Xiao, L.; Fan, J. Platinum nanoparticles supported on Ca (Mg)-zeolites for efficient room-temperature alcohol oxidation under aqueous conditions. Chem. Commun. 2014, 50, 9679–9682. [Google Scholar] [CrossRef]
- Su, H.; Yang, C. Selective oxidation of benzyl alcohol catalyzed by (TEAH) nH3-nPW12O40 and its reaction mechanism. Chin. J. Catal. 2014, 35, 1224–1234. [Google Scholar] [CrossRef]
- Peterson, B.M.; Herried, M.E.; Neve, R.L.; McGaff, R.W. Oxidation of primary and secondary benzylic alcohols with hydrogen peroxide and tert-butyl hydroperoxide catalyzed by a “helmet” phthalocyaninato iron complex in the absence of added organic solvent. Dalton Trans. 2014, 43, 17899–17903. [Google Scholar] [CrossRef]
- Sadri, F.; Ramazani, A.; Massoudi, A.; Khoobi, M.; Tarasi, R.; Shafiee, A.; Azizkhani, V.; Dolatyari, L.; Joo, S.W. Green oxidation of alcohols by using hydrogen peroxide in water in the presence of magnetic Fe3O4 nanoparticles as recoverable catalyst. Green Chem. Lett. Rev. 2014, 7, 257–264. [Google Scholar] [CrossRef]
- Mu, B.; Liu, P.; Dong, Y.; Lu, C.; Wu, X. Superparamagnetic pH-sensitive multilayer hybrid hollow microspheres for targeted controlled release. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3135–3144. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Z.; Lei, Y.; Du, Y.; Li, H.; Yang, H.; Nie, Y.; Ma, J. Highly selective oxidation of alcohols catalyzed by Cu (II)-Schiff base-SBA-15 with hydrogen peroxide in water. J. Porous Mater. 2013, 20, 277–284. [Google Scholar] [CrossRef]
- Atashin, H.; Malakooti, R. Magnetic iron oxide nanoparticles embedded in SBA-15 silica wall as a green and recoverable catalyst for the oxidation of alcohols and sulfides. J. Saudi Chem. Soc. 2017, 21, S17–S24. [Google Scholar] [CrossRef]
- Davis, S.E.; Ide, M.S.; Davis, R.J. Selective oxidation of alcohols and aldehydes over supported metal nanoparticles. Green Chem. 2013, 15, 17–45. [Google Scholar] [CrossRef]
- Wei, Q.; James, S.L. A metal–organic gel used as a template for a porous organic polymer. Chem. Commun. 2005, 3, 1555–1556. [Google Scholar] [CrossRef]
- Hosseini-Monfared, H.; Näther, C.; Winkler, H.; Janiak, C. Highly selective and “green” alcohol oxidations in water using aqueous 10% H2O2 and iron-benzenetricarboxylate metal–organic gel. Inorg. Chim. Acta 2012, 391, 75–82. [Google Scholar] [CrossRef]
- Kharat, A.N.; Bakhoda, A.; Jahromi, B.T. Green and chemoselective oxidation of alcohols with hydrogen peroxide: A comparative study on Co (II) and Co (III) activity toward oxidation of alcohols. Polyhedron 2011, 30, 2768–2775. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Ma, B.; Ding, Y.; Qiu, W. Oxidation of alcohols with hydrogen peroxide in water catalyzed by recyclable keggin-type tungstoborate catalyst. Catal. Commun. 2010, 11, 527–531. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, Y.; Ding, Y.; Zhao, W. A water-soluble dilacunary silicotungstate as an effective catalyst for oxidation alcohols in water with hydrogen peroxide. Catal. Commun. 2010, 11, 853–857. [Google Scholar] [CrossRef]
- Tundo, P.; Romanelli, G.P.; Vázquez, P.G.; Aricò, F. Multiphase oxidation of alcohols and sulfides with hydrogen peroxide catalyzed by heteropolyacids. Catal. Commun. 2010, 11, 1181–1184. [Google Scholar] [CrossRef]
- Maity, P.; Bhaduri, S.; Lahiri, G.K. A water soluble heteropolyoxotungstate as a selective, efficient and environment friendly oxidation catalyst. J. Chem. Sci. 2009, 121, 377–385. [Google Scholar] [CrossRef][Green Version]
- Sato, K.; Aoki, M.; Takagi, J.; Noyori, R. Organic solvent-and halide-free oxidation of alcohols with aqueous hydrogen peroxide. J. Amer. Chem. Soc. 1997, 119, 12386–12387. [Google Scholar] [CrossRef]
- Sato, K.; Takagi, J.; Aoki, M.; Noyori, R. Hydrogen peroxide oxidation of benzylic alcohols to benzaldehydes and benzoic acids under halide-free conditions. Tetrahedron Lett. 1998, 39, 7549–7552. [Google Scholar] [CrossRef]
- Jia, A.; Lou, L.-L.; Zhang, C.; Zhang, Y.; Liu, S. Selective oxidation of benzyl alcohol to benzaldehyde with hydrogen peroxide over alkali-treated ZSM-5 zeolite catalysts. J. Mol. Catal A. Chem 2009, 306, 123–129. [Google Scholar] [CrossRef]
- Tayebee, R.; Alizadeh, M.H. Water as an efficient solvent for oxygenation transformations with 34% hydrogen peroxide catalyzed by some heteropolyoxometalates. Monatsh. Chem. 2007, 138, 763–769. [Google Scholar] [CrossRef]
- Hulce, M.; Marks, D.W. Organic-solvent-free phase-transfer oxidation of alcohols using hydrogen peroxide. J. Chem. Educ. 2001, 78, 66. [Google Scholar] [CrossRef]
- Lampman, G.M.; Sharpe, S.D. A phase transfer catalyzed permanganate oxidation: Preparation of vanillin from isoeugenol acetate. J. Chem. Educ. 1983, 60, 503. [Google Scholar] [CrossRef]
- Naeimi, A.; Honarmand, M.; Sedri, A. Ultrasonic assisted fabrication of first MoO3/copper complex bio-nanocomposite based on Sesbania sesban plant for green oxidation of alcohols. Ultrason. Sonochem. 2019, 50, 331–338. [Google Scholar] [CrossRef]
- Boruah, J.J.; Das, S.P. Solventless, selective and catalytic oxidation of primary, secondary and benzylic alcohols by a Merrifield resin supported molybdenum (vi) complex with H2O2 as an oxidant. RSC Adv. 2018, 8, 34491–34504. [Google Scholar] [CrossRef]
- Dimitratos, N.; Lopez-Sanchez, J.A.; Morgan, D.; Carley, A.F.; Tiruvalam, R.; Kiely, C.J.; Bethell, D.; Hutchings, G.J. Solvent-free oxidation of benzyl alcohol using Au–Pd catalysts prepared by sol immobilisation. PCCP 2009, 11, 5142–5153. [Google Scholar] [CrossRef]
- Tareq, S.S.; Saiman, M.I.; Hin, T.-Y.Y.; Abdullah, A.H.; Rashid, U. The impact of hydrogen peroxide as an oxidant for solvent-free liquid phase oxidation of benzyl alcohol using Au-Pd supported carbon and titanium catalysts. Bull. Chem. React. Eng. 2018, 13, 373–385. [Google Scholar] [CrossRef]
- Farhadi, S.; Hakimi, M.; Maleki, M. 12-Molybdophosphoric acid anchored on aminopropylsilanized magnetic graphene oxide nanosheets (Fe3O4/GrOSi(CH2)3–NH2/H3 PMo12O40): A novel magnetically recoverable solid catalyst for H2O2-mediated oxidation of benzylic alcohols under solvent-free conditions. RSC Adv. 2018, 8, 6768–6780. [Google Scholar]
- D Kurbah, S.; Kumar, A.; Shangpung, S.; Asthana, M.; Syiemlieh, I.; A Lal, R. Oxidation of Alcohols by Hydrogen Peroxide Catalyzed by Trinuclear Copper (II) Complex [Cu3(slmh)(μ-Cl)2(CH3OH)3]. 0.5 CH3OH derived from Disalicylaldehyde Malonoyldihydrazone. Curr. Organocatalysis 2017, 4, 62–68. [Google Scholar] [CrossRef]
- Malakooti, R.; Feghhi, A. MoO x–pyridine organic–inorganic hybrid wires as a reusable and highly selective catalyst for the oxidation of alcohols: A comparison study between reaction-controlled phase-transfer catalysis and heterogeneous catalysis. New J. Chem. 2017, 41, 3405–3413. [Google Scholar] [CrossRef]
- D Kurbah, S.; Kumar, A.; Asthana, M.; Shangpung, S.; Koch, A.; A Lal, R. Water Soluble Heterobimetallic Complex [CuZn (bz)3(bpy)2] BF4 a Catalyzed Selective Oxidation of Alcohols to Aldehydes Mediated by Hydrogen Peroxide in Aqueous Medium in the Absence of a Base and Co-Catalyst. Curr. Organocatalysis 2016, 3, 45–51. [Google Scholar] [CrossRef]
- Saeednia, S.; Ardakani, M.H.; Pakdin-Parizi, Z.; Iranmanesh, P.; Sinaei, S. Solvent-free chemoselective oxidation of alcohols by hydrogen peroxide using a new synthesized copper complex as reusable heterogeneous nanocatalyst. J. Iran. Chem. Soc. 2016, 13, 1963–1975. [Google Scholar] [CrossRef]
- Saxena, S.K.; Viswanadham, N.; Ala’a, H. Enhanced selective oxidation of benzyl alcohol to benzaldehyde on mesopore created mordenite catalyst. J. Porous Mater. 2016, 23, 1671–1678. [Google Scholar] [CrossRef]
- Wang, B.; Hu, Y.; Fang, D.; Wu, L.; Xing, R. Efficient and Reusable Sn (II)-containing Imidazolium-based Ionic Liquid as a Catalyst for the Oxidation of Benzyl Alcohol. J. Chin. Chem. Soc. 2016, 63, 991–999. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Bagherzadeh, M.; Karimi, H. Preparation, characterization and catalytic activity of CoFe2O4 nanoparticles as a magnetically recoverable catalyst for selective oxidation of benzyl alcohol to benzaldehyde and reduction of organic dyes. J. Colloid Interface Sci. 2016, 465, 271–278. [Google Scholar] [CrossRef]
- Trakarnpruk, W. Mn-and Co-substituted polyoxotungstates on MCM-41 and layered double hydroxide as catalysts in the oxidation of benzyl alcohol and cyclohexane. Mendeleev. Commun. 2016, 3, 256–258. [Google Scholar] [CrossRef]
- Varadwaj, G.B.B.; Rana, S.; Parida, K. Cs salt of Co substituted lacunary phosphotungstate supported K10 montmorillonite showing binary catalytic activity. Biochem. Eng. J. 2013, 215, 849–858. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, J.; Pan, J.; Sun, F.a.; He, M.; Chen, Q. Effect of Mg2+ on the catalytic activities of CoMgAl hydrotalcites in the selective oxidation of benzyl alcohol to benzaldehyde. Catal. Commun. 2015, 69, 1–4. [Google Scholar] [CrossRef]
- Hasannia, S.; Yadollahi, B. Zn–Al LDH nanostructures pillared by Fe substituted Keggin type polyoxometalate: Synthesis, characterization and catalytic effect in green oxidation of alcohols. Polyhedron 2015, 99, 260–265. [Google Scholar] [CrossRef]
- Li, X.; Cao, R.; Lin, Q. Selective oxidation of alcohols with H2O2 catalyzed by long chain multi-SO3H functionalized heteropolyanion-based ionic liquids under solvent-free conditions. Catal. Commun. 2015, 69, 5–10. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Yang, Y.; Cao, X.-H.; Lu, M. Highly selective oxidation of alcohols with hydrogen peroxide and polyethylene oxide-supported long-chain imidazolium polyoxometalate hybrid catalyst. J. Iran. Chem. Soc. 2015, 12, 1765–1770. [Google Scholar] [CrossRef]
- Judy-Azar, A.-R.; Mohebbi, S. A novel magnetic hybrid nanomaterial as a highly efficient and selective catalyst for alcohol oxidation based on new Schiff base complexes of transition metal ions. J. Mol. Catal A Chem. 2015, 397, 158–165. [Google Scholar] [CrossRef]
- Han, M.-K.; Kim, S.; Kim, S.T.; Lee, J.C. Bismuth Tribromide Catalyzed Oxidation of Alcohols with Aqueous Hydrogen Peroxide. Synlett 2015, 26, 2434–2436. [Google Scholar]
- Wu, G.; Wang, X.; Liu, X.; Ding, K.; Zhang, F.; Zhang, X. Environmental benign oxidation of benzyl alcohol catalyzed by sulphonato-salphen–chromium (III) complexes immobilized on MCM-41. Catal. Lett. 2014, 144, 364–371. [Google Scholar] [CrossRef]
- Pathan, S.; Patel, A. Solvent free clean selective oxidation of alcohols catalyzed by mono transition metal (Co, Mn, Ni)-substituted Keggin-phosphomolybdates using hydrogen peroxide. App. Catal A. Gen. 2013, 459, 59–64. [Google Scholar] [CrossRef]
- Jing, L.; Shi, J.; Zhang, F.; Zhong, Y.; Zhu, W. Polyoxometalate-based amphiphilic catalysts for selective oxidation of benzyl alcohol with hydrogen peroxide under organic solvent-free conditions. Ind. Eng. Chem. Res. 2013, 52, 10095–10104. [Google Scholar] [CrossRef]
- Zamani, F.; Izadi, E. Synthesis and Characterization of Copper (II)–Cysteine/SiO2–Al2O3 as an Efficient and Reusable Heterogeneous Catalyst for the Oxidation of Aromatic Alcohols. J. Inorg. Organomet. P 2013, 23, 1501–1510. [Google Scholar] [CrossRef]
- Borthakur, R.; Asthana, M.; Kumar, A.; Koch, A.; Lal, R. Solvent free selective oxidation of alcohols catalyzed by a trinuclear complex with a dicopper (II)–monozinc (II) centre using hydrogen peroxide as an oxidant. RSC Adv. 2013, 3, 22957–22962. [Google Scholar] [CrossRef]
- Zhou, X.-T.; Ji, H.-B.; Liu, S.-G. Solvent-free selective oxidation of primary and secondary alcohols catalyzed by ruthenium-bis (benzimidazole) pyridinedicarboxylate complex using hydrogen peroxide as an oxidant. Tetrahedron Lett. 2013, 54, 3882–3885. [Google Scholar] [CrossRef][Green Version]
- Tian, W.; Hou, Y.; Wang, X.; Lu, B.; Zhao, J.; Cai, Q. A Simple Polyoxometallate for Selective Oxidation of Benzyl Alcohol to Benzaldehyde with Hydrogen Peroxide. Chin. J. Chem. 2012, 30, 433–437. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Arunrao, A.S.; Narayan, M.P.; Kumar, S.S.; Kumar, S.S.; Bhagat, P.R. Selective oxidation of alcohol to carbonyl compound catalyzed by l-aspartic acid coupled imidazolium based ionic liquid. J. Mol. Liq. 2012, 173, 180–183. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, B.; Wang, X.; Zhao, J.; Wang, X.; Cai, Q. Highly selective oxidation of benzyl alcohol to benzaldehyde with hydrogen peroxide by biphasic catalysis. Biochem. Eng. J. 2010, 162, 738–742. [Google Scholar] [CrossRef]
- Cui, S.; Lu, B.; Xiao, X.; Han, Y.; Cai, Q. Synthesis of diphenylmethane from benzene and paraformaldehyde catalyzed by acidic ionic liquids. Catal. Lett. 2007, 119, 277–282. [Google Scholar] [CrossRef]
- Wang, X.; Wu, G.; Wei, W.; Sun, Y. Solvent-free oxidation of alcohols by hydrogen peroxide over chromium Schiff base complexes immobilized on MCM-41. Transit. Met. Chem. 2010, 35, 213–220. [Google Scholar] [CrossRef]
- Biradar, A.V.; Dongare, M.K.; Umbarkar, S.B. Selective oxidation of aromatic primary alcohols to aldehydes using molybdenum acetylide oxo-peroxo complex as catalyst. Tetrahedron Lett. 2009, 50, 2885–2888. [Google Scholar] [CrossRef]
- Shi, F.; Tse, M.K.; Pohl, M.-M.; Radnik, J.; Brückner, A.; Zhang, S.; Beller, M. Nano-iron oxide-catalyzed selective oxidations of alcohols and olefins with hydrogen peroxide. J. Mol. Catal A. Chem 2008, 292, 28–35. [Google Scholar] [CrossRef]
- Wang, X.; Wu, G.; Li, J.; Zhao, N.; Wei, W.; Sun, Y. Surface-modified improvement in catalytic performance of Cr (salen) complexes immobilized on MCM-41 in solvent-free selective oxidation of benzyl alcohol. Catal. Lett. 2007, 119, 87–94. [Google Scholar] [CrossRef]
- Wang, G.; Feng, L.; Luck, R.L.; Evans, D.G.; Wang, Z.; Duan, X. Sol–gel synthesis, characterization and catalytic property of silicas modified with oxomolybdenum complexes. J. Mol. Catal A Chem. 2005, 241, 8–14. [Google Scholar] [CrossRef]
- Ming-Lin, G.; Hui-Zhen, L. Selective oxidation of benzyl alcohol to benzaldehyde with hydrogen peroxide over tetra-alkylpyridinium octamolybdate catalysts. Green Chem. 2007, 9, 421–423. [Google Scholar] [CrossRef]
- Wang, J.; Yan, L.; Qian, G.; Li, S.; Yang, K.; Liu, H.; Wang, X. Na4H3[SiW9Al3(H2O)3O37]·12H2O/H2O: A new system for selective oxidation of alcohols with H2O2 as oxidant. Tetrahedron 2007, 63, 1826–1832. [Google Scholar] [CrossRef]
- Hajian, R.; Jafari, F. Zinc polyoxometalate immobilized on ionic liquid-modified MCM-41: An efficient reusable catalyst for the selective oxidation of alcohols with hydrogen peroxide. J. Iran. Chem. Soc. 2019, 16, 563–570. [Google Scholar] [CrossRef]
- Sedighipoor, M.; Kianfar, A.H.; Mahmood, W.A.K.; Azarian, M.H. Epoxidation of alkenes by an oxidovanadium (IV) tetradentate Schiff base complex as an efficient catalyst with tert-butyl hydroperoxide. Inorg. Chim. Acta 2017, 457, 116–121. [Google Scholar] [CrossRef]
- Adam, M.S.S. Catalytic activity of nickel (II), copper (II) and oxovanadium (II)-dihydroindolone complexes towards homogeneous oxidation reactions. Appl. Organomet. Chem. 2018, 32, e4234. [Google Scholar] [CrossRef]
- Mangin, F.; Prinsen, P.; Yepez, A.; Gilani, M.R.H.S.; Xu, G.; Len, C.; Luque, R. Microwave assisted benzyl alcohol oxidation using iron particles on furfuryl alcohol derived supports. Catal. Commun. 2018, 104, 67–70. [Google Scholar] [CrossRef]
- Chuanfeng, H.; Jianhao, Z.; Zhida, H.; Huihui, F.; Xinhua, P. Metal Molybdate Catalysts for the Selective Oxidation of Olefins and Alcohols Using Hydrogen Peroxide as Oxidant. Chin. J. Org. Chem. 2018, 38, 486–491. [Google Scholar]
- Maleki, M.; Baghbanian, S.M.; Tajbakhsh, M. Heteropolyacid immobilized on polymer/magnetic zeolite nanocomposite as a new and recyclable catalyst for the selective oxidation of alcohols. J. Iran.Chem. Soc. 2018, 15, 359–368. [Google Scholar] [CrossRef]
- Kurbah, S.D.; Asthana, M.; Syiemlieh, I.; Lal, R.A. Peroxidative catalytic oxidation of alcohols catalyzed by heterobinuclear vanadium (V) complexes using H2O2 as terminal oxidizing agents. Appl. Organomet. Chem. 2018, 32, e4299. [Google Scholar] [CrossRef]
- Cánepa, A.L.; Elías, V.R.; Vaschetti, V.M.; Sabre, E.V.; Eimer, G.A.; Casuscelli, S.G. Selective oxidation of benzyl alcohol through eco-friendly processes using mesoporous V-MCM-41, Fe-MCM-41 and Co-MCM-41 materials. App. Catal A. Gen. 2017, 545, 72–78. [Google Scholar] [CrossRef]
- Miao, C.; Li, X.-X.; Lee, Y.-M.; Xia, C.; Wang, Y.; Nam, W.; Sun, W. Manganese complex-catalyzed oxidation and oxidative kinetic resolution of secondary alcohols by hydrogen peroxide. Chem. Sci. 2017, 8, 7476–7482. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kon, Y.; Ogawa, A.; Uesaka, Y.; Tamura, M.; Sato, K. Mixed Picolinate and Quinaldinate Iron (III) Complexes for the Catalytic Oxidation of Alcohols with Hydrogen Peroxide. ChemCatChem 2016, 8, 2930–2938. [Google Scholar] [CrossRef]
- Maleki, A.; Rahimi, R.; Maleki, S. Efficient oxidation and epoxidation using a chromium (VI)-based magnetic nanocomposite. Environ. Chem. Lett. 2016, 14, 195–199. [Google Scholar] [CrossRef]
- Assady, E.; Yadollahi, B.; Riahi Farsani, M.; Moghadam, M. Zinc polyoxometalate on activated carbon: An efficient catalyst for selective oxidation of alcohols with hydrogen peroxide. Appl. Organomet. Chem 2015, 29, 561–565. [Google Scholar] [CrossRef]
- Tayebani, M.; Shafaat, B.; Iravani, M. Hydrogen peroxide oxidation of primary alcohols by thiosemicarbazide Schiff base metal complexes. Iran. J. Catal. 2015, 5, 213–221. [Google Scholar]
- Ragupathi, C.; Vijaya, J.J.; Narayanan, S.; Jesudoss, S.; Kennedy, L.J. Highly selective oxidation of benzyl alcohol to benzaldehyde with hydrogen peroxide by cobalt aluminate catalysis: A comparison of conventional and microwave methods. Ceram. Int. 2015, 41, 2069–2080. [Google Scholar] [CrossRef]
- Prakash, S.; Charan, C.; Singh, A.K.; Shahi, V.K. Mixed metal nanoparticles loaded catalytic polymer membrane for solvent free selective oxidation of benzyl alcohol to benzaldehyde in a reactor. App. Catal B: Environ. 2013, 132, 62–69. [Google Scholar] [CrossRef]
- Szávuly, M.; Szilvási, S.D.; Csonka, R.; Klesitz, D.; Speier, G.; Giorgi, M.; Kaizer, J. Catalytic oxidation of alcohols and sulfides with hydrogen peroxide using isoindoline and phthalazine-based diiron complexes. J. Mol.Catal A. Chem 2014, 393, 317–324. [Google Scholar] [CrossRef]
- Saisaha, P.; Buettner, L.; van der Meer, M.; Hage, R.; Feringa, B.L.; Browne, W.R.; de Boer, J.W. Selective catalytic oxidation of alcohols, aldehydes, alkanes and alkenes employing manganese catalysts and hydrogen peroxide. Adv. Synth. Catal 2013, 355, 2591–2603. [Google Scholar] [CrossRef]
- de Boer, J.W.; Brinksma, J.; Browne, W.R.; Meetsma, A.; Alsters, P.L.; Hage, R.; Feringa, B.L. Cis-dihydroxylation and epoxidation of alkenes by [Mn2O(RCO2)2(tmtacn)2]: Tailoring the selectivity of a highly H2O2-efficient catalyst. J. Am. Chem. Soc. 2005, 127, 7990–7991. [Google Scholar] [CrossRef] [PubMed]
- de Boer, J.W.; Browne, W.R.; Brinksma, J.; Alsters, P.L.; Hage, R.; Feringa, B.L. Mechanism of cis-dihydroxylation and epoxidation of alkenes by highly H2O2 efficient dinuclear manganese catalysts. Inorg. Chem. 2007, 46, 6353–6372. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.J.; Unjaroen, D.; Mecozzi, F.; Harvey, E.C.; Saisaha, P.; Pijper, D.; de Boer, J.W.; Alsters, P.; Feringa, B.L.; Browne, W.R. Manganese-Catalyzed Selective Oxidation of Aliphatic C–H groups and Secondary Alcohols to Ketones with Hydrogen Peroxide. ChemSusChem 2013, 6, 1774–1778. [Google Scholar] [CrossRef]
- Masoudian, S.; Monfared, H.H.; Aghaei, A. Silica aerogel–iron oxide nanocomposites: Recoverable catalysts for the oxidation of alcohols with hydrogen peroxide. Transit. Met. Chem 2011, 36, 521–530. [Google Scholar] [CrossRef]
- Weng, Z.; Liao, G.; Wang, J.; Jian, X. Selective oxidation of benzyl alcohol with hydrogen peroxide over reaction-controlled phase-transfer catalyst. Catal. Commun. 2007, 8, 1493–1496. [Google Scholar] [CrossRef]
- Pillai, U.R.; Sahle-Demessie, E. Selective oxidation of alcohols over vanadium phosphorus oxide catalyst using hydrogen peroxide. App. Catal A. Gen. 2004, 276, 139–144. [Google Scholar] [CrossRef]
- Pillai, U.R.; Sahle-Demessie, E. Oxidation of alcohols over Fe3+/montmorillonite-K10 using hydrogen peroxide. App. Catal A. Gen. 2003, 245, 103–109. [Google Scholar] [CrossRef]
- Veisi, H.; Safarimehr, P.; Hemmati, S. Oxo-vanadium immobilized on polydopamine coated-magnetic nanoparticles (Fe3O4): A heterogeneous nanocatalyst for selective oxidation of sulfides and benzylic alcohols with H2O2. J. Taiwan. Inst. Chem. E 2018, 88, 8–17. [Google Scholar] [CrossRef]
- Kimi, M.; Jaidie, M.M.H.; Pang, S.C. Bimetallic Cu-Ni nanoparticles supported on activated carbon for catalytic oxidation of benzyl alcohol. J. Phys. Chem. Solids 2018, 112, 50–53. [Google Scholar] [CrossRef]
- Sarmah, K.; Pal, J.; Maji, T.K.; Pratihar, S. Magnetically Recoverable Heterobimetallic Co2Mn3O8: Selective and Sustainable Oxidation and Reduction Reactions. Acs Sustain. Chem. Eng. 2017, 5, 11504–11515. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, X.; Wu, P.; Zhang, Y.; Liu, B.; Hu, H.; Xue, G. Divanadium-Substituted Phosphotungstate Supported on Magnetic Mesoporous Silica Nanoparticles as Effective and Recyclable Catalysts for the Selective Oxidation of Alcohols. ChemCatChem 2016, 8, 3680–3687. [Google Scholar] [CrossRef]
- Pääkkönen, S.; Pursiainen, J.; Lajunen, M. Room-temperature oxidation of secondary alcohols by bromate–bromide coupling in acidic water. Synth. Commun. 2012, 42, 534–540. [Google Scholar] [CrossRef]
- Kuroboshi, M.; Goto, K.; Tanaka, H. Electrooxidation of alcohols in N-oxyl-immobilized silica gel/water disperse system: Approach to totally closed system. Synthesis 2009, 2009, 903–908. [Google Scholar] [CrossRef]
- Moriyama, K.; Takemura, M.; Togo, H. Selective oxidation of alcohols with alkali metal bromides as bromide catalysts: Experimental study of the reaction mechanism. J. Org. Chem. 2014, 79, 6094–6104. [Google Scholar] [CrossRef]
- Komagawa, H.; Maejima, Y.; Nagano, T. Sodium bromide-catalyzed oxidation of secondary benzylic alcohols using aqueous hydrogen peroxide as terminal oxidant. Synlett 2016, 27, 789–793. [Google Scholar] [CrossRef]
- Weng, Z.; Wang, J.; Zhang, S.; Jian, X. Selective oxidation of benzyl alcohol by heteropolytungstate as reaction-controlled phase-transfer catalyst with hydrogen peroxide. Bull. Chem. Soc. Jpn 2008, 81, 525–529. [Google Scholar] [CrossRef]
- Dabiri, M.; Koohshari, M.; Shafipour, F.; Kasmaei, M.; Salari, P.; MaGee, D. Supported vanadium Schiff bases complex on nano silica: A heterogeneous catalyst for the selective oxidation of sulfides and alcohols. J. Iran. Chem. Soc. 2016, 13, 1265–1272. [Google Scholar] [CrossRef]
- Chhikara, B.S.; Chandra, R.; Tandon, V. A versatile method for the hydrogen peroxide oxidation of alcohols using ptc condition in tert-butanol. Synlett 2005, 2005, 0872–0874. [Google Scholar] [CrossRef]
- Nishida, S.; Hayashi, M. Oxidation of secondary benzylic alcohols to ketones and benzylic oxygenation of alkylarenes with hydrogen peroxide in the presence of activated carbon. Synlett 2012, 23, 1683–1685. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, C.; Wu, S.; Zhang, W.; Xue, W.; Zeng, Z. Synthesis of Benzaldehyde and Benzoic Acid by Selective Oxidation of Benzyl Alcohol with Iron (III) Tosylate and Hydrogen Peroxide: A Solvent-Controlled Reaction. Catal. Lett. 2018, 148, 3082–3092. [Google Scholar] [CrossRef]
- Karimi, B.; Rostami, F.B.; Khorasani, M.; Elhamifar, D.; Vali, H. Selective oxidation of alcohols with hydrogen peroxide catalyzed by tungstate ions (WO4=) supported on periodic mesoporous organosilica with imidazolium frameworks (PMO-IL). Tetrahedron 2014, 70, 6114–6119. [Google Scholar] [CrossRef]
- Masoudian, S.; Yahyaei, H. Oxidation of Alcohols with Hydrogen Peroxide Catalyzed by Supported Fe (III) Porphyrins; NISCAIR-CSIR: New Delhi, India, 2011. [Google Scholar]
- Galica, M.; Kasprzyk, W.; Bednarz, S.; Bogdał, D. Microwave-assisted oxidation of alcohols by hydrogen peroxide catalysed by tetrabutylammonium decatungstate. Chem. Pap. 2013, 67, 1240–1244. [Google Scholar] [CrossRef]
- Hayashi, H.; Kikawa, K.; Murai, Y.; Shigemoto, N.; Sugiyama, S.; Kawashioro, K. Competitive oxidation of 1-and 2-propanol catalyzed by titanium silicalite-1 and the application for selective oxidation of 1-methoxy-2-propanol to 1-methoxy-2-propanone. Catal. Lett. 1996, 36, 99–102. [Google Scholar] [CrossRef]
- Choi, K.-M.; Ikeda, S.; Ishino, S.; Ikeue, K.; Matsumura, M.; Ohtani, B. Oxidation of hydrophobic alcohols using aqueous hydrogen peroxide over amphiphilic silica particles loaded with titanium (IV) oxide as a liquid–liquid phase-boundary catalyst. Appl. Catal A. Gen. 2005, 278, 269–274. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).