Abstract
Lignocellulosic residues represent a promising source of raw material for obtaining several high-value bioproducts, including cellulose and derivatives. One of the main barriers to cellulose extraction from these residues is the presence of other components associated with the cellulose matrix, such as lignin and hemicellulose. To overcome this limitation, it is necessary to apply specific treatments to remove these constituents. In this study, the effectiveness of three chemical treatment methods in the purification of cellulose extracted from urban pruning biomass of the species Clitoria fairchildiana were evaluated, namely (i) alkaline treatment using dilute sodium hydroxide solution; (ii) alkaline treatment followed by bleaching with hydrogen peroxide; and (iii) alkaline treatment followed by bleaching with hydrogen peroxide and sodium hydroxide combined. The changes in chemical composition and thermal properties caused by each method were analyzed using techniques such as Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), and scanning electron microscopy (SEM). The results demonstrated that the biomass pretreatment reduced the content of impurities, lignin, and hemicellulose, increasing the cellulose content to 37.16% in the combined treatment (H2O2 + NaOH). Furthermore, the FTIR spectra revealed characteristic bands of important functional groups, which reaffirmed the chemical structure of the extracted cellulose through the identification of hydroxyl, carbonyl groups, and C-H bending vibrations. Additionally, the SEM results indicated an increase in specific surface area and greater exposure of fibrils, providing visual confirmation of the removal of constituents from the cellulosic matrix. Collectively, these results demonstrate the potential of combined chemical treatments for the valorization of Clitoria fairchildiana biomass and indicate its technical feasibility for obtaining cellulose with a higher degree of purity.
1. Introduction
In recent years, the demand for new products obtained from sustainable raw materials and processes has grown, leading the industry to seek practices with less environmental impact, mainly using biomass, and as an example, lignocellulosic biomass is standing out, from which cellulose, hemicellulose, and lignin can be extracted []. In this scenario, cellulose stands out as a bioproduct and input for the development of renewable products, especially in biomaterials and carriers’ development with better thermal and/or mechanical properties that align with technological innovation and sustainability [,].
Lignocellulosic biomass has gained prominence in this context due to its wide availability and low cost []. As previously mentioned, lignocellulosic biomass—whether from urban or agro-industrial sources—is primarily composed of cellulose, hemicellulose, and lignin. These components are intricately bound through covalent and hydrogen bonds, forming a recalcitrant lignocellulosic matrix that hinders cellulose purification []. The proportions of these constituents vary depending on the source biomass type, species, and plant part [], making specific treatments necessary to enhance the purity of extracted cellulose [].
In addition to agricultural residues such as corn cobs and straw [,], rice husks [], sugarcane bagasse [], and soybean hulls [], other relevant sources of lignocellulosic materials include forest residues and softwood materials like sawdust, wood chips, and tree bark []. Urban solid wastes rich in plant fibers, such as recyclable paper and cardboard, also represent significant resources [].
Alternative sources of this biomass include residues from agricultural, industrial, and urban processes [,]. Among these, urban lignocellulosic waste from tree pruning and removal remains relatively underexplored. A relevant species in this context is Clitoria fairchildiana (commonly known as “sombreiro” or “palheteira”), widely used for shading in urban green areas due to its rapid adaptation to nutrient-poor soils and fast growth rate [,]. Urban pruning waste typically consists of trunks, branches, and bark—lignocellulosic materials containing high concentrations of lignin, cellulose, and hemicellulose [,].
In Brazil, urban solid waste generation reached approximately 77.1 million tons in 2022 []. It is estimated that 7–10% of this total consists of pruning and vegetation removal waste, corresponding to 5.4–7.7 million tons [], of which only about 16% is currently reused [].
Pretreatment processes disrupt the hydrogen bonds within the lignocellulosic matrix, facilitating the removal of non-cellulosic components such as lignin and hemicellulose, thereby preparing the fibrous matrix for cellulose extraction [].
Given the structural complexity of these biomass materials, various techniques have been investigated, including physical, biological, chemical, and physical–chemical treatments, and their combinations, to enhance cellulose accessibility [,]. Among available methods, chemical pretreatment—encompassing alkaline, acid, oxidative, and ionic liquid treatments—stands out for its ability to depolymerize the lignocellulosic structure and degrade impurities, thereby exposing cellulose fibers and preparing them for subsequent steps such as hydrolysis [,]. This process yields materials with higher purity and improved structural accessibility, enhancing both the yield and quality of the obtained cellulose.
In this context, integrated chemical pretreatment using traditional agents such as hydrogen peroxide in combination with alkaline compounds has attracted significant interest []. This approach offers notable technoeconomic advantages, including high efficiency, minimal sugar degradation compared to acid pretreatment, reduced temperature and pressure requirements, and the use of environmentally benign organic solvents [,]. However, several challenges must be addressed before large-scale industrial implementation can be achieved. These include the inherent instability of hydrogen peroxide, which decomposes readily and requires strict storage conditions [,]. Additional challenges relate to mixing efficiency, interaction between peroxide and the organic matrix, and the selectivity of the combined hydrogen peroxide–sodium hydroxide system toward contaminants []. Furthermore, increased water consumption due to additional washing stages may undermine the process sustainability [].
This study aimed to evaluate the influence of different pretreatment stages applied to urban pruning waste from Clitoria fairchildiana for cellulose extraction. The originality of this work lies not only in the choice of this specific and underexplored biomass source but also in the detailed characterization of its lignocellulosic matrix and in the evaluation of treatment protocol efficacy, constituting a contribution for future applications, such as the production of cellulose nanoparticles.
2. Methodology
2.1. Materials
Clitoria fairchildiana prunings were collected at the Federal University of Pernambuco (UFPE) (Recife-PE, Brazil) during the campus cutting and cleaning activity. The biomass, composed of branches, trunks, and bark, was initially manually reduced in size and then processed in a Willye knife mill (Tecnal, R-TE-650/1), using a 32-mesh sieve. After grinding, the material was stored in a dry and ventilated environment in order to preserve its physicochemical characteristics until the tests were performed.
For the determination of ash content, a mass of approximately 2 g of plant material was weighed in a previously tared porcelain crucible. The sample was then heated in a muffle furnace at 550 °C for 5 h. For moisture content, the material was transferred to a thermostated circulating air oven and maintained at 105 °C until constant weight [,]. The chemical composition of the in natura biomass was determined in percentage terms (w/w). The quantification of cellulose and hemicellulose followed the methods described by the Technical Association of the Pulp and Paper Industry (TAPPI) [], while the determination of lignin content was performed based on the methodology described by Gouveia []. The determination of extractives was carried out by weighing 5 g of biomass sample in filter paper cartridges and subjecting it to extraction in a Soxhlet apparatus, using a hexane/ethanol solvent mixture in a 2:1 ratio for 7 h [].
2.2. Chemical Treatment Steps
The first step consisted of an alkaline treatment (mercerization) with a 2% (w/v) NaOH solution. A total of 50 g of biomass was mixed with 1000 mL of the solution (resulting in a 1:20 solid-to-liquid ratio, w/v). The mixture was maintained at 80 °C for 2 h. The resulting solid was then filtered, washed with distilled water until a neutral pH of 7 was achieved, and subsequently oven-dried at 55 °C for 24 h with air circulation [].
After being subjected to alkaline treatment, the biomass underwent two different bleaching processes. In the first, 30% H2O2 (v/v) was used in a ratio of 1 g of mercerized biomass to 20 mL of hydrogen peroxide, and the mixture was kept heated at 80 °C for 1.5 h. The solid was filtered and washed until it reached a pH close to 7. This process was performed twice, under the same conditions [].
The second bleaching treatment was performed by combining a 5% (w/v) NaOH solution and 30% (v/v) H2O2, maintaining the same operational conditions described in the first bleaching [].
2.3. Biomass Yield
The mass yield of the materials after each treatment step was calculated using Equation (1), expressed as a percentage of the mass of the raw biomass. This method enables a standardized evaluation of the extraction efficiency relative to the raw material [].
where
Yield (%) = (m2/m1) × 100
- m1: initial mass of the raw biomass in dry weight (g);
- m2: final mass of the treated and dried material (g).
2.4. Biomass Characterization After the Treatments
The treated biomasses were chemically characterized according to the methodologies presented in Section 2.1. The treated and in natura biomass samples were also subjected to scanning electron microscopy (SEM), thermogravimetric analysis (TG), and Fourier transform infrared spectroscopy (FTIR).
For scanning electron microscopy—SEM (Vegan3, Tescan, Brno, Czech Republic), the samples were subjected to metallization using a platinum target for 3 min, followed by a gold target for 5 min at a current of 10 mA. The SEM was operated at 5 kV, with a focus magnification between 457 and 1800 times. Fields of view from 454 μm to 115 μm were used, with scales of 100 μm and 20 μm for the photomicrographs. The material was scanned at multiple points, with magnifications ranging from 300 to 4000 times.
Thermogravimetric (TG) analyses of the treated samples were evaluated using a TGA-51/51H (Shimadzu, Kyoto, Japan). The analysis was performed at a continuous heating rate of 10 °C per minute until reaching 900 °C in a nitrogen (N2) atmosphere to avoid undesired reactions. Thermal decomposition curves were obtained by means of a microcomputer connected to the instrument, using the TA-60 WS Shimadzu software (version 2.21), which captured data at a rate of one point every 0.5 s, ensuring high resolution in the results.
Fourier transform infrared spectroscopy (FTIR) spectra were obtained using a spectrophotometer (FTIR IRAffinity-1, Shimadzu, Kyoto, Japan) and were used to analyze the samples isolated from the raw and treated biomass. The spectroscope operated over a wavelength range of 4000 to 600 cm−1 and performed 64 scans. For the analysis, a mass of 2 mg of each sample (raw and treated biomass) was weighed using a high-precision analytical balance and homogenized with 200 mg of KBr for the preparation of each pellet.
3. Results and Discussion
3.1. Biomass Characterization and Yield After the Treatments
Chemical characterization of Clitoria fairchildiana biomass was enabled to evaluate the treatment efficacy through comparative analysis of lignocellulosic composition, using raw biomass as a reference. The biomass yield and lignocellulosic contents after each treatment are presented in Table 1, and visually, in Figure 1.

Table 1.
Chemical composition and yield of raw material and pretreated biomass from tree-pruning C. fairchildiana.

Figure 1.
Stages of preparation and processing of Clitoria fairchildiana biomass: (A) fragmented raw biomass; (B) ground biomass; (C) cellulosic pulp after delignification; (D) bleached cellulose.
As shown in Figure 1, image (A) displays the raw biomass from urban pruning, consisting of heterogeneous fragments of branches and bark. In image (B), it can be observed that the biomass, after grinding, underwent a color change from dark brown to yellow following treatment with H2O2. This same result is shown in greater detail in image (C). Finally, in image (D), the coloration turned white after bleaching with the H2O2 + NaOH solution, an effect attributed to the removal of non-cellulosic components, such as lignin.
The chromatic alteration indicates the efficient removal of lignocellulosic components through degradation of lignin chromophore groups []. The application of H2O2 in two cycles demonstrated limited delignification efficiency, with a discrete reduction in lignin content [,], as demonstrated in Table 1.
The alkaline treatment with NaOH/H2O2 proved superior, where the generation of hydroperoxide anions (HOO−) simultaneously promoted oxidation and depolymerization of lignin, in addition to effective hemicellulose removal [,]. This protocol resulted in material with high cellulose purity and visually detectable whitening, indicating effective fibrillar purification [,,,].
The results presented in Table 1 indicate that the raw biomass of Clitoria fairchildiana exhibits a cellulose content of 29.78%, a value close to those reported in the literature for other lignocellulosic biomasses, such as bamboo (36% to 45.9%) []. Similarly, the observed cellulose (30–50%), lignin (27–32%), and hemicellulose (15–30%) contents fall within the ranges documented for conventional plant species [,,], corroborating the representativeness of the analyzed sample within the context of lignocellulosic biomasses.
It is observed that both treatments promoted the deconstruction of the lignocellulosic matrix through the rupture of crucial chemical bonds, resulting in a significant percentage reduction of the components. This indicates physical/chemical removal, not merely a change in molecular interactions: hemicellulose (7.87% and 4.80%, respectively) and lignin (19.34% and 15.62%, respectively). As a consequence, exposure of the cellulose fibers and a consequent relative increase in their content (33.33% and 35.43%, respectively) were observed [,].
The mass yields obtained for each protocol were 61.80% (mercerization), 57.33% (H2O2 bleaching), and 55.60% (combined H2O2 + NaOH treatment). The superiority of the combined treatment is evident, as it promoted higher removal of non-cellulosic components such as hemicellulose and lignin. The isolated use of H2O2 would require multiple cycles for satisfactory delignification due to its limited oxidative capacity in the absence of an alkaline enhancing agent; the combined protocol demonstrated superior effectiveness in removing non-cellulosic components (lignin and hemicelluloses) in a single cycle.
The cellulose content in the treated biomasses showed values of 33.30% for the mercerized sample, 35.43% for bleaching with H2O2 alone, and 37.16% for the combined H2O2 + NaOH treatment (if these % are based on the biomass yield, a treated biomass with more than 65% of cellulose content was obtained in both treatments). The chemical composition of the Clitoria fairchildiana biomass obtained in this study resulted in yields comparable to those reported in previous works [,,]. Notably, the relatively reduction in hemicellulose and lignin contents is a relevant aspect when compared to data from other lignocellulosic biomasses treated with alkaline peroxide [].
The cellulose content of approximately 37.16% achieved by a single-step combined H2O2/NaOH treatment is considered comparable when compared to other mild chemical routes applied to residual biomass. For context, a study with oil palm mesocarp fibers, using a similar alkaline–oxidative sequence, obtained a purity of 39.1% []. On the other hand, higher values, such as the 76.4% reported for pineapple crown fibers, were achieved through a multi-step process that included additional bleaching on previously alkalized fibers [].
The performance of the method used here demonstrates that the synergistic action of NaOH (which promotes fiber swelling and breaks crucial chemical bonds (such as aryl–ether, carbon–carbon, aryl–aryl, and esters of cell wall carbohydrates)) and H2O2 (which acts as an oxidizing agent in lignin degradation) is effective for a single-step process, positioning it favorably in terms of procedural simplicity versus delignification efficiency [,].
The efficacy of the H2O2/NaOH system, widely recognized in the literature for its efficiency in depolymerizing non-cellulosic components such as lignin, is supported by chemical mechanisms. These mechanisms, essential for elucidating biomass transformation, are initiated when NaOH accelerates the decomposition of H2O2 []. These ions, in turn, attack the bonds and aromatic structures in lignin, breaking down its matrix and releasing the cellulose within the fiber. The main chemical reactions involved in this process can be described as follows:
H2O2 + H2O ⇌ HOO− + H3O+ (Reaction 1.1)
H2O2 + HO− ⇌ H2O + HOO• (Reaction 1.2)
H2O2 + HOO− ⇌ HO• + O2•− + H2O (Reaction 1.3)
H2O2 + HOO− + H3O+ ⇌ O2 + 3H2O (Reaction 1.4)
The first step (Reaction 1.1) represents the initiation of hydrogen peroxide degradation through its interaction with water. Reaction 1.2 demonstrates the generation of reactive species, such as the hydroperoxyl radical (HOO•), while Reaction 1.3 results in the formation of hydroxyl radicals (•OH), known for their high oxidative reactivity. Reaction 1.4 marks the end of the process, with the release of oxygen and water []. The oxidative effect of peroxide is intensified in an alkaline medium, such as in NaOH solutions, due to the formation of the hydroperoxide anion, considered the primary active species responsible for modifying the aromatic structures of lignin []. However, this species is unstable under such conditions, decomposing into hydroxyl radicals (•OH) and superoxide (O2•−), which play a key role in the lignocellulosic degradation process [,,,,].
3.2. Fourier Transform Infrared (FTIR) Analysis
FTIR analysis revealed significant structural modifications in the biomass following chemical treatments, corroborating delignification and exposure of the cellulosic fraction. The intensification of the broad band at ~3346 cm−1 (Figure 2) is associated with O-H stretching of hydroxyl groups, related to moisture and inter- and intramolecular interactions [,]. After treatment, the emergence and intensification of this band were observed, indicating greater exposure of hydroxyl groups []. The absence of this visible band in the native biomass can be attributed to the coverage of hydroxyl groups by the lignin and hemicellulose matrix, as well as the pre-existing strong hydrogen bonding interactions [,,] Additionally, prominent C-H stretching absorptions in the ~3017 cm−1 region also contribute to the spectral complexity in this area.
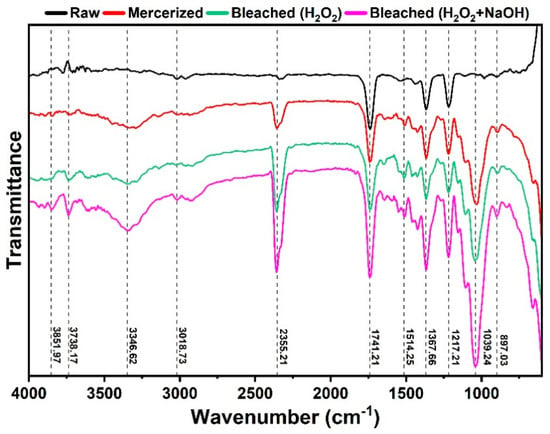
Figure 2.
Infrared spectra of the raw, mercerized, and bleached biomasses from pruning of C. fairchildiana.
Changes in the carbonyl region (1741 cm−1) provide important insights into the chemical reactions involved. This band, attributed to C=O stretching vibrations of esters, carboxylic acids, and ketones [,], is related to the exposure, release, and generation of carbonyl groups via oxidative processes during delignification []. A low-intensity band at 1514 cm−1, observed in the fibers, is associated with the deformation of C-C bonds [].
A medium-intensity band at 1373 cm−1 is identified as angular C-H deformation of methylene or methyl groups, often overlapping with vibrations near carbonyl regions. Weak absorptions at 1420 cm−1 are due to the bending mode (in-plane angular deformation) of the CH2 group, characteristic of carbohydrates such as cellulose [,].
A band of increased intensity observed at 1039 cm−1 corresponds to C–O stretching, typical of polysaccharides, overlapped with the band at 1055 cm−1 related to C–O stretching of the glucose ring. The C–O band may originate from various fractions such as alcohols, carboxylic acids, and esters; its intensification therefore confirms the exposure of internal carbohydrate structures [,].
Finally, a low-intensity band at 897 cm−1 is attributed to the characteristic deformation of the β-glycosidic ring, common to polysaccharides such as cellulose and hemicelluloses in natura [,]. This band showed progressive relative intensification with increasing severity of delignification treatments. This behavior was particularly evident in the sample bleached with H2O2 + NaOH, indicating greater exposure of the cellulosic fraction due to selective removal of lignocellulosic components.
Fourier transform infrared (FTIR) spectroscopy was used to characterize the structure and chemical changes in the biomass constituents, both in their in natura form and after chemical treatments. These treatments, applied for cellulose purification in pruning fibers, are also capable of inducing morphological changes.
3.3. Scanning Electron Microscopy (SEM)
Microscopic analyses of particles on the surface of the urban pruning material were performed using micrographs with reference scales of 20 µm and 50 µm. The biomass morphology, analyzed by scanning electron microscopy (SEM), is shown in Figure 3, where significant structural differences on the fiber surfaces can be observed. These morphological alterations clearly demonstrate the impact of the applied chemical treatments, revealing the progressive de-structuring of the lignocellulosic matrix and the exposure of cellulosic fibrils after each processing stage.
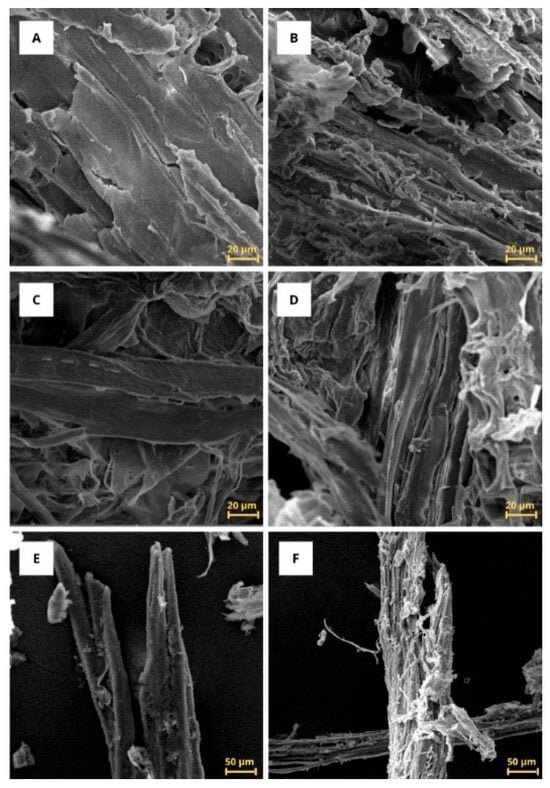
Figure 3.
Scanning electron microscopy. Scale bar of 20 µm ((A): raw; (B): mercerized; (C): dual H2O2 bleaching; (D): H2O2 + NaOH bleaching) and 50 µm ((E): raw; (F): mercerized) of C. fairchildiana biomass.
The analyses suggested a distinct morphological evolution, where each processing phase promoted morphological changes on the fiber surface. It is possible to observe in Figure 3A,E (20 µm and 50 µm, respectively) that, before treatment, the raw material in natura has a rough surface, already somewhat irregular, but with smoother and more compacted fibers as observed in other studies [,]. After the mercerization treatment, in Figure 3B,F (20 µm and 50 µm, respectively), it is observed that the surface is more elongated and fibrous, with more evident bundles of fibrils, which resulted from the partial removal of non-cellulosic components and the reorganization of the fibrillar structure due to the chemical treatment. In addition, an opening and exposure of several thin bundles of fibrils is identified [].
In Figure 3C,D, structural changes in the cellulose fibrils after the bleaching process become evident, with them more exposed, separated, and swollen. In addition, cracks appear on the surface of the fibers and an increase in roughness [,,]. These changes result from the removal of components of the lignocellulosic matrix, such as lignin and hemicellulose, as well as other substances present in the raw material, including proteins, extractives, and impurities. The morphological characteristics observed in the fibers resemble morphologies already described in the literature [,].
3.4. Thermogravimetric Analysis (TG/DTG)
Thermogravimetric analysis (TGA) was used to evaluate the thermal stability of fibers from urban pruning waste, both in their natural state and after chemical treatments. Figure 4A,B show the thermogravimetry (TG) and derivative thermogravimetry (DTG) curves obtained for the in natura sample, the mercerized sample, and those bleached with hydrogen peroxide and with a mixture of hydrogen peroxide and sodium hydroxide. These curves represent the mass loss of the samples during continuous heating up to 700 °C.
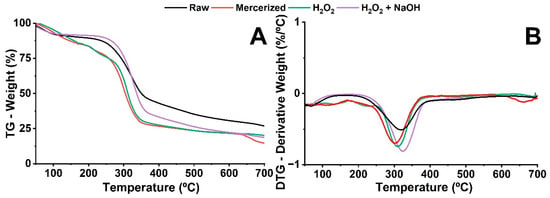
Figure 4.
(A) TG and (B) DTG curves of raw biomass and chemically treated biomasses.
An initial mass loss (<100 °C) was observed in all fibers, regardless of treatment, and was mainly attributed to the evaporation of adsorbed water [,]. The onset of biomass thermal degradation occurred at approximately 250 °C with mass loss, primarily associated with the depolymerization of hemicelluloses. The DTG thermogram of the raw sample (Figure 4B) exhibited a pronounced decomposition peak at 319 °C, corresponding to the thermal degradation of cellulose, where the highest mass loss rate was recorded. A low-intensity peak at 425 °C, in the carbonization region, is associated with the degradation of carbon-rich solid residues (char formation and release of gases) []. Lignin degradation occurs significantly in this temperature range []. The raw sample showed a higher final residual mass compared to the treated fibers, indicating a higher content of inorganic components (ash) and the presence of lignin and other non-cellulosic components [].
Compared to raw fibers, the chemical treatments of mercerization and bleaching promoted an increase in thermal stability. This effect is explained by the progressive reduction of components that have a lower decomposition temperature than cellulose []. Equally significant is the emergence of a low-intensity peak above 600 °C in the DTG curve, specifically induced by the hydrogen peroxide treatment. This behavior is consistent with the nature of the residual lignin, which under these conditions preferentially contributes to the formation of carbonaceous residue rather than volatile mass loss [], demonstrating that the bleaching process, while selectively removing most of this component, promotes the condensation of the remaining fractions. The lower thermal stability of the mercerized fiber compared to the bleached one is attributed to its higher residual content of lignin and hemicellulose, which were more effectively reduced during bleaching [,].
The thermogravimetric analysis of the sample bleached with H2O2 (Figure 4B) reveals two fundamental aspects of the structural modification promoted by the treatment. Firstly, a significant mass loss is observed in the TG curve in the 260 °C range, with a pronounced degradation peak at 365.63 °C in the DTG curve, corresponding to the thermal decomposition of cellulose.
The bleaching with H2O2 + NaOH produced a distinct thermal profile, characterized by a significant mass loss initiating at 290 °C and a broad peak at 330 °C in the DTG curve, corresponding to the thermal decomposition of cellulose.
The thermogravimetric analyses consistently confirmed the efficient extraction of cellulose through the chemical treatments, a result that corroborates the data presented in Table 1, demonstrating the increase in cellulose content. Additionally, the results indicate that the intensification of the carbonyl and ether bands in the FTIR is attributed to the greater exposure of the cellulosic fraction in the treated samples. The thermal stability of cellulose is therefore high, with the decomposition of its crystalline part being particularly difficult due to the high structural organization, which confers greater thermal resistance []. These data demonstrate that the treatments decisively modify the thermal behavior of the fibers, providing valuable information for process optimization and future applications.
Interesting directions are regarding the exploration of advanced lignocellulosic fractionation techniques, notably the Acetosolv process, which employs acetic acid and an acid catalyst under heating to obtain high-purity lignin and a cellulosic solid residue [,]. Preliminary trials with ionic liquids, such as 2-hydroxyethylammonium ([2-HEA]), aim to evaluate their efficiency as solvents for cellulose []. These solvents are distinguished by their capacity for selective dissolution of biomass components, and their strategic combination with conventional pretreatments may enhance biomass accessibility, enabling the isolation of high-quality cellulose. The last goal is to guide the production of high-value cellulosic materials, such as cellulose nanocrystals (CNCs) and cellulose nanofibrils (CNFs), whose morphological and surface properties are critically dependent on isolation conditions and biomass source, for application in nanocomposites and nanomaterials [,].
4. Conclusions
Urban pruning residues demonstrated technical feasibility for cellulose extraction through sequential treatments with NaOH and H2O2. Characterization revealed a complementary and synergistic action of the reagents: the NaOH treatment was crucial for initial fiber swelling and hemicellulose removal, while H2O2 acted effectively in delignification through oxidation. This combined action was decisive in achieving a cellulose content of 37.16%. FTIR analyses indicated greater exposure of hydroxyl groups after the removal of extractives from the in natura biomass during treatment. The O-H band, previously broad and masked by other components in the in natura biomass, became more defined and intense after the treatments; however, an increase in absorbance intensity was recorded in the characteristic regions of carbonyls, C-O, and C-H bonds. SEM (scanning electron microscopy) images revealed significant morphological modifications, including fiber destructuring and exposure of cellulosic fibrils. Thermogravimetric analysis (TGA) corroborated the process efficiency, exhibiting a degradation profile characteristic of cellulose and higher thermal stability. Subsequent studies will be focused on optimizing process parameters (temperature, time, and concentration) and assessing the life cycle of the solvents, ensuring the technical and environmental feasibility of the proposed procedure. The characterization of the products using SEM, FTIR, and TGA techniques will be essential to correlate processing conditions with material properties.
Author Contributions
Conceptualization, M.B.d.S., R.M.R.G.A. and R.R.d.L.A.; methodology, M.B.d.S., R.M.R.G.A. and R.R.d.L.A.; formal analysis, M.B.d.S., R.R.d.L.A., J.M.D.d.F., J.D.d.F. and L.N.L., investigation, M.B.d.S. and R.R.d.L.A.; writing—original draft preparation., M.B.d.S., R.R.d.L.A. and.; resources, R.M.R.G.A., C.E.d.F.S. and J.D.d.F.; visualization, R.R.d.L.A., R.M.R.G.A. and C.E.d.F.S. and T.d.M.B.; software, C.E.d.F.S. and R.R.d.L.A.; validation, R.M.R.G.A. and C.E.d.F.S.; data curation, R.M.R.G.A., R.R.d.L.A., M.R.P.B. and T.d.M.B.; writing—review and editing, M.B.d.S., R.M.R.G.A., R.R.d.L.A., C.E.d.F.S. and M.R.P.B.; supervision, R.M.R.G.A.; project administration, R.M.R.G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from MCTI—Brazil through CNPq/BRICS-ST Call n° 28/2023—process 440026/2024-5 and CNPq/CT-BIOTEC Call n° 31/2022 —process 440444/2022-5 (VALORA-FORSU). In addition, funding was provided by the Research Support Foundation of Alagoas—FAPEAL (Project Numbers: E:60030.0000000463/2020 and E:00030.000002067/2023—PDPG/CAPES/FAPEAL through Call n° 38/2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors express their sincere gratitude to the Coordination for the Improvement of Higher Education Personnel (CAPES) and the Alagoas State Research Support Foundation (FAPEAL) for their financial support through the scholarships awarded. They also thank the Federal Institute of Alagoas (IFAL) for technical support in the analyses performed, as well as the Federal University of Alagoas and the Food and Beverage Technology (LTBA) and Bioprocesses (LaBio) Laboratories for allowing the use of their facilities and for their technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Artilha-Mesquita, C.A.F.; Stafussa, A.P.; Paraiso, C.M.; Rodrigues, L.M.; Silva, L.A.; Santos, S.S.; Marins, A.R.; Madrona, G.S. Evaluation of quality management and its tools: Applicability in the animal food industry. Res. Soc. Dev. 2021, 10, e20210111248. [Google Scholar]
- Zhu, S.; Sun, H.; Um, T.; Li, Q.; Richel, A. Preparation of cellulose nanocrystals from purple sweet potato peels by ultrasound-assisted maleic acid hydrolysis. Food Chem. 2022, 403, 134496. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, F.; Chen, J.; Wang, Y.; Zhou, Z.; Lian, R. Development of seaweed-derived polysaccharide/cellulose nanocrystal-based antiifogging labels loaded with alizarin for monitoring aquatic products freshness. Int. J. Biol. 2023, 235, 126640. [Google Scholar]
- Baraka, F.; Langari, M.M.; Beitia, I.; Dávila, I.; Labidi, J.; Morales, A.; Sillero, L. Enhancing lignocellulosic biomass pretreatment with choline chloride-based deep eutetic solvents. J. Environ. Chem. Eng. 2025, 13, 117087. [Google Scholar] [CrossRef]
- Tiwari, A.; Sanjog, J. Morphological, structural, and thermal properties of cellulose nanocrystals extracted from Indian water chestnut shells (agricultural waste). Next Mater. 2025, 8, 100653. [Google Scholar] [CrossRef]
- Mateo, S.; Peinado, S.; Morillas-Gutiérrez, F.; La Rubia, M.D.; Moya, A.J. Nanocellulose from agricultural wastes: Products and applications—A review. Processes 2021, 9, 1594. [Google Scholar] [CrossRef]
- Zeng, Q.; Ma, J.; Liu, T.; Shi, Y.; Hua, R.; Zhu, Y.; Xu, Y.; Zhu, J. Extraction of xylo-oligosaccharides and carboxylated cellulose nanocrystals from corncob using stepwise treatment with ammonium persulfate. Int. J. Biol. Macromol. 2025, 322, 147142. [Google Scholar] [CrossRef]
- Nyerere, G.; Kyokusiima, S.; Nabaterega, R.; Tumusiime, G.; Kavuma, C. The synergy of maize straw cellulose and sugarcane bagasse fibre on the characteristics of bioplastic packaging film. Bioresour. Technol. Rep. 2024, 28, 102007. [Google Scholar] [CrossRef]
- Techawinyutham, L.; Sundaram, R.S.; Suyambulingam, I.; Mo-on, S.; Srisuk, R.; Divakaran, D.; Rangappa, S.M.; Siengchin, S. Rice husk biowaste derived microcrystalline cellulose reinforced sustainable green composites: A comprehensive characterization for lightweight applications. Int. J. Biol. Macromol. 2025, 299, 140153. [Google Scholar] [CrossRef]
- Bortolatto, R.; Bittencourt, P.R.S.; Yamashita, F. Biodegradable starch/polyvinyl alcohol composites produced by thermoplastic injection containing cellulose extracted from soybean hulls (Glycine max L.). Ind. Crops Prod. 2022, 176, 114383. [Google Scholar] [CrossRef]
- Moriana, R.; Vilaplana, F.; Ek, M. Cellulose Nanocrystals from Forest Residues as Reinforcing Agents for Composites: A Study from Macro- to Nano-Dimensions. Carbohydr. Polym. 2016, 139, 139–149. [Google Scholar] [CrossRef]
- Jaffur, N.; Jeetah, P. Produção de papel de baixo custo a partir de fibras Pandanus utilis em substituição à madeira. Pesqui. Em Meio Ambiente Sustentável 2019, 29, 20. [Google Scholar]
- Alves, M.M.; Alves, E.U.; Bruno, R.L.A.; Silva, K.R.G.; Santos-Moura, S.S.; Barrozo, L.M.; Araújo, L.R. Potencial fisiológico de sementes de Clitoriafairchildiana R.A. Howard.-Fabaceae submetidas a diferentes regimes de luz e temperatura. Ciência Rural 2012, 42, 2199–2205. [Google Scholar] [CrossRef]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, Chemical Modification, and Application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
- Nelo, L.M.A.; Ribeiro, A.P. Arborização urbana: Uma perspectiva sobre o direcionamento dos resíduos em cidades brasileiras. Periódico Técnico e Científico Cidades Verdes 2023, 11, 80–93. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and sustainable pretreatment methods for cellulose extraction from lignocellulosic biomass and its applications: A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Abrema. Panorama dos Resíduos Sólidos no Brasil; ABREPEL: São Paulo, Brazil, 2023. [Google Scholar]
- Correia, G.R.; Machado, J.C.; Almeida, S.S.; Santos, L.A.C.; Costa, R.L.; Calil, F.N.; Silva-Neto, C.M. Scientific Production on Urban Pruning Waste: A Scientometric Analysis. Braz. J. Phys. Geogr. 2022, 15, 1701–1714. [Google Scholar]
- Li, Y.; Hua, D.; Xu, H.; Zhao, Y.; Jin, F.; Fang, X. Improving biodegradability of corn stover pretreated by different organic acids: Investigation on the hydrolysis/acidification and methanogenic performance. Ind. Crops Prod. 2022, 177, 114395. [Google Scholar] [CrossRef]
- Alizadeh, H.-R.; Kansedo, J.; Tan, I.S.; Tan, Y.H.; Suali, E.; Dini, A. Recent advances on two-step and combined multi-step pretreatment of lignocellulosic biomass for cellulose extraction. Bioresour. Technol. Rep. 2025, 31, 102243. [Google Scholar] [CrossRef]
- Gil-López, D.I.L.; Lois-Correa, J.A.; Sánchez-Pardo, M.E.; Domínguez-Crespo, M.A.; Torres-Huerta, A.M.; Rodríguez-Salazar, A.E.; Orta-Guzmán, V.N. Production of dietary fibers from sugarcane bagasse and sugarcane tops using microwave-assisted alkaline treatments. Ind. Crops Prod. 2019, 135, 159–169. [Google Scholar] [CrossRef]
- Meng, X.; Liu, F.; Xiao, Y.; Cao, J.; Wang, M.; Duan, X. Alterações nas propriedades físico-químicas e funcionais da fibra alimentar insolúvel da palha de trigo sarraceno por tratamento com peróxido de hidrogênio alcalino. Food Chem. X 2019, 3, 100029. [Google Scholar]
- Nair, L.G.; Agrawal, K.; Verma, P. Organosolv pretreatment: An in-depth purview of mechanics of the system. Bioresour. Bioprocess. 2023, 10, 50. [Google Scholar] [CrossRef]
- Jiang, Y.; Ni, P.; Chen, C.; Lu, Y.; Yang, P.; Kong, B.; Fisher, A.; Wang, X. Selective Electrochemical H2O2 Production through Two-Electron Oxygen Electrochemistry. Adv. Energy Mater. 2018, 8, 1801909. [Google Scholar] [CrossRef]
- Ranganathan, S.; Sieber, V. Recent Advances in the Direct Synthesis of Hydrogen Peroxide Using Chemical Catalysis—A Review. Catalysts 2018, 8, 379. [Google Scholar] [CrossRef]
- Araújo, A.A.D.S.; Mercuri, L.P.; Seixas, S.R.S.; Storpirtis, S.; Matos, J.D.R. Determinação dos teores de umidade e cinzas de amostras comerciais de guaraná utilizando métodos convencionais e análise térmica. Revista Brasileira de Ciências Farmacêuticas 2006, 42, 269–277. [Google Scholar] [CrossRef]
- Tappi. T 203 CM-99. In Alpha-, Beta- and Gamma-Cellulose in Pulp; TAPPI Press: Atlanta, GA, USA, 2009; 7p. [Google Scholar]
- Gouveia, E.R.; Nascimento, R.T.; Souto-Maior, A.M.; Rocha, G.J.D.M. Validação de metodologia para a caracterização química de bagaço de cana-de-açúcar. Química Nova 2009, 32, 1500–1503. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, H.V.; Juan, J.C.; Phang, S.M. Production of new cellulose nanomaterial from red algae marine biomass Gelidium elegans. Carbohydr. Polym. 2016, 151, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Tanpichai, S.; Subir, K.B.; Suteera Witayakran, E.H.Y. Water Hyacinth: A Sustainable Lignin-Poor Cellulose Source for the Production of Cellulose Nanofibers. ACS Sustain. Chem. Eng. 2019, 7, 18884–18893. [Google Scholar] [CrossRef]
- Vu, A.N.; Nguyen, L.H.; Tran, H.-C.V.; Yoshimura, K.; Tran, T.D.; Van Le, H.; Nguyen, N.-U.T. Cellulose nanocrystals extracted from rice husk using the formic/peroxyformic acid process: Isolation and structural characterization. RSC Adv. 2024, 14, 2048–2060. [Google Scholar] [CrossRef]
- Tran, M.H.; Phan, D.-P.; Lee, E.Y. Revisão sobre modificações de lignina em relação a ingredientes naturais de proteção UV para protetores solares à base de lignina. Green Chem. 2021, 23, 4633–4646. [Google Scholar] [CrossRef]
- Ji, C.; Wang, Y. Lignin-containing cellulose nanocrystals from maple leaves: A natural Pickering emulsion stabilizer for food preservation. Food Chem. 2025, 463, 141407. [Google Scholar] [CrossRef] [PubMed]
- Tofani, G.; Cornet, I.; Tavernier, S. Multiple Linear Regression to Predict the Brightness of Waste Fibres Mixtures before Bleaching. Chem. Pap. 2022, 76, 4351–4365. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Y.; Qian, Y.; Qiu, X.; Ren, Y.; Yang, D. Reduction of lignin color via one-step UV irradiation. Green Chem. 2016, 18, 695–699. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.; Zhou, Z.; Li, A.; Zhu, S.; Li, J.; Zhang, W.; Zhang, F.; Chen, K. A feasible approach to chromophores removal and color reduction in industrial lignin via deep eutectic solvent/isopropanol treatment. Int. J. Biol. Macromol. 2025, 290, 138857. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Kantola, A.M.; Ämmälä, A. Nanofibras de celulose de serragem tratada com tioureia ácida, não branqueada e branqueada com peróxido de hidrogênio. J. Clean. Prod. 2023, 423, 138824. [Google Scholar]
- Biswas, S.; Rahaman, T.; Gupta, P.; Mitra, R.; Dutta, S.; Kharlyngdoh, E.; Guha, S.; Ganguly, J.; Pal, A.; Das, M. Cellulose and lignin profiling in seven, economically important bamboo species of India by anatomical, biochemical, FTIR spectroscopy and thermogravimetric analysis. Biomass Bioenergy 2022, 158, 106362. [Google Scholar] [CrossRef]
- Ciftci, D.; Flores, R.A.; Saldaña, M.D. Cellulose Fiber Isolation and Characterization from Sweet Blue Lupin Hull and Canola Straw. J. Polym. Environ. 2018, 26, 2773–2781. [Google Scholar] [CrossRef]
- Sobri, N.S.A.; Harun, S.; Abdul, P.M.; Feng, A.W.; Salleh, M.Z.M. Xylan Solubilisation from Oil Palm Frond and Sago Palm Bark via In-Situ Reductant-Aided Alkaline Peroxide Pretreatment. Ind. Crops Prod. 2025, 232, 121309. [Google Scholar] [CrossRef]
- Díaz, A.B.; Blandino, A.; Belleli, C.; Caro, I. An Effective Process for Pretreating Rice Husk to Enhance Enzyme Hydrolysis. Ind. Eng. Chem. Res. 2014, 53, 10870–10875. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Ornaghi, H.L.; Arantes, V.; Cioffi, M.O.H. Effect of chemical treatment of pineapple crown fiber in the production, chemical composition, crystalline structure, thermal stability and thermal degradation kinetic properties of cellulosic materials. Carbohydr. Res. 2021, 499, 108227. [Google Scholar] [CrossRef]
- Song, Z.; Yang, G.; Liu, X.; Yan, Z.; Yuan, Y.; Liao, Y. Comparison of Seven Chemical Pretreatments of Corn Straw for Improving Methane Yield by Anaerobic Digestion. PLoS ONE 2014, 9, e93801. [Google Scholar] [CrossRef]
- He, Z.-J.; Chen, K.; Liu, Z.-H.; Li, B.-Z.; Yuan, Y.-J. Valorizing renewable cellulose from lignocellulosic biomass toward functional products. J. Clean. Prod. 2023, 414, 137708. [Google Scholar] [CrossRef]
- Jantarat, C.; Kaewpradit, S.; Chingunpitak, J.; Srivaro, S. Characteristics of microcrystalline cellulose derived from oil palm trunk slabs and its potential for use as tablet diluent. Heliyon 2025, 11, e42902. [Google Scholar] [CrossRef]
- Othman, J.A.S.; Ilyas, R.A.; Nordin, A.H.; Ngadi, N.; Alkbir, M.F.M.; Knight, V.F.; Norrrahim, M.N.F. Optimization of delignification and mercerization processes for high-purity cellulose extraction from Semantan bamboo (Gigantochloa scortechinii) using Response Surface Modelling. Carbohydr. Polym. Technol. Appl. 2025, 10, 100784. [Google Scholar] [CrossRef]
- Alam, M.M.; Greco, A.; Rajabimashhadi, Z.; Esposito Corcione, C. Efficient and environmentally friendly techniques for extracting lignin from lignocellulose biomass and subsequent uses: A review. Clean. Mater. 2024, 13, 100253. [Google Scholar] [CrossRef]
- Moura, H.O.M.A.; Campos, L.M.A.; da Silva, V.L.; de Andrade, J.C.F.; de Assumpção, S.M.N.; Pontes, L.A.M.; de Carvalho, L.S. Investigating Acid/Peroxide-Alkali Pretreatment of Sugarcane Bagasse to Isolate High Accessibility Cellulose Applied in Acetylation Reactions. Cellulose 2018, 25, 5669–5685. [Google Scholar] [CrossRef]
- Abderrahim, B.; Abderrahman, E.; Mohamed, A.; Fatima, T.; Abdesselam, T.; Krim, O. Kinetic Thermal Degradation of Cellulose, Polybutylene Succinate and a Green Composite: Comparative Study. World J. Environ. Eng. 2015, 3, 95–110. [Google Scholar]
- Oliveira, L.M.T.M.; Oliveira, L.F.A.M.; Sonsin, A.F.; Duarte, J.L.S.; Soletti, J.I.; Fonseca, E.J.S.; Ribeiro, L.M.O.; Meili, L. Ultrafast diesel oil spill removal by fibers from silk-cotton tree: Characterization and sorption potential evaluation. J. Clean. Prod. 2020, 263, 121448. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, K.; Qian, H.; Ramachandran, B.; Jiang, F. The advances of characterization and evaluation methods for the compatibility and assembly structure stability of food soft matter. Trends Food Sci. Technol. 2021, 112, 753–763. [Google Scholar] [CrossRef]
- Oliveira, L.M.T.M.; Fonseca, E.J.S.; Bernardo, V.B.; Zanta, C.L.P.S.; Oliveira, L.F.A.M.; Oliveira, J.N.S.R.D.; Souza, S.T.D.; Duarte, J.L.D.S. Modified kapok fibers (Ceiba pentandra (L.) Gaerth) for oil spill remediation. Appl. Sci. 2024, 14, 11995. [Google Scholar] [CrossRef]
- Norfarhana, A.S.; Ilyas, R.A.; Ngadi, N.; Othman, M.H.D.; Misenan, M.S.M.; Norrrahim, M.N.F. Revolutionizing lignocellulosic biomass: A review of harnessing the power of ionic liquids for sustainable utilization and extraction. Int. J. Biol. Macromol. 2023, 256, 128256. [Google Scholar] [CrossRef] [PubMed]
- Celina, M.C.; Linde, E.; Martinez, E. Carbonyl Identification and Quantification Uncertainties for Oxidative Polymer Degradation. Polym. Degrad. Stab. 2021, 188, 109550. [Google Scholar] [CrossRef]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef]
- Thi Thuy Van, N.; Gaspillo, P.; Thanh, H.G.T.; Nhi, N.H.T.; Long, H.N.; Tri, N.; Thi Truc Van, N.; Nguyen, T.-T.; Ky Phuong Ha, H. Cellulose from the banana stem: Optimization of extraction by response surface methodology (RSM) and charaterization. Heliyon 2022, 8, e11845. [Google Scholar] [CrossRef]
- Shinde, S.D.; Meng, X.; Kumar, R.; Ragauskas, A.J. Recent advances in understanding the pseudo-lignin formation in a lignocellulosic biorefinery. Green Chem. 2018, 20, 2192–2205. [Google Scholar] [CrossRef]
- Ren, M.; Fakayode, O.A.; Kong, F.; Zhou, C.; Chen, L.; Fan, X.; Liang, J.; Li, H. Characterization of cellulose nanocrystals prepared by different delignification methods and application of ultra-light, hydrophobic aerogels as oil absorbent in food systems. Ind. Crops Prod. 2023, 197, 116653. [Google Scholar] [CrossRef]
- Luchese, C.L.; Engel, J.B.; Tessaro, I.C. A Review on the Mercerization of Natural Fibers: Parameters and Effects. Korean J. Chem. Eng. 2024, 41, 571–587. [Google Scholar] [CrossRef]
- Den, W.; Sharma, V.K.; Lee, M.; Nadadur, G.; Varma, R.S. Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value-Added Chemicals. Front. Chem. 2018, 6, 141. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Fathi, M.; Ghoddusi, H.B. Nanoencapsulation of oregano essential oil using cellulose nanocrystals extracted from hazelnut shell to enhance shelf life of fruits: Case study: Pears. Int. J. Biol. Macromol. 2023, 242, 124704. [Google Scholar] [CrossRef]
- Lamaming, J.; Hashim, R.; Sulaiman, O.; Leh, C.P.; Sugimoto, T.; Nordin, N.A. Cellulose nanocrystals isolated from oil palm trunk. Carbohydr. Polym. 2015, 127, 202–208. [Google Scholar] [CrossRef]
- Brindha, R.; Narayana, C.K.; Vijayalakshmi, V.; Nachane, R.P. Effect of different retting processes on yield and quality of banana pseudostem fiber. J. Nat. Fibers 2019, 16, 58–67. [Google Scholar] [CrossRef]
- Pinheiro, M.A.; Ribeiro, M.M.; Rosa, D.L.S.; Nascimento, D.D.C.B.; Da Silva, A.C.R.; Dos Reis, M.A.L.; Monteiro, S.N.; Candido, V.S. Periquiteira (Cochlospermum orinocense): A Promising Amazon Fiber for Application in Composite Materials. Polymers 2023, 15, 2120. [Google Scholar] [CrossRef] [PubMed]
- Apaydın Varol, E.; Mutlu, Ü. TGA-FTIR Analysis of Biomass Samples Based on the Thermal Decomposition Behavior of Hemicellulose, Cellulose, and Lignin. Energies 2023, 16, 3674. [Google Scholar] [CrossRef]
- Raza, M.; Abu-Jdayil, B. Extraction of cellulose nanocrystals from date seeds using transition metal complex-assisted hydrochloric acid hydrolysis. Int. J. Biol. Macromol. 2025, 294, 139477. [Google Scholar] [CrossRef] [PubMed]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of Cellulose and Preparation of Nanocellulose from Sisal Fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.; Wang, S.; Luo, Z. Interactions of biomass components during pyrolysis: A TG-FTIR study. J. Anal. Appl. Pyrolysis 2011, 90, 213–218. [Google Scholar] [CrossRef]
- Zortea, L.F.; Pinheiro, I.R.; Mulin, L.B.; Mascarenhas, A.R.P.; Nascimento, J.N.; Tonoli, G.H.D.; Moulin, J.C.; Monteiro, S.N.; Oliveira, M.P. Improved non-woven surgical masks with nanostructured cellulosic reinforcement from sugarcane bagasse waste. J. Mater. Res. Technol. 2024, 30, 580–588. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Zattera, A.J. Structural Characteristics and thermal Properties of Native Cellulose. In Em cellulose-Fundamental Aspects; van de Ven, T., Godbout, L., Eds.; Intech: London, UK, 2013. [Google Scholar]
- Huamani-Palomino, R.G.; Mayta, S.; Córdova, B.M.; Yáñez-S, M.; Venâncio, T.; Rivera, E.; Quintana, M. Estudo do efeito de agentes branqueadores no índice cristalino de materiais à base de celulose derivados da casca de milho por espectroscopias de RMN de 13C CP/MAS e FT-IR. Carbohydr. Polym. 2024, 346, 122593. [Google Scholar]
- Norfarhana, A.S.; Ilyas, R.A.; Ngadi, N.; Othman, M.H.D.O. Optimization of Ionic Liquid Pretreatment of Sugar Palm Fiber for Cellulose Extraction. J. Mol. Liq. 2024, 398, 124256. [Google Scholar] [CrossRef]
- Burhani, D.; Septevani, A.A. Isolation of nanocellulose from oil palm empty fruit bunches using strong acid hydrolysis. AIP Conf. Proc. 2018, 2024, 020005. [Google Scholar]
- Antony Jose, S.; Cowan, N.; Davidson, M.; Godina, G.; Smith, I.; Xin, J.; Menezes, P.L. A Comprehensive Review on Cellulose Nanofibers, Nanomaterials, and Composites: Manufacturing, Properties, and Applications. Nanomaterials 2025, 15, 356. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).