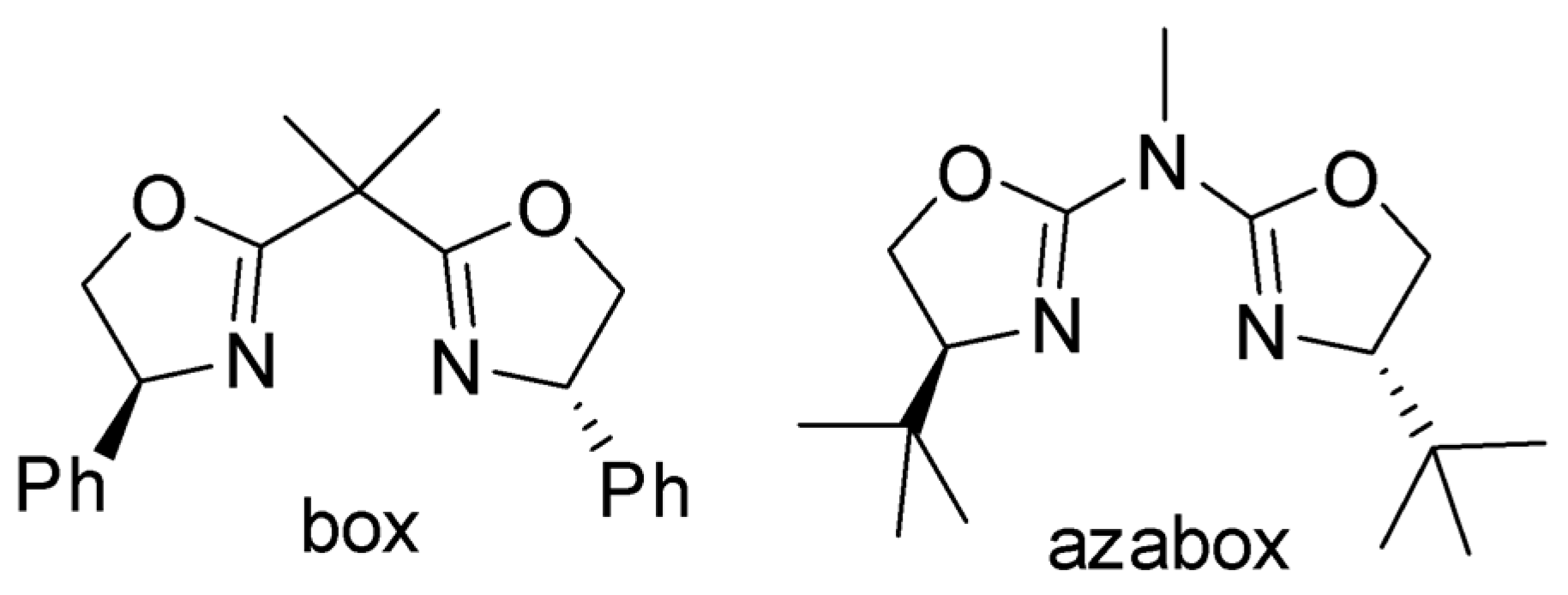
Bis- and Azabis(oxazoline)–Copper–Tungstophosphate Immobilized on Mesoporous Silica: Preparation and Use as Catalyst in Enantioselective Cyclopropanation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Mesoporous SiO2
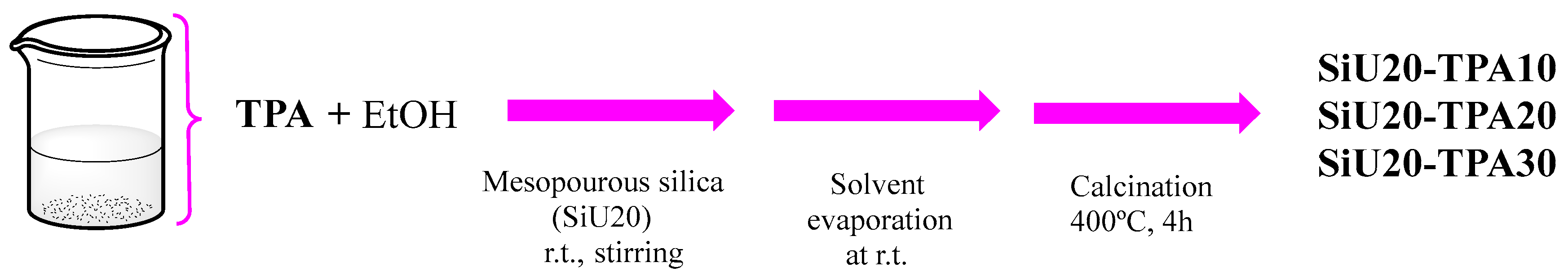
2.2. Immobilization of TPA on Mesoporous Silicas
2.3. Preparation of Box- and Azabox-Cu/SiUX-TPAY
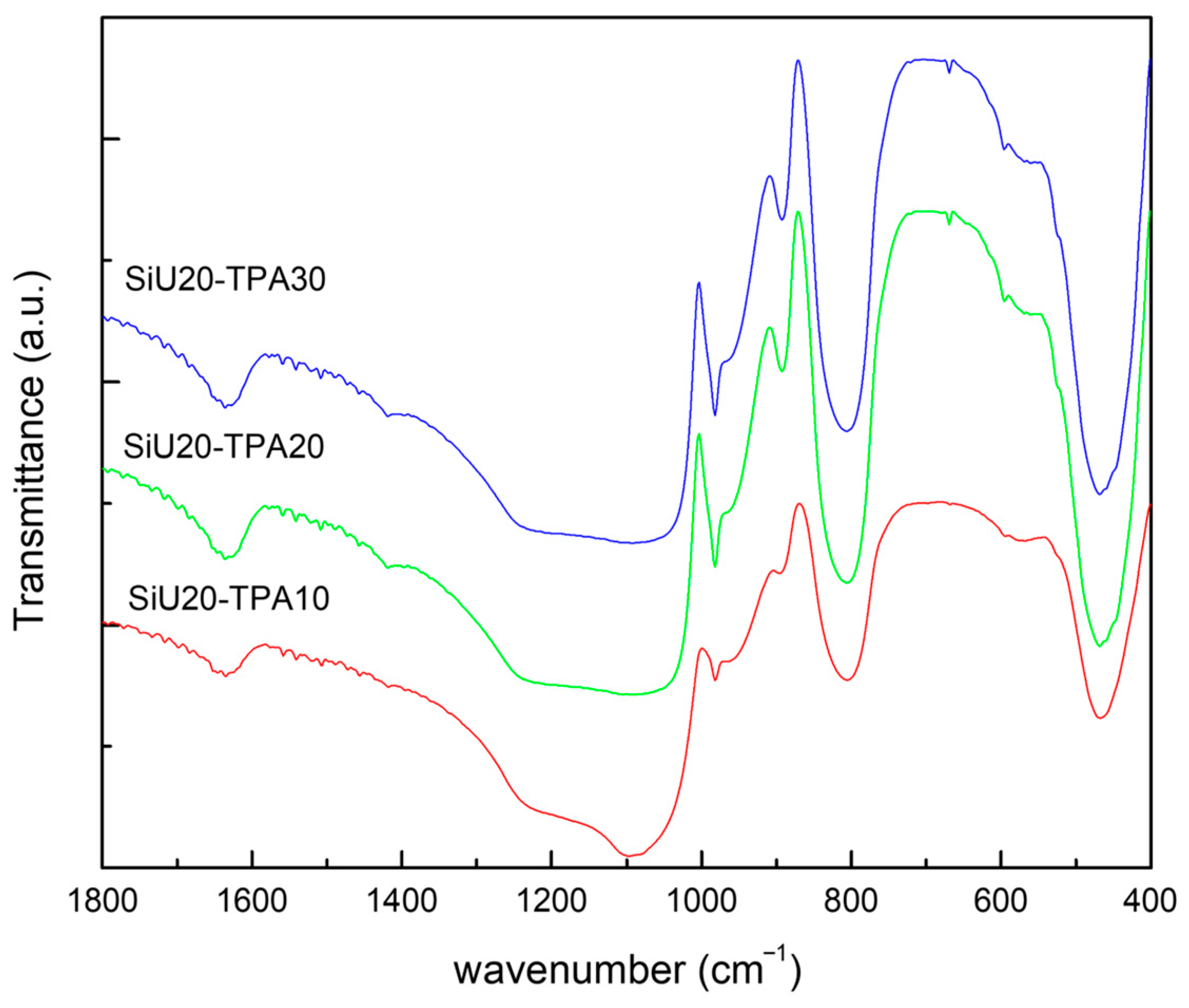
2.4. Characterization of Catalysts
2.5. Cyclopropanation Reaction
3. Results and Discussion
3.1. Preparation and Characterization of the Catalysts
3.2. Catalytic Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAS | Atomic Absorbation Spectrometry |
| EDX | Energy Disperse X-ray Analysis |
| FT-IR | Fourier Transform Infrared Spectrometry |
| HPAs | Heteropolyacids |
| ICP | Inductively Coupled Plasma |
| SBET | Specific Surface Area |
| SEM | Scanning Electron Microsoccopy |
| SMIC | Micropore Specific Surface Area |
| SiUX | Silice prepared using X amount of urea |
| SiUX-TPAY | TPA supported on SiUX with Y loading |
| TPA | Tungstophosphoric acid |
| Vp | Total Pore Volume |
| Dp | Pore Diameter |
| Ei | Initial electrode potential |
| NAS | Number of acid sites |
References
- Blaser, H.-U. Enantioselective synthesis using chiral heterogeneous catalysts. Tetrahedron Asymmetry 1991, 2, 843–866. [Google Scholar] [CrossRef]
- Blaser, H.-U.; Pugin, B. Scope and Limitations of the Application of Heterogeneous Enantioselective Catalysts. In Chiral Reactions in Heterogeneous Catalysis; Jannes, G., Dubois, V., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 33–57. [Google Scholar] [CrossRef]
- Heitbaum, M.; Glorius, F.; Escher, I. Asymmetric Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2006, 45, 4732–4762. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, A.; Liu, C.; Cheng, J.; Hu, M. Helical polyether-immobilized chiral aza-bis(oxazolines): Synthesis and synergistic effect on the enantioselectivity of Zn-catalyzed Henry reaction. Eur. Polym. J. 2023, 194, 112160. [Google Scholar] [CrossRef]
- Fraile, J.M.; García, J.I.; Mayoral, J.A. Noncovalent Immobilization of Enantioselective Catalysts. Chem. Rev. 2009, 109, 360–417. [Google Scholar] [CrossRef]
- Fraile, J.M.; García, J.I.; Mayoral, J.A.; Tarnai, T. Solvent and counterion effects in the asymmetric cyclopropanation catalyzed by bis(oxazoline)–copper complexes. J. Mol. Catal. A Chem. 1999, 144, 85–89. [Google Scholar] [CrossRef]
- Fraile, J.M.; García, J.I.; Herrerías, C.I.; Mayoral, J.A.; Harmer, M.A. Bis(oxazoline)–copper complexes supported by electrostatic interactions: Scope and limitations. J. Catal. 2004, 221, 532–540. [Google Scholar] [CrossRef]
- Torviso, R.; Mansilla, D.; Belizán, A.; Alesso, E.; Moltrasio, G.; Vázquez, P.; Pizzio, L.; Blanco, M.; Cáceres, C. Catalytic activity of Keggin heteropolycompounds in the Pechmann reaction. Appl. Catal. A Gen. 2008, 339, 53–60. [Google Scholar] [CrossRef]
- Mansilla, D.; Torviso, M.R.; Alesso, E.; Cáceres, C. Synthesis of chromanols using the eco-friendly catalyst AlPMo12O40/Carbon: The role of support. Curr. Top. Catal. 2016, 12, 61–74. [Google Scholar]
- Rengifo-Herrera, J.A.; Blanco, M.N.; Pizzio, L.R. Photocatalytic bleaching of aqueous malachite green solutions by UV-A and blue-light-illuminated TiO2 spherical nanoparticles modified with tungstophosphoric acid. Appl. Catal. B Environ. 2011, 110, 126–132. [Google Scholar] [CrossRef]
- Zsigmond, Á.; Undrala, S.; Notheisz, F.; Szöllősy, Á.; Bakos, J. Substituent effects in enantioselective hydrogenations catalyzed by immobilized Rh complexes. Appl. Catal. A Gen. 2006, 303, 29–34. [Google Scholar] [CrossRef]
- Brandts, J.A.M.; Berben, P.H. Application of Immobilized Rhodium Catalyst Precursors in Enantio- and Chemoselective Hydrogenation Reactions. Org. Proc. Res. Dev. 2003, 7, 393–398. [Google Scholar] [CrossRef]
- Zsigmond, Á.; Bogár, K.; Notheisz, F. Comparative study of “ship-in-a-bottle” and anchored heterogenized Rh complexes. J. Catal. 2003, 213, 103–108. [Google Scholar] [CrossRef]
- Desimoni, G.; Faita, G.; Jørgensen, K.A. Update 1 of: C2-Symmetric Chiral Bis(oxazoline) Ligands in Asymmetric Catalysis. Chem. Rev. 2011, 111, PR284–PR437. [Google Scholar] [CrossRef] [PubMed]
- Fraile, J.M.; García, J.I.; Herrerías, C.I.; Mayoral, J.A.; Pires, E.; Salvatella, L. Beyond reuse in chiral immobilized catalysis: The bis(oxazoline) case. Catal. Today 2009, 140, 44–50. [Google Scholar] [CrossRef]
- Fraile, J.; García, J.I.; Mayoral, J. Recent advances in the immobilization of chiral catalysts containing bis(oxazolines) and related ligands. Coord. Chem. Rev. 2008, 252, 624–646. [Google Scholar] [CrossRef]
- Zid, T.B.; Fahdli, M.; Khedher, I.; Fraile, J.M. New bis(oxazoline)-vanadyl complexes, supported by electrostatic interaction in laponite clay, as heterogeneous catalysts for asymmetric oxidation of methyl phenyl sulfide. Micropor. Mesopor. Mater. 2017, 239, 167–172. [Google Scholar] [CrossRef]
- Fadhli, M.; Khedher, I.; Fraile, J.M. Enantioselective epoxidation of styrene with TBHP catalyzed by bis(oxazoline)-vanadyl-laponite materials. Catal. Commun. 2018, 117, 90–93. [Google Scholar] [CrossRef]
- Feldman, R.A.; Fraile, J.M. Non-covalent immobilization of chiral copper complexes on Al-MCM41: Effect of the nature of the ligand. Catal. Commun. 2016, 83, 74–77. [Google Scholar] [CrossRef]
- Werner, H.; Vicha, R.; Gissibl, A.; Reiser, O. Improved Synthesis of Aza-bis(oxazoline) Ligands. J. Org. Chem. 2003, 68, 10166–10168. [Google Scholar] [CrossRef]
- Fraile, J.M.; García, J.I.; Herrerías, C.I.; Mayoral, J.A.; Reiser, O.; Socuéllamos, A.; Werner, H. The Role of Binding Constants in the Efficiency of Chiral Catalysts Immobilized by Electrostatic Interactions: The Case of Azabis(oxazoline)–Copper Complexes. Chem. Eur. J. 2004, 10, 2997–3005. [Google Scholar] [CrossRef]
- Fraile, J.M.; Pérez, I.; Mayoral, J.A.; Reiser, O. Multipurpose box- and azabox-Based Immobilized Chiral Catalysts. Adv. Synth. Catal. 2006, 348, 1680–1688. [Google Scholar] [CrossRef]
- Feldman, R.A.; Fraile, J.M. Improved methodology for non-covalent immobilization of tert-butyl-azabis(oxazoline)–copper complex on Al-MCM41. Appl. Catal A Gen. 2015, 502, 166–173. [Google Scholar] [CrossRef]
- Silva, A.R.; Guimarães, V.; Carneiro, L.; Nunes, N.; Borges, S.; Pires, J.; Martins, Â.; Carvalho, A.P. Copper(II) aza-bis(oxazoline) complex immobilized onto ITQ-2 and MCM-22 based materials as heterogeneous catalysts for the cyclopropanation of styrene. Micropor. Mesopor. Mater. 2013, 179, 231–241. [Google Scholar] [CrossRef]
- Schätz, A.; Grass, R.N.; Kainz, Q.; Stark, W.J.; Reiser, O. Cu(II)−Azabis(oxazoline) Complexes Immobilized on Magnetic Co/C Nanoparticles: Kinetic Resolution of 1,2-Diphenylethane-1,2-diol under Batch and Continuous-Flow Conditions. Chem. Mater. 2009, 22, 305–310. [Google Scholar] [CrossRef]
- Torviso, M.R.; Blanco, M.N.; Cáceres, C.V.; Fraile, J.M.; Mayoral, J.A. Supported heteropolyanions as solid counterions for the electrostatic immobilization of chiral copper complexes. J. Catal. 2010, 275, 70–77. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Shimizu, T.; Kuroda, K.; Kato, C. The Preparation of Alkyltrimethylammonium–Kanemite Complexes and Their Conversion to Microporous Materials. Bull. Chem. Soc. Jpn. 1990, 63, 988–992. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, D.; Ding, T.; Shih, W.-H.; Liu, X.; Cheng, S.Z.D.; Fu, Q. A Non-surfactant Templating Route to Mesoporous Silica Materials. Adv. Mater. 1998, 10, 313–316. [Google Scholar] [CrossRef]
- Zheng, J.-Y.; Pang, J.-B.; Qiu, K.-Y.; Wei, Y. Synthesis and characterization of mesoporous titania and silica–titania materials by urea templated sol–gel reactions. Micropor. Mesopor. Mater. 2001, 49, 189–195. [Google Scholar] [CrossRef]
- Shukla, M.S.; Hande, P.E.; Chandra, S. Porous Silica Support for Immobilizing Chiral Metal Catalyst: Unravelling the Activity of Catalyst on Asymmetric Organic Transformations. ChemistrySelect 2022, 7, 1–23. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Rocchiccioli-Deltcheff, C.; Thouvenot, R.; Franck, R. Spectres i.r. et Raman d’hétéropolyanions α—XM12O40n− de structure de type Keggin (X = BIII, SiIV, GeIV, PV, AsV et M = WVI et MoVI). Spectrochim. Acta Part A Mol. Spectrosc. 1976, 32, 587–597. [Google Scholar] [CrossRef]
- Vázquez, P.; Pizzio, L.; Cáceres, C.; Blanco, M.; Thomas, H.; Alesso, E.; Finkielsztein, L.; Lantaño, B.; Moltrasio, G.; Aguirre, J. 2-Methoxynaphthalene acylation using aluminum or copper salts of tungstophosphoric and tungstosilicic acids as catalysts. Catal. Today 2011, 173, 32–37. [Google Scholar] [CrossRef]
- Pizzio, L.R.; Vázquez, P.G.; Cáceres, C.V.; Blanco, M.N.; Alesso, E.N.; Erlich, M.I.; Torviso, R.; Finkielsztein, L.; Lantaño, B.; Moltrasio, G.Y.; et al. Influence of the Alcohol Molecular Size in the Dehydration Reaction Catalyzed by Carbon-Supported Heteropolyacids. Catal. Lett. 2004, 93, 67–73. [Google Scholar] [CrossRef]
- Méndez, L.; Torviso, R.; Pizzio, L.; Blanco, M. Tungstophosphoric acid supported on core-shell polystyrene-silica microspheres or hollow silica spheres catalyzed trisubstituted imidazole synthesis by multicomponent reaction. J. Molec. Catal. A Chem. 2015, 420, 294–302. [Google Scholar] [CrossRef]
- Torviso, M.R.; Mansilla, D.S.; Fraile, J.M.; Mayoral, J.A. The importance of copper placement in chiral catalysts supported on heteropolyanions: Lacunary vs external exchanged. Molec. Catal. 2020, 489, 110935. [Google Scholar] [CrossRef]
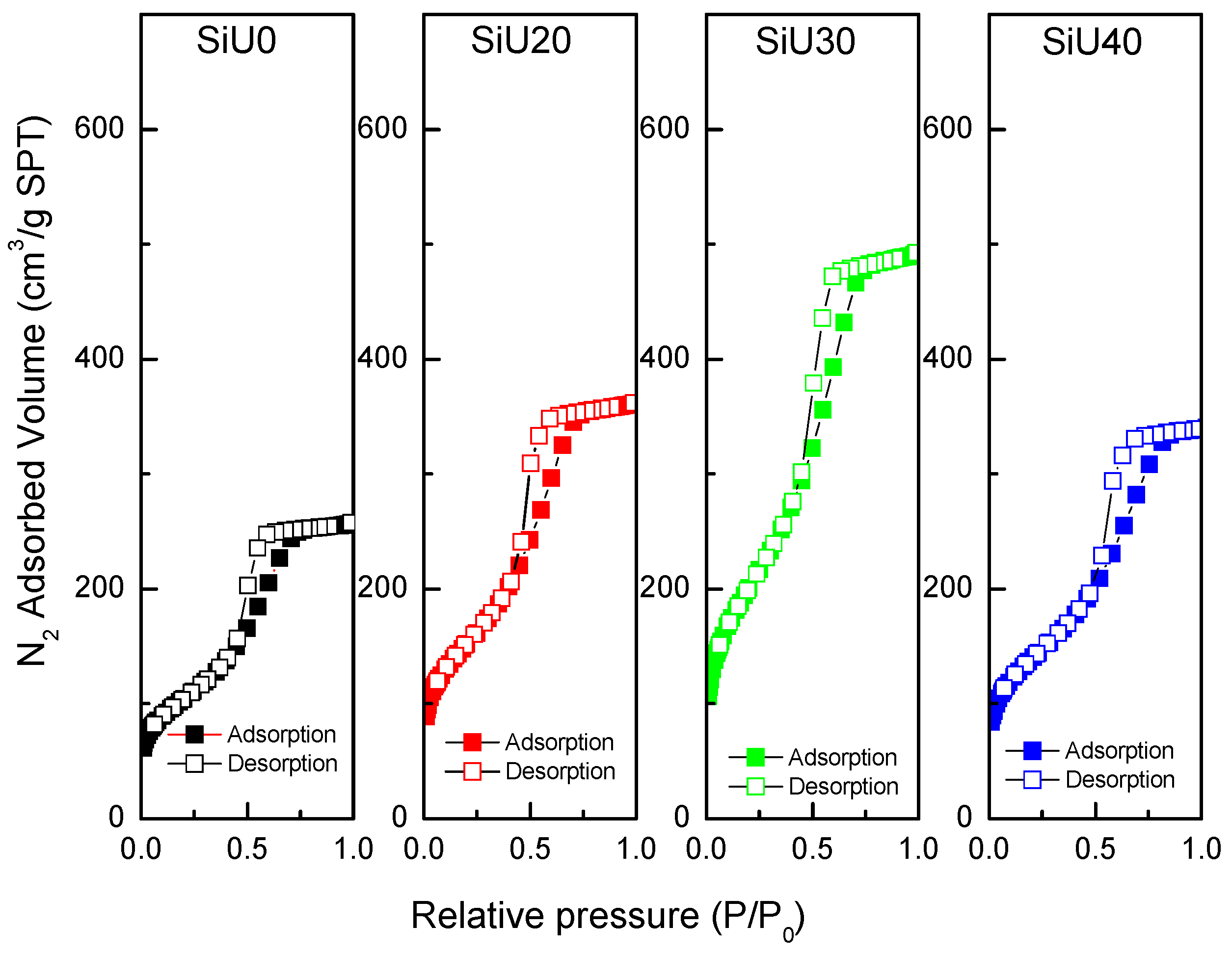

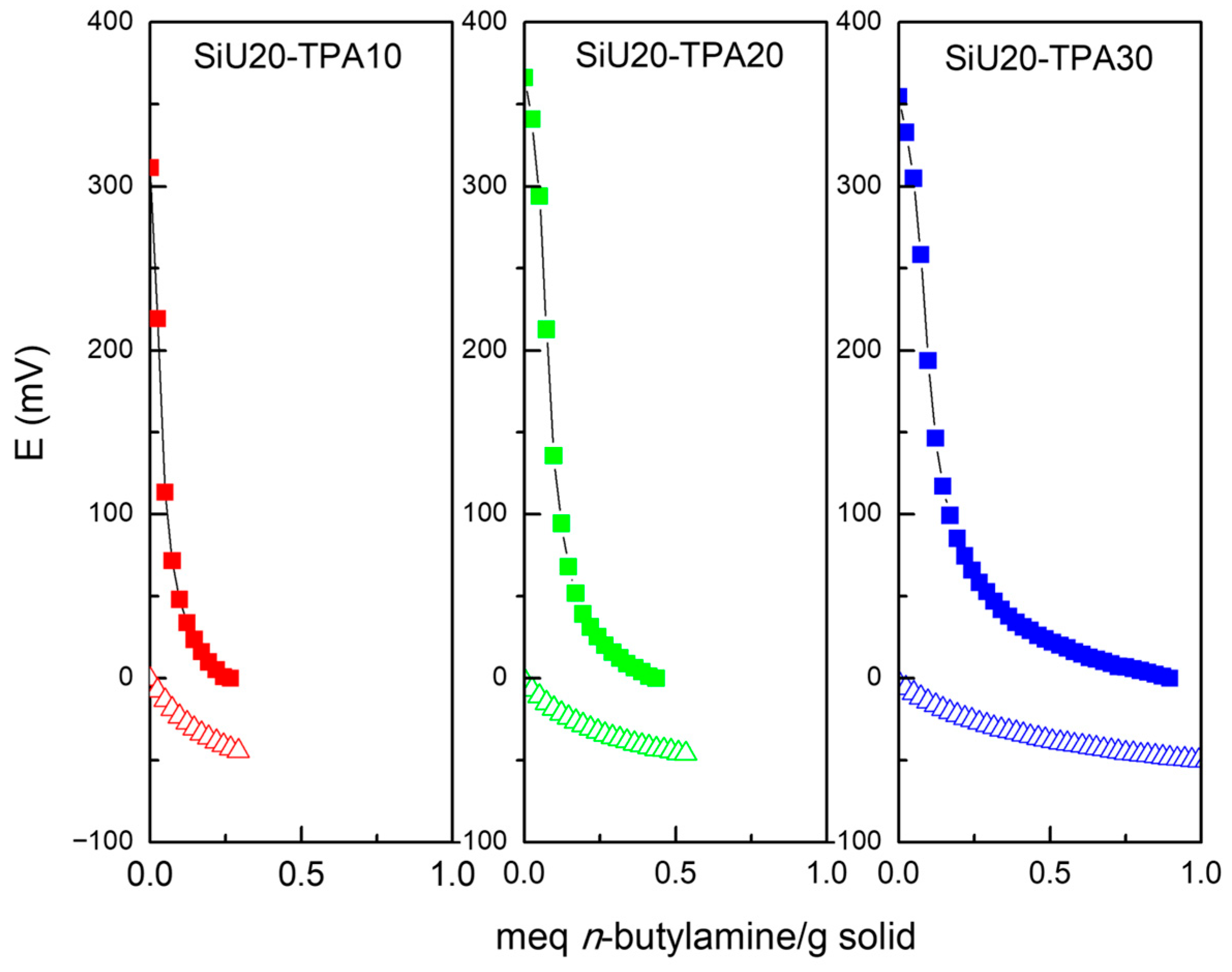

| Entry | Sample | SBET (m2/g) | SMIC (m2/g) | SMIC/SBET (%) | Vp (cm3/g) | Dp (nm) |
|---|---|---|---|---|---|---|
| 1 | SiU0 | 380 | 52 | 13.7 | 0.42 | 3.2 |
| 2 | SiU20 | 464 | 51 | 11.0 | 0.74 | 5.6 |
| 3 | SiU30 | 523 | 70 | 13.4 | 0.55 | 4.7 |
| 4 | SiU40 | 440 | 42 | 9.5 | 0.53 | 4.9 |
| Entry | Sample | % TPA (w/w) | SBET (m2/g) | SMIC (m2/g) | Vp (cm3/g) |
|---|---|---|---|---|---|
| 1 | SiU20-TPA10 | 9.6 | 259 | 67 | 0.74 |
| 2 | SiU20-TPA20 | 18.1 | 233 | 58 | 0.69 |
| 3 | SiU20-TPA30 | 29.3 | 201 | 54 | 0.60 |
| mmol/g | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Cu | W | P | Cu/TPA |
| 1 | azabox-Cu/SiU20-TPA10 | 0.032 | 0.422 | 0.032 | 1.00 |
| 2 | azabox-Cu/SiU20-TPA20 | 0.054 | 0.586 | 0.046 | 1.17 |
| 3 | azabox-Cu/SiU-TPA30 | 0.057 | 0.740 | 0.062 | 0.93 |
| 4 | box-Cu/SiU20-TPA10 | 0.057 | 0.431 | 0.034 | 1.67 |
| 5 | box-Cu/SiU20-TPA20 | 0.055 | 0.553 | 0.044 | 1.26 |
| 6 | box-Cu/SiU20-TPA30 | 0.072 | 0.797 | 0.063 | 1.14 |
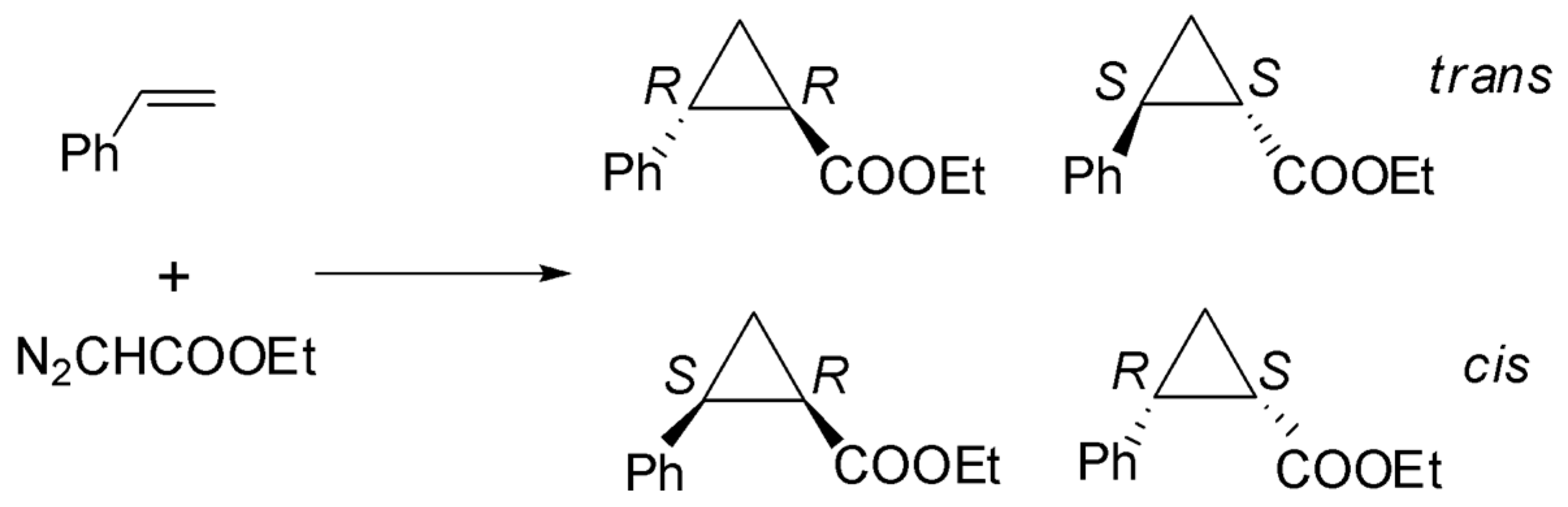
| Entry | Catalyst | Yield (%) (TON) c | trans/cis | % ee trans | % ee cis |
|---|---|---|---|---|---|
| 1 | azaboxCu/SiU20-TPA10 | 10 | 53/47 | 42 | 36 |
| cycle 2 | 8 | 57/43 | 18 | 15 | |
| 2 | azaboxCu/SiU20-TPA20 | 40 | 70/30 | 65 | 49 |
| 3 | box-Cu/SiU20-TPA20 | 22 | 65/35 | 60 | 46 |
| cycle 2 | 20 | 61/39 | 56 | 40 | |
| cycle 3 | 18 | 67/33 | 48 | 32 | |
| 4 | azaboxCu/SiU20-TPA30 | 7 | 54/45 | 35 | 34 |
| cycle 2 | 3 | 57/43 | 24 | 19 | |
| 5 | azabox2Cu/SiU20-TPA20 b | 42 | 71/29 | 90 | 71 |
| cycle 2 | 35 | 70/30 | 88 | 70 | |
| cycle 3 | 37 | 71/29 | 87 | 71 | |
| cycle 4 | 35 | 70/30 | 80 | 72 | |
| 6 | box2Cu/SiU20-TPA20 b | 25 | 70/30 | 64 | 52 |
| cycle 2 | 26 | 69/31 | 60 | 47 | |
| cycle 3 | 11 | 69/31 | 61 | 50 | |
| 7 | box2Cu/SiU20-TPA10 b | 11 | 50/49 | 41 | 32 |
| 8 | box2Cu/SiU20-TPA30 b | 11 | 53/47 | 34 | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansilla, D.S.; Pizzio, L.R.; Mayoral, J.A.; Fraile, J.M.; Torviso, M.R. Bis- and Azabis(oxazoline)–Copper–Tungstophosphate Immobilized on Mesoporous Silica: Preparation and Use as Catalyst in Enantioselective Cyclopropanation. Reactions 2025, 6, 59. https://doi.org/10.3390/reactions6040059
Mansilla DS, Pizzio LR, Mayoral JA, Fraile JM, Torviso MR. Bis- and Azabis(oxazoline)–Copper–Tungstophosphate Immobilized on Mesoporous Silica: Preparation and Use as Catalyst in Enantioselective Cyclopropanation. Reactions. 2025; 6(4):59. https://doi.org/10.3390/reactions6040059
Chicago/Turabian StyleMansilla, Daniela S., Luis R. Pizzio, José A. Mayoral, José M. Fraile, and M. Rosario Torviso. 2025. "Bis- and Azabis(oxazoline)–Copper–Tungstophosphate Immobilized on Mesoporous Silica: Preparation and Use as Catalyst in Enantioselective Cyclopropanation" Reactions 6, no. 4: 59. https://doi.org/10.3390/reactions6040059
APA StyleMansilla, D. S., Pizzio, L. R., Mayoral, J. A., Fraile, J. M., & Torviso, M. R. (2025). Bis- and Azabis(oxazoline)–Copper–Tungstophosphate Immobilized on Mesoporous Silica: Preparation and Use as Catalyst in Enantioselective Cyclopropanation. Reactions, 6(4), 59. https://doi.org/10.3390/reactions6040059







