Abstract
Edible oils are one of the renewable sources that enable the possibility of producing biodiesel sustainably. The transesterification of canola oil with methanol using cesium-modified phosphotungstic acid (Cs2.5H0.5PW12O40) as a heterogeneous catalyst was studied. Reaction conditions, specifically reaction time, catalyst loading, and the ratio of methanol to canola oil, were systematically explored. The canola oil conversion reached 55% at room temperature after 24 h. The reusability tests showed that the conversion of canola oil to biodiesel was maintained.
1. Introduction
Biodiesel is a fatty acid methyl ester produced from transesterification, where triglycerides found in oils, animal fats, and used grease react with methanol using acid or base catalysts. Biodiesel has many advantages over its petroleum counterpart, including close to zero sulfur emissions and biodegradability [1]. Biodiesel also has similar characteristics to conventional diesel, which allows it to directly replace diesel fuels with little to no modification of combustion engines. Therefore, biodiesel is a promising fuel that can mitigate the emissions produced from internal combustion. The choice of feedstock plays an important role in synthesizing biodiesel as it controls the quality of the fuel [2]. There are debates on choosing edible oils over nonedible oils due to the increasing concerns about food supply. Meanwhile, vegetable oils have been one of the traditional sources for biodiesel synthesis because of the presence of fatty acids as a major component [3].
Base catalysts, particularly sodium hydroxide, have been widely used in industry for biodiesel production [4]. Compared with acid catalysts, particularly hydrochloric acid and sulfuric acid, base catalysts have enhanced reaction rates. However, base catalysts suffer from a variety of limitations. One limitation is that the free fatty acid concentration cannot exceed 0.5 weight%, otherwise, saponification will hinder the formation of desired products. The alcohols must also be anhydrous because the existence of water would promote the hydrolysis of the products back to free fatty acids. On the other hand, acid catalysts can catalyze both esterification and transesterification reactions, which points to the direction of biodiesel production from low-cost lipid feedstocks that are associated with high free fatty acid concentrations [5]. Furthermore, acid-catalyzed transesterification shows good tolerance to water, which indicates that acid catalysts have promising practical applications [5]. Because sodium hydroxide and liquid acids are homogenous catalysts, purification and separation of products from outlet streams cannot be avoided. For example, multiple rounds of washing steps are required, which creates wastewater and high demands for reactors because of the corrosive properties of strong acid/base catalysts.
Heterogeneous solid catalysts could provide an alternative solution to overcome these challenges arising from homogeneous catalysts. When utilizing easy-operated separation techniques, the whole cost could be decreased, especially when taking into consideration the reusability potential of the catalyst. Heteropoly acids, particularly because of their strong Brønsted acidity, prevail over other heterogeneous catalysts when applied to esterification and transesterification processes [6]. The aqueous mixtures of heteropoly acids and methanol [7] or ethanol [8] have been studied for the production of biodiesel at room temperature. The reaction of methanol and canola oil using H3PW12O40 was studied for biodiesel production, but the reaction was conducted at 125 °C [9]. Those previous reports paved the direction for using heteropoly acids as catalysts for biodiesel synthesis. However, the inefficiency of post-reaction separation may lead to an increase in cost when catalysts and reactants are in the same phase.
Partial proton substitution from heteropoly acids with large cations has been considered an effective technique to increase the surface area and tune pore structures while maintaining the required acidity and increasing the insolubility [10]. Zhang [11] reported a 96.1% conversion in the esterification of oleic acid and methanol using Ag1(NH4)2PW12O40 at 70 °C. Chai [12] demonstrated an almost 100% conversion in the transesterification of vegetable oil (specifically Eruce Sativa Gar. Oil) and methanol using Cs2.5PW12O40 at 60 °C. Lee [13] further investigated the performance of a series of CsxP3−xW12O40 compounds for the transesterification of tributyrin and methanol under the same reaction temperature. They concluded that the heteropoly acid catalyst prevailed over other solid acid catalysts, including sulfate zirconia and zeolites, and did not lose activity in recycle tests.
In this study, we investigated the application of cesium-modified phosphotungstic acid, specifically Cs2.5H0.5PW12O40, for the transesterification of canola oil and methanol. Canola oil is a suitable feedstock for biodiesel production given that the majority of canola oil components are oleic acid (65%) and alfa-linolenic acid (12%). Its low saturated fat content makes it possible to withstand colder temperatures. Canola oil also meets the 50 percent greenhouse gas reduction requirements of the U.S. EPA as a raw material [14]. The choice of Cs2.5H0.5PW12O40 as the catalyst is mainly because the addition of cesium could greatly increase the insolubility and surface area of heteropoly acids [10]. We investigated the reaction conditions to maximize biodiesel production and focused on the molar ratio of methanol/oil, the catalyst loading, and the reaction time. Our results showed the feasibility of biodiesel synthesis using solid acid catalysts at room temperature.
2. Experimental Section
2.1. Materials
All chemicals, including phosphotungstic acid hydrate (reagent grade, Sigma-Aldrich, St. Louis, MI, USA), cesium carbonate (99.994%, Alfa Aesar, Haverhill, MA, USA), and methanol (HPLC grade, Fisher Scientific, Hampton, NH, USA), were used as received. The canola oil used for the reactions was purchased from the Hannaford store.
2.2. Catalyst Preparation
The synthesis of cesium-modified phosphotunstic acid (Cs2.5H0.5PW12O40) was adapted from the literature [15]. Cesium carbonate aqueous solution (82.6 mM) was added dropwise to a phosphotungstic acid aqueous solution (9.9 μM) under vigorous stirring. The synthesis process was carried out in an ice-water bath. The mixture was left to stir for one hour in the ice-water bath, followed by stirring under room temperature without ice for another 30 min. The as-prepared precipitates were separated from the solution by centrifugation. Precipitates were dried in a vacuum at 40 °C overnight. The surface area of Cs2.5H0.5PW12O40 was measured as 100 m2/g, which agreed with that of previous reports [16].
2.3. Characterization
Raman spectra were collected with an NT-MDT Raman spectrometer using a diode laser beam. An excitation wavelength of 532 nm was used. The spectra were collected by co-adding five scans of 10 s and using a laser power of 22 ± 2 mW under ambient conditions.
2.4. Transesterification Reaction
All the transesterification reactions were carried out in a 60 mL high-pressure glass tube (Ace Glass Inc., Vineland, NJ, USA). In a typical procedure, 5 mL of canola oil was added to the reactor, followed by designated amounts of the catalyst and methanol. The mixture was left to stir for certain time periods; typically, the reaction was set for 6 h. After the reaction was complete, the heterogeneous mixture was centrifuged and the solutions were used for activity testing. For reusability tests, the used catalyst was washed using 2-propanol three times, followed by centrifugation. The final washed sample was dried under vacuum at room temperature overnight.
The acid value (AV) was calculated based on the following equation [17].
where and are the volume (mL) and molar concentration (M) of potassium hydroxide, respectively. The unit of acid value is .
The conversion of canola oil was calculated using the following equation:
where AV1 and AV2 are the acid values of fresh canola oil and the liquid solutions separated from the product mixtures.
3. Results and Discussion
3.1. Effect of Reaction Time
All activity tests were performed at room temperature. Various time intervals, ranging from 3 h to 24 h, were applied to investigate how the reaction time affects the conversion of canola oil. Reaction conditions, particularly the ratio of 20:1 moles of methanol to oil and 7 wt% catalyst, were kept constant in order to strictly evaluate the role of reaction time. As shown in Figure 1a, the conversion of canola oil gradually increased as the reaction time increased. The conversion is 25% just after a 3 h reaction. The conversion reaches 55% after 24 h. It is worth emphasizing that more than 50% conversion was achieved under room temperature.
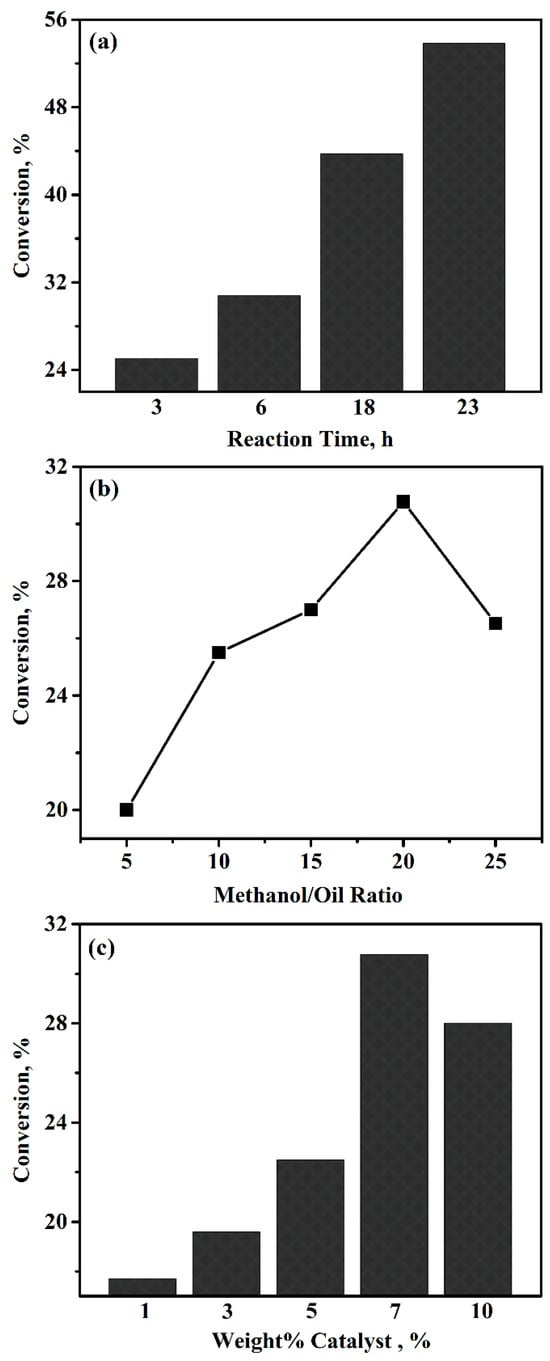
Figure 1.
The conversion of canola oil with various operation conditions: (a) reaction time (molar ratio of methanol/canola oil = 20:1, 7.0 wt% catalysts, 25 °C); (b) molar ratio of methanol to canola oil (7.0 wt% catalysts, 6 h, 25 °C); (c) amount of catalyst (molar ratio of methanol/canola oil = 20:1, 6 h, 25 °C).
3.2. Effect of Methanol/Oil Ratio
The ratio of methanol and canola oil greatly affects the conversion. Particularly, tuning the amount of methanol above the stoichiometric ratio favors the formation of biodiesel. Different molar ratios of methanol/canola oil, ranging from 10:1, 15:1, 20:1, to 25:1, were investigated. Starting with the value of 5:1, the ratio was increased to a high end while the reaction time was chosen as 6 h. As shown in Figure 1b, there is a clear increase in conversion with higher ratios of methanol to canola oil. However, the conversion of canola oil started to drop after the ratio passed the value of 20. The decrease in activity could be attributed to the “over-saturation” of the reaction, causing conversion to only occur at the catalyst’s surface [17]. Given that the molar ratio of methanol/oil at 20:1 achieved the highest conversion, this ratio was used in subsequent studies.
3.3. Effect of Catalyst Weight
The methanol-to-oil ratio was kept constant for all the runs at 20:1, while the reaction time was 6 h and the temperature was 25 °C. Meanwhile, the weight percentages of the catalyst in the reaction mixture varied with values of 1.0, 3.0, 5.0, 7.0, and 10.0. It was shown in Figure 1c that there is a steady increase in conversion up to 7.0 wt%. The highest conversion of 30.8% was achieved while the percentage was 7.0 wt%. This suggests that the transesterification reaction is completed under the reaction conditions we investigated. We also observed that at 10 wt%, the solution started to emulsify. This could be explained by the fact that the extra heteropoly acids could facilitate the emulsion reaction. Therefore, 7.0 wt% was chosen for all catalytic tests.
3.4. Catalyst Reusability
Transesterification reactions could benefit more from using solid catalysts if those catalysts could maintain high activity in sequential tests. To study the reusability of heteropoly acid catalysts, three tests were performed under the optimized reaction conditions. After each run, the liquid products were separated from the solid catalysts by centrifugation. Our results showed that the conversion of canola oil to biodiesel was maintained at 55% at room temperature under optimized reaction conditions. We also observed that the choice of solvents could affect the efficiency of removing leftover liquid components from the catalyst. Among different alcohols, including methanol, ethanol, and propanol, our results showed that 2-propanol enables us to efficiently clean catalysts in terms of washing time and the amount of solvent being applied.
3.5. Characterization
Raman spectroscopy was applied to study the structure of Cs2.5H0.5PW12O40. Figure 2 shows the characteristic Keggin structure with bands located at 530 cm−1, 898 cm−1, 985 cm−1, and 1001 cm−1 [18,19]. The lower wavelength bands, 530 cm−1 and 898 cm−1, were assigned to the W-Ob-W bridge between corner-sharing octahedra, and the W-Oc-W bridges between edge-sharing octahedra, respectively [18]. The bands at 985 cm−1 and 1001 cm−1 were attributed to symmetric and asymmetric W = Ot within WO6 octahedra [19]. These characteristic bands were observed with the fresh catalyst and the catalyst after three cyclic tests. Although the intensities of the Raman bands decreased in the samples after three reusability tests, Raman spectra confirmed that the structure of heteropoly acids was maintained. This explains why the activity did not drop after three test runs.
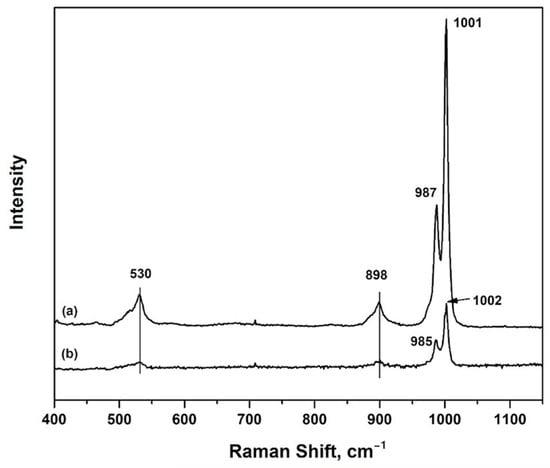
Figure 2.
Raman spectroscopy of Cs2.5H0.5PW12O40 (a) as-prepared; (b) after the 3rd recycle reactions.
Compared with previous studies [20] using cesium-modified heteropoly acid catalysts, our catalysts demonstrated that the transesterification of canola oil can occur at room temperature. The heterogenous reaction and activity maintained after reusability tests showcased the economic advantage of solid acid catalysts. To further improve conversion and yield, photocatalysis-assisted biodiesel production may prevail over other options based on the promising applications of heteropoly acids as photocatalysts [21].
4. Conclusions
We show that heteropoly acid-based catalysts can effectively transform edible oil into biodiesel at room temperature. Reaction conditions, including the molar ratio of methanol/oil, the catalyst loadings, and the reaction time, were optimized. Room temperature conversion was achieved using the Cs2.5H0.5PW12O40 catalyst. Particularly, 55% conversion was reported after 24 h. Under the optimized reaction conditions, our results also demonstrated that cesium-modified heteropoly acids show good reusability characteristics.
Author Contributions
Conceptualization, N.Y. and N.L.F.; methodology, N.Y. and N.L.F.; software, N.L.F. and G.C.; validation, N.L.F., G.C. and N.Y.; formal analysis, N.L.F., G.C. and N.Y.; investigation, N.L.F.; resources, N.Y.; data curation, N.L.F., G.C. and N.Y.; writing—original draft preparation, N.L.F. and N.Y.; writing—review and editing, N.Y.; visualization, N.L.F., G.C. and N.Y.; supervision, N.Y.; project administration, N.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available upon request from the corresponding author.
Acknowledgments
N.F. is grateful for the Undergraduate Research Award provided by the Hamel Undergraduate Researcher Center. N.Y. is supported by the University of New Hampshire’s start-up funds. The authors thank Darcy Silver for technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lee, A.F.; Bennet, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef] [PubMed]
- Melero, J.A.; Iglesias, J.; Morales, G. Heterogeneous acid catalysts for biodiesel production: Current status and future challenges. Green Chem. 2009, 11, 1285–1308. [Google Scholar] [CrossRef]
- Endalew, A.K.; Kiros, Y.; Zanzi, R. Inorganic heterogeneous catalysts for biodiesel production from vegetable oils. Biomass Bioenerg. 2011, 35, 3787–3809. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Lotero, E.; Liu, Y.; Lopez, D.E.; Suwannakarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of Biodiesel via Acid Catalysis. Ind. Eng. Chem. Res. 2005, 44, 5353–5363. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Rashid, U. Supported solid and heteropoly acid catalysts for production of biodiesel. Catal. Rev. 2017, 59, 165–188. [Google Scholar] [CrossRef]
- de Godói Silva, V.W.; Laier, L.O.; Silva, M.J.d. Novel H3PW12O40: Catalysed Esterification Reactions of Fatty Acids at Room Temperature for Biodiesel Production. Catal. Lett. 2010, 135, 207–211. [Google Scholar] [CrossRef]
- Hamad, B.; Lopes de Souza, G.; Sapaly, G.; Carneiro Rocha, M.G.; Pries de Oliveira, P.G.; Gonzalez, W.A.; Andrade Sales, E.; Essayem, N. Transesterification of rapeseed oil with ethanol over heterogeneous heteropolycids. Catal. Commun. 2008, 10, 92–97. [Google Scholar] [CrossRef]
- Narasimharao, K.; Brown, D.R.; Lee, A.F.; Newman, A.D.; Siril, P.F.; Tavener, S.J.; Wilson, K. Structure-activity relations in Cs-doped heteropolyacid catalysts for biodiesel production. J. Catal. 2007, 248, 226–234. [Google Scholar] [CrossRef]
- Timofeeva, M.N. Acid catalysis by heteropoly acids. Appl. Catal. A 2003, 256, 19–35. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Wei, F.F.; Li, Q.; Huang, J.S.; Feng, Y.M.; Zhang, Y.T. Mesoporous Ag1(NH4)2PW12O40 heteropolyacids as effective catalysts for the esterification of oleic acid to biodiesel. RSC Adv. 2017, 7, 51090–51095. [Google Scholar] [CrossRef]
- Chai, F.; Cao, F.; Zhai, F.; Chen, Y.; Wang, X.; Su, Z. Transesterification of Vegetable Oil to Biodiesel using a Heteropolyacid Solid Catalyst. Adv. Synth. Catal. 2007, 349, 1057–1065. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, M.S.; Hong, S.S. Transesterification of Canola Oil with Methanol Over Heteropolyacids. J. Biobased Mater. Bioenergy 2014, 8, 202–207. [Google Scholar] [CrossRef]
- Su, F.; Guo, Y. Advancements in solida cid catalysts for biodiesel production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Dias, J.A.; Caliman, E.; Dias, S.C.L. Effects of cesium ion exchange on acidity of 12-tungstophosphoric acid. Microporous Mesoporous Mater. 2004, 76, 221–232. [Google Scholar] [CrossRef]
- Tatematsu, S.; Hibi, Y.; Okuhara, T.; Misono, M. Preparation process and catalytic activity of CsxH3-xPW12O40. Chem. Lett. 1984, 13, 865–868. [Google Scholar] [CrossRef]
- Doyle, A.M.; Albayati, T.M.; Abbas, A.S.; Alismaeel, Z.T. Biodiesel production by esterification of oleic acid over zeolite Y prepared from kaolin. Renew. Energy 2016, 97, 19–23. [Google Scholar] [CrossRef]
- Rocchiccioli-Deltcheff, C.; Fournier, M.; Franck, R.; Thouvenot, R. Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum (VI) and tungsten (VI) compounds related to the Keggin structure. Inorg. Chem. 1983, 22, 207–216. [Google Scholar] [CrossRef]
- Matkovic, S.R.; Briand, L.E.; Bañares, M.Á. Investigation of the thermal stability of phosphotungstic Wells-Dawson heteropolyc-acid through in situ Raman spectroscopy. Mater. Res. Bull. 2011, 46, 1946–1948. [Google Scholar] [CrossRef]
- Sheikh, R.; Choi, M.S.; Im, J.S.; Park, Y.H.J. Study on the solid acid catalysts in biodiesel production from high acid value oil. Ind. Eng. Chem. 2013, 19, 1413–1419. [Google Scholar] [CrossRef]
- Hiskia, A.; Mylonas, A.; Papaconstantinou, E. Comparison of the photoredox properties of polyoxometallates and semiconducting particles. Chem. Soc. Rev. 2001, 30, 62–69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).