10.1. General Methods for the Preparation of Catalysts
Method A: preparation of 7a–e: CDI (2.3 equiv.) was added to a stirred suspension of N,N-dimethyl-l-phenylalanine (2.0 equiv.) in dry DMF (15 mL per mmol of N,N-dimethyl-l-phenylalanine), and the mixture was stirred with gentle warming until dissolved and stirring continued for 1 h. The mixture was then cooled (0 °C), and the diamine (1.0 equiv.) was added. After stirring for 24 h at room temperature, the reaction mixture was rotary evaporated under reduced pressure then co-evaporated with heptane (3 times) to remove DMF. The products 7a–e were purified by repeated column chromatography (0–20% MeOH/CHCl3).
(2S,2′S)-N,N′-(ethane-1,2-diyl)bis(2-(dimethylamino)-3-phenylpropanamide) 7a.
Method A: Dimethyl-l-phenylalanine (500 mg, 2.60 mmol), 1,2-diaminoethane (78 mg, 1.30 mmol), CDI (484 mg, 2.3 mmol) gave 7a (144 mg, 27%) as a white solid. Rf 0.32 (10% MeOH in CHCl3); Mp. 117 °C; [α]D20 5.2 (c = 1.0, CHCl3); δH 7.09–7.26 (10H, m, 2 × Ph), 6.18 (2H, br s, 2 × NH), 3.12–3.22 (4H, m, 2 × CH2), 3.03 (2H, dd, J 8.4, 12.9, 2 × CH), 2.85–2.96 (2H, m, 2 × CH), 2.79 (2H, dd, J 4.7, 13 Hz, 2 × CH), 2.25 (12H, s, 4 × Me); δC 172.2, 139.6, 129.6, 128.5, 126.3, 71.1, 42.4, 39.0, 33.9; vmax 3303, 2926, 2859, 2828, 2782, 1648, 1536, 1454, 1238. MS (ESI) m/z 206.1 (100%, [M+2H]2+), 411.3, (15%, [M+H]+); HRMS (ESI) m/z found 411.2752, C24H35N4O2+ ([M+H]+) requires 411.2755.
(2S,2′S)-N,N′-(propane-1,3-diyl)bis(2-(dimethylamino)-3-phenylpropanamide) 7b.
Method A: Dimethyl-l-phenylalanine (750 mg, 3.89 mmol), 1,3-diaminopropane (144 mg, 1.94 mmol), CDI (882 mg, 5.44 mmol) gave 7b (192 mg, 23%) as a white solid. Rf 0.23 (10% MeOH in CHCl3); Mp. 106 °C; [α]D20 +22.4 (c = 1.1, CHCl3); δH 7.24–7.25 (8H, m, 8 × CH), 7.13–7.19 (2H, m, 2 × CH), 6.97 (2H, br t, J 6.2 Hz, 2 × NH) 3.22 (2H, dd, J 5.4, 7.6 Hz, 2 × CH), 3.16 (2H, dd, J 13.3, 7.6 Hz, 2 × CH), 2.92–3.10 (4H, m, 2 × CH2), 2.89 (2H, dd, J 13.3, 5.4 Hz, 2 × CH) 2.32 (12H, s 4 × Me), 1.43 (2H, pentet, J 6.4 Hz, CH2); δC 172.3, 139.7, 129.4, 128.4, 126.2, 71.2, 42.4, 35.8, 33.6, 29.8; vmax 3310, 2973, 2933, 2775, 1639, 1533, 1494, 1266; MS (ESI) m/z 213.1 (100%, [M+2H]2+), (425.3, (98%, [M+H]+); HRMS (ESI) m/z found 425.2910, C24H37N4O2+ ([M+H]+) requires 425.2911.
(2S,2′S)-N,N′-(butane-1,4-diyl)bis(2-(dimethylamino)-3-phenylpropanamide) 7c.
Method A: Dimethyl-l-phenylalanine (500 mg, 2.59 mmol), 1,4-diaminobutane (114 mg, 1.29 mmol), CDI (503 mg, 3.10 mmol) gave 7c (189 mg, 33%) as a white solid. Rf 0.26 (10% MeOH in CHCl3); Mp. 123 °C; [α]D20 +10.0 (c = 1.0, CHCl3); δH 7.18–7.20 (8H, m, 8 × CH), 7.07–7.13 (2H, m, 2 × CH), 6.80 (2H, br t, J 6.0 Hz, 2 × NH), 3.18 (2H, dd, J 5.4, 7.5 Hz, 2 × CH), 3.02–3.13 (6H, m, 2 × CH, 2 × CH2), 2.81 (2H, dd, J 5.4, 13.5 Hz, 2 × CH), 2.25 (12H, s, 4 × Me), 1.23–1.30 (4H, m, 2 × CH2); δC 172.1, 139.9, 129.4, 128.4, 126.2, 71.0, 42.3, 38.8, 33.0, 27.0; vmax 3310, 2933, 2865, 2827, 2775, 1642, 1535, 1494, 1248; MS (ESI) m/z 220.1 (4%, [M+2H]2+), 439.3, (100%, [M+H]+); HRMS (ESI) m/z found 439.3065, C24H39N4O2+ ([M+H]+) requires 439.3068.
(2S,2′S)-N,N′-(pentane-1,5-diyl)bis(2-(dimethylamino)-3-phenylpropanamide) 7d.
Method A: Dimethyl-l-phenylalanine (500 mg, 2.58 mmol), 1,5-diaminopentane (132 mg, 1.29 mmol), CDI (502 mg, 3.10 mmol) gave 7d (191 mg, 33%) as a white solid. Rf 0.23 (10% MeOH in CHCl3); Mp. 76 °C; [α]D20 +16.0 (c = 0.96, CHCl3,); δH 7.16–7.19 (8H, m, 8 × CH), 7.08–7.13 (2H, m, 2 × CH), 6.75 (2H, br t, J 6.1 Hz, 2 × NH), 3.05–3.19 (8H, m 4 × CH, 2 × CH2), 2.81 (2H, dd, J 13.4, 5.3 Hz, 2H, 2 × CH); 2.24 (12H, s, 4 × Me), 1.28–1.36 (4H, m, 2 × CH2), 1.07–1.14 (2H, m, CH2); δC 172.0, 139.9, 129.3, 128.4, 126.2, 71.0, 42.3, 38.9, 33.0, 29.3, 24.2; vmax 3310, 2940, 1640, 1536, 1454, 1254; MS (ESI) m/z 227.1 (4%, [M+2H]2+), 445.3, (100%, [M+H]+); HRMS (ESI) m/z found 453.3221, C27H41N4O2+ ([M+H]+) requires 453.3224.
(2S,2′S)-N,N′-(hexane-1,6-diyl)bis(2-(dimethylamino)-3-phenylpropanamide) 7e.
Method A: Dimethyl-l-phenylalanine (496 mg, 1.29 mmol), 1,6-diaminopropane (150 mg, 1.94 mmol), CDI (502 mg, 3.10 mmol) gave 7b (193 mg, 31%) as a white solid. Rf 0.25 (10% MeOH in CHCl3); Mp. 98 °C; [α]D20 +12 (c = 1.1, CHCl3); δH 7.15–7.23 (8H, m, 8 × CH), 7.06–7.13 (2H, m, 2 × CH), 6.74 (2H, br t, J 5.2 Hz, 2 × NH), 3.02–3.18 (8H, m, 4 × CH2), 2.81 (2H, dd, J 13.1, 5.0 Hz, 2 × CH), 2.24 (12H, s, 4 × Me), 1.25–1.35 (4H, m, 2 × CH2), 1.07–1.21 (4H, m. 2 × CH2); δC 172.1, 140.1, 129.4, 128.4, 126.2, 71.1, 42.4, 39.0, 33.0, 29.5, 26.4; vmax 3306, 2932, 2774, 1643, 1536, 1454, 1249. MS (ESI) 467.3, (100%, [M+H]+). HRMS (ESI) m/z found 467.3378, C28H42N4O2+ ([M+H]+) requires 467.3381.
Method B, preparation of 7a, 7b, 7f, and 10.
General method: (i) CDI (2.2 equiv.) was added in portions over 5 min to a stirred solution of Z-Phe-OH (2.0 equiv.) dissolved in CH
2Cl
2 (150 mL). After 10 min, the required amine (1.0 equiv.) was added dropwise as a liquid or dissolved in a small volume of CH
2Cl
2. After 24 h, diethyl ether (100 mL) was added to the solidified mass, which was then filtered through a sinter and the solid washed with 1:1 dichloromethane:diethyl ether until the product was free of imidazole. Drying the product under vacuum gave the required intermediates in 94%, 89%, and 72% yields, respectively. In the case of the intermediate for
10, the product did not precipitate, and thus the reaction was washed with citric acid solution (aq. 10%, 4 × 50 mL), sodium bicarbonate (aq. sat., 2 × 50 mL), and brine (3 × 50 mL), dried (MgSO
4) to give the required product in 96% yield. Data for the first two bis-Cbz-protected intermediates was in full agreement with the literature [
32].
(ii) The bis-Cbz-protected intermediate (1 equiv.) was suspended in methanol (100 mL per gram), and formaldehyde solution (aq. 37%, 13 equiv.) and Pd/C (10%
w/
w, 0.50 g per g of starting material) were added. The mixture was vigorously stirred under a hydrogen atmosphere for 5–14 days. The reaction mixture was filtered through celite, which was washed with methanol and evaporated. The mass obtained was co-evaporated with water (3 × 25 mL) to remove excess formaldehyde, then co-evaporated with toluene (2 × 25 mL) to remove water. The residue was dissolved in chloroform, dried (MgSO
4), filtered, and evaporated to give the crude products. The products
7a,
7b,
7f, and
10 were purified by column chromatography (0–20% MeOH/CHCl
3) and were obtained as solids in 30%, 13%, and 76% yield, respectively. Data for
7a and
7b were identical to that reported above.
(2S,2′S)-N,N′-(heptane-1,7-diyl)bis(2-(dimethylamino)-3-phenylpropanamide) 7f.
Method B: (i) Cbz-l-phenylalanine (2.50 g, 8.35 mmol), 1,7-diaminopropane (530 mg, 4.07 mmol), and CDI (1.50 g, 9.25 mmol) gave dibenzyl ((2S,2′S)-(heptane-1,7-diylbis(azanediyl))bis(1-oxo-3-phenylpropane-1,2-diyl))dicarbamate (intermediate) (2.05 g, 2.90 mmol, 72%) as a white solid. Mp. 195–8 °C; [α]D20 +17.1 (c 4.1, DMSO); δH (d6-DMSO) 7.95 (2H, br t, J 5.8 Hz, 2 × NH), 7.48 (2H, br d, J 8.7 Hz, 2 × NH), 7.05–7.35 (20H, m, 4 × Ph), 4.89–4.97 (4H, m, 2 × CH2), 4.16–4.22 (2H, m, 2 × CH), 2.97–3.11 (4H, m, 2 × CH2), 2.93 (2H, dd, J 13.7, 4.9 Hz, 2 × CH), 2.75 (2H, dd, J 13.7, 10.0 Hz, 2 × CH), 1.16–1.50 (10H, m. 5 × CH2); δC (d6-DMSO) 171.1, 155.8, 138.1, 137.1, 129.2, 128.3, 128.0, 127.7, 127.5, 126.2, 65.2, 56.3, 38.5, 37.8, 29.0, 28.5, 26.3; vmax 3299, 3062, 3031, 2936, 2854, 1692, 1648, 1532, 1286, 1258, 1239; MS (ESI) m/z (693.4 (100%, [M+H]+); HRMS (ESI) m/z found 693.3660, C41H49N4O6+ ([M+H]+) requires 693.3647. (ii) The above Cbz-protected intermediate (1.00 g, 1.44 mmol) and formaldehyde (aq. 37%, 1.51 mL, 18.7 mmol) on hydrogenation (Pd/C 10% w/w, 0.50 g) for 5 days gave 7f (0.53 g, 1.10 mmol) in 76% yield as a white solid. Rf 0.25 (10% MeOH in CHCl3); Mp. 93–5 °C; [α]D20 +7.2 (c = 1.0, CHCl3); δH 7.17–7.41 (10H, m, 2 × Ph), 6.91 (2H, br s, 2 × NH), 3.35–3.44 (2H, m, 2 × CH), 3.14–3.26 (6H, m, 2 × CH, 2 × CH2), 2.95 (2H, dd, J 13.7, 4.7 Hz, 2 × CH), 2.40 (12H, s, 4 × Me), 1.37–1.44 (4H, m, 2 × CH2), 1.18–1.31 (6H, m. 3 × CH2); δC 171.5, 139.6, 129.4, 128.5, 126.3, 70.8, 42.2, 39.2, 33.1, 29.5, 28.8, 26.8; vmax 3268, 3086, 3028, 2928, 2858, 2826, 2772, 1641, 1557, 1250; MS (ESI) 241.2 (100%, [M+2H]2+), 481.4, (4%, [M+H]+); HRMS (ESI) m/z found 241.1808, C28H46N4O22+ ([M+2H]2+) requires 241.1805, m/z found 481.3540, C28H45N4O2+ ([M+H]+) requires 481.3537.
(2S,2′S)-N,N′-(2,2-dimethylpropane-1,3-diyl)bis(2-(dimethylamino)-3-phenylpropanamide) 10.
Method B (i) Cbz-l-phenylalanine (3.69 g, 12.33 mmol), 1,3-diamino-2,2-dimethylpropane 9 (0.60 g, 5.87 mmol), CDI (2.19 g, 13.51 mmol) gave dibenzyl ((2S,2′S)-((2,2-dimethylpropane-1,3-diyl)bis(azanediyl))bis(1-oxo-3-phenylpropane-1,2-diyl))dicarbamate (3.76 g, 5.66 mmol) in 96% yield as a white solid. Mp. 91–94 °C; [α]D20 -7.3 (c = 4.0, CHCl3); δH 7.04–7.26 (20H, m, 4 × Ph), 7.00 (2H, br s, 2 × NH), 5.51 (2H, br d, J 8.0 Hz, 2 × NH), 4.90–5.02 (4H, m, 2 × CH2), 4.30–4.47 (2H, m, 2 × CH), 2.79–3.13 (4H, m, 2 × CH2), 2.54–2.93 (2H, m, 2 × CH2) 0.55 (6H, s, 2 × CH3); δC 172.0, 156.1, 136.5, 136.2, 129.4, 128.8, 128.6, 128.3, 128.1, 127.1, 67.1, 56.5, 46.1, 34.8, 36.3, 23.5; vmax 3308, 3063, 3031, 2958, 1701, 1654, 1526, 1497, 1234, 1027, 738, 695; MS (ESI) m/z (665.3 (100%, [M+H]+); HRMS (ESI) m/z found 665.3336, C39H45N4O6+ ([M+H]+) requires 665.3334. (ii) The above Cbz-protected intermediate (1.59 g, 2.39 mmol) and formaldehyde solution (aq. 37%, 2.6 mL, 35.0 mmol) on hydrogenation (Pd/C 10% w/w, 0.50 g) for 5 days gave 10 (0.688 g, 1.48 mmol) in 63% yield as a white solid. Mp. 81–84 °C; [α]D20 +22.5 (c = 4.2, CHCl3); δH 7.07–7.23 (12H, m, 2 × Ph, 2 × NH), 3.22 (2H, dd, J 8.1, 5.5 Hz 2 × CH), 3.08 (2H, dd, J 13.7, 8.1 Hz 2 × CH), 2.86 (2H, dd, J 13.7, 5.5 Hz 2 × CH), 2.62–2.72 (4H, m, 2 × CH2), 2.30 (12H, s, 4 × Me), 0.58 (6H, m. 2 × Me); δC 172.0, 139.2, 129.3, 128.4, 126.2, 71.0, 45.6, 42.3, 36.0, 34.1, 23.6; vmax 3304, 3063, 3030, 2959, 1700, 1653, 1526, 1235, 739, 695; MS (ESI) 227.2 (100%, [M+2H]2+); HRMS (ESI) m/z found 227.1648, C27H42N4O22+ ([M+H]2+) requires 227.1648.
Method C: preparation of 7b. (i) Dibenzyl ((2
S,2′
S)-(propane-1,3-diylbis(azanediyl))bis(1-oxo-3-phenylpropane-1,2-diyl))dicarbamate [
32] (8.12 g, 12.75 mmol, prepared as in Method B, part (i)) was added in portions to HBr in AcOH (35% 100 mL, excess) and the mixture stirred for 4 h. Diethyl ether (100 mL) was added and the supernatant liquid decanted from the precipitated solid. This solid was dissolved in water (100 mL) which was extracted with diethyl ether (3 × 50 mL) then basified (NaOH to pH 12) and then further extracted with chloroform (3 × 100 mL). The combined chloroform extracts were dried (MgSO
4), filtered and evaporated to give (2
S,2′
S)-N,N’-(propane-1,3-diyl)bis(2-amino-3-phenylpropanamide) [
32] as a white solid (4.14 g, 11.24 mmol).
(ii) This product was dissolved in methanol (50 mL), formaldehyde solution (aq. 37%, 12.1 mL, 0.15 mol) and Pd/C (10%
w/
w, 1.0 g) were added, and the mixture stirred under a hydrogen atmosphere for 5 days. The mixture was filtered through a celite© pad which was washed with MeOH, and the filtrate evaporated under reduced pressure. After co-evaporation with water (3 × 50 mL) and toluene (2 × 50 mL) the solid residue was dissolved in chloroform, dried (MgSO
4), filtered and evaporated. The residue was dissolved in chloroform (100 mL) and extracted with hydrochloric acid (aq. 2N, 40 mL) and the aqueous phase separated and extracted with chloroform (2 × 50 mL) then basified with NaOH (aq. 2M to pH 14) and extracted with chloroform (3 × 50 mL). These extracts were dried and evaporated to give
7b (4.34 g, 10.22 mmol) in 80% yield as a white solid which gave identical data to that reported above.
Method D: Preparation of 10. (i) CDI (2.55 g, 15.8 mmol) was added to a stirred solution of Boc-l-phenylalanine (3.82 g, 14.39 mmol) in CH2Cl2 (50 mL). After 10 min, 1,3-diamino-2,2-dimethylpropane 9 (0.70 g, 6.85 mmol) was added and the mixture stirred for 48 h. The reaction was filtered, and the filtrate washed with citric acid solution (aq. 10%, 4 × 50 mL), sodium bicarbonate (aq. sat., 2 × 50 mL) and brine (3 × 50 mL), dried (MgSO4), filtered and evaporated to give 20 (2.98 g, 4.99 mmol) in 72% yield as a white solid. Mp. 78–82 °C; [α]D20 -9.6 (c = 4.0, CHCl3); δH 7.09–7.24 (10H, m, 4 × Ph), 6.75–6.93 (2H, m, 2 × NH), 5.08 (2H, br d, J 7.7 Hz, 2 × NH), 4.26–4.31 (2H, m, 2 × CH), 2.93–3.03 (4H, m, 2 × CH2), 2.60–2.77 (4H, m, 2 × CH2) 1.34 (18H, s, 6 × CH3), 0.62 (6H, s, 2 × CH3); δC 172.2, 155.6, 136.8, 129.4, 128.7, 127.0, 80.2, 56.2, 46.0, 38.4, 36.4, 36.4, 28.4, 23.6; vmax 3305, 3062, 2974, 2930, 1654, 1524, 1496, 1247, 1164, 698; MS (ESI) m/z (579.4 (100%, [M+H]+); HRMS (ESI) m/z found 597.3648, C33H49N4O6+ ([M+H]+) requires 597.3647. (ii) Compound 20 (1.00 g, 1.67 mmol) was dissolved in dichloromethane (10 mL), cooled (0 °C) and trifluoroacetic acid (5 mL) was added. After stirring overnight, the mixture was evaporated and dissolved in water (15 mL) following which excess NaOH (aq. 2M) was added, and the mixture extracted with chloroform (3 × 50 mL). The combined organic extract were dried (MgSO4), filtered and evaporated to give the diamine which was used in the next step without further purification. (iii) The diamine was dissolved in methanol (5 mL per gram) and formaldehyde solution (aq. 37%, 2.50 mL, 33.6 mmol, 13.0 equiv.) and RaneyNi (10% w/w, 0.25 g) were added. The mixture was vigorously stirred under a hydrogen atmosphere for 5 days and the reaction mixture was filtered through celite under a blanket of nitrogen (CAUTION RaneyNi is prone to ignition in oxygen) which was washed with further methanol. The filtrate was evaporated, and the residue obtained was co-evaporated with water (3 × 25 mL) to remove excess formaldehyde then co-evaporated with toluene (2 × 25 mL) to remove water. The residue was redissolved in chloroform, dried (MgSO4), filtered and evaporated to give 10 (0.55 g, 1.22 mmol) in 73% yield as a gum. Data for 10 was identical to that reported above.
(2S,2′S)-N,N′-(ethane-1,2-diyl)bis(1-methylpyrrolidine-2-carboxamide) 11a.
(2
S,2′
S)-
N,
N′-(Ethane-1,2-diyl)bis(pyrrolidine-2-carboxamide) [
29] (0.87 g, 3.42 mmol (Prepared by coupling Cbz-
l-Pro-OH and ethylene diamine (Method C i), 88%) then HBr/AcOH deprotection (Method D
(i), 65%)), formaldehyde solution (aq. 37%, 3.28 mL, 44.0 mmol, 13.0 equiv.) and RaneyNi (10%
w/
w, 0.25 g) in methanol (5 mL) using
Method D (iii) over 7 days (as reported above for
10) gave
11a (0.63 g, 2.23 mmol) in 65% yield as a white solid.
Mp. 154–155 °C;
Rf 0.23 (10% MeOH in CHCl
3); [α]
D20 −120.4 (c = 4, CHCl
3);
δH 7.60 (2H, br s, 2 × NH), 3.29–3.46 (4H, m, 2 × CH
2), 3.04–3.12 (2H, m, 2 × CH), 2.88 (2H, dd,
J 5.2, 10.2 Hz, 2 × CH), 2.33 (6H, s, 2 × Me), 2.30–2.37 (2H, m, 2 × CH), 2.12–2.24 (2H, m, 2 × CH), 1.63–1.84 (6H, m, 2 × CH, 2 × CH
2);
δC 175.2, 68.9, 56.7, 41.8, 38.8, 31.1, 24.3;
vmax 3275, 2964, 2938, 2872, 3840, 2782, 2763, 1658, 1510, 1457, 1427, 1226, 1048, 745;
MS (ESI) 283.2, (10%, [M+H]
+);
HRMS (ESI)
m/
z found 283.2126, C
14H
27N
4O
2+ ([M+H]
+) requires 283.2129.
(2S,2′S)-N,N′-(propane-1,2-diyl)bis(1-methylpyrrolidine-2-carboxamide) 11b.
(2
S,2′
S)-
N,
N′-(propane-1,3-diyl)bis(pyrrolidine-2-carboxamide) [
28] (Prepared by coupling Cbz-Pro-OH and propane-1,3-diamine (Method C (i), 77%) then HBr/AcOH deprotection (Method D (i), 83%). data for dibenzyl 2,2′-((propane-1,3-diylbis- (azanediyl))bis(carbonyl))(2S,2′S)-bis(pyrrolidine-1-carboxylate; gum, [α]
D20 −24 (c = 4.0 CHCl
3);
δH (d
6-DMSO) 7.83–8.02 (2H, m, 2 × NH), 7.19–7.42 (10H, m, 2 × Ph), 4.94–5.13 (4H, m, 2 × CH
2), 4.06–4.21 (2H, m, 2 × CH), 3.28–3.55 (4H, m, 2 × CH
2), 2.88–3.12 (4H, m, 2 × CH
2), 1.91–1.99 (2H, m, 2 × CH), 1.68–1.91 (6H, m, 2 × CH, 2 × CH
2), 1.37–1.56 (2H, m. CH
2);
δC (d
6-DMSO) 172.1/172.0/171.8/171.8, 145.1/153.9, 137.0, 128.4/128.3/128.2, 127.8/127.6/127.6/127.5, 127.1/127.0, 65.9/65.8/65.2, 60.3/59.7, 47.1/46.5, 36.1/36.0/36.0/35.8, 31.3/30.2, 29.2, 23.9/23.1;
vmax 3292, 3065, 2952, 2879, 1692, 1655, 1532, 1411, 1354, 1239, 1209, 1175, 1118, 1090, 917, 728, 696;
MS (ESI)
m/
z 537.3 (100%, [M+H]
+);
HRMS (ESI)
m/
z found 537.2708, C
29H
37N
4O
6+ ([M+H]
+) requires 537.2708.), 0.63 g, 2.35 mmol), formaldehyde solution (aq. 37%, 2.45 mL, 32.6 mmol, 13.9 equiv.) and RaneyNi (10%
w/
w, 0.25 g) in methanol (4 mL) using Method D
(iii) gave
11b (0.41 g, 1.38 mmol) in 59% yield as a white solid.
Mp. 99–102 °C;
Rf 0.29 (10% MeOH in CHCl
3); [α]
D20 −132.0 (c = 4, CHCl
3);
δH 7.57 (2H, br s, 2 × NH), 3.17–3.31 (4H, m, 2 × CH
2), 3.03–3.15 (2H, m, 2 × CH), 2.88 (2H, dd,
J 4.8, 9.9 Hz, 2 × CH), 2.35 (6H, s, 2 × Me), 2.28–2.41 (2H, m, 2 × CH), 2.13–2.25 (2H, m, 2 × CH), 1.61–1.86 (8H, m, 2 × CH, 3 × CH
2);
δC 174.8, 69.0, 56.7, 41.8, 35.7, 31.1, 30.1, 24.2;
vmax 3290, 2956, 2940, 2926, 2878, 2744, 1651, 1523, 1457, 1152, 774:
MS (ESI) 227.2, (10%, [M+H]
+);
HRMS (ESI)
m/
z found 297.2283, C
15H
29N
4O
2+ ([M+H]
+) requires 297.2285.
(2S,2′S)-N,N′-(2,2-dimethylpropane-1,3-diyl)bis(1-methylpyrrolidine-2-carboxamide) 12.
(i) Cbz-l-proline (2.28 g, 9.15 mmol, 2.10 equiv.), diamine 9 (0.44 g, 4.31 mmol, 1.0 equiv.) and CDI (1.92 g, 11.84 mmol, 2.6 equiv.) gave dibenzyl 2,2′-(((2,2-dimethylpropane-1,3-diyl)bis(azanediyl)) bis(carbonyl))(2S,2′S)-bis(pyrrolidine-1-carboxylate) (2.44 g, 4.32 mmol) in 95% yield as a gum using Method B, part (i). [α]D20 -51 (c = 3.0, CHCl3); δH (mixture of rotamers) 8.08/8.13/8.24/8.39/8.52 (2H, br s, NH), 7.04–7.58 (11H, m, 2 × Ph, NH), 4.90–5.19 (4H, m, 2 × CH2), 4.29–4.38 (2H, m, 2 × CH), 3.39–3.76 (4H, m, 2 × CH2), 2.61–3.10 (4H, m, 2 × CH2) 1.86–2.25 (8H, m, 4 × CH2), 0.61/0.69/0.80/0.88 (6H, br s, 2 × Me); δC 172.6/173.0, 154.9/155.6, 149.3, 148.5, 136.3/136.5, 136.2/137.1, 133.7/133.9, 127.7/127.8/128.0/128.4/128.5/128.7/128.8/129.2/130.0/130.5, 119.7, 117.2, 116.0, 69.9, 67.1, 60.8/61.0, 45.2/45.9/46.6/46.9/47.4, 36.6, 31.4, 29.1/29.4, 24.5/24.7, 23.4/23.6. vmax 3292, 3056, 2952, 2880, 1692, 1655, 1411, 1354, 1090, 728, 696; MS (ESI) 565.3, (100%, [M+H]+); HRMS (ESI) m/z found 565.3020, C31H41N4O6+ ([M+H]+) requires 565.3021. (ii) The above compound (2.33 g, 4.13 mmol) was deprotected with HBr/AcOH as in method C part (ii) to give (2S,2′S)-N,N′-(2,2-dimethylpropane-1,3-diyl)bis(pyrrolidine-2-carboxamide) (1.00 g, 3.38 mmol) in 82% yield as a gum which was used in the next step without further purification. [α]D20 -73.8 (c 2.75, CHCl3); δH (d6-DMSO) 8.13 (2H, t, J 6.8 Hz, 2 × NH), 3.52 (2H, dd, J 5.3, 8.6 2 × CH), 3.33 (2H, br s, 2 × NH obscured), 2.65–2.95 (8H, m, 4 × CH2), 1.83–2.04 (2H, m, 2 × CH) 1.55–1.74 (6H, m, 2 × CH, 2 × CH2), 0.74 (6H, s, 2 × Me); δC 174.8, 60.3, 46.7, 45.0, 36.5, 30.7, 25.8, 23.2; vmax 3359, 3281, 2959, 2870, 1639, 1523; MS (ESI) 149.1, (100%, [M+H]2+); 297.2, (5%, [M+H]+); HRMS (ESI) m/z found 297.2282, C15H29N4O2+ ([M+H]+) requires 297.2285. (ii) The above compound (0.88 g, 2.97 mmol), formaldehyde solution (aq. 37%, 4.7 mL, 63.0 mmol, 21.0 equiv.) and RaneyNi (10% w/w, 0.25 g) in methanol (5 mL) using Method D (iii) (as reported above for 10) gave crude material which was purified by column chromatography (0–2% MeOH in chloroform) gave 12 (0.38 g, 1.14 mmol) in 38% yield as a gum. Rf 0.38 (10% MeOH in CHCl3); [α]D20 -24 (c = 4, CHCl3); δH 7.78 (2H, t, J 7.1 Hz, 2 × NH), 3.10–3.15 (2H, m, 2 × CH), 3.00 (2H, dd, J 6.9, 13.7 Hz, 2 × CH), 2.96 (2H, dd, J 6.9, 13.7 Hz, 2 × CH), 2.88 (2H, dd, J 5.2, 10.2 Hz, 2 × CH), 2.37 (6H, s, 2 × Me), 2.29–2.35 (2H, m, 2 × CH), 2.13–2.24 (2H, m, 2 × CH), 1.71–1.83 (6H, m, 2 × CH, 2 × CH2) 0.85 (6H, s, 2 × CH3); δC 175.1, 69.1, 56.7, 45.4, 41.8, 36.8, 31.3, 24.3, 23.6; vmax 3360, 3283, 2959, 2870, 2845, 2785, 1648, 1518, 1451, 1198, 1179, 979; MS (ESI) 163.1 (100%, [M+2H]2+); 325.3, (15%, [M+H]+); HRMS m/z found 163.1334, C17H32N4O22+ ([M+2H]2+) requires 163.1335; (ESI) m/z found 325.2596, C17H32N4O2+ ([M+H]+) requires 325.2598.
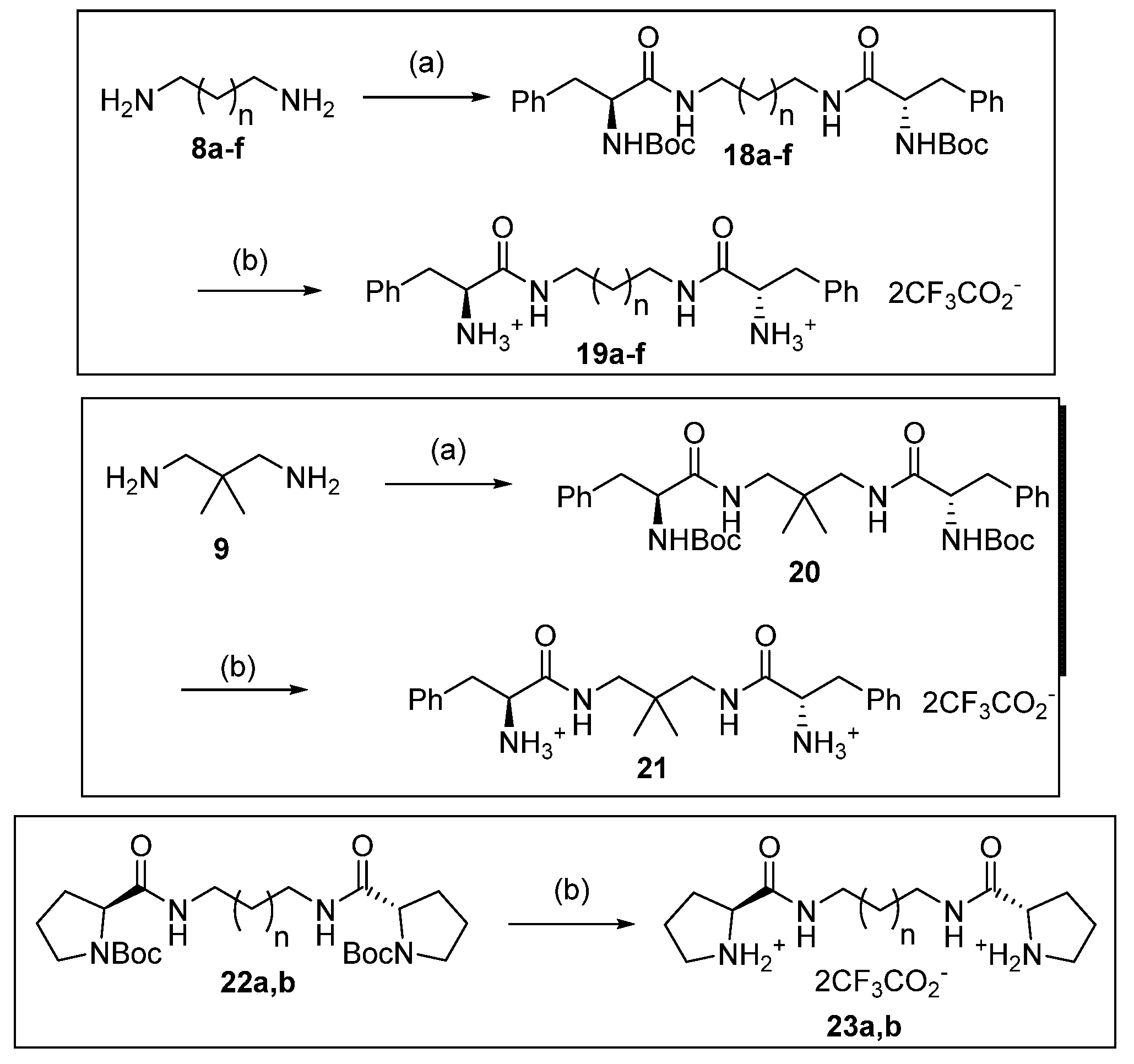
10.3. Preparation of 19a–f, 21, 23a,b and 25
Compounds
19a–f, 21 and
23a,b were prepared by dissolving the corresponding Boc-protected precursor
18a–f, [
25,
26,
27]
20 or
22a,b [
28,
29] (0.17 mmol) in chloroform (3 mL) which was cooled (0°C) and trifluoroacetic acid (1–1.5 mL) was added. After stirring at rt for 3 h the reaction was evaporated to dryness and the product dried under vacuum for 24 h. Compound
25 was prepared from diamine
24 (50 mg, 0.17 mmol) by dissolving in chloroform (3 mL) which was cooled (0°C) and trifluoroacetic acid (1 mL) added, then evaporated to dryness under vacuum for 24 h. The catalysts were used directly in the aldol reaction without further purification.
(2S,2′S)-1,1′-(ethane-1,2-diylbis(azanediyl))bis(1-oxo-3-phenylpropan-2-aminium) bistrifluoroacetate salt 19a.
White solid, Mp. 155–159 °C; [α]D20 +36.1 (c 6.1, MeOH); δH (d6-DMSO) 8.61 (2H, br s 2 × NH), 8.31 (6H, br s, 6 × NH), 7.03–7.51 (10H, m, 2 × Ph), 3.86–4.01 (2H, m, 2 × CH), 2.84–3.11 (8H, m, 4 × CH2); δC 168.1, 158.7 (C, q, 2JCF 33.6 Hz) 135.1, 129.5, 128.6, 127.2, 116.6 (C, q, JCF 296.5 Hz), 53.8, 38.0, 37.0; vmax 3321, 3112, 2981, 2867, 2823, 1743, 1671, 1552, 1496, 1058, 655; MS (ESI) 178.1, (100%, [M]2+); HRMS (ESI) m/z found 178.1100, C20H28N4O22+ ([M]2+) requires 178.1101.
(2S,2′S)-1,1′-(propane-1,2-diylbis(azanediyl))bis(1-oxo-3-phenylpropan-2-aminium) bistrifluoroacetate salt 19b.
Gum; [α]D20 +19.3 (c 10.3, MeOH); δH (d6-DMSO, mixture of rotamers) 8.40 (2H, t, J 5.7 Hz, 2 × NH), 8.03/8.31 (6H, br s, 6 × NH), 7.19–7.32 (10H, m, 2 × Ph), 3.86–4.03 (2H, m, 2 × CH), 2.73–3.07 (8H, m, 4 × CH2), 1.35/1.86 (2H, 2 × pentet, J 7.6/6.7 Hz CH2); δC 167.8, 158.7 (C, q, 2JCF 33.6 Hz) 135.1, 129.5, 128.6, 127.2, 116.5 (C, q, JCF 296.6 Hz), 53.7, 37.2, 36.3/36.4, 25.3/28.3; vmax 3095, 2980, 2873, 1764, 1664, 1499, 1140, 629; MS (ESI) 185.1, (100%, [M]2+); HRMS (ESI) m/z found 185.1179, C21H30N4O22+ ([M]2+) requires 185.1179.
(2S,2′S)-1,1′-(butane-1,2-diylbis(azanediyl))bis(1-oxo-3-phenylpropan-2-aminium) bistrifluoroacetate salt 19c.
Gum; [α]D20 +46.5 (c 4.0, MeOH); δH (d6-DMSO) 8.40 (2H, t, J 5.7 Hz, 2 × NH), 8.31 (6H, br s, 6 × NH), 7.19–7.35 (10H, m, 2 × Ph), 3.89–4.03 (2H, m, 2 × CH), 2.81–3.14 (8H, m, 4 × CH2), 1.05–1.26 (4H, m, 2 × CH2); δC 167.8, 158.7 (C, q, 2JCF 34.7 Hz) 135.2, 129.6, 128.6, 127.2, 116.4 (C, q, JCF 294.0 Hz), 53.7, 38.3, 37.3, 26.0; vmax 2941, 1776, 1663, 1137, 700; MS (ESI) 192.1, (100%, [M]2+); HRMS (ESI) m/z found 192.1256, C21H30N4O22+ ([M]2+) requires 192.1257.
(2S,2′S)-1,1′-(pentane-1,2-diylbis(azanediyl))bis(1-oxo-3-phenylpropan-2-aminium) bistrifluoroacetate salt 19d.
Gum; [α]D20 +54.2 (c 4.0, MeOH); δH (d6-DMSO) 8.40 (2H, t, J 5.5 Hz, 2 × NH), 8.27 (6H, br s, 6 × NH), 7.19–7.34 (10H, m, 2 × Ph), 3.86–3.98 (2H, m, 2 × CH), 2.93–3.12 (4H, m, 2 × CH, 2 × CH2), 2.81–2.92 (2H, m, 2 × CH), 1.12–1.26 (4H, m, 2 × CH2), 0.90–1.01 (2H, m, CH2); δC 167.5, 158.5 (C, q, 2JCF 35.1 Hz) 135.0, 129.5, 128.5, 127.2, 116.2 (C, q, JCF 293.4 Hz), 53.6, 38.5, 37.2, 28.4, 23.5; vmax 2945, 1763, 1664, 1147, 700; MS (ESI) 199.1, (100%, [M]2+); HRMS (ESI) m/z found 199.1335, C21H30N4O22+ ([M]2+) requires 199.1335.
(2S,2′S)-1,1′-(hexane-1,2-diylbis(azanediyl))bis(1-oxo-3-phenylpropan-2-aminium) bistrifluoroacetate salt 19e.
Gum; [α]D20 +39.0 (c 4.0, MeOH); δH (d6-DMSO) 8.34 (2H, t, J 5.5 Hz, 2 × NH), 8.28 (6H, br s, 6 × NH), 7.20–7.33 (10H, m, 2 × Ph), 3.87–4.00 (2H, m, 2 × CH), 3.04–3.14 (2H, m, 2 × CH), 2.99 (4H, d, J 6.3 Hz, 2 × CH2), 2.86–2.96 (2H, m, 2 × CH), 1.12–1.32 (4H, m, 2 × CH2), 0.96–1.12 (4H, m, 2 × CH2); δC 167.9, 158.8 (C, q, 2JCF 35.0 Hz) 139.8, 129.9, 128.9, 127.6, 117.1 (C, q, JCF 296.8 Hz), 54.0, 39.1, 37.6, 29.1, 26.4; vmax 2940, 1763, 1663, 1146, 699; MS (ESI) 206.1, (100%, [M]2+); HRMS (ESI) m/z found 206.1415, C21H30N4O22+ ([M]2+) requires 206.1414.
(2S,2′S)-1,1′-(heptane-1,2-diylbis(azanediyl))bis(1-oxo-3-phenylpropan-2-aminium) bistrifluoroacetate salt 19f.
Gum; [α]D20 +42.9 (c 4.0, MeOH); δH (d6-DMSO) 8.34 (2H, t, J 5.6 Hz, 2 × NH), 8.29 (6H, br s, 6 × NH), 7.20–7.33 (10H, m, 2 × Ph), 3.88–4.00 (2H, m, 2 × CH), 3.06–3.15 (2H, m, 2 × CH), 3.00 (4H, d, J 6.9 Hz, 2 × CH2), 2.86–2.96 (2H, m, 2 × CH), 1.18–1.35 (4H, m, 2 × CH2), 1.02–1.17 (6H, m, 3 × CH2); δC 167.6, 158.6 (C, q, 2JCF 35.8 Hz) 135.1, 129.5, 128.5, 127.2, 116.1 (C, q, JCF 296.0 Hz), 53.6, 38.7, 37.2, 37.6, 28.7, 28.5, 26.3; vmax 2938, 2863, 1776, 1662, 1141, 700; MS (ESI) 213.2, (100%, [M]2+); HRMS (ESI) m/z found 213.1492, C21H30N4O22+ ([M]2+) requires 213.1492.
(2S,2′S)-1,1′-((2,2-Dimethylpropane-1,3-diyl)bis(azanediyl))bis(1-oxo-3-phenylpropan-2-aminium) bistrifluoroacetate salt 21.
Gum; [α]D20 +45.8 (c 4.0, MeOH); δH (d6-DMSO) 8.21 (6H, s 6 × NH), 8.22 (2H, t, J 5.4 Hz, 2 × NH), 7.20–7.42 (10H, m, 2 × Ph), 3.99–4.15 (2H, m, 2 × CH), 3.01 (4H, d, J 7.3 Hz, 2 × CH2), 2.85 (2H, dd, J 6.7, 13.5 Hz, 2 × CH), 2.85 (2H, dd, J 5.7, 13.5 Hz, 2 × CH), 0.56 (6H, s, 2 × Me); δC 168.5, 158.8 (C, q, 2JCF 35.7 Hz), 135.1, 129.6, 128.8, 127.4, 116.1 (C, d, JCF 269.6 Hz), 53.8, 46.7, 37.4, 36.2, 25.9; vmax 2968, 1764, 1666, 1145, 699; MS (ESI) 199.1, (100%, [M]2+); HRMS (ESI) m/z found 199.1334, C23H36N4O22+ ([M]2+) requires 199.1335.
(2S,2′S)-2,2′-((ethane-1,2-diylbis(azanediyl))bis(carbonyl))bis(pyrrolidin-1-ium) bistrifluoroacetate salt 23a.
Gum; [α]D20 −22.8 (c 4.0, MeOH); δH (d6-DMSO) 9.81 (2H, br s, 2 × NH), 8.68–8.78 (2H, m, 2 × NH), 8.53 (2H, br s, 2 × NH), 4.09–4.19 (2H, m, 2 × CH), 3.12–3.30 (8H, m, 2 × CH2), 2.18–2.30 (2H, m, 2 × CH), 1.80–1.92 (6H, m, 2 × CH, 2 × CH2); δC 168.4, 158.8 (C, q, 2JCF 34.7 Hz), 116.8 (C, d, JCF 295.1 Hz), 59.1, 45.7, 38.5, 29.5, 23.6; vmax 3290, 3085, 2992, 2958, 1665, 1572, 1169, 1131, 835, 796, 720, 678; MS (ESI) 128.1 (100%, [M]2+); HRMS (ESI) m/z found 128.0942, C12H24N4O22+ ([M]2+) requires 128.0944.
(2S,2′S)-2,2′-((ethane-1,2-diylbis(azanediyl))bis(carbonyl))bis(pyrrolidin-1-ium) bistrifluoroacetate salt 23b.
Gum; [α]D20 −36.7 (c 4.0, MeOH); δH (d6-DMSO) 9.63–9.96 (2H, br m, 2 × NH), 8.63 (2H, t, J 5.6 Hz, 2 × NH), 8.44–8.59 (2H, br m, 2 × NH), 4.08–4.27 (2H, m, 2 × CH), 3.06–3.33 (8H, m, 4 × CH2), 2.18–2.39 (2H, m, 2 × CH), 1.75–1.97 (6H, m, 2 × CH, 2 × CH2), 1.53–1.70 (2H, m, CH2); δC 168.2, 159.0 (C, q, 2JCF 35.4 Hz), 116.5 (C, q, JCF 292.8 Hz), 59.2, 45.7, 36.7, 29.8, 28.7, 23.7; vmax 3290, 3092, 2967, 1779, 1665, 1567, 1168, 1132, 835, 797, 721, 704; MS (ESI) 135.1 (100%, [M]2+); HRMS (ESI) m/z found 135.1021, C13H26N4O22+ ([M]2+) requires 135.1022.
Di-tert-butyl ((2S, 2′S, 3R, 3′R)-(ethane-1,2-diylbis(azanediyl))bis(3-hydroxy-1-oxobutane-1,2-diyl))dicarbamate 25.
EDC.HCl (887 mg, 4.63 mmol, 2.30 equiv.), HOBt.H2O (840 mg, 6.50 mmol, 3.23 equiv.), DIPEA (0.79 mL, 585 mg, 4.52 mmol, 2.25 equiv.) and DMAP (50 mg, 0.41 mmol, 0.20 equiv.) were added sequentially to a cooled (0 °C) solution of l-Boc-Thr 24 (908 mg, 4.14 mmol, 2.06 equiv.) in dry DMF (40 mL) After 1 h, ethylene diamine 8a (n = 0, 121 mg, 0.134 mL, 2.01 mmol, 1.00 equiv.) was added and the resulting mixture was stirred to rt for 48 h. The mixture was diluted with EtOAc (150 mL), washed with NaHCO3 solution (aq., sat. 2 × 50 mL) and brine (2 × 100 mL), then dried (MgSO4) and evaporated under reduced pressure. Column chromatography of the crude product (0–2% MeOH in CH2Cl2) gave 25 (645 mg, 1.39 mmol) in 69% yield as a white solid. [α]D20 +14.5 (c 4.0, CHCl3); Mp. 82–85 °C; δH 7.19–7.36 (2H, br m, 2 × NH), 5.72–5.94 (2H, br m, 2 × NH), 4.28–5.39 (2H, br m, 2 × CH), 4.18 (2H, br s, 2 × OH) 4.04 (2H, br d, J 6.0 Hz, 2 × CH), 3.44–3.61 (2H, br m, 2 × CH), 3.19–3.26 (2H, br m, 2 × CH), 1.43 (18H, s, 6 × Me), 1.17 (6H, d, J 6.0 Hz, 2 × Me); δC 172.9, 156.5, 80.5, 67.2, 59.8, 39.8, 28.4, 19.3; vmax 3326, 2978, 2935, 1688, 1649, 1497, 1366, 1248, 1161, 910, 729; MS (ESI) 463.3 (100%, [M+H]+); HRMS (ESI) m/z found 463.2753, C20H38N4O8+ ([M+H]+) 463.2762.
(2S, 2′S, 3R, 3′R)-1,1′-(ethane-1,2-diylbis(azanediyl))bis(3-hydroxy-1-oxobutan-2-aminium) bistrifluoroacetate 26.
Compound 25 (105 mg, 0.228 mmol) was dissolved in chloroform (4 mL), cooled (0 °C) and trifluoroacetic acid (2 mL) was added. After stirring to rt over 24 h the reaction was evaporated to dryness and the product dried under vacuum for 24 h to give 26 (109 mg) as a gum. This was used directly in the aldol reaction without further purification. [α]D20 +3.6 (c 2.7, MeOH); δH 8.63–8.70 (2H, br m, 2 × NH), 8.01–8.22 (4H, br m, 2 × NH2), 6.73–8.34 (2H, br s, 2 × OH), 3.85–3.93 (2H, m, 2 × CH), 3.42–3.54 (2H, m, 2 × CH), 3.08–3.31 (4H, m, 2 × CH2), 1.12 (6H, d, J 6.3 Hz); δC 167.2, 158.6 (C, q, 2JCF 35.8 Hz), 116.3 (C, q, JCF 296.0 Hz), 65.7, 58.5, 38.2, 20.0; vmax 3254, 3087, 2983, 1662, 1534, 1169, 1181, 1130, 839, 799, 722; MS (ESI) 132.1 (100%, [M]2+), 263.2 (25%, [M-H]+); HRMS (ESI) m/z found 103.0892, C21H30N4O22+ ([M]2+) requires 103.0893; m/z found 263.1712, C21H30N4O22+ ([M-H]+) requires 263.1714.