Abstract
The goal of finding efficient and safe hydrogen storage material motivated researchers to develop several materials to fulfil the demand of the U.S. Department of Energy (DOE). In the past few years, several metal hydrides, complex hydrides such as borohydrides and alanates, have been researched and found efficient due to their high gravimetric and volumetric density. However, the development of these materials is still limited by their high thermodynamic stability and sluggish kinetics. One of the methods to improve the kinetics is to use catalysts. Among the known catalysts for this purpose, transition metals and their compounds are known as the leading contender. The present article reviews the d-block transition metals including Ni, Co, V, Ti, Fe and Nb as catalysts to boost up the kinetics of several hydride systems. Various binary and ternary metal oxides, halides and their combinations, porous structured hybrid designs and metal-based Mxenes have been discussed as catalysts to enhance the de/rehydrogenation kinetics and cycling performance of hydrogen storage systems.
Keywords:
metal hydrides; complex hydrides; hydrogen storage; kinetics; catalysts; activation energy 1. Introduction
The search for clean and abundant energy sources to compete with fossil fuels brings us to hydrogen as a next-generation energy carrier. Hydrogen is a promising alternative energy carrier as a long-term solution with the only product as water and not CO2. To establish hydrogen infrastructure, its efficient storage is warranted. There are several technologies available in the market for hydrogen storage, but these technologies are still not good in terms of cost and handling, which are critical issues. To guide the technological and scientific community, the U.S. Department of Energy (DOE) set targets for onboard hydrogen storage to achieve the required capacity by the year. For example, 5.5 wt% gravimetric capacity or a 40 g H2/L volumetric capacity is the target by the end of 2025, whereas it is set to be 6.5 wt% gravimetric capacity or a 55 g H2/L volumetric capacity as ultimate target [1]. The technologies involved in hydrogen storage are liquefied hydrogen storage, gaseous hydrogen storage and solid-state hydrogen storage as shown in Figure 1. Hydrogen in the liquid state requires more than twice the space in comparison to gasoline to run the car up to 300 miles. In the gaseous state, hydrogen is even more complicated to handle, with the cost more than 4–5 times of the gasoline storage [2]. Hydrogen storage in the compressed state needs heavy and large cylinders with special coatings which add more cost to the method. In addition, the safety risks due to high pressure cannot be avoided. Thus, both techniques, i.e., compressed hydrogen gas and low-temperature liquefied hydrogen gas, have their own disadvantages and are unrealistic for commercial use. Consequently, researchers have been attracted by solid-state hydrogen storage in materials due to its high gravimetric and volumetric capacities, ease of handling and safety. In the last few years, researchers have been more focused to achieve the DOE targets and to understand the hydrogen de/adsorption properties of physically and chemically bounded hydrogen in solid materials. The mechanism of solid-state hydrogen storage materials can be classified based on the state of hydrogen in the materials, i.e., physisorption (physically bounded hydrogen) and chemisorption (chemically bounded hydrogen). In physisorption, molecular hydrogen is adsorbed onto the surface of the material by the weak van der Waals interactions. Due to the weak interaction of the nonpolar hydrogen molecule with the adsorbent surface, it is easy to desorb hydrogen at high temperature. To avoid this quick release of hydrogen, cryogenic temperatures (~77 K) are usually applied for the hydrogen storage process [3]. However, room temperature hydrogen desorption for storage and onboard vehicular applications is desired and difficult to achieve. As we can see in Figure 2, physisorption systems commonly attract attention due to their porous structures and high surface area. For example, carbon-related materials and carbon nanotubes (CNTs) were investigated because of their high surface area and high capacity up to 6.5 wt%, but due to desorption at low temperature, these are not suitable practically [4]. Zeolites and metal organic frameworks (MOFs) [5,6] and organic polymers [7,8] have been investigated due to their good porosity and high surface area (low density) which is helpful to increase hydrogen storage capacity. However, the requirement of cryogenic temperature makes them unsuitable for commercial use. On the other hand, in chemisorption, strong chemical interaction occurs between the atomic hydrogen and the solid material (high interaction energy 100–200 kJ mol−1). Hydrogen is stored by strong bonding with the solid material and is released at specified conditions through a chemical reaction. As shown in Figure 2, chemisorption systems include two broad categories, i.e., metal hydrides and complex hydrides.
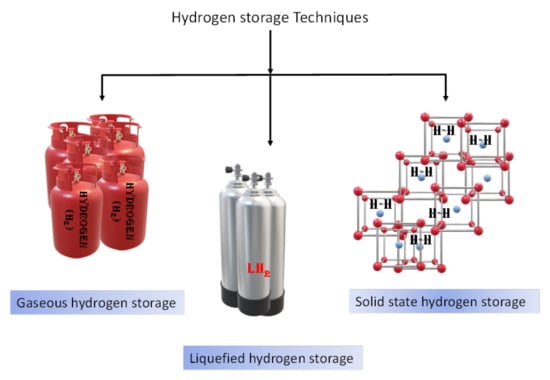
Figure 1.
Different hydrogen storage methods.
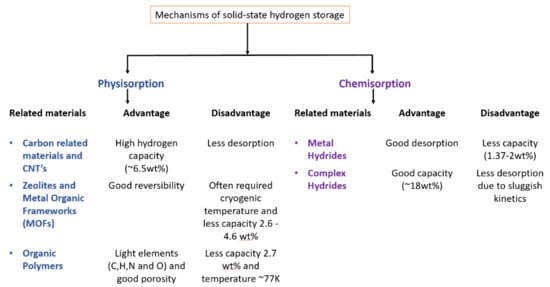
Figure 2.
Solid-state hydrogen storage mechanisms, related materials and their advantages and disadvantages.
Metal hydrides are commonly in the form of MHx where M = metal, H is hydrogen atom and x is the number of hydrogen atoms. To form metal hydride, hydrogen reacts with the metal (or metal alloy) and transfers the H- (hydride ion) as per Equation (1).
Typical metal hydrides (interstitial hydrides) such as LaNi5, TiCrMn, VTiCr, TiFe and vanadium-based hydrogen storage alloys have been studied for a long time and are reversible under moderate conditions [9,10,11,12,13]. However, due to the lower (up to 2 wt%) gravimetric capacity of such conventional metal hydrides, they are restricted to use in stationary applications only, such as hydrogen tanks and compressors. On the other hand, light metal hydrides (LiH, NaH and MgH2) and complex hydrides such as borohydrides and alanates (for e.g., LiBH4, NaBH4, NaAlH4, LiAlH4, Na3AlH6 and Mg2FeH6) have high gravimetric capacities (7–18.6 wt%). Regardless of the high gravimetric capacity, these materials possess serious thermodynamic and kinetic issues that create problems such as high stability (requiring high operating temperature) and slow reaction rate (causing slow charging/discharging). The research on metal/complex hydrides has been carried out for a long time. However, the material selection and design of metal hydrides are still important to optimize the thermodynamics and kinetics. To solve the thermodynamics issues, several approaches are described by many researchers. One of them is nanosizing (<10 nm) as well as nanoconfinement (support on matrix) [14]. Nanosizing greatly influences the size of particles (generally by using the high-energy ball milling technique) and nanoconfinement is where the nanoparticles are supported on the porous catalyst. These techniques not only improve the thermodynamics and reversibility of the system but are also helpful to enhance the rate of reaction. However, high sensitivity towards oxidation and less cyclic stability (due to agglomeration, which loses the benefits of nanostructure) are the main disadvantages of such techniques [14]. Thus, people investigated other techniques to improve the thermodynamics of the hydrogen systems such as insertion of a third element as described well by Jain et al. in 2018 [15]. On the other hand, many metal/complex hydrides have high gravimetric capacity but due to the complex nature and existence of multiple phases after dehydrogenation they often face kinetic and reversibility issues. Several noble metal catalysts have been employed to resolve these issues; however, the high cost and lower abundancy of such catalysts make them irrelevant [16,17,18,19,20]. Therefore, the choice of d-block elements is appropriate due to their abundance on earth, feasible cost and high activity, which make them a suitable candidate to improve the sluggish kinetics of hydrogen storage materials [21,22,23]. There are many factors which affect the kinetics of the hydrogen storage system. To understand the problems related to kinetics in detail, we will move towards the next section of this article.
2. Basic Understanding of Kinetics and a Light on the Kinetics of Metal Hydride
Kinetics is not easy to understand and is something which defines the rate of hydrogen absorption and desorption by the metal. It is clearly different from the thermodynamics of the hydride materials. Thermodynamics can be understood by two factors: enthalpy of formation (△H) and entropy of hydride (△S), whereas kinetics can be understood by the activation energy (Ea) of the reaction. There are two equations which can help to calculate the activation of reaction (Ea) as mentioned below, (2) and (3):
(1). Arrhenius equation:
(2). Kissinger equation:
where Ea= activation energy, k= rate constant, A= frequency factor, R = gas constant (8.3145 J mol−1 K−1), T = temperature, β = heating rate and Tp = peak temperature.
The issue of kinetics in hydrogen storage materials has been investigated for a long time by continuous inventions of different techniques such as (nanosizing) ball milling and nanoconfinement, use of catalyst and thin films [24,25,26,27]. The problem of kinetics occurs because of the complexity of these hydride materials. These complex structures made of different elements have a different-sized ionic radius which creates highly directional bonding in these hydride materials [28]. Due to this, a diffusion barrier may form which will enhance the very slow rate of reaction for hydrogen uptake and release [15]. Moreover, other than complexity, many other reasons that may affect the kinetics of hydrogen absorption and desorption will be discussed later. Light metal hydrides such as Li, Na and Mg have high capacity (in comparison with the other metal hydrides) due to their light weight, abundance and recyclability of parent metal/materials [29]. These metals/materials are connected with hydrogen via different bonds including ionic, polar covalent and metallic. Due to the high strength and stability in ionic and covalent bonds, the metal hydride does not easily show good reversibility. However, in the case of metallic bonding, it is possible to alter the strength by providing the required high temperature and other properties of hydrogen storage materials. There are several issues one needs to understand with the metal hydride reaction system. Firstly, hydrogen absorption is an exothermic reaction, i.e., intake of hydrogen by metal hydride evolves some heat during the reaction. That simply indicates that during the absorption process, the generated heat increases the operating temperature. If this heat does not dissipate in a timely manner, the absorption rate is reduced. On the other hand, in the case of desorption, the rate is affected by the desorption of hydrogen by the metal hydride system due to the endothermic reaction [30]. It is easy to understand that stronger bonding between hydrogen and metal enhances the requirement of temperature to desorb hydrogen [31,32]. Many factors are involved that affect the kinetics of metal hydride; one of them is the hydrogen dissociation that happens on the outer surface of the metal. For a specific temperature and pressure, the hydrogen absorption/desorption rate is influenced by the intrinsic properties of the material. The metal-hydrogen interaction through several steps is explained in the next section. Mg-based materials that are not only cheap and abundant, but also have large mass and volume densities for hydrogen storage, are useful for understanding the kinetic problem. Mg-based materials often have sluggish kinetics due to the surface oxidation in the air, resulting in the formation of oxides and hydroxides which creates a barrier to diffuse hydrogen. Usually, the activation process is employed to break the oxide layer on the surface of the material which provides the absorption of hydrogen [33]. For example, TiFe alloy is superior because of its low cost and abundancy but it requires activation to remove the cover of the oxide layer [11,34,35]. As discussed above, Mg-based materials require high temperature because their kinetics is slow with hydrogen and requires activation before absorption of hydrogen. Addition of a tiny amount of catalyst dramatically increases the rate of dissociation of H2 molecule to H atom [33]. Another reason for slow kinetics is illustrated through the diffusion coefficient of MgH2 (1.5 × 10−16 m2/s), which is smaller than Mg (4 × 10−13 m2/s). After the formation of the hydride layer (MgH2), it prevents the diffusion of hydrogen further into the metal. After this, hydrogen is not absorbed from the upper layer, but it absorbs through the interface of Mg-MgH2, which limits the kinetics. To overcome this problem, many researchers have suggested the use of small particles in place of bulk magnesium to accomplish easy absorption and desorption of H atom from the stable nanocluster [36,37,38]. Researchers have proposed many steps to illustrate the absorption and desorption steps for hydrogen storage materials as described in the next section.
3. Mechanism of Hydrogen Absorption/Desorption and Need of Catalyst
The mechanism of hydrogen absorption and desorption can be understood through several steps as shown in Figure 3. The hydride phase is formed from the metal/gas interface to the center as shown in Figure 3 (left). Initially, hydrogen forms a solid solution (α phase) when a small amount of hydrogen occupies the interstitial sites of the host metal M as shown in the figure. When the hydrogen content is increased further, β-phase is formed with the saturation of the solid solution and hydride phase is formed.
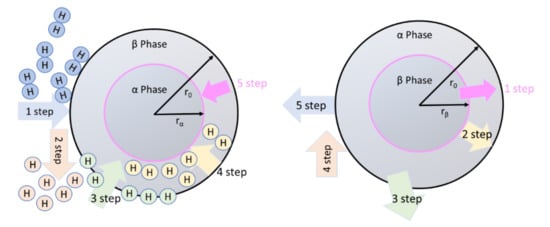
Figure 3.
Hydrogen absorption steps (left) and desorption (right) by a metal/hydride.
In the case of absorption, hydrogen gas is absorbed by the solid material by following these steps [15,39]:
- (1).
- Physisorption;
- (2).
- Chemisorption;
- (3).
- Surface penetration;
- (4).
- Diffusion through hydride layer to the metal/hydride interface;
- (5).
- Formation of hydride at interface of metal hydride.
In the case of desorption, reverse steps are followed as shown in Figure 3 (right) after chemical reaction. These steps are as follows:
- (1).
- Hydride decomposition;
- (2).
- Diffusion of hydrogen atom through metal;
- (3).
- Surface penetration;
- (4).
- Recombination of hydrogen atom into molecule;
- (5).
- Desorption to the gas phase.
These steps indicate that in the desorption process, hydrogen diffuses through the metal phase and the diffusion coefficient is higher than that of the hydride phase (absorption case). After that, hydrogen recombines instead of dissociating and in this recombination step, the hydrogen atom does not need to cross any energy barrier. This is the simple reason for why it does not face a high kinetic barrier in dehydrogenation (in comparison to hydrogenation). However, due to the exothermic nature of hydrogenation (such as hydrogen absorption by Mg), it is thermodynamically possible. To understand the hydrogenation steps, let us take an example of MgH2. Mg absorbs hydrogen by simply following the equation and the model shown in Figure 4 [40]:
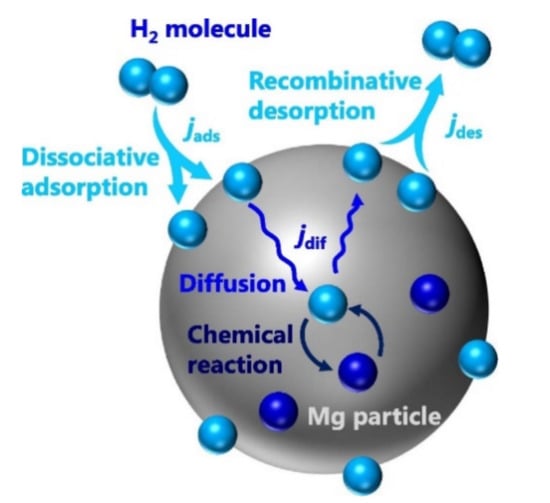
Figure 4.
Conceptual schematic of our hydrogen-dynamic model. jads, jdes, and jdif denote the fluxes of hydrogen dissociative adsorption, recombinative desorption and diffusion, respectively [40]. (Reprinted with permission from Elsevier).
Dissociation of the hydrogen molecule into the hydrogen atom in the absorption process and recombination in the case of desorption are the first steps of hydrogen ab/desorption by Mg. The second step is the transportation between the surface and bulk regions/surface penetration of magnesium metal. It follows with the diffusion in the bulk of magnesium as the third step and then the fourth step is hydriding and dehydriding reactions, becoming MgH2/Mg. The rate-limiting step is the one with the largest energy barrier between the dissociation and diffusion and could be any of them. Physisorption of H2 molecule onto the metal surface only requires a small amount of activation energy and consequently it is not often considered as the rate-limiting step. For hydrogen absorption, steps 2, 3, 4 and 5 could be rate-limiting steps and thus will be elucidated here. Physisorption (step 1) is usually a fast process and hence it is not a rate-limiting step. However, concentration of hydrogen is an important parameter which affects the metal surface and is directly proportional to the applied hydrogen pressure. When nucleation plays an important role at the initial stage towards the hydride formation, then steps 3 and 5 could be a rate-limiting step, which happens rarely. In most of the cases, diffusion of hydrogen through the hydride to the interface with the metallic phase is a rate-limiting step. Similar steps can be considered as rate-limiting steps for hydrogen dehydrogenation, such as steps 1, 2 and 3 (see Figure 3 (right)) [14,41]. There is a phenomenon called “hydrogen spillover” that also happens on the surface of metal while hydrogen dissociates into atom and tries to insert itself into bulk from the catalyzed surface. During this process, some barriers still exist to stop or slow down the process of hydrogen migration to bulk, thus realizing the hydrogen spillover effect. However, it is often difficult to analyze such effects easily. Many people used spillover agents (carbonaceous allotropes, activated carbon and carbon nanotubes) to improve the kinetics due to spillover effect [42,43,44]. Efrat Ruse et al. investigated the Mg de/hydriding kinetics by using a spillover catalyst and found that nanometric Pd/CNT was superior to microsized Pd/AC for accelerating Mg de/hydriding kinetics [42]. So, how should one decide which element or catalyst should be added to improve the kinetics of hydrogen de/absorption materials? It is still a difficult question, which motivates researchers to develop more catalysts. To design the optimum catalyst, Sabatier explained that the absorption energy or bonding between catalyst and adsorbed gas (hydrogen) should have an intermediate strength that is neither too strong nor too weak [45]. This leads to a volcano plot between rate and bond strength as illustrated in Figure 5.
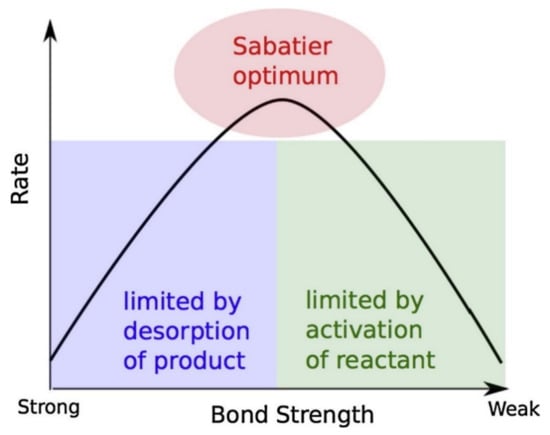
Figure 5.
Schematic representation of the qualitative Sabatier principle [45]. (Reprinted with permission from Springer).
Sergio Trasatti proved such a volcano plot for hydrogen evolution reaction experimentally (see Equation (5)), i.e., the M-H bond strength is directly related to the rate of electrolytic evolution of hydrogen (see Figure 6) [46].
H+ + e− → H2
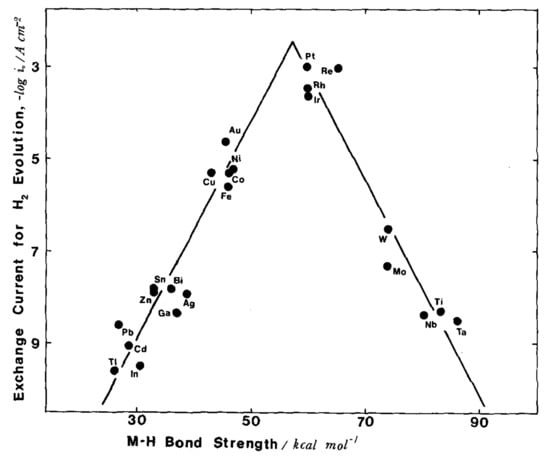
Figure 6.
Volcano plot for the hydrogen evolution reaction as a function of the M-H bond strength [46]. (Reprinted with permission from Elsevier).
Such a model could help to design more effective catalysts and it has been investigated by many researchers [47,48]. However, experimental evidence showed the reverse of the volcano plot in the case of Mg hydride for hydrogen storage due to several reasons. These include the formation of intermediate magnesium/catalyst phases [49,50], and transportation of atomic hydrogen to the Mg phase by intermediate catalytic phase [51,52] or restricting hydrogen diffusion elsewhere from the catalytic site. Pozzo et al. [53] proposed an inverse volcano plot (Figure 7), combining the effects of hydrogen dissociation and hydrogen diffusion energy barriers mentioned on the y-axis in the figure which is strongly related to the x-axis, i.e., d band center. From the figure, it could be analyzed that Ni and Pd have good catalytic activities but experimentally Ti and V have been found to have good catalytic activity like Ni and Pd [53]. Moreover, we could observe that Ni, Fe and Rh are sitting near the peak of the inverse volcano plot and have the lowest energy barriers, thus the most active catalyst. Therefore, one still needs to analyze and understand the catalytic behavior to develop the effective catalyst for hydrogen de/absorption. Coming towards the second step of hydrogen de/adsorption, i.e., surface penetration, there is an oxide layer (creating MgO/Mg(OH)2) on the surface of Mg/MgH2 due to the small traces of O2/H2O. However, this oxide layer could be caused to vanish during several cycles or by using the efficient catalyst doping. The catalyst could provide active sites to the hydride surface and create a clear path to transport H2 from surface to bulk Mg/MgH2. The process is usually known as the gateway effect as recently discussed by Biasetti et al. [54]. They mentioned that the combined effect of nanosizing and addition of transition metal (TM) as catalyst could improve the kinetics via gateway effect. TM successfully generates an interface of Mg/TM and MgH2/TM that could act as a gateway or spillover mechanism during the de/hydrogenation reactions for Mg/MgH2 [55,56]. Addition of catalyst is not only helpful in providing the path to allow H2 to penetrate into the surface but also helps in accelerating the hydrogen rate in the next step. Due to the partial hydride formation layer, the hydrogen diffusion rate becomes slow in the case of MgH2. For de/hydrogenation it is believed that nanosized catalytic components could alter the sluggish kinetics involved in the hydrogen diffusion step. This mechanism is often famed as the “hydrogen pathway” effect. Friedrichs et al. proposed a “pathway model” through MgH2/Nb2O5 nanopowder system by lowering the oxidation state of Nb2O5 which could help form a pathway to facilitate hydrogen transportation into the sample [57]. There are two important factors to consider here, reduction in particle size and grain size. The reduction of particle size could reduce the diffusion distance which is helpful to enhance hydrogen absorption. However, reducing the grain size may help to obtain more grain boundaries. A greater number of grain boundaries itself could act as a pathway to transport hydrogen, which directly helps to control the slow process of diffusion and enhance the rate of reaction [58]. Moving towards the last step of hydrogen absorption, i.e., nucleation and growth of MgH2 phase, it is still not clear if this is a rate-controlling step or not. Many studies have strongly recommended Johnson–Mehl–Avrami–Kolmogorov (JMAK), which is a suitable nucleation and growth model for many catalyzed MgH2 systems [59,60]. Other diffusion models include “Jander diffusion model” which has been used to fit the kinetics of catalyzed MgH2 systems [41,61,62]. Interestingly, Mooij et al. explained the nucleation and growth mechanisms of nano MgH2 by using the JMAK model and claimed that the absorption and desorption mechanisms are not exactly reverse to each other, i.e., asymmetry exists between desorption and absorption [63]. They showed that the energy barrier for nucleation of Mg is smaller than that for the nucleation of MgH2.
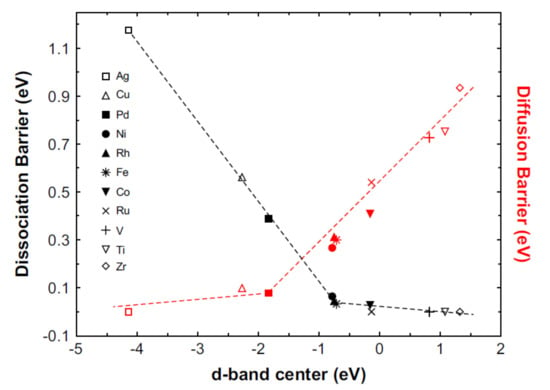
Figure 7.
Activation energy barrier for hydrogen dissociation (black) and diffusion (red) of hydrogen on pure Mg and metal-doped Mg surfaces as a function of the d-band center positions [53]. (Reprinted with permission from Elsevier).
In summary, it can be understood that improving the hydrogen de/absorption kinetics means reducing the activation barrier involved with the rate-limiting steps. There are many techniques and methods to improve kinetics reported by different groups such as nanoscaling, nanoconfinement, use of catalyst, doping and alloying [24,25,26,27,64]. However, in our perspective, alloying is basically a way to alter the thermodynamics of the system and does not affect the kinetics directly. Alloying is one of the best strategies to improve the thermodynamics and to destabilize the hydrogen storage systems [65,66]. The remaining two strategies, i.e., nanosizing and use of catalyst, are both effective techniques. However, our focus in this review is on the use of catalyst, especially d-block elements as catalyst to improve the kinetics of metal hydrides. The role of catalyst in reducing the activation barrier can be seen in Figure 8, where the black color curve shows the activation energy for the non-catalyzed sample, which is reduced for the catalyzed sample (shown by the red curve). The next section sheds light on the role of transition metals and their alloys as catalyst for hydrides.
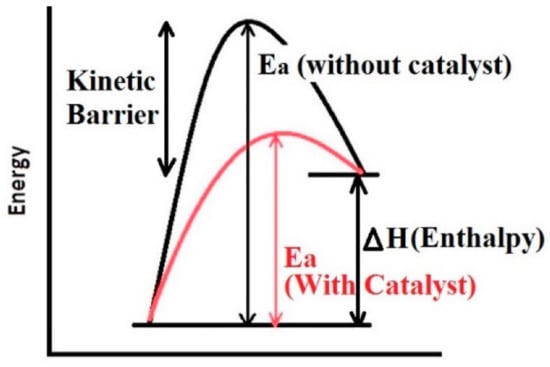
Figure 8.
Representation of the kinetic barrier of the reaction and lowering the activation energy (Ea) using catalyst [15].
4. D-block (Transition Metal) Elements as Catalyst
It is well known that many different materials, alloys, complex hydrides and composites have been used for the purpose of hydrogen storage [11,15,24,26,67,68,69,70,71,72]. Even if surface oxidation can be fixed by sample activation and other related methods, it takes a long time to desorb/absorb hydrogen by hydride materials and hence this makes it almost impossible for practical usage. Besides, the use of nanosizing technique is also not practical as it is not easy to prepare such small particles when it comes to preparing material in tons. Due to the above reasons, use of catalyst is the most decent choice to enhance the kinetics of these hydrides. Usually d-block elements have the presence of vacant d-orbital, thus they have the ability to exhibit variable valencies [73]. Induced vacancies may help to absorb hydrogen in blank space and release them when needed at certain temperatures and pressures. The d-block elements have a tendency to form complex compounds and have been used in various applications related to energy harvesting, storage and transportation [73,74]. In this section, the use of d-block elements and their alloys for improving the kinetics of hydride materials will be reviewed one by one.
4.1. Transition Unary Metals as Catalyst
Unary catalyst is the single metal catalyst, and many transition metal unary catalysts show good performance in improving the hydrogen storage properties of MgH2. Transition metals including Ni, Ti, Fe, Zr, Nb, V, Cr, Co, Mo, Rh, Pd, Cu, Ag and their nano forms have been developed to improve the hydrogen storage properties of MgH2 [53,72,75,76,77,78,79,80,81,82,83,84,85,86,87,88]. In 2005, Haneda et al. mixed Ni nanoparticles as catalyst with MgH2 by adopting a ball milling technique [89]. They confirmed that the desorption peak was found at 270 °C on addition of Ni nanoparticles into the MgH2 which is much lower than the pure MgH2. Superior catalytic effect of Ni was confirmed in terms of several parameters such as particle size and amount of catalyst. Later, in 2010, Yang et al. studied the effect of fine Ni particles (90–200 nm) on hydrogen desorption of MgH2 [90]. MgH2 mixed with 2 at% Ni particles started hydrogen desorption rapidly at 200 °C and around 6.5 wt% hydrogen was released up to 340 °C temperature. In this study, they concluded that site density of the catalyst over the MgH2 particle was the key factor to enhance the efficiency of the catalyst and improve the sorption kinetics of MgH2. Later, Liu et al. also reported their investigation on doping Ni in Mg/MgH2 system by synthesizing Mg-Ni nanocomposite by the coprecipitation method. According to the results, Mg-Ni nanocomposite revealed quite fast hydrogen absorption, 85% of its maximum capacity was achieved at lower temperature at 125 °C within 45 s. They also calculated activation energy of Mg-Ni nanocomposite as 57.4 kJ/mol H2. It was concluded that the nano size of Mg, formation of gama-MgH2 phase and Mg2Ni formation after de/hydrogenation cycles were the main factors to obtain the superior hydrogen sorption properties of Mg-Ni [91]. In 2018, Sun et al. investigated the properties of Ni nanobelts as catalyst with the aim of improving the hydrogen storage properties of magnesium [92]. Hydrogen was observed to release at 174 °C temperature and they also calculated activation energy 69.2 ± 2.5 kJ mol–1 H2 which was found to be lower in comparison to the reported values for pure Mg/H2 reaction. This was considered to be evidence of the catalytic properties of the Ni nanobelts. More recently, Yang et al. put their efforts towards improving the kinetics of MgH2 via doping with a flake Ni nanocatalyst [93]. They claimed 6.7 wt% of hydrogen release at 300 °C within 3 min starting from 180 °C by MgH2 + 5 wt% Ni composites. They also checked the absorption, which was started below 50 °C after complete dehydrogenation and found that 4.6 wt% of hydrogen was absorbed by the composite at 125 °C within 20 min at 3MPa hydrogen pressure. They put forth Ni nanoflakes as a catalyst which covered MgH2 and acted as a “hydrogen pump” to accelerate the rates of hydrogen absorption and desorption. Besides MgH2, several other hydrogen storage materials were explored to observe the effect of transition metals on their hydrogen storage properties. In 2015, our group investigated the effect of different nanometals (Ni, Co, Nb) as catalyst on the absorption–desorption properties of KSiH3 [78]. Among them, nano Ni was found to be the most effective catalyst, having minimum activation energy 106 kJ mol−1 compared with the others. Recently, the effect of Fe was also found to be exciting for the improvement of the sorption properties of KSiH3 [24]. Pozzo et al. verified the superior catalytic properties by DFT calculations of Ni and Fe from d-block elements and announced Ni and Fe as the most active catalysts [53]. Recently, Gasnier et al. designed a catalyst containing d-block elements combined with N-doped graphene-rich aerogels to improve the sorption kinetics of LiBH4 [94]. Obviously, carbon- or graphene-rich components could help to spread nanomaterials homogeneously and to avoid any kind of agglomeration. The presence of metallic nanoparticles such as Ni and Co helped to shift desorption at lower temperature. It was mentioned that the presence of Ni is more favorable (70%) than the Co (30%), as Ni could desorb 6.5 wt% and Co releases 1 wt% of hydrogen at temperature 325 °C and 226 °C, respectively. Meng et al. also mentioned that Ni nanoparticles play a crucial role in lowering the onset temperature (around 180 °C) of LiBH4 and reported porous Ni@C derived from bimetallic metal organic frameworks (MOF) catalyst as shown in Figure 9 [95].
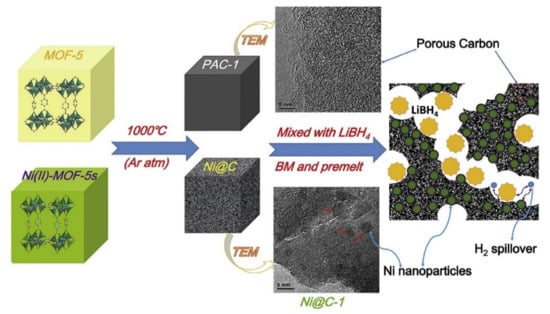
Figure 9.
The schematic illustration of the products derived from MOF-5 and Ni(II)-MOF-5s and application in LiBH4 hydrogen release [95]. (Reprinted with permission from Elsevier).
High specific surface area and pore size distribution were found to play the key roles to boost up the kinetics and 11 wt% H2 was released at 375 °C by LiBH4/Ni@C-1. Iron (Fe), which is the most common metal element in life, has been broadly exercised for many applications for a long time. In 1999, Zaluska et al. studied the catalytic effect of Pd and Fe on magnesium surface for hydrogen storage [85]. They stated that by using a small amount of catalyst (palladium or iron) the need for activation can be eliminated because the catalyst offsets the negative impact of surface oxidation. They also suggested the use of other catalysts such as V and Zr and a mixture of Mn and Zr for the improvement of sorption conditions of Mg. In 2012, Montone et al. studied the hydrogenation of 5 wt% Fe-added MgH2 and doped MgH2 nanocomposite was cycled under maximum hydrogen pressure up to 47 de/absorption cycles at 300 °C [96]. They concluded that the rate-limiting step was affected during hydrogen de/absorption, due to the morphological and structural growth of the material; however, the maximum storage capacity was mostly unaffected. Recently, Gattia et al. reported reduced activation energy of MgH2 + 5 wt% Fe sample, which was found to be the main reason for the improvement of the sorption kinetics [97]. In 2020, Antiqueira et al. also reported extremely fast kinetics of MgH2 catalyzed by Fe [98]. They claimed that 10 h and 24 h nanocomposites showed fast kinetics under mild conditions: 300–350 °C temperature under 10 bar H2 for absorption and 0.13 bar H2 for desorption. Besides Ni and Fe, there are many unary metal catalysts such as Ti, Nb, V, Co, Mo and Zr [99]. In 2004, Wang et al. revealed that NaAlH4 endures the kinetics and cycling properties when transition metal, especially Ti, is added as a catalyst to it. The 4 mol% Ti-doped NaAlH4 was recharged at 120 °C under 12 MPa hydrogen within 8–10 h; however, hydrogen capacity faded with the number of increasing cycles and was found as 2.8 wt% after eight cycles. They investigated and proposed that the improved kinetics was possible due to the localized surface of the catalytic species containing Ti [100]. Chaudhuri et al. also confirmed that the Ti atoms and local arrangement of Ti atoms have an important catalytic role to play in the chemisorption of the molecular hydrogen process [101]. Blomqvist et al. studied the dehydrogenation from 3d-(TM) transition-metals (Sc, Ti, V, Cr, Mn, Fe, Co, Ni and Cu)-doped NaAlH4 [102]. They came up with two important findings related to bond length (TM-Al) and binding energy (TM-NaAlH4). The shortening of the TM–Al bond has an impact on lowering the hydrogen binding energy due to the common inclination towards formation of new phase and thus the kinetics is improved in this process. They concluded that the binding energy in TM-doped NaAlH4 was lower than that of undoped NaAlH4. They also suggested that Fe and Cr as a catalyst are better than Ti for efficient hydrogen desorption [102]. Huang et al. reported the influence of transition metals (TMs) and reported a significant improvement in this sequence (Pd > Co > Zr > Ni > Nb > Hf > Ti > Mn > Fe > V > Cu > Cr) on the de/hydriding critical point of NaAlH4 [103]. Moreover, Cui el al. proposed Mg-TM core shell nanostructures (where TM: Ti, Nb, V, Co, Mo or Ni) prepared by a wet-chemical method [104]. They have reported Mg-TM dehydrogenation curves for all samples below 225 °C as shown in Figure 10. The reported performance was arranged in higher rank order (superior catalyst) as Mg-Ti > Mg-Nb > Mg-Ni > Mg-V > Mg-Co > Mg-Mo. Recently, Dmytro Korablov et al. also proposed Mg-25%TM (where TM = Ti, Nb or V) composites and their kinetic studies [105]. They suggested that 0.75 Mg-0.25 V composite showed extraordinarily fast kinetics of hydrogen absorption at room temperature as shown in Figure 11. Pd was also reported as a superior catalyst by many researchers [106]. Liu et al. used Pd nanoparticles with Mg, i.e., Mg-Pd that could absorb up to 3 wt% hydrogen in 2 h at the lowest temperature 50 °C [107]. However, due to the cost of Pd, it is recommended to preferentially design the abundant and cost-effective metal catalyst for the long term. Apart from the hydride/complex hydrides, transition metal (catalyst) mixed metal organic frameworks (TM-MOFs), due to a large specific surface area, are also effective for hydrogen storage application [108,109,110,111]. The Co, Fe, Ni, Cu, Pd and Nb metals have been reported as efficient catalysts to improve the catalytic properties of MOFs. Recently, Wang et al. manifested the synergistic and catalytic effect of Mg-TM/ZIF-67 (TM = Ni, Cu, Pd, Nb) nanocomposites to achieve excellent hydrogen storage properties [87]. The calculated dehydrogenation activation energy was reduced up to 75.8 kJ mol−1 H2 for Mg-Nb/ZIF-67 nanocomposite and thus Nb was found to be the most effective catalyst for hydrogen storage performance. It was claimed that the core-shell structure was helpful to suppress the activation barrier of Mg and blocked the grain growth of the particle. Therefore, this kind of single metal catalyst is also helpful along with the combinatorial designed structure to inhibit the lattice expansion and this could enhance the retention rate of the hydrogen absorption desorption cycle [87]. In summary, it could be understood that different d-block elements are effective due to the above discussed reasons depending on the systems. However costly elements such as Pd should be avoided while designing the efficient catalyst. The interesting and cost-effective elements include Ni, Co, V, Ti, Fe and Nb, which were commonly used as the unary catalyst to enhance the sluggish kinetics of various hydrogen storage systems.
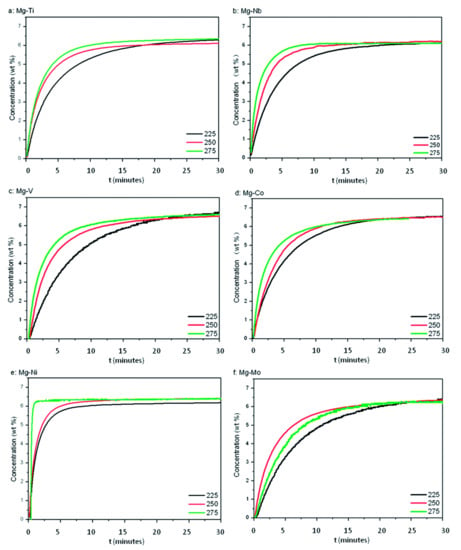
Figure 10.
Isothermal hydrogenation curves of Mg–TM samples at 225, 250 and 275 °C. (a) Mg–Ti; (b) Mg–Nb; (c) Mg–V; (d) Mg–Co; (e) Mg–Ni; (f) Mg–Mo [104]. (Reprinted with permission from the Royal Society of Chemistry).
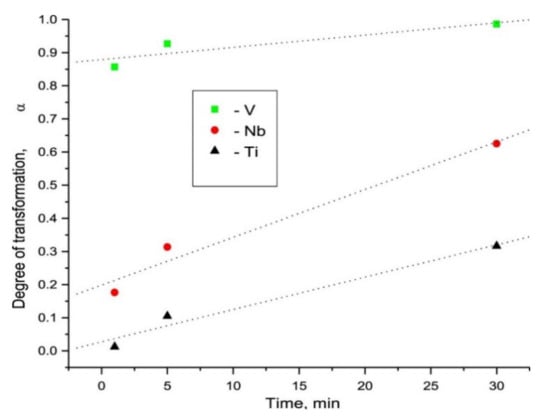
Figure 11.
Dependences of the degree of transformation of Mg into its hydride on hydrogenation time during RT absorption with Ti, Nb or V additives [105]. (Reprinted with permission from Elsevier).
4.2. D-block Binary Metal Catalyst (Metal-Metal, Metal Oxides, Metal Halides, etc.)
Transition metals are good catalysts, not only in their elemental form, but also in compound form. Several compounds such as metal oxides, halides and carbides have been used to improve the sorption kinetics of almost all the families of hydrides, which will be reviewed in this section. Recently, several reports have been published seeking to improve the hydrogen sorption properties of MgH2 by using binary transition metals as catalysts such as NiCu [112], ZrH2 [113], HfCl4 [114], Nb2O5 [115], [116], Mn3O4 [117], FeCo [118], TiO2 [119], Ti3C2 [120], FeCl3 [121], Nb(V) ethoxide [122], ZrCo [123], Fe2O3 [124], YH2 [125], TiH2 [54] and many more. Zhang et al. used NiCu catalyst with different molar ratios and found that among them 5h milled MgH2/Ni-50%Cu system desorbed hydrogen at 205.8 °C, which is 96.9 °C lower than the pure MgH2 as shown in Figure 12 [112]. Many researchers predicted the effect of these transition metal catalysts by theoretical calculations in order to support and find the possible reason for such improvements observed in experimental studies. Zhang et al. proposed that 10 wt% ZrCo-nanosheets-added MgH2 could desorb around 6.3 wt% H2 within 5 min at 300 °C and absorb 4.4 wt% H2 under 3 MPa hydrogen pressure in 10 min even at 120 °C [123]. Theoretical calculations performed for this study revealed the stable adsorption configurations of MgH2 molecule on ZrCo (110) surface. They concluded that the ss orbital overlap of Mg and H bond was weakened, which was suggested to be a boon for enhancing the sorption kinetics of MgH2 (see Figure 13). Chen et al. proposed and confirmed by experimental means as well as DFT calculations that ZrH2 nanocatalyst homogeneously spread on the surface of MgH2 (see Figure 14), which exhibited fast kinetics due to the lattice distortion between ZrH2 and MgH2/Mg phases [113]. They claimed that ZrH2-MgH2 was able to absorb 5.90 wt% hydrogen at 65 °C under 65 bar H2 back pressure within 100 min. Rehydrogenation pressure was decreased down to 6 bars; however, ZrH2-MgH2 could still absorb 3.96 wt% hydrogen at 65 °C within 120 min, which confirms the excellent hydrogen absorption properties of catalyzed MgH2. Iron and Co mixed binary catalysts in the form of nanosheets were investigated for their catalytic effectiveness and sorption properties of catalyzed MgH2 system by Yang et al. [118]. The nanocatalyst FeCo with MgH2 desorbed 6 wt% hydrogen within 9.5 min at 300 °C. In the case of absorption, FeCo-MgH2 started taking up hydrogen at room temperature and absorbed a total 5.4 wt% hydrogen while heating up to 200 °C temperature. They found reduced activation energies for dehydrogenation (65.3 ± 4.7 kJ mol−1) and rehydrogenation (53.4 ± 1.0 kJ mol−1) for this system. In 2014, Cai et al. mentioned the benefits of combining d-block element Co (electron-rich) with boron (electron-deficient) and used this combination to catalyze LiBH4 [125]. The electronic structure of CoB, its morphology and specific surface area have been suggested to play the main role in improving the sorption properties of LiBH4 (see Figure 15). The best kinetics was shown by the mulberry-like CoB-catalyzed LiBH4, which desorbed 10.4 wt% hydrogen within 1 h at 350 °C. Many halides and oxides were also investigated as efficient catalysts. Iron chloride was considered to be an excellent catalyst for MgH2 and reduced desorption temperature up to 90 °C lower than the pure MgH2. M. Ismail [121] reported 10 wt% FeCl3-doped MgH2 system which displayed a faster dehydrogenation rate than the pristine MgH2. Iron oxides (Fe2O3) were found capable of increasing the rate of hydrogen absorption and desorption of MgH2. Song et al. [124] demonstrated the increased rate of hydrogen charging and discharging by the addition of Fe2O3 catalyst to Mg (MgH2-forming mechanical milling). They also confirmed that the chemisorption of hydrogen molecule was the rate-determining step in the case of charging of Mg-10Fe2O3. Apart from these, Nb2O5 has secured its own place, being an excellent catalyst for many hydrogen storage systems. Recently, Gi et al. shed light on the effective factors of Nb2O5 catalyst for Mg [116]. It was revealed that metastable amorphous Nb2O5 was easily converted to the reduced state and could provide more catalytic active sites for the reaction. Later, Zhang et al. also demonstrated synthesis of Nb2O5 hollow spheres (o-Nb2O5) and showed high catalytic activity on MgH2 for hydrogen storage application [115]. The enhanced catalytic activity could be seen by fast hydrogen desorption, i.e., a total 5.5 wt% within 5 min at 300 °C. Rafi-ud-din et al. reported Nb2O5, TiO2 and Cr2O3 nanoparticles as catalyst to improve the hydrogen sorption properties of NaAlH4 [126]. Among them, Cr2O3 was not found to be that much more effective in terms of enhancing dehydriding/rehydriding kinetics and reducing the dehydrogenation temperature; however, the other two oxide-added systems showed kinetic improvement, i.e., 5 mol% TiO2-mixed NaAlH4 (onset desorption temperature 100 °C) and 5 mol% Nb2O5-added NaAlH4 (onset desorption temperature 80 °C). It was explained that Cr2O3 acted as a surface catalyst and stayed stable in the agglomerated form during the milling and cycling process as well; thus, it was shown to have very limited catalytic activity. On the other hand, hydrogen cycling has confirmed the reduction of TiO2 and Nb2O5, which promoted the formation of the oxygen-deficient reduced niobium and titanium oxide species. These oxygen-deficient reduced species contributed to enhancing the kinetics by facilitating the diffusion of hydrogen through the barriers during de/hydrogenation [126]. The catalytic enhancement by Nb2O5 and TiO2 was also attributed to their reduced particle size with high dispersion. In 2016, Khan et al. also studied Nb2O5-doped NaAlH4 and found the reduced desorption temperature ranging from 285 °C (pure NaAlH4) to 250 °C for doped NaAlH4 [127]. They mentioned the four regions of the cycle at 100 °C, 125 °C, 150 °C and 175 °C; the amount of hydrogen desorbed was up to 1.2 wt%. The hydrogen desorption was increased in the second region up to 4.0 wt% in just 30 min, and within 60 min a maximum of 4.5 wt% H2 was released. Rafi-ud-din et al. also reported d-block metal oxides such as Cr2O3 and Nb2O5 in which Nb2O5 was found to be more effective than Cr2O3 [128]. In addition, 1 mol% and 2 mol% doped LiAlH4 samples showed lower desorption temperature with little reduction in hydrogen capacity and approx. 6.9 wt% H2 was released at 193 °C. While talking about oxides, TiO2 cannot be ignored as a superior catalyst to improve the sorption kinetics of metal/complex hydride. In 2011, Ismail et al. reported a significant improvement in kinetics by releasing 5.2 wt% hydrogen within 30 min at 100 °C from 5 wt% TiO2-mixed LiAlH4 [129]. Activation energy was reduced to 49 kJ/mol for 5 wt% TiO2-added LiAlH4 in comparison to 114 kJ/mol for pristine LiAlH4. The improved desorption was achieved by the catalytic properties on the surface of TiO2. Recently, Berezovets et al. reported nano-TiO2 and some other Ti-related catalysts for Mg [119]. According to them, 5 mol% TiO2-added Mg showed 5.7 wt% hydrogenation. Desorption was found to occur at a 90 °C lower temperature than the pure MgH2. In 2017, our group also investigated the effect of TiF4 on decomposition of MgH2 and related compounds [130]. We investigated the fact that 10 wt% TiF4-added MgH2 started desorption around 150 °C temperature and thus enhanced the sorption kinetics. We also investigated the mechanism of this enhancement using XPS and it was revealed that Ti4+ was reduced to Ti3+ and Ti2+ (lower oxidation state) in this process. Other than Ti-based binary catalysts, Mn3O4 showed good catalytic activities for MgH2. Zhang et al. stated that by adding 10 wt% Mn3O4 nanoparticles, the catalyzed MgH2 started desorbing hydrogen at 200 °C and approx. 6.8 wt% H2 was released within 8 min at 300 °C [117]. The same group also studied the effect of ZrMn2 nanoparticles on MgH2 and prepared MgH2 + 10 wt% nano-ZrMn2 composite, which released hydrogen at 181.9 °C [131]. Moreover, El-Eskandarany et al. [132] claimed MgH2 catalyzed with nano-LaNi3, i.e., MgH2 -7 wt% LaNi3 sample, could release 5.6 wt% H2 within 37 min at 225 °C. In addition, the MgH2-LaNi3 sample showed an extremely long cycle-life (2000 h) at 225 °C with no degradation of its hydrogen storage capacity. As discussed above, the development of binary transition metal catalysts is quite useful and beneficial to enhance the catalytic effect on the sorption properties of various systems for hydrogen storage.
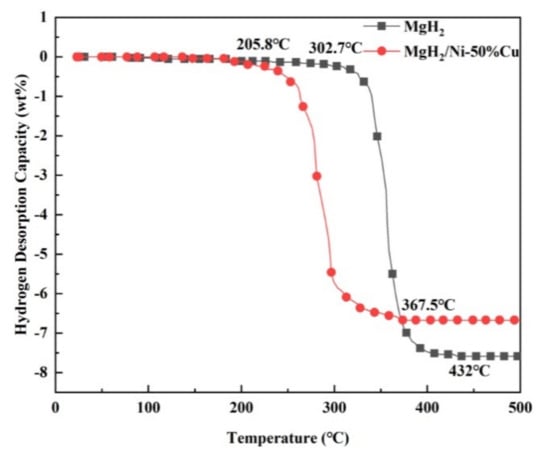
Figure 12.
TPD curves of the as-milled MgH2 and MgH2/Ni–50% Cu systems with a heating rate of 3 °C/min [112]. (Reprinted with permission from Elsevier).
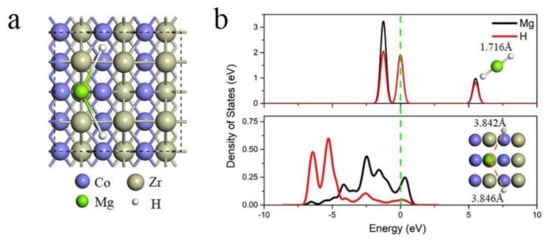
Figure 13.
The MgH2 absorption on ZrCo (110). The adsorption configuration (a) and the corresponding density of states (b) [123]. (Reprinted with permission from Elsevier).
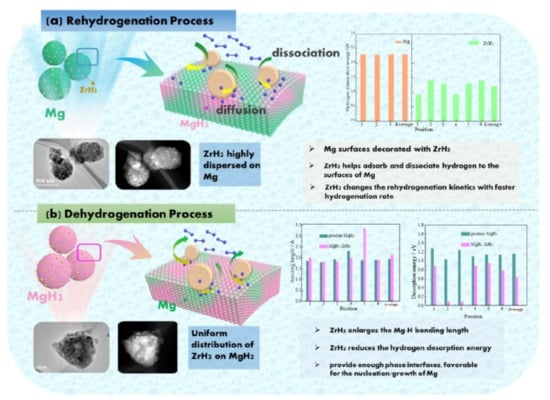
Figure 14.
(a) The schematic of the rehydrogenation and (b) dehydrogenation processes of MgH2-ZrH2 [113]. (Reprinted with permission from Elsevier).
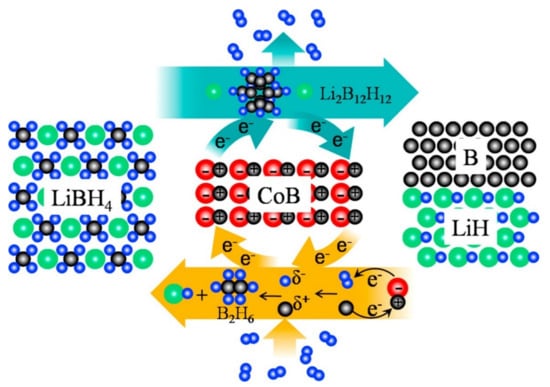
Figure 15.
Schematic illustration of dehydrogenation and rehydrogenation mechanism of CoB-catalyzed LiBH4 [125]. (Reprinted with permission from Elsevier).
4.3. D-block Ternary Metal Catalysts and Miscellaneous Catalysts
After unary and binary metal catalysts, many other combinations such as ternary, quaternary and miscellaneous catalysts in the form of alloys and composites were also developed. In this section, we have focused on the use of such miscellaneous catalysts to improve the sorption kinetics of different hydrogen storage systems. As discussed for the binary metal catalyst system above, the Ti-based catalyst has always fascinated researchers due to its extremely good catalytic activity. Nafiseh Mahmoudi et al. synthesized nanosized particles and grain structures by addition of 5 at% (TiCr1.2Fe0.6) in MgH2 powder using mechanical milling [133]. The 4 h mechanically milled catalyzed MgH2 released 4 wt% hydrogen and reduced the decomposition temperature from 327 °C to 241 °C. As shown in Figure 16a, Zhou et al. studied a series of Ti-based additives to improve the hydrogen storage properties of MgH2 [134]. A Ti-based ternary catalyst, TiVMn, showed the minimum desorption temperature (see Figure 16b) as 216.7 °C. They emphasized the difference between the dehydrogenation temperature of TiVMn-mixed MgH2 and milled MgH2 which was significant, i.e., approx. 120 °C. Recently, Berezovets et al. [119] reported the effect of Ti-based nanosized additives on the hydrogen storage properties of MgH2. As shown in Figure 17, Ti4Fe2Ox additive has the most significant effect among all. Wang et al. [135] demonstrated the unary (Ti, Al, C), binary (TiAl, Ti3Al, Ti3C2) and ternary (Ti3AlC2) catalysts for improving the reversible hydrogen storage properties of MgH2. It was noticed that the ternary Ti3AlC2 catalyst was better than the other unary catalysts in reducing the desorption temperature of MgH2. As shown in Figure 18, the hydrogen desorption temperature for Ti3AlC2-catalyst-mixed MgH2 was 250 °C, which was 12–57 °C lower than those of the 7 wt% unary and binary catalysts. However, the catalytic effect of ternary Ti3AlC2 was found to be lower in comparison to Ti3C2 because of the unique 2D layered structure of Ti3C2 which helped it to maintain a large active surface area. Besides Ti-based additives, other catalysts such as NiMnAl [136], Ni3FeMn [137], FeCoNi [138], CuFe2O4 [139], MgAgZn [140], LaFeO3 [141], ZrCrMn [141], ZrCrCu [142], ZrCrM [143], ZrCrNi [144], Zr0.4Ti0.6Co [145], MgNiO2 [146] and SrTiO3 [147] have been reported for a long time. We would like to focus on some of the effective ternary catalysts recently used to improve the sorption kinetics of MgH2. Singh et al. [138] developed a ternary catalyst, FeCoNi, supported on graphene to improve the hydrogen sorption properties of MgH2. As can be seen in Figure 19i, they reported the earliest desorption temperature for MgH2 + 5 wt% FeCoNi@GS as 100 °C, 25 °C lower than that of milled uncatalyzed (B.M) MgH2 and FeCoNi-catalyzed MgH2, respectively.
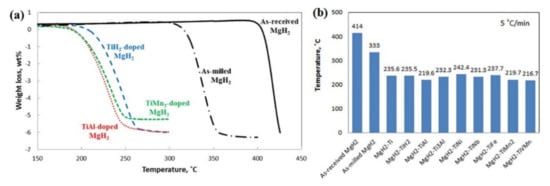
Figure 16.
(a) TGA curves of as-received, as-milled, TiH2-doped, TiMn2-doped and TiAl-doped MgH2 systems. (b) Dehydrogenation temperatures of different Ti-based catalyst-doped systems, determined by TGA profiles at fractional conversion α = 0.4 [134]. (Reprinted with permission from the American Chemical Society).
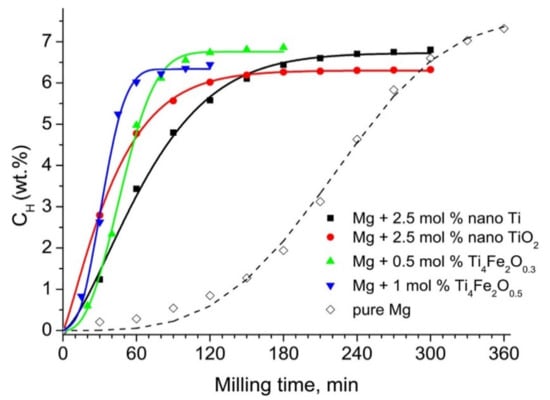
Figure 17.
Hydrogen absorption by powder mixtures of Mg with Ti-based additives during reactive ball milling in hydrogen [119]. (Reprinted with permission from Elsevier).
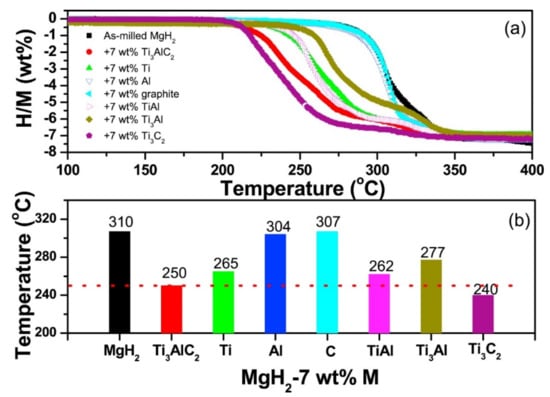
Figure 18.
(a) Volumetric release and (b) midpoint dehydrogenation temperature of MgH2-7 wt% M (M = Ti3AlC2, Ti, Al, graphite, Ti3C2, TiAl and Ti3Al) composites [135]. (Reprinted with permission from Elsevier).
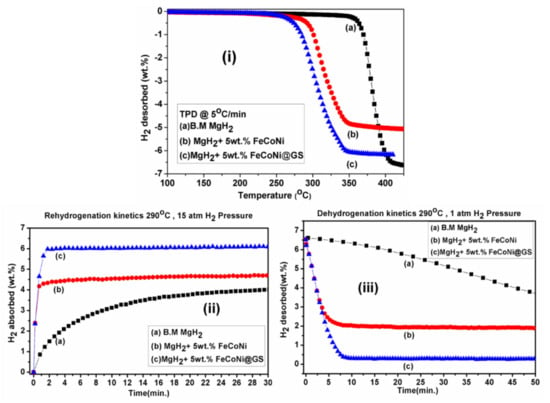
Figure 19.
(i) TPD profile of (a) B.M MgH2 (uncatalyzed sample); (b) MgH2 + 5 wt% FeCoNi; (c) MgH2 + 5 wt% FeCoNi@GS. (ii) Rehydrogenation kinetics curve of (a) B.M MgH2; (b) MgH2 + 5 wt% FeCoNi; (c) MgH2 + 5 wt% FeCoNi@GS at 290 °C at 15 atm. (iii) Dehydrogenation kinetics curve of (a) B.M MgH2; (b) MgH2 + 5 wt% FeCoNi; (c) MgH2 + 5 wt% FeCoNi@GS at 290 °C at 1 atm [138]. (Reprinted with permission from Elsevier.)
The rehydrogenation reversibility of MgH2 + 5 wt% FeCoNi@GS was also found to be superior (see Figure 19ii). It was also found that MgH2 + 5 wt% FeCoNi@GS desorbed 6.14 wt% hydrogen (highest in comparison to others) within 8.5 min under similar conditions (Figure 19iii). Other than ternary metals catalysts, oxides catalysts have always been fascinating due to their extremely good performance with various hydrogen storage systems. M. Ismail et al. achieved excellent dehydrogenation and hydrogenation kinetics of MgH2 by doping with CuFe2O4 catalyst [139]. They concluded that the 10 wt% CuFe2O4-doped MgH2 desorbed about 5.3 wt% H2 within 10 min at 320 °C and 5.0 wt% H2 was absorbed at 250 °C in 30 min by the catalyzed sample. The same group reported the study on LaFeO3 synthesized through the ball milling technique [141]. They reported that LaFeO3-catalyzed MgH2 sample was able to desorb about 3.7 wt% of H2 within 15 min at 320 °C. Zhang et al. reported the synergistic catalytic effects of Zr-based ternary metal catalyst Zr0.4Ti0.6Co nanosheets and carbon nanotubes (see Figure 20) [145]. It was found that MgH2 + 10 wt% Zr0.4Ti0.6Co/5 wt% CNTs composite quickly released 90% hydrogen within 10 min at 300 °C. Moreover, after complete dehydrogenation, the same sample could absorb 3.51 wt% hydrogen within 20 min under 3 MPa pressure at 125 °C temperature. Ali et al. synthesized MgNiO2 nanoflakes via the hydrothermal method and investigated their catalytic roles on the hydrogen sorption performance of MgH2 [146]. The enhanced absorption kinetics was found for the MgNiO2-nanoflakes-doped MgH2 sample with the capacity of hydrogen up to 6.1 wt% that could be reached within 10 min at 200 °C. Yahya et al. studied the catalytic effects of SrTiO3 on the hydrogen storage properties of MgH2 [147]. The onset temperature was reduced by 55 °C in comparison to as-milled MgH2 by the addition of 10 wt% SrTiO3. The composite MgH2-10 wt% SrTiO3 was able to absorb 4.3 wt% of hydrogen within 60 min. Besides MgH2, other systems such as LiBH4, LiAlH4 and NaAlH4 were also improved in their hydrogenation properties by the addition of oxide catalysts. Nanosized catalyst NiFeO4 was found suitable to lower the onset and peak temperature by 226 °C and 260 °C with respect to pristine LiBH4 [148]. Approximately 5 wt% hydrogen was released at 300 °C within 20 min. First principle calculation suggested the reduction of energy required to release H atom by addition of d-block transition metals and hence promoted desorption of hydrogen [149]. Moreover, Wan et al. introduced MnFe2O4 nanoparticles to improve the catalytic activity of NaAlH4 [150]. Improved kinetics was shown for isothermal dehydrogenation from 7 mol% MnFe2O4-doped NaAlH4 with slightly reduced capacity. The onset decomposition temperatures for the 7 mol% MnFe2O4-doped NaAlH4 were significantly reduced to 95 °C, 152 °C and 327 °C for the three decomposition steps which were lowered by 84 °C, 88 °C and 84 °C, respectively, lower than that of undoped NaAlH4. Huang et al. demonstrated NaAlH4 + 3 mol% NiFe2O4 having good cycle stability at only 150 °C with a slight capacity loss after five cycles [151]. They revealed that the enhanced catalytic performance of NaAlH4 by the addition of 3 mol% NiFe2O4 might be due to the small-sized particles. Nanosized particles might have provided active sites at the surface of NaAlH4, resulting in the improved kinetics. In addition to this, other d-block bimetallic oxides were also introduced to enhance the kinetics of LiAlH4 such as CoFe2O4 [152], NiFe2O4 [153] and MgFe2O4 [154]. Li et al. demonstrated 3 mol% nanosized NiFe2O4-doped LiAlH4 sample could desorb 7 wt% H2 within 91 s under 0.1 MPa pressure at 120 °C, which was 6.3 wt% higher than the pristine LiAlH4 [153]. The same group investigated the catalytic effects of CoFe2O4 nanoparticles and successfully achieved a lower onset temperature of 65 °C, which was reduced by 90 °C in comparison to the as-received LiAlH4 [152]. In 2019, Ali et al. checked the catalytic effects of MgFe2O4 in LiAlH4 and obtained faster 3.5 wt% H2 desorption within 30 min at 90 °C [154]. The synergistic effect of two metal combinations and formation of active species were found to be the reasons responsible for improving the dehydrogenation kinetics of LiAlH4. However, agglomeration remains a challenge sometimes to represent the fast kinetics and low desorption temperature. Some researchers tried to design d-block elemental catalysts decorated or mixed with graphene to solve such related issues. Tan et al. mentioned that the desorption temperature for 20 wt% MWCNTs/0.4Ni (multiwalled carbon nanotubes/0.4Ni)-mixed LiAlH4 could be decreased to 80 °C, whereas 20 wt% MWCNTs/0.4-Co-doped LiAlH4 could desorb hydrogen at 100 °C [155]. Hence, they confirmed the superiority of Ni metal over Co for the dehydrogenation behavior of LiAlH4. In their point of view, carbon materials were also helpful for the confinement of hydrides; therefore, these were able to provide a relatively clear path for hydrogen diffusion. Moreover, the electron affinity of carbon materials could affect the hydrogen removal energy in the dehydrogenation reaction [155]. Jiao et al. designed the NiCo nanoalloy (4–6 nm) encapsulated in grapheme layers (NiCo@G) to investigate the catalytic effect of catalyst on LiAlH4 [156]. The 1 wt% NiCo@G-doped LiAlH4 sample started releasing hydrogen at 43 °C (onset temperature) and 7.3 wt% hydrogen was liberated below 200 °C with reduced activation energy 54.8 kJ mol−1. Recently, NiCo2O4 nanorods anchored on rGO, i.e., NiCo2O4@rGO, was synthesized by Xia et al. and dehydrogenation of LiAlH4 was evaluated [157]. For the isothermal dehydrogenation, 7 wt% NiCo2O4@rGO-mixed LiAlH4 sample could release approx. 4 wt% hydrogen within 20 min at 150 °C. This catalyzed sample showed 21.9% and 37.1% reduced activation energy of the two-step dehydrogenation of pure LiAlH4. DFT calculations were performed to investigate the mechanism of improved kinetics. They revealed that interfacial charge transfer and dehybridization of Al-H cluster provided the weakness in Al-H bonding. They also concluded that large surface area, mesoporous structure, cluster-surface interface and synergistic effects were the responsible events in facilitating the dehydrogenation of NiCo2O4@rGO-mixed LiAlH4 [157]. More recently, NiFe2O4 was supported on two-dimensional hexagonal boron nitride (NiFe2O4@h-BN) to increase the catalytic activity of LiAlH4 [158]. The onset desorption temperature for 7 wt% NiFe2O4@h-BN-doped LiAlH4 was observed to be 77.1 °C with 6.74 wt% hydrogen liberation. After dehydrogenation, 0.6 wt% H2 was absorbed under 300 bar pressure at 300 °C temperature in 11 h by the dehydrogenated product. Zhao et al. designed a catalyst containing core-shell structure CoNi@C via hydrothermal and calcination reduction to improve the catalytic activity of MgH2 [159]. Prepared catalyst was found to efficiently desorb 5.83 wt% hydrogen within 1800 s started at 173 °C up to max 275 °C temperature and released 4.83 wt% H2 within 1800 s at the lowest temperature 100 °C. Meng et al. investigated the superiority of designed 3D flower-like TiO2@C nanostructured catalyst towards MgH2 [160]. They reported that the hydrogen sorption kinetics of MgH2 was enhanced by the flower-like TiO2@C catalyst (6 wt% hydrogen desorption within 7 min). Liu et al. recently reported an interesting design for a catalyst, i.e., Ni3Fe was homogeneously loaded on reduced graphene oxide (Ni3Fe/rGO) based on layered double hydroxide (LDH) precursor as shown in Figure 21 (upper panel) [161]. As shown in Figure 21a,b, MgH2-5 wt% Ni3Fe/rGO composite could absorb 6 wt% hydrogen within 80 s at 100 °C and 6.2 wt% hydrogen could be achieved within 60 s at 125 °C. The mechanism was revealed for the catalysis and the proposed synergistic effect was attributed to rGo as well as in situ formation of active species Mg2Ni and Fe. In short, they happened to design such a catalyst with high efficiency based on LDH precursors. Ding et al. reported two different catalysts, MgCNi3 [162] in 2019 and MgCCo1.5Ni1.5 [160] in 2020, to improve the sorption kinetics of MgH2. They called it anti-perovskite material MgCNi3 [162]; it was doped with Mg and approx. 4.42 wt% hydrogen was absorbed within 20 min at 423 K, whereas 4.81 wt% hydrogen was reversibly released within 20 min at 593 K. They revealed the formation of Mg2NiH4 hydride and carbon material during hydrogenation of composite where Mg2NiH4 induced dehydrogenation and carbonaceous material inhibited the growth and agglomeration of MgH2. Later they realized that addition of Co in the same composite could be beneficial to enhance the sorption kinetics of MgH2 due to the superior catalytic activity of Co. Then further, they reported Mg/MgH2-MgCCo1.5Ni1.5 composite releasing H2 at 217 °C, 160 °C lower than that of the MgH2, and it quickly absorbed 5.5 wt% hydrogen within 60 min at 150 °C temperature [163]. Moreover, they reported good cycling performance of Mg/MgH2-MgCCo1.5Ni1.5 composite and emphasized the formation of active species MgC0·5Co3 and carbon materials during de/absorption, which was the main reason for having good catalytic effects. Another multielement NiMn9.3Al4.0Co14.1Fe3.6 alloy was introduced by Meena et al. to enhance the dehydrogenation kinetics of MgH2 [164]. They reported that 50 wt% NiMn9.3Al4.0Co14.1Fe3.6-alloy-doped MgH2 decreases desorption temperature by about 80 °C compared to the as-milled MgH2 sample at onset temperature 220 °C. Some recent reports show that Mxenes-based materials have high potential to improve the kinetic sorption of MgH2 [165]. The Mxenes are basically a set of controlled spacious interlayers that can adsorb a number of hydrogen atoms/molecules on the (like 2D materials) structure by physisorption, chemisorption or Kubas interaction. It is believed that the unique 2D structure and formation of active species play an important role in destabilizing the MgH2 for hydrogen storage application. In 2019, Li et al. [166] prepared Ti2C Mxene by selective etching of Al layer from Ti2AlC. The results showed that the starting dehydrogenation temperature and activation energies of MgH2-5 wt%Ti2C were decreased by 37 °C and 36.5%, respectively. In 2020, Zhu et al. [167] reported a novel Ti3C2 Mxene-based catalyst (Ni@Ti-MX) (see Figure 22) which could enable Mg to absorb 5.4 wt% hydrogen in 25 s at 125 °C temperature and release 5.2 wt% hydrogen in 15 min at 250 °C. The interesting point about this catalyst was that it enabled the absorption of 4 wt% H2 in 5 h even at room temperature. Reduced kinetic energy barrier was found (56 ± 4 and 73 ± 3.5 kJ/mol H2 for hydrogenation and dehydrogenation, respectively) for improved kinetics of catalyzed MgH2. The enhanced hydrogen sorption kinetics is basically attributed to the hybrid design and coupling of many metals together. Furthermore, two kinds of Mxenes were mixed: 2D vanadium carbide (V2C) and titanium carbide (Ti3C2), in order to check the synergistic effects on decomposition of MgH2 (see Figure 23) [168]. According to this report, hydrogen atoms or molecules during the desorption process may pass through the MgH2/V2C/Ti3C2 triple-grain boundaries and through the Mg/Ti3C2 interfaces during the absorption process.
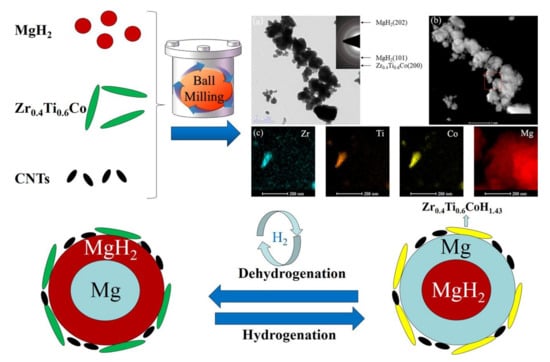
Figure 20.
Preparation and investigation of MgH2-Zr0.4Ti0.6Co nanosheets and carbon nanotubes [145]. (Reprinted with permission from Elsevier.)
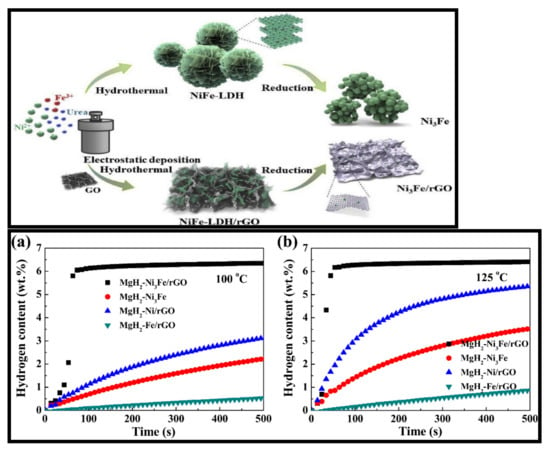
Figure 21.
Synthesis procedure for the Ni3Fe and Ni3Fe/rGO. The strong electrostatic effect between GO and LDH results in the formation of a load structure rather than a flower shape (upper panel). Isothermal rehydrogenation curves of MgH2-5 wt% Ni3Fe/rGO, MgH2-5 wt% Ni3Fe, MgH2-5 wt% Ni/rGO and MgH2-5 wt% Fe/rGO at 100 °C (a) and 125 °C (b) under 3.0 MPa hydrogen pressure [161]. (Reprinted with permission from Elsevier.)
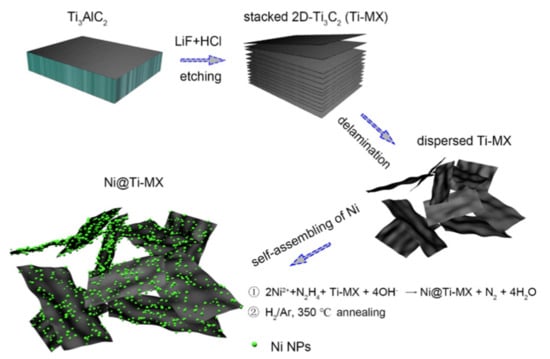
Figure 22.
Schematic illustrations of the synthetic strategy applied for the Ni@Ti-MX catalyst [167]. (Reprinted with permission from American Chemical Society.)
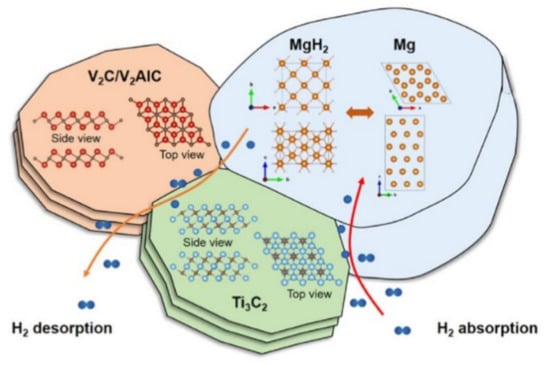
Figure 23.
Schematic pictures showing the hydrogen desorption and absorption mechanisms of MgH2 with the addition of V2C/Ti3C2 [168]. (Reprinted with permission from the American Chemical Society.)
Figure 24 shows the excellent cycling stability of MgH2/V2C/Ti3C2 up to 10 cycles with reversible capacity 6.3 wt% hydrogen. These catalysts were found to be good for other complex hydrides also. As an example, the onset temperature was reduced for 40 wt% Ti3C2-Mxene-catalyzed LiBH4 to 120 °C and a total 5.37 wt% H2 was liberated within 1 h at 350 °C with a decreased activation energy (70.3 kJ mol−1 H2) [169]. The two-dimensional layered titanium carbide (2D Mxene) was studied in order to facilitate the surface functionalization and to study the catalytic activity of hydrogen de/adsorption for NaAlH4 [170]. The NaAlH4 + 10 wt% Mxene/A-TiO2 sample was found to be superior (than the NaAlH4) with the dehydrogenation temperature 90 °C. At isothermal conditions (140 °C), NaAlH4 + 10 wt% Mxene/A-TiO2 sample could release more than 3 wt% of hydrogen within 7 min, and a total of 4.8 wt% of hydrogen could be released within 200 min. The activation of Mxene/A-TiO2 sample was attained by the synergistic effect of homogeneously spread Ti-H and TiC originating from Mxene/A-TiO2. Apart from these specific families, several other catalysts based on transition metals have been reported. As an example, LiBH4-0.04(Li3BO3 + NbH) composite was designed to achieve good reversibility and cyclic stability in comparison to pristine LiBH4 [171]. Li et al. reported the onset temperature as 190 °C and 8.2 wt% H2 was released at 400 °C for this composite. Moreover, the dehydrogenated product was able to absorb 7.9 wt% H2 in just 20 min under 50 bar at 500 °C in the rehydrogenation process. Excellent capacity retention of 7.2 wt% was found for 30 cycles. In another work, Yuan et al. studied the carbon-coated titanium dioxide supported on two-dimensional titanium carbide (C@TiO2/Ti3C2) [172]. The desorption temperature was reduced by 70 °C in comparison to the pristine NaAlH4 sample. Approximately 4 wt% H2 was liberated within 13 min at 140 °C temperature. The reduced activation energies (Ea) were found as 72.41 and 64.27 kJ mol−1 for the first two steps of the catalyzed NaAlH4 sample. More recently, Jiang et al. also demonstrated the superior catalytic efficiency of Ti3C2-doped NaH/Al composite which could desorb 4.2 wt% H2 at 110 °C within 4.5 min [173]. They reported one of the lowest initial desorption temperatures, 76 °C, for Ti-doped NaAlH4. The activation energies of the first and second steps were calculated as 92.5 kJ/mol and 58.1 kJ/mol, respectively, for Ti3C2-doped NaH/Al composite, which were found to be lower than the uncatalyzed NaAlH4. It was revealed that titanium acted as a fast channel pathway for hydrogen absorption and desorption. Moreover, high-valence Ti was found to be the responsible event in Ti3C2-doped NaH/Al composite, showing better storage properties in comparison to Ti3C2-doped NaAlH4 [173]. A balancing and coupling between nanoconfinement and catalysis were recently focused on to improve the hydrogen sorption properties of NaAlH4. Li et al. succeeded in a strategy to combine the confinement and catalysis (catalyzed sample denoted as NaAlH4/Raney Ni) to make possible NaAlH4 desorption at nearly 100 °C [174]. The NaAlH4/Raney Ni desorbed H2 at initial temperature 85 °C and finished at 260 °C with the lowest activation energy ~20 kJ mol−1. Dehydrogenated product NaH + Al could be able to regenerate NaAlH4 at 150 °C and under 7 MPa hydrogen pressure. The absorbed hydrogen could be released again at 70 °C upon second dehydrogenation. The remarkable performance was attributed to the lowering of the diffusion path by nanoconfinement on porous support and catalytic sites of Ni, which could ensure an effective route for improving the sorption properties of complex hydride (NaAlH4). Moreover, Chen et al. also developed a similar strategy to balance the synergistic effect between the catalyst and nanoconfinement using porous carbon scaffolds via the controllable etching of Co nanoparticles [175]. A 3.7 wt% hydrogen was released at 164 °C (peak temperature) with a loading ratio as high as 67%. The reversibility was achieved for 3.3 wt% hydrogen up to five cycles at 170 °C desorption temperature. By designing such a combination of Co-doped nanoporous carbon as scaffolds, the formation of void space near the active Co nanoparticles could be achieved, leading to the enhanced catalytic effects and achieving the superior hydrogen storage performance of NaAlH4.
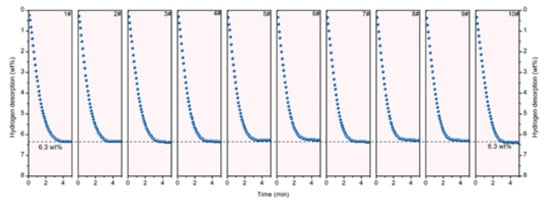
Figure 24.
Cycling hydrogen desorption curves of MgH2–2V2C/Ti3C2 at 300 °C for up to 10 cycles [168]. (Reprinted with permission from the American Chemical Society.)
Thus, in summary, it can be understood that easy synthesized nanoparticles, hybrid structure, selection of suitable metal, core-shell designs, metal catalysts supported on a matrix which can provide high surface area and addition of graphene and carbon to prohibit the agglomeration of material are the key factors in designing the suitable catalysts for hydrogen storage application.
5. Summary and Future Perspective
Hydrogen storage is one of the biggest challenges in using hydrogen as a practical energy carrier. For onboard hydrogen storage, many factors need consideration, such as safety, affordability, handling and cost. The solid-state hydrogen storage technique is beneficial from a safety point of view, uses mild operating temperature and pressure and maintains a high capacity for storing hydrogen. Several metal hydrides and complex hydrides are promising candidates for solid-state hydrogen storage due to their high gravimetric and volumetric density. However, sluggish sorption kinetics and low reversibility put limitations on using them for hydrogen storage. The use of a suitable catalyst can improve the kinetics of hydride materials. The d-block transition metals play an important role as catalyst in the field of hydrogen storage. Thus, we discussed various types of d-block elements and their related compounds as a suitable catalyst to improve the hydrogen ab/desorption performances of various hydrogen storage systems. It can be concluded that by the addition of unary (metal) and binary catalysts (metal oxides, metal halides) and their combinations, hybrid designs containing porous structures, various metal-based Mxenes and designed nanoconfined catalysts could be used to enhance the dehydrogenation/rehydrogenation kinetics and cyclic performance of the abovementioned hydride materials. Moreover, the addition of the catalyst significantly reduces the activation energy of the composite in comparison to the pristine hydride materials. Reduced activation energy can lead to a decrease in the hydrogen desorption temperature. Several other factors such as nanosizing, synergistic effects and formation of active species are responsible for activating the catalyst and providing more nucleation sites at the surface of the material along with providing the clear path to hydrogen diffusion resulting in the enhancement of the early dehydrogenation. However, numerous efforts are still needed to achieve the DOE goal in order to satisfy the thirst for suitable hydrogen storage material in terms of fast desorption. This review paper is helpful for designing a new composite hydrogen system by the addition of d-block metals and their derivatives as suitable catalysts to improve the sorption properties for hydrogen storage material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fcto_Targets_onboard_Hydro_Storage_Explanation. Available online: http://www.doc88.com/p-7324389963306.html (accessed on 16 August 2021).
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Mater. Sustain. Energy 2010, 265–270. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, C.G.; Zhou, H.; Ye, E.; Xu, J.; Li, Z.; Loh, X.J. Current Research Trends and Perspectives on Solid-State Nanomaterials in Hydrogen Storage. Research 2021, 1–39. [Google Scholar] [CrossRef]
- Mohan, M.; Sharma, V.K.; Kumar, E.A.; Gayathri, V. Hydrogen storage in carbon materials—A review. Energy Storage 2019, 1, e35. [Google Scholar] [CrossRef]
- Jia, J.; Lin, X.; Wilson, C.; Blake, A.J.; Champness, N.R.; Hubberstey, P.; Walker, G.; Cussena, E.J.; Schröder, M. Twelve-connected porous metal–organic frameworks with high H2 adsorption. Chem. Commun. 2007, 8, 840–842. [Google Scholar] [CrossRef]
- Van den Berg, A.W.C.; Areán, C.O. Materials for hydrogen storage: Current research trends and perspectives. Chem. Commun. 2008, 6, 668–681. [Google Scholar] [CrossRef]
- Budd, P.M.; Butler, A.; Selbie, J.; Mahmood, K.; McKeown, N.B.; Ghanem, B.; Msayib, K.; Book, D.; Waltonc, A. The potential of organic polymer-based hydrogen storage materials. Phys. Chem. Chem. Phys. 2007, 9, 1802–1808. [Google Scholar] [CrossRef]
- Tedds, S.; Walton, A.; Broom, D.P.; Book, D. Characterisation of porous hydrogen storage materials: Carbons, zeolites, MOFs and PIMs. Faraday Discuss. 2011, 151, 75–94. [Google Scholar] [CrossRef]
- Selvaraj, S.; Jain, A.; Miyaoka, H.; Kojima, Y.; Ichikawa, T. Hydrogen Sorption and Cyclic Compressor Performance of V40Ti21.5Cr33.5M5 (M= Nb, Zr, Fe) Alloys. J. Jpn. Inst. Energy 2019, 98, 157–164. [Google Scholar] [CrossRef]
- Guo, F.; Jain, A.; Miyaoka, H.; Kojima, Y.; Ichikawa, T. Critical Temperature and Pressure Conditions of Degradation during Thermochemical Hydrogen Compression: A Case Study of V-Based Hydrogen Storage Alloy. Energies 2020, 13, 2324. [Google Scholar] [CrossRef]
- Guo, F.; Namba, K.; Miyaoka, H.; Jain, A.; Ichikawa, T. Hydrogen storage behavior of TiFe alloy activated by different methods. Mater. Lett. X 2021, 9, 100061. [Google Scholar]
- Liang, G.; Huot, J.; Schulz, R. Hydrogen storage properties of the mechanically alloyed LaNi5-based materials. J. Alloys Compd. 2001, 320, 133–139. [Google Scholar] [CrossRef]
- Grochala, W.; Edwards, P.P. Thermal Decomposition of the Non-Interstitial Hydrides for the Storage and Production of Hy-drogen. Chem. Rev. 2004, 104, 1283–1316. [Google Scholar] [CrossRef]
- De Jongh, P.E.; Adelhelm, P. Nanosizing and Nanoconfinement: New Strategies Towards Meeting Hydrogen Storage Goals. ChemSusChem 2010, 3, 1332–1348. [Google Scholar] [CrossRef]
- Jain, A.; Agarwal, S.; Ichikawa, T. Catalytic Tuning of Sorption Kinetics of Lightweight Hydrides: A Review of the Materials and Mechanism. Catalysts 2018, 8, 651. [Google Scholar] [CrossRef]
- Adams, B.D.; Chen, A. The role of palladium in a hydrogen economy. Mater. Today 2011, 14, 282–289. [Google Scholar] [CrossRef]
- Konda, S.K.; Chen, A. Palladium based nanomaterials for enhanced hydrogen spillover and storage. Mater. Today 2016, 19, 100–108. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, H.; Guo, Y.; Jiang, X.; Qi, Y.; Sun, B.; Li, H.; Zheng, J.; Li, X. A rare earth hydride supported ruthenium catalyst for the hydrogenation of N-heterocycles: Boosting the activity via a new hydrogen transfer path and controlling the stereoselectivity. Chem. Sci. 2019, 10, 10459–10465. [Google Scholar] [CrossRef]
- Chen, J.; Fu, J.; Fu, K.; Xiao, R.; Wu, Y.; Zheng, X.; Liu, Z.; Zheng, J.; Li, X. Combining catalysis and hydrogen storage in direct borohydride fuel cells: Towards more efficient energy utilization. J. Mater. Chem. A 2017, 5, 14310–14318. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; Fei, S.; Ke, H.; Cheng, H. A comparative study of catalytic dehydrogenation of perhydro-N-ethylcarbazole over noble metal catalysts. Int. J. Hydrogen Energy 2014, 39, 18976–18983. [Google Scholar]
- Huang, Y.; An, C.; Zhang, Q.; Zhag, L.; Shao, H.; Liu, Y.; Zhang, Y.; Yuan, H.; Wang, C.; Wang, Y. Cost-effective mechanochemical synthesis of highly dispersed supported transition metal catalysts for hydrogen storage. Nano Energy 2021, 80, 105535. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, A.; Wang, W.; Huang, Y.; Liu, X.; Miao, S.; Liu, J.; Zhang, T. Co–N–C Catalyst for C–C Coupling Reactions: On the Catalytic Performance and Active Sites. ACS Catal. 2015, 5, 6563–6572. [Google Scholar]
- Zhang, J.; Yan, S.; Xia, G.; Zhou, X.; Lu, X.; Yu, L.; Yu, X.; Peng, P. Stabilization of low-valence transition metal towards advanced catalytic effects on the hydrogen storage performance of magnesium hydride. J. Magnes. Alloy. 2021, 9, 647–657. [Google Scholar] [CrossRef]
- Sharma, S.; Guo, F.; Ichikawa, T.; Kojima, Y.; Agarwal, S.; Jain, A. Iron based catalyst for the improvement of the sorption properties of KSiH3. Int. J. Hydrogen Energy 2020, 45, 33681–33686. [Google Scholar] [CrossRef]
- Agarwal, S.; Mangal, R.K.; Kumar, M.; Awasthi, K.; Kumar, S.; Jain, A. Hydrogen Sorption Characteristics of ZrCrAl Ternary Alloy as a Function of Milling Time. Macromol. Symp. 2017, 376, 1700047. [Google Scholar]
- Pal, P.; Jain, A.; Miyaoka, H.; Kojima, Y.; Ichikawa, T. Eutectic melting in x(2LiBH4-MgH2) hydrogen storage system by the addition of KH. Int. J. Hydrogen Energy 2020, 45, 17000–17005. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, A.; Kojima, Y. Thermodynamics and kinetics of hydrogen absorption–desorption of vanadium synthesized by aluminothermy. J. Therm. Anal. Calorim. 2017, 130, 721–726. [Google Scholar] [CrossRef]
- Yoshino, M.; Komiya, K.; Takahashi, Y.; Shinzato, Y.; Yukawa, H.; Morinaga, M. Nature of the chemical bond in complex hydrides, NaAlH4, LiAlH4, LiBH4 and LiNH2. J. Alloys Compd. 2005, 404–406, 185–190. [Google Scholar] [CrossRef]
- Schüth, F.; Bogdanović, B.; Felderhoff, M. Light metal hydrides and complex hydrides for hydrogen storage. Chem. Commun. 2004, 20, 2249–2258. [Google Scholar] [CrossRef]
- Modi, P.; Aguey-Zinsou, K.-F. Room Temperature Metal Hydrides for Stationary and Heat Storage Applications: A Review. Front. Energy Res. 2021, 9, 128. [Google Scholar] [CrossRef]
- Jain, I.P.; Jain, P.; Jain, A. Novel hydrogen storage materials: A review of lightweight complex hydrides. J. Alloys Compd. 2010, 503, 303–339. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Bouaricha, S.; Dodelet, J.P.; Guay, D.; Huot, J.; Schulz, R. Study of the activation process of Mg-based hydrogen storage materials modified by graphite and other carbonaceous compounds. J. Mater. Res. 2001, 16, 2893–2905. [Google Scholar] [CrossRef]
- Bellosta von Colbe, J.M.; Puszkiel, J.; Capurso, G.; Franz, A.; Ulrich Benz, H.; Zoz, H.; Klassen, T.; Dornheim, M. Scale-up of milling in a 100 L device for processing of TiFeMn alloy for hydrogen storage applications: Procedure and characterization. Int. J. Hydrogen Energy 2019, 44, 29282–29290. [Google Scholar] [CrossRef]
- Shinzato, K.; Hamamoto, S.; Miyaoka, H.; Ichikawa, T. Room-Temperature Hydrogen Absorption of Titanium with Surface Modification by Organic Solvents. J. Phys. Chem. C 2019, 123, 19269–19274. [Google Scholar] [CrossRef]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Vajeeston, P.; Ravindran, P.; Fichtner, M.; Fjellvåg, H. Influence of Crystal Structure of Bulk Phase on the Stability of Nanoscale Phases: Investigation on MgH2 Derived Nanostructures. J. Phys. Chem. 2012, 116, 18965–18972. [Google Scholar] [CrossRef]
- Nogita, K.; Tran, X.Q.; Yamamoto, T.; Tanaka, E.; McDonald, S.D.; Gourlay, C.M.; Yasuda, K.; Matsumura, S. Evidence of the hydrogen release mechanism in bulk MgH2. Sci. Rep. 2015, 5, 8450. [Google Scholar]
- Wang, H.; Lin, H.J.; Cai, W.T.; Ouyang, L.Z.; Zhu, M. Tuning kinetics and thermodynamics of hydrogen storage in light metal element based systems—A review of recent progress. J. Alloys Compd. 2016, 658, 280–300. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Tanabe, K. Development of a kinetic model of hydrogen absorption and desorption in magnesium and analysis of the rate-determining step. Chem. Phys. Lett. 2018, 699, 132–138. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, J.; Bowman, R.C., Jr.; Fang, Z.Z. Roles of Ti-Based Catalysts on Magnesium Hydride and Its Hydrogen Storage Properties. Inorganics 2021, 9, 36. [Google Scholar] [CrossRef]
- Ruse, E.; Pevzner, S.; Bar, I.P.; Nadiv, R.M.; Skripnyuk, V.; Rabkin, E.; Regev, O. Hydrogen storage and spillover kinetics in carbon nanotube-Mg composites. Int. J. Hydrogen Energy 2016, 41, 2814–2819. [Google Scholar] [CrossRef]
- Pevzner, S.; Pri-Bar, I.; Lutzky, I.; Ben-Yehuda, E.; Ruse, E.; Regev, O. Carbon Allotropes Accelerate Hydrogenation via Spillover Mechanism. J. Phys.Chem C 2014, 118, 27164–27169. [Google Scholar]
- Zhou, W.; Zhao, Y.; Wang, Y.; Wang, S.; Ma, X. Glycerol Hydrogenolysis to 1,3-Propanediol on Tungstate/Zirconia-Supported Platinum: Hydrogen Spillover Facilitated by Pt(1 1 1) Formation. ChemCatChem 2016, 8, 3663–3671. [Google Scholar] [CrossRef]
- Medford, J.A.; Vojvodic, A.S.; Hummelshøj, J.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Nørskov, J.K. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 2015, 328, 36–42. [Google Scholar] [CrossRef]
- Trasatti, S. Work function, electronegativity, and electrochemical behaviour of metals: III. Electrolytic hydrogen evolution in acid solutions. J. Electroanal. Chem. Interfacial Electrochem. 1972, 39, 163–184. [Google Scholar] [CrossRef]
- Litovchenko, V.G.; Efremov, A.A. The enhanced catalytic dissociation of adsorbed hydrogen containing molecules. Condens. Matter Phys. 1999, 2, 561. [Google Scholar] [CrossRef][Green Version]
- Zeradjanin, A.R.; Grote, J.P.; Polymeros, G.; Mayrhofer, K.J.J. A Critical Review on Hydrogen Evolution Electrocatalysis: Re-exploring the Volcano-relationship. Electroanalysis 2016, 28, 2256–2269. [Google Scholar] [CrossRef]
- Pelletier, J.F.; Huot, J.; Sutton, M.; Schulz, R.; Sandy, A.R.; Lurio, L.B.; Mochrie, S.G.J. Hydrogen desorption mechanism in MgH2−Nb nanocomposites. Phys. Rev. B 2001, 63, 052103. [Google Scholar] [CrossRef]
- Borgschulte, A.; Bösenberg, U.; Barkhordarian, G.; Dornheim, M.; Bormann, R. Enhanced hydrogen sorption kinetics of magnesium by destabilized MgH2−δ. Catal. Today 2007, 120, 262–269. [Google Scholar] [CrossRef]
- Charbonnier, J.; Rango, P.D.; Fruchart, D.; Miraglia, S.; Skryabina, N.; Huot, J.; Hauback, B.; Pitt, M.; Rivoirard, S. Structural analysis of activated Mg(Nb)H2. J. Alloys Compd. 2005, 404–406, 541–544. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, Y.F.; Zhang, X.; Hu, J.J.; Gao, M.X.; Pan, H.G. Empowering hydrogen storage performance of MgH2 by nanoengineering and nanocatalysis. Mater. Today Nano 2020, 9, 100064. [Google Scholar] [CrossRef]
- Pozzo, M.; Alfè, D. Hydrogen dissociation and diffusion on transition metal (=Ti, Zr, V, Fe, Ru, Co, Rh, Ni, Pd, Cu, Ag)-doped Mg(0001) surfaces. Int. J. Hydrogen Energy 2009, 34, 1922–1930. [Google Scholar] [CrossRef]
- Andrés, T.B.; Zélis Luis, M.; Marcos, M. Differences in the heterogeneous nature of hydriding/dehydriding kinetics of MgH2—TiH2 nanocomposites. Int. J. Hydrogen Energy 2020, 45, 27421–27433. [Google Scholar] [CrossRef]
- Ren, C.; Fang, Z.Z.; Zhou, C.; Lu, J.; Ren, Y.; Zhang, X.; Luo, X. In situ X-ray diffraction study of dehydrogenation of MgH2 with Ti-based additives. Int. J. Hydrogen Energy 2014, 39, 5868–5873. [Google Scholar] [CrossRef]
- Ponthieu, M.; Calizzi, M.; Pasquini, L.; Fernandez, J.F.; Cuevas, F. Synthesis by reactive ball milling and cycling properties of MgH2–TiH2 nanocomposites: Kinetics and isotopic effects. Int. J. Hydrogen Energy 2014, 39, 9918–9923. [Google Scholar] [CrossRef]
- Friedrichs, O.; Sánchez-López, J.C.; López-Cartes, C.; Klassen, T.; Bormann, R.; Fernández, A. Nb2O5 “Pathway Effect” on Hydrogen Sorption in Mg. J. Phys. Chem. B 2006, 110, 7845–7850. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; Jongh, P.D.; Milanese, C.; Milčius, D.; et al. Review of magnesium hydride-based materials: Development and optimisation. Appl. Phys. A 2016, 122, 97. [Google Scholar] [CrossRef]
- Yin, Y.; Qi, Y.; Li, B.; Gu, H.; Zhao, J.; Ji, L.; Zhang, B.; Yuan, Z.; Zhang, Y. A comparative study of NbF5 catalytic effects on hydrogenation/dehydrogenation kinetics of Mg-Zn-Ni and Mg-Cu-Ni systems. Mater. Charact. 2021, 174, 110993. [Google Scholar] [CrossRef]
- Lakhnik, A.M.; Kirian, I.M.; Rud, A.D. The Mg/MAX-phase composite for hydrogen storage. Int. J. Hydrogen Energy 2021, in press. [Google Scholar] [CrossRef]
- Luo, Q.; An, X.H.; Pan, Y.B.; Zhang, X.; Zhang, J.-Y.; Li, Q. The hydriding kinetics of Mg–Ni based hydrogen storage alloys: A comparative study on Chou model and Jander model. Int. J. Hydrogen Energy 2010, 35, 7842–7849. [Google Scholar] [CrossRef]
- Luo, Q.; Li, J.; Li, B.; Liu, B.; Shao, H.; Li, Q. Kinetics in Mg-based hydrogen storage materials: Enhancement and mechanism. J. Magnes. Alloy 2019, 7, 58–71. [Google Scholar] [CrossRef]
- Mooij, L.; Dam, B. Nucleation and growth mechanisms of nano magnesium hydride from the hydrogen sorption kinetics. Phys. Chem. Chem. Phys. 2013, 15, 11501–11510. [Google Scholar] [CrossRef]
- Ouyang, L.; Liu, F.; Wang, H.; Liu, J.; Yang, X.-S.; Sun, L.; Zhu, M. Magnesium-based hydrogen storage compounds: A review. J. Alloys Compd. 2020, 832, 154865. [Google Scholar] [CrossRef]
- Jain, A.; Jain, R.K.; Agarwal, S.; Jain, I.P. Structural and thermodynamical investigations of La0.23Ni0.34Co0.33Nd0.08Ti0.01Al0.01 hydrogen storage alloy. Int. J. Hydrogen Energy 2008, 33, 356–359. [Google Scholar] [CrossRef]
- Pal, P.; Kumari, P.; Wang, Y.; Isobe, S.; Kumar, M.; Ichikawa, T.; Jain, A. Destabilization of LiBH4 by the infusion of Bi2X3 (X = S, Se, Te): An in situ TEM investigation. J. Mater. Chem. A 2020, 8, 25706–25715. [Google Scholar] [CrossRef]
- Jangir, M.; Jain, A.; Yamaguchi, S.; Ichikawa, T.; Lal, C.; Jain, I.P. Catalytic effect of TiF4 in improving hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2016, 41, 14178–14183. [Google Scholar] [CrossRef]
- Jain, A.; Miyaoka, H.; Ichikawa, T. Destabilization of lithium hydride by the substitution of group 14 elements: A review. Int. J. Hydrogen Energy 2016, 41, 5969–5978. [Google Scholar] [CrossRef]
- Jain, A.; Miyaoka, H.; Ichikawa, T. Two-Peak Mystery of LiNH2–NaH Dehydrogenation Is Solved? A Study of the Analogous Sodium Amide/Lithium Hydride System. J. Phys. Chem. C 2016, 120, 27903–27909. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, A.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Study on the thermal decomposition of NaBH4 catalyzed by ZrCl4. Int. J. Hydrogen Energy 2017, 42, 22432–22437. [Google Scholar] [CrossRef]
- Selvaraj, S.; Jain, A.; Kumar, S.; Zhang, T.; Isobe, S.; Miyaoka, H.; Kojima, Y.; Ichikawa, T. Study of cyclic performance of V-Ti-Cr alloys employed for hydrogen compressor. Int. J. Hydrogen Energy 2018, 43, 2881–2889. [Google Scholar] [CrossRef]
- Vyas, D.; Jain, P.; Khan, J.; Kulshrestha, V.; Jain, A.; Jain, I.P. Effect of Cu catalyst on the hydrogenation and thermodynamic properties of Mg2Ni. Int. J. Hydrogen Energy 2012, 37, 3755–3760. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Catalytic Mechanism of Transition-Metal Compounds on Mg Hydrogen Sorption Reaction. J. Phys. Chem. B 2006, 110, 11020–11024. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Z.; Zheng, H.; Cao, R. Recent Progress on Defect-rich Transition Metal Oxides and Their Energy-Related Applications. Chem. Asian J. 2020, 15, 3717–3736. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jain, A.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Catalytic effect of bis (cyclopentadienyl) nickel II on the improvement of the hydrogenation-dehydrogenation of Mg-MgH2 system. Int. J. Hydrogen Energy 2017, 42, 17178–17183. [Google Scholar] [CrossRef]
- Vyas, D.; Jain, P.; Agarwal, G.; Jain, A.; Jain, I.P. Hydrogen storage properties of Mg2Ni affected by Cr catalyst. Int. J. Hydrogen Energy 2012, 37, 16013–16017. [Google Scholar] [CrossRef]
- Ma, X.; Liu, S.; Huang, S. Hydrogen adsorption and dissociation on the TM-doped (TM=Ti, Nb) Mg55 nanoclusters: A DFT study. Int. J. Hydrogen Energy 2017, 42, 24797–24810. [Google Scholar] [CrossRef]
- Jain, A.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Tailoring the absorption–desorption properties of KSiH3 compound using nano-metals (Ni, Co, Nb) as catalyst. J. Alloys Compd. 2015, 645, S144–S147. [Google Scholar] [CrossRef]
- Mao, J.F.; Wu, Z.; Chen, T.J.; Weng, B.C.; Xu, N.X.; Huang, T.S.; Guo, Z.P.; Liu, H.K.; Grant, D.M.; Walker, G.S.; et al. Improved Hydrogen Storage of LiBH4 Catalyzed Magnesium. J. Phys. Chem. C 2007, 111, 12495–12498. [Google Scholar] [CrossRef][Green Version]
- Lillo-Ródenas, M.A.; Aguey-Zinsou, K.F.; Cazorla-Amorós, D.; Linares-Solano, A.; Guo, Z.X. Effects of Carbon-Supported Nickel Catalysts on MgH2 Decomposition. J. Phys. Chem. C 2008, 112, 5984–5992. [Google Scholar] [CrossRef]
- Kaupp, G. Reactive milling with metals for environmentally benign sustainable production. CrystEngCom. 2011, 13, 3108–3121. [Google Scholar] [CrossRef]
- Hudson, M.S.L.; Takahashi, K.; Ramesh, A.; Awasthi, S.; Ghosh, A.K.; Ravindran, P.; Srivastava, O.N. Graphene decorated with Fe nanoclusters for improving the hydrogen sorption kinetics of MgH2—Experimental and theoretical evidence. Catal. Sci. Technol. 2016, 6, 261–268. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, L.; Yao, Z.; Yan, N.; Sun, Z.; Yang, X.; Zhu, X.; Hu, S.; Chen, L. Facile synthesized Fe nanosheets as superior active catalyst for hydrogen storage in MgH2. Int. J. Hydrogen Energy 2019, 44, 21955–21964. [Google Scholar] [CrossRef]
- Mao, J.; Guo, Z.; Yu, X.; Liu, H.; Wu, Z.; Ni, J. Enhanced hydrogen sorption properties of Ni and Co-catalyzed MgH2. Int. J. Hydrogen Energy 2010, 35, 4569–4575. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Ström–Olsen, J.O. Nanocrystalline magnesium for hydrogen storage. J. Alloys Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Webb, C.J. A review of catalyst-enhanced magnesium hydride as a hydrogen storage material. J. Phys. Chem. Solids 2015, 84, 96–106. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, Z.; Fu, H.; Liu, H.; Guo, J. Synergistic catalytic effects of ZIF-67 and transition metals (Ni, Cu, Pd, and Nb) on hydrogen storage properties of magnesium. Int. J. Hydrogen Energy 2020, 45, 13376–13386. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Nasani, N.; Pérez, J.; Hortigüela, M.J.; Yang, T.; Bdikin, I.; Fagg, D.P. Two step mechanochemical synthesis of Nb doped MgO rock salt nanoparticles and its application for hydrogen storage in MgH2. Int. J. Hydrogen Energy 2016, 41, 11716–11722. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic effect of Ni nano-particle and Nb oxide on H-desorption properties in MgH2 prepared by ball milling. J. Alloys Compd. 2005, 404–406, 716–719. [Google Scholar] [CrossRef]
- Yang, W.N.; Shang, C.X.; Guo, Z.X. Site density effect of Ni particles on hydrogen desorption of MgH2. Int. J. Hydrogen Energy 2010, 35, 4534–4542. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, J.; Zeng, X.; Wu, X.; Li, D.; Ding, W. Hydrogen Storage Properties of a Mg–Ni Nanocomposite Coprecipitated from Solution. J. Phys. Chem. C 2014, 118, 18401–18411. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, T.; Aguey-Zinsou, K.-F. Magnesium Supported on Nickel Nanobelts for Hydrogen Storage: Coupling Nanosizing and Catalysis. ACS Appl. Nano Mater. 2018, 1, 1272–1279. [Google Scholar] [CrossRef]
- Yang, X.; Hou, Q.; Yu, L.; Zhang, J. Improvement of the hydrogen storage characteristics of MgH2 with a flake Ni nano-catalyst composite. Dalton Trans. 2021, 50, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Gasnier, A.; Amica, G.; Juan, J.; Troiani, H.; Gennari, F.C. N-Doped Graphene-Rich Aerogels Decorated with Nickel and Cobalt Nanoparticles: Effect on Hydrogen Storage Properties of Nanoconfined LiBH4. J. Phys. Chem. C 2020, 124, 115–125. [Google Scholar] [CrossRef]
- Meng, X.; Wan, C.B.; Wang, Y.T.; Ju, X. Porous Ni@C derived from bimetallic Metal–Organic Frameworks and its application for improving LiBH4 dehydrogenation. J. Alloys Compd. 2018, 735, 1637–1647. [Google Scholar] [CrossRef]
- Montone, A.; Aurora, A.; Mirabile Gattia, D.; Vittori Antisari, M. Microstructural and Kinetic Evolution of Fe Doped MgH2 during H2 Cycling. Catalysts 2012, 2, 400–411. [Google Scholar] [CrossRef]
- Gattia, D.M.; Jangir, M.; Jain, I.P. Study on nanostructured MgH2 with Fe and its oxides for hydrogen storage applications. J. Alloys Compd. 2019, 801, 188–191. [Google Scholar] [CrossRef]
- Antiqueira, F.J.; Leiva, D.R.; Zepon, G.; de Cunha, B.F.R.F.; Figueroa, S.J.A.; Botta, W.J. Fast hydrogen absorption/desorption kinetics in reactive milled Mg-8 mol% Fe nanocomposites. Int. J. Hydrogen Energy 2020, 45, 12408–12418. [Google Scholar] [CrossRef]
- Liu, T.; Ma, X.; Chen, C.; Xu, L.; Li, X. Catalytic Effect of Nb Nanoparticles for Improving the Hydrogen Storage Properties of Mg-Based Nanocomposite. J. Phys. Chem. C 2015, 119, 14029–14037. [Google Scholar] [CrossRef]
- Wang, P.; Jensen, C.M. Method for preparing Ti-doped NaAlH4 using Ti powder: Observation of an unusual reversible dehydrogenation behavior. J. Alloys Compd. 2004, 379, 99–102. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Muckerman, J.T. First-Principles Study of Ti-Catalyzed Hydrogen Chemisorption on an Al Surface: A Critical First Step for Reversible Hydrogen Storage in NaAlH4. J. Phys. Chem. B 2005, 109, 6952–6957. [Google Scholar] [CrossRef]
- Blomqvist, A.; Araujo, C.M. Dehydrogenation from 3d-transition-metal-doped NaAlH4: Prediction of catalysts. Appl. Phys. Lett. 2007, 90, 141904. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, Y.-J.; Sun, T.; Guo, J.; Sun, L.-X.; Zhu, M. Influence of Transition Metal Additives on the Hydriding/Dehydriding Critical Point of NaAlH4. J. Phys. Chem. C 2009, 113, 9936–9943. [Google Scholar] [CrossRef]
- Cui, J.; Liu, J.; Wang, H.; Ouyang, L.; Sun, D.; Zhu, M.; Yao, X. Mg–TM (TM: Ti, Nb, V, Co, Mo or Ni) core–shell like nanostructures: Synthesis, hydrogen storage performance and catalytic mechanism. J. Mater. Chem. 2014, 2, 9645–9655. [Google Scholar] [CrossRef]
- Korablov, D.; Besenbacher, F.; Jensen, T.R. Kinetics and thermodynamics of hydrogenation-dehydrogenation for Mg-25%TM (TM = Ti, Nb or V) composites synthesized by reactive ball milling in hydrogen. Int. J. Hydrogen Energy 2018, 43, 16804–16814. [Google Scholar] [CrossRef]
- Xie, W.; West, D.J.; Sun, Y.; Zhang, S. Role of nano in catalysis: Palladium catalyzed hydrogen desorption from nanosized magnesium hydride. Nano Energy 2013, 2, 742–748. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.; Liu, Z.; Zhu, Y.; Zhang, J.; Li, L. Magnesium Nanoparticles with Pd Decoration for Hydrogen Storage. Front. Chem. 2020, 7, 949. [Google Scholar] [CrossRef]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen Storage in Microporous Metal-Organic Frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lan, Z.; Huang, X.; Liu, H.; Guo, J. Study on catalytic effect and mechanism of MOF (MOF = ZIF-8, ZIF-67, MOF-74) on hydrogen storage properties of magnesium. Int. J. Hydrogen Energy 2019, 44, 28863–28873. [Google Scholar] [CrossRef]
- Ma, Z.; Zou, J.; Hu, C.; Zhu, W.; Khan, D.; Zeng, X.; Ding, W. Effects of trimesic acid-Ni based metal organic framework on the hydrogen sorption performances of MgH2. Int. J. Hydrogen Energy 2019, 44, 29235–29248. [Google Scholar] [CrossRef]
- Ma, Z.; Zou, J.; Khan, D.; Zhu, W.; Hu, C.; Zeng, X.; Ding, W. Preparation and hydrogen storage properties of MgH2-trimesic acid-TM MOF (TM=Co, Fe) composites. J. Mater. Sci. Technol. 2019, 35, 2132–2143. [Google Scholar] [CrossRef]
- Zhang, J.; He, L.; Yao, Y.; Zhou, X.J.; Yu, L.P.; Lu, X.Z.; Zhou, D.W. Catalytic effect and mechanism of NiCu solid solutions on hydrogen storage properties of MgH2. Renew. Energy 2020, 154, 1229–1239. [Google Scholar]
- Chen, M.; Wang, Y.; Xiao, X.; Lu, Y.; Zhang, M.; Zheng, J.; Chen, L. Highly efficient ZrH2 nanocatalyst for the superior hydrogenation kinetics of magnesium hydride under moderate conditions: Investigation and mechanistic insights. Appl. Surf. Sci. 2021, 541, 148375. [Google Scholar] [CrossRef]
- Ismail, M. Effect of adding different percentages of HfCl4 on the hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2021, 46, 8621–8628. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.; Zhang, X.; Hu, J.; Gao, M.; Pan, H.; Liu, Y. Synthesis process and catalytic activity of Nb2O5 hollow spheres for reversible hydrogen storage of MgH2. Int. J. Energy Res. 2021, 45, 3129–3141. [Google Scholar] [CrossRef]
- Gi, H.; Shinzato, K.; Balgis, R.; Ogi, T.; Sadakane, M.; Wang, Y.; Isobe, S.; Miyaoka, H.; Ichikawa, T. Effective Factor on Catalysis of Niobium Oxide for Magnesium. ACS Omega 2020, 5, 21906–21912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, Z.; Yao, Z.; Yang, L.; Yan, N.; Lu, X.; Xiao, B.; Zhu, X.; Chen, L. Excellent catalysis of Mn3O4 nanoparticles on the hydrogen storage properties of MgH2: An experimental and theoretical study. Nanoscale Adv. 2020, 2, 1666–1675. [Google Scholar] [CrossRef]
- Yang, X.; Ji, L.; Yan, N.; Sun, Z.; Lu, X.; Zhang, L.; Zhu, X.; Chen, L. Superior catalytic effects of FeCo nanosheets on MgH2 for hydrogen storage. Dalton Trans. 2019, 48, 12699–12706. [Google Scholar] [CrossRef] [PubMed]
- Berezovets, V.V.; Denys, R.V.; Zavaliy, I.Y.; Kosarchyn, Y.V. Effect of Ti-based nanosized additives on the hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2021, 19. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Zhang, X.; Yang, Y.; Gao, M.; Pan, H. Superior catalytic activity derived from a two-dimensional Ti3C2 precursor towards the hydrogen storage reaction of magnesium hydride. Chem. Commun. 2016, 52, 705–708. [Google Scholar] [CrossRef]
- Ismail, M. Influence of different amounts of FeCl3 on decomposition and hydrogen sorption kinetics of MgH2. Int. J. Hydrogen Energy 2014, 39, 2567–2574. [Google Scholar] [CrossRef]
- Ojeda, X.A.; Castro, F.J.; Pighin, S.A.; Troiani, H.E.; Moreno, M.S.; Urretavizcaya, G. Hydrogen absorption and desorption properties of Mg/MgH2 with nanometric dispersion of small amounts of Nb(V) ethoxide. Int. J. Hydrogen Energy 2021, 46, 4126–4136. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Z.; Zhu, X.; Yao, Z.; Sun, Z.; Ji, L.; Yan, N.; Xiao, B.; Chen, L. Two-dimensional ZrCo nanosheets as highly effective catalyst for hydrogen storage in MgH2. J. Alloys Compd. 2019, 805, 295–302. [Google Scholar] [CrossRef]
- Song, M.Y.; Kwak, Y.J. Hydrogen charging kinetics of Mg—10wt% Fe2O3 prepared via MgH2-forming mechanical milling. Mater. Res. Bull. 2021, 140, 111304. [Google Scholar] [CrossRef]
- Cai, W.; Wang, H.; Liu, J.; Jiao, L.; Wang, Y.; Ouyang, L.; Sun, T.; Sun, D.; Wang, H.; Yao, X.; et al. Towards easy reversible dehydrogenation of LiBH4 by catalyzing hierarchic nanostructured CoB. Nano Energy 2014, 10, 235–244. [Google Scholar] [CrossRef]
- Rafi-ud-din, Xuanhui, Q.; Ping, L.; Zhang, L.; Qi, W.; Iqbal, M.Z.; Rafique, M.Y.; Farooq, M.H.; Islam-ud-din. Superior Catalytic Effects of Nb2O5, TiO2, and Cr2O3 Nanoparticles in Improving the Hydrogen Sorption Properties of NaAlH4. J. Phys. Chem. C 2012, 116, 11924–11938. [Google Scholar] [CrossRef]
- Khan, J.; Jain, I.P. Catalytic effect of Nb2O5 on dehydrogenation kinetics of NaAlH4. Int. J. Hydrogen Energy 2016, 41, 8264–8270. [Google Scholar] [CrossRef]
- Rafi-ud-din, Xuanhui, Q.; Ping, L.; Zhang, L.; Ahmad, M. Hydrogen Sorption Improvement of LiAlH4 Catalyzed by Nb2O5 and Cr2O3 Nanoparticles. J. Phys. Chem. C 2011, 115, 13088–13099. [Google Scholar] [CrossRef]
- Ismail, M.; Zhao, Y.; Yu, X.B.; Nevirkovets, I.P.; Dou, S.X. Significantly improved dehydrogenation of LiAlH4 catalysed with TiO2 nanopowder. Int. J. Hydrogen Energy 2011, 36, 8327–8334. [Google Scholar] [CrossRef]
- Jain, A.; Agarwal, S.; Kumar, S.; Yamaguchi, S.; Miyaoka, H.; Kojimaa, Y.; Ichikawa, T. How does TiF4 affect the decomposition of MgH2 and its complex variants?—An XPS investigation. J. Mater. Chem. A. 2017, 5, 15543–15551. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Z.; Yao, Z.; Ji, L.; Sun, Z.; Yan, N.; Zhang, B.; Xiao, B.; Du, J.; Zhu, X.; et al. A striking catalytic effect of facile synthesized ZrMn2 nanoparticles on the de/rehydrogenation properties of MgH2. J. Mater. Chem. A 2019, 7, 5626–5634. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Saeed, M.; Al-Nasrallah, E.; Al-Ajmi, F.; Banyan, M. Effect of LaNi3 Amorphous Alloy Nanopowders on the Performance and Hydrogen Storage Properties of MgH2. Energies 2019, 12, 1005. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Kaflou, A.; Simchi, A. Hydrogen desorption properties of MgH2–TiCr1.2Fe0.6 nanocomposite prepared by high-energy mechanical alloying. J. Power Sources 2011, 196, 4604–4608. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, Z.-Z.; Ren, C.; Li, J.; Lu, J. Effect of Ti Intermetallic Catalysts on Hydrogen Storage Properties of Magnesium Hydride. J. Phys. Chem. C 2013, 117, 12973–12980. [Google Scholar] [CrossRef]
- Wang, K.; Du, H.; Wang, Z.; Gao, M.; Pan, H.; Liu, Y. Novel MAX-phase Ti3AlC2 catalyst for improving the reversible hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2017, 42, 4244–4251. [Google Scholar] [CrossRef]
- Meena, P.; Jangir, M.; Kumar, A.; Singh, R.; Sharma, V.K.; Jain, I.P. Improved dehydrogenation kinetics of MgH2 due to NiMnAl. Mater. Res. Express 2017, 4, 116520. [Google Scholar] [CrossRef]
- Motavalli, A.; Rajabi, M. Catalytic effect of melt-spun Ni3FeMn alloy on hydrogen desorption properties of nanocrystalline MgH2 synthesized by mechanical alloying. Int. J. Hydrogen Energy 2014, 39, 17047–17053. [Google Scholar] [CrossRef]
- Singh, S.; Bhatnagar, A.; Shukla, V.; Vishwakarma, A.K.; Soni, P.K.; Verma, S.K.; Shaz, M.A.; Sinha, A.S.K.; Srivastavaa, O.N. Ternary transition metal alloy FeCoNi nanoparticles on graphene as new catalyst for hydrogen sorption in MgH2. Int. J. Hydrogen Energy 2020, 45, 774–786. [Google Scholar] [CrossRef]
- Ismail, M.; Mustafa, N.S.; Ali, N.A.; Sazelee, N.A.; Yahya, M.S. The hydrogen storage properties and catalytic mechanism of the CuFe2O4-doped MgH2 composite system. Int. J. Hydrogen Energy 2019, 44, 318–324. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, H.; Liu, J.; Ouyang, L.; Zhu, M. Destabilizing the dehydriding thermodynamics of MgH2 by reversible intermetallics formation in Mg−Ag−Zn ternary alloys. J. Power Sources 2018, 396, 796–802. [Google Scholar] [CrossRef]
- Sazelee, N.A.; Idris, N.H.; Md Din, M.F.; Yahya, M.S.; Ali, N.A.; Ismaila, M. LaFeO3 synthesised by solid-state method for enhanced sorption properties of MgH2. Results Physics. 2020, 16, 102844. [Google Scholar] [CrossRef]
- Agarwal, S.; Jain, A.; Jain, P.; Jangir, M.; Jain, I.P. Kinetic Enhancement in the Sorption Properties by Forming Mg–x wt % ZrCrCu Composites. J. Phys. Chem. C 2013, 117, 11953–11959. [Google Scholar] [CrossRef]
- Agarwal, S.; Aurora, A.; Jain, A.; Montone, A. Structural and H2 sorption properties of MgH2–10 wt%ZrCrM (M = Cu, Ni) nano-composites. J. Nanoparticle Res. 2011, 13, 5719–5726. [Google Scholar] [CrossRef]
- Agarwal, S.; Aurora, A.; Jain, A.; Jain, I.P.; Montone, A. Catalytic effect of ZrCrNi alloy on hydriding properties of MgH2. Int. J. Hydrogen Energy 2009, 34, 9157–9162. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Z.; Cai, Z.; Yan, N.; Lu, X.; Zhu, X.; Chen, L. Enhanced hydrogen storage properties of MgH2 by the synergetic catalysis of Zr0.4Ti0.6Co nanosheets and carbon nanotubes. Appl. Surf. Sci. 2020, 504, 144465. [Google Scholar] [CrossRef]
- Ali, N.A.; Idrisa, N.H.; Md Din, M.F.; Yahya, M.S.; Ismail, M. Nanoflakes MgNiO2 synthesised via a simple hydrothermal method and its catalytic roles on the hydrogen sorption performance of MgH2. J. Alloys Compd. 2019, 796, 279–286. [Google Scholar] [CrossRef]
- Yahya, M.S.; Ismail, M. Catalytic effect of SrTiO3 on the hydrogen storage behaviour of MgH2. J. Energy Chem. 2019, 28, 46–53. [Google Scholar] [CrossRef]
- Zhang, J.; Li, P.; Wan, Q.; Zhai, F.; Volinskyd, A.A.; Qu, X. Superior destabilization effects of LiBH4 with the addition of nano-sized nickel ferrite NiFe2O4. RSC Adv. 2015, 5, 81212–81219. [Google Scholar] [CrossRef]
- Mo, X.; Jiang, W.; Cao, S. First-principles study on the dehydrogenation characteristics of LiBH4 modified by Ti. Results Phys. 2017, 7, 3236–3242. [Google Scholar] [CrossRef]
- Wan, Q.; Li, P.; Li, Z.; Zhao, K.; Liu, Z.; Wang, L.; Zhai, F.; Qu, X.; Volinsky, A.A. NaAlH4 dehydrogenation properties enhanced by MnFe2O4 nanoparticles. J. Power Sources 2014, 248, 388–395. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.; Wan, Q.; Zhang, J.; Li, Y.; Li, R.; Dong, R.; Qu, X. Improved dehydrogenation performance of NaAlH4 using NiFe2O4 nanoparticles. J. Alloys Compd. 2017, 709, 850–856. [Google Scholar] [CrossRef]
- Li, Z.; Zhai, F.; Wan, Q.; Liu, Z.; Shan, J.; Li, P.; Volinskyc, A.A.; Qua, X. Enhanced hydrogen storage properties of LiAlH4 catalyzed by CoFe2O4 nanoparticles. RSC Adv. 2014, 4, 18989–18997. [Google Scholar] [CrossRef]
- Li, P.; Li, Z.; Zhai, F.; Wan, Q.; Li, X.; Qu, X.; Volinsky, A.A. NiFe2O4 Nanoparticles Catalytic Effects of Improving LiAlH4 Dehydrogenation Properties. J. Phys. Chem. C 2013, 117, 25917–25925. [Google Scholar] [CrossRef]
- Ali, N.A.; Idris, N.H.; Sazelee, N.A.; Yahya, M.S.; Halim Yap, F.A.; Ismaila, M. Catalytic effects of MgFe2O4 addition on the dehydrogenation properties of LiAlH4. Int. J. Hydrogen Energy 2019, 44, 28227–28234. [Google Scholar] [CrossRef]
- Tan, C.-Y.; Tsai, W.-T. Effects of Ni and Co-decorated MWCNTs addition on the dehydrogenation behavior and stability of LiAlH4. Int. J. Hydrogen Energy 2015, 40, 14064–14071. [Google Scholar] [CrossRef]
- Jiao, C.; Sun, L.; Xu, F.; Liu, S.-S.; Zhang, J.; Jiang, X.; Yang, L. NiCo nanoalloy encapsulated in graphene layers for improving hydrogen storage properties of LiAlH4. Sci. Rep. 2016, 6, 27429. [Google Scholar] [CrossRef]
- Xia, Y.; Wei, S.; Huang, Q.; Li, J.; Cen, X.; Zhang, H.; Chu, H.; Sun, L.; Xu, L.; Huang, P. Facile synthesis of NiCo2O4-anchored reduced graphene oxide nanocomposites as efficient additives for improving the dehydrogenation behavior of lithium alanate. Inorg. Chem. Front. 2020, 7, 1257–1272. [Google Scholar] [CrossRef]
- Wei, S.; Xue, S.; Huang, C.; Che, B.; Zhang, H.; Sun, L.; Xu, F.; Xia, Y.; Cheng, R.; Zhang, C.; et al. Multielement synergetic effect of NiFe2O4 and h-BN for improving the dehydrogenation properties of LiAlH4. Inorg. Chem. Front. 2021, 8, 3111–3126. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Liu, J.; Ma, Z.; Zhang, J.; Liu, Y.; Li, Y.; Li, L. Enhancing hydrogen storage properties of MgH2 by core-shell CoNi@C. J. Alloys Compd. 2021, 862, 158004. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, X.; Bosang, L.; Meijia, L.; Man, C.; Lixin, C. Superior de/hydrogenation performances of MgH2 catalyzed by 3D flower-like TiO2@C nanostructures. J. Energy Chem. 2020, 46, 191–198. [Google Scholar]
- Liu, J.; Ma, Z.; Liu, Z.; Tang, Q.; Zhu, Y.; Lin, H.; Zhang, Y.; Zhang, J.; Liu, Y.; Li, L. Synergistic effect of rGO supported Ni3Fe on hydrogen storage performance of MgH2. Int. J. Hydrogen Energy 2020, 45, 16622–16633. [Google Scholar] [CrossRef]
- Ding, Z.; Fu, Y.; Wang, Y.; Bi, J.; Zhang, L.; Peng, D.; Li, Y.; Han, S. MgCNi3 prepared by powder metallurgy for improved hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2019, 44, 8347–8356. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, L.; Fu, Y.; Wang, W.; Wang, Y.; Bi, J.; Li, Y.; Han, S. Enhanced kinetics of MgH2 via in situ formed catalysts derived from MgCCo1.5Ni1.5. J. Alloys Compd. 2020, 822, 153621. [Google Scholar] [CrossRef]
- Meena, P.; Singh, R.; Sharma, V.K.; Jain, I.P. Role of NiMn9.3Al4.0Co14.1Fe3.6 alloy on dehydrogenation kinetics of MgH2. J. Magnes. Alloy. 2018, 6, 318–325. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Hashmi, S.A.R.; Kim, K.-H. MXenes: Emerging 2D materials for hydrogen storage. Nano Energy 2021, 85, 105989. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Du, Y.; Liao, W. Catalytic effect of Ti2C MXene on the dehydrogenation of MgH2. Int. J. Hydrogen Energy 2019, 44, 6787–6794. [Google Scholar] [CrossRef]
- Zhu, W.; Panda, S.; Lu, C.; Ma, Z.; Khan, D.; Dong, J.; Sun, F.; Xu, H.; Zhang, Q.; Zou, J. Using a Self-Assembled Two-Dimensional MXene-Based Catalyst (2D-Ni@Ti3C2) to Enhance Hydrogen Storage Properties of MgH2. ACS Appl. Mater. Interfaces 2020, 12, 50333–50343. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, C.; Wang, X.; Xu, L.; Huang, X.; Wang, X.; Ning, H.; Lan, Z.; Guo, J. Combinations of V2C and Ti3C2 MXenes for Boosting the Hydrogen Storage Performances of MgH2. ACS Appl. Mater. Interfaces 2021, 13, 13235–13247. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, D.; Liu, X.; Fan, G.; Liu, B. Improving the hydrogen storage performance of lithium borohydride by Ti3C2 MXene. Int. J. Hydrogen Energy 2019, 44, 29297–29303. [Google Scholar] [CrossRef]
- Fan, Y.; Yuan, Z.; Zou, G.; Zhang, Q.; Liu, B.; Peng, Q. Two-dimensional MXene/A-TiO2 composite with unprecedented catalytic activation for sodium alanate. Catal. Today 2018, 318, 167–174. [Google Scholar] [CrossRef]
- Li, Z.; Gao, M.; Gu, J.; Xian, K.; Yao, Z.; Shang, C.; Liu, Y.; Guo, Z.; Pan, H. In Situ Introduction of Li3BO3 and NbH Leads to Superior Cyclic Stability and Kinetics of a LiBH4-Based Hydrogen Storage System. ACS Appl. Mater. Interfaces 2020, 12, 893–903. [Google Scholar] [CrossRef]
- Yuan, Z.; Fan, Y.; Chen, Y.; Liu, X.; Liu, B.; Han, S. Two-dimensional C@TiO2/Ti3C2 composite with superior catalytic performance for NaAlH4. Int. J. Hydrogen Energy 2020, 45, 21666–21675. [Google Scholar] [CrossRef]
- Jiang, R.; Xiao, X.; Zheng, J.; Chen, M.; Chen, L. Remarkable hydrogen absorption/desorption behaviors and mechanism of sodium alanates in-situ doped with Ti-based 2D MXene. Mater. Chem. Phys. 2020, 242, 122529. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.Z.; Zhang, Y.; Liu, D.M.; Wang, C.Y.; Si, T.Z.; Li, Y.T.; Zhang, Q.A. Coupling of nanoconfinement with metallic catalysis in supported NaAlH4 for low-temperature hydrogen storage. J. Power Sources 2021, 491, 229611. [Google Scholar] [CrossRef]
- Chen, W.; You, L.; Xia, G.; Yu, X. A balance between catalysis and nanoconfinement towards enhanced hydrogen storage performance of NaAlH4. J. Mater. Sci. Technol. 2021, 79, 205–211. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).