Abstract
In this study, new mono- and di-alkoxido zirconium(IV) complexes supported by tetradentate dianionic cyclam ligands were synthesized and characterized. These compounds were obtained by reaction of the parent Zr(IV) dichlorido species with one or two equivalents of the corresponding lithium alkoxido, whereas (3,5-Me2Bn2Cyclam)Zr(OPh)2 was prepared by protonolysis of the orthometallated species (3,5-Me-C6H4CH2)2Cyclam)Zr with phenol. The solid-state molecular structures of (Bn2Cyclam)ZrCl(OtBu) and (4-tBuBn2Cyclam)Zr(OiPr)2 show a trigonal prismatic geometry around the metal centers. (Bn2Cyclam)Zr(SPh)(OtBu) and (Bn2Cyclam)ZrMe(OiPr) were prepared by reaction of (Bn2Cyclam)ZrCl(OR) (R = iPr, tBu) with one equivalent of LiSPh or MeMgCl, respectively. The reactions of (Bn2Cyclam)Zr(OiPr)2 and (4-tBuBn2Cyclam)Zr(OiPr)2 with carbon dioxide suggested the formation of species that correspond to the addition of four CO2 molecules.
1. Introduction
The use of alkoxide ligands as alternatives to the cyclopentadienyl ligand started in the 1980s. Considering electron-count rules, alkoxide and cyclopentadienyl ligands may be formally considered electronically equivalent, bonding to transition metals through one σ- and two π-donor orbitals. Regardless of this analogy, cyclopentadienyl is a carbon based and soft ligand, while alkoxides are hard ligands that form with Group 4 metals high polar and strong bonds. The latter feature was largely responsible for the extensive use of titanium and zirconium alkoxidos in catalysis [1,2]. The high polarity of M-OR bonds led to the application of several families of early transition metal complexes in the ring opening polymerization (ROP) of cyclic esters [3,4,5,6,7,8,9,10,11,12]. Our previous research on cyclam-based Zr(IV) alkoxido, phenoxido and thiophenoxido derivatives [13,14,15] also disclosed new catalytic systems for the ROP of rac-lactide strongly influenced by the nature of the OR ligands [13]. The characterization of the polymers obtained and DFT calculations clarified the important features that govern the catalytic activity and the role of OR ligands as initiators of the polymerization reactions or as supporting ligands that direct the insertion of the monomers into the Zr-N bonds of the cyclam ligand with concomitant formation of cyclam functionalized polylactide [13]. The selective insertion of heteroallenes into the Zr-Namido bonds of (Bn2Cyclam)Zr(OR)2 (R = iPr, tBu) revealed to be a suitable procedure for the N-functionalization of the cyclam ring [14]. In view of the importance of N-functionalized cyclams in several chemical [16,17,18,19,20,21] and biological [22,23,24,25,26] applications, we present here the reactivity of di-alkoxido zirconium(IV) complexes with carbon dioxide. In this manuscript, we also describe the synthesis and characterization of new mono-alkoxido zirconium(IV) complexes supported by tetradentate dianionic cyclam ligands and mixed complexes bearing one alkoxide and one methyl or thiophenoxide ligands. The formation of asymmetrically substituted cyclam-based Zr(IV) complexes of general formula (Bn2Cyclam)Zr(X)(Y) is foreseen to display an important role in diverse catalytic transformations.
2. Materials and Methods
2.1. General Considerations
(Bn2Cyclam)ZrCl2, 1, [27] (Bn2Cyclam)ZrCl(OiPr), 3, [13] (3,5-Me2Bn2Cyclam)ZrCl2, 7, [16] (4-tBuBn2Cyclam)ZrCl2, 8, [16] (3,5-Me-C6H4CH2)2Cyclam)Zr, 9 [19] (Bn2Cyclam)Zr(OiPr)2, 11 [14] and (4-tBuBn2Cyclam)Zr(OiPr)2, 12 [15] were prepared according to previously described procedures. Lithium salts were prepared by lithiation of thiophenol or the corresponding alcohols with LinBu. All other reagents were commercial grade and used without further purification. All manipulations were performed under an atmosphere of dry oxygen-free nitrogen by means of standard Schlenk and glovebox techniques. Carbon dioxide was supplied by Air Liquide and passed over a bed of molecular sieves before use. Solvents were pre-dried using 4 Å molecular sieves and refluxed over sodium-benzophenone under an atmosphere of N2 and collected by distillation. Deuterated solvents were dried with 4 Å molecular sieves and freeze-pump-thaw degassed prior to use. NMR spectra were recorded in a Bruker AVANCE II 300 or 400 MHz spectrometer, at 296 K, referenced internally to residual proton-solvent (1H) or solvent (13C) resonances, and reported relative to tetramethylsilane (0 ppm). 2D NMR experiments such as 1H-13C{1H} HSQC and 1H-1H COSY were performed to make all assignments. IR spectra were obtained on a Jasco FT/IR-4100 spectrometer. Elemental analyses were performed in a Fisons CHNS/O analyser Carlo Erba Instruments EA-1108 equipment (Thermo Scientific, Waltham, MA, USA) at the Analytical Laboratory of the Instituto Superior Técnico.
2.2. Synthesis and Characterization
(Bn2Cyclam)ZrCl(OtBu), 2: A THF solution of LiOtBu (103 mg, 1.29 mmol) was added to a suspension of 1 (700 mg, 1.29 mmol) in the same solvent. The mixture was stirred overnight, and the yellowish suspension turned into a colorless solution. The solvent was evaporated, and the product was extracted with small volumes of warm toluene. Evaporation to dryness afforded a white solid in 62% yield (463 mg, 0.80 mmol). Crystalline material suitable for X-ray diffraction was obtained from a concentrated toluene solution at −20 °C. 1H NMR (C6D6, 300.1 MHz, 296 K): δ (ppm) 7.16–7.10 (overlapping, 10H, total, PhCH2N), 4.93 (m, 2JH-H = 14 Hz, 1H, PhCH2N), 4.63 (m, 1H, [C3]CH2N), 4.52 (d, 2JH-H = 14 Hz, 1H, PhCH2N), 4.32 (d, 2JH-H = 14 Hz, 1H, PhCH2N), 4.26–4.14 (overlapping, 2H total, 2JH-H = 14 Hz, 1H, PhCH2N and 1H, [C3]CH2N), 3.69 (m, 1H, [C3]CH2N), 3.56–3.45 (overlapping, 2H total, [C2]CH2N), 3.16 (m, 1H, [C3]CH2N), 2.99 (m, 1H, [C2]CH2N), 2.85–2.77 (overlapping, 2H total, 1H, [C3]CH2N and 1H, [C2]CH2N), 2.72–2.63 (overlapping, 3H total, 1H, [C3]CH2N and 2H, [C2]CH2N), 2.37–2.33 (overlapping, 2H total, [C3]CH2N), 2.17 (m, 1H, [C2]CH2N), 2.09 (m, 1H, [C2]CH2N), 1.73–1.64 (overlapping, 10H total, 9H, C(CH3)3 and 1H, CH2CH2CH2), 1.55 (m, 1H, CH2CH2CH2), 1.16 (m, 1H, CH2CH2CH2), 1.04 (m, 1H, CH2CH2CH2). 13C{1H} NMR (C6D6, 75.5 MHz, 296 K): δ (ppm) 133.0 (Ph), 132.9 (Ph), 128.6 (Ph), 127.9 (Ph), 76.8 (C(CH3)3), 57.0 (PhCH2N), 56.7 ([C3]CH2N), 56.4 ([C3]CH2N), 56.2 (PhCH2N), 56.0 ([C3]CH2N), 54.5 ([C3]CH2N), 53.1 ([C2]CH2N), 51.8 ([C2]CH2N), 48.4 ([C2]CH2N), 48.2 ([C2]CH2N), 33.3 (C(CH3)3), 25.0 (CH2CH2CH2), 24.6 (CH2CH2CH2). Anal. calcd for C28H47ClN4OZr: C, 58.15; H, 7.49; N, 9.69. Found: C, 58.15; H, 7.55; N, 9.96.
(Bn2Cyclam)ZrCl(OPh), 4: A THF solution of LiOPh (138 mg, 1.38 mmol) was added to a suspension of 1 (747 mg, 1.38 mmol) in the same solvent. The mixture was stirred overnight, and the yellowish suspension turned into a colorless solution. The solvent was evaporated, and the product was extracted with small volumes of warm toluene. Evaporation to dryness afforded a white solid in 56% yield (460 mg, 0.77 mmol). 1H NMR (C6D6, 300.1 MHz, 296 K): δ (ppm) 7.42 (dd, 3JH-H = 8 Hz, 4JH-H = 1 Hz, 2H, o-PhOZr), 7.34 (t, 3JH-H = 8 Hz, 2H, m-PhOZr), 7.13–7.00 (overlapping, 10H total, PhCH2N), 6.90 (t, 3JH-H = 8 Hz, 1H, p-PhOZr), 4.79 (m, 2JH-H = 14 Hz, 1H, PhCH2N), 4.59 (m, 1H, [C3]CH2N), 4.32 (d, 2JH-H = 14 Hz, 1H, PhCH2N), 4.25–4.11 (overlapping, 3H total, 2JH-H = 14 Hz, 2H, PhCH2N and 1H, [C3]CH2N), 3.58 (m, 1H, [C3]CH2N), 3.54–3.41 (overlapping, 2H total, [C2]CH2N), 3.11 (m, 1H, [C3]CH2N), 2.99 (m, 1H, [C2]CH2N), 2.80–2.66 (overlapping, 5H total, 2H, [C3]CH2N and 3H, [C2]CH2N), 2.39–2.28 (overlapping, 2H total, [C3]CH2N), 2.18–2.05 (overlapping, 2H total, [C2]CH2N), 1.63 (m, 1H, CH2CH2CH2), 1.50 (m, 1H, CH2CH2CH2), 1.17 (m, 1H, CH2CH2CH2), 1.03 (m, 1H, CH2CH2CH2). 13C{1H} NMR (C6D6, 75.5 MHz, 296 K): δ (ppm) 164.7 (i-PhOZr), 133.0 (PhCH2N), 132.8 (PhCH2N), 132.5 (i-PhCH2N), 132.2 (i-PhCH2N), 129.8 (m-PhOZr), 128.3 (PhCH2N), 120.0 (o-PhOZr), 119.0 (p-PhOZr), 56.8 ([C3]CH2N), 56.5 (PhCH2N), 56.3 ([C3]CH2N and PhCH2N), 55.2 ([C3]CH2N), 54.8 ([C3]CH2N), 53.3 ([C2]CH2N), 52.2 ([C2]CH2N), 48.7 ([C2]CH2N), 48.4 ([C2]CH2N), 25.2 (CH2CH2CH2), 24.9 (CH2CH2CH2). Anal. calcd for C30H39ClN4OZr: C, 60.22; H, 6.57; N, 9.36. Found: C, 60.25; H, 6.63; N, 9.46.
(Bn2Cyclam)Zr(SPh)(OtBu), 5: A THF solution of LiSPh (65 mg, 0.56 mmol) was added to a suspension of 2 (300 mg, 0.55 mmol) in the same solvent. The mixture was stirred overnight, and the yellowish suspension turned into a colorless solution. The solvent was evaporated, and the product was extracted with small volumes of warm toluene. Evaporation to dryness afforded a white solid in 64% yield (228 mg, 0.35 mmol). 1H NMR (C6D6, 400.1 MHz, 296 K): δ (ppm) 7.96 (d, 3JH-H = 6 Hz, 2H, o-PhSZr), 7.18–6.92 (overlapping, 14H total, 10H, PhCH2N, 2H, m-PhSZr and 2H, p-PhSZr), 4.78–4.71 (overlapping, 4H total, 3H, PhCH2N and 1H, [C3]CH2N), 4.33–4.23 (overlapping, 2H total, 1H, PhCH2N and 1H, [C3]CH2N), 4.09 (m, 1H, [C3]CH2N), 3.72 (m, 1H, [C3]CH2N), 3.52 (m, 1H, [C2]CH2N), 3.20 (m, 1H, [C3]CH2N), 3.03 (m, 1H, [C2]CH2N), 2.88–2.78 (overlapping, 3H total, 1H, [C2]CH2N and 2H, [C3]CH2N), 2.72–2.61 (overlapping, 2H total, [C2]CH2N), 2.40–2.31 (overlapping, 2H total, 1H, [C2]CH2N and 1H, [C3]CH2N), 2.19–2.17 (overlapping, 2H total, [C2]CH2N), 1.76 (m, 1H, CH2CH2CH2), 1.57 (m, 1H, CH2CH2CH2), 1.46 (s, 9H, C(CH3)3), 1.20 (m, 1H, CH2CH2CH2), 1.03 (m, 1H, CH2CH2CH2). 13C{1H} NMR (C6D6, 100.6 MHz, 296 K): δ (ppm) 133.3 (Ph), 133.0 (Ph), 132.9 (Ph), 132.6 (Ph), 128.6 (Ph), 128.4 (Ph), 127.9 (Ph), 122.7 (Ph), 77.5 (C(CH3)3), 57.0 (PhCH2N), 56.3 ([C3]CH2N), 56.1 ([C3]CH2N and [C2]CH2N), 55.6 (PhCH2N), 54.5 ([C3]CH2N), 53.4 ([C3]CH2N), 52.3 ([C2]CH2N), 49.4 ([C2]CH2N), 48.5 ([C2]CH2N), 32.4 (C(CH3)3), 25.0 (CH2CH2CH2), 24.5 (CH2CH2CH2). Anal. calcd for C34H48N4OSZr: C, 62.63; H, 7.42; N, 8.59; S, 4.92. Found: C, 59.50; H, 7.72; N, 8.10; S, 5.33.
(Bn2Cyclam)ZrMe(OiPr), 6: To a THF solution of 3 (112 mg, 0.20 mmol), 0.1 mL of a MeMgCl solution (2.18 M in THF) was slowly added. The mixture was refluxed overnight. The solvent was evaporated, and the light beige residue was extracted with toluene. To the toluene extract a few drops of dioxane was added. The suspension was filtered, and the solvents were evaporated to dryness affording a beige solid in 60% yield (65.3 mg, 0.12 mmol). 1H NMR (toluene-d8, 300.1 MHz, 296 K): δ (ppm) 7.14–6.96 (overlapping, 10H total, Ph), 4.99 (m, 3JH-H = 6 Hz, 1H, CH(CH3)2), 4.78 (d, 2JH-H = 14 Hz, 1H, PhCH2N), 4.49–4.37 (overlapping, 2H total, 1H, [C3]CH2N) and 1H, PhCH2N), 4.24–4.03 (overlapping, 3H total, 1H, [C3]CH2N) and 2H, PhCH2N), 3.58–3.40 (overlapping, 3H total, 1H, [C3]CH2N and 2H, [C2]CH2N), 3.09–2.98 (overlapping, 2H total, 1H, [C3]CH2N and 1H, [C2]CH2N), 2.83–2.78 (overlapping, 2H total, 1H, [C3]CH2N and 1H, [C2]CH2N), 2.73–2.65 (overlapping, 3H total, 1H, [C3]CH2N and 2H, [C2]CH2N), 2.36–2.31 (overlapping, 2H total, [C3]CH2N), 2.17–2.12 (overlapping, 2H total, [C2]CH2N), 1.74–1.60 (overlapping, 2H total, CH2CH2CH2), 1.54 (d, 2JH-H = 6 Hz, 3H, CH(CH3)2), 1.48 (d, 2JH-H = 6 Hz, 3H, CH(CH3)2), 1.16–1.03 (overlapping, 2H total, CH2CH2CH2), 0.26 (s, 3H, Zr-CH3). 13C{1H} NMR (toluene-d8, 75.5 MHz, 296 K): δ (ppm) 133.1 (Ph), 132.9 (Ph), 132.8 (Ph), 129.3 (Ph), 128.4 (Ph), 128.2 (Ph), 128.0 (Ph), 72.6 (CH(CH3)2), 56.9 (PhCH2N or [C3]CH2N), 56.8 (PhCH2N or [C3]CH2N), 56.3 (PhCH2N or [C3]CH2N), 56.2 (PhCH2N or [C3]CH2N), 56.0 (PhCH2N or [C3]CH2N), 54.0 ([C3]CH2N), 52.9 ([C2]CH2N), 51.7 ([C2]CH2N), 48.3 ([C2]CH2N), 48.0 ([C2]CH2N), 27.8 (CH(CH3)2), 27.7 (CH(CH3)2), 24.8 CH2CH2CH2), 24.5 (CH2CH2CH2), 2.5 (Zr-CH3) Anal. calcd for C28H44N4OZr: C, 61.83; H, 8.15; N, 10.30. Found: C, 61.91; H, 8.18; N, 10.23.
(3,5-Me2Bn2Cyclam)Zr(OPh)2, 10: In a J-Young NMR tube, phenol (14 mg, 0.15 mmol) was added to a toluene-d8 solution of 9 (40 mg, 0.076 mmol). The formation of 10 was observed by NMR after 24 h. 1H NMR (toluene-d8, 300.1 MHz, 296 K): δ (ppm) 7.13–6.67 (overlapping, 16H total, 10H, PhOZr and 6H, PhCH2N), 4.41 (d, 2JH-H = 12Hz, 2H, PhCH2N), 4.34 (d, 2JH-H = 12 Hz, 2H, PhCH2N), 4.22 (m, 2H, [C3]CH2N), 3.62 (m, 2H, [C2]CH2N), 3.18 (m, 2H, [C3]CH2N), 2.82 (m, 2H, [C2]CH2N), 2.69-2.61 (overlapping, 4H total, 2H, [C2]CH2N and 2H, [C3]CH2N), 2.54 (m, 2H, [C3]CH2N), 2.43 (m, 2H, [C2]CH2N), 2.14 (s, 12H, CH3), 1.67 (m, 2H, CH2CH2CH2), 1.08 (m, 2H, CH2CH2CH2). 13C{1H} NMR (toluene-d8, 75.5 MHz, 296 K): δ (ppm) 164.4 (i-PhOZr), 137.7 (Ph), 130.7 (Ph), 129.1 (Ph), 128.8 (Ph), 128.5 (Ph), 125.6 (Ph), 119.9 (Ph), 56.7 ([C3]CH2N), 56.5 (PhCH2N), 54.6 ([C3]CH2N), 52.3 ([C2]CH2N), 48.5 ([C2]CH2N), 25.0 (CH2CH2CH2), 21.3 (CH3).
{Bn2(OCO)2Cyclam}Zr(OCOOiPr)2, 13: Carbon dioxide was bubbled into a toluene solution of 11 (125 mg, 0.21 mmol) at room temperature for 1h. The precipitate was filtered off and dried under vacuum to afford a white solid quantitatively. Anal. calculated for C34H48N4O10Zr: C, 53.45; H, 6.33; N, 7.33. Found: C, 53.78; H, 7.22; N, 7.36. FT-IR (KBr, cm−1): 1377 and 1463 (νC-O).
{4-tBuBn2(OCO)2Cyclam}Zr(OCOOiPr)2, 14: Carbon dioxide was bubbled into a toluene solution of 12 (130 mg, 0.18 mmol) at room temperature for 1 h. The precipitate was filtered off and dried under vacuum to afford a white solid quantitatively. Anal. calculated for C42H64N4O10Zr: C, 57.57; H, 7.36; N, 6.39. Found: C, 57.90; H, 7.96; N, 6.34. FT-IR (KBr, cm−1): 1377 and 1458 (νC-O).
2.3. General Procedure for X-ray Crystallography
Suitable crystals of compounds 2 and 12 were coated and selected in Fomblin® oil under an inert atmosphere of nitrogen. Crystals were then mounted on a loop external to the glovebox environment and data was collected using graphite monochromated Mo-Kα radiation (λ = 0.71073 Å) on a Bruker AXS-KAPPA APEX II diffractometer (Bruker AXS Inc., Madison, WI, USA) equipped with an Oxford Cryosystem open-flow nitrogen cryostat. Cell parameters were retrieved using Bruker SMART and refined using Bruker SAINT software on all observed reflections [28]. Absorption corrections were applied using SADABS [29]. The structures were solved by direct methods using SIR97 [30]. Structure refinements were done using SHELXL [31], included in the WinGX-Version 1.80.01 system of programs [32]. Hydrogen atoms were inserted in calculated positions and allowed to refine in the parent atoms. Torsion angles, mean square planes, and other geometrical parameters were calculated using SHELXL [31]. Compound 2 crystalized with disordered molecules of the solvent in the asymmetric unit. As all attempts to model the disorder did not lead to acceptable solutions, the Squeeze/PLATON [33] sequence was applied. Crystallographic and experimental details of data collection and crystal structure determinations are available in Table 1. Illustrations of the molecular structures were made with ORTEP-3 for Windows [34].

Table 1.
Crystal data and details of structure refinement for compounds 2 and 12.
3. Results and Discussion
Monoalkoxido Zr(IV) complexes supported by diamido diamine cyclam based ligands were obtained by reaction of (Bn2Cyclam)ZrCl2, 1, with one equivalent of LiOR (R = tBu, 2, iPr, 3, Ph, 4). The substitution of the remaining chloride in complexes 2 and 3 by thiophenoxide or methyl ligands was carried out using LiSPh or MeMgCl to afford (Bn2Cyclam)Zr(SPh)(OtBu), 5, and (Bn2Cyclam)ZrMe(OiPr), 6, respectively. The synthetic route for the preparation of complexes 2–6 is shown in Scheme 1.
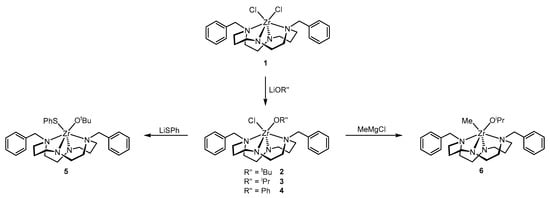
Scheme 1.
Synthetic route for the preparation of complexes 2–6.
The presence of two different substituent groups in the adjacent positions of the zirconium coordination sphere in complexes 2–6 led to a reduction of symmetry from C2 (in 1) to C1. In accordance, the 1H NMR spectra of complexes 2–6 reveal 20 resonances integrating to 1 proton each and 2 AB systems corresponding to the benzylic protons. In addition to the ancillary ligand resonances, the iPr groups in 3 and 6 show two diastereotopic methyl resonances and one septet with 3JH-H = 6 Hz. The tBu groups in 2 and 5 appear as a singlet at 1.73 and 1.46 ppm, respectively. The methyl groups coordinated to zirconium in 6 appear as a singlet at 0.26 ppm. In the 13C{1H} NMR spectra, 12 resonances and 2 sets of aromatic signals attributed to the ancillary ligand carbons are observed as well as the resonances assigned to the other ligands. The 1H and 13C NMR spectra of complexes 2–6 are presented in Figures S1–S4, respectively, in Supplementary Information.
Crystals of 2 suitable for single crystal X-ray diffraction were obtained from a concentrated toluene solution at −20 °C. An ORTEP depiction of its molecular structure along with selected bond lengths and angles are shown in Figure 1.
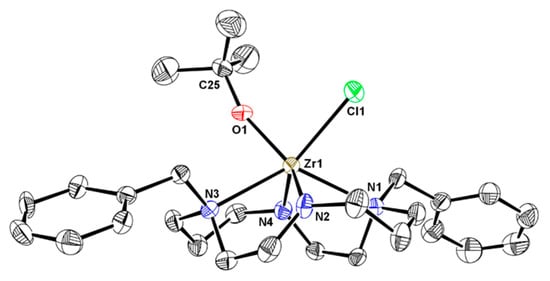
Figure 1.
ORTEP diagram of 2 with thermal ellipsoids at 40% probability level. Hydrogen atoms are omitted for clarity. Selected bond lengths (Å) and angles (º): Zr(1)-Cl(1) 2.528(2), Zr(1)-O(1) 1.958(4), Zr(1)-N(1) 2.453(5), Zr(1)-N(2) 2.101(5), Zr(1)-N(3) 2.508(4), Zr(1)-N(4) 2.068(5); O(1)-Zr(1)-Cl(1) 87.0(1), Zr(1)-O(1)-C(25) 161.8(4).
In complex 2, the zirconium is coordinated to the four nitrogen atoms of the cyclam ring, one chloride ligand and one oxygen atom of the OtBu ligand in a trigonal prismatic geometry. The metal is located above the macrocycle at 1.098(2) Å from the average plane defined by the four nitrogen atoms. The Zr-Namido and the Zr-Namine bond lengths lie within the expected ranges for similar bonds in hexa-coordinated Zr(IV) complexes [13,14,15,16,18,19,27,35,36,37,38]. The Zr-Cl and Zr-O bond lengths of 2.528(3) and 1.958(4) Å, respectively, and the O(1)-Zr(1)-Cl(1) angle at 87.0(1)° are comparable with other Zr(IV) complexes based on Bn2Cyclam ligands [13,14,16,18,19,27,38].
Dialkoxido derivatives can be obtained from the reaction of the dichlorido precursor with two equivalents of a suitable lithium alkoxido [13,14] or by protonolysis of an orthometallated species with an alcohol [18]. (3,5-Me2Bn2Cyclam)Zr(OPh)2, 10, was prepared using the latter strategy by reaction of (3,5-Me-C6H4CH2)2Cyclam)Zr, 9, with two equivalents of phenol. The synthesis of (Bn2Cyclam)Zr(OiPr)2, 11, and (4-tBuBn2Cyclam)Zr(OiPr)2, 12, was carried out by reaction of (Bn2Cyclam)ZrCl2, 1, and (4-tBuBn2Cyclam)ZrCl2, 8, with two equivalents of LiOiPr, respectively. The metathesis reaction is the unique route for the synthesis of 12 because an orthometallated species similar to 9 cannot be formed in view of the stereochemical constraints imposed by the tert-butyl substituents on the para positions of the benzylic pendant arms of the macrocycle. The synthetic route for the preparation of complexes 10–12 is shown in Scheme 2.
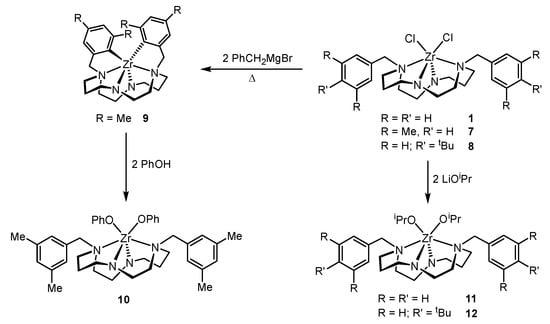
Scheme 2.
Synthetic route for the preparation of complexes 10–12.
The 1H NMR spectra of complexes 10–12 show 10 resonances integrating for 2 protons each and corresponding to the Hanti and Hsyn methylene protons of the macrocycle backbone. The AB system assigned to the benzylic protons of the cyclam pendant arms in 10 appears at 4.41 and 4.34 ppm. Two sets of aromatic resonances are observed in the spectrum of 10, which correspond to the OPh and NCH2Ph groups. The 13C{1H} NMR spectrum of 10 exhibit six resonances attributed to the ancillary ligand methylene carbons and the OPh ligand give raise to one set of resonances in agreement with a C2 symmetry. The 1H and 13C NMR spectrum of 10 is presented in Figure S5 in Supplementary Information.
Crystals of 12 suitable for single crystal X-ray diffraction were obtained from a concentrated toluene solution at −20 °C. An ORTEP depiction of its molecular structure along with selected bond lengths and angles are shown in Figure 2.
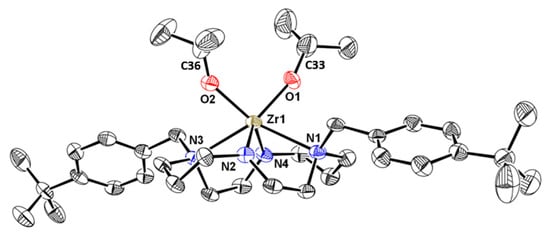
Figure 2.
ORTEP diagram of 12 with thermal ellipsoids at 40% probability level. Hydrogen atoms are omitted for clarity. Selected bond lengths (Å) and angles (º): Zr(1)-O(1) 2.001(4), Zr(1)-O(2) 2.021(5), Zr(1)-N(1) 2.490(5), Zr(1)-N(2) 2.101(5), Zr(1)-N(3) 2.518(5), Zr(1)-N(4) 2.100(5); O(1)-Zr(1)-O(2) 92.1(2), Zr(1)-O(1)-C(33) 161.3(5), Zr(1)-O(2)-C(36) 142.0(5).
In complex 12, the zirconium is coordinated to the four nitrogen atoms of the macrocycle, and to the oxygen atoms of the isopropoxido ligands in a trigonal prismatic geometry. The four nitrogen atoms of the macrocycle define one rectangular face, as commonly reported for other cyclam based complexes [13,14,15,16,18,19,27,35,36,37,38]. The metal is located above the macrocycle at 1.222(2) Å from the average plane defined by the cyclam nitrogen atoms. The Zr-Namido, Zr-Namine and Zr-O bond lengths are within the ranges usually observed for this type of bonds in Zr(IV) complexes of formula (Bn2Cyclam)Zr(OR)2 (R = iPr, tBu, Ph) [13,14,18,19]. The O(1)-Zr(1)-O(2) angle is in agreement with the values found in other Zr(IV) trigonal prismatic complexes supported by a tetradentate dianionic cyclam-based ligand [13,14,18,19]. The wide Zr(1)-O(1)-C(33) and Zr(1)-O(2)-C(36) angles of 161.3(5)° and 142.0(5)°, respectively, reflect the substantial π-donation of the oxygen atoms to the Zr metal center.
A preliminary study on the reactivity of (Bn2Cyclam)Zr(OiPr)2, 11, or the more soluble analogue (4-tBuBn2Cyclam)Zr(OiPr)2, 12, with 2 equivalents of CO2 revealed the formation of a mixture of products that were not possible to fully characterize [15]. Bubbling carbon dioxide in a toluene solution of 11 or 12 led to the formation of very insoluble white precipitates. The insolubility of the products in the most common deuterated solvents did not allow their characterization by NMR. The C, H, N elemental analyses suggest the formation of a species of empirical formulas C34H48N4O10Zr and C42H64N4O10Zr that corresponds to the addition of four CO2 molecules to (Bn2Cyclam)Zr(OiPr)2, 11, and (4-tBuBn2Cyclam)Zr(OiPr)2, 12, respectively. The IR spectra of both compounds (see Figures S6 and S7 in Supplementary Information) show strong absorption bands in the range 1377–1463 cm−1 that were attributed to the presence of carbamate and carbonate fragments. The X-ray powder diffractogram of the solids also revealed the presence of zirconium carbonate confirming CO2 insertion into the Zr-O bonds. Although the insolubility of the products did not allow further characterization and the data obtained do not provide detailed structural information, one may speculate that CO2 added to both Zr-OiPr and Zr-Namido bonds of the di-alkoxido Zr(IV) derivatives 11 and 12 give {Bn2(OCO)2Cyclam}Zr(OCOOiPr)2, 13, and {4-tBuBn2(OCO)2Cyclam}Zr(OCOOiPr)2, 14, respectively. This reactivity pattern contrasts with that observed for the reaction of other heteroallenes with cyclam-based Zr(IV) alkoxidos. Isocyanates and CS2 selectively insert into Zr-Namido bonds of (Bn2Cyclam)Zr(OiPr)2 to form N-bonded ureate and dithiocarbamate fragments, respectively [14,15]. The insertion of carbon dioxide into Zr-OiPr bonds to form carbonate fragments may be attributed to the high oxophilicity of zirconium. The hydrolysis of compounds 13 and 14 was not possible due to their stability in protic solvents, which prevented the isolation of the corresponding N-functionalized cyclams.
4. Conclusions
Various cyclam-based Zr(IV) alkoxido derivatives have been synthesized and fully characterized. The structural characterization of the complexes in solution and in the solid state are consistent and reveals that the cyclam ligand remains tetracoordinated to the zirconium in the solution. Complexes (Bn2Cyclam)ZrCl(OtBu) and (4-tBuBn2Cyclam)Zr(OiPr)2 display trigonal prismatic geometries with X-Zr-X’ angles of 87.0(1)° and 92.1(2)° and distances between the zirconium and the plane defined by the four nitrogen atoms of the cyclam of 1.098(2) and 1.222(2) Å, respectively. The Zr-Namine and Zr-Namido bond lengths are unaffected by the type of ligands that complete the zirconium coordination sphere (2.453(5) < Zr-Namine < 2.518(5) Å and 2.068(5) < Zr-Namido < 2.101(5) Å). Although in both complexes the Zr-O distances are comprised in the small range 1.958(4)–2.021(5) Å, the Zr-O-C angles assume values from 142.0(5)° to 161.8(4)°. There is no correlation between the decreasing of the Zr-O distances and the widening of the Zr-O-C angles. All compounds are thermally stable and, surprisingly, (Bn2Cyclam)ZrMe(OiPr) does not undergo C-H activation of the benzyl pendant arms of the cyclam ligand with formation of orthometalated species as observed in other Zr(IV) alkyl derivatives supported by Bn2Cyclam [17,18,19,35,38]. This observation points out that the bonding of the isopropoxide ligand to the (Bn2Cyclam)Zr core stabilizes the Zr-C bond, a feature that may have important implications in catalysis and deserves further research. The reaction of (Bn2Cyclam)Zr(OiPr)2 and (4-tBuBn2Cyclam)Zr(OiPr)2 with excess of carbon dioxide led to the formation of very insoluble products that are tentatively assigned as {Bn2(OCO)2Cyclam}Zr(OCOOiPr)2 and {4-tBuBn2(OCO)2Cyclam}Zr(OCOOiPr)2, respectively, which might be formed from the addition of CO2 molecules to both Zr-OiPr and Zr-Namido bonds.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/reactions2030021/s1, Figures S1–S5: 1H and 13C NMR spectra of complexes 2, 4, 5, 6 and 10, Figures S6–S7: FT-IR spectra of complexes 11–14. Data for structures 2 and 12 were deposited in CCDC under the deposit numbers 2092600 and 2092601 and can be obtained free of charge from the Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/conts/retrieving.html.
Author Contributions
L.G.A. performed the synthesis and characterization of the compounds and wrote the manuscript; A.M.M. supervised the experiments and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação para a Ciência e a Tecnologia, Portugal.
Acknowledgments
The authors thank to Vânia André (CQE-IST) for the X-ray powder diffractograms of {Bn2(OCO)2Cyclam}Zr(OCOOiPr)2, 13, and {4-tBuBn2(OCO)2Cyclam}Zr(OCOOiPr)2, 14.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hegedus, L.; Lipshutz, B.; Nozaki, H.; Reetz, M.; Rittmeyer, P.; Smith, K.; Totter, F.; Yamamoto, H. Organometallics in Synthesis: A Manual; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1994; pp. 195–282. [Google Scholar]
- Yamasaki, S.; Kanai, M.; Shibasaki, M. Zirconium Alkoxides in Catalysis. Chem. Eur. J. 2001, 7, 4066–4072. [Google Scholar] [CrossRef]
- Stopper, A.; Rosen, T.; Venditto, V.; Goldberg, I.; Kol, M. Group 4 Metal Complexes of Phenylene-Salalen Ligands in rac-Lactide Polymerization Giving High Molecular Weight Stereoblock Poly (lactic acid). Chem. Eur. J. 2017, 23, 11540–11548. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.D.; Hancock, S.L.; McKeown, P.; Schäfer, P.M.; Buchard, A.; Thomas, L.H.; Mahon, M.F.; Lowe, J.P. Zirconium complexes of bipyrrolidine derived salan ligands for the isoselective polymerisation of rac-lactide. Chem. Commun. 2014, 50, 15967–15970. [Google Scholar] [CrossRef] [Green Version]
- Romain, C.; Heinrich, B.; Laponnaz, S.B.; Dagorne, S. A robust zirconium N-heterocyclic carbene complex for the living and highly stereoselective ring-opening polymerization of rac-lactide. Chem. Commun. 2012, 48, 2213–2215. [Google Scholar] [CrossRef] [PubMed]
- Doerr, A.M.; Burroughs, J.M.; Legaux, N.M.; Long, B.K. Redox-switchable ring-opening polymerization by tridentate ONN-type titanium and zirconium catalysts. Cat. Sci. Tech. 2020, 10, 6501–6510. [Google Scholar] [CrossRef]
- Wang, X.; Thevenon, A.; Brosmer, J.L.; Yu, I.; Khan, S.I.; Mehrkhodavandi, P.; Diaconescu, P.L. Redox Control of Group 4 Metal Ring-Opening Polymerization Activity toward L-Lactide and ε-Caprolactone. J. Am. Chem. Soc. 2014, 136, 11264–11267. [Google Scholar] [CrossRef] [Green Version]
- Normand, A.T.; Malacea-Kabbara, R.; Lapenta, R.; Dajnak, A.; Richard, P.; Cattey, H.; Bolley, A.; Grassi, A.; Milione, S.; Auffrant, A.; et al. Phosphasalen group IV metal complexes: Synthesis, characterization and ring opening polymerization of lactide. Dalton Trans. 2020, 49, 6989–7004. [Google Scholar] [CrossRef]
- Stopper, A.; Press, K.; Okuda, J.; Goldberg, I.; Kol, M. Zirconium Complexes of Phenylene-Bridged {ONSO} Ligands: Coordination Chemistry and Stereoselective Polymerization of rac-Lactide. Inorg. Chem. 2014, 53, 9140–9150. [Google Scholar] [CrossRef]
- McKeown, P.; Brown-Humes, J.; Davidson, M.G.; Mahon, M.F.; Woodman, T.J.; Jones, M.D. Ligands and complexes based on piperidine and their exploitation of the ring opening polymerisation of rac-lactide. Dalton Trans. 2017, 46, 5048–5057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapenta, R.; Buonerba, A.; Nisi, A.D.; Monari, M.; Grassi, A.; Milione, S.; Capacchione, C. Stereorigid OSSO-Type Group 4 Metal Complexes in the Ring-Opening Polymerization of rac-lactide. Inorg. Chem. 2017, 56, 3447–3458. [Google Scholar] [CrossRef]
- Turner, Z.R.; Lamb, J.V.; Robinson, T.P.; Mandal, D.; Buffet, J.-C.; O’Hare, D. Ring-opening polymerization of L- and rac-lactide using group 4 permethylpentalene aryloxides and alkoxides. Dalton Trans. 2021, 50, 4805–4818. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.G.; Hild, F.; Munhá, R.F.; Veiros, L.F.; Dagorne, S.; Martins, A.M. Synthesis and structural characterization of novel cyclam-based zirconium complexes and their use in the controlled ROP of rac-lactide: Access to cyclam-functionalized polylactide materials. Dalton Trans. 2012, 41, 14288–14298. [Google Scholar] [CrossRef]
- Alves, L.G.; Martins, A.M. Cyclam Functionalization through Isocyanate Insertion in Zr-N Bonds. Inorg. Chem. 2012, 51, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.G.; Madeira, F.; Munhá, R.F.; Barroso, S.; Veiros, L.F.; Martins, A.M. Reactions of heteroallenes with cyclam-based Zr(IV) complexes. Dalton Trans. 2015, 44, 1441–1455. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.G.; Antunes, M.A.; Matos, I.; Munhá, R.F.; Duarte, M.T.; Fernandes, A.C.; Marques, M.M.; Martins, A.M. Reactivity of a new family of diamido-diamine cyclam-based zirconium complexes in ethylene polymerization. Inorg. Chim. Acta 2010, 363, 1823–1830. [Google Scholar] [CrossRef]
- Antunes, M.A.; Munhá, R.F.; Alves, L.G.; Schafer, L.L.; Martins, A.M. Intramolecular hydroamination catalysis using trans-N,N’-dibenzylcyclam zirconium complexes. J. Organomet. Chem. 2011, 696, 2–6. [Google Scholar] [CrossRef]
- Alves, L.G.; Madeira, F.; Munhá, R.F.; Maulide, N.; Veiros, L.F.; Martins, A.M. Cooperative Metal-Ligand Hydroamination Catalysis Supported by C-H Activation in Cyclam Zr(IV) Complexes. Inorg. Chem. 2018, 57, 13034–13045. [Google Scholar] [CrossRef]
- Munhá, R.F.; Alves, L.G.; Bharathi, S.; Martins, A.M. A New Family of Zirconium Complexes Anchored on Dianionic Cyclam-based Ligands: Syntheses, Structures and Catalytic Applications. In Advances in Organometallic Chemistry: The Silver/Gold Jubilee International Conference on Organometallic Chemistry Celebratory Book; Pombeiro, A.J.L., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; Volume 2, pp. 315–323. [Google Scholar]
- Wong, J.K.-H.; Ast, S.; Yu, M.; Flehr, R.; Counsell, A.J.; Turner, P.; Crisologo, P.; Todd, M.H.; Rutledge, P.J. Synthesis and Evaluation of 1,8-Disubstituted-Cyclam/Naphtalimide Conjugates as Probes for Metal Ions. ChemistryOpen 2016, 5, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Voutsadaki, S.; Tsikalas, G.K.; Klontzas, E.; Froudakis, G.E.; Pergantis, S.A.; Demadis, K.D.; Katerinopoulos, H.E. A cyclam-type “turn on” fluorescent sensor selective for mercury ions in aqueous media. RSC Adv. 2012, 2, 12679–12682. [Google Scholar] [CrossRef]
- Liang, X.; Sadler, P.J. Cyclam complexes and their applications in medicine. Chem. Soc. Rev. 2004, 33, 246–266. [Google Scholar] [CrossRef]
- Yu, M.; Nagalingam, G.; Ellis, S.; Martinez, E.; Sintchenko, V.; Spain, M.; Rutledge, P.J.; Todd, M.H.; Triccas, J.A. Nontoxic Metal-Cyclam Complexes, a New Class of Compounds with Potency against Drug-Resistant Mycobacterium tuberculosis. J. Med. Chem. 2016, 59, 5917–5921. [Google Scholar] [CrossRef]
- Alves, L.G.; Pinheiro, P.F.; Feliciano, J.R.; Dâmaso, D.P.; Leitão, J.H.; Martins, A.M. Synthesis, antimicrobial activity and toxicity to nematodes of cyclam derivatives. Int. J. Antimicrob. Agents 2017, 49, 646–649. [Google Scholar] [CrossRef]
- Alves, L.G.; Portel, J.F.; Sousa, S.A.; Ferreira, O.; Almada, S.; Silva, E.R.; Martins, A.M.; Leitão, J.H. Investigations into the Structure/Antibacterial Activity Relationships of Cyclam and Cyclen Derivatives. Antibiotics 2019, 8, 224. [Google Scholar] [CrossRef] [Green Version]
- Pilon, A.; Lorenzo, J.; Rodriguez-Calado, S.; Adão, P.; Martins, A.M.; Valente, A.; Alves, L.G. New Cyclams and Their Copper(II) and Iron(III) Complexes: Synthesis and Potential Apllication as Anticancer Agents. ChemMedChem 2019, 14, 770–778. [Google Scholar] [CrossRef]
- Munhá, R.F.; Alves, L.G.; Maulide, N.; Duarte, M.T.; Markó, I.E.; Fryzuk, M.D.; Martins, A.M. trans-Disubstituted diamido-diamine cyclam zirconium complexes. Inorg. Chem. Commun. 2008, 11, 1174–1176. [Google Scholar] [CrossRef]
- SAINT. Version 7.03A; Bruker AXS Inc.: Madison, WI, USA, 1997–2003. [Google Scholar]
- Sheldrick, G.M. SADABS, Software for Empirical Absorption Corrections; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. 2015, C71, 9–18. [Google Scholar]
- Farrugia, L.J. ORTEP-3 for Windows—A version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- Munhá, R.F.; Antunes, M.A.; Alves, L.G.; Veiros, L.F.; Fryzuk, M.D.; Martins, A.M. Structure and Reactivity of Neutral and Cationic trans-N,N′-Dibenzylcyclam Zirconium Alkyl Complexes. Organometallics 2010, 29, 3753–3764. [Google Scholar] [CrossRef]
- Munhá, R.F.; Veiros, L.F.; Duarte, M.T.; Fryzuk, M.D.; Martins, A.M. Synthesis and structural studies of amido, hydrazido and imido zirconium(IV) complexes incorporating a diamido/diamine cyclam-based ligand. Dalton Trans. 2009, 36, 7494–7508. [Google Scholar] [CrossRef] [PubMed]
- Munhá, R.F.; Ballman, J.; Veiros, L.F.; Patrick, B.O.; Fryzuk, M.D.; Martins, A.M. Dinuclear Cationic Zirconium Hydrides Stabilized by the N,N-Dibenzylcyclam Ancillary Ligand. Organometallics 2012, 31, 4937–4940. [Google Scholar] [CrossRef]
- Alves, L.G.; Munhá, R.F.; Martins, A.M. Synthesis and reactivity of cyclam-based Zr(IV) complexes. Inorg. Chim. Acta 2019, 490, 204–214. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).