Abstract
The gut microbiota has emerged as a critical immune–metabolic interface, orchestrating a complex network of interactions that extend well beyond digestion. This highly diverse community of bacteria, viruses, archaea, and eukaryotic microbes modulates host immunometabolism, metabolic reprogramming, and systemic inflammatory responses, thereby shaping human health and disease trajectories. Dysbiosis, or disruption of microbial homeostasis, has been implicated in inflammatory bowel disease, cardiometabolic disorders, neurodegeneration, dermatological conditions, and tumorigenesis. Through the biosynthesis of short-chain fatty acids (SCFAs), bile acid derivatives, tryptophan metabolites, and microbial-derived indoles, the gut microbiota regulates epigenetic programming, barrier integrity, and host–microbe cross-talk, thereby influencing disease onset and progression. In oncology, specific microbial taxa and oncomicrobiotics (cancer-modulating microbes) are increasingly recognized as key determinants of immune checkpoint inhibitor (ICI) responsiveness, chemotherapeutic efficacy, and resistance mechanisms. Microbiota-targeted strategies such as fecal microbiota transplantation (FMT), precision probiotics, prebiotics, synbiotics, and engineered microbial consortia are being explored to recalibrate microbial networks and enhance therapeutic outcomes. At the systems level, the integration of multi-omics platforms (metagenomics, transcriptomics, proteomics, and metabolomics) combined with network analysis and machine learning-based predictive modeling is advancing personalized medicine by linking microbial signatures to clinical phenotypes. Despite remarkable progress, challenges remain, including the standardization of microbiome therapeutics, longitudinal monitoring of host–microbe interactions, and the establishment of robust ethical and regulatory frameworks for clinical translation. Future directions should prioritize understanding the causal mechanisms of microbial metabolites in immunometabolic regulation, exploring microbial niche engineering, and developing precision microbiome editing technologies (CRISPR, synthetic biology).
1. Introduction
1.1. Overview of Gut Microbiota and Microecology
The human gut microbiota is a vast and complex ecosystem composed of trillions of microorganisms, including bacteria, archaea, fungi, viruses, and protozoa, residing primarily in the gastrointestinal tract [1]. These microbes interact intricately with both the host and each other, forming a dynamic network that influences numerous physiological functions, such as digestion, immune modulation, metabolic processes, and neurological functions [2]. Due to its extensive influence on health and disease, the gut microbiota is often referred to as a “forgotten organ” [3].
Microecology, a subfield of microbial ecology, focuses on understanding the interactions among microorganisms within specific microenvironments such as the human gut. It examines how microbial populations coexist, compete, and cooperate within their habitat [4]. In the gut, micro-ecological studies provide insights into microbial colonization patterns, niche differentiation, symbiotic relationships, and the effects of external influences such as diet, antibiotics, and environmental changes [5].
1.2. Microbial Diversity and Composition of Gut Microbiota
1.2.1. Diversity and Complexity
The gut microbiome represents one of the most diverse microbial ecosystems in the human body, composed of bacteria, fungi, viruses, and protozoa. This ecosystem encompasses thousands of bacterial species, viruses, archaea, and eukaryotic microorganisms and plays a fundamental role in maintaining host physiology and health [6]. The bacterial component is predominantly made up of the phyla Firmicutes and Bacteroidetes, although species-level composition shows considerable variability between individuals. The colon is a particular microbial hotspot, harboring up to 1012 cells per gram of intestinal content. The gut microbiome encompasses over 1000 bacterial species across approximately 50 bacterial phyla, though most belong to a few dominant phyla including Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria [7].
1.2.2. Composition and Variability
The gut microbiota is primarily dominated by two bacterial phyla: Bacteroidetes and Firmicutes, together accounting for more than 90% of the total microbial population [8]. Additional phyla such as Actinobacteria, Fusobacteria, Proteobacteria, Verrucomicrobia, and Cyanobacteria also contribute to gut ecology [9]. The collective genetic material of gut microbes referred to as the gut microbiome is estimated to be 150 times larger than the human genome, underscoring the extensive metabolic and functional potential of these organisms [10,11]. Despite the dominance of specific phyla, the overall composition varies significantly between individuals, particularly at the genus and species levels [12].
Key determinants of gut microbiota composition include:
- Diet: Nutrient intake influences microbial diversity; fiber-rich diets promote beneficial bacteria, while high-fat or processed diets encourage the growth of potentially pathogenic species.
- Age: The microbiome undergoes dynamic shifts across the human lifespan, from infancy to old age.
- Antibiotic Use: Antibiotics can profoundly disrupt microbial balance, decreasing diversity and promoting dysbiosis.
- Environmental Exposures: Factors such as geography, hygiene, lifestyle, and contact with animals impact microbial acquisition and composition.
These variables contribute to the uniqueness of each individual’s gut microbiome and highlight the need for personalized approaches in microbiome research and clinical applications.
1.3. Aims and Significance of Microbiome Research
Microbiome research aims to unravel the complex interactions between gut microbes and human health, disease mechanisms, and therapeutic responses. Major objectives include [13]:
- Understanding Disease Mechanisms: Investigating how microbial communities influence the onset and progression of diseases.
- Developing Therapeutic Strategies: Exploring the potential of probiotics, prebiotics, fecal microbiota transplantation (FMT), and microbial-based drug formulations for gut microbiota modulation [14].
- Advancing Precision Medicine: Integrating microbiome profiles with metagenomics and multi-omics technologies to develop personalized healthcare strategies.
- Identifying Research Gaps: Synthesizing current knowledge to illuminate areas requiring further investigation and innovation.
- Enhancing Cancer Treatment: Studying how the gut microbiota affects the tumor microenvironment and modulates the efficacy of immunotherapy and chemotherapy in patients.
The integration of microbiome insights into medical practice has the potential to transform personalized medicine by tailoring treatments to an individual’s unique microbiome profile.
1.4. Conceptual Framework
The novelty of this review lies in its integrative perspective—linking microbial community composition with immunemetabolic crosstalk and disease outcomes. We emphasize the bidirectional nature of host–microbe communication, where immune regulation, nutrient signaling, and metabolite networks function as a unified system. Furthermore, we discuss how recent multi-omics and machine learning approaches are reshaping mechanistic understanding and guiding the design of precision microbiome-based interventions.
All bacterial taxa cited in this review were updated to reflect current classification according to the List of Prokaryotic names with Standing in Nomenclature (LPSN 2025) and the NCBI Taxonomy Database [15,16]. Recently reclassified Lactobacillus species (e.g., Lacticaseibacillus casei, Limosilactobacillus reuteri) and Eubacterium rectale (now Agathobacter rectalis) have been corrected to reflect current nomenclature.
2. Composition and Functional Diversity of the Gut Microbiota
2.1. Significant Microbial Phyla
The human gut microbiota is predominantly composed of two major bacterial phyla: Firmicutes and Bacteroidetes, which together constitute over 90% of the gut microbial population [3]. Other phyla, including Actinobacteria, Proteobacteria, Fusobacteria, Verrucomicrobia, and Cyanobacteria, also play critical roles in the structure and function of the gut ecosystem. The relative abundance of these groups varies among individuals and across different segments of the gastrointestinal tract [17].
2.2. Functions of the Gut Microbiota
2.2.1. Nutrient Metabolism and SCFA Production
The gut microbiota plays a central role in the metabolism of complex carbohydrates and dietary fibers that are otherwise indigestible by human enzymes. Microbial fermentation of these substrates yields short-chain fatty acids (SCFAs) such as acetate (Table 1), propionate, and butyrate. These SCFAs serve as primary energy sources for colonocytes, contribute to maintaining colonic pH, and exhibit anti-inflammatory and immunomodulatory properties [18].

Table 1.
Immune–Metabolic Roles of Gut Microbiota in Disease Modulation.
Table 1.
Immune–Metabolic Roles of Gut Microbiota in Disease Modulation.
| Microbial Factor/Species | Immune–Metabolic Function | Disease Context/Effect | Reference |
|---|---|---|---|
| Faecalibacterium prausnitzii | Produces butyrate and anti-inflammatory metabolites; induces IL-10 and Treg cells; suppresses NF-κB signaling. | Mitigates colitis inflammation and enhances gut barrier integrity. | [19,20] |
| F. prausnitzii (live strain) | Reduces expression of pro-inflammatory cytokines (IFN-γ, TNF-α, IL-6, IL-12); inhibits IL-8 via NF-κB | Reduces severity in patients with IBD, stabilizes gut homeostasis | [13] |
| Akkermansia muciniphila | Supports mucosal integrity; modulates metabolic inflammation. | Linked to improved obesity and metabolic health outcomes | [21,22] |
| SCFAs & metabolites (Butyrate) | Signal through GPCRs; support gut barrier, immune balance, and systemic metabolism; Inhibits histone deacetylases (HDACs). | Protective for patients with asthma, IBD, and colon inflammation, systemic inflammation. | [18,23] |
| Bacteroides species (e.g., B. uniformis) | Ferment carbohydrates into acetate/propionate; regulate gut microenvironment and metabolism. | Potential obesity alleviation and immune modulation in patients. | [24] |
| Dysbiosis (Microbial Imbalance) | Loss of beneficial organisms, overgrowth of pathobionts, reduced diversity (Figure 1). | Associated with patients with IBD, obesity, cancer, and neuro-inflammation. | [20,25] |
2.2.2. Immune System Modulation
The gut microbiota is crucial in developing and regulating the host immune system. It supports immune tolerance, educates immune cells to distinguish between pathogens and commensals, and influences both innate and adaptive immune responses [26]. Gut microbes promote the maturation of immune components such as dendritic cells (Figure 1), macrophages, T-helper cells, and regulatory T cells (Tregs), often via the activation of Toll-like receptor (TLR) signaling [27].
2.2.3. Protection Against Pathogens
The gut microbiota acts as a powerful safeguard against invading pathogens by maintaining a dynamic balance within the intestinal environment. Rather than allowing harmful microbes to freely colonize, resident bacteria establish colonization resistance a process in which they occupy ecological niches, consume available nutrients, and adhere to mucosal surfaces, leaving little opportunity for pathogens to gain a foothold [28].
This protection extends beyond simple competition. Commensal microbes actively reinforce the intestinal barrier by stimulating mucus production and tightening epithelial junctions, thereby reducing the risk of pathogen entry into deeper tissues. They also trigger the release of antimicrobial compounds, such as bacteriocins and defensins, which directly suppress or kill harmful organisms [29].
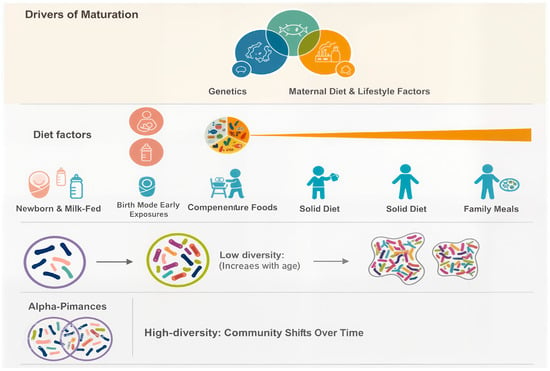
Figure 1.
Developmental Trajectories of the Gut Microbiota during the First Three Years of Life [adapted from [30]].
2.2.4. Gut–Organ Communication Axes
The influence of the gut microbiota extends well beyond the intestinal tract, shaping the physiology and health of multiple organ systems through highly integrated communication networks [31]. These connections, often referred to as gut–organ axes, highlight the systemic role of microbial communities in maintaining homeostasis and influencing disease outcomes [32].
- Gut–Brain Axis:The gut–brain axis represents a complex, bidirectional communication system that operates through neural, hormonal, metabolic, and immune pathways. Gut microbes synthesize a variety of neuroactive compounds, including serotonin, dopamine precursors, γ-aminobutyric acid (GABA), and short-chain fatty acids [25], all of which influence gastrointestinal motility, stress response, mood regulation, and cognitive processes. Disruptions in this axis have been linked to neuropsychiatric and neurodegenerative disorders. For example, alterations in microbial composition have been associated with heightened risk of Parkinson’s disease and Alzheimer’s disease, where gut-derived signals may influence neuroinflammation and protein aggregation in the brain [33].
- Gut–Liver Axis:The liver and gut are intimately connected, as nearly 70% of hepatic blood flow is supplied directly from the gut via the portal vein. This anatomical relationship exposes the liver to microbial metabolites and components such as lipopolysaccharides (LPSs), peptidoglycans, and bile acids. The gut microbiota plays a critical role in bile acid metabolism, which in turn modulates immune cell function in the liver, particularly natural killer T (NKT) cells [34]. Interestingly, primary bile acids can support protective immune activity and even suppress tumor growth by enhancing NKT responses, while secondary bile acids, produced by microbial transformation, may contribute to chronic inflammation and carcinogenesis. This delicate balance illustrates how gut–liver interactions can determine outcomes ranging from metabolic homeostasis to liver disease progression [35].
- Gut–Skin Axis:The relationship between the gut microbiota and skin health has gained increasing attention, especially in the context of chronic inflammatory skin conditions. Dysbiosis in the gut can lead to systemic immune dysregulation (Figure 2), increased intestinal permeability, and circulation of pro-inflammatory metabolites that affect skin physiology [36]. Conditions such as psoriasis, rosacea, and atopic dermatitis have all been linked to gut microbial imbalances. Likewise, gut-derived metabolites, including short-chain fatty acids, can influence skin barrier integrity, hydration, and immune responses, underscoring the systemic nature of microbial communication. These insights highlight that maintaining gut microbial balance is not only essential for internal organ function but also for visible markers of health such as skin integrity and appearance [37].
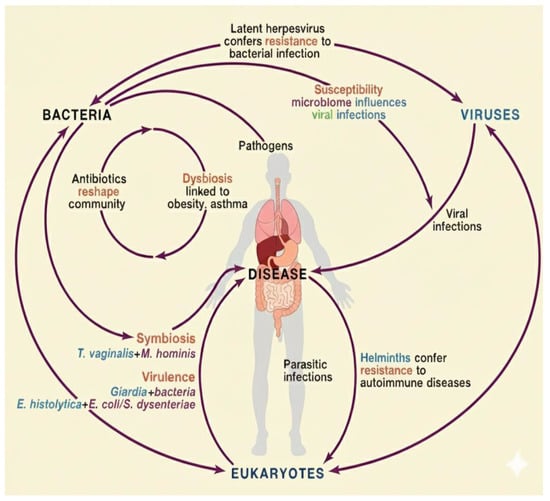
Figure 2.
Complex interactions between bacterial, viral, and eukaryotic components of the gut microbiota regulate immune responses, metabolic pathways, and susceptibility to disease [adapted from [38]].
2.3. The Role of Gut Microbiota in Human Health
2.3.1. The Microbiota as a Central Regulator of Host Physiology
This is regularly referred to as the “second genome” or even the “forgotten organ”, the gut microbiota plays an indispensable role in regulating host physiology, metabolism, and immunity. This dense and diverse microbial ecosystem co-evolves with its host, creating a symbiotic relationship that underpins many aspects of human health. A balanced microbiota supports mucosal barrier integrity, fine-tunes inflammatory responses, and sustains metabolic homeostasis [39]. In contrast, disruption of this delicate balance—known as dysbiosis—has been strongly linked to the onset and progression of a wide range of disorders in patients, including obesity, diabetes, inflammatory bowel disease, neurodegenerative conditions, and even certain cancers [40,41].
2.3.2. Key Functional Contributions
- I. Nutrient Absorption and Metabolism: Gut microbes facilitate the breakdown of dietary fibers and complex carbohydrates, leading to SCFA production, which fuels colonocytes, modulates immune signaling, and contributes to anti-inflammatory processes [42].
- II. Immune System Modulation: The gut microbiota plays a critical role in educating and shaping the host immune system. It stimulates the secretion of protective mucins and antimicrobial peptides while also fostering immune tolerance to harmless antigens. Through interactions with macrophages, dendritic cells, and regulatory T cells (Tregs), commensal microbes ensure a balanced immune response that is effective against pathogens but restrained enough to prevent excessive inflammation or autoimmunity. This immunomodulatory role is central to preventing conditions such as allergies, autoimmune diseases, and chronic inflammatory disorders [43].
- III. Barrier Integrity and Pathogen Defense: Commensal microbes maintain the gut mucosal barrier and inhibit pathogenic overgrowth by producing antimicrobial peptides and modifying the intestinal environment [44].
- IV. Xenobiotic and Drug Metabolism: Gut microbes also influence the fate of therapeutic drugs and xenobiotics. Microbial enzymes can activate, deactivate, or even toxify pharmaceutical compounds, thereby altering their bioavailability and efficacy. For example, certain bacteria metabolize digoxin, reducing its therapeutic effect, while others enhance the activity of specific chemotherapeutic agents [45].
2.4. Importance of Gut Microbiota Balance
A well-balanced gut microbiome is essential for sustaining physiological functions and resisting disease. The dynamic equilibrium between beneficial and potentially harmful microbes regulates immune competence, metabolic activity, and intestinal barrier function. Many factors contribute to dysbiosis, including poor diet, antibiotic use, gastrointestinal infections, surgical interventions, chronic inflammation, and sedentary lifestyle. Correcting microbial imbalances through targeted strategies can restore gut homeostasis and improve overall health [46].
3. Microbiota–Immune and Metabolic Interactions
3.1. Overview of Host–Microbiota Interactions
The gut microbiome engages in intricate, bidirectional communication with its host, influencing a wide range of physiological processes through immune, metabolic, and neuroendocrine networks. These interactions are mediated by an array of microbial components, including metabolites, cell-surface antigens, and secreted signaling molecules, which collectively shape host biology [47].
Microbial metabolites, such as short-chain fatty acids, secondary bile acids, and tryptophan derivatives, serve as chemical messengers that modulate energy metabolism, maintain epithelial barrier integrity, and regulate inflammatory responses. Surface antigens and microbial-associated molecular patterns (MAMPs) interact with host pattern recognition receptors [48], fine-tuning immune responses to distinguish between harmless commensals and potential pathogens. Additionally, secreted molecules such as bacteriocins and neurotransmitter-like compounds can influence gut motility, neural signaling, and even systemic hormonal pathways, demonstrating the extensive reach of gut microbes beyond the intestine [49].
3.2. Immune System Crosstalk
The gut microbiota is a central architect of host immune function (Figure 1), shaping both local and systemic immunity through continuous bidirectional interactions.
- Recognition of Microbial Signals: The host immune system detects microbial presence via pattern recognition receptors (PRRs), including Toll-like receptors (TLRs) and NOD-like receptors (NLRs). These receptors recognize microbial-associated molecular patterns (MAMPs) such as lipopolysaccharides (LPS), flagellin, and peptidoglycans, initiating tailored immune responses that distinguish between beneficial commensals and potential pathogens [50].
- Mucosal Immunity: Commensal microbes actively promote the secretion of antimicrobial peptides (AMPs), immunoglobulin A (IgA), and mucus, forming a multifaceted defense that protects against invading pathogens while maintaining immune tolerance to harmless microbes. These mechanisms establish a controlled environment that balances protection with symbiosis [51].
- Immune Homeostasis: Gut microbial signals modulate the equilibrium between pro-inflammatory and regulatory pathways. They influence the differentiation and function of T cells—including regulatory T cells (Tregs)—as well as dendritic cells and macrophages, shaping immune responses that are precise and proportionate [52].
- Epithelial Barrier Support: The microbiota reinforces gut barrier integrity by enhancing tight junction protein expression and stimulating mucus production (Table 1), preventing the translocation of pathogens and their toxins into systemic circulation [52].
Disruption of these finely tuned interactions contributes to immune dysregulation and is implicated in inflammatory and autoimmune diseases, such as patients with inflammatory bowel disease (IBD) and patients with colorectal cancer [19].
3.3. Gut Barrier Integrity
The host’s intestinal barrier serves as a critical line of defense, separating the external environment within the gut lumen from the body’s internal systems (Table 1). A healthy gut microbiota plays a pivotal role in preserving the integrity of this barrier through multiple complementary mechanisms [53]. One key function is the regulation of tight junction proteins, which seal the spaces between epithelial cells and prevent unwanted leakage of microbes and toxins. Beneficial bacteria also stimulate the production of mucus by goblet cells, creating a physical and biochemical shield that limits direct microbial contact with the intestinal epithelium [54].
Beyond structural reinforcement, the gut microbiota actively modulates the host’s immune system to maintain a state of controlled tolerance. By regulating inflammatory signaling, commensal microbes help avoid excessive immune activation that could otherwise damage the barrier. In addition, microbial metabolites—particularly short-chain fatty acids such as butyrate—serve as energy sources for intestinal epithelial cells and further promote barrier repair and resilience [28].
3.4. Microbial Metabolites and Host Physiology
Gut microbes produce a wide spectrum of metabolites in the hosts that exert systemic effects, linking intestinal microbial activity to host physiology across multiple organs.
- Short-Chain Fatty Acids (SCFAs): Fermentation of dietary fibers generates SCFAs—primarily butyrate, acetate, and propionate—that serve as energy sources for colonocytes, modulate immune signaling (e.g., Treg differentiation), and strengthen epithelial barrier function [55].
- Bile Acid Metabolites: Microbial transformation of primary bile acids into secondary bile acids influences lipid metabolism, immune pathways, and hepatic function through receptors such as FXR and TGR5. These metabolites play key roles in the gut–liver axis and in systemic metabolic regulation [56].
- Tryptophan Derivatives: Indole and related compounds activate the aryl hydrocarbon receptor (AhR), contributing to mucosal immunity, epithelial repair, and modulation of inflammatory responses [57].
- Vitamins: Certain gut bacteria synthesize essential vitamins, including vitamin K and B-group vitamins, supporting coagulation, DNA synthesis, energy metabolism, and overall cellular function [58].
Collectively, these metabolites link the host’s gut microbiota to systemic organ function, participating in pathways such as the gut–brain, gut–liver, and gut–immune axes, illustrating the microbiome’s integral role in overall health.
3.5. Host Regulation of Microbiota
The host environment profoundly shapes the composition, diversity, and functional output of the gut microbiota, creating a dynamic equilibrium between microbial communities and host physiology.
- Diet: Macronutrient composition and fiber intake strongly influence microbial diversity and metabolic activity. High-fiber diets enrich SCFA-producing bacteria, while high-fat or low-fiber diets may promote dysbiosis and inflammation [59].
- Immune Regulation: Host defenses, including IgA secretion, antimicrobial peptide production, and continuous epithelial renewal, maintain microbial balance and prevent overgrowth of potentially harmful organisms.
- Medications: Antibiotics, nonsteroidal anti-inflammatory drugs (NSAIDs), proton pump inhibitors, and other pharmaceuticals can disrupt microbial ecosystems, often leading to reduced diversity and altered metabolic outputs [60].
- Environmental and Lifestyle Factors: Urbanization, stress, hygiene practices, toxin exposure, and early-life microbial exposures shape the microbiota, influencing immune development and long-term health outcomes.
- Genetic Background: Host genetics determine susceptibility to microbial colonization, immune responsiveness, and predisposition to dysbiosis, highlighting the interplay between hereditary factors and microbial ecology [21].
These host-driven factors modulate the flexibility of the gut environment, dictating how microbial communities influence metabolism, immunity, and overall health.
4. Gut Microbial Symbiosis and the Health Consequences of Dysbiosis
4.1. The Functional Benefits of Gut Symbiosis
The human gastrointestinal tract hosts a complex, diverse, and metabolically active microbial community that exists in a mutualistic relationship with its host. This gut symbiosis is essential for numerous physiological processes (Figure 2), including nutrient metabolism, immune system development, mucosal integrity, neurobehavioral regulation, and protection against pathogens. Disruption of this balance, termed dysbiosis, is associated with a wide spectrum of chronic, inflammatory, metabolic, and neoplastic disorders [61].
4.1.1. Nutrient Metabolism and Energy Production
The gut microbiota plays a central role in human nutrient metabolism and energy homeostasis. Commensal microbes efficiently break down dietary fibers and complex carbohydrates that are indigestible by the host alone, fermenting them into short-chain fatty acids (SCFAs) such as acetate (Table 1), propionate, and butyrate. These SCFAs serve multiple vital functions [23]):
- Energy Supply: Butyrate acts as a primary energy source for colonocytes, supporting epithelial health and promoting efficient nutrient absorption.
- Immune Modulation: SCFAs influence the differentiation and function of immune cells, including regulatory T cells, helping to maintain a balanced inflammatory response [62].
- Metabolic Regulation: These metabolites play key roles in lipid metabolism and glucose homeostasis, contributing to overall energy balance and metabolic health.
Beyond SCFA production, the gut microbiota contributes to the synthesis of essential vitamins, including B-group vitamins and vitamin K, which support cellular metabolism, coagulation, and DNA synthesis. Additionally, microbial enzymatic activity enables the biotransformation of xenobiotics and pharmaceutical compounds, thereby affecting their bioavailability, therapeutic efficacy, and potential toxicity [63].
4.1.2. Immune System Development and Regulation
The host’s gut microbiota plays a pivotal role in shaping and regulating both the innate and adaptive branches of the host immune system. Through constant interaction with the intestinal epithelium and immune cells, commensal microbes help educate the immune system to respond appropriately to pathogens while maintaining tolerance to harmless antigens [64]. Key mechanisms include:
- Promotion of Immune Tolerance: Commensal microbes prevent excessive immune activation, reducing the risk of chronic inflammation and autoimmune responses.
- Modulation of Regulatory T Cells (Tregs): Microbial signals influence the differentiation and function of Tregs, which are critical for maintaining immune homeostasis and controlling inflammatory responses [64].
- Production of Immunoregulatory Metabolites: Short-chain fatty acids (SCFAs), aryl hydrocarbon receptor (AhR) ligands, and polyamines produced by gut bacteria serve as signaling molecules that fine-tune immune responses, support mucosal integrity, and regulate inflammatory pathways.
Specific microbial species of hosts contribute uniquely to host immunity (Table 1). For example, Bifidobacterium and Akkermansia muciniphila have been shown to enhance antitumor immune responses and improve the effectiveness of immune checkpoint inhibitors (ICIs), demonstrating the therapeutic potential of modulating gut microbial composition [22].
4.1.3. Maintenance of Gut Barrier Integrity and Pathogen Defense
A balanced and diverse gut microbiota is essential for maintaining the integrity of the intestinal barrier and protecting the host from enteric pathogens. Commensal microbes employ multiple complementary strategies to achieve this defense [19]:
- Competitive Exclusion: Beneficial microbes occupy ecological niches within the gut, consuming available nutrients and adhering to mucosal surfaces. This limits the ability of pathogenic organisms to colonize and establish infections [65]).
- Induction of Antimicrobial Peptides and Mucin Production: Gut bacteria stimulate the secretion of antimicrobial peptides (AMPs) and mucus, creating both chemical and physical barriers that inhibit pathogen growth and adherence to the epithelium [66].
- Reinforcement of Epithelial Tight Junctions: Microbial signals enhance the expression and assembly of tight junction proteins, strengthening the epithelial barrier. This reduces intestinal permeability, prevents the translocation of pathogens and toxins into the systemic circulation, and mitigates inflammation [67].
4.1.4. Gut–Brain Axis and Mental Health
The gut–brain axis of hosts represents a dynamic, bidirectional communication network between the gastrointestinal tract and the central nervous system, mediated through neural, immune, and endocrine pathways. The gut microbiota plays a critical role in this crosstalk, influencing both brain function and behavior [68].
- Production of Neuroactive Compounds: Gut microbes synthesize and modulate the availability of neuroactive molecules, including gamma-aminobutyric acid (GABA), serotonin precursors, dopamine intermediates, and short-chain fatty acids (SCFAs). These compounds can directly or indirectly affect neurotransmission, mood, and cognitive processes [69].
- Regulation of Neuro-inflammation: Microbial signals influence neuroimmune pathways, modulating microglial activation and inflammatory responses within the brain. This interaction can impact susceptibility to neuroinflammatory and neurodegenerative conditions [70].
- Implications for Mental Health: Dysbiosis and alterations in microbial composition have been linked to mood disorders such as depression and anxiety, as well as neurodegenerative diseases including Alzheimer’s and Parkinson’s disease. Gut microbial balance, therefore, emerges as a potential target for therapeutic interventions aimed at improving mental and neurological health [71].
4.1.5. Metabolite Production and Systemic Regulation
The gut microbiota produces a diverse array of bioactive metabolites that exert profound effects on host physiology, extending well beyond the gastrointestinal tract. These microbial products serve as critical signaling molecules, influencing systemic metabolic, immune, and even oncological processes [72].
- Regulation of Systemic Inflammation: Microbial metabolites, including short-chain fatty acids (SCFAs), indole derivatives, and polyamines, modulate immune cell activity and cytokine production. This helps maintain a balanced inflammatory state, preventing chronic low-grade inflammation that is implicated in metabolic, cardiovascular, and autoimmune diseases [73].
- Modulation of Hepatic Metabolism and Energy Balance: Gut-derived metabolites, such as secondary bile acids and SCFAs, interact with hepatic receptors like FXR and TGR5 to regulate lipid and glucose metabolism, energy homeostasis, and insulin sensitivity. These interactions link microbial activity to systemic metabolic health [74].
- Alteration of the Tumor Microenvironment (TME): Microbial metabolites can influence the immune landscape within the TME of patients by modulating cytokine profiles, immune cell infiltration, and local inflammation. Such effects may either suppress or promote tumor progression and can impact the efficacy of chemotherapy and immunotherapy, highlighting the microbiota’s role in cancer biology [75].
4.1.6. Drug Metabolism and Therapeutic Response
The gut microbiota plays a crucial role in modulating the host’s metabolism and efficacy of numerous pharmaceutical agents, acting as an additional layer of host metabolism. Microbial enzymes can directly alter drug structures, impacting both their activity and safety profile [45].
- Activation or Inactivation of Therapeutic Agents: Certain gut bacteria can chemically modify drugs, either activating prodrugs into their therapeutically active forms or inactivating medications, thereby reducing their effectiveness.
- Modification of Drug Pharmacokinetics: Microbial activity can influence drug absorption in the gut, affect systemic half-life, and alter elimination pathways. This can result in variations in drug concentration and therapeutic outcomes among individuals [45].
- Regulation of Host Metabolic Pathways: The microbiota can modulate the expression of host metabolic enzymes and transporters, further shaping drug metabolism and response.
Dysbiosis may reduce chemotherapy efficacy or increase toxicity, and it has been shown to affect the outcomes of immunotherapy.
4.1.7. Modulation of the Tumor Microenvironment (TME)
The gut microbiota exerts profound influence on the tumor microenvironment (TME) in patients, shaping its immune, metabolic, and inflammatory landscape. Through a combination of microbial metabolites, immune modulation, and systemic signaling, microbial communities can either foster an anti-tumor environment or support tumor progression [75].
Key mechanisms include:
- Cytokine Regulation and Immune Infiltration: Commensal microbes and their metabolites influence the production of cytokines such as IL-10, IL-17, and IFN-γ, which in turn regulate the recruitment and activation of immune cells including T cells, natural killer (NK) cells, and dendritic cells within the TME [45].
- Inflammation Control: Depending on microbial composition, the microbiota can suppress chronic low-grade inflammation that promotes tumor growth or, conversely, exacerbate pro-inflammatory pathways that support cancer progression [76].
- Therapeutic Modulation: Specific bacterial species have been shown to enhance the efficacy of conventional therapies in patients, such as chemotherapy and immune checkpoint inhibitors (ICIs), by improving antigen presentation, T-cell activation, and overall immune responsiveness [77].
Thus, the microbiota acts not only as a passive bystander but as an active participant in patient’s tumor biology, with its composition and functional activity critically determining cancer outcomes and therapeutic success.
4.2. Gut Dysbiosis: Etiology and Clinical Consequences
4.2.1. Definition and Causes
Gut dysbiosis refers to a disruption in microbial equilibrium, characterized by reduced microbial diversity, loss of beneficial taxa, and overgrowth of opportunistic pathogens [65].
Contributing factors include:
- Dietary Factors
- High-fat, low-fiber diets reduce beneficial microbial species.
- Medical Interventions.
- Antibiotic overuse, chemotherapy, surgery, and radiation alter gut ecology.
- Pathological Conditions.
- Chronic inflammation and infections drive dysbiosis.
- Environmental & Lifestyle Stressors.
- Toxins and psychological stress impact microbial function and host resilience.
4.2.2. Disease Associations
- Inflammatory DisordersIn Patients with IBD, depletion of Faecalibacterium prausnitzii and expansion of adherent-invasive E. coli drive mucosal inflammation. Patients with Irritable Bowel Syndrome (IBS) are linked to reduced Bifidobacterium and Lacticaseibacillus casei, causing increased permeability and visceral hypersensitivity. Psoriasis is associated with loss of SCFA producers and immune imbalance [19,20].
- Cancer and Tumor ProgressionDysbiosis promotes oncogenic inflammation and immune evasion. Fusobacterium nucleatum is strongly linked to colorectal cancer progression in patients. Microbial composition also shapes response to chemotherapy and immunotherapy [78].
- Metabolic DisordersIn patients with obesity, a higher Firmicutes/Bacteroidetes ratio enhances energy harvest. In Type 2 Diabetes, reduced diversity and SCFAs contribute to insulin resistance and inflammation.
- Neuropsychiatric DisordersDysbiosis alters neurotransmitter synthesis and neuroimmune signaling, contributing to depression, anxiety, and neurodegenerative diseases such as Alzheimer’s and Parkinson’s [79].
- Drug Metabolism and Therapy ResponseAntibiotic-induced dysbiosis reduces the efficacy of immune checkpoint inhibitors, while altered microbial enzymes impair drug metabolism and treatment outcomes.
5. Microbiota-Derived Metabolites and Therapeutic Implications
Modulation of the gut microbiota has emerged as a promising therapeutic strategy across a spectrum of diseases in patients, including cancer, metabolic disorders, autoimmune conditions, and gastrointestinal pathologies. These interventions aim to restore microbial homeostasis, enhance mucosal immunity, and optimize host–microbe interactions [71].
5.1. Prebiotics
Prebiotics are non-digestible dietary substrates that selectively stimulate the growth and activity of host’s beneficial gut microorganisms, thereby contributing to host health. Common prebiotics include inulin (Table 1), fructo-oligo-saccharides (FOS), galacto-oligo-saccharides (GOS), resistant starches (RS), pectic oligo-saccharides (POS), and flavanol-rich compounds [14].
- These substrates promote the proliferation of commensal taxa such as Bifidobacterium and Lacticaseibacillus casei, enhancing microbial diversity and intestinal barrier function [19,20].
- Fermentation of prebiotics yields short-chain fatty acids (SCFAs), including butyrate and propionate, which regulate inflammation, improve epithelial integrity, and exert systemic immunomodulatory effects [55].
- Prebiotic intake has been associated with metabolic improvements, including enhanced insulin sensitivity and body weight regulation in overweight individuals.
- In oncology, dietary fiber and ginseng polysaccharides have been shown to augment PD-1 blockade efficacy by suppressing regulatory T cells (Tregs) and activating cytotoxic T cells within the tumor microenvironment (TME) [74].
- Efficacy depends on the presence of specific microbial taxa; without a sufficient baseline population of target microbes, prebiotics may yield minimal benefit.
5.2. Probiotics
Probiotics are live microorganisms that, when administered in adequate amounts, confer health benefits through their interactions with the host and native microbiota.
- Predominantly from the genera Lacticaseibacillus casei, Lactiplantibacillus plantarum, Limosilactobacillus reuteri and Bifidobacterium, probiotics can modulate the microbiota, enhance epithelial barrier integrity, produce antimicrobial compounds, and regulate host immune responses [20].
- Specific strains, such as L. helveticus and L. plantarum, have been shown to augment natural killer (NK) cell activity and induce T-helper cell responses that support tumor suppression [34,75].
- Clinical applications span a range of Patients with inflammatory bowel disease (IBD) conditions, including irritable bowel syndrome (IBS), antibiotic-associated diarrhea, and even neuropsychiatric disorders such as depression [53].
- Bifidobacterium infantis has demonstrated the symptom of relief in patients with IBS, while certain strains are linked to remission maintenance in patients with ulcerative colitis (though less effective in patients with Crohn’s disease) [19].
- In the context of cancer immunotherapy for the patient, probiotics may enhance immune checkpoint blockade (ICB) efficacy by restoring microbial diversity and promoting immune activation.
- Next-generation probiotics (NGPs), such as Faecalibacterium prausnitzii and Akkermansia muciniphila, are being developed for precision microbiome therapies due to their endogenous origin and anti-inflammatory potential [13].
5.3. Synbiotics
Synbiotics are synergistic formulations combining probiotics and prebiotics to potentiate mutual benefits and enhance colonization and metabolic activity of beneficial microbes of the hosts [59].
- These formulations improve microbial resilience and host–microbe interactions, especially in gastrointestinal and metabolic diseases.
- Synbiotics have demonstrated efficacy in reducing the incidence of necrotizing enterocolitis in neonates and lowering blood pressure in hypertensive patients [59].
- In patients with ulcerative colitis, combinations such as Bifidobacterium breve with GOS have yielded improved clinical outcomes.
- Emerging evidence suggests synbiotics may augment the therapeutic response to ICBs in hepatocellular carcinoma by modulating the TME.
5.4. Fecal Microbiota Transplantation (FMT)
FMT involves the transfer of processed fecal material from a screened healthy donor into a recipient’s gastrointestinal tract (Table 1), with the goal of restoring eubiotic microbial composition in the patients [3].
- Rigorous donor screening is essential, excluding individuals with a personal or familial history of autoimmune, metabolic, or oncologic conditions.
- Delivery routes include colonoscopy, enema, nasoduodenal tubes, and encapsulated oral formulations.
- FMT is an established treatment for patients with repeated Clostridioides difficile infections, and it is under investigation for a wide range of conditions, including patients with IBD, graft-versus-host disease (GVHD), hepatic encephalopathy, multiple sclerosis, autism, and metabolic syndrome [19].
- In oncology, FMT has been shown to modulate tumor-resident microbiota, enhance systemic and intratumoral immune responses, and improve responses to ICB therapy, particularly when derived from donors who previously responded favorably to immunotherapy [75].
- Potential mechanisms include the regulation of intestinal and circulating miRNAs, metabolic remodeling, and TME reprogramming.
- Challenges include inter-individual variability in response, uncertainty about long-term efficacy, and logistical complexities regarding donor matching, route of administration, and frequency of delivery.
5.5. Dietary Modifications
Diet plays a pivotal role in shaping the gut microbiome. Specific dietary components can selectively enrich beneficial microbes and their metabolic outputs.
- High-fiber diets promote saccharolytic fermentation and SCFA production, which support mucosal health and anti-inflammatory pathways [55].
- Polyphenols—bioactive compounds in fruits, vegetables, tea, and wine exhibit prebiotic properties and contribute to microbial diversity [80].
- Fermented foods, including yogurt, kefir, sauerkraut, and kimchi, introduce live microbial cultures that reinforce microbial resilience and immune tolerance [80].
- Dietary strategies have been linked to cancer prevention in patients, improved gut barrier function, and enhanced responsiveness to cancer therapies [81].
- Personalized dietary interventions, based on individual microbial profiles, may further optimize outcomes.
5.6. Microbiome-Based Drug Development
Advances in synthetic biology have enabled the development of engineered microbial therapeutics that manipulate gut ecosystem function for therapeutic gain [17].
- Engineered probiotics can be programmed to secrete bioactive compounds, degrade immunosuppressive molecules, modulate immune checkpoints, or deliver tumor-specific antigens.
- Bacteria equipped with CRISPR-based genome editing or type III secretion systems (T3SS) enable targeted interventions within the host or tumor microenvironment [82].
- Microbiome-based approaches can also influence systemic drug metabolism, thereby modulating pharmacokinetics and improving therapeutic indices.
- Bacteriophage therapy offers a precise method to selectively eliminate pathogenic strains without disrupting commensal microbiota [83].
- These tools offer highly specific, customizable treatments but require stringent safety evaluations and regulatory frameworks prior to clinical application.
6. Gut Microbiota in Cancer and Immunotherapy
The gut microbiota has emerged as a critical determinant of cancer progression and therapeutic outcomes in patients, particularly in the context of Patient immune checkpoint inhibitors (ICIs). Recent studies have highlighted the pivotal role of specific beneficial bacteria in modulating the tumor microenvironment (TME) and enhancing the efficacy of immunotherapy [84]. For instance, Akkermansia muciniphila and Bifidobacterium longum have been shown to significantly elevate ICI responsiveness by promoting the infiltration and activation of intratumoral CD8+ T cells, which are essential for anti-tumor immunity [85].
6.1. Influence on Immune Checkpoint Inhibitors (ICIs) and Immunotherapy Response
The gut microbiota significantly influences cancer development and the effectiveness of immunotherapy in patients. The composition of the gut microbiome can affect how patients respond to cancer therapies (Table 2), particularly immune checkpoint inhibitors (ICIs) [22]. Research indicates that specific bacterial species and their metabolites can either promote or inhibit tumor growth and also modulate the efficacy of cancer treatments [75].
- Certain bacteria enhance tumor-infiltrating T-cell activity.
- Dysbiosis can lead to resistance against ICIs [75].
- Microbiota-derived metabolites reshape the tumor microenvironment (TME).
- FMT improves outcomes in patients who do not initially respond to ICIs [86].
6.2. Key Bacteria Associated with Improved Cancer Treatment Outcomes
Specific bacterial taxa are strongly associated with favorable responses to cancer immunotherapy in patients. Akkermansia muciniphila improves epithelial integrity and enhances ICI efficacy, while Bifidobacterium species increase anti-PD-1 effectiveness in preclinical models (Table 2). Similarly, taxa such as Lachnospiraceae, Ruminococcaceae, and Faecalibacterium prausnitzii contribute to improved outcomes by promoting immune balance and anti-inflammatory effects [87].
- Akkermansia muciniphila: Enhances ICI efficacy and lymphocyte infiltration.
- Bifidobacterium spp.: Improve anti-PD-1 therap=y, especially in breast cancer models [87].
- Lachnospiraceae and Alistipes: Linked with longer survival in Patients with HCC.
- Faecalibacterium prausnitzii: Supports anti-PD-L1 therapy through butyrate production [88].
- Other commensals (Collinsella, Enterococcus, Blautia) support immune homeostasis.
6.3. Role of Microbial Metabolites in Tumor Progression and Therapy
Microbial metabolites act as molecular messengers between the microbiota and host, affecting patients’ tumor growth and therapy response on the patients. Short-chain fatty acids (SCFAs) such as butyrate improve gut homeostasis and reduce angiogenesis, while inosine enhances PD-L1 therapy. Conversely, metabolites like hydrogen sulfide and secondary bile acids can promote cancer progression [63,89].
- SCFAs strengthen the gut barrier and limit tumor angiogenesis.
- Inosine boosts anti-PD-L1 therapy effectiveness [89].
- Hydrogen sulfide and bile acids promote DNA damage and colorectal cancer.
- Indole derivatives (e.g., 3-IAA) modulate chemotherapy in pancreatic cancer.
6.4. Antibiotic Impact on Cancer Therapy
Antibiotics can disrupt the delicate balance of the host’s gut microbiome, leading to reduced microbial diversity and poorer therapy outcomes. Patients who receive antibiotics during ICI therapy generally show lower survival rates and weaker responses. Experimental models also confirm that antibiotic use reduces the efficacy of chemotherapies such as cyclophosphamide [90].
- Broad-spectrum antibiotics cause dysbiosis and weaken immune responses.
- Antibiotic use during ICI therapy lowers survival and response rates.
- Disruption of gut balance increases intestinal permeability and inflammation.
- Germ-free or antibiotic-treated mice show poor chemotherapy outcomes.

Table 2.
Microbiota-Targeted Therapeutic Strategies and Their Immune–Metabolic Effects.
Table 2.
Microbiota-Targeted Therapeutic Strategies and Their Immune–Metabolic Effects.
| Strategy | Mechanism of Action | Immune Effects | Metabolic Effects | References |
|---|---|---|---|---|
| Probiotics (Bifidobacterium, Lacticaseibacillus casei, Akkermansia) | Enhance gut barrier, modulate cytokine production, compete with pathogens | Increase Treg activation, reduce pro-inflammatory cytokines (IL-6, TNF-α), promote antitumor immunity in patients | Improve insulin sensitivity, lipid metabolism, and SCFA production. | [91] |
| Prebiotics (inulin, resistant starch, fibers) | Provide substrates for beneficial microbes, increase SCFA production | Promote anti-inflammatory immune responses; enhance IgA secretion in patients | Boost SCFA levels (butyrate, propionate), improve glucose and lipid metabolism. | [92] |
| Synbiotics (Probiotic + Prebiotic) | Synergistic effect improving colonization of beneficial microbes (Figure 2) | Enhance immune tolerance and lower inflammatory markers of the patients | Support nutrient absorption and improve metabolic homeostasis. | [93] |
| Fecal Microbiota Transplantation (FMT) | Restores microbial diversity using donor stool | Re-establishes immune balance, restores Treg/Th17 ratio, improves response to immunotherapy in patients | Enhances metabolic function, reduces endotoxemia and systemic inflammation. | [3] |
| Dietary Interventions (Mediterranean diet, fermented foods) | Increase microbial richness and diversity | Promote immune tolerance, decrease pro-inflammatory pathways in hosts | Increase SCFA levels, improve cardiovascular and metabolic outcomes. | [94] |
| Microbiome-based Drugs (live biotherapeutics, engineered bacteria) | Target-specific pathways with microbial strains or engineered metabolites | Modulate tumor immunity, balance immune dysregulation in patients | Correct metabolic disorders by modulating bile acids and SCFAs. | [75] |
| Antibiotic Stewardship | Prevents broad-spectrum disruption of microbiota | Reduces dysbiosis-related immune dysfunction in patients | Preserves metabolic stability by maintaining microbial diversity | [95] |
7. The Role of Gut Microbiota in Other Health Conditions
The gut microbiota exerts far-reaching effects beyond gastrointestinal health of the hosts, playing an important role in cardiovascular, neurological, dermatological, and hormonal systems of host. Its influence on metabolism, inflammation, and immune regulation demonstrates its central role in maintaining systemic homeostasis. Dysbiosis in these microbial communities is increasingly linked to chronic diseases, suggesting that microbiota-targeted interventions could provide novel therapeutic avenues [96].
7.1. Cardiovascular Diseases
Gut microbiota composition strongly influences cardiovascular health by modulating lipid metabolism, bile acid transformation, and systemic inflammation. One key mechanism involves the microbial conversion of dietary phosphatidylcholine into trimethylamine (TMA), later oxidized into trimethylamine-N-oxide (TMAO) in hosts [97]), a metabolite associated with atherosclerosis and cardiovascular events. Elevated TMAO levels have been linked with increased risk of myocardial infarction and stroke, while a more diverse microbiota may counterbalance these harmful pathways. Dysbiosis of the patients also promotes gut permeability, allowing endotoxins such as LPS into circulation [98], thereby triggering chronic inflammation and accelerating vascular disease. Additionally, microbial disturbances alter cholesterol absorption and bile acid metabolism, contributing to hyperlipidemia and non-alcoholic fatty liver disease [99]. Dysbiosis increases production of TMAO, a microbial metabolite strongly associated with atherosclerosis severity and thrombosis. Targeting TMAO-producing bacteria and enhancing SCFA-producing strains show Cardioprotective Potential [94].
- Key points:
- Gut microbes produce TMAO, strongly linked to atherosclerosis and stroke.
- Dysbiosis promotes gut leakiness and systemic inflammation.
- Microbiota influence cholesterol and bile acid metabolism, affecting lipid profiles.
- Reduced microbial diversity may worsen cardiovascular risk.
7.2. Neurological Disorders
Through the gut–brain axis, the microbiota communicates bidirectionally with the central nervous system, influencing cognitive and emotional health. Beneficial microbes produce neurotransmitters such as serotonin and gamma-aminobutyric acid (GABA), which regulate mood and behavior [100]. Dysbiosis disrupts this production, contributing to depression, anxiety, and neurodegenerative conditions. Increasing evidence suggests that microbial changes precede the onset of Alzheimer’s and Parkinson’s disease, pointing toward microbiota modulation as a potential preventive strategy. Microbial metabolites, particularly SCFAs [101], play an additional role by influencing neuroinflammation, gut motility, and visceral sensitivity, all of which are important in patients with disorders like irritable bowel syndrome (IBS) [19]. Over 90% serotonin production in the gut demonstrates microbiota’s role in neuro-regulation. Microbial imbalance promotes neuroinflammation and cognitive decline through the vagus nerve and immune signaling, contributing to Parkinson’s and Alzheimer’s pathology [33].
- Key points:
- Gut microbes produce neurotransmitters (serotonin, GABA) critical for mood regulation in patients.
- Dysbiosis contributes to depression, anxiety, and neurodegeneration.
- Microbial changes may precede Alzheimer’s and Parkinson’s symptoms.
- SCFAs affect patients’ brain signaling, inflammation, and IBS development.
7.3. Psoriasis and Skin Disorders
The gut–skin axis highlights the microbiota’s role in dermatological health. Dysbiosis is frequently observed in patients with inflammatory skin conditions, including psoriasis, acne, atopic dermatitis, and rosacea. In psoriasis, reduced populations of SCFA-producing bacteria weaken regulatory T cell (Treg) function, fueling systemic inflammation and immune dysregulation [36]. Low dietary fiber intake further reduces SCFA levels, activating dendritic cells and perpetuating chronic inflammation. This altered microbial-immune interaction may also increase susceptibility to systemic comorbidities, such as arthritis and cardiovascular disease, often seen in psoriasis patients [102]. Reduced SCFA-producing bacteria contribute to hyperactivation of Th17 cells and systemic inflammation, characteristic of psoriasis and atopic dermatitis. Microbiota modulation is emerging as a complementary therapeutic approach [103].
- Key points:
- Gut dysbiosis is linked to psoriasis, acne, dermatitis, and rosacea.
- Loss of SCFA-producing bacteria weakens Treg function and fuels inflammation.
- Low-fiber diets reduce SCFAs, worsening skin inflammation.
- Psoriasis is a systemic inflammatory condition tied to gut microbiome imbalance.
7.4. Hormonal Regulation
The gut microbiota significantly influences patients’ hormonal regulation through the estrobolome, which controls estrogen metabolism. By deconjugating estrogens, gut bacteria regulate their reabsorption, thereby impacting conditions for patients such as breast cancer, endometriosis, and cardiometabolic risk [104]. Additionally, microbial activity modulates bile acid metabolism, affecting lipid and glucose regulation. Secondary bile acids produced by gut bacteria can circulate systemically into the host’s body, altering insulin sensitivity and contributing to metabolic diseases such as obesity, diabetes, and polycystic ovary syndrome (PCOS) in patients [105].
- Key points:
- The estrobolome regulates estrogen reabsorption, influencing breast cancer and metabolic risk.
- Gut microbes shape bile acid metabolism, affecting lipid and glucose homeostasis.
- Secondary bile acids influence insulin sensitivity and fat storage.
- Dysbiosis contributes to PCOS, obesity, and diabetes.
8. Personalized and Translational Approaches
Recent advances in microbiome research point toward major breakthroughs in therapeutic strategies, diagnostics, and our overall understanding of how the gut microbiota influences health and disease [82]. Promising trends include genome editing of microbial communities, precision-based therapies, development of robust ethical and regulatory frameworks, and the urgent need for standardization in clinical microbiome applications. These innovations aim to move microbiome-based treatments from experimental to mainstream medicine while ensuring safety and efficacy [82].
8.1. Recent Methods & Technologies
Advancements in shotgun metagenomics, metabolomics, spatial transcriptomics, and single-cell sequencing now enable high-resolution functional mapping of microbe-host interactions. Machine learning and network analysis help predict responders to immunotherapy and model dysbiosis-driven metabolic pathways [106,107].
8.2. Microbiome Editing (CRISPR, Synthetic Biology)
Microbiome editing is one of the most exciting frontiers in this field, involving targeted manipulation of microbial communities to achieve therapeutic benefits. Tools such as CRISPR-Cas9, base editing, and synthetic biology are enabling precise modification of bacterial genomes, opening the door to highly specific interventions against antibiotic-resistant pathogens, metabolic dysfunction, and even cancer [82]). Engineered bacteriophages and CRISPR-equipped viruses can selectively eliminate harmful bacteria, while synthetic biology approaches allow the design of microbial strains capable of producing therapeutic compounds or modulating host metabolism. Although promising, these technologies face challenges related to stability, delivery, and biosafety, and their long-term effects are not yet fully understood [14]. Advancements in shotgun metagenomics, metabolomics, spatial transcriptomics, and single-cell sequencing now enable high-resolution functional mapping of microbe-host interactions. Machine learning and network analysis help predict responders to immunotherapy and model dysbiosis-driven metabolic pathways [106,107].
- Key points:
- CRISPR and base editing enable precise genetic modification of gut bacteria.
- Engineered bacteriophages target antibiotic-resistant microbes.
- Synthetic biology allows microbes to produce therapeutic metabolites.
- In situ microbiome engineering manipulates bacteria within their natural habitat.
- Challenges: delivery, stability, safety, and long-term controllability.
8.3. Personalized Microbiome-Based Therapies
Personalized approaches are rapidly becoming a central theme in microbiome research. Unlike one-size-fits-all treatments, these therapies are tailored to the unique microbial profiles of individual patients, taking into account diet, genetics, demographics, and lifestyle. Personalized microbiome profiling through metagenomics allows clinicians to predict treatment outcomes and optimize interventions [108]. For example, patient-specific microbial signatures can guide the use of probiotics, prebiotics, or microbiota-derived drugs, improving chemotherapy efficacy while minimizing toxicity. Dietary habits, which can quickly reshape gut microbial populations, also form an integral part of precision medicine strategies [109]. Personalized microbiome-based therapy incorporates an individual’s microbial profile, genetics, lifestyle, and diet to predict therapeutic responses. Precision probiotics, AI-guided diet modification, and responder-derived FMT are advancing toward personalized interventions for cancer and metabolic disorders [110].
- Key points:
- Interventions are tailored to individual microbial profiles.
- Incorporates genetic, dietary, and lifestyle factors into therapy.
- Microbiome profiling guides personalized probiotics and microbiota-targeted drugs.
- Precision medicine integrates microbiome data to predict treatment response.
- Personalized approaches reduce variability in drug absorption and toxicity.
8.4. Ethical and Regulatory Challenges in Microbiome Research
As microbiome-based therapies move toward clinical translation, ethical and regulatory challenges remain significant. Defining whether such therapies should be categorized as drugs, biologics, or dietary supplements has direct implications for approval and regulation [111,112]. Treatments involving live organisms, such as FMT or probiotics, require strict oversight to prevent risks such as pathogen transfer. Informed consent and clear communication of risks and benefits are essential in clinical trials. Additionally, the societal impact of such interventions, including equitable access and potential misuse, must be carefully considered [14].
- Key points:
- Classification of microbiome therapies (drug vs. supplement) affects regulation.
- Live-organism therapies (FMT, probiotics) require rigorous screening.
- Risks include pathogen transfer and variable safety outcomes.
- Ethical concerns involve informed consent and equitable access.
- Societal benefits must be weighed against risks in clinical application.
8.5. Long-Term Implications of Gut Microbiota Modulation
The long-term impact of microbiome modulation on treatment outcomes, side effects, and overall host health remains poorly understood. FMT and probiotic therapies show promise, but longitudinal studies are needed to track microbiome changes and patient outcomes over years [14,75]. For instance, breast cancer studies suggest that shifts in microbial composition during therapy could affect disease recurrence or secondary complications. Standardized sampling methods, robust control groups, and multi-omics integration will be vital to understanding how microbiome modulation influences human health across the lifespan [113].
- Key points:
- Long-term effects of FMT and probiotics are not fully known.
- More longitudinal studies are required across diverse populations.
- Standardized sampling and clinical trial methods ensure reliable data.
- Microbiome changes during therapy may affect recurrence risk and side effects.
9. Limitations and Future Directions
9.1. Limitations of Microbiome Research
Current microbiome research faces significant limitations, as much of the existing knowledge remains correlative rather than causal, hindered by inter-individual variability, inconsistent sampling methods, and short follow-up periods. The challenges of culturing anaerobic species further complicate mechanistic insights [114,115]. Additionally, the clinical reproducibility of microbiota-targeted therapies is highly variable due to the profound influence of host genetics, diet, age, and environmental factors on microbial behavior to address these gaps, more longitudinal studies integrating multi-omic approaches are essential for advancing robust clinical translation and establishing causal relationships in various diseases [114].
9.2. Future Directions
Future research should move beyond descriptive correlations toward mechanistic and personalized models. Integration of metagenomics, metatranscriptomics, metabolomics, and AI-based prediction systems will be essential to elucidate cause–effect relationships and translate microbiome knowledge into clinical benefit.
10. Conclusions
The gut microbiome plays a pivotal role in human health, and its modulation presents a promising avenue for therapeutic interventions, particularly in cancer treatment. Current research underscores the intricate relationship between the gut microbiome, the tumor microenvironment (TME) [75], and the host immune system, influencing treatment outcomes and overall disease progression.
Microbiome-driven therapies, including fecal microbiota transplantation (FMT), probiotics, and prebiotics, are being actively explored to enhance cancer immunotherapy. FMT, especially in combination with immune checkpoint inhibitors (ICIs), has shown potential in improving treatment response in various cancers, including hepatocellular carcinoma (HCC). Ongoing clinical trials are evaluating these interventions, focusing on key outcomes such as objective response rate (ORR), disease control rate (DCR), and adverse events (AEs).
Specific microbial compositions have been identified as predictive markers of immunotherapy efficacy. For instance, increased levels of Lachnospiraceae bacterium-GAM79, Alistipes sp. Marseille-P5997, Ruminococcus, and Klebsiella have been linked to better outcomes in Patients with HCC receiving anti-PD-1 therapy. Conversely, gut dysbiosis—characterized by an imbalance in microbial communities, including a reduced Prevotella/Bacteroides ratio—has been associated with poor response to nivolumab.
Dietary interventions with prebiotics, such as resistant starches and dietary fiber, are also being investigated for their ability to modulate the gut microbiome and enhance ICI efficacy. Increased dietary fiber intake has been linked to improved sensitivity to PD-1 inhibitors by suppressing regulatory T (Treg) cells and activating cytotoxic T cells. Additionally, probiotics like Lacticaseibacillus rhamnosus and synbiotics (a combination of probiotics and prebiotics) are being evaluated for their role in microbiome modulation during cancer treatment.
Short-chain fatty acids (SCFAs), including butyrate, acetate, and propionate, are key microbial metabolites with significant therapeutic potential. These SCFAs contribute to immune regulation, enhance intestinal barrier function, and have demonstrated potential anticancer effects. Furthermore, bioactive compounds such as nisin have been explored for their apoptogenic properties, offering novel approaches for cancer therapy.
Network analysis has emerged as a powerful tool for deciphering the complexity of microbiome interactions. By analyzing microbial co-occurrence patterns, differential networks, and causal relationships, researchers can identify critical microorganisms, biomarkers, and regulatory pathways involved in disease progression and treatment response.
However, factors such as antibiotic use can disrupt the gut microbiome, leading to dysbiosis, inflammatory disorders, and diminished response to cancer therapy. As such, strategic manipulation of the gut microbiome—through dietary interventions, FMT, or microbiome-based therapies—holds immense potential in developing personalized medical strategies.
Author Contributions
I.M. led the conceptualization, writing, and editing of the manuscript while supervising the overall project. M.R.A. contributed to data curation and drafting key sections. M.S.K. focused on formal analysis and visualization, ensuring clarity in presentation. M.N.B. provided methodological expertise and validation of content. M.A.K. played a pivotal role in resource acquisition and securing funding, alongside contributing to revisions. M.M.P. managed project administration and enhanced the manuscript through critical review and refinement. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study did not involve human or animal subjects; therefore, ethical approval was not required.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Acknowledgments
This study is supported via funding from Prince Sattam bin Abdulaziz University, Al-Kharj, Saudi Arabia, and Project number (PSAU/2025/R/1447).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hugon, P.; Lagier, J.C.; Colson, P.; Bittar, F.; Raoult, D. Repertoire of human gut microbes. Microb. Pathog. 2017, 106, 103–112. [Google Scholar] [CrossRef]
- Lushchak, V.I. Symphony of Digestion: Coordinated Host–Microbiome Enzymatic Interplay in Gut Ecosystem. Biomolecules 2025, 15, 1151. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787. [Google Scholar] [CrossRef]
- Virgo, M.; Mostowy, S.; Ho, B.T. Emerging models to study competitive interactions within bacterial communities. Trends Microbiol. 2025, 33, 688–700. [Google Scholar] [CrossRef]
- Moran, N.A.; Ochman, H.; Hammer, T.J. Evolutionary and ecological consequences of gut microbial communities. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 451–475. [Google Scholar] [CrossRef] [PubMed]
- Matijašić, M.; Meštrović, T.; Čipčić Paljetak, H.; Perić, M.; Barešić, A.; Verbanac, D. Gut microbiota beyond bacteria—Mycobiome, virome, archaeome, and eukaryotic parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef] [PubMed]
- Deering, K.E.; Devine, A.; O’Sullivan, T.A.; Lo, J.; Boyce, M.C.; Christophersen, C.T. Characterizing the composition of the pediatric gut microbiome: A systematic review. Nutrients 2019, 12, 16. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Kovatcheva-Datchary, P.; Tremaroli, V.; Bäckhed, F. The gut microbiota. In The Prokaryotes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–24. [Google Scholar]
- Zhu, B.; Wang, X.; Li, L. Human gut microbiome: The second genome of human body. Protein Cell 2010, 1, 718–725. [Google Scholar] [CrossRef]
- Dave, M.; Higgins, P.D.; Middha, S.; Rioux, K.P. The human gut microbiome: Current knowledge, challenges, and future directions. Transl. Res. 2012, 160, 246–257. [Google Scholar] [CrossRef]
- Khalil, M.; Di Ciaula, A.; Mahdi, L.; Jaber, N.; Di Palo, D.M.; Graziani, A.; Gyorgy, B.; Portincasa, P. Unraveling the role of the human gut microbiome in health and diseases. Microorganisms 2024, 12, 2333. [Google Scholar] [CrossRef]
- Olteanu, G.; Ciucă-Pană, M.A.; Busnatu, Ș.S.; Lupuliasa, D.; Neacșu, S.M.; Mititelu, M.; Musuc, A.M.; Ioniță-Mîndrican, C.-B.; Boroghină, S.C. Unraveling the microbiome–human body axis: A comprehensive examination of therapeutic strategies, interactions and implications. Int. J. Mol. Sci. 2024, 25, 5561. [Google Scholar] [CrossRef]
- Jaswal, A.S.; Mishra, S.; Elangovan, R. Prebiotic Oligosaccharide Production in Microbial Cells. In Microbial Nutraceuticals: Products and Processes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2025; pp. 81–113. [Google Scholar]
- NCBI Taxonomy Database. National Center for Biotechnology Information. 2025. Available online: https://www.ncbi.nlm.nih.gov/taxonomy (accessed on 10 October 2025).
- List of Prokaryotic Names with Standing in Nomenclature (LPSN). 2023. Available online: https://lpsn.dsmz.de/ (accessed on 10 October 2025).
- Ahlawat, S.; Asha, N.; Sharma, K.K. Gut–organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut microbiota and short chain fatty acids: Implications in glucose homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Shu, L.Z.; Ding, Y.D.; Xue, Q.M.; Cai, W.; Deng, H. Direct and indirect effects of pathogenic bacteria on the integrity of intestinal barrier. Ther. Adv. Gastroenterol. 2023, 16, 17562848231176427. [Google Scholar] [CrossRef] [PubMed]
- Mc Neil, V.; Lee, S.W. Advancing Cancer Treatment: A Review of Immune Checkpoint Inhibitors and Combination Strategies. Cancers 2025, 17, 1408. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, Y.; Xiao, H.; Sun, H. Advancements in understanding the role of intestinal dysbacteriosis mediated mucosal immunity in IgA nephropathy. BMC Nephrol. 2024, 25, 203. [Google Scholar] [CrossRef]
- Pandiyan, P.; Bhaskaran, N.; Zou, M.; Schneider, E.; Jayaraman, S.; Huehn, J. Microbiome dependent regulation of Tregs and Th17 cells in mucosa. Front. Immunol. 2019, 10, 426. [Google Scholar] [CrossRef]
- Pires, L.; Gonzalez-Paramás, A.M.; Heleno, S.A.; Calhelha, R.C. Gut microbiota as an endocrine organ: Unveiling its role in human physiology and health. Appl. Sci. 2024, 14, 9383. [Google Scholar] [CrossRef]
- Maslowski, K.M. Metabolism at the centre of the host–microbe relationship. Clin. Exp. Immunol. 2019, 197, 193–204. [Google Scholar] [CrossRef]
- Dicks, L.M. Gut bacteria and neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut microbiota and immune system interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, K.; Yan, L.; Wang, P.; Zhao, F.; Hu, S. The role of toll-like receptors in immune tolerance induced by Helicobacter pylori infection. Helicobacter 2023, 28, e13020. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Flores, G.; Pickard, J.M.; Núñez, G. Microbiota-mediated colonization resistance: Mechanisms and regulation. Nat. Rev. Microbiol. 2023, 21, 347–360. [Google Scholar] [CrossRef]
- Rose, A.E.; Fansler, R.T.; Zhu, W. Commensal resilience: Ancient ecological lessons for the modern microbiota. Infect. Immun. 2025, 93, e00502-24. [Google Scholar] [CrossRef]
- Derrien, M.; Alvarez, A.S.; de Vos, W.M. The gut microbiota in the first decade of life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef]
- Shang, Z.; Pai, L.; Patil, S. Unveiling the dynamics of gut microbial interactions: A review of dietary impact and precision nutrition in gastrointestinal health. Front. Nutr. 2024, 11, 1395664. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef]
- Giau, V.V.; Wu, S.Y.; Jamerlan, A.; An, S.S.A.; Kim, S.; Hulme, J. Gut microbiota and their neuroinflammatory implications in Alzheimer’s disease. Nutrients 2018, 10, 1765. [Google Scholar] [CrossRef]
- Scarpellini, E.; Fagoonee, S.; Rinninella, E.; Rasetti, C.; Aquila, I.; Larussa, T.; Ricci, P.; Luzza, F.; Abenavoli, L. Gut microbiota and liver interaction through immune system cross-talk: A comprehensive review at the time of the SARS-CoV-2 pandemic. J. Clin. Med. 2020, 9, 2488. [Google Scholar] [CrossRef]
- Song, Y.; Lau, H.C.; Zhang, X.; Yu, J. Bile acids, gut microbiota, and therapeutic insights in hepatocellular carcinoma. Cancer Biol. Med. 2024, 21, 144–162. [Google Scholar] [CrossRef]
- Munteanu, C.; Turti, S.; Marza, S.M. Unraveling the Gut–Skin Axis: The Role of Microbiota in Skin Health and Disease. Cosmetics 2025, 12, 167. [Google Scholar] [CrossRef]
- Khan, I.M.; Nassar, N.; Chang, H.; Khan, S.; Cheng, M.; Wang, Z.; Xiang, X. The microbiota: A key regulator of health, productivity, and reproductive success in mammals. Front. Microbiol. 2024, 15, 1480811. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.; Ali, K.; Khan, A.U. It’s all relative: Analyzing microbiome compositions, its significance, pathogenesis and microbiota derived biofilms: Challenges and opportunities for disease intervention. Arch. Microbiol. 2023, 205, 257. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Azad, M.B.; Bäckhed, F.; Blaser, M.J.; Byndloss, M.; Chiu, C.Y.; Chu, H.; Dugas, L.R.; Elinav, E.; Gibbons, S.M.; et al. Clinical translation of microbiome research. Nat. Med. 2025, 31, 1099–1113. [Google Scholar] [CrossRef]
- Arnold, W.M.; Hill, E.S.; Fei, N.; Yee, A.L.; Garcia, M.S.; Cralle, L.E.; Gilbert, J.A. The human microbiome in health and disease. In Genomic Applications in Pathology; Springer International Publishing: Cham, Switzerland, 2018; pp. 607–618. [Google Scholar]
- Fernández, J.; Redondo-Blanco, S.; Gutiérrez-del-Río, I.; Miguélez, E.M.; Villar, C.J.; Lombo, F. Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: A review. J. Funct. Foods 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Fan, J.; Zhu, J.; Xu, H. Strategies of Helicobacter pylori in evading host innate and adaptive immunity: Insights and prospects for therapeutic targeting. Front. Cell. Infect. Microbiol. 2024, 14, 1342913. [Google Scholar] [CrossRef]
- Baindara, P.; Mandal, S.M. Gut-antimicrobial peptides: Synergistic co-evolution with antibiotics to combat multi-antibiotic resistance. Antibiotics 2023, 12, 1732. [Google Scholar] [CrossRef]
- Pant, A.; Maiti, T.K.; Mahajan, D.; Das, B. Human gut microbiota and drug metabolism. Microb. Ecol. 2023, 86, 97–111. [Google Scholar] [CrossRef]
- Jena, R.; Singh, N.A.; Ahmed, N.; Choudhury, P.K. Bifidobacteria in antibiotic-associated dysbiosis: Restoring balance in the gut microbiome. World J. Microbiol. Biotechnol. 2025, 41, 297. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Bu, X.; Chen, D.; Wu, X.; Wu, H.; Caiyin, Q.; Qiao, J. Molecules-mediated bidirectional interactions between microbes and human cells. npj Biofilms Microbiomes 2025, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, Z.M.; Zhang, L.L.; Li, J.M.; Lv, W.L. The role of gut microbiota in some liver diseases: From an immunological perspective. Front. Immunol. 2022, 13, 923599. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Somaratne, G. Functional food and nutraceuticals for the prevention of gastrointestinal disorders. In Industrial Application of Functional Foods, Ingredients and Nutraceuticals; Academic Press: Cambridge, MA, USA, 2023; pp. 501–534. [Google Scholar]
- Tiruvayipati, S.; Hameed, D.S.; Ahmed, N. Play the plug: How bacteria modify recognition by host receptors? Front. Microbiol. 2022, 13, 960326. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, F.R.; Eguileor, A.; Llorente, C. Goblet cells: Guardians of gut immunity and their role in gastrointestinal diseases. Egastroenterology 2024, 2, e100098. [Google Scholar] [CrossRef]
- Brescia, C.; Audia, S.; Pugliano, A.; Scaglione, F.; Iuliano, R.; Trapasso, F.; Perrotti, N.; Chiarella, E.; Amato, R. Metabolic drives affecting Th17/Treg gene expression changes and differentiation: Impact on immune-microenvironment regulation. Apmis 2024, 132, 1026–1045. [Google Scholar] [CrossRef]
- Bastida, G.; Mínguez, A.; Nos, P.; Moret-Tatay, I. Immunoepigenetic regulation of inflammatory bowel disease: Current insights into novel epigenetic modulations of the systemic immune response. Genes 2023, 14, 554. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Alonso-Cotoner, C.; Abril-Gil, M.; Albert-Bayo, M.; Mall, J.P.G.; Expósito, E.; Gonzalez-Castro, A.M.; Lobo, B.; Santos, J. The role of purported mucoprotectants in dealing with irritable bowel syndrome, functional diarrhea, and other chronic diarrheal disorders in adults. Adv. Ther. 2021, 38, 2054–2076. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Tong, Y.; Lou, X. Interplay Between Bile Acids, Gut Microbiota, and the Tumor Immune Microenvironment: Mechanistic Insights and Therapeutic Strategies. Front. Immunol. 2025, 16, 1638352. [Google Scholar] [CrossRef]
- Marafini, I.; Monteleone, I.; Laudisi, F.; Monteleone, G. Aryl hydrocarbon receptor signalling in the control of gut inflammation. Int. J. Mol. Sci. 2024, 25, 4527. [Google Scholar] [CrossRef] [PubMed]
- Tarracchini, C.; Lordan, C.; Milani, C.; Moreira, L.P.; Alabedallat, Q.M.; de Moreno de LeBlanc, A.; Turroni, F.; Lugli, G.A.; Mancabelli, L.; Longhi, G.; et al. Vitamin biosynthesis in the gut: Interplay between mammalian host and its resident microbiota. Microbiol. Mol. Biol. Rev. 2025, 89, e00184-23. [Google Scholar] [CrossRef] [PubMed]
- Mantri, A.; Klümpen, L.; Seel, W.; Krawitz, P.; Stehle, P.; Weber, B.; Koban, L.; Plassmann, H.; Simon, M.C. Beneficial effects of Synbiotics on the gut microbiome in individuals with low Fiber intake: Secondary analysis of a double-blind, randomized controlled trial. Nutrients 2024, 16, 2082. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cui, J.; Jiang, S.; Zheng, C.; Zhao, J.; Zhang, H.; Zhai, Q. Early life gut microbiota: Consequences for health and opportunities for prevention. Crit. Rev. Food Sci. Nutr. 2024, 64, 5793–5817. [Google Scholar] [CrossRef]
- Munteanu, C.; Schwartz, B. Interactions between dietary antioxidants, dietary Fiber and the gut microbiome: Their putative role in inflammation and Cancer. Int. J. Mol. Sci. 2024, 25, 8250. [Google Scholar] [CrossRef]
- Paddison Rees, N. Investigating the Potential Senomorphic Properties of SCFA Butyrate on Aged T Cells. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2024. [Google Scholar]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Q. Gut microbiota influences the efficiency of immune checkpoint inhibitors by modulating the immune system. Oncol. Lett. 2024, 27, 87. [Google Scholar] [CrossRef]
- Dey, P.; Ray Chaudhuri, S. The opportunistic nature of gut commensal microbiota. Crit. Rev. Microbiol. 2023, 49, 739–763. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. The role of the mucosal barrier system in maintaining gut symbiosis to prevent intestinal inflammation. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2025; Volume 47, p. 2. [Google Scholar]
- Aburto, M.R.; Cryan, J.F. Gastrointestinal and brain barriers: Unlocking gates of communication across the microbiota–gut–brain axis. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 222–247. [Google Scholar] [CrossRef]
- Tao, W.; Yu, Y.; Tan, D.; Huang, X.; Huang, J.; Lin, C.; Yu, R. Microbiota and enteric nervous system crosstalk in diabetic gastroenteropathy: Bridging mechanistic insights to microbiome-based therapies. Front. Cell. Infect. Microbiol. 2025, 15, 1603442. [Google Scholar] [CrossRef]
- Dezfouli, M.A.; Rashidi, S.K.; Yazdanfar, N.; Khalili, H.; Goudarzi, M.; Saadi, A.; Kiani Deh Kiani, A. The emerging roles of neuroactive components produced by gut microbiota. Mol. Biol. Rep. 2025, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Di Benedetto, S. Neuroimmune crosstalk in chronic neuroinflammation: Microglial interactions and immune modulation. Front. Cell. Neurosci. 2025, 19, 1575022. [Google Scholar] [CrossRef]
- Kustrimovic, N.; Balkhi, S.; Bilato, G.; Mortara, L. Gut microbiota and immune system dynamics in Parkinson’s and Alzheimer’s diseases. Int. J. Mol. Sci. 2024, 25, 12164. [Google Scholar] [CrossRef] [PubMed]
- Anwer, E.K.; Ajagbe, M.; Sherif, M.; Musaibah, A.S.; Mahmoud, S.; ElBanbi, A.; Abdelnaser, A. Gut microbiota secondary metabolites: Key roles in GI tract cancers and infectious diseases. Biomedicines 2025, 13, 100. [Google Scholar] [CrossRef]
- Mafe, A.N.; Büsselberg, D. The Effect of Microbiome-Derived Metabolites in Inflammation-Related Cancer Prevention and Treatment. Biomolecules 2025, 15, 688. [Google Scholar] [CrossRef]
- Marroncini, G.; Naldi, L.; Martinelli, S.; Amedei, A. Gut–liver–pancreas axis crosstalk in health and disease: From the role of microbial metabolites to innovative microbiota manipulating strategies. Biomedicines 2024, 12, 1398. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.A.; Patel, V.P.; Bhosle, K.P.; Nagare, S.D.; Thombare, K.C. The tumor microenvironment: Shaping cancer progression and treatment response. J. Chemother. 2025, 37, 15–44. [Google Scholar] [CrossRef]
- Nishida, A.; Andoh, A. The role of inflammation in cancer: Mechanisms of tumor initiation, progression, and metastasis. Cells 2025, 14, 488. [Google Scholar] [CrossRef]
- Garabatos, N.; Angelats, E.; Santamaria, P. Mechanistic and therapeutic advances in immune-mediated gastrointestinal disorders. J. Allergy Clin. Immunol. 2025, 165, 1133–1159. [Google Scholar] [CrossRef]
- Pignatelli, P.; Nuccio, F.; Piattelli, A.; Curia, M.C. The role of Fusobacterium nucleatum in oral and colorectal carcinogenesis. Microorganisms 2023, 11, 2358. [Google Scholar] [CrossRef]
- Peterson, C.T. Dysfunction of the microbiota-gut-brain axis in neurodegenerative disease: The promise of therapeutic modulation with prebiotics, medicinal herbs, probiotics, and synbiotics. J. Evid.-Based Integr. Med. 2020, 25, 2515690X20957225. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Tarfeen, N.; Mohamed, H.; Song, Y. Fermented foods: Their health-promoting components and potential effects on gut microbiota. Fermentation 2023, 9, 118. [Google Scholar] [CrossRef]
- Fong, W.; Li, Q.; Yu, J. Gut microbiota modulation: A novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef]
- Amen, R.A.; Hassan, Y.M.; Essmat, R.A.; Ahmed, R.H.; Azab, M.M.; Shehata, N.R.; Elgazzar, M.M.; El-Sayed, W.M. Harnessing the Microbiome: CRISPR-Based Gene Editing and Antimicrobial Peptides in Combating Antibiotic Resistance and Cancer. Probiotics Antimicrob. Proteins 2025, 17, 1938–1968. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, M.O.; Jain, A.; Joseph, C.E.; Edison, L.K. Microbiome-driven therapeutics: From gut health to precision medicine. Gastrointest. Disord. 2025, 7, 7. [Google Scholar] [CrossRef]
- Simpson, R.C.; Shanahan, E.R.; Scolyer, R.A.; Long, G.V. Towards modulating the gut microbiota to enhance the efficacy of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2023, 20, 697–715. [Google Scholar] [CrossRef]
- Lin, A.; Xiong, M.; Jiang, A.; Huang, L.; Wong, H.Z.; Feng, S.; Zhang, C.; Li, Y.; Chen, L.; Chi, H.; et al. The microbiome in cancer. iMeta 2025, 4, e70070. [Google Scholar] [CrossRef]
- Ciernikova, S.; Sevcikova, A.; Novisedlakova, M.; Mego, M. Insights into the Relationship Between the Gut Microbiome and Immune Checkpoint Inhibitors in Solid Tumors. Cancers 2024, 16, 4271. [Google Scholar] [CrossRef]
- Wang, S.; Xu, B.; Zhang, Y.; Chen, G.; Zhao, P.; Gao, Q.; Yuan, L. The role of intestinal flora on tumorigenesis, progression, and the efficacy of PD-1/PD-L1 antibodies in colorectal cancer. Cancer Biol. Med. 2024, 21, 65–82. [Google Scholar] [CrossRef]
- Xin, Y.; Peng, G.; Song, W.; Zhou, X.; Huang, X.; Cao, X. Gut microbiota as a prognostic biomarker for unresectable hepatocellular carcinoma treated with anti-PD-1 therapy. Front. Genet. 2024, 15, 1366131. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Yang, M.; Chen, M. Metabolic interactions: How gut microbial metabolites influence colorectal cancer. Front. Microbiol. 2025, 16, 1611698. [Google Scholar] [CrossRef] [PubMed]
- Jyoti; Dey, P. Mechanisms and implications of the gut microbial modulation of intestinal metabolic processes. npj Metab. Health Dis. 2025, 3, 24. [Google Scholar] [CrossRef]
- Shahini, A.; Shahini, A. Role of interleukin-6-mediated inflammation in the pathogenesis of inflammatory bowel disease: Focus on the available therapeutic approaches and gut microbiome. J. Cell Commun. Signal. 2023, 17, 55–74. [Google Scholar] [CrossRef]
- Sheng, W.; Ji, G.; Zhang, L. Immunomodulatory effects of inulin and its intestinal metabolites. Front. Immunol. 2023, 14, 1224092. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.M.; Shekhar, A.C. Lipopolysaccharide, arbiter of the gut–liver axis, modulates hepatic cell pathophysiology in alcoholism. Anat. Rec. 2025, 308, 975–1004. [Google Scholar] [CrossRef]
- Al-Habsi, N.; Al-Khalili, M.; Haque, S.A.; Elias, M.; Olqi, N.A.; Al Uraimi, T. Health benefits of prebiotics, probiotics, synbiotics, and postbiotics. Nutrients 2024, 16, 3955. [Google Scholar] [CrossRef]
- Mafe, A.N.; Büsselberg, D. Microbiome integrity enhances the efficacy and safety of anticancer drug. Biomedicines 2025, 13, 422. [Google Scholar] [CrossRef]
- Cheemala, S.C.; Syed, S.; Bibi, R.; Suhail, M.B.; Dhakecha, M.D.; Subhan, M.; Mahmood, M.S.; Shabbir, A.; Islam, H.; Islam, R. Unraveling the gut microbiota: Key insights into its role in gastrointestinal and cardiovascular health. J. Adv. Med. Med. Res. 2024, 36, 34–47. [Google Scholar] [CrossRef]
- Leng, X.; Wei, X.; Wang, J.; Yao, X.; Zhang, M.; Sun, D.; Liang, J.; Chi, L.; Cheng, Y. Impacts of intestinal microbiota metabolite trimethylamine N-oxide on cardiovascular disease: A bibliometric analysis. Front. Microbiol. 2025, 15, 1491731. [Google Scholar] [CrossRef]
- Muttiah, B.; Hanafiah, A. Gut Microbiota and Cardiovascular Diseases: Unraveling the Role of Dysbiosis and Microbial Metabolites. Int. J. Mol. Sci. 2025, 26, 4264. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Lev-Ari, S. Targeting the Gut Microbiome to Improve Immunotherapy Outcomes: A Review. Integr. Cancer Ther. 2024, 23, 15347354241269870. [Google Scholar] [CrossRef] [PubMed]
- Mehta, I.; Juneja, K.; Nimmakayala, T.; Bansal, L.; Pulekar, S.; Duggineni, D.; Ghori, H.K.; Modi, N.; Younas, S. Gut Microbiota and Mental Health: A Comprehensive Review of Gut-Brain Interactions in Mood Disorders. Cureus 2025, 17, e81447. [Google Scholar] [CrossRef]
- Jagodic, A.; Krsek, A.; Schleicher, L.M.S.; Baticic, L. Microbiome Dysbiosis as a Driver of Neurodegeneration: Insights into Alzheimer’s and Parkinson’s Diseases. Gastrointest. Disord. 2025, 7, 28. [Google Scholar] [CrossRef]
- Yoshida, M.; Funasaka, Y.; Saeki, H.; Yamamoto, M.; Kanda, N. Dietary fiber inulin improves murine imiquimod-induced psoriasis-like dermatitis. Int. J. Mol. Sci. 2023, 24, 14197. [Google Scholar] [CrossRef]
- Fallahi, N.; Rafiee, M.; Hosseini, S.S.; Sereshki, N.; Anani Sarab, G.; Erfanian, N. Short-Chain Fatty Acids and Their Role in Modulating Autoimmune Responses in Psoriasis: Insights from Recent Microbiota Research. Lett. Appl. Microbiol. 2025, 78, ovaf091. [Google Scholar] [CrossRef] [PubMed]
- Larnder, A.H.; Manges, A.R.; Murphy, R.A. The estrobolome: Estrogen-metabolizing pathways of the gut microbiome and their relation to breast cancer. Int. J. Cancer 2025, 157, 599–613. [Google Scholar] [CrossRef]
- Babu, A.; Devi Rajeswari, V.; Ganesh, V.; Das, S.; Dhanasekaran, S.; Usha Rani, G.; Ramanathan, G. Gut microbiome and polycystic ovary syndrome: Interplay of associated microbial-metabolite pathways and therapeutic strategies. Reprod. Sci. 2024, 31, 1508–1520. [Google Scholar] [CrossRef]
- O’Sullivan, S.; Qi, L.; Zalloua, P. From omics to AI—Mapping the pathogenic pathways in type 2 diabetes. FEBS Lett. 2025, 599, 3244–3280. [Google Scholar] [CrossRef]
- Northen, T.R.; Kleiner, M.; Torres, M.; Kovács, Á.T.; Nicolaisen, M.H.; Krzyżanowska, D.M.; Sharma, S.; Lund, G.; Jelsbak, L.; Baars, O.; et al. Community standards and future opportunities for synthetic communities in plant–microbiota research. Nat. Microbiol. 2024, 9, 2774–2784. [Google Scholar] [CrossRef]
- Shukla, V.; Singh, S.; Verma, S.; Verma, S.; Rizvi, A.A.; Abbas, M. Targeting the microbiome to improve human health with the approach of personalized medicine: Latest aspects and current updates. Clin. Nutr. ESPEN 2024, 63, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Ayesiga, I.; Iqbal, S.; Kyejjusa, Y.; Okoboi, J.; Omara, T.; Adelina, T.; Deogratias, D.; Anyole, A.A.; Kagimu, B.B.; Odongo, D.; et al. Key regulatory aspects of prebiotics, probiotics, and synbiotics in the management of metabolic disorders. In Synbiotics in Metabolic Disorders; CRC Press: Boca Raton, FL, USA, 2024; pp. 214–244. [Google Scholar]
- Ejtahed, H.S.; Parsa, M.; Larijani, B. Ethical challenges in conducting and the clinical application of human microbiome research. J. Med. Ethics Hist. Med. 2023, 16, 5. [Google Scholar] [CrossRef]
- Rodriguez, J.; Cordaillat-Simmons, M.; Badalato, N.; Berger, B.; Breton, H.; de Lahondès, R.; Deschasaux-Tanguy, M.; Desvignes, C.; D’humières, C.; Kampshoff, S.; et al. Microbiome testing in Europe: Navigating analytical, ethical and regulatory challenges. Microbiome 2024, 12, 258. [Google Scholar] [CrossRef]
- Dongre, D.S.; Saha, U.B.; Saroj, S.D. Exploring the role of gut microbiota in antibiotic resistance and prevention. Ann. Med. 2025, 57, 2478317. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.C.; Chia, N.; Nelson, H.; Segal, E.; Elinav, E. Microbiome at the frontier of personalized medicine. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2017; Volume 92, pp. 1855–1864. [Google Scholar]
- Wilson, B.C.; Zuppi, M.; Derraik, J.G.; Albert, B.B.; Tweedie-Cullen, R.Y.; Leong, K.S.; Beck, K.L.; Vatanen, T.; O’Sullivan, J.M.; Cutfield, W.S. Long-term health outcomes in adolescents with obesity treated with faecal microbiota transplantation: 4-year follow-up. Nat. Commun. 2025, 16, 7786. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).