Fluorination to Convert the Surface of Lignocellulosic Materials from Hydrophilic to Hydrophobic
Abstract
1. Introduction
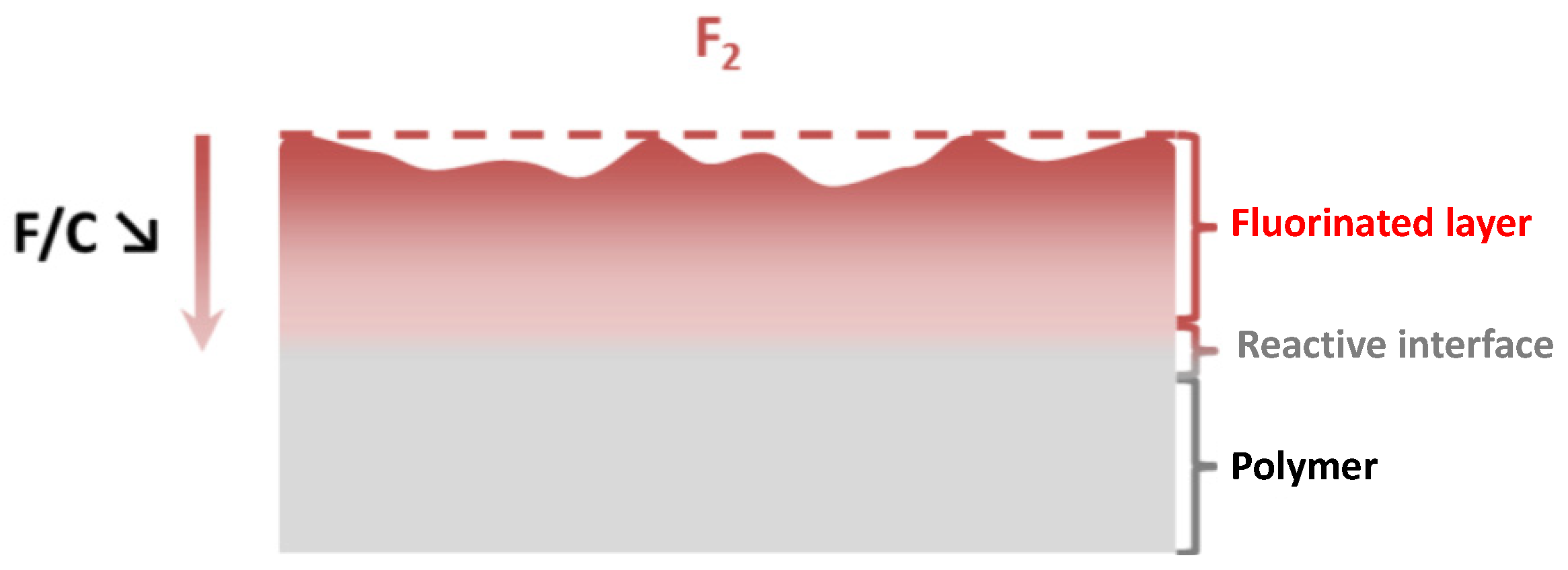
2. General Trends for Gas/Solid and Plasma Fluorination
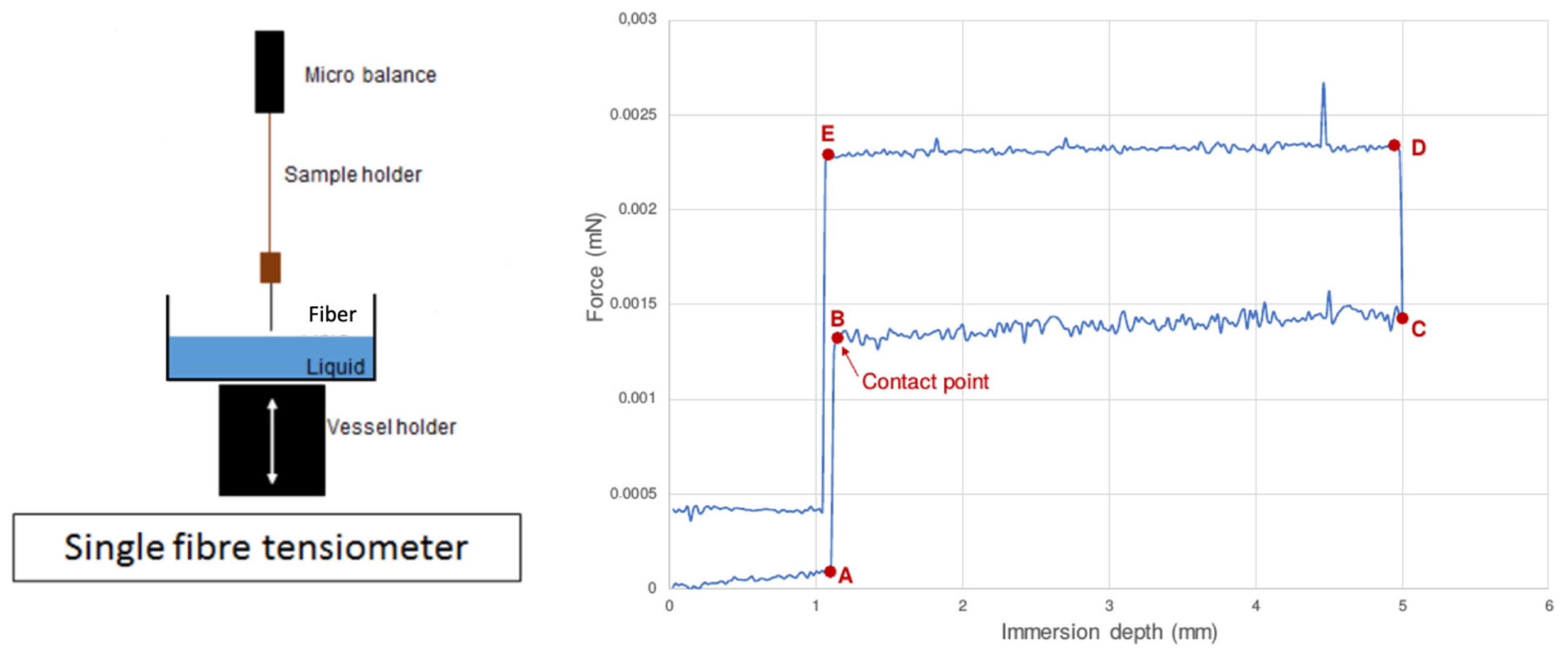
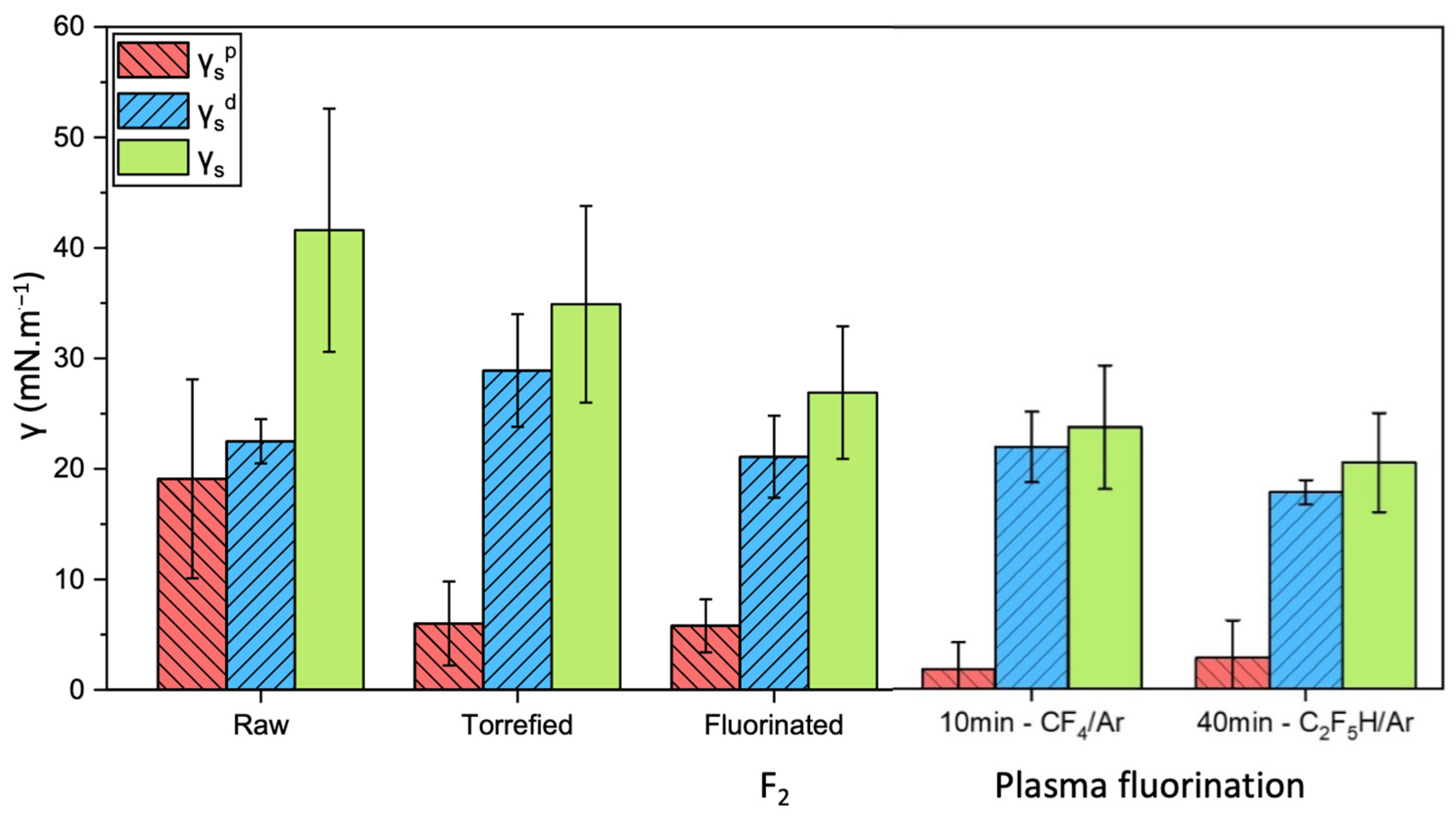
3. Surface Energy Investigation Techniques
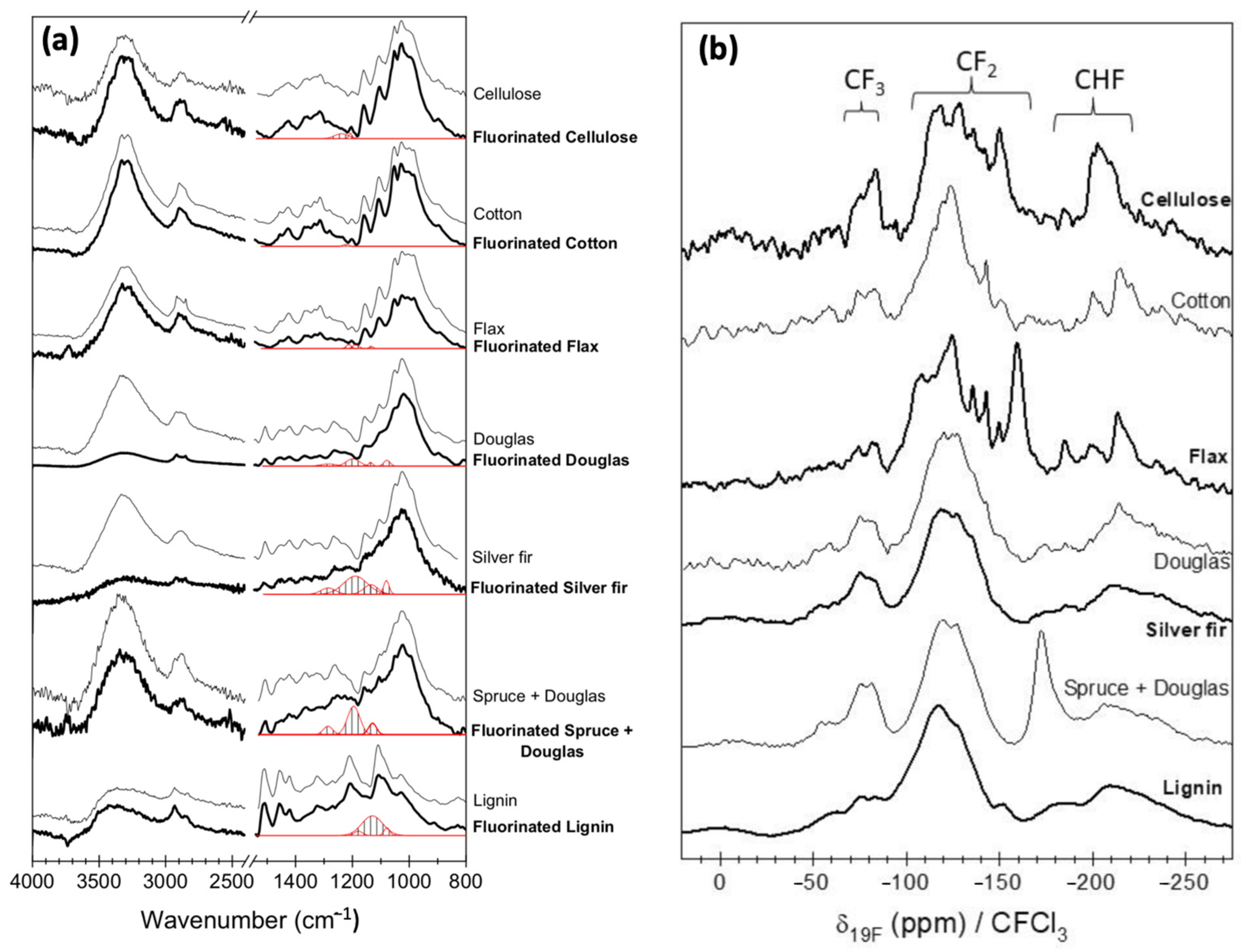
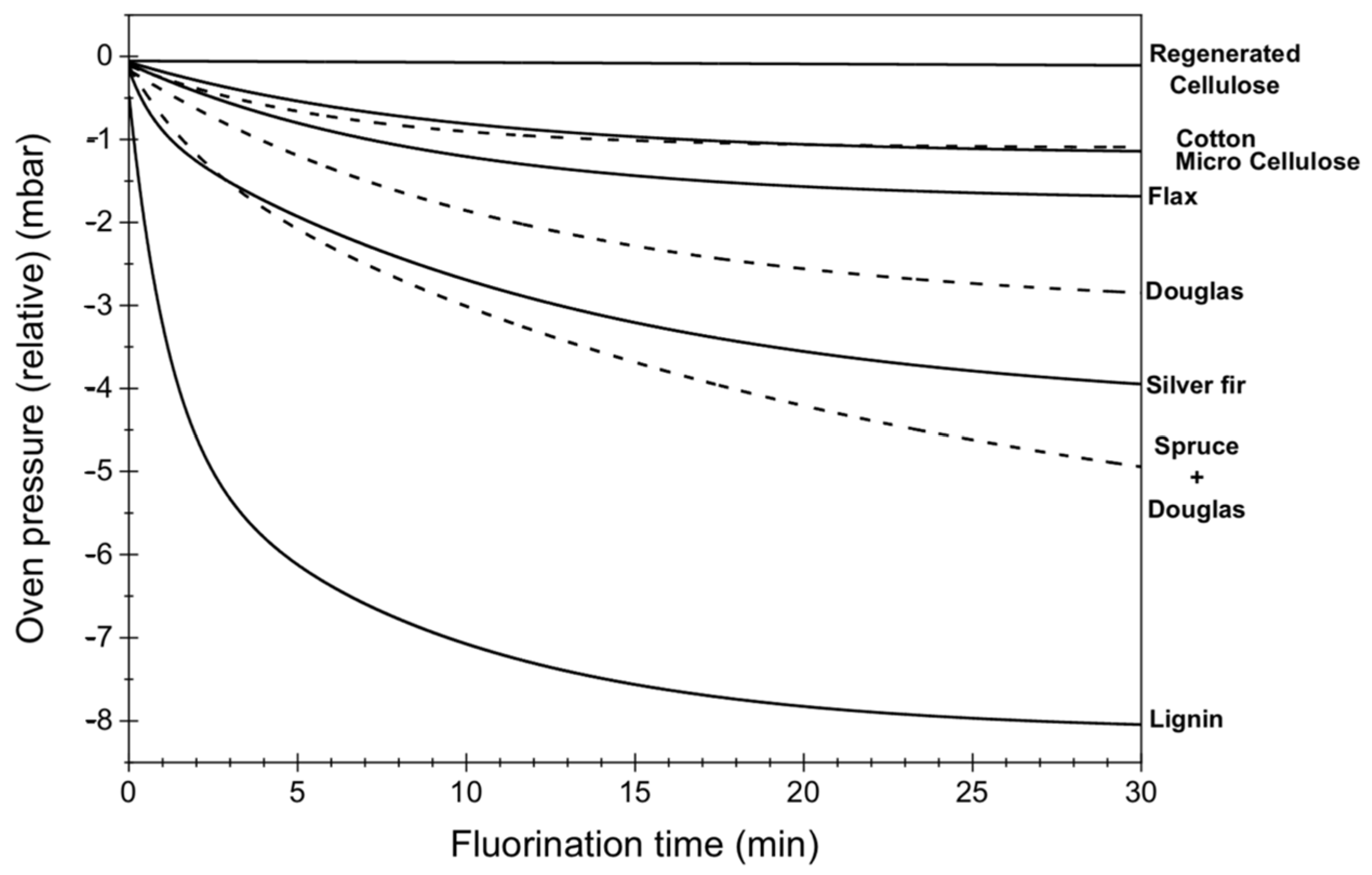
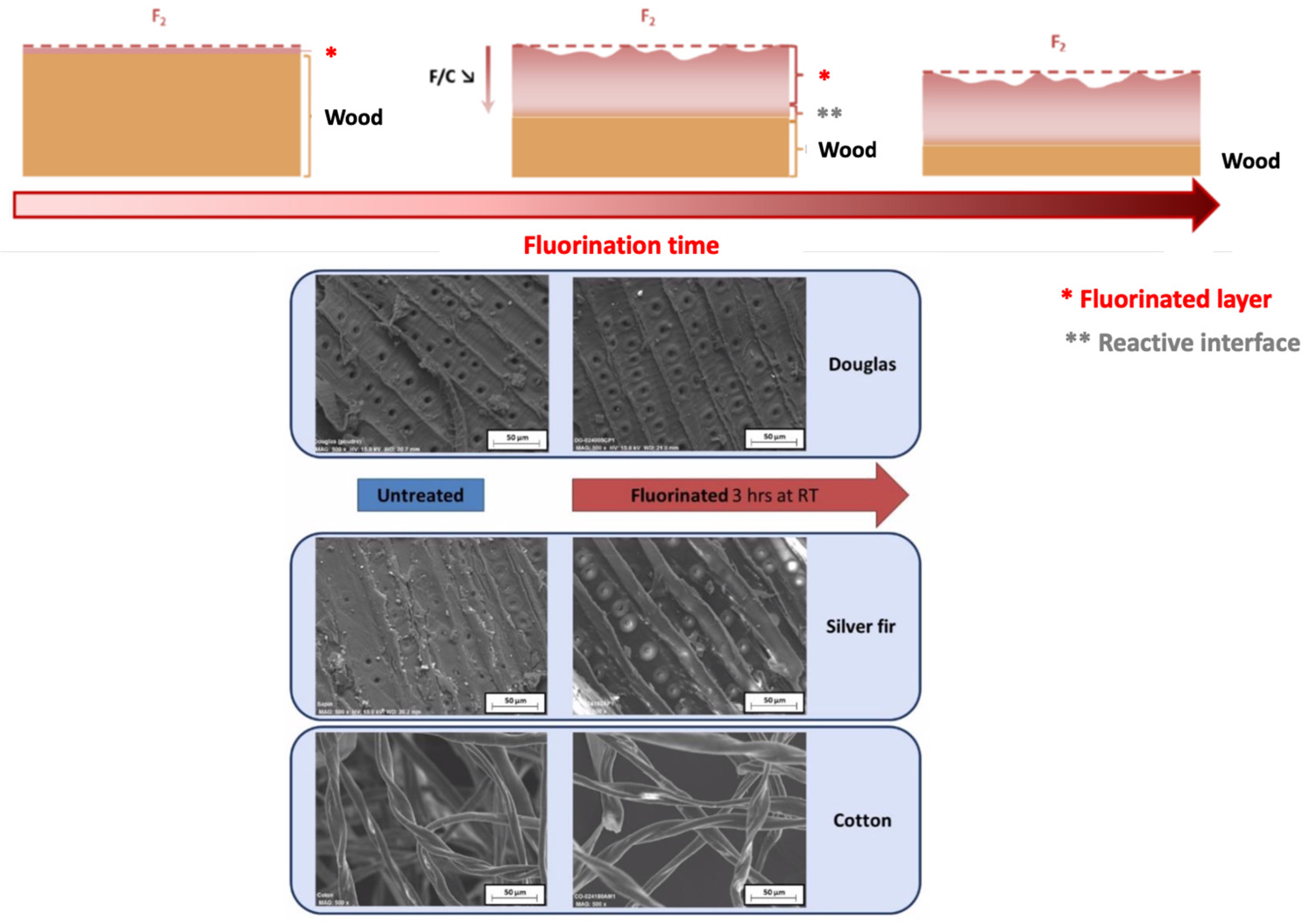
4. Fluorination of Wood; Lignin as a Key Component
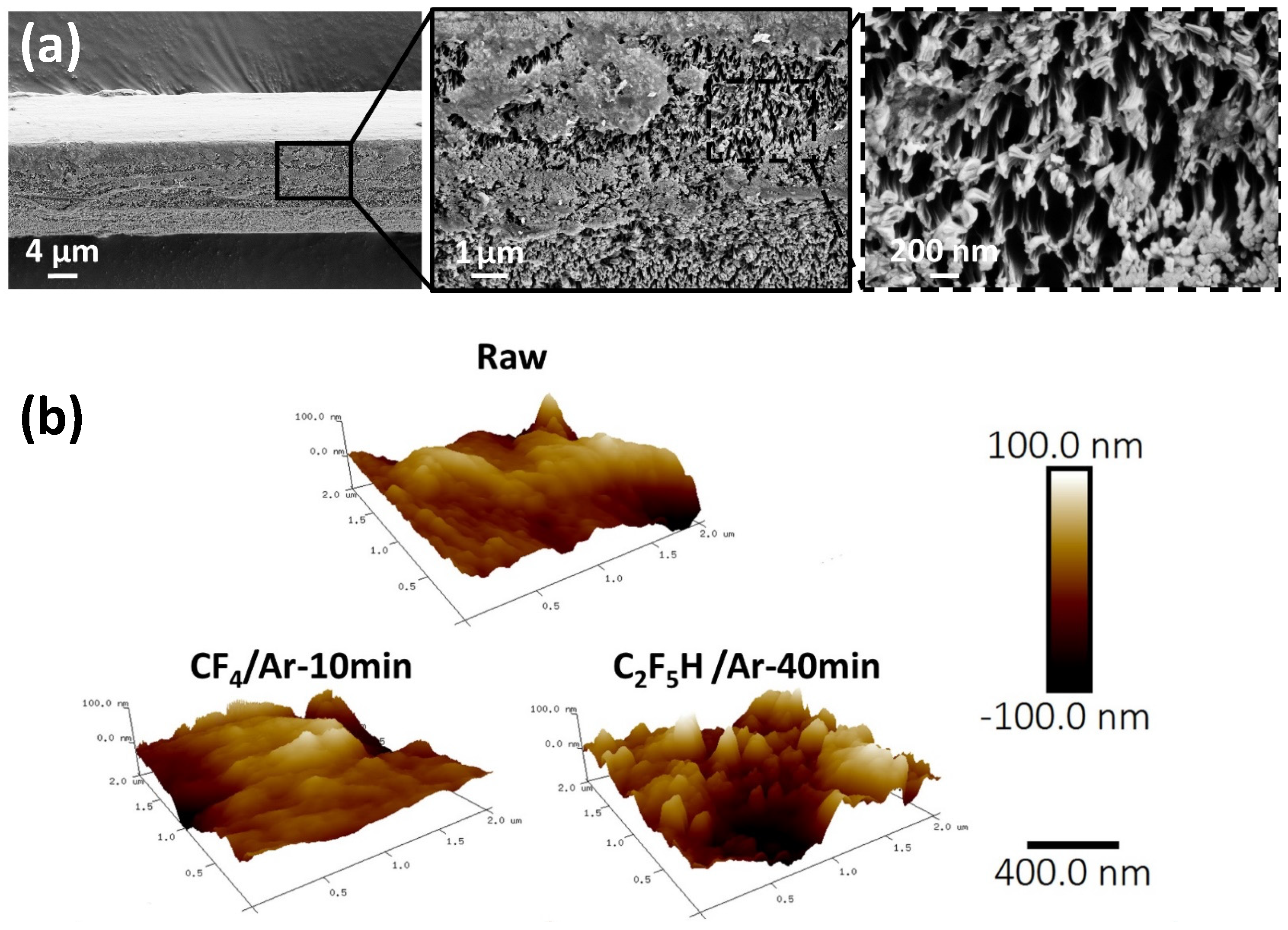
5. Fluorination of Natural Fibers, a Representative Example of Flax
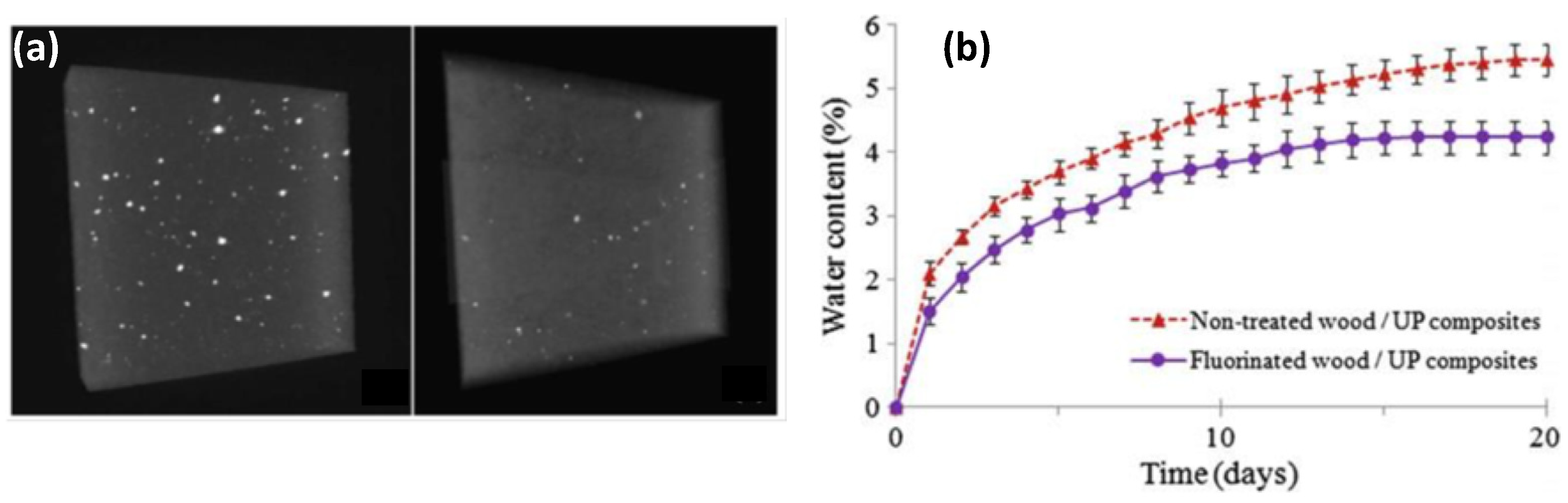
6. Applications
7. Scalability, Durability of the Fluorination Treatment and Possible Release of Fluorinated Species in the Environment
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Charlet, K. Contribution à l’étude de Composites Unidirectionnels renforcés par des fi-bres de lin: Relation entre la Microstructure de la fibre et ses propriétés mécaniques. Ph.D. Thesis, Université de Caen, Caen, France, 2006. [Google Scholar]
- Al-Oqla, F.M.; Salit, M.S. Natural Fiber Composites. In Materials Selection for Natural Fiber Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–48. [Google Scholar]
- Dhaliwal, J.S. Natural Fibers: Applications. In Generation, Development and Modifications of Natural Fibers; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Holbery, J.; Houston, D. Natural-fiber-reinforced polymer composites in automotive applications. JOM 2006, 58, 80–86. [Google Scholar] [CrossRef]
- Wambua, P.; Ivens, J.; Verpoest, I. Natural fibres: Can they replace glass in fibre reinforced plastics? Compos. Sci. Technol. 2003, 63, 1259–1264. [Google Scholar] [CrossRef]
- Moudood, A.; Rahman, A.; Öchsner, A.; Islam, M.; Francucci, G. Flax fiber and its composites: An overview of water and moisture absorption impact on their performance. J. Reinf. Plast. Compos. 2019, 38, 323–339. [Google Scholar] [CrossRef]
- Wu, C.-M.; Lai, W.-Y.; Wang, C.-Y. Effects of Surface Modification on the Mechanical Properties of Flax/β-Polypropylene Composites. Materials 2016, 9, 314. [Google Scholar] [CrossRef]
- Asim, M.; Abdan, K.; Jawaid, M.; Nasir, M.; Dashtizadeh, Z.; Ishak, M.R.; Hoque, M.E. A Review on Pineapple Leaves Fibre and Its Composites. Int. J. Polym. Sci. 2015, 2015, 950567. [Google Scholar] [CrossRef]
- Abbas, M.; Jeon, H.-Y. (Eds.) Generation, Development and Modifications of Natural Fibers; IntechOpen: London, UK, 2019. [Google Scholar]
- Yan, L.; Chouw, N.; Jayaraman, K. Flax fibre and its composites—A review. Compos. Part B Eng. 2014, 56, 296–317. [Google Scholar] [CrossRef]
- Charlet, K.; Baley, C.; Morvan, C.; Jernot, J.P.; Gomina, M.; Bréard, J. Characteristics of Hermès flax fibres as a function of their location in the stem and properties of the derived unidirectional composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1912–1921. [Google Scholar] [CrossRef]
- Charlet, K.; Jernot, J.-P.; Gomina, M.; Bizet, L.; Bréard, J. Mechanical Properties of Flax Fibers and of the Derived Unidirectional Composites. J. Compos. Mater. 2010, 44, 2887–2896. [Google Scholar] [CrossRef]
- Baley, C.; Bourmaud, A. Average tensile properties of French elementary flax fibers. Mater. Lett. 2014, 122, 159–161. [Google Scholar] [CrossRef]
- Pucci, M.F.; Liotier, P.-J.; Seveno, D.; Fuentes, C.; Van Vuure, A.; Drapier, S. Wetting and swelling property modifications of elementary flax fibres and their effects on the Liquid Composite Molding process. Compos. Part A Appl. Sci. Manuf. 2017, 97, 31–40. [Google Scholar] [CrossRef]
- Dhakal, H.N.; Zhang, Z.Y.; Richardson, M.O.W. Effect of water absorption on the mechanical properties of hemp fibre reinforced unsaturated polyester composites. Compos. Sci. Technol. 2007, 67, 1674–1683. [Google Scholar] [CrossRef]
- Chotirat, L.; Chaochanchaikul, K.; Sombatsompop, N. On adhesion mechanisms and interfacial strength in acrylonitrile–butadiene–styrene/wood sawdust composites. Int. J. Adhes. Adhes. 2007, 27, 669–678. [Google Scholar] [CrossRef]
- Kazayawoko, M.; Balatinecz, J.J.; Matuana, L.M. Surface modification and adhesion mechanisms in woodfiber-polypropylene composites. J. Mater. Sci. 1999, 34, 6189–6199. [Google Scholar] [CrossRef]
- Klason, C.; Kubát, J.; Strömvall, H.E. The Efficiency of Cellulosic Fillers in Common Thermoplastics. Part 1. Filling without Processing Aids or Coupling Agents. Int. J. Polym. Mater. Polym. Biomater. 1984, 10, 159–187. [Google Scholar] [CrossRef]
- Célino, A.; Freour, S.; Jacquemin, F.; Casari, P. The hygroscopic behavior of plant fibers: A review. Front. Chem. 2014, 1, 43. [Google Scholar] [CrossRef]
- Zhao, X. Biomass-Based Formaldehyde-Free Bio-Resin for Wood Panel Process. In Handbook of Composites from Renewable Materials; Scrivener Publishing LLC: Beverly, MA, USA, 2017; pp. 129–149. [Google Scholar]
- Ratna, D. Handbook of Thermoset Resins; iSmithers: Shawbury, UK, 2009. [Google Scholar]
- Mark, J.E. Polymer Data Handbook, 2nd ed.; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Park, S.-J. Recent Uses of Carbon Fibers. In Carbon Fibers; Park, S.-J., Ed.; Springer: Singapore, 2018; pp. 241–277. [Google Scholar]
- Kesarwani, S. Polymer Composites in Aviation Sector. Int. J. Eng. Res. Technol. 2017, 6. [Google Scholar] [CrossRef]
- Skita, A.; Keil, F. Basenbildung aus Carbonylverbindungen. Ber. Dtsch. Chem. Ges. 1928, 61, 1452–1459. [Google Scholar] [CrossRef]
- Wang, C.; Piao, C. From hydrophilicity to hydrophobicity: A critical review-part II: Hydrophobic conversion. Wood Fiber Sci. 2011, 43, 41–56. [Google Scholar]
- Paul, A.; Joseph, K.; Thomas, S. Effect of surface treatments on the electrical properties of low-density polyethylene composites reinforced with short sisal fibers. Compos. Sci. Technol. 1997, 57, 67–79. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Rozman, H.D.; Ahmad, M.N.; Ismail, H. Acetylated plant-fiber-reinforced polyester composites: A study of mechanical, hygrothermal, and aging characteristics. Polym.-Plast. Technol. Eng. 2000, 39, 757–781. [Google Scholar] [CrossRef]
- Rowell, R.M.; Dickerson, J.P. Acetylation of Wood. In Deterioration and Protection of Sustainable Biomaterials; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2014; Volume 1158, pp. 301–327. [Google Scholar]
- Li, X.; Tabil, L.G.; Panigrahi, S. Chemical Treatments of Natural Fiber for Use in Natural Fiber-Reinforced Composites: A Review. J. Polym. Environ. 2007, 15, 25–33. [Google Scholar] [CrossRef]
- Peças, P.; Carvalho, H.; Salman, H.; Leite, M. Natural Fibre Composites and Their Applications: A Review. J. Compos. Sci. 2018, 2, 66. [Google Scholar] [CrossRef]
- Fridman, A. Plasma Chemistry; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Ali, A.; Shaker, K.; Nawab, Y.; Jabbar, M.; Hussain, T.; Militky, J.; Baheti, V. Hydrophobic treatment of natural fibers and their composites—A review. J. Ind. Text. 2018, 47, 2153–2183. [Google Scholar] [CrossRef]
- Lee, S.G.; Choi, S.-S.; Park, W.H.; Cho, D. Characterization of surface modified flax fibers and their biocomposites with PHB. Macromol. Symp. 2003, 197, 089–100. [Google Scholar] [CrossRef]
- Martin, A.R.; Manolache, S.; Denes, F.S.; Mattoso, L.H.C. Functionalization of sisal fibers and high-density polyethylene by cold plasma treatment. J. Appl. Polym. Sci. 2002, 85, 2145–2154. [Google Scholar] [CrossRef]
- Wakida, T. Surface modification of fibre and polymeric materials by discharge treatment and its application to textile processing. Indian J. Fibre Text. Res. 1996, 21, 69–78. [Google Scholar]
- Wong, K.K.; Tao, X.M.; Yuen, C.W.M.; Yeung, K.W. Topographical Study of Low Temperature Plasma Treated Flax Fibers. Text. Res. J. 2000, 70, 886–893. [Google Scholar] [CrossRef]
- Sun, D. Surface Modification of Natural Fibers Using Plasma Treatment. In Biodegradable Green Composites; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 18–39. [Google Scholar]
- Sinha, E.; Panigrahi, S. Effect of Plasma Treatment on Structure, Wettability of Jute Fiber and Flexural Strength of its Composite. J. Compos. Mater. 2009, 43, 1791–1802. [Google Scholar] [CrossRef]
- Sun, D.; Stylios, G. Investigating the Plasma Modification of Natural Fiber Fabrics-The Effect on Fabric Surface and Mechanical Properties. Text. Res. J. 2005, 75, 639–644. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Sokhansanj, S.; Hess, J.; Wright, C.; Boardman, R. A Review on Biomass Torrefaction Process and Product Properties for Energy Applications. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar] [CrossRef]
- Bergman, P.; Boersma, A.; Kiel, J.; Prins, M.; Ptasinski, K.; Janssen, F. Torrefaction for Entrained Flow Gasification of Biomass. ECN-C-05-067; Energy Research Centre of the Netherlands (ECN): Petten, The Netherlands, 2005; 51p.
- Shoulaifar, T. Chemical Changes in Biomass During Torrefaction. Ph.D. Thesis, Åbo Akademi University, Turku, Finland, 2016. [Google Scholar]
- Mburu, F.; Dumarçay, S.; Bocquet, J.F.; Petrissans, M.; Gérardin, P. Effect of chemical modifications caused by heat treatment on mechanical properties of Grevillea robusta wood. Polym. Degrad. Stab. 2008, 93, 401–405. [Google Scholar] [CrossRef]
- Kabir, M.M.; Wang, H.; Lau, K.T.; Cardona, F. Chemical treatments on plant-based natural fibre reinforced polymer composites: An overview. Compos. Part B Eng. 2012, 43, 2883–2892. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, M.; Chen, L. Interface and bonding mechanisms of plant fibre composites: An overview. Compos. Part B Eng. 2016, 101, 31–45. [Google Scholar] [CrossRef]
- Ichazo, M.N.; Albano, C.; González, J.; Perera, R.; Candal, M.V. Polypropylene/wood flour composites: Treatments and properties. Compos. Struct. 2001, 54, 207–214. [Google Scholar] [CrossRef]
- Hassan, A.; Abd Rahman, N.M.M.; Yahya, R. Extrusion and injection-molding of glass fiber/MAPP/polypropylene: Effect of coupling agent on DSC, DMA, and mechanical properties. J. Reinf. Plast. Compos. 2011, 30, 1223–1232. [Google Scholar] [CrossRef]
- Mohebby, B.; Fallah-Moghadam, P.; Ghotbifar, A.R.; Kazemi-Najafi, S. Influence of Maleic-Anhydride-Polypropylene (MAPP) on Wettability of Polypropylene/Wood Flour/Glass Fiber Hybrid Composites. J. Agric. Sci. Technol. 2011, 13, 877–884. [Google Scholar]
- Dai, D.; Fan, M. Wood fibres as reinforcements in natural fibre composites: Structure, properties, processing and applications. In Natural Fibre Composites; Hodzic, A., Shanks, R., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 3–65. [Google Scholar]
- Wang, Q.; Xiao, S.; Shi, S.Q.; Xu, S.; Cai, L. Self-bonded natural fiber product with high hydrophobic and EMI shielding performance via magnetron sputtering Cu film. Appl. Surf. Sci. 2019, 475, 947–952. [Google Scholar] [CrossRef]
- Thakur, K.; Kalia, S.; Pathania, D.; Kumar, A.; Sharma, N.; Schauer, C.L. Surface functionalization of lignin constituent of coconut fibers via laccase-catalyzed biografting for development of antibacterial and hydrophobic properties. J. Clean. Prod. 2016, 113, 176–182. [Google Scholar] [CrossRef]
- Dong, A.; Fan, X.; Wang, Q.; Yu, Y.; Cavaco-Paulo, A. Hydrophobic surface functionalization of lignocellulosic jute fabrics by enzymatic grafting of octadecylamine. Int. J. Biol. Macromol. 2015, 79, 353–362. [Google Scholar] [CrossRef]
- Dong, A.; Yu, Y.; Yuan, J.; Wang, Q.; Fan, X. Hydrophobic modification of jute fiber used for composite reinforcement via laccase-mediated grafting. Appl. Surf. Sci. 2014, 301, 418–427. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Nicoletti, F.; Vitale, G.; Prestipino, M.; Valenza, A. A new eco-friendly chemical treatment of natural fibres: Effect of sodium bicarbonate on properties of sisal fibre and its epoxy composites. Compos. Part B Eng. 2016, 85, 150–160. [Google Scholar] [CrossRef]
- Gulati, D.; Sain, M. Fungal-modification of Natural Fibers: A Novel Method of Treating Natural Fibers for Composite Reinforcement. J. Polym. Environ. 2006, 14, 347–352. [Google Scholar] [CrossRef]
- Huang, X.; Wang, A.; Xu, X.; Liu, H.; Shang, S. Enhancement of Hydrophobic Properties of Cellulose Fibers via Grafting with Polymeric Epoxidized Soybean Oil. ACS Sustain. Chem. Eng. 2017, 5, 1619–1627. [Google Scholar] [CrossRef]
- Kick, T.; Grethe, T.; Mahltig, B. A Natural Based Method for Hydrophobic Treatment of Natural Fiber Material. Acta Chim. Slov. 2017, 64, 373–380. [Google Scholar] [CrossRef]
- Lee, K.; Jur, J.; Kim, D.H.; Parsons, G. Mechanisms for hydrophilic/hydrophobic wetting transitions on cellulose cotton fibers coated using Al2O3 atomic layer deposition. J. Vac. Sci. Technol. A 2012, 30, 01A163. [Google Scholar] [CrossRef]
- DeCorpo, J.J.; Steiger, R.P.; Franklin, J.L.; Margrave, J.L. Dissociation Energy of F2. J. Chem. Phys. 1970, 53, 936–938. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 86th ed.; Taylor & Francis: Abingdon, UK, 2005. [Google Scholar]
- Brauns, F.E.; Brauns, D.A. The Chemistry of Lignin: Covering the Literature for the Years 1949–1958; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Sapieha, S.; Verreault, M.; Klemberg-Sapieha, J.E.; Sacher, E.; Wertheimer, M.R. X-Ray photoelectron study of the plasma fluorination of lignocellulose. Appl. Surf. Sci. 1990, 44, 165–169. [Google Scholar] [CrossRef]
- Sahin, H.T.; Manolache, S.; Young, R.A.; Denes, F. Surface fluorination of paper in CF4-RF plasma environments. Cellulose 2002, 9, 171–181. [Google Scholar] [CrossRef]
- Yamaguchi, F.; Sakamoto, E. Process for Fluorinating Cellulosic Materials and Fluorinated Cellulosic Materials. European Patent No. EP0890579A1, 10 February 1999. [Google Scholar]
- Otsuka, A.J.; Lagow, R.J. The direct fluorination of hydrocarbon polymers. J. Fluor. Chem. 1974, 4, 371–380. [Google Scholar] [CrossRef]
- Lagow, R.J.; Margrave, J.L. Direct Fluorination: A “New” Approach to Fluorine Chemistry. In Progress in Inorganic Chemistry; Wiley: Hoboken, NJ, USA, 1979; pp. 161–210. [Google Scholar]
- Lippard, S.J. Progress in Inorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 1992. [Google Scholar]
- Kharitonov, A.P. Practical applications of the direct fluorination of polymers. J. Fluor. Chem. 2000, 103, 123–127. [Google Scholar] [CrossRef]
- Tressaud, A.; Durand, E.; Labrugère, C.; Kharitonov, A.P.; Kharitonova, L.N. Modification of surface properties of carbon-based and polymeric materials through fluorination routes: From fundamental research to industrial applications. J. Fluor. Chem. 2007, 128, 378–391. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Taege, R.; Ferrier, G.; Teplyakov, V.V.; Syrtsova, D.A.; Koops, G.H. Direct fluorination—Useful tool to enhance commercial properties of polymer articles. J. Fluor. Chem. 2005, 126, 251–263. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Kharitonova, L.N. Surface modification of polymers by direct fluorination: A convenient approach to improve commercial properties of polymeric articles. Pure Appl. Chem. 2009, 81, 451–471. [Google Scholar] [CrossRef]
- Cardinaud, C. Fluorine-based plasmas: Main features and application in micro-and nanotechnology and in surface treatment. C. R. Chim. 2018, 21, 723–739. [Google Scholar] [CrossRef]
- Han, Y.; Manolach, S.O.; Denes, F.; Rowell, R.M. Cold plasma treatment on starch foam reinforced with wood fiber for its surface hydrophobicity. Carbohydr. Polym. 2011, 86, 1031–1037. [Google Scholar] [CrossRef]
- Shul, R.; Pearton, S. Handbook of Advanced Plasma Processing Techniques; Springer Science & Business Media: New York, NY, USA, 2000. [Google Scholar]
- Vallan, A.; Carullo, A.; Casalicchio, M.L.; Penna, A.; Perrone, G.; Vietro, N.D.; Milella, A.; Fracassi, F. A plasma modified fiber sensor for breath rate monitoring. In Proceedings of the 2014 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Lisbon, Portugal, 11–12 June 2014; pp. 1–5. [Google Scholar]
- Vourdas, N.; Tserepi, A.; Gogolides, E. Nanotextured super-hydrophobic transparent poly(methyl methacrylate) surfaces using high-density plasma processing. Nanotechnology 2007, 18, 125304. [Google Scholar] [CrossRef]
- Sparavigna, A.C. Plasma treatment advantages for textiles. arXiv 2008, arXiv:0801.3727. [Google Scholar] [CrossRef]
- Xie, L.; Tang, Z.; Jiang, L.; Breedveld, V.; Hess, D.W. Creation of superhydrophobic wood surfaces by plasma etching and thin-film deposition. Surf. Coat. Technol. 2015, 281, 125–132. [Google Scholar] [CrossRef]
- Parveen, S.; Rana, S.; Goswami, P. Developing Super-Hydrophobic and Abrasion-Resistant Wool Fabrics Using Low-Pressure Hexafluoroethane Plasma Treatment. Materials 2021, 14, 3228. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Stylios, G.K. Fabric surface properties affected by low temperature plasma treatment. J. Mater. Process. Technol. 2006, 173, 172–177. [Google Scholar] [CrossRef]
- Navarro, F.; Dávalos, F.; Denes, F.; Cruz, L.E.; Young, R.A.; Ramos, J. Highly hydrophobic sisal chemithermomechanical pulp (CTMP) paper by fluorotrimethylsilane plasma treatment. Cellulose 2003, 10, 411–424. [Google Scholar] [CrossRef]
- Thomas, Y. An essay on the cohesion of fluids. Proc. R. Soc. Lond. 1832, 95, 171–172. [Google Scholar]
- Schellbach, S.L.; Monteiro, S.N.; Drelich, J.W. A novel method for contact angle measurements on natural fibers. Mater. Lett. 2016, 164, 599–604. [Google Scholar] [CrossRef]
- Hodzic, A.; Stachurski, Z.H. Droplet on a fibre: Surface tension and geometry. Compos. Interfaces 2001, 8, 415–425. [Google Scholar] [CrossRef]
- van Hazendonk, J.M.; van der Putten, J.C.; Keurentjes, J.T.F.; Prins, A. A simple experimental method for the measurement of the surface tension of cellulosic fibres and its relation with chemical composition. Colloids Surf. A 1993, 81, 251–261. [Google Scholar] [CrossRef]
- Garat, W.; Pucci, M.F.; Leger, R.; Govignon, Q.; Berthet, F.; Perrin, D.; Ienny, P.; Liotier, P.-J. Surface energy determination of fibres for Liquid Composite Moulding processes: Method to estimate equilibrium contact angles from static and quasi-static data. Colloids Surf. A 2021, 611, 125787. [Google Scholar] [CrossRef]
- Pucci, M.F.; Liotier, P.-J.; Drapier, S. Tensiometric method to reliably assess wetting properties of single fibers with resins: Validation on cellulosic reinforcements for composites. Colloids Surf. A 2017, 512, 26–33. [Google Scholar] [CrossRef]
- Téraube, O.; Agopian, J.-C.; Pucci, M.F.; Liotier, P.-J.; Hajjar-Garreau, S.; Batisse, N.; Charlet, K.; Dubois, M. Fluorination of flax fibers for improving the interfacial compatibility of eco-composites. Sustain. Mater. Technol. 2022, 33, e00467. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Pouzet, M.; Dubois, M.; Charlet, K.; Béakou, A. The effect of lignin on the reactivity of natural fibres towards molecular fluorine. Mater. Des. 2017, 120, 66–74. [Google Scholar] [CrossRef]
- Dubois, M.; Giraudet, J.; Guérin, K.; Hamwi, A.; Fawal, Z.; Pirotte, P.; Masin, F. EPR and Solid-State NMR Studies of Poly(dicarbon monofluoride) (C2F)n. J. Phys. Chem. B 2006, 110, 11800–11808. [Google Scholar] [CrossRef]
- Ahmad, Y.; Disa, E.; Guérin, K.; Dubois, M.; Petit, E.; Hamwi, A.; Thomas, P.; Mansot, J.L. Structure control at the nanoscale in fluorinated graphitized carbon blacks through the fluorination route. J. Fluor. Chem. 2014, 168, 163–172. [Google Scholar] [CrossRef]
- Ahmad, Y.; Disa, E.; Dubois, M.; Guérin, K.; Dubois, V.; Zhang, W.; Bonnet, P.; Masin, F.; Vidal, L.; Ivanov, D.A.; et al. The synthesis of multilayer graphene materials by the fluorination of carbon nanodiscs/nanocones. Carbon 2012, 50, 3897–3908. [Google Scholar] [CrossRef]
- Zhang, W.; Dubois, M.; Guérin, K.; Hamwi, A.; Giraudet, J.; Masin, F. Solid-state NMR and EPR study of fluorinated carbon nanofibers. J. Solid State Chem. 2008, 181, 1915–1924. [Google Scholar] [CrossRef]
- Teraube, O.; Agopian, J.-C.; Petit, E.; Metz, F.; Batisse, N.; Charlet, K.; Dubois, M. Surface modification of sized vegetal fibers through direct fluorination for eco-composites. J. Fluor. Chem. 2020, 238, 109618. [Google Scholar] [CrossRef]
- Agopian, J.-C.; Teraube, O.; Dubois, M.; Charlet, K. Fluorination of carbon fibre sizing without mechanical or chemical loss of the fibre. Appl. Surf. Sci. 2020, 534, 147647. [Google Scholar] [CrossRef]
- Pouzet, M.; Dubois, M.; Charlet, K.; Béakou, A.; Leban, J.M.; Baba, M. Fluorination renders the wood surface hydrophobic without any loss of physical and mechanical properties. Ind. Crops Prod. 2019, 133, 133–141. [Google Scholar] [CrossRef]
- Belov, N.A.; Blinov, I.A.; Alentiev, A.Y.; Belokhvostov, V.M.; Mukhortov, D.A.; Chirkov, S.V.; Mazur, A.S.; Kostina, Y.V.; Vozniuk, O.N.; Kurapova, E.S.; et al. Direct fluorination of acetyl and ethyl celluloses in perfluorinated liquid medium. J. Polym. Res. 2020, 27, 290. [Google Scholar] [CrossRef]
- Turku, I.; Rohumaa, A.; Tirri, T.; Pulkkinen, L. Progress in Achieving Fire-Retarding Cellulose-Derived Nano/Micromaterial-Based Thin Films/Coatings and Aerogels: A Review. Fire 2024, 7, 31. [Google Scholar] [CrossRef]
- Kharitonov, A.P. Direct Fluorination of Polymers; Nova Science Publishers: New York, NY, USA, 2008. [Google Scholar]
- Pouzet, M. Modification de l’énergie de surface du bois par fluoration. Ph.D. Thesis, Université Clermont Auvergne, Clermont-Ferrand, France, 2017. [Google Scholar]
- Boonstra, M.J.; Van Acker, J.; Tjeerdsma, B.F.; Kegel, E.V. Strength properties of thermally modified softwoods and its relation to polymeric structural wood constituents. Ann. For. Sci. 2007, 64, 679–690. [Google Scholar] [CrossRef]
- Téraube, O.; Agopian, J.-C.; Pucci, M.F.; Liotier, P.-J.; Conchon, P.; Badel, É.; Hajjar-Garreau, S.; Leleu, H.; Baylac, J.-B.; Batisse, N.; et al. Optimization of interfacial adhesion and mechanical performance of flax fiber-based eco-composites through fiber fluorination treatment. Compos. Part B Eng. 2025, 296, 112228. [Google Scholar] [CrossRef]
- Kharitonov, A.P.; Simbirtseva, G.V.; Tressaud, A.; Durand, E.; Labrugère, C.; Dubois, M. Comparison of the surface modifications of polymers induced by direct fluorination and rf-plasma using fluorinated gases. J. Fluor. Chem. 2014, 165, 49–60. [Google Scholar] [CrossRef]
- van Krevelen, D.W.; te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier Science: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Téraube, O.; Gratier, L.; Agopian, J.-C.; Pucci, M.F.; Liotier, P.-J.; Hajjar-Garreau, S.; Petit, E.; Batisse, N.; Bousquet, A.; Charlet, K.; et al. Elaboration of hydrophobic flax fibers through fluorine plasma treatment. Appl. Surf. Sci. 2023, 611, 155615. [Google Scholar] [CrossRef]
- Téraube, O.; Choupas, S.; El Feggouri, L.; Agopian, J.-C.; Charlet, K.; Dubois, M. Towards natural fibers resistant to mold and termites thanks to fluorine. Colloids Surf. A 2024, 702, 134926. [Google Scholar] [CrossRef]
- Kouicem, M.M.; Tomasella, E.; Bousquet, A.; Batisse, N.; Monier, G.; Robert-Goumet, C.; Dubost, L. An investigation of adhesion mechanisms between plasma-treated PMMA support and aluminum thin films deposited by PVD. Appl. Surf. Sci. 2021, 564, 150322. [Google Scholar] [CrossRef]
- Tursi, A.; De Vietro, N.; Beneduci, A.; Milella, A.; Chidichimo, F.; Fracassi, F.; Chidichimo, G. Low pressure plasma functionalized cellulose fiber for the remediation of petroleum hydrocarbons polluted water. J. Hazard. Mater. 2019, 373, 773–782. [Google Scholar] [CrossRef]
- Saulnier, F.; Dubois, M.; Charlet, K.; Frezet, L.; Beakou, A. Direct fluorination applied to wood flour used as a reinforcement for polymers. Carbohydr. Polym. 2013, 94, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Charlet, K.; Saulnier, F.; Gautier, D.; Pouzet, M.; Dubois, M.; Béakou, A. Fluorination as an Effective Way to Reduce Natural Fibers Hydrophilicity. In Proceedings of the Natural Fibres: Advances in Science and Technology Towards Industrial Applications, Dordrecht, The Netherlands, 27–29 April 2016; pp. 211–229. [Google Scholar]
- Garcia, R. Amélioration de la Stabilité Dimensionnelle des Panneaux de Fibre de Bois MDF par Traitements Physico-Chimiques; Université de Laval: Québec, QC, Canada, 2005. [Google Scholar]
- Nachtigall, S.M.B.; Cerveira, G.S.; Rosa, S.M.L. New polymeric-coupling agent for polypropylene/wood-flour composites. Polym. Test. 2007, 26, 619–628. [Google Scholar] [CrossRef]
- Stamatakis, G.; Knuutinen, U.; Laitinen, K.; Spyros, A. Analysis and aging of unsaturated polyester resins in contemporary art installations by NMR spectroscopy. Anal. Bioanal. Chem. 2010, 398, 3203–3214. [Google Scholar] [CrossRef]
- Odegard, G.M.; Bandyopadhyay, A. Physical aging of epoxy polymers and their composites. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1695–1716. [Google Scholar] [CrossRef]
- Pouzet, M.; Gautier, D.; Charlet, K.; Dubois, M.; Béakou, A. How to decrease the hydrophilicity of wood flour to process efficient composite materials. Appl. Surf. Sci. 2015, 353, 1234–1241. [Google Scholar] [CrossRef]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. ilastik: Interactive machine learning for (bio)image analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Berthet, M.A.; Commandré, J.M.; Rouau, X.; Gontard, N.; Angellier-Coussy, H. Torrefaction treatment of lignocellulosic fibres for improving fibre/matrix adhesion in a biocomposite. Mater. Des. 2016, 92, 223–232. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Craig, L.; Lutz, A.; Berry, K.A.; Yang, W. Recommendations for fluoride limits in drinking water based on estimated daily fluoride intake in the Upper East Region, Ghana. Sci. Total Environ. 2015, 532, 127–137. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). National Primary Drinking Water Regulations; EPA: Washington, DC, USA, 2022. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 1 November 2025).
- Suchet, N.; Batisse, N.; Dubois, M.; Guieu, B.; Sanquer, F. Surface Chemistry Stabilization by Esterification of Polypropylene Treated by Direct Fluorination. ChemPlusChem 2025, 90, e202500128. [Google Scholar] [CrossRef] [PubMed]
- Maity, J.; Jacob, C.; Das, C.K.; Singh, R.P. Direct fluorination of Twaron fiber and investigation of mechanical thermal and morphological properties of high density polyethylene and Twaron fiber composites. J. Appl. Polym. Sci. 2008, 107, 3739–3749. [Google Scholar] [CrossRef]
- Pouzet, M.; Dubois, M.; Charlet, K.; Béakou, A. From hydrophilic to hydrophobic wood using direct fluorination: A localized treatment. C. R. Chim. 2018, 21, 800–807. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Reihmane, S.; Gassan, J. Properties and modification methods for vegetable fibers for natural fiber composites. J. Appl. Polym. Sci. 1996, 59, 1329–1336. [Google Scholar] [CrossRef]
- Frollini, E.; Leao, A.L.; Mattoso, L.H.C. Natural Polymers and Agrofibers Based Composites; Embrapa Instrumentacao Agropecuaria: São Carlos, Brazil, 2000. [Google Scholar]
- Frollini, E.; Leao, A.; Mattoso, L.; Rowell, R.; Han, J.; Rowell, J. Characterization and Factors Effecting Fiber Properties. Nat. Polym. Agrofibers Compos. 2000. [Google Scholar]
- Mohammed, L.; Ansari, M.N.M.; Pua, G.; Jawaid, M.; Islam, M.S. A Review on Natural Fiber Reinforced Polymer Composite and Its Applications. Int. J. Polym. Sci. 2015, 2015, 243947. [Google Scholar] [CrossRef]
- Thapliyal, D.; Verma, S.; Sen, P.; Kumar, R.; Thakur, A.; Tiwari, A.K.; Singh, D.; Verros, G.D.; Arya, R.K. Natural Fibers Composites: Origin, Importance, Consumption Pattern, and Challenges. J. Compos. Sci. 2023, 7, 506. [Google Scholar] [CrossRef]
- Zamora-Mendoza, L.; Gushque, F.; Yanez, S.; Jara, N.; Álvarez-Barreto, J.F.; Zamora-Ledezma, C.; Dahoumane, S.A.; Alexis, F. Plant Fibers as Composite Reinforcements for Biomedical Applications. Bioengineering 2023, 10, 804. [Google Scholar] [CrossRef] [PubMed]
- Ogale, A.A.; Zhang, M.; Jin, J. Recent advances in carbon fibers derived from biobased precursors. J. Appl. Polym. Sci. 2016, 133, 43794. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, X.; Nguyen, T.T.; Wu, J.; Guo, M.; Liu, W.; Du, C. Green and Low-Cost Natural Lignocellulosic Biomass-Based Carbon Fibers—Processing, Properties, and Applications in Sports Equipment: A Review. Polymers 2022, 14, 2591. [Google Scholar] [CrossRef] [PubMed]
- Mano, N.; Camus, J.; Guedon, E.; Guilloton, M.; Lemaire, M. Modification Enzymatique de la Lignine Pour sa Solubilisation et Applications. WO Patent No. 2019/076893, 25 April 2019. [Google Scholar]



| Sample | Raw | Fluorinated |
|---|---|---|
| Cellulose | 28° | 30° |
| Cotton | Non-measurable | 20° |
| Douglas | 70° | 85° |
| Silver fir | 51° | 98° |
| Spruce + Douglas | 80° | 95° |
| Lignin | 48° | 77° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Dumontel, A.; Téraube, O.; Falcon, T.; Bousquet, A.; Tomasella, E.; Pucci, M.F.; Liotier, P.-J.; Ahmad, Y.; Charlet, K.; Dubois, M. Fluorination to Convert the Surface of Lignocellulosic Materials from Hydrophilic to Hydrophobic. Surfaces 2026, 9, 3. https://doi.org/10.3390/surfaces9010003
Dumontel A, Téraube O, Falcon T, Bousquet A, Tomasella E, Pucci MF, Liotier P-J, Ahmad Y, Charlet K, Dubois M. Fluorination to Convert the Surface of Lignocellulosic Materials from Hydrophilic to Hydrophobic. Surfaces. 2026; 9(1):3. https://doi.org/10.3390/surfaces9010003
Chicago/Turabian StyleDumontel, Alexandre, Olivier Téraube, Tomy Falcon, Angélique Bousquet, Eric Tomasella, Monica Francesca Pucci, Pierre-Jacques Liotier, Yasser Ahmad, Karine Charlet, and Marc Dubois. 2026. "Fluorination to Convert the Surface of Lignocellulosic Materials from Hydrophilic to Hydrophobic" Surfaces 9, no. 1: 3. https://doi.org/10.3390/surfaces9010003
APA StyleDumontel, A., Téraube, O., Falcon, T., Bousquet, A., Tomasella, E., Pucci, M. F., Liotier, P.-J., Ahmad, Y., Charlet, K., & Dubois, M. (2026). Fluorination to Convert the Surface of Lignocellulosic Materials from Hydrophilic to Hydrophobic. Surfaces, 9(1), 3. https://doi.org/10.3390/surfaces9010003






