Effects of Temperature on Anti-Seepage Coating During Vapor Phase Aluminizing of K4125 Ni-Based Superalloy
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Initial Microstructure of the Anti-Seepage Coating
3.2. Masking Performance of the Anti-Seepage Coating
3.3. Microstructure Evolution of the Anti-Seepage Coating
4. Discussion
4.1. Protection Mechanism of the Composite Coating
4.2. Effect of Aluminizing Temperature on Anti-Seepage Performance
5. Conclusions
- The anti-seepage coating provides effective protection through dual mechanisms that combine physical sealing induced by high-temperature densification with diffusion inhibition from the formation of the NiAl/Al2O3 composite structure.
- The anti-seepage capability is highly dependent on temperature. The coating exhibited superior protection at 1080 °C, where the thickness of the transition zone is reduced to 42 μm, compared with 158 μm at 880 °C. Elevated temperature significantly enhances particle sintering and accelerates the formation of a denser and more continuous NiAl phase, thereby establishing a more efficient and stable protective system.
- The coating also offers a practical advantage in post-aluminizing removal. The inherent mismatch of the coefficient of thermal expansion between the coating and the substrate enables the solidified coating to be mechanically removed without damaging the substrate.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abkenar, A.K.; Malekan, A.; Bozorg, M.; Nematipour, K. Hot corrosion and oxidation behavior of Pt–aluminide and Pt–Rh–aluminide coatings applied on nickle-base and cobalt-base substrates. Met. Mater.-Int. 2024, 30, 2466–2489. [Google Scholar] [CrossRef]
- Barjesteh, M.M.; Zangeneh-Madar, K.; Abbasi, S.M.; Shirvani, K. The effect of platinum-aluminide coating features on high-temperature fatigue life of nickel-based superalloy rene®80. J. Min. Metall. Sect. B-Metall. 2019, 55, 235–251. [Google Scholar] [CrossRef]
- Rezaee, B.; Rastegari, S.; Eyvazjamadi, H. Formation mechanism of Pt-modified aluminide coating structure by out-of-the-pack aluminizing. Surf. Eng. 2021, 37, 343–350. [Google Scholar] [CrossRef]
- Guo, L.; He, W.; Chen, W.; Xue, Z.; He, J.; Guo, Y.; Wu, Y.; Gao, L.; Li, D.; Zhang, Z.; et al. Progress on high-temperature protective coatings for aero-engines. Surf. Sci. Technol. 2023, 1, 6. [Google Scholar] [CrossRef]
- Mateusz, K.; Dominik, K.; Xin, Y.; Wojciech, R.; Kowalewski, Z.L.; Cezary, S. Aluminide thermal barrier coating for high temperature performance of MAR 247 nickel based superalloy. Coatings 2021, 11, 48. [Google Scholar] [CrossRef]
- Pytel, M.; Tokarski, T.; Goral; Fillip, R. Structure of Pd-Zr and Pt-Zr modified aluminide coatings deposited by a CVD method on nickel superalloys. Kov. Mater.-Met. Mater. 2019, 57, 343–354. [Google Scholar] [CrossRef]
- Firouzi, A.; Shirvani, K. Internal surface protection of gas turbine blade by Si-aluminide coating. Mater. Corros. 2011, 62, 681–686. [Google Scholar] [CrossRef]
- Xu, Z.; Dai, J.; Niu, J.; He, L.; Mu, R.; Wang, Z. Isothermal oxidation and hot corrosion behaviors of diffusion aluminide coatings deposited by chemical vapor deposition. J. Alloys Compd. 2015, 637, 343–349. [Google Scholar] [CrossRef]
- Zahedi, H.; Nogorani, F.S.; Safari, M. Microstructure analysis of the pack cementation aluminide coatings modified by CeO2 addition. Met. Mater.-Int. 2021, 27, 922–930. [Google Scholar] [CrossRef]
- Kopec, M. Recent advances in the deposition of aluminide coatings on nickel-based superalloys: A synthetic review. Coatings 2024, 14, 630. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Sayre, G. Factors affecting the microstructure of platinum-modified aluminide coatings during a vapor phase aluminizing process. Surf. Coat. Technol. 2009, 203, 1264–1272. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Matsunaga, Y.; Nakagawa, K.; Tuda, Y.; Taniguchi, S. Growth behavior of coatings formed by vapor phase aluminizing using Fe-Al pellets of varying composition. Mater. Trans. 2006, 47, 2341–2347. [Google Scholar] [CrossRef]
- Taghipour, M.; Eslami, A.; Bahrami, A. High temperature oxidation behavior of aluminide coatings applied on HP-MA heat resistant steel using a gas-phase aluminizing process. Surf. Coat. Technol. 2022, 434, 128181. [Google Scholar] [CrossRef]
- Dai, Y.; Zou, J.; Ning, X.; Wei, H.; Zhan, W.; Li, F. Effect of the anti-seepage masking layer on the microstructure evolution of Ni-based superalloy during co-deposition of the Al-Si coating. Mater. Today Commun. 2023, 37, 107227. [Google Scholar] [CrossRef]
- Han, L.; Zheng, S.; Tao, M.; Fei, C.; Hu, Y.; Huang, B.; Yuan, L. Service damage mechanism and interface cracking behavior of Ni-based superalloy turbine blades with aluminized coating. Int. J. Fatigue 2021, 153, 106500. [Google Scholar] [CrossRef]
- Texier, D.; Monceau, D.; Hervier, Z.; Andrieu, E. Effect of interdiffusion on mechanical and thermal expansion properties at high temperature of a MCrAlY coated Ni-based superalloy. Surf. Coat. Technol. 2016, 307, 81–90. [Google Scholar] [CrossRef]
- Dai, Y.; Zou, J.; Shi, Q.; Li, X.; Wei, H. The evolution mechanism of ethylene-based and glass-ceramic as composited anti-seepage masking layer for Ni-based superalloy during aluminizing. Colloid Surf. A-Physicochem. Eng. Asp. 2025, 705, 135732. [Google Scholar]
- Kovtun, G.; Cuberes, T. Impact of glycerol and heating rate on the thermal decomposition of PVA films. Polymers 2025, 17, 2095. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Han, L.; Xiong, G.; Guo, Z.H.; Huang, J.W.; Zhang, Y.J.; Shen, Z.; Ge, C.C. Impact of sintering aid type and content on the mechanical properties of digital light processing 3D-printed Si3N4 ceramics. Materials 2024, 17, 5830. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, Z.; Niu, J.; He, L.; Mu, R.; Wang, K. Effects of deposition temperature on the kinetics growth and protective properties of aluminide coatings. J. Alloys Compd. 2015, 632, 238–245. [Google Scholar] [CrossRef]
- Arda, I.A.; Gökhan, G.; Kaan, D.; Havva, K.Z.; Metin, U. The Effects of Chemical Vapor Aluminizing Process Time and Post-processing for Nickel Aluminide Coating on CMSX-4 Alloy. J. Mater. Eng. Perform. 2021, 31, 2341–2353. [Google Scholar] [CrossRef]
- Goward, G.; Boone, D. Mechanisms of formation of diffusion aluminide coatings on nickel-base superalloys. Oxid. Met. 1971, 3, 475–495. [Google Scholar] [CrossRef]
- Brossard, J.M.; Panicaud, B.; Balmain, J.; Bonnet, G. Modelling of aluminized coating growth on nickel. Acta Mater. 2007, 55, 6586–6595. [Google Scholar] [CrossRef]
- Rajendran, R. Gas turbine coatings–An overview. Eng. Fail. Anal. 2012, 26, 355–369. [Google Scholar] [CrossRef]
- Angenete, J.; Stiller, K. Comparison of inward and outward grown Pt modified aluminide diffusion coatings on a Ni based single crystal superalloy. Surf. Coat. Technol. 2002, 150, 107–118. [Google Scholar] [CrossRef]
- Zuo, J.D.; Wang, Y.Q.; Sun, Q.Y.; Zhang, J.Y.; Liu, G.; Sun, J. Structure controlling strategy towards the high-temperature oxidation in multilayered coatings: An experience from Cr/CrAlN system. NPJ Mater. Degrad. 2025, 9, 22. [Google Scholar] [CrossRef]
- Rafiee, H.; Arabi, H.; Rastegari, S. Effects of temperature and Al-concentration on formation mechanism of an aluminide coating applied on superalloy IN738LC through a single step low activity gas diffusion process. J. Alloys Compd. 2010, 505, 206–212. [Google Scholar] [CrossRef]
- Costas, T.; Dimitrios, F.; Xanthi, N.; Ioannis, T.; Costas, P. Thermal Behavior of Poly(vinyl alcohol) in the Form of Physically Crosslinked Film. Polymers 2023, 15, 1843. [Google Scholar] [CrossRef]
- Bendaoudi, S.-E.; Bounazef, M.; Djeffal, A. The effect of sintering temperature on the porosity and compressive strength of corundum. J. Mech. Behav. Mater. 2018, 27, 0018. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Wang, S.; Zhang, M.; Gong, P.; Hu, Z.; Li, B. Effect of Cr content on the high temperature oxidation behavior of FeCoNiMnCrx porous high-entropy alloys. J. Mater. Res. Technol. 2024, 33, 3324–3333. [Google Scholar] [CrossRef]
- Zhou, R.; Huang, T.; Lu, Y.; Hong, M. Tunable coloring via post-thermal annealing of laser-processed metal surface. Appl. Sci. 2018, 8, 1716. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, X.; Cao, X.; Jiang, H.; Yan, D.; Rong, L. Effect of oxidation temperature on microstructure and liquid lead-bismuth eutectic corrosion resistance of pre-oxidized film on high-silicon ferritic/martensitic steel. J. Nucl. Mater. 2025, 615, 155931. [Google Scholar] [CrossRef]
- Peter, R.; Saric, I.; Piltaver, I.K.; Badovinac, I.J.; Petravic, M. Oxide formation on chromium metal surfaces by low-energy oxygen implantation at room temperature. Thin Solid Films 2017, 636, 225–231. [Google Scholar] [CrossRef]
- Pollock, T.M.; Tin, S. Nickel-based superalloys for advanced turbine engines: Chemistry, microstructure and properties. J. Propul. Power 2006, 22, 361–374. [Google Scholar] [CrossRef]
- Katsumi, O.; Hirosi, H.; Toshihiro, Y.; Michio, Y.; Kazumasa, O. X-ray diffractometric determination of lattice misfit between γ and γ’ phases in Ni-base superalloys. Adv. X-Ray Anal. 1988, 32, 365–375. [Google Scholar]
- Jerzy, M.; Maryana, Z.-Y.; Maciej, Z.; Jolanta, R. SEM/TEM Investigation of aluminide coating Co-doped with Pt and Hf deposited on Inconel 625. Materials 2018, 11, 898. [Google Scholar]
- Ma, S.; Ding, Q.; Wei, X.; Zhang, Z.; Bei, H. The effects of alloying elements Cr, Al, and Si on oxidation behaviors of Ni-based superalloys. Materials 2022, 15, 7352. [Google Scholar] [CrossRef]
- Zheng, H.; Li, L.; Jin, S.; Fan, P.; Zhong, M. Effect of tiny amount of DMC on thermal, mechanical, optical, and water resistance properties of poly(vinyl alcohol). J. Polym. Eng. 2022, 42, 846–855. [Google Scholar] [CrossRef]
- Senkevich, S.I.; Druzhinina, T.V.; Kharchenko, I.M.; Kryazhev, Y.G. Thermal transformations of polyvinyl alcohol as a source for the preparation of carbon materials. Solid Fuel Chem. 2007, 41, 45–51. [Google Scholar] [CrossRef]
- Wang, L.-S.; Song, J.-B.; Dong, H.; Yao, J.-T. Sintering-induced failure mechanism of thermal barrier coatings and sintering-resistant design. Coatings 2022, 12, 1083. [Google Scholar] [CrossRef]
- Song, Z.; Feng, L.J.; Chen, C.; Yan, M.; Wen, Z.C.; Chun, H.X.; Wang, B.Y.; Bin, Z.H. Improved oxidation resistance of Cr-Si coated Zircaloy with an in-situ formed Zr2Si diffusion barrier. NPJ Mater. Degrad. 2023, 7, 56. [Google Scholar]
- Peng, Z.; Kong, L.X. A thermal degradation mechanism of polyvinyl alcohol/silica nanocomposites. Polym. Degrad. Stabil. 2007, 92, 1061–1071. [Google Scholar] [CrossRef]
- Mugwagwa, L.; Yadroitsev, I.; Matope, S. Effect of process parameters on residual stresses, distortions, and porosity in selective laser melting of maraging steel 300. Metals 2019, 9, 1042. [Google Scholar] [CrossRef]
- Andritschky, M.; Alpuim, P.; Stöver, D.; Funke, C. Study of the mechanics of the delamination of ceramic functional coatings. Mater. Sci. Eng. A 1999, 271, 62–69. [Google Scholar] [CrossRef]
- Kyudeok, O.; Sunhyung, K.; Zhenghui, S.; Hwan, J.M.; Martti, T.; Lae, L.H. Effect of carboxymethyl cellulose and polyvinyl alcohol on the cracking of particulate coating layers. Prog. Org. Coat. 2022, 170, 106951. [Google Scholar]
- Wang, Y.; Guo, H.-B.; Peng, H.; Peng, L.-Q.; Gong, S.-K. Diffusion barrier behaviors of (Ru,Ni)Al/NiAl coatings on Ni-based superalloy substrate. Intermetallics 2010, 19, 191–195. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Fukaya, H.; Murata, Y.; Tanaka, K.; Inui, H. Diffusion of Al and Al-substituting elements in Ni3Al at elevated temperatures. Mater. Trans. 2012, 53, 2111–2118. [Google Scholar] [CrossRef]
- Xiao, S.; Meng, X.; Shi, K.; Liu, L.; Wu, H.; Lian, W.; Zhou, C.; Lyu, Y.; Chu, P.K. Hydrogen permeation barriers and preparation techniques: A review. J. Vac. Sci. Technol. A 2022, 40, 060803. [Google Scholar] [CrossRef]
- Müller, J.; Neuschütz, D. Efficiency of α-alumina as diffusion barrier between bond coat and bulk material of gas turbine blades. Vacuum 2003, 71, 247–251. [Google Scholar] [CrossRef]
- Wang, X.; Atkinson, A.; Chirivì, L.; Nicholls, J.R. Evolution of stress and morphology in thermal barrier coatings. Surf. Coat. Technol. 2010, 204, 3851–3857. [Google Scholar] [CrossRef]
- Elizarova, Y.A.; Zakharov, A.I. High-Temperature Functional Protective Coatings. Refract. Ind. Ceram. 2021, 61, 592–599. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, Z.; Zhao, S.; He, P.; Duan, X.; Jia, D.; Zhou, Y. Kinetics and properties of porous alumina with a dense surface layer prepared via bimodal powder sintering. Ceram. Int. 2025, 51, 8446–8453. [Google Scholar] [CrossRef]
- Santosh, S.; Pandian, R.V.; Srivatsan, S. An overview on synthesis, processing and applications of nickel aluminides: From fundamentals to current prospects. Crystals 2023, 13, 435. [Google Scholar] [CrossRef]


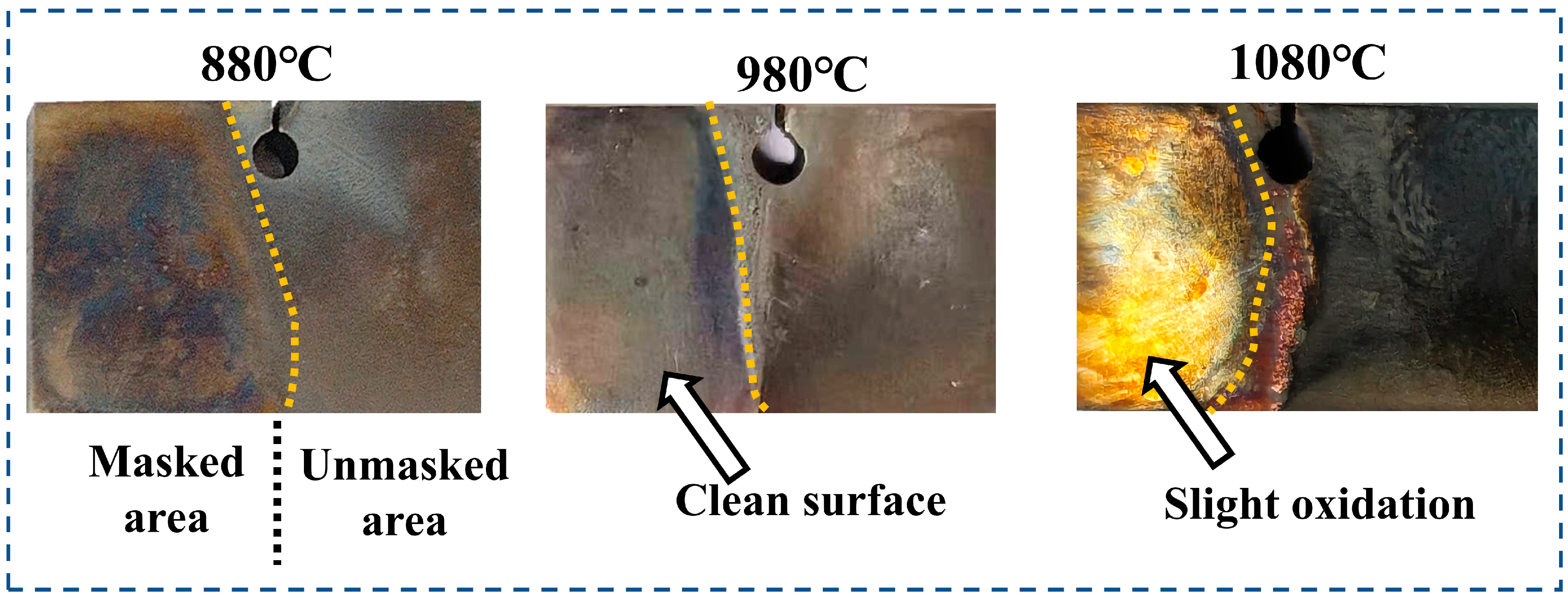
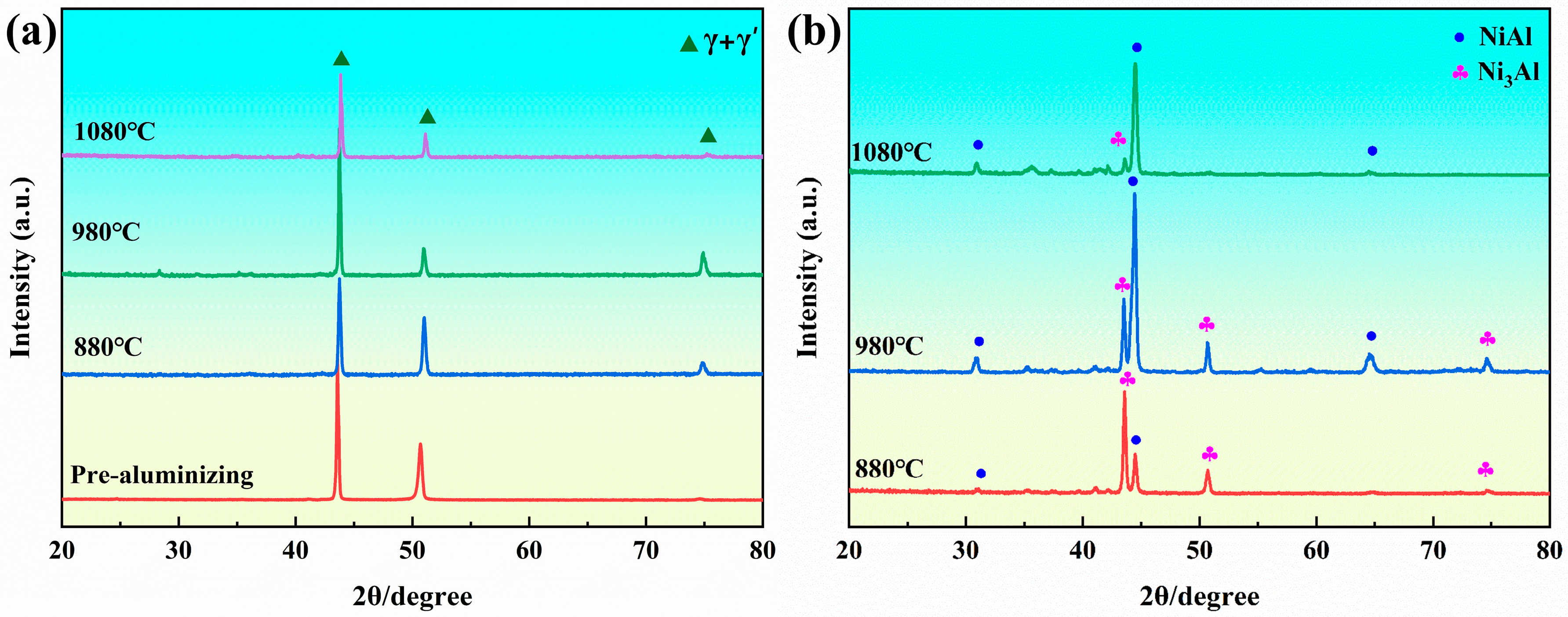



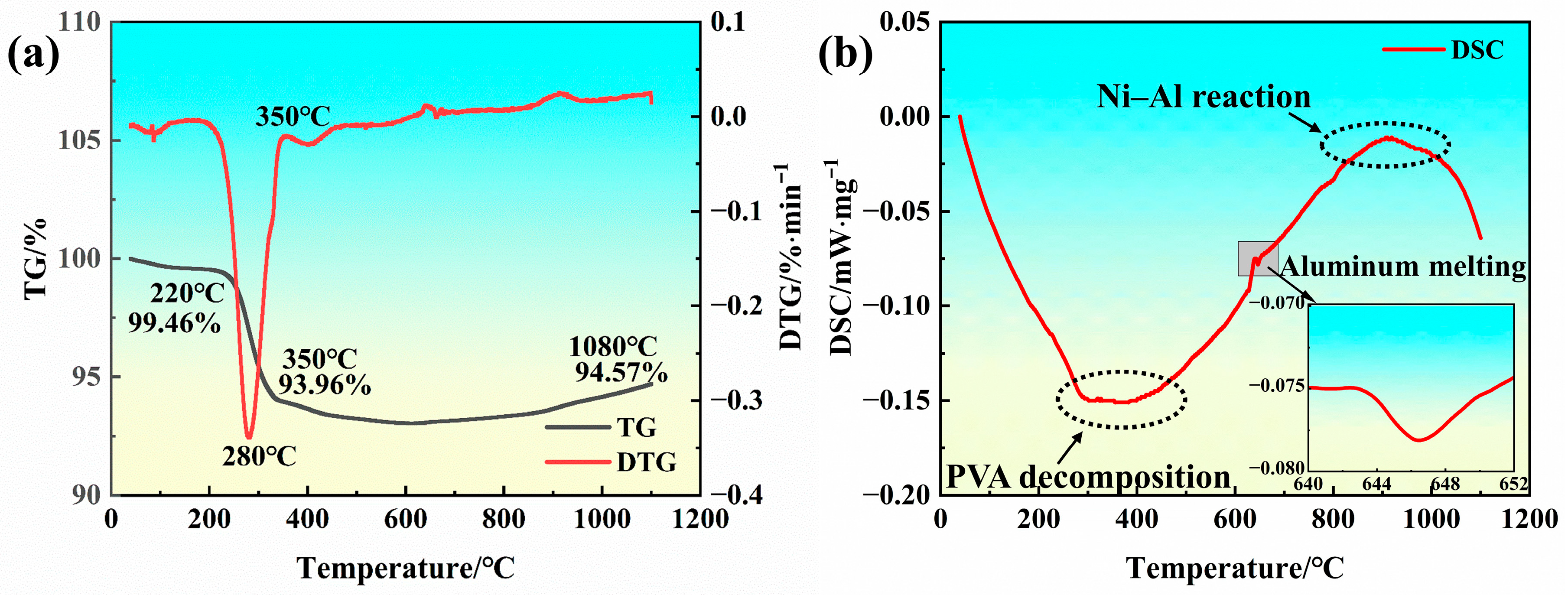



| Elements | W | Ta | Cr | Ti | Co | Al | Mo | Si | Ni |
|---|---|---|---|---|---|---|---|---|---|
| K4125 | 5.7 | 5.3 | 9.1 | 2.6 | 10.1 | 3.2 | 1.6 | 0.16 | Bal. |
| Position | Elements (at. %) | Phases | ||
|---|---|---|---|---|
| Ni | Al | O | ||
| p1 | 69.6 | 23.0 | 7.4 | Ni3Al + Ni |
| p2 | 3.6 | 43.1 | 53.3 | Al2O3 |
| p3 | 69.2 | 27.5 | 3.3 | Ni3Al + Ni |
| p4 | 55.0 | 43.3 | 1.7 | Ni3Al + NiAl + Ni |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Zhou, X.; Xie, C.; Li, Y.; Li, Y. Effects of Temperature on Anti-Seepage Coating During Vapor Phase Aluminizing of K4125 Ni-Based Superalloy. Surfaces 2026, 9, 2. https://doi.org/10.3390/surfaces9010002
Zhou X, Xie C, Li Y, Li Y. Effects of Temperature on Anti-Seepage Coating During Vapor Phase Aluminizing of K4125 Ni-Based Superalloy. Surfaces. 2026; 9(1):2. https://doi.org/10.3390/surfaces9010002
Chicago/Turabian StyleZhou, Xuxian, Cheng Xie, Yidi Li, and Yunping Li. 2026. "Effects of Temperature on Anti-Seepage Coating During Vapor Phase Aluminizing of K4125 Ni-Based Superalloy" Surfaces 9, no. 1: 2. https://doi.org/10.3390/surfaces9010002
APA StyleZhou, X., Xie, C., Li, Y., & Li, Y. (2026). Effects of Temperature on Anti-Seepage Coating During Vapor Phase Aluminizing of K4125 Ni-Based Superalloy. Surfaces, 9(1), 2. https://doi.org/10.3390/surfaces9010002







