“And Hence Have Been a Thousand Mistakes”: Marble or Alabaster? Resolving an Old Problem of Material Identification with Ultra-Portable Near-Infrared Spectroscopy
Abstract
1. Introduction
1.1. Historical Aspects
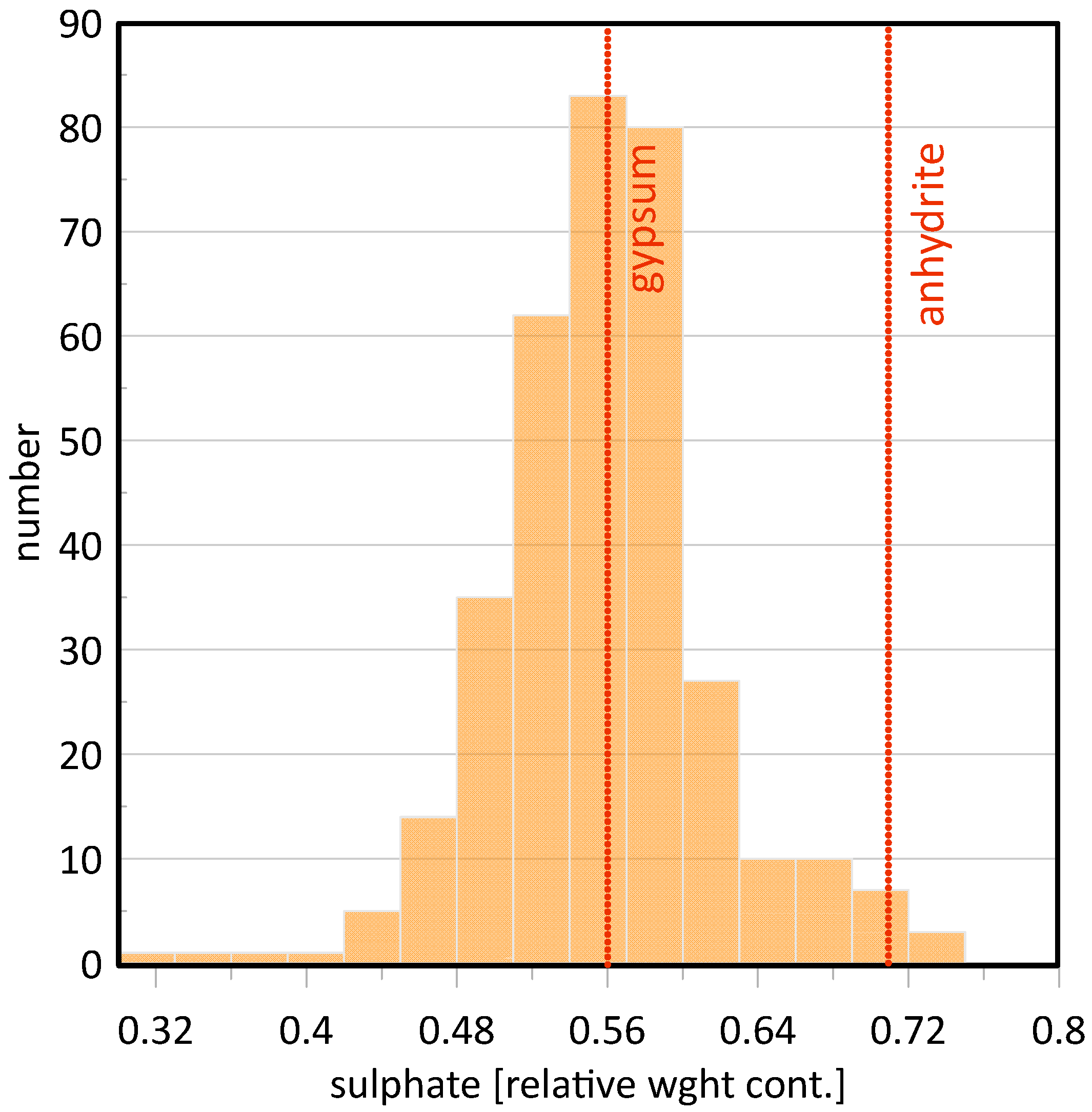
1.2. Mineralogical, Physical and Chemical Aspects
1.3. The Marble–Alabaster Problem in Modern Collections
2. Materials and Methods
3. Results and Discussion
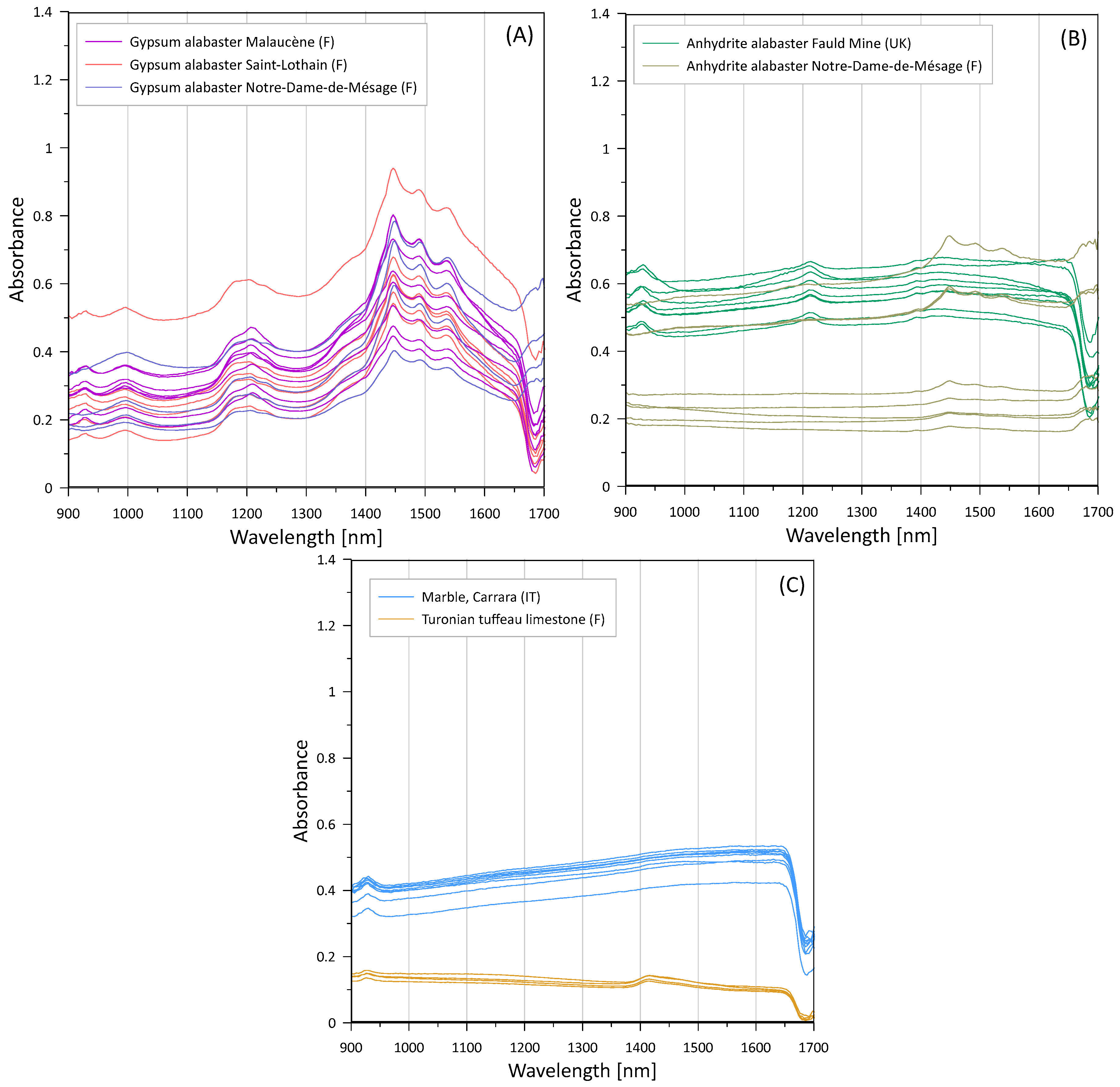
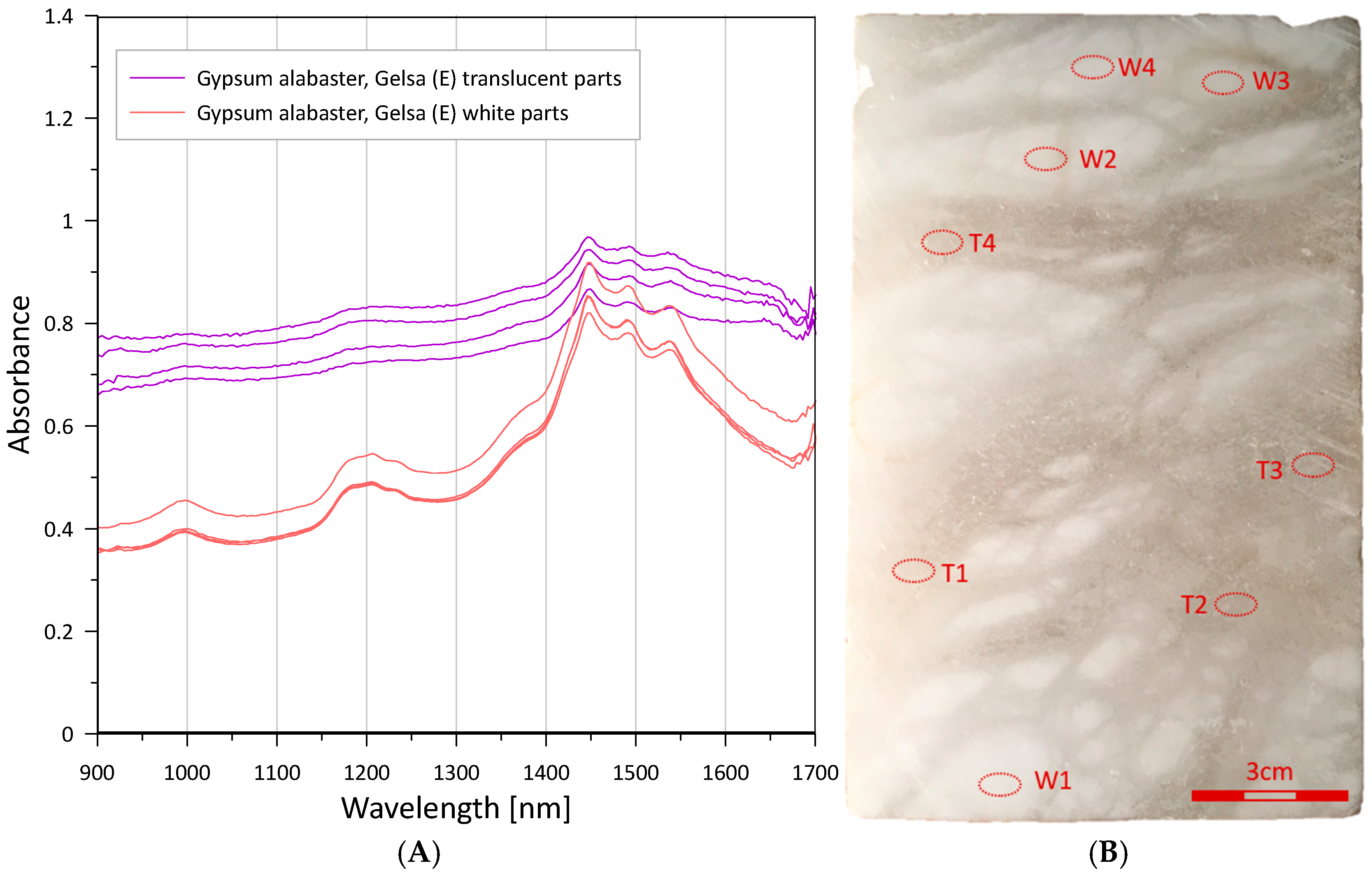
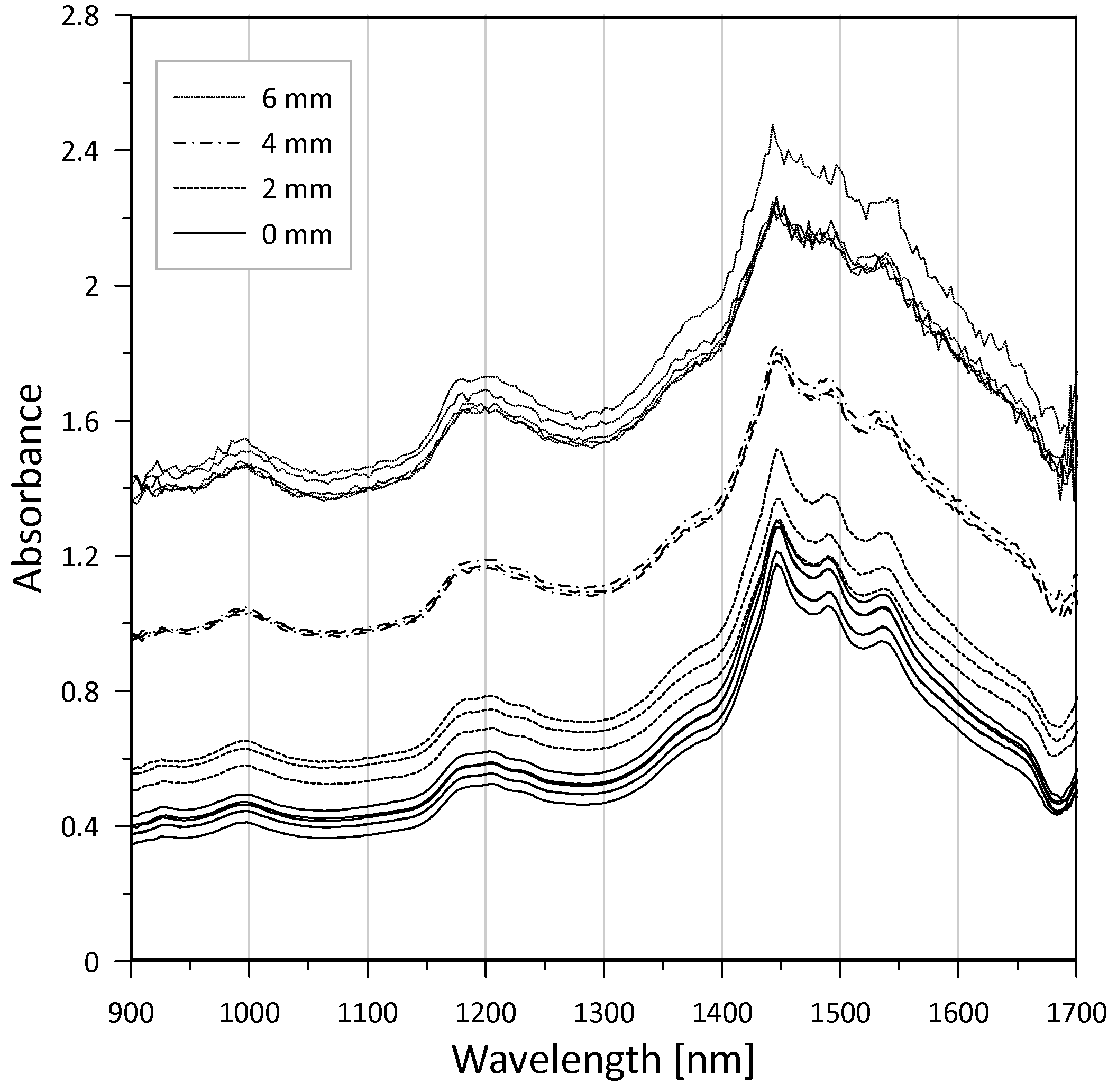
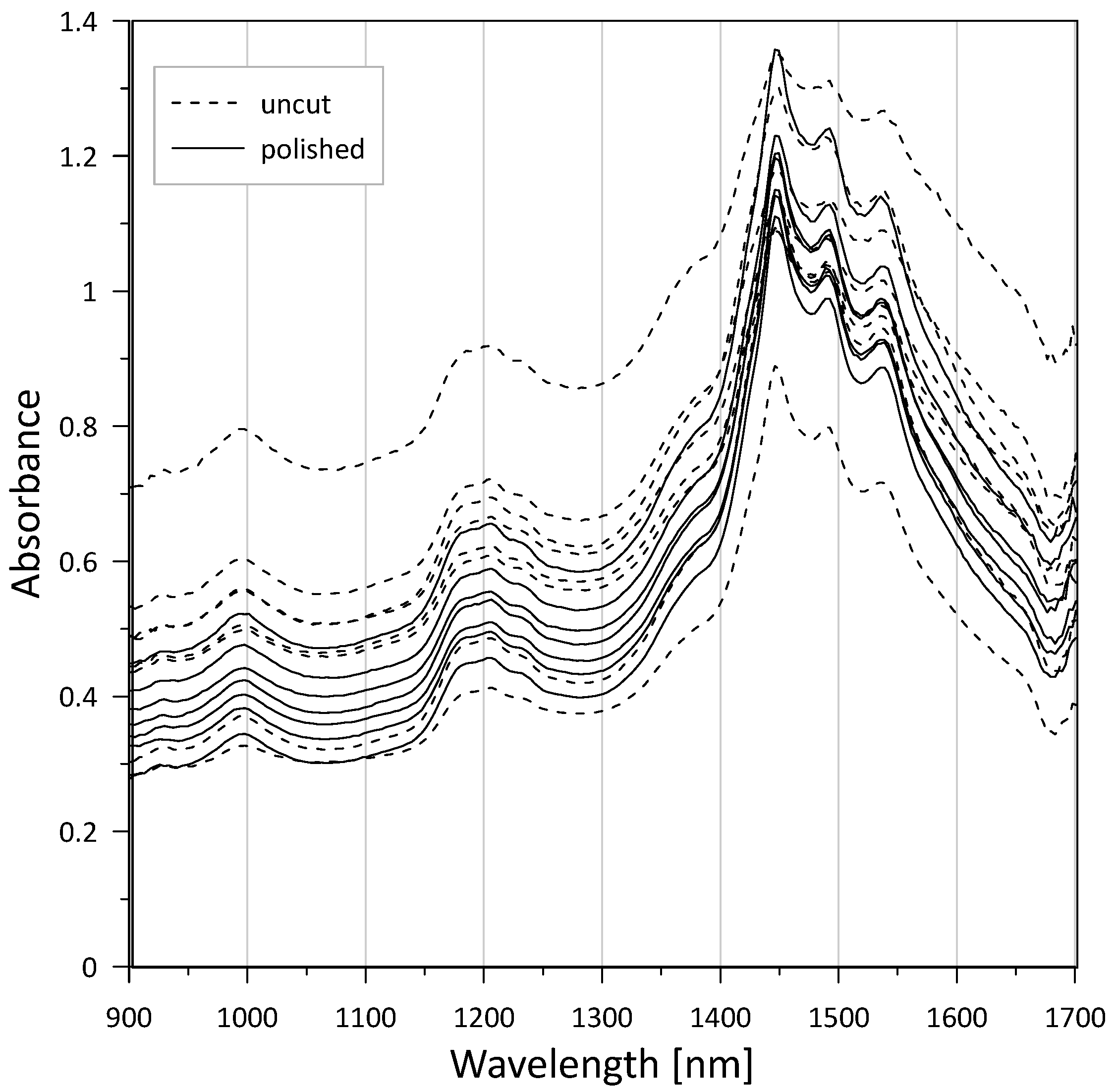
3.1. Raw Materials
| Approx. λ (nm) | Assignment/Physical Origin | Observed in | Reference(s) |
|---|---|---|---|
| ~930 | Suspected presence of goethite (α-FeOOH), ligand field transitions. | Gypsum alabaster (Malaucène, Saint Lothain), Fauld Mine anhydrite | [59,64] |
| ~990–1010 | Combination/overtone modes involving H–O–H/O–H (first overtone O-H stretch + first overtone H–O–H bending [60]). | Gypsum alabaster (Malaucène, Saint Lothain) | [48,49,60,66,69] |
| ~1150–1250 | Combination/overtone modes involving H–O–H/O–H (H–O–H bending fundamental + first overtone O–H stretch [60]). | All gypsum alabasters, Fauld Mine anhydrite | [49,60,69] |
| ~1400 | Clay minerals (smectite, kaolinite, illite, …), overtones of O–H stretch. | Tuffeau limestone | [64,65,66] |
| ~1360–1440 | H–O–H/O–H first overtone/H–O–H related combination features. | All gypsum alabasters | [48,49,61] |
| 1440–1540 (diagnostic triplet: 1446, 1490, 1538 nm [48]) | First overtone(s) of O–H/structurally bound water in gypsum—the characteristic 1.4–1.5 µm triplet discriminates hydrated Ca-sulphates (gypsum) from anhydrous phases. | All gypsum alabasters, Notre-Dame-de-Mésage partly hydrated anhydrite | [48,49,60,61,69] |
3.2. Case Study 1: Saint Catherine
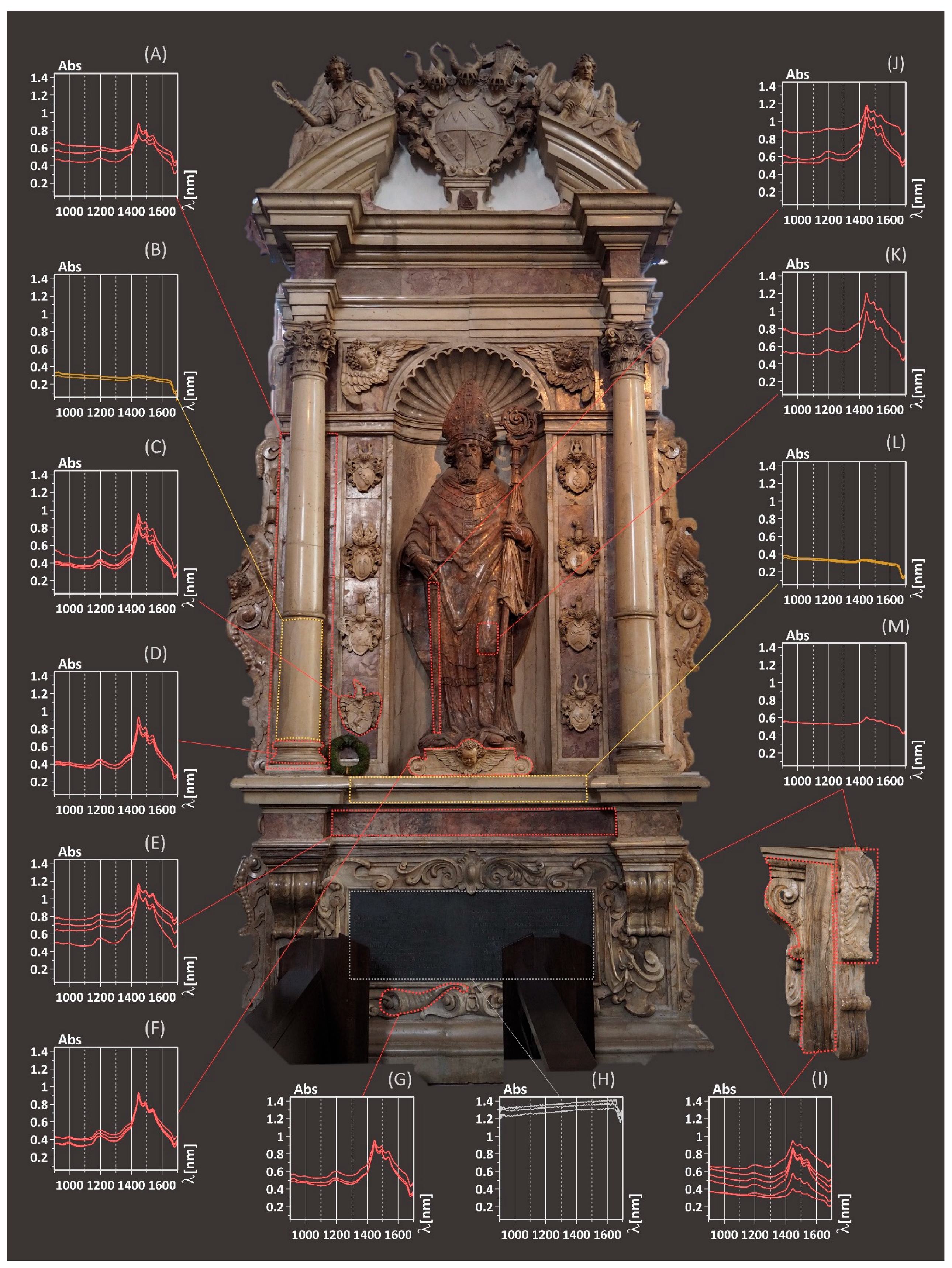
3.3. Case Study 2: Funeral Monument of Bishop Julius Echter, Würzburg Cathedral (Bavaria, Germany)
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMD | Digital micro-mirror device |
| FTIR | Fourier-transform infrared spectroscopy |
| LIBS | Laser-induced breakdown spectroscopy |
| NIRS | Near-infrared spectroscopy |
| VNIR | Visible-to-near-infrared |
| XRF | X-ray fluorescence spectroscopy |
References
- Primavori, P. Carrara Marble: A nomination for ‘Global Heritage Stone Resource’ from Italy. Geol. Soc. Lond. Spec. Publ. 2015, 407, 137–154. [Google Scholar] [CrossRef]
- Klapisch-Zuber, C. Les Maîtres du Marbre: Carrare, 1300–1600; Éditions de l’École des Hautes Etudes en Sciences Sociales: Paris, France, 1969. [Google Scholar]
- St John Hope, W.H. On the early working of alabaster in England. In Illustrated Catalogue of the Exhibition of English Medieval Alabaster Work: Held in the Rooms of the Society of Antiquaries, 26th May to 30th June, 1910; St John Hope, W.H., Ed.; Society of Antiquaries of London: London, UK, 1913. [Google Scholar]
- Espanol, F. L’exploitation des carrières d’albâtre en Catalogne au Moyen Âge. In Proceedings of the Relations, échanges et Coopération en Méditerranée, 128° Congrès National des Sociétés Historiques et Scientifiques, Bastia, France, 14–21 April 2003. [Google Scholar]
- Aillaud, R.; Anheim, E. L’albâtre de Notre-Dame de-Mésage. Exploitation, circulation et usages (XIVe-XVIe siècle). Rev. de l’Art 2018, 200/2018-2, 31–36. [Google Scholar]
- Kloppmann, W.; Leroux, L.; Bromblet, P.; Le Pogam, P.Y.; Cooper, A.H.; Worley, N.; Guerrot, C.; Montech, A.T.; Gallas, A.M.; Aillaud, R. Competing English, Spanish, and French alabaster trade in Europe over five centuries as evidenced by isotope fingerprinting. Proc. Natl. Acad. Sci. USA 2017, 114, 11856–11860. [Google Scholar] [CrossRef]
- Theiss, H.; Roller, S.; Gonzalez de Quevedo Ibanez, M.; Klopmann, W.; Wille, G. „Der Alabaster will aber etwas anders behandelt seyn…” Überlegungen zu Möglichkeiten der Oberflächenreinigung von unbehandelten Alabaster. In Handbuch der Oberflächenreinigung, 7th ed.; Eipper, P.-B., Ed.; Verlag Dr. Christian Müller-Straten: München, Germany, 2021; Volume 3, pp. 117–207. [Google Scholar]
- Lipińska, A. Alabastrum, id EST, corpus hominis: Alabaster in the Low Countries, a cultural history. Neth. Yearb. Hist. Art/Ned. Kunsthist. Jaarb. Online 2013, 62, 84–115. [Google Scholar] [CrossRef]
- Bruchet, M.P.M. Marguerite d’Autriche, Duchesse de Savoie: Ouvrage Publié Sous les Auspices du Comité Flamand de France; IMPR. L. Danel: Lille, France, 1927. [Google Scholar]
- Tritenne, D. Le marbre de Carrare utilisé à Brou. In Brou, un Monument Européen à l’aube de la Renaissance = Brou, a European Monument in the Early Renaissance; Editions du Patrimoine, Centre des Monuments Nationaux: Paris, France, 2009; pp. 183–189. [Google Scholar]
- Jugie, S.; Leroux, L.; Bromblet, P.; Kloppmann, W. L’albâtre et ses sources: Incertitudes historiques et ambiguïtés de la documentation levées grâce aux analyses. Technè 2024, 57, 49–59. [Google Scholar] [CrossRef]
- Pliny the Elder; Eichholz, D.E. Pliny, Natural History, Volume X: Books 36–37; Eichholz, D.E., Translator; Heinemann, W., Ed.; Harvard University Press: Cambridge, MA, USA, 1962. [Google Scholar]
- Caley, E.R.; Richards, J.F. Theophrastus on Stones: Introduction, Greek Text, English Translation, and Commentary; The Ohio State University Press: Columbus, OH, USA, 1956. [Google Scholar]
- Hill, J. Theophrastus’s History of Stones: With an English Version, and Critical and Philosophical Notes, Including the Modern History of the Gems Described by That Author and of Many Other of the Native Fossils; C. Davis: London, UK, 1746. [Google Scholar]
- de Boodt, A. Gemmarum et Lapidum Historia Quam Olim Edidit Anselmus Boetius de Boot, Brugensis, Rudolphi II. Imperatoris Medicus; Ioannis Maire: Leyden, Netherlands, 1647. [Google Scholar]
- Schröder, J. Pharmacopoeia Medico-Chymica Sive Thesaurus Pharmacologicus: Quo Composita Quaeque Celebriora, Hinc Mineralia, Vegetabilia & Animalia Chymico-Medice Describuntur, Atque Insuper Principia Physicae Hermetico-Hippocraticae Candide Exhibentur; Opus, Non Minus Utile Physicis Quam Medicis; Ioannis Gerlin: Ulm, Germany, 1644. [Google Scholar]
- Plot, R. Natural History of Staffordshire; Printed at the Theater: Oxford, UK, 1686. [Google Scholar]
- Richelet, P. Albatre, albastre. In Dictionnaire François; Jean Herman Widerhold: Genève, Switzerland, 1680. [Google Scholar]
- Aldrovandi, U. Musaeum Metallicum in Libros IV; Bernia, Marcus Antonius: Bologna, Italy, 1648. [Google Scholar]
- Holinshed, R.; Harrison, W.; Stanyhurst, R.; Hooker, J.; Thynne, F.; Fleming, A.; Stow, J.; Ellis, H. Holinshed’s Chronicles of England, Scotland and Ireland/[edited 1807 by Sir Henry Ellis]; J. Johnson: London, UK, 1587. [Google Scholar]
- Pott, J.H. Chymische Untersuchungen Welche fürnehmlich von der Lithogeognosia oder Erkäntniß und Bearbeitung der Gemeinen Einfacheren Steine und Erden Ingleichen von Feuer und Licht Handeln; Christian Friedrich Voß: Potsdam, Germany, 1746. [Google Scholar]
- Daubenton, L.J.-M. Mémoire sur l’albâtre. Mémoires de mathématiques et de physique tirés des registres de l’Académie royale des sciences 1759, 1754, 237–249. [Google Scholar]
- Lucas, J.-A.-H. Albâtre calcaire, Albâtre gypseux. Nouveau Dictionnaire d’Histoire Naturelle, Appliquée aux arts, à l’Agriculture, à l’Economie Rurale et Domestique, à la Médecine, etc.; Aba-Ani; Deterville: Paris, France, 1816; pp. 283–285. [Google Scholar]
- Bomford, D. Introduction. In Looking Through Paintings. The Study of Painting Techniques and Materials in Support of Art Historical Research, Hermens, E., Ed.; Archetype Publication: London, UK, 1998. [Google Scholar][Green Version]
- Roberts, J.L. Things. Material Turn, Transnational Turn. Am. Art 2017, 61, 64–69. [Google Scholar] [CrossRef]
- Attanasio, D.; Brilli, M.; Ogle, N. The Isotopic Signature of Classical Marbles; L’Erma di Bretschneider: Rome, Italy, 2006. [Google Scholar]
- Antonelli, F.; Lazzarini, L. An updated petrographic and isotopic reference database for white marbles used in antiquity. Rend. Lincei 2015, 26, 399–413. [Google Scholar]
- Craig, H.; Craig, V. Greek Marbles: Determination of Provenance by Isotopic Analysis. Science 1972, 176, 401–403. [Google Scholar] [CrossRef]
- Kloppmann, W.; Leroux, L.; Bromblet, P.; Le Pogam, P.-Y.; Montech, A.T.; Guerrot, C. A pan-European art trade in the late middle ages: Isotopic evidence on the master of Rimini enigma. PLoS ONE 2022, 17, e0265242. [Google Scholar] [CrossRef]
- Kloppmann, W.; Leroux, L.; Bromblet, P.; Guerrot, C.; Proust, E.; Cooper, A.H.; Worley, N.; Smeds, S.A.; Bengtsson, H. Tracing Medieval and Renaissance Alabaster Works of Art Back to Quarries: A Multi-Isotope (Sr, S, O) Approach. Archaeometry 2014, 56, 203–219. [Google Scholar] [CrossRef]
- Kloppmann, W.; Le Pogam, P.-Y.; Leroux, L. La sculpture sur albâtre en France du xive au xvie siècle: Enjeux, méthodes et résultats d’un programme de recherche. Rev. de l’Art 2018, 200/2018-2, 9–20. [Google Scholar]
- de Roy, J.; Fontaine, L.; Patigny, G. ‘Marbre d’albastre’: Status of the interdisciplinary research project on marble and alabaster. In Alabaster Sculpture in Europe 1300–1650; Debaene, M., Ed.; Harvey Miller Publishers: London, UK; Turnhout, Belgium, 2022; pp. 31–37. [Google Scholar]
- González de Quevedo Ibáñez, M.; Hildenbrand, T.; Theiss, H. Material und Werktechnik, Kunstechnologischer Befund des Rimini-Altars–Experimente zur Bildhauertechnik–Studien zur Oberflächenveredlung und Polychromie mittelalterlicher Alabasterskuplturen. In Mission Rimini: Material, Geschichte, Restaurierung. Der Rimini-Altar, Roller, S., Theiss, H., Eds.; Deutscher Kunstverlag: Berlin, Germany, 2021; pp. 185–277. [Google Scholar]
- Seymour, T. The EU law that’s threatening to up-end the antiquities market. Financial Times, 5 March 2025. Available online: https://www.ft.com/content/9a35cde6-c5f1-4a72-8795-59410f04753a (accessed on 23 October 2025).
- Prieto-Taboada, N.; Gómez-Laserna, O.; Martínez-Arkarazo, I.; Olazabal, M.Á.; Madariaga, J.M. Raman Spectra of the Different Phases in the CaSO4–H2O System. Anal. Chem. 2014, 86, 10131–10137. [Google Scholar] [CrossRef]
- Jehlička, J.; Culka, A. Critical evaluation of portable Raman spectrometers: From rock outcrops and planetary analogs to cultural heritage—A review. Anal. Chim. Acta 2022, 1209, 339027. [Google Scholar] [CrossRef]
- de Roy, J.; Fontaine, L.; Patigny, G. New Insights on St Catherine of Alexandria by André Beauneveu. Correct Identification and Provenance Analysis of the Original Sculpture Material and Latest Findings on the Polychromy. In Alabaster as a Material for Medieval and Renaissance Sculpture, 8th Annual Ards Conference, Paris, France, 18–20 January 2022; Brepols: Turnhout, Belgium, 2024; Submitted. [Google Scholar]
- Blanco, M.; Villarroya, I. NIR spectroscopy: A rapid-response analytical tool. TrAC Trends Anal. Chem. 2002, 21, 240–250. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Siesler, H.W.; Huck, C.W. Handheld near-infrared spectrometers: Where are we heading? NIR News 2020, 31, 28–35. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Principles and Applications of Miniaturized Near-Infrared (NIR) Spectrometers. Chem.–Eur. J. 2021, 27, 1514–1532. [Google Scholar] [CrossRef]
- Singh, H.; Sridhar, A.; Saini, S.S. Ultra-Low-Cost Self-Referencing Multispectral Detector for Non-Destructive Measurement of Fruit Quality. Food Anal. Methods 2020, 13, 1879–1893. [Google Scholar] [CrossRef]
- Wolfrum, E.J.; Payne, C.; Schwartz, A.; Jacobs, J.; Kressin, R.W. A Performance Comparison of Low-Cost Near-Infrared (NIR) Spectrometers to a Conventional Laboratory Spectrometer for Rapid Biomass Compositional Analysis. BioEnergy Res. 2020, 13, 1121–1129. [Google Scholar] [CrossRef]
- Cura, K.; Rintala, N.; Kamppuri, T.; Saarimäki, E.; Heikkilä, P. Textile recognition and sorting for recycling at an automated line using near infrared spectroscopy. Recycling 2021, 6, 11. [Google Scholar] [CrossRef]
- Chavan, R.B.; Bhargavi, N.; Lodagekar, A.; Shastri, N.R. Near infra red spectroscopy: A tool for solid state characterization. Drug Discov. Today 2017, 22, 1835–1843. [Google Scholar] [CrossRef]
- Tang, Y.; Jones, E.; Minasny, B. Evaluating low-cost portable near infrared sensors for rapid analysis of soils from South Eastern Australia. Geoderma Reg. 2020, 20, e00240. [Google Scholar] [CrossRef]
- Kim, Y.; Caumon, M.-C.; Barres, O.; Sall, A.; Cauzid, J. Identification and composition of carbonate minerals of the calcite structure by Raman and infrared spectroscopies using portable devices. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 261, 119980. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, A.Z.; Freemen, J.J. Raman, MIR, and NIR Spectroscopic Study of Calcium Sulfates: Gypsum, Bassanite, and Anhydrite. In Proceedings of the 40th Lunar and Planetary Science Conference, (Lunar and Planetary Science XL), The Woodlands, TX, USA, 23–27 March 2009. [Google Scholar]
- Bishop, J.L.; Lane, M.D.; Dyar, M.D.; King, S.J.; Brown, A.J.; Swayze, G.A. Spectral properties of Ca-sulfates: Gypsum, bassanite, and anhydrite. Am. Mineral. 2014, 99, 2105–2115. [Google Scholar] [CrossRef]
- Harrison, T.N. Experimental VNIR reflectance spectroscopy of gypsum dehydration: Investigating the gypsum to bassanite transition. Am. Mineral. 2012, 97, 598–609. [Google Scholar] [CrossRef]
- InnoSpectra. Product Specification. NIR-S-G1, Version 1.2, 2021/1/5; InnoSpectra Corporation: Hsinchu, Taiwan, 2021; Available online: https://www.innospectra.com/ueditor/php/upload/file/20220928/1664336948908375.pdf (accessed on 22 October 2025).
- InnoSpectra. InnoSpectra NIRScan Spectrometer & Module User Manual Ver. 1.2 2021/09/06; InnoSpectra Corporation: Hsinchu, Taiwan, 2006. [Google Scholar]
- Labsphere Inc. Spectralon® Diffuse Reflectance Material–SRM-99, Data Sheet. 2023. Available online: https://www.labsphere.com/wp-content/uploads/2023/01/Spectralon.pdf (accessed on 23 October 2025).
- Jugie, S.; Kloppmann, W.; Leroux, L. Les albâtres du Jura au XVe et au XVIe siècle: Des archives aux carrières, des carrières aux œuvres. In Proceedings of the L’albâtre, Matériau de la Sculpture du Moyen Âge et de la Renaissance, Huitième Colloque d’ARDS sur L’actualité de la Recherche sur la Sculpture du Moyen Âge et de la Renaissance, Paris, France, 18–19 January 2022. [Google Scholar]
- Kloppmann, W.; Le Pogam, P.-Y.; Leroux, L.; Bromblet, P. De la carrière de Malaucène vers tout l’Occident du XIVe siècle. Un groupe d’œuvres en albâtre au temps de la papauté d’Avignon. In Proceedings of the El Lujo en las Artes, Zaragoza, Spain, 9–11 May 2019; pp. 236–246. [Google Scholar]
- Beck, K.; Al-Mukhtar, M.; Rozenbaum, O.; Rautureau, M. Characterization, water transfer properties and deterioration in tuffeau: Building material in the Loire valley—France. Build. Environ. 2003, 38, 1151–1162. [Google Scholar] [CrossRef]
- Cloutis, E.; Craig, M.; Kruzelecky, R.; Jamroz, W.; Scott, A.; Hawthorne, F.; Mertzman, S. Spectral reflectance properties of minerals exposed to simulated Mars surface conditions. Icarus 2008, 195, 140–168. [Google Scholar] [CrossRef]
- Hunt, G.R.; Salisbury, J.W.; Lenhoff, C.J. Visible and near-infrared spectra of minerals and rocks: IV. Sulphides and sulphates. Mod. Geol. 1971, 3, 1–14. [Google Scholar]
- Kokaly, R.F.; Clark, R.N.; Swayze, G.A.; Livo, K.E.; Hoefen, T.M.; Pearson, N.C.; Wise, R.A.; Benzel, W.; Lowers, H.A.; Driscoll, R.L.; et al. USGS Spectral Library Version 7; 1035; USGS: Reston, VA, USA, 2017; p. 68. [Google Scholar]
- Clark, R.N. Reflectance spectra. In AGU Handbook of Physical Constants; Ahrens, T.J., Ed.; American Geophysical Union: Washington DC, 1995; Volume 178. [Google Scholar]
- Crowley, J.K. Visible and near-infrared (0.4–2.5 μm) reflectance spectra of playa evaporite minerals. J. Geophys. Res. Solid Earth 1991, 96, 16231–16240. [Google Scholar] [CrossRef]
- Cloutis, E.A.; Hawthorne, F.C.; Mertzman, S.A.; Krenn, K.; Craig, M.A.; Marcino, D.; Methot, M.; Strong, J.; Mustard, J.F.; Blaney, D.L. Detection and discrimination of sulfate minerals using reflectance spectroscopy. Icarus 2006, 184, 121–157. [Google Scholar] [CrossRef]
- Young, J.A. Alabaster; Derbyshire Museum Service: Nottingham, UK, 1990; p. 68. [Google Scholar]
- Firman, R.J. A geological approach to the history of English Alabaster. Mercian Geol. 1984, 9, 161–178. [Google Scholar]
- Stenberg, B.; Rossel, R.A.V.; Mouazen, A.M.; Wetterlind, J. Visible and near infrared spectroscopy in soil science. Adv. Agron. 2010, 107, 163–215. [Google Scholar] [CrossRef]
- Lindberg, J.D.; Snyder, D.G. Diffuse reflectance spectra of several clay minerals. Am. Mineral. J. Earth Planet. Mater. 1972, 57, 485–493. [Google Scholar]
- Clark, R.N.; King, T.V.; Klejwa, M.; Swayze, G.A.; Vergo, N. High spectral resolution reflectance spectroscopy of minerals. J. Geophys. Res. Solid Earth 1990, 95, 12653–12680. [Google Scholar] [CrossRef]
- Hunt, G.R.; Ashley, R.P. Altered Rock Spectra in the Visible and Near Infrared; E79-10256; U.S. Geological Survey: Denver, CO, USA, 1979. Available online: https://ntrs.nasa.gov/citations/19790023545 (accessed on 23 October 2025).
- Ortí, F.; Rosell, L.; PlayÀ, E.; Salvany, J.M. Meganodular anhydritization: A new mechanism of gypsum to anhydrite conversion (Palaeogene-Neogene, Ebro Basin, North-east Spain). Sedimentology 2012, 59, 1257–1277. [Google Scholar] [CrossRef]
- Khayamim, F.; Wetterlind, J.; Khademi, H.; Robertson, A.H.J.; Cano, A.F.; Stenberg, B. Using Visible and near Infrared Spectroscopy to Estimate Carbonates and Gypsum in Soils in Arid and Subhumid Regions of Isfahan, Iran. J. Near Infrared Spectrosc. 2015, 23, 155–165. [Google Scholar] [CrossRef]
- Woelk, M. 2 St Catherine of Alexandria. In Alabaster Sculpture in Europe 1300–1650; Debaene, M., Ed.; Harvey Miller Publishers: London, UK; Turnhout, Belgium, 2022; pp. 40–41. [Google Scholar]
- Le Pogam, P.-Y.; Jugie, S. La Sculpture Gothique. 1140–1430; Hazan: Paris, Italy, 2020. [Google Scholar]
- Van de Putte, F. La Chapelle des Comtes de Flandre à Courtrai; Beyaert: Belgium, Brussel, 1875. [Google Scholar]
- Nash, S. ‘No Equal in Any Land’. André Beauneveu, Artist to the Courts of France and Flanders; Paul Holberton Publishing Ltd.: London, UK, 2007. [Google Scholar]
- Dehaisnes, C.C.A. Histoire de l’art Dans la Flandre, l’Artois & le Hainaut Avant le XVe Siècle; L. Quarré: Lille, France, 1886. [Google Scholar]
- Casier, J.; Bergmans, P. L’art Ancien Dans les Flandres (région de l’Escaut): Mémorial de L’exposition Rétrospective Organisée à Gand en 1913; G. van Oest & cie: Brussels, Belgium; Paris, France, 1914. [Google Scholar]
- Scharold, C.G. Geschichte und Beschreibung des St. Kilians-Doms zu Würzburg; Thein: Würzburg, Germany, 1837. [Google Scholar]
- Gradmann, G.; Michael Kern, B. Inaugural-Dissertation zur Erlangung der Doktorwürde, Universität zu Tübingen. Ph.D. Thesis, Universität zu Tübingen, Universitäts-Buchdruckerei J.H.Ed. Heitz (Heitz & Mündel), Strassburg, France, 1916. [Google Scholar]
- Bruhns, L. Würzburger Bildhauer der Renaissance und des Werdenden Barock, 1540–1650; Verlag für Praktische Kunstwissenschaft, Dr. F.X. Weizinger: Munich, Germany, 1923. [Google Scholar]
- Dombrowski, D. Cat. No 21.7. In Julius Echter: Patron Der Künste. Konturen Eines Fürsten Und Bischofs Der Renaissance; Dombrowski, D., Maier, M., Müller, F., Eds.; De Gruyter: Berlin, Germany, 2017; pp. 385–387. [Google Scholar]
- Moshammer, B.; Uhlir, C.; Rohatsch, A.; Unterwurzacher, M. Adnet ‘Marble’, Untersberg ‘Marble’and Leitha limestone—Best examples expressing Austria’s physical cultural heritage. In Engineering Geology for Society and Territory-Volume 5: Urban Geology, Sustainable Planning and Landscape Exploitation; Springer: Cham, Switzerland, 2015; pp. 253–257. [Google Scholar]
- Golloch, A.; Heidbreder, S.; Lühr, C. Identification of amber and imitations by near infrared reflection spectroscopy. Fresenius’ J. Anal. Chem. 1998, 361, 545–546. [Google Scholar] [CrossRef]
- Dubelaar, W. Albast uit Nottingham geologie, winning en verspreiding in de Lage Landen. Gea 2009, 42, 72–77. [Google Scholar]

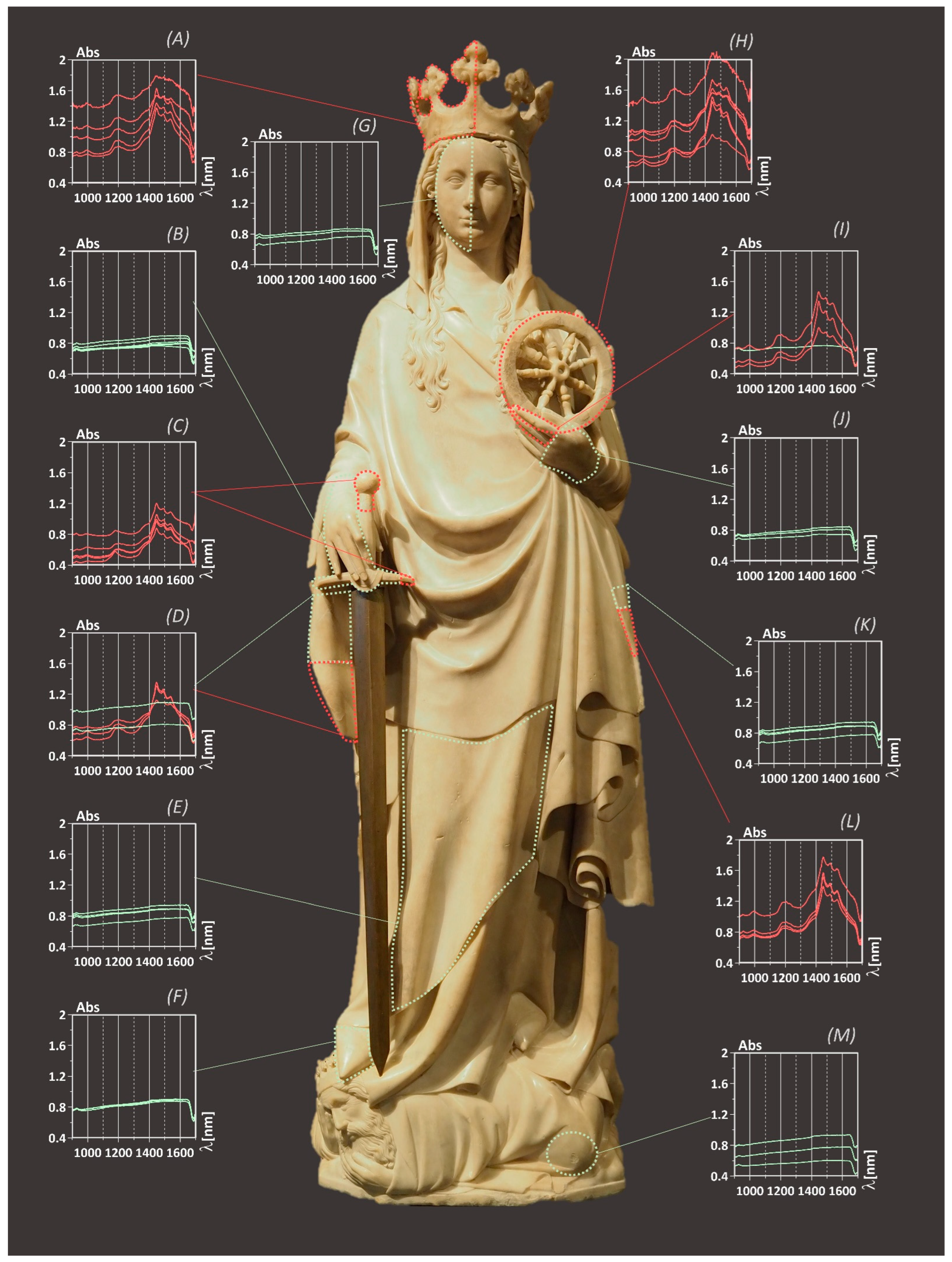

| Parts | Zone Figure 7 | Material | Original/Restoration |
|---|---|---|---|
| Face, backs of the hands, mantle folds, foot, king | G, B, E, F, J, K, M | Marble | Original |
| Crown, wheel, index and middle fingertips of the left hand | A, H, I | Alabaster | Replacements |
| Parts of mantle folds | D, L | Alabaster | Replacements |
| Sword pommel, parts of sword hilt | C | Alabaster | Replacements |
| Parts of sword hilt | B | Marble | Replacements |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kloppmann, W.; Lipińska, A.; Rolland, O. “And Hence Have Been a Thousand Mistakes”: Marble or Alabaster? Resolving an Old Problem of Material Identification with Ultra-Portable Near-Infrared Spectroscopy. Heritage 2025, 8, 455. https://doi.org/10.3390/heritage8110455
Kloppmann W, Lipińska A, Rolland O. “And Hence Have Been a Thousand Mistakes”: Marble or Alabaster? Resolving an Old Problem of Material Identification with Ultra-Portable Near-Infrared Spectroscopy. Heritage. 2025; 8(11):455. https://doi.org/10.3390/heritage8110455
Chicago/Turabian StyleKloppmann, Wolfram, Aleksandra Lipińska, and Olivier Rolland. 2025. "“And Hence Have Been a Thousand Mistakes”: Marble or Alabaster? Resolving an Old Problem of Material Identification with Ultra-Portable Near-Infrared Spectroscopy" Heritage 8, no. 11: 455. https://doi.org/10.3390/heritage8110455
APA StyleKloppmann, W., Lipińska, A., & Rolland, O. (2025). “And Hence Have Been a Thousand Mistakes”: Marble or Alabaster? Resolving an Old Problem of Material Identification with Ultra-Portable Near-Infrared Spectroscopy. Heritage, 8(11), 455. https://doi.org/10.3390/heritage8110455






