Abstract
This study evaluates the enzymatic hydrolysis of pretreated corn cobs (PCCs) using a blend of commercial enzymes (Cellulase enzyme blend and Viscozyme L), followed by simultaneous saccharification and fermentation (SSF) with Mucor indicus DSM 2185 for ethanol production. A combination of 2% (vol vol−1) Cellulase enzyme blend and 5.18% (vol vol−1) Viscozyme L, corresponding to an enzyme loading of 48.9 FPU/gPCCs, enabled near-complete hydrolysis of 40 g L−1 PCCs within 6–48 h, achieving 92.66% total carbohydrate conversion into fermentable sugars. In SSF experiments conducted in Erlenmeyer flasks, optimal ethanol production in matrix nutrient medium (MNM) reached 14.95 g L−1, with a conversion coefficient of 0.373 g g−1 at 30 °C over a 48 h period. Scale-up of the bioprocess in a 1.5 L stirred-tank bioreactor at 30 °C resulted in an ethanol concentration of 16.46 g L−1, a total carbohydrate conversion of 86.27%, and a substrate-to-ethanol conversion coefficient of 0.44 g g−1 within 22 h. Minor secondary metabolites, including 0.88 g L−1 xylitol and 0.26 g L−1 glycerol, were also detected. Overall, the results demonstrate the potential of M. indicus in combination with commercial enzyme blends as a scalable strategy for industrial ethanol production.
1. Introduction
Over 95% of carbon-based chemicals used in modern industry are still derived from fossil resources [1,2]. As concerns regarding climate change, resource depletion, and environmental sustainability intensify, the transition toward renewable feedstocks has become a central objective in advancing a sustainable bioeconomy. In this context, lignocellulosic biomass has emerged as one of the most promising renewable sources of organic carbon [3,4,5]. Derived from plant materials via photosynthesis, lignocellulosic biomass represents the most abundant renewable source of organic matter on Earth. It is primarily composed of three structural polymers, namely cellulose, hemicellulose, and lignin, along with minor constituents such as proteins, pigments, and minerals [6,7].
Despite its significant potential, a substantial portion of lignocellulosic biomass generated globally, particularly in the form of agricultural residues, is either discarded or incinerated. This practice results in the loss of valuable opportunities to convert such biomass into high-value products, including biofuels, biochemicals, and biopolymers [8,9]. Among agricultural residues, corn residues (e.g., stalks, cobs, leaves, husks, silks, etc.) are among the most prevalent, owing to corn’s status as the world’s leading grain crop. Global maize production is estimated at approximately 1.2 billion metric tons annually, with the United States and China accounting for more than half of this total. Other major corn-producing countries include Brazil, Argentina, Ukraine, Mexico, South Africa, and Canada [10].
Compared to conventional sugar, and starch-based feedstocks, lignocellulose offers greater economic viability due to its lower cost, non-competition with food resources, and broad geographic availability [11,12]. However, the principal challenge in utilising lignocellulosic biomass lies in its complex and recalcitrant structure. Lignin forms a protective barrier around cellulose fibers, while cellulose is tightly bound to hemicellulose, creating a matrix characterised by high crystallinity and limited surface accessibility factors that collectively impede enzymatic hydrolysis [13].
To overcome the recalcitrant nature of lignocellulosic biomass, a pretreatment step is essential prior to enzymatic saccharification. Pretreatment disrupts the lignocellulosic matrix, enhancing enzyme accessibility, and may involve physical (e.g., grinding, milling), chemical (e.g., dilute acid or alkaline treatments), physico-chemical (e.g., steam explosion, ammonia fiber expansion), or biological approaches [14,15]. Among these, dilute acid pretreatment is widely employed at the industrial scale due to its effectiveness in hydrolysing hemicellulose, swelling cellulose fibers, and partially removing lignin. This process is typically conducted at temperatures between 140 and 215 °C with acid concentrations ranging from 0.5 to 10.0% (w w−1) [16].
Following pretreatment, the biomass undergoes enzymatic hydrolysis using cellulase-based enzyme cocktails to depolymerise cellulose and hemicellulose into fermentable sugars. The efficiency of hydrolysis depends on several parameters, including temperature, pH, enzyme loading, and substrate characteristics [17,18]. Commercial enzyme formulations commonly used include Celluclast (from Trichoderma reesei) and Novozym 188 (rich in β-glucosidase), along with more advanced preparations such as Cellic CTec2, HTec2, and the Accellerase product line [19].
The resulting sugar-rich hydrolysate serves as the substrate for microbial fermentation to produce ethanol, one of the most prominent biofuels. The conventional lignocellulosic ethanol production process comprises four stages: pretreatment, enzymatic saccharification, fermentation, and ethanol recovery [20,21]. Several bioconversion strategies have been developed to convert lignocellulosic biomass into ethanol, each with specific advantages and limitations. The traditional approach, Separate Hydrolysis and Fermentation (SHF), involves two discrete steps: enzymatic hydrolysis of the pretreated biomass, followed by microbial fermentation of the resulting sugars. While SHF allows for independent optimisation of each step, it increases processing time, energy consumption, and equipment demand, and is susceptible to product inhibition due to sugar accumulation during hydrolysis [21].
In contrast, Simultaneous Saccharification and Fermentation (SSF) combines enzymatic hydrolysis and fermentation in a single bioreactor, reducing sugar accumulation and enzyme inhibition. This integration simplifies process control and lowers capital costs but requires a compromise in operational parameters to accommodate both enzyme activity and microbial growth [18,22]. An extension of SSF is Simultaneous Saccharification and Co-Fermentation (SScF), which enables concurrent fermentation of both hexose and pentose sugars alongside enzymatic hydrolysis. This approach aims to maximise sugar utilisation and ethanol yield; however, it is constrained by the limited pentose-fermenting capacity of conventional industrial yeasts such as Saccharomyces cerevisiae. Consequently, mixed cultures or genetically engineered strains capable of co-fermenting glucose and xylose are often required [21,23].
A more integrated approach, Consolidated Bioprocessing (CBP), merges enzyme production, saccharification, and fermentation into a single step using a single microorganism. CBP offers significant potential for process simplification and cost reduction but remains limited by the scarcity of naturally occurring microorganisms that possess both efficient enzyme-producing capabilities and high ethanol productivity [24].
A major challenge in lignocellulosic biomass fermentation is the efficient conversion of both hexose and pentose sugars. While Saccharomyces cerevisiae is the industrial benchmark for hexose fermentation due to its high ethanol yield and inhibitor tolerance, it lacks the metabolic capacity to ferment pentoses such as xylose and arabinose. To address this limitation, co-cultures incorporating pentose-fermenting yeasts such as Pichia stipitis or Candida shehatae have been explored. However, these yeasts typically demonstrate lower ethanol productivity and greater sensitivity to fermentation inhibitors [25,26].
A particularly promising microorganism for the integrated bioconversion of lignocellulosic biomass is Mucor indicus, a filamentous fungus capable of fermenting both hexoses and pentoses at elevated temperatures that are compatible with enzymatic hydrolysis [27]. In addition to ethanol, M. indicus can produce high-value co-products such as chitosan and lipids, which have applications in pharmaceuticals, agriculture, and biodiesel production [28]. Notably, M. indicus also possesses the capacity to produce xylitol, a sugar alcohol extensively used as a sugar substitute. Xylitol offers health benefits for individuals with diabetes and oral health conditions and is widely applied in the pharmaceutical and nutraceutical industries. The global xylitol market is expanding, driven by increasing health awareness and the demand for sugar-free products [29]. Under aerobic conditions, M. indicus primarily exhibits filamentous growth, which is typically associated with low xylitol yields. However, xylitol productivity improves markedly under oxygen-limited conditions that induce a mixed morphology comprising both yeast-like and filamentous forms [30].
The objective of this study is to optimise the enzymatic hydrolysis of dilute-acid-pretreated corn cobs using a mixture of commercial enzyme preparations, namely the Cellulase enzyme blend (Cellic CTec2) and Viscozyme L, to maximise the release of fermentable sugars. Subsequently, the study aims to optimise simultaneous saccharification and fermentation (SSF), using this enzyme system in combination with M. indicus in order to maximise the efficiency of ethanol production. Finally, the optimised SSF process is scaled up in a stirred-tank bioreactor to assess its feasibility for industrial application.
2. Materials and Methods
2.1. Feedstock and Working Microorganism
In this study, corn cobs obtained from a commercial corn producer located in the northern region of Croatia were employed as the lignocellulosic feedstock. The cobs were first size-reduced using a hammer mill (NA45; Megametal d.o.o., Kotoriba, Croatia) and subsequently sieved through a 5 mm mesh. Dilute acid pretreatment was performed in a high-pressure reactor at 180 °C for 5 min, following the protocol established by Marđetko et al. [16]. The pretreatment resulted in the formation of solid and liquid phases, of which only the solid fraction, referred to as pretreated corn cobs (PCCs), was utilised in this study.
The composition of the solid phase of PCCs was determined using the standard procedure developed by the National Renewable Energy Laboratory (NREL) [31] and expressed as percentage of dry weight (w w−1): 55.58 ± 3.48% glucans, 1.11 ± 0.07% xylans, 0.48 ± 0.03% arabinans, 4.32 ± 0.27% acetic acid, 4.31 ± 0.27% formic acid, 0.21 ± 0.01% acid-soluble lignin, and 27.30 ± 1.71% lignin.
The filamentous fungus Mucor indicus DSM 2185 was used as the working microorganism. The strain was obtained from the culture collection of the Laboratory for Biochemical Engineering, Industrial Microbiology, and Malting and Brewing Technology at the University of Zagreb, Faculty of Food Technology and Biotechnology, Croatia. Pure cultures were maintained on solid medium composed of (g L−1): 25 glucose, 5 meat extract, 2 diammonium sulfate (DAS), 2 diammonium phosphate (DAP), and 20 agar. The medium was sterilised at 121 °C for 15 min in an autoclave. Unless otherwise specified, all chemicals used in this study were procured from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Preparation of Acetate Buffer and Matrix Nutrient Medium
To prepare the acetate buffer, a 0.2 M acetic acid solution (Solution A) and a sodium acetate trihydrate solution (C2H3O2Na·3H2O; Solution B) were used. Solution A was prepared by adding 5.78 mL of glacial acetic acid to demineralised water and adjusting the final volume to 0.5 L. Solution B was prepared by dissolving 13.6 g of sodium acetate trihydrate in demineralised water, also adjusted to a final volume of 0.5 L. The acetate buffer was then formulated by mixing 74 mL of Solution A with 176 mL of Solution B in a 250 mL Erlenmeyer flask, yielding a final pH of 5.0.
The matrix nutrient medium (MNM), designed to simulate conditions for simultaneous saccharification and fermentation, was prepared using the following components (g L−1): 5 yeast extract, 2 diammonium phosphate (DAP), 2 diammonium sulfate (DAS), and 40 g L−1 pretreated corn cobs (PCCs) as the substrate. The pH of the medium was adjusted to 5.0 prior to sterilisation at 121 °C for 15 min in an autoclave.
2.3. Enzymatic Hydrolysis of PCCs
Enzymatic hydrolysis of pretreated corn cobs (PCCs) was performed using a combination of the Cellulase enzyme blend (synonym: Cellic CTec2; Sigma-Aldrich, St. Louis, MO, USA) and Viscozyme L (Sigma-Aldrich, St. Louis, MO, USA) in a 50 mL acetate buffer (pH 5.0). To optimise hydrolysis conditions, experiments were designed to evaluate the effects of varying substrate and enzyme concentrations. PCCs concentrations of 10, 20, and 40 g L−1 were tested. Reaction mixtures were prepared in sealed flasks and sterilised by autoclaving at 121 °C for 15 min.
Enzyme loadings were applied in three combinations of Cellulase/Viscozyme L (1/1%, 2/5%, and 5/10% vol vol−1) across the different PCCs concentrations, resulting in a range of enzyme loadings per gram of PCCs (Table 1). Hydrolysis was carried out at 50 °C with continuous magnetic stirring at 160 rpm. Samples were withdrawn at predetermined time intervals and analysed using ultra-performance liquid chromatography (UPLC).

Table 1.
Enzyme-to-substrate (E/PCCs) load used in PCCs hydrolysis experiments.
Enzymatic Hydrolysis Optimisation Using DesignExpert Software
To further optimise the enzymatic hydrolysis of pretreated corn cobs (PCCs), a statistical design of experiments (DoE) approach was employed using DesignExpert v11 software (Stat-Ease, Inc., Minneapolis, MN, USA). Response Surface Methodology (RSM) was applied using a Box–Behnken design (randomised, without blocks), consisting of 24 experimental runs. A quadratic model was selected to enable the evaluation of nonlinear effects and interactions between the independent variables.
The concentrations of the Cellulase enzyme blend and Viscozyme L (vol vol−1) were defined as independent variables, both to be minimised, while hydrolysis time was fixed at 48 h and the PCCs concentration was maintained at 40 g L−1. The response variable, PCCs hydrolysis efficiency (PCCs-HE), was set as the target for maximisation. Hydrolysis experiments were conducted in flasks containing 50 mL of either acetate buffer or matrix nutrient medium (MNM).
Based on the experimental results, the DoE software (DesignExpert v11 software, Stat-Ease, Inc., Minneapolis, MN, USA) generated a predictive quadratic model to identify the optimal enzyme concentrations for maximising PCCs-HE under the specified conditions. The resulting predictive model is expressed as follows:
where Cell and Vis are enzymes concentrations [vol vol−1] and t is time [h].
w (PCCs-HE) = 11.02981 − 8.71089 (Cell) + 6.56444 (Vis) + 1.17608 (t) [%]
The results of the predictive model indicated that the optimal enzyme combination for maximising PCCs hydrolysis efficiency consisted of 2% (vol vol−1) Cellulase enzyme blend and 5.18% (vol vol−1) Viscozyme L, corresponding to enzyme loadings of 48.9 FPU g−1 PCCs and 171.8 U g−1 PCCs, respectively. This enzyme combination was subsequently used in further experiments.
To validate the findings from the optimisation study, hydrolysis of PCCs was conducted at four different temperatures (20 °C, 30 °C, 40 °C, and 50 °C) over a 48 h period, under continuous magnetic stirring at 160 rpm. Samples were collected at defined time points and analysed using ultra-performance liquid chromatography (UPLC).
2.4. Preparation of Nutrient Media for Cultivation of Mucor indicus
For inoculum preparation, a nutrient medium was prepared containing the following components (g L−1): 20 glucose, 5 yeast extract, 2 diammonium phosphate (DAP), and 2 diammonium sulfate (DAS). The medium was sterilised at 121 °C for 15 min.
In the subsequent simultaneous saccharification and fermentation (SSF) experiments, pretreated corn cobs (PCCs) were used as the carbon source at a concentration of 40 g L−1. Two different media were evaluated: one based on acetate buffer (pH 5.0), and the other on matrix nutrient medium (MNM).
For bioreactor cultivations, MNM was employed. The SSF process was carried out in a 1.5 L stirred-tank bioreactor (Biostat MD, B. Braun Biotech International, Melsungen, Hessen, Germany), with the pH of the medium adjusted to 5.0 prior to sterilisation. The bioreactor was filled with demineralized water to reach the final working volume and then sterilised at 121 °C for 15 min.
2.5. Simultaneous Saccharification and Fermentation of PCCs by M. indicus for Ethanol Production
The inoculum of Mucor indicus was prepared using the nutrient medium described in Section 2.4. Fungal spores were harvested from agar plates containing pure culture using 0.1% (vol vol−1) Tween 80 solution. Spore concentration was determined using a Thoma counting chamber, and 106 spores were inoculated into 250 mL of sterile medium in 500 mL Erlenmeyer flasks. Cultivation was carried out under aerobic conditions at 30 °C with shaking at 140 rpm for 24 h.
2.5.1. Simultaneous Saccharification and Fermentation in Flasks
SSF was conducted in 500 mL Erlenmeyer flasks containing 250 mL of medium (either acetate buffer or matrix nutrient medium, MNM) supplemented with 40 g L−1 PCCs. Each flask was inoculated with 8% (vol vol−1) of the prepared M. indicus culture. In addition, 2% (vol vol−1) Cellulase enzyme blend and 5.18% (vol vol−1) Viscozyme L were added, corresponding to enzyme loadings of 48.9 FPU g−1 PCCs and 171.5 U g−1 PCCs, respectively. The flasks were incubated at three different temperatures (20 °C, 30 °C, and 40 °C) on a rotary shaker at 200 rpm for 48 h. Samples were taken at regular intervals during cultivation and analysed using ultra-performance liquid chromatography (UPLC) for sugar release, ethanol, and other secondary metabolites production.
2.5.2. Simultaneous Saccharification and Fermentation in Stirred Tank Bioreactor
SSF of PCCs was also performed in a 1.5 L stirred-tank bioreactor using MNM as the fermentation medium. The working volume was maintained at 1.5 L, and the system was inoculated with 8% (vol vol−1) M. indicus culture. To facilitate saccharification, 2% (vol vol−1) Cellulase enzyme blend and 5.18% (vol vol−1) Viscozyme L were added (48.9 FPU g−1 PCCs; 171.5 U g−1 PCCs). The process was conducted under aerobic conditions at 30 °C up to 48 h, with an aeration rate of 2 L min−1 and an agitation speed of 50 rpm. To minimise foam formation, which can interfere with bioprocess efficiency, Antifoam 204 was added at a concentration of 1 mL L−1. Samples were collected periodically throughout the SSF process and analysed via UPLC to monitor saccharification, ethanol production, and other metabolites synthesis.
2.6. Analytical Methods
2.6.1. Determination of Cellulase and Xylanase Enzyme Activities
To determine cellulase activity (CA), the method described by Ghose [32] was adapted. Filter paper strips (50 mg, 1 × 6 cm) were placed in Eppendorf cuvettes along with 1 mL of acetate buffer (pH 5.0). The cuvettes were pre-incubated in a thermostatic bath at 50 °C for 10 min. Serial dilutions of the commercial enzyme preparations were made using the same buffer: for Viscozyme L at 0.1%, 0.4%, 0.6%, 0.8%, and 5% (vol vol−1); and for the Cellulase enzyme blend at 0.1%, 0.2%, 0.4%, 0.6%, and 1% (vol vol−1).
To each cuvette, 0.5 mL of the corresponding diluted enzyme solution was added. The mixtures were incubated in a Thermo-Shaker (TS 100, Biosan, Riga, Latvia) at 50 °C for 60 min. The reaction was terminated by heating the samples at 95 °C for 15 min. All samples were subsequently analysed using ultra-performance liquid chromatography (UPLC).
Cellulase activity (CA) was calculated according to Equation (2) [32]. For the enzyme control, the same procedure was followed using 1 mL of buffer and 0.5 mL of the 2% (vol vol−1) diluted enzyme solution, while for the substrate control, 1.5 mL of buffer and 50 mg of filter paper were used.
where wenzyme represents the volumetric percentage of enzyme required to release 2 g L−1 of glucose under the assay conditions. One filter paper unit (FPU mL−1) is defined as the amount of enzyme that liberates 1 μmol of glucose per min per mL of enzyme solution under the specified test conditions.
To determine xylanase activity (XA), 50 mg of xylan was used as the substrate. Serial dilutions of the commercial enzyme preparations were prepared using acetate buffer (pH 5.0). For Viscozyme L, the dilutions were 5%, 1%, 0.8%, 0.6%, 0.4%, 0.2%, and 0.1% (vol vol−1), while for the Cellulase enzyme blend, the dilutions were 5%, 1%, 0.8%, 0.6%, and 0.4% (vol vol−1). All other experimental steps followed the same procedure as described for the determination of cellulase activity. Xylanase activity was calculated according to Equation (3).
where wenzyme represents the volumetric percentage of enzyme required to release 2 g L−1 of xylose under the assay conditions. One unit (U mL−1) is defined as the amount of enzyme that liberates 1 μmol of xylose per min per mL of enzyme solution under the specified test conditions.
2.6.2. UPLC Analysis
The ultra-performance liquid chromatography (UPLC) analysis was conducted using an Agilent Technologies 1290 Infinity II system (Santa Clara, CA, USA), comprising a G7104A 1290 Flexible Pump, a G7129B 1290 Vialsampler, a temperature-controlled oven, a Rezex ROA-Organic Acid H+ analytical column (15 cm × 7.2 mm; Phenomenex, Torrance, CA, USA) with corresponding pre-columns, a G7162A 1260 RID refractive index detector, and OpenLAB CDS chromatography software (Rev. C. 01. 08 [210]). The mobile phase consisted of 0.0025 M sulfuric acid, delivered at a flow rate of 0.6 mL min−1, with an injection volume of 10 µL, following the method described by Marđetko et al. [33]. Prior to analysis, the samples were centrifuged to isolate the supernatant, which was then mixed in a 1:1 (vol vol−1) ratio with a 100 g L−1 zinc sulfate (ZnSO4·7H2O) solution to precipitate proteins. After 10 min of incubation, the precipitated proteins were removed by centrifugation at 4000 rpm for 10 min. The resulting supernatant was filtered through a 0.2 μm nylon syringe filter before injection. Chromatographic separation was performed on the Rezex ROA-Organic Acid H+ column, with the mobile phase maintained at 60 °C in the oven and the RID detector set to 40 °C.
2.6.3. Calculation of Bioprocess Efficiency Parameters
Bioprocess efficiency parameters were calculated according to the literature as follows [34,35]:
where CS0, CS are the initial and final substrate concentration (g L−1); CPO, CP are the initial and final product concentration (g L−1); YS is the total consumption of the substrates (g L−1); YP is the total product yield (g L−1); YP/S is the conversion coefficient of the substrate into the product (g g−1); YP/ST is the theoretical conversion coefficient of the substrate into the product (g g−1); E is the bioprocess efficiency (%), Pr is the bioprocess productivity (g L−1 h−1) and t is time (h).
2.6.4. Statistical Analysis
The standard deviation of the experimental data was calculated using the default statistical procedures implemented in Statistica software, version 12.0 (StatSoft, Tulsa, OK, USA). All experiments were conducted at least in duplicate, and the results are reported as mean values in the corresponding tables and figures.
3. Results and Discussion
3.1. Enzymatic Hydrolysis of PCCs in Different Experimantal Conditions
Given the limited publicly available information on the specific enzymatic activities of the commercial enzyme preparations, specifically Viscozyme L and the Cellulase enzyme blend (Cellic CTec2), the experimental determination of both cellulase and xylanase activities was undertaken to support rational optimisation of enzyme loading for lignocellulosic biomass hydrolysis. Due to the differing enzyme compositions specified by the manufacturer, considerable variability in enzymatic performance was expected.
Protein concentrations of the commercial formulations were quantified as 103.89 ± 1.12 g L−1 for Viscozyme L and 198.25 ± 2.21 g L−1 for the Cellulase enzyme blend consistent with data from the literature [36,37]. Based on these values, specific enzyme activities were calculated. The Cellulase enzyme blend demonstrated a cellulase activity of 76.45 ± 0.91 FPU mL−1, compared to 8.18 ± 0.07 FPU mL−1 for Viscozyme L. These findings confirm Viscozyme L’s low cellulase activity, while the Cellulase enzyme blend compares well with Celluclast 1.5 FG L (84 FPU mL−1) [38] and surpasses BTXL from T. reesei (55 FPU mL−1) [39]. These results align with the functional roles of the enzymes: the Cellulase enzyme blend is specialised for cellulose degradation, whereas Viscozyme L contains a broader spectrum of carbohydrases, including hemicellulases. According to the available literature, the Cellulase enzyme blend is 1.5 times less effective for the conversion of lignocellulosic biomass into ethanol than the new upgraded enzyme blend Cellic CTec3 [40]. Assessment of xylanase activity further confirmed this distinction. Viscozyme L exhibited xylanase activity of 128.02 ± 1.13 U mL−1, while the Cellulase enzyme blend showed significantly lower activity at 12.04 ± 0.08 U mL−1. Though both are lower than the activities reported for high-performance commercial xylanases such as Celluclast 1.5 L (905 U mL−1) and Novozym 188 (605 U mL−1) [41], the differences in activity profiles underscore the complementary nature of the two enzymes.
To explore their synergistic hydrolytic potential, a systematic investigation of enzymatic hydrolysis was carried out using pretreated corn cobs (PCCs) as the substrate and various combinations of the two enzyme preparations across a range of substrate concentrations (Figure 1). In all experiments conducted in this study, enzyme dosages were expressed as volumetric ratios (vol vol−1) rather than standardised activity units (e.g., FPU or U). This approach was chosen due to the complex, multi-enzyme nature of the commercial formulations and the absence of universally defined activity values for these preparations. Volumetric dosing ensured consistency and reproducibility across all experimental setups. Nevertheless, the corresponding enzyme activities, expressed in FPU g−1 PCCs and U g−1 PCCs, were independently determined and reported to facilitate process comparison and support future scale-up efforts (Table 1).
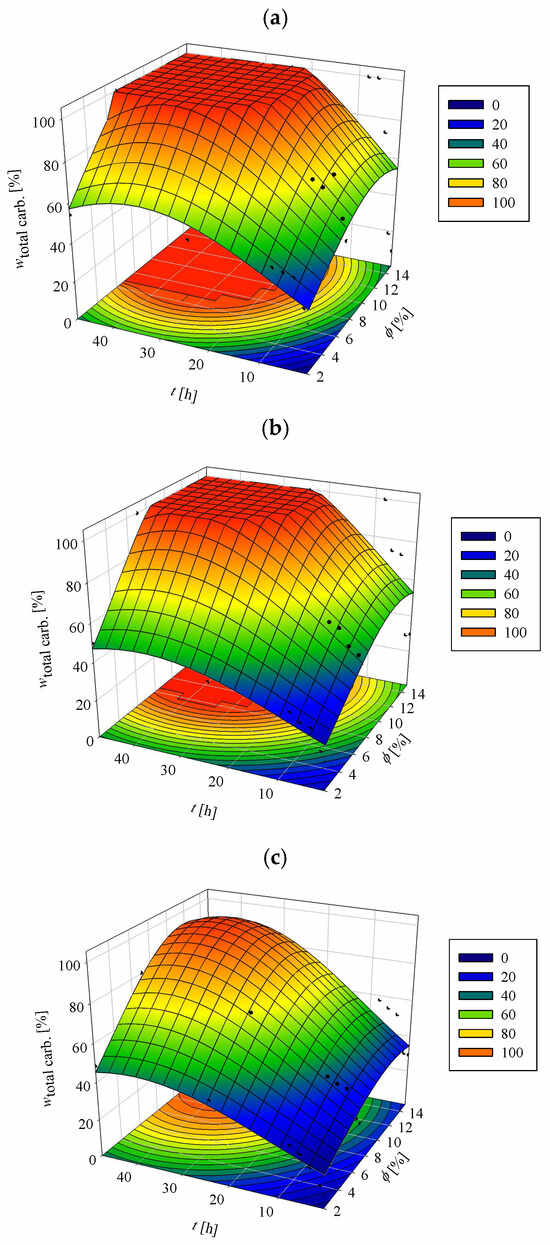
Figure 1.
Enzymatic hydrolysis efficiency of PCCs at different substrate concentrations: (a) 10 g L−1, (b) 20 g L−1, (c) 40 g L−1. Hydrolysis was performed in acetate buffer (50 mM, pH 4.8) at 50 °C with varying enzyme ratios (Cellulase enzyme blend/Viscozyme L) and hydrolysis time. Black dots in the figure represent the average values of the experimental data.
At the lowest substrate concentration tested (10 g L−1; Figure 1a), the combination of 1% (vol vol−1) Cellulase enzyme blend and 1% (vol vol−1) Viscozyme L, corresponding to enzyme loadings of 84.7 FPU g−1 PCCs and 140.06 U g−1 PCCs, respectively, achieved a total carbohydrate conversion of 54.12 ± 2.05% after 48 h. Under these conditions, glucan and xylan conversions reached 57.38 ± 1.60% and 100%, respectively. Increasing the enzyme concentrations to 2% Cellulase enzyme blend and 5% Viscozyme L (194.0 FPU g−1 PCCs; 664.18 U g−1 PCCs) resulted in complete hydrolysis of all carbohydrates within the same time period. At the highest enzyme loading tested (5%/10%; 464.5 FPU g−1 PCCs; 1340.4 U g−1 PCCs), full carbohydrate conversion was achieved in just 6 h, with xylan hydrolysis completed as early as 2 h.
At the intermediate substrate concentration (20 g L−1; Figure 1b), the 1%/1% enzyme combination (42.35 FPU g−1 PCCs; 70.03 U g−1 PCCs) yielded a total carbohydrate conversion of 49.76 ± 1.04% after 48 h, with glucan and xylan conversions of 56.61 ± 1.30% and 61.52 ± 1.07%, respectively. The 2%/5% enzyme mixture (97.0 FPU g−1 PCCs; 332.09 U g−1 PCCs) again enabled complete hydrolysis within 48 h, while the 5%/10% combination (232.25 FPU g−1 PCCs; 670.2 U g−1 PCCs) reduced the required time to just 8 h. In both cases, full glucan hydrolysis required the entire 48 h period, whereas xylan was completely hydrolysed within 4 h.
At the highest substrate concentration tested (40 g L−1; Figure 1c), overall hydrolysis efficiencies decreased, likely due to process limitations at high solids loadings. The 1%/1% enzyme formulation (21.18 FPU g−1 PCCs; 35.02 U g−1 PCCs) achieved a total carbohydrate conversion of 48.29 ± 1.65%. This was significantly improved by the 2%/5% (48.5 FPU g−1 PCCs; 166.05 U g−1 PCCs) and 5%/10% (116.13 FPU g−1 PCCs; 335.1 U g−1 PCCs) formulations, which resulted in conversion efficiencies of 84.88 ± 0.90%, and 80.67 ± 1.10%, respectively. Glucan conversion followed a similar pattern, rising from 54.37 ± 0.90% to 94.25 ± 1.23% and 99.72 ± 2.01% with increasing enzyme loading. However, the marginal improvement between the medium and the highest enzyme concentrations suggests diminishing returns, likely due to mass transfer limitations caused by higher viscosity and reduced enzyme–substrate accessibility [42]. In contrast, xylan hydrolysis remained efficient across all enzyme levels, reaching completion within 4–8 h at the higher dosages.
Among all tested conditions, the combination of 2% (vol vol−1) Cellulase enzyme blend and 5% (vol vol−1) Viscozyme L consistently demonstrated the best performance. It achieved near-complete hydrolysis (80–100% total carbohydrate conversion depending on substrate concentration) with enzyme loadings of 48.9 FPU g−1 PCCs and 171.5 U g−1 PCCs. This formulation provided the most favourable balance between enzymatic efficiency and economic feasibility. As reported by Althuri and Banerjee [42], exceeding the optimal enzyme dose typically leads to disproportionate cost increases without meaningful improvements in conversion. Persistent lignin in PCCs likely contributed to reduced hydrolysis efficiency at higher substrate concentrations through mechanisms such as physical occlusion, non-productive enzyme binding, and phenolic inhibition [37,43]. Nevertheless, the strategic pairing of enzymes in this study effectively mitigated these constraints, enabling high saccharification yields across all substrate levels.
Comparison with recent literature further supports the advantages of the present approach. Kleingesinds et al. [44] reported glucose yields of approximately 80–85% by incorporating Tween 80 during pretreatment and saccharification, while also reducing Cellic CTec2 usage by 41.67%. The addition of this non-ionic surfactant facilitated lignin removal and disrupted biomass structure, enhancing enzyme accessibility and contributing to improved hydrolysis. However, despite these enhancements, their final yields remained 20–25% lower than those achieved in the current study, which relied solely on the synergistic action of a dual-enzyme cocktail. Moreover, the formulation used in this work enabled rapid and high saccharification efficiency without the inclusion of surfactants or additional additives. While the use of surfactants could potentially allow further reductions in enzyme loading and remains a promising strategy for improving cost-efficiency, it was not within the scope of this study and will be considered in future investigations aimed at process optimisation and economic feasibility.
In a related study, Sahare et al. [45] reported conversion efficiencies ranging from 64% to 100% during the enzymatic hydrolysis of alkali-pretreated corn cobs using Penicillium pinophilum cellulases at enzyme loadings of 5–20 FPU g−1 over 8–48 h. While the final yields are comparable to those obtained in the present study, several key differences must be considered. The current study utilised dilute-acid-pretreated corn cobs as the substrate, which typically retain a higher proportion of lignin compared to alkali-pretreated biomass. This residual lignin is known to inhibit enzymatic hydrolysis by promoting non-productive enzyme binding, physically shielding polysaccharides, and releasing phenolic compounds that interfere with enzyme activity. Consequently, dilute-acid-pretreated biomass presents greater hydrolytic resistance. To overcome these challenges, our study employed a broader range of enzyme loadings, as high as 116.1 FPU g−1 PCCs and 335.1 U g−1 PCCs, substantially exceeding the enzyme concentrations used in Sahare et al.’s work [45]. Despite the more uncooperative nature of the substrate, the optimised dual-enzyme formulation in the present study enabled significantly faster hydrolysis kinetics and consistently high conversion efficiencies across a broad range of substrate concentrations. These results highlight the robustness, adaptability, and industrial relevance of the enzyme system used, particularly for processing lignin-rich lignocellulosic feedstocks.
Similarly, Chen et al. [46] investigated fed-batch enzymatic hydrolysis of sulfuric-acid-pretreated corn cobs using T. reesei cellulase (20 FPU g−1) and A. niger cellobiase, achieving a hydrolysis yield of 79.5% and 116.3 g L−1 of reducing sugars at 200 g L−1 substrate after 60 h. Their approach benefited from improved mixing and reduced inhibition but required multi-step feeding and enzyme supplementation. In comparison, the present study employed a simpler batch process at 40 g L−1 substrate using a co-applied dual-enzyme system, achieving a higher yield of 92.66% in 48 h. Although this required a higher enzyme load, it avoided fed-batch complexity and additional enzyme components. The integration of in situ enzyme production in future work could offset enzyme costs and enhance process feasibility.
In summary, the dual-enzyme system demonstrated consistently high saccharification efficiency across all tested substrate concentrations, with particularly strong performance at 40 g L−1 PCCs. Higher substrate concentrations (≥40 g L−1 PCCs) were also explored; however, these trials proved unsuccessful due to excessive viscosity, which limited mixing efficiency and reduced enzyme accessibility. This enzyme combination achieved near-complete hydrolysis within 48 h in batch mode, without the need for surfactants, accessory enzymes, or fed-batch configurations. While the enzyme loading is relatively high, the simplicity and speed of the process, combined with potential future implementation of in situ enzyme production, underscore its feasibility for scalable, cost-effective integration into lignocellulosic biorefinery systems.
Building upon these results, enzymatic hydrolysis at the highest substrate concentration (40 g L−1 PCCs) was further optimised using DesignExpert v 11 software (Stat-Ease, Inc., Minneapolis, MN, USA). The analysis identified the optimal enzyme formulation as 2% (vol vol−1) Cellulase enzyme blend and 5.18% (vol vol−1) Viscozyme L, corresponding to enzyme loadings of 48.9 FPU g−1PCCs and 171.5 U g−1PCCs, and protein loadings of 0.099 g g−1 PCCs and 0.135 g g−1 PCCs for the Cellulase enzyme blend and Viscozyme L, respectively. Under these optimised conditions, the model predicted a maximum total carbohydrate conversion of 92.66% after 48 h, representing a 1.15-fold improvement compared to the already effective 2%/5% formulation.
To validate the model predictions, confirmatory hydrolysis experiments were performed under the optimised enzyme conditions in both acetate buffer and matrix nutrient medium (MNM) over a 48 h period (Figure 2). While buffered systems are generally recommended by enzyme suppliers to maintain optimal catalytic performance, MNM was included to simulate conditions more representative of integrated biorefinery operations, particularly those compatible with downstream microbial fermentation. Interestingly, the enzyme system performed equally well or better in MNM. The enhancement of PCCs hydrolysis in the matrix nutrient medium (MNM), compared to the acetate buffer, is likely due to the more complex composition of MNM, which contains additional nutrients, minerals, and growth-supporting factors that can improve enzyme stability, catalytic performance, and substrate-enzyme interactions. These components may mitigate inhibitory effects from lignin-derived phenolics, or other degradation byproducts present in the dilute-acid-pretreated PCCs. Moreover, certain ions and cofactors present in MNM could stabilise the enzyme structure or modulate the pH locally, contributing to more favourable reaction kinetics These findings show that the optimised dual-enzyme system performs efficiently even with residual lignin, highlighting the value of using media that reflect real biorefinery conditions, especially in integrated SSF processes.
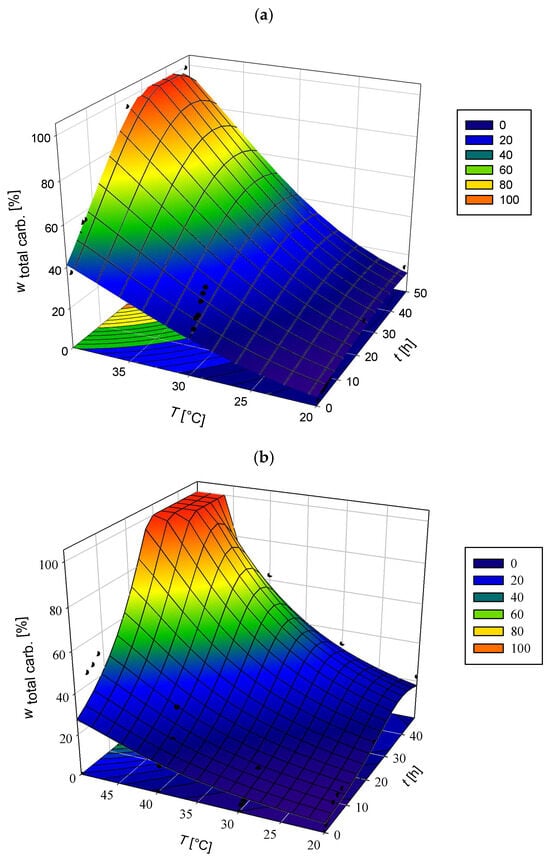
Figure 2.
Effect of temperature and hydrolysis time on enzymatic saccharification efficiency of 40 g L−1-pretreated corn cobs. Hydrolysis was performed in (a) acetate buffer (50 mM, pH 4.8) and (b) MNM, using an optimised enzyme cocktail (2% vol vol−1 Cellulase enzyme blend and 5.18% vol vol−1 Viscozyme L). Black dots in the figure represent the average values of the experimental data.
In acetate buffer, PCCs hydrolysis efficiency exhibited a clear dependence on temperature (Figure 2a). At 50 °C, complete carbohydrate conversion (100%) was achieved within 48 h, outperforming previous studies, where glucan and xylan conversions reached only 52.69% and 62.84%, respectively, under similar conditions [47]. As the temperature decreased, hydrolysis efficiency declined considerably: at 40 °C, total carbohydrate conversion dropped to 62.68%; at 30 °C-within the optimal range for microbial growth—conversion decreased further to 32.98 ± 1.90%; and at 20 °C, it fell to 22.43 ± 1.41%. These trends reflect the temperature sensitivity of enzymatic activity, which is further challenged by the structural recalcitrance and lignin-rich composition of dilute-acid-pretreated corn cobs (PCCs).
Parallel experiments conducted in matrix nutrient medium (MNM) revealed distinct performance trends (Figure 2b). The total carbohydrate conversion (100%) was achieved at 40 °C-surpassing the 62.68% obtained in the buffer under the same conditions. At 30 °C, MNM supported a 40.03 ± 3.04% conversion, which was 7.05% higher than that achieved in buffer. However, at 20 °C, MNM underperformed slightly, yielding 15.29 ± 0.96–7.14% lower than the conversion observed in buffer. It is important to note that MNM hydrolysis was not conducted at 50 °C, as Mucor indicus does not tolerate such elevated temperatures. Thus, the experimental design prioritised conditions relevant to integrated SSF processes.
These findings indicate that although PCCs hydrolysis at 20 °C remains inefficient in both systems, MNM confers a clear advantage at intermediate temperatures (30–40 °C). The complete hydrolysis achieved at 40 °C in MNM demonstrates its suitability for bioprocesses requiring compatibility with microbial activity. Additionally, the improved performance at 30 °C suggests that MNM enhances enzymatic functionality under conditions aligned with microbial growth, supporting more effective integration of saccharification and fermentation in industrial-scale bioconversion workflows.
Overall, these results provide key insights for bioprocess scale-up, illustrating the interplay between medium composition and temperature in determining hydrolysis efficiency, particularly for lignin-rich, dilute-acid-pretreated biomass. While 50 °C remains optimal for stand-alone enzymatic hydrolysis in buffered systems, MNM enables high conversion yields under more biologically relevant conditions. This is a critical consideration for the development of economically viable biorefineries, where maintaining both enzymatic activity and microbial viability is essential for efficient, integrated process performance.
3.2. Simultaneous Saccharification and Fermentation of PCCs for Ethanol Production by M. indicus DSM 2185 in Erlenmeyer Flasks
The cultivation of Mucor indicus DSM 2185 was performed on the solid fraction of corn cobs remaining after dilute acid pretreatment in a high-pressure reactor. Simultaneous saccharification and fermentation (SSF) was conducted under aerobic conditions using two media systems: an acetate buffer and matrix nutrient medium (MNM). Enzymatic saccharification was facilitated by the addition of 2% (vol vol−1) Cellulase enzyme blend and 5.18% (vol vol−1) Viscozyme L, corresponding to enzymatic loadings of 48.9 FPU g−1 PCCs and 171.5 U g−1 PCCs, respectively (Figure 3 and Figure 4).
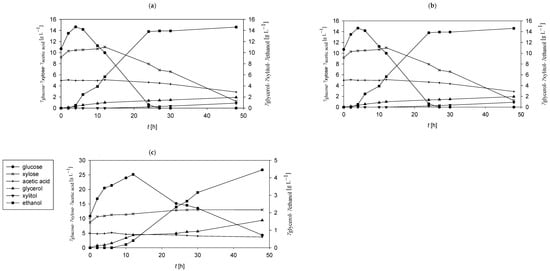
Figure 3.
Concentration profiles of glucose (●), xylose (×), glycerol (▲), xylitol (▼), and ethanol (■) during SSF of PCCs in an acetate buffer (pH = 5) using Mucor indicus under varying temperature conditions. The enzymatic hydrolysis was initiated with 2% (vol vol−1) Cellulase enzyme blend and 5.18% (vol vol−1) Viscozyme L. SSF was conducted under aerobic conditions with orbital agitation (200 rpm) at (a) 20 °C, (b) 30 °C, (c) 40 °C.
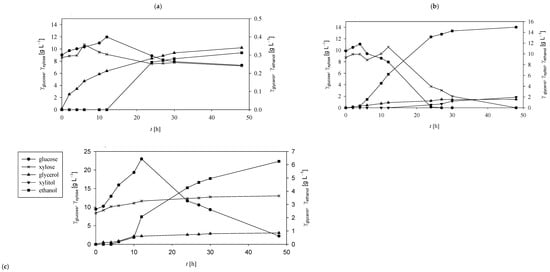
Figure 4.
Concentration profiles of glucose (●), xylose (×), glycerol (▲), xylitol (▼), and ethanol (■) during SSF of PCCs in MNM using Mucor indicus under varying temperature conditions. The enzymatic hydrolysis was initiated with 2% (vol vol−1) Cellulase enzyme blend and 5.18% (vol vol−1) Viscozyme L. SSF was conducted under aerobic conditions with orbital agitation (200 rpm) at (a) 20 °C, (b) 30 °C, (c) 40 °C.
In the acetate buffer system (Figure 3), SSF at varying temperatures resulted in distinct profiles of carbohydrate release, metabolite formation, and substrate utilisation. At 20 °C, glucose concentration peaked at 11.67 ± 1.15 g L−1 after 12 h, while xylose reached 11.22 ± 1.08 g L−1 at 48 h (Figure 3a). Increasing the temperature to 30 °C enhanced hydrolysis, with glucose peaking at 14.65 ± 0.85 g L−1 within 4 h, representing a 24.44% increase compared to the MNM system (Figure 3b). At 40 °C, the maximum glucose concentration reached 25.16 ± 1.21 g L−1 at 12 h, exceeding the corresponding MNM value by 8.66% (Figure 3c).
Acetate utilisation varied with temperature, with assimilation levels of 1.10 ± 0.10 g L−1, 2.18 ± 0.08 g L−1, and 1.47 ± 0.11 g L−1 observed at 20 °C, 30 °C, and 40 °C, respectively. Ethanol production exhibited pronounced temperature dependence, with final concentrations of 0.68 ± 0.01 g L−1 at 20 °C, 14.61 ± 1.08 g L−1 at 30 °C, and 4.46 ± 0.31 g L−1 at 40 °C. These correspond to substrate-to-ethanol conversion coefficients of 0.032, 0.376, and 0.206 g g−1, respectively (Table 2). Xylitol production was only detected at 30 °C, reaching 0.89 ± 0.01 g L−1, although at a lower yield than observed in MNM-based fermentations. The highest ethanol productivity within the acetate buffer system was recorded at 30 °C, reaching 0.304 ± 0.02 g L−1 h−1.

Table 2.
Bioprocess efficiency parameters of the SSF process using Mucor indicus, supplemented with 2% vol−1 vol−1 Cellulase enzyme blend and 5.18% vol−1 vol−1 Viscozyme L, under varying temperatures and nutrient medium compositions.
Experiments conducted in the matrix nutrient medium (MNM; Figure 4) revealed similar yet distinct trends compared to the acetate buffer system. In all tested conditions, the initial stages of SSF were characterised by increasing concentrations of glucose and xylose. At 20 °C, glucose rose from 9.03 ± 0.41 g L−1 to a peak of 11.98 ± 0.65 g L−1 at 12 h, before declining to 7.32 ± 0.65 g L−1 by the end of the process. Xylose peaked earlier, at 10.72 ± 1.01 g L−1 after 6 h (Figure 4a). Temperature had a pronounced effect on both sugar release and consumption. At 30 °C, the glucose concentration peaked at 11.07 ± 0.98 g L−1 after 4 h, while at 40 °C it reached 22.98 ± 1.65 g L−1 after 12 h-more than double the maximum at 20 °C. Xylose concentrations also peaked at 30 °C and 40 °C, reaching 10.56 ± 0.78 g L−1 and 10.75 ± 0.69 g L−1 after 6 and 12 h, respectively. Complete sugar consumption was only observed at 30 °C, whereas residual glucose and xylose were detected at both 20 °C and 40 °C.
Ethanol production followed a similar temperature-dependent trend. The highest concentration (14.95 ± 1.20 g L−1) was recorded at 30 °C, significantly outperforming yields at 40 °C (6.26 ± 0.09 g L−1) and 20 °C (0.31 ± 0.01 g L−1). Based on theoretical sugar availability (glucose: 31.26 g L−1; xylose: 11.12 g L−1), the maximum substrate-to-ethanol conversion coefficient was 0.373 g g−1 (equivalent to 73.14% of theoretical yield), with a corresponding ethanol productivity of 0.312 g L−1 h−1, both attained at 30 °C (Table 2).
These results are consistent with findings from previous studies. For example, Molaverdi et al. [48] reported ethanol concentrations of 99.4 g L−1 (89.5% yield) from rice straw using M. indicus in SSF following sodium carbonate pretreatment and enzyme supplementation at 20 FPU g−1. Even at lower enzyme loading (5 FPU g−1), ethanol production remained high (90.9 g L−1). Similarly, Karimi et al. [49] observed ethanol conversion coefficients of 0.34–0.35 g g−1 during anaerobic SSF of rice straw. Although our study employed higher enzyme loadings (48.9 FPU g−1 PCCs and 171.5 U g−1 PCCs), the use of dilute-acid-pretreated corn cobs, a more robust substrate due to higher lignin and inhibitor content, presents greater hydrolytic and fermentative challenges. Nonetheless, our aerobic system achieved comparable or slightly higher ethanol conversion coefficients (0.370–0.376 g g−1), highlighting the robustness of the selected enzyme formulation and the effectiveness of M. indicus under integrated SSF conditions using acid-pretreated lignocellulosic biomass. Although the application of Mucor indicus in SSF processes remains relatively underexplored, related studies support the outcomes of the present work. For instance, Kleingesinds et al. [44] optimised Tween 80 concentrations and reduced enzyme dosages, achieving ethanol yields of 0.37 g g−1 sugars and a productivity of 1.02 g L−1 h−1 using Scheffersomyces stipitis. Sataris et al. [50] demonstrated that yeast-like morphologies of filamentous fungi favour higher ethanol concentrations (78–92% of theoretical maximum) and productivities (0.43–0.64 g L−1 h−1), while filamentous forms promoted chitosan and fatty acid accumulation, particularly linoleic acid. Similarly, Itelima et al. [51] reported ethanol production of 10.08% (vol vol−1) with 0.63 mg mL−1 reducing sugars after 7 days using a co-culture of Aspergillus niger and Saccharomyces cerevisiae.
Temperature was a decisive factor in determining SSF performance. While enzymatic hydrolysis peaked at 40 °C, near the enzyme’s optimum (50 °C), the highest ethanol concentrations (14.95 ± 1.20 g L−1), conversion coefficients (0.373–0.376 g g−1), and productivities (0.312–0.304 g L−1 h−1) were consistently achieved at 30 °C. This outcome reflects a favourable compromise between enzymatic efficiency and the metabolic activity of M. indicus. Higher temperatures were not tested in MNM due to the organism’s thermosensitivity [27]. Additionally, the MNM system, designed to simulate fermentation-compatible conditions, showed enhanced compatibility with microbial growth and did not impair enzymatic performance, underscoring its suitability for integrated bioprocess applications.
Minor concentrations of glycerol and xylitol were also detected, with xylitol reaching up to 1.95 g L−1. The relatively low xylitol production is attributable to the low xylan content of the pretreated corn cobs (1.11 ± 0.07% w w−1), which limits xylose availability, and by extension, xylitol biosynthesis. As xylitol is derived from xylose via reductive pathways, limited substrate availability and process-dependent fermentation efficiency significantly influence the final yield [52].
Collectively, these findings highlight the importance of optimising process parameters, particularly temperature, enzyme loading, and media formulation, to maximise ethanol production from lignocellulosic feedstocks. Based on this study, 30 °C was identified as the optimal temperature for integrated SSF with M. indicus DSM 2185 using acid-pretreated corn cobs and a dual-enzyme system (48.9 FPU g−1 PCCs and 171.5 U g−1 PCCs).
3.3. Simultaneous Saccharification and Fermentation of PCCs for Ethanol Production by M. indicus DSM 2185 in a Stirred Tank Bioreactor
The scale-up of Mucor indicus DSM 2185 cultivation was performed using simultaneous saccharification and fermentation (SSF) in a 1.5 L stirred tank bioreactor, based on optimised conditions identified in earlier research phases. The cultivation medium consisted of matrix nutrient medium (MNM) supplemented with 40 g L−1 of dilute-acid-pretreated corn cobs (PCCs) hydrolysate. To initiate enzymatic saccharification, 2% (vol vol−1) Cellulase enzyme blend and 5.18% (vol vol−1) Viscozyme L, corresponding to 48.9 FPU g−1 PCCs and 171.5 U g−1 PCCs, were added at the start of cultivation along with the fungal inoculum. Substrate utilisation and product formation over time are presented in Figure 5.
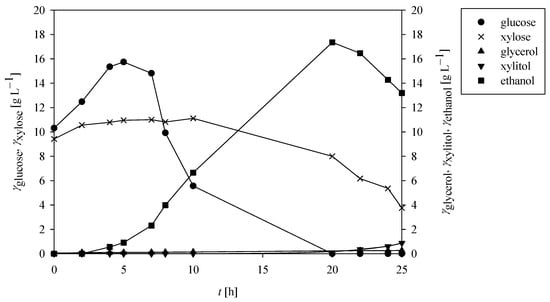
Figure 5.
Concentration profiles of glucose (●), xylose (×), glycerol (▲), xylitol (▼), and ethanol (■) during SSF of 40 g L−1 PCCs using M. indicus DSM 2185 in a stirred tank bioreactor with 2% (vol vol−1) Cellulase enzyme blend and 5.18% (vol vol−1) Viscozyme L.
During scale-up SSF in the stirred tank bioreactor, glucose concentration increased from 10.31 g L−1 to a peak of 15.74 g L−1 within the first 5 h, indicating rapid enzymatic saccharification. Subsequently, glucose levels declined due to fungal consumption and became undetectable after 20 h. However, the earlier reduction in xylose concentration suggests that glucose depletion occurred prior to this point. Xylose reached its theoretical maximum concentration (11.00 g L−1) at 7 h, corresponding to complete hydrolysis of the xylan fraction in PCCs. Approximately 65% of this xylose was assimilated by M. indicus within 25 h. Although complete xylose consumption was not achieved, the bioprocess was terminated to prevent ethanol degradation, which was observed to initiate toward the end of cultivation.
Ethanol production peaked at 22 h, reaching 16.46 g L−1, followed by a gradual decline. In addition to ethanol, minor concentrations of xylitol (0.88 g L−1) and glycerol (0.26 g L−1) were also detected at the end of the bioprocess.
The highest ethanol conversion coefficient (YEtOH/S) was 0.44 g g−1 at 22 h. Conversion coefficients for xylitol and glycerol were 0.13 g g−1 (YXOH/S) and 0.007 g g−1 (YGly/S), respectively, calculated based on the assumption of complete (100%) saccharification of the raw PCCs.
Final biomass concentration was not determined due to the integrated nature of SSF and the complexity of biomass quantification in the presence of residual solids. Nevertheless, process dynamics in the stirred tank bioreactor demonstrated significantly improved performance compared to flask-scale SSF. Notably, the bioprocess proceeded nearly twice as fast in the stirred tank bioreactor, likely due to improved control of key bioprocess parameters such as pH, substrate availability, oxygen transfer, and system homogeneity [27]. However, extending the cultivation beyond 25 h led to ethanol and sugar depletion, as these compounds were increasingly utilised for fungal maintenance metabolism and growth.
In comparison, de Oliveira et al. [53] carried out SSF of pretreated sugarcane straw in a 1 L bioreactor using Saccharomyces cerevisiae, reporting ethanol yields of 48–52% and a maximum productivity of 0.51 g L−1 h−1 at 24 h. In contrast, the present study achieved an ethanol yield of 86.27% and a peak productivity of 0.75 g L−1 h−1 at 22 h under similar conditions. Despite the promising performance, a notable limitation is the relatively high enzyme loading required to achieve such yields, specifically, 48.9 FPU g−1 PCCs and 171.5 U g−1 PCCs. These enzyme doses, although effective, contribute significantly to overall process costs, especially when applied to substrates such as dilute-acid-pretreated corn cobs, which contain substantial lignin fractions that can inhibit enzymatic activity and reduce hydrolysis efficiency. Furthermore, the separation of fungal biomass from the residual lignocellulosic matrix remains a technical challenge in SSF processes, particularly when considering downstream valorisation pathways such as the isolation of chitin and chitosan from M. indicus. These issues highlight the need for further optimisation of enzyme efficiency and biomass recovery strategies to enhance the overall economic and operational viability of integrated biorefinery systems. One potential solution is the incorporation of in situ enzyme production, which could substantially reduce external enzyme costs while simultaneously generating an additional value-added product within the biorefinery framework, thus improving overall process sustainability and profitability.
4. Conclusions
The filamentous fungus Mucor indicus is recognised for its metabolic versatility, including the production of biofuels and other value-added compounds. In this study, it was evaluated for its ethanol production through simultaneous saccharification and fermentation (SSF) of the solid phase of dilute-acid-pretreated corn cobs (PCCs). The enzymatic hydrolysis of PCCs was performed using various combinations of commercial enzyme cocktails-specifically, Cellulase enzyme blend (Cellic CTec2) and Viscozyme L. Among the tested formulations, the combination of 2% (vol vol−1) Cellulase and 5.18% (vol vol−1) Viscozyme L, corresponding to enzyme loads of 48.9 FPU g−1 PCCs and 171.5 U g−1 PCCs, achieved the highest total carbohydrate conversion, exceeding 92.66% after 48 h.
SSF conducted in a stirred tank bioreactor demonstrated superior performance compared to Erlenmeyer flask cultures, with bioprocess kinetics that were approximately twice as fast, attributable to more controlled environmental parameters such as oxygen availability, substrate dispersion, temperature and pH regulation. The maximum ethanol conversion coefficient was observed at 30 °C, reaching 0.44 g g−1 at 22 h of cultivation. Although secondary metabolites such as xylitol (0.88 g L−1) and glycerol (0.26 g L−1) were also detected, their yields remained low under the applied conditions.
These results highlight the critical influence of medium composition, enzyme formulation, and cultivation conditions on overall SSF efficiency. The integration of optimised enzymatic hydrolysis with SSF for the bioconversion of lignocellulosic biomass such as PCCs represents a viable pathway for sustainable ethanol production. However, to enhance industrial feasibility, further strategies are needed, particularly in reducing enzyme costs and improving downstream separation of microbial biomass and coproducts. Specific strategies for enzyme cost reduction must include the following: (i) exploring the addition of surfactants to enhance hydrolysis efficiency and reduce enzyme demand, as demonstrated in comparable studies; and (ii) implementing in situ enzyme production, which could minimise reliance on commercial enzyme preparations while introducing a new product stream into the biorefinery system. Both strategies offer promising approach to improve the economic viability of SSF based on lignocellulosic biomass conversion.
Future research will be focused on improving xylitol yields from pretreated lignocellulosic residues (including corn cobs, leaves, and stalks), researching the addition of surfactants, and implementing in situ enzyme production as a cost-reduction measure. This dual approach could reduce dependency on commercial enzyme preparations while simultaneously generating additional value streams within an integrated biorefinery framework.
Author Contributions
Conceptualization, N.M. and A.T.; methodology, N.M. and M.P.; software, N.M. and A.T.; validation, N.M., A.T. and M.N.; formal analysis, N.M. and A.T.; investigation, N.M. and A.T.; resources, B.Š.; data curation, A.D.; writing—original draft preparation, N.M.; writing—review and editing, A.T., V.P.T. and M.N.; visualization, N.M.; supervision, B.Š. and V.P.T.; project administration, B.Š.; funding acquisition, B.Š. and A.T.; had equal contribution to the research as a first author N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Croatian Science Foundation, grant number HRZZ-3075, “Biorefinery system for biofuels and biochemicals production from non-food lignocellulosic raw materials”, Biorefinery-NFLRM.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Levi, P.G.; Cullen, M.C. Mapping Global Flows of Chemicals: From Fossil Fuel Feedstocks to Chemical Products. Environ. Sci. Technol. 2018, 52, 1725–1734. [Google Scholar] [CrossRef]
- Rass-Hansen, J.; Falsig, H.; Jorgensen, B.; Christensen, C.H. Perspective Bioethanol: Fuel or feedstock. J. Chem. Technol. Biotechnol. 2007, 82, 329–333. [Google Scholar] [CrossRef]
- Ingle, A.P.; Kumar Chandel, A.; da Silva, S.S. Biorefinig of lignocellulose into Valuable Products. In Lignocellulosic Biorefinig Technologies; Ingle, A.P., Kumar Chandel, A., da Silva, S.S., Eds.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Fatma, S.; Amir, H.; Noman, M.; Temoor, A.; Muhammad, S.; Mohsin, T.; Imran, S.; Tabassum, R. Lignocellulosic Biomass: A Sustainable Bioenergy Source for the Future. Protein Pept. Lett. 2018, 25, 148–163. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Shah, A.A.; Seehar, T.H.; Sharma, K.; Toor, S.S. Chapter 7—Biomass pretreatment technologies. In Hydrocarbon Biorefinery; Maity, S.K., Gayen, K., Bhowmick, T.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 203–228. [Google Scholar] [CrossRef]
- Chen, H. Biotechnology of Lignocellulose: Theory and Practice; Springer: London, UK, 2014; pp. 1–185. [Google Scholar]
- Ingle, A.P.; Saxena, S.; Moharil, M.P.; Rivaldi, J.D.; Ramos, L.; Chandel, A.K. Production of biomaterials and biochemicals from lignocellulosic biomass through sustainable approaches: Current scenario and future perspectives. Biotechnol. Sustain. Mater. 2025, 2, 3. [Google Scholar] [CrossRef]
- Mutjaba, M.; Fraceto, L.F.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Foreign Agricultural Service—U.S. Department of Agriculture. Available online: https://ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=0440000 (accessed on 23 July 2025).[Green Version]
- Huo, J.; Wang, Z.; Lauri, P.; Medrano-Garcia, J.D.; Guillen-Gosalbez, G.; Hellweg, S. Region-Specific Sourcing of Lignocellulose Residues as Renewable Feedstocks for a Net-Zero Chemical Industry. Environ. Sci. Technol. 2024, 58, 13748–13759. [Google Scholar] [CrossRef]
- Zhi-Guo, Z.; Hong-Zhang, C. Enhancement of the enzymatic hydrolysis of wheat straw by pretreatment with 1-allyl-3-methylimidazolium chloride ([Amim] Cl). Afr. J. Biotechnol. 2012, 11, 8032–8037. [Google Scholar] [CrossRef]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Marđetko, N.; Novak, M.; Trontel, A.; Grubišić, M.; Galić, M.; Šantek, B. Bioethanol Production from Dilute-acid Pre-treated Wheat Straw Liquor Hydrolysate by Genetically Engineered Saccharomyces cerevisiae. Chem. Biochem. Eng. Q. 2018, 32, 483–499. [Google Scholar] [CrossRef]
- Zhang, H.; Han, L.; Dong, H. An insight to pretreatment, enzyme adsorption and enzymatic hydrolysis of lignocellulosic biomass: Experimental and modeling studies. Renew. Sustain. Energy Rev. 2021, 140, 110758. [Google Scholar] [CrossRef]
- Pinaki, D.; Lhakpa, W.; Joginder, S. Simultaneous Saccharification and Fermentation (SSF), An Efficient Process for Bio-Ethanol Production: An overview. Biosci. Biotech. Res. Asia 2015, 12, 87–100. [Google Scholar] [CrossRef]
- Rosales-Calderon, O.; Trajano, H.L.; Duff, S.J. Stability of commercial glucanase and β-glucosidase preparations under hydrolysis conditions. PeerJ 2014, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Kululo, W.W.; Habtu, N.G.; Abera, M.K.; Sendekie, Z.B.; Fanta, S.W.; Yemata, T.A. Advances in various pretreatment strategies of lignocellulosic substrates for the production of bioethanol: A comprehensive review. Discov. Appl. Sci. 2025, 7, 476. [Google Scholar] [CrossRef]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Šantek, M.I.; Komes, D.; Novak, S.; Šantek, B. Bioethanol Production from Renewable Raw Materials and Its Separation and Purification: A Review. Food Technol. Biotechol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Olofsson, K.; Bertilsson, M.; Lidén, G. A short review on SSF—An interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 7. [Google Scholar] [CrossRef]
- Asemoloye, M.D.; Bello, T.S.; Oladoye, P.O.; Remilekun Gbadamosi, M.; Babarinde, S.O.; Ebenezer Adebami, G.; Olowe, O.M.; Temporiti, M.E.E.; Wanek, W.; Marchisio, M.A. Engineered yeasts and lignocellulosic biomaterials: Shaping a new dimension for biorefinery and global bioeconomy. Bioengineered 2023, 14, 2269328. [Google Scholar] [CrossRef]
- Banner, A.; Toogood, H.S.; Scrutton, N.S. Consolidated Bioprocessing: Synthetic Biology Routes to Fuels and Fine Chemicals. Microorganisms 2021, 9, 1079. [Google Scholar] [CrossRef]
- Lyu, Q.; Dar, R.A.; Baganz, F.; Smoliński, A.; Rasmey, A.-H.M.; Liu, R.; Zhang, L. Effects of Lignocellulosic Biomass-Derived Hydrolysate Inhibitors on Cell Growth and Lipid Production During Microbial Fermentation of Oleaginous Microorganisms—A Review. Fermentation 2025, 11, 121. [Google Scholar] [CrossRef]
- Aditiya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second generation bioethanol production: A critical review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Karimi, K.; Zamani, A. Mucor indicus: Biology and industrial application perspectives: A review. Biotechnol. Adv. 2013, 31, 466–481. [Google Scholar] [CrossRef]
- Sharifyazd, S.; Karimi, K. Effects of fermentation conditions on valuable products of ethanolic fungus Mucor indicus. Electron. J. Biotechnol. 2017, 30, 77–82. [Google Scholar] [CrossRef]
- Umai, D.; Kayalvizhi, R.; Kumar, V.; Jacob, S. Xylitol: Bioproduction and Applications-A Review. Front. Sustain. 2022, 3, 826190. [Google Scholar] [CrossRef]
- Dasgupta, D.; Bandhu, S.; Adhikari, D.K.; Ghosh, D. Challenges and prospects of xylitol production with whole cell bio-catalysis: A review. Microbiol. Res. 2017, 197, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL Technical Report; Laboratory Analytical Procedure (LAP): Singapore, 2012. [Google Scholar]
- Adney, B.; Baker, J. Measurement of Cellulase Activities; NREL Technical Report; Laboratory Analytical Procedure (LAP): Singapore, 1996. [Google Scholar]
- Marđetko, N.; Kolakušić, A.; Trontel, A.; Novak, M.; Pavlečić, M.; Dobrinčić, A.; Petravić Tominac, V.; Šantek, B. Usage of the Fungus Mucor indicus and the Bacterium Rhodovulum adriaticum in a Biorefinery System for Biochemical Production on Grass Hydrolysates. Polymers 2025, 17, 369. [Google Scholar] [CrossRef]
- Pavlečić, M.; Novak, M.; Trontel, A.; Marđetko, N.; Grubišić, M.; Ljubas, B.D.; Tominac, V.P.; Rakovac, R.C.; Šantek, B. Mathematical Modelling of Bioethanol Production from Raw Sugar Beet Cossettes in a Horizontal Rotating Tubular Bioreactor. Fermentation 2022, 8, 13. [Google Scholar] [CrossRef]
- Doran, P. Presentation and Analysis of Data. In Bioprocess Engineering Principles; Doran, P., Ed.; Academic Press Limited: London, UK, 1998; pp. 27–48. [Google Scholar]
- Liu, Y.; Angelov, A.; Übelacker, M.; Baudrexl, M.; Ludwig, B.; Rühmann, B.; Sieber, V.; Liebl, W. Proteomic analysis of Viscozyme L and its major enzyme components for pectic substrate degradation. Int. J. Biol. Macromol. 2024, 266, 131309. [Google Scholar] [CrossRef]
- Yang, B.; Dai, Z.; Ding, S.-Y.; Wyman, C.E. Enzymatic hydrolysis of cellulosic biomass. Biofuels 2011, 2, 421–449. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Haven, M.O.; Lindedam, J.; Felby, C.; Gama, M. Celluclast and Cellic CTec2: Saccharification/fermentation of wheat straw, solid-liquid partition and potential of enzyme recycling by alkaline washing. Enzym. Microb. Tech. 2015, 79, 70–77. [Google Scholar] [CrossRef]
- Abedinifar, S.; Karimi, K.; Khanahmadic, M.; Taherzadeh, M.J. Ethanol production by Mucor indicus and Rhizopus oryzae from rice straw by separate hydrolysis and fermentation. Biomass Bioenergy 2009, 33, 828–833. [Google Scholar] [CrossRef]
- Synthetic Biology Project: Cellic CTec. Available online: https://www.synbioproject.tech/cpi/applications/cellic-ctec/ (accessed on 28 July 2025).
- Saha, B.C.; Iten, L.B.; Cotta, M.A.; Wu, Y.V. Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process. Biochem. 2005, 40, 3693–3700. [Google Scholar] [CrossRef]
- Althuri, A.; Banerjee, R. Separate and simultaneous saccharification and fermentation of a pretreated mixture of lignocellulosic biomass for ethanol production. Biofuels 2017, 10, 61–72. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energ. Combust. Sci. 2012, 38, 449–469. [Google Scholar] [CrossRef]
- Kleingesinds, E.K.; José, A.H.M.; Brumano, L.P.; Silva-Fernandes, T.; Rodrigues, D.; Rodrigues, R.L.B. Intensification of bioethanol production by using Tween 80 to enhance dilute acid pretreatment and enzymatic saccharification of corncob. Ind. Crops Prod. 2018, 124, 166–176. [Google Scholar] [CrossRef]
- Sahare, P.; Singh, R.; Laxman, R.S.; Rao, M. Effect of Alkali Pretreatment on the Structural Properties and Enzymatic Hydrolysis of Corn Cob. Appl. Biochem. Biotechnol. 2012, 168, 1806–1819. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xia, L.; Xue, P. Enzymatic hydrolysis of corncob and ethanol production from cellulosic hydrolysate. Int. Biodeterior. Biodegrad. 2007, 59, 85–89. [Google Scholar] [CrossRef]
- Šantek, M.I.; Zvonar, I.; Beluhan, S.; Šantek, B. Proizvodnja bioetanola iz kukuruznih oklasaka. Kem. Ind. 2018, 67, 297–308. [Google Scholar] [CrossRef]
- Molaverdi, M.; Karimi, K.; Mirmohamadsadeghi, S.; Galbe, M. High titer ethanol production from rice straw via solid-state simultaneous saccharification and fermentation by Mucor indicus at low enzyme loading. Energy Convers. Manag. 2019, 182, 520–529. [Google Scholar] [CrossRef]
- Karimi, K.; Emtiazi, G.; Taherzadeh, M.J. Production of ethanol and mycelial biomass from rice straw hemicellulose hydrolyzate by Mucor indicus. Process. Biochem. 2006, 41, 653–658. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K.; Zamani, A. Oil, chitosan, and ethanol production by dimorphic fungus Mucor indicus from different lignocelluloses. J. Chem. Technol. Biotechnol. 2016, 91, 1835–1843. [Google Scholar] [CrossRef]
- Itelima, J.; Ogbonna, A.; Pandukur, S.; Egbere, J.; Salami, A. Simultaneous Saccharification and Fermentation of Corn Cobs to Bio-Ethanol by Co-Culture of Aspergillus niger and Saccharomyces cerevisiae. Int. J. Environ. Sci. Dev. 2013, 4, 239–242. [Google Scholar] [CrossRef]
- Hilpmann, G.; Kurzhals, P.; Reuter, T.; Ayubi, M.M. Reaction Kinetics of One-Pot Xylan Conversion to Xylitol via Precious Metal Catalyst. Front. Chem. Eng. 2020, 2, 600936. [Google Scholar] [CrossRef]
- de Oliveira, R.A.; Gottschalk, L.M.F.; Freitas, S.P.; da Silva Bon, E.P. One-vessel saccharification and fermentation of pretreated sugarcane bagasse using a helical impeller bioreactor. Biomass Conv. Bioref. 2018, 8, 1–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).