Abstract
Soil reflects ecosystem processes and is influenced by gradual biospheric changes, which can affect its biotic components. In fairy rings, soil morphology, physicochemical properties, and biota are interconnected within a shared environmental space. In La Bertolina grasslands, while fungal and bacterial genomics have been investigated, the micromorphological soil effects of these rings have not. This study micromorphologically analyzed thin sections of three fairy rings at four zones: the ring center, the zone of peak growth in 2013 (R13), the predicted growth zone for 2019 (R19), and outside the ring. From each zone, two thin soil sections were prepared, totaling 24 samples. Fungal structures were exhaustively described according to morphological criteria following reference by multiple authors. The soil was a calcareous, loamy Regosol, and showed moderately developed crumb or laminar microstructures. Nine types of fungal structures were identified, consistent with genomic findings in the zone. Although fungal abundance did not vary across zones, mesofauna droppings were more frequent in R13 and R19, which was related to higher nutrient or water availability due to the fungal activity. Regarding the groundmass of the topsoil, neither the composition nor the microstructure of the surface horizons varied according to the moment of appearance of the ring at the sampled points.
1. Introduction
Fairy rings occur when fungi grow radially in the soil, raising from a central point, progressively degrading organic matter and thus affecting vegetation. These patterns are found in several natural plant communities, for example on sand dunes and in temperate forests but especially in grasslands of the US, Europe and Japan [1,2]. They are most frequently observed in areas with annual rainfall ranging from 900 to 1300 mm and at altitudes between 500 and 2200 m.a.s.l, with average annual temperatures ranging from 3 to 13 °C.
Fairy rings are classified by mycelial nutrient source geometry into “free” (unrestricted growth) and “tethered” (growth limited by a fixed nutrient source) types [3]. Based on vegetative impact, they are grouped as Type I (vegetation death or damage), Type II (stimulated growth at the margins), and Type III (no visible plant effect but fruiting bodies form a ring) [4]. In general, these formations have been attributed to the radial growth of the mycelia of Ascomycota and saprophytic Basidiomycota [5,6,7].
Fungal species significantly alter soil properties through radial mycelial expansion, leading to organic carbon mineralization in inner ring zones and elevated levels of K, P, and Ca compared with surrounding unaffected grassland [8,9]. Fairy rings also modify soil pH, increase salinity, and induce pronounced hydrophobicity [9]. Additionally, they enhance nitrogen availability by transforming soil organic proteins and other nitrogenous compounds into forms readily assimilable by vascular plants [4].
Fairy rings can influence plant growth through several mechanisms beyond simple nutrient enrichment. They play a role as ecosystem engineers, influencing the composition and diversity of vegetation and the effects on soil microbiota.
In La Bertolina (a montane grassland in the Catalan Pyrenees), a project on fairy ring dynamics was carried out from 2013 to 2019, where the composition of the soil fungal community was determined with genetic analysis (metabarcoding with amplified DNA markers) [6,7].
In the same grassland of La Bertolina, we found no significant differences regarding exchangeable phosphorous content, pH and available calcium in the different vegetation zones associated with fairy rings. However, significant differences were found in terms of moisture content and extractable potassium [10]. However, it is unknown whether the appearance of fairy rings generates any changes at the micromorphological level in the topsoil.
Soil micromorphology has many possibilities of use, from the analysis of soil mineral components and organic components to the classification of humus and organic horizons, as well as changes at the microstructural level [11], which has never been considered in the study of soils under fairy rings.
The study of soil micromorphology through thin sections of undisturbed soil provided information on microstructure, aggregates, pedofeatures and biological characteristics, as well as the contents and relative distribution of organic matter, fungal activity, fauna activity at a microscopical scale, among other aspects.
Our objectives were to determine the effect of the appearance of fairy rings on the micromorphological characteristics of grassland soils in the mountainous region of the Catalan Pyrenees and to analyze the various biological structures of the fungi present in the soil layers. In order to investigate if there were micromorphological differences in the soil associated with fairy ring emergence, we sampled four ring zones: the center of the ring, the zone where the ring emerging in 2013 was located, the zone where the rings were expected to appear in 2019 based on fairy ring growth ratios, and the outer grassland without fairy ring affectation.
The hypothesis was that in the ground mass of the topsoil layer, neither the composition nor the microstructure of the surface horizons varied according to the timing of ring emergence, but a greater presence of fungal structures and mesofauna was expected in the ring emergence zones.
2. Materials and Methods
Study Site and Sampling
La Bertolina is an area of ecological interest, located NE of the Iberian Peninsula (42°05′56″ N y 1°39′40″ E), 1276 m.a.s.l. in the Catalan Pyrenees (Municipality of Navés, Catalonia) (Figure 1). The mean annual temperature is 8.7 °C, and the mean annual precipitation is 954.8 mm [12]. The parent material of the soils corresponds to slope colluvium from polygenic calcareous conglomerates [13]. The land use is extensive pasture, with a cattle intensity of 0.44 LSU (livestock unit) ha−1 and a grazing period from May or June to November since 1998; it had a prior agricultural use for a cereal crop until an extensive wildfire affected the area in 1998.
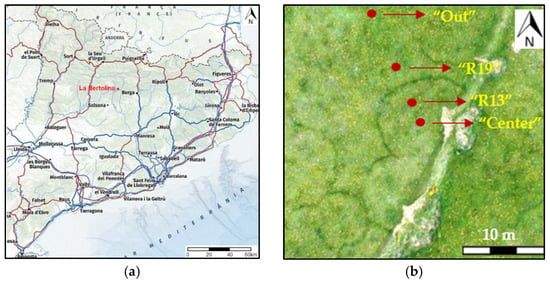
Figure 1.
(a) Map location of La Bertolina; (b) example of sampled. Ring 1. Dark vegetation is the fungal front in 2017.
The survey was conducted in March 2019 before the rings were visible in the vegetation. The selection of the rings (1, 2 and 3) and sampling in this grassland area were made possible by geo-referencing these fairy rings from a mosaic of aerial images from previous studies [6].
Profile Description and Macromorphological and Physicochemical Analysis
The soil of the area was described following SINEDARES criteria [14]. The different horizons were sampled and analyzed following the methods described by [15]. Soil samples were air-dried and sieved to 2 mm. The following analyses were undertaken on all samples: pH (1:2.5), electrical conductivity (EC) (1:5), carbonates, soil organic matter (SOM), and phosphorous and particle size distribution.
Soil Micromorphological Analyses
Three fairy rings (1, 2 and 3) were sampled in four ring zones, designated in a radial transect across each ring: (1) in the center of the ring (“Center”); (2) in the geo-referenced 2013 ring zone (“R13”); (3) in the geo-referenced estimated apparition zone 2019 (“R19”); and (4) outside the ring without any possible ring influence (>2 m outside the ring) (“Out”) (Figure 1).
Twelve undisturbed blocks of 20 cm in length, 10 cm in width and 10 cm in depth were taken from the soil surface (one at each sampling point, including vegetation) (Figure 1); they were wrapped in adhesive plastic and contained in tetrabrik containers to ensure that they maintained their shape during the drying process. They were air-dried and impregnated with a polyester resin with a fluorescent dye (Uvitex© manufactured by Bayer, Leverkusen, Germany, the reagent was sourced in Barcelona, Spain), and two thin sections were obtained from each undisturbed soil block for a total of 24 thin horizontal sections of 5 × 13 cm in size. They were studied using an Olympus petrographic microscope (BX51), manufactured by Olympus Corporation, Tokyo, Japan, the equipment was sourced in Spain. True color scans of the sections were made with a high-resolution Epson scanner (Figure 2).

Figure 2.
Process to create thin sections. (a) Undisturbed block impregnated with polyester resin with a fluorescent dye (Uvitex©). (b) Thin slice of soil cut from the undisturbed block placed in temporary glass. (c) First machine polishing to place the final glass. (d) Machine for cutting the undisturbed sample of temporary glass and obtaining the thin section. (e) Final polishing to obtain thin sections. (f) Olympus petrographic microscope (BX51) used to validate thickness.
The guidelines of [16,17,18] were followed for the thin section description. The plant residues, different excrements, and other organic components were described according to [19].
Fungal structures were exhaustively described according to morphological criteria and tentatively classified at the taxonomic level of order following reference image collections by multiple authors. Their relative abundance was estimated after the exploration of the entire area of each or the thin sections following this procedure: from a reference thin section with the highest content of fungal structures (one for all fungal structures), the relative abundance of structures was visually estimated, and this corresponded to approximately 5% of the total surface or apparent volume. Six classes based on the visual estimation of relative abundance with respect to the occupied percentage of the solid fraction were established: for each fungal feature, the thin section with the highest abundance was chosen as a reference, the volume % occupied by the feature was visually estimated, and lower % classes were determined for the rest of the sections.
Absent (−): 0% structural content; Rare (+): <1% of the total surface area or apparent volume of the solid fraction of the lamina; Common (++): 1–2% of the total surface area; Frequent (+++): 2–3% of the total surface area; Abundant (++++): 3–4% of the total surface area; Very Abundant (+++++): >4% of the total surface area.
3. Results
Soil Macromorphology, Analysis and Classification
The soil profile described in the field was composed of four horizons—A1 (0–2 cm), A2 (2–10/12 cm), A3 (10/12–27 cm), and Bw (27–55/999 cm)—and classified as Typic Ustorthent [20] or Regosol [21] (Figure 3).
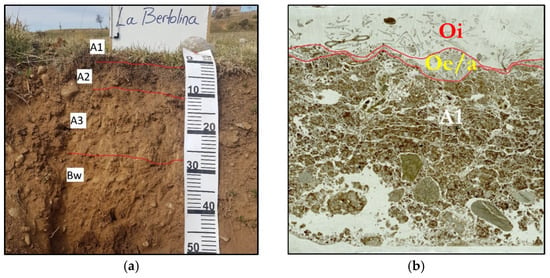
Figure 3.
Soil profile and thin section scans using parallel polarizers. (a) Horizons described in the field. (b) Laminar structure and subangular blocks. Ring 3, R13.
When performing the thin section analysis with the microscope, two additional horizons were determined over the A1 horizon: one Oi horizon and another Oe/Oa horizon, with average thicknesses of 0.5 and 1 cm, respectively (Figure 3).
The soil was well-drained, slightly stony, moderately deep, had a strong reaction to HCl 11% due to the presence of CaCO3, basic (pH: 7.8–8.5), non-saline, and had an organic matter content of 8.7% and a phosphorus content (Olsen) = 12.3 ppm in the surface horizons. The texture of the A1 and A2 horizons was loam, while in the A3 and Bw horizons, it was sandy loam [22].
Micromorphological Analysis
The groundmass of all the sections has a single spaced porphyric c/f related distribution. The coarse components were fragments of quartz, carbonates, calcareous conglomerates, quarzitic sandstones and calcium carbonate biospheroids in some thin sections. The micromass was light brown in color, mottled, and composed of clay, fine silt and amorphous organic matter, with a weak crystallitic micritic b-fabric. The organic material was randomly distributed throughout the thin section, while the presence of charcoal, fungal structures, and pedofeatures varied in abundance and distribution [22].
Description of Fungal Structures
The fungal structures normally appeared clustered and were associated with organic residues or organic material (Figure 4 and Figure 5) [22].
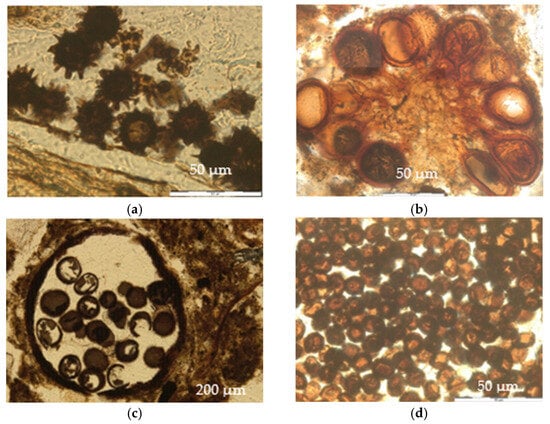
Figure 4.
Fungal structures. (a) Echinulate spores. Ring 3, zone “R13”, thin section 2, PPL (parallel polarizers). (b) Orange spores. Ring 3, zone “R13”, thin section 1, PPL. (c) Brown rough spores and structure in which they were contained. Ring 2, “Out”, thin section 2, PPL. (d) Brown smooth spores. Ring 2, zone “R13”, thin Section 1, PPL.
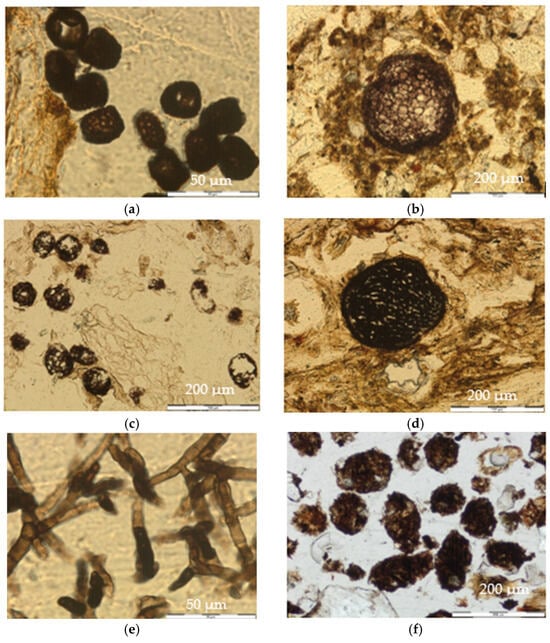
Figure 5.
Fungal structures and pedofeatures. (a) Brown square spores. Ring 3, zone “R13”, thin section 1, PPL. (b) Large sclerotia. Ring 1, zone “R13”, thin section 1, PPL. (c) Small sclerotia. Ring 2, “Out”, thin section 2, PPL. (d) Perithecia. Ring 1, “Center”, thin section 2, PPL. (e) Ascomycota fungal hyphae. Ring 2, zone “R13”, thin section 1, PPL. (f) Large droppings. Ring 1, zone “R13”, thin section 2, PPL.
- (a)
- Echinulate Spores
They were dark brown, globose ornamented unicellular structures, with diameters between 20 and 25 µm. The ornamentation was composed of numerous thorny endings 2–3 µm long that completely covered the spores (Figure 4a).
- (b)
- Orange Spores
They were unicellular structures, globose or ovate in shape, with a diameter of 25 to 40 µm or 25 µm wide and 40 µm long (the elongated ones). Those spores were mainly orange in color, and their cell wall was thick and smooth with a more intense coloration. They were generally found in a round-shaped sporocarp, which could contain from 3 to more than 10 spores (Figure 4b).
- (c)
- Brown Rough Spores
They were globose ornamented unicellular structures with a rough outer layer, a dark brown color, and diameters from 35 to 50 µm. They were found in groups of 3 to 17 units contained in a structure with either a rounded shape or accommodated to spaces between aggregates and the organic material of the A1 horizon (Figure 4c).
- (d)
- Brown Smooth Spores
They were unicellular structures, globose to ovate in shape, not ornamented, and brown in color. Their diameter was 10 to 15 µm, and they were usually found in large groups outside of the remains of plant tissues or amorphous organic material, enclosed in a rounded structure, or conformed to the shape of the plant residue (Figure 4d).
- (e)
- Brown Square Spores
They were ornamented unicellular structures, with a rough outer thick wall and dark brown color. Their shape varied between semi-square and semi-rectangular, and their size was approximately 15 × 25 µm. They were found in groups of more than 15 units close to the decomposing plant material or in spaces between the aggregates and the amorphous organic material of the A1 horizon (Figure 5a).
- (f)
- Large Sclerotia
They had a rounded shape, with an outer layer more intense in color than the center. They contained cells, from a few to many, and their diameters were from 125 to 150 µm. They were found isolated within the groundmass, mostly located in the fine material (Figure 5b).
- (g)
- Small Sclerotia
They had a rounded shape and contained cells, from a few to many, and they had diameters from 25 to 75 µm. They were found in groups and were located in the remains of plant material in horizon A1 (Figure 5c).
- (h)
- Perithecia
They had a spherical or globose shape with diameters between 130 and 170 µm. Their cortex was dark in color and more intense than the center (Figure 5d).
- (i)
- Hyphae
They were dark brown in color, and their size was variable, but the width never exceeded 10 µm. They had septate asci. They corresponded to hyphae of Ascomycota, because they had a septate mycelium and produced endogenous ascospores (Figure 5e).
Pedofeatures are reorganizations of soil materials that cannot be attributed to an inheritance from the parental material [16].
- (a)
- Excrement Pedofeatures
These are organic remains resulting from faunal activity. Their size, shape, location, and degree of processing vary depending on the organisms that generated them. Their color is generally brown and varies from light to dark [19]. We differentiated large excrements (from 150 μm to 1 mm in diameter) from small excrements (less than 150 μm) [19].
Large droppings were mainly found in the Oe/Oa horizon, had sections shaped from rounded to oval, with a rough edge, brown color (light and dark tones), and heterogeneous compositions (organic rest and mineral material). They were often observed in clusters and had an average size of between 200 and 400 µm (Figure 5f).
Mite droppings are depositions with an ovoid (rounded) shape and a smooth exterior located in or around remains of plant tissues, with variable sizes between 40 and 150 µm. They are made of dotted amorphous organic matter and dark brown in color (Figure 6a).
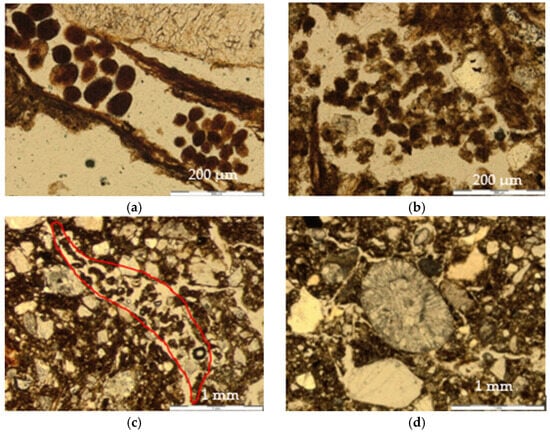
Figure 6.
Pedofeatures and other traits. (a) Mite droppings. Ring 2, “Out”, thin section 2, PPL. (b) Enchytraeid droppings. Ring 2, “Center”, thin section 1, PPL. (c) Loose continuous infilling of a channel with of enchytraeid excrements. Ring 2, “Out”, thin section 1, PPL. (d) Biospheroid. Ring 1, “Out”, thin section 1, PPL.
Enchytraeid droppings are more irregular in shape (sub-rounded to subangular) with a rough boundary, 40–100 µm in size, and located in the spaces between the aggregates, with different degrees of decay and generally filling channels (Figure 6b).
- (b)
- Excrement Infillings
They were also observed in all thin sections. The main type corresponded to channel infillings by enchytraeid droppings in different degrees of decomposition, plant remains and fine amorphous organic material (Figure 6c).
- (c)
- Nodules
They corresponded to impregnating an anorthic nodules of iron oxides. They had a rounded shape, and their diameter size was from 0.50 to 0.80 mm. They were only observed in three of the studied thin sections, without any distribution pattern associated with the rings. Their anorthic character excluded a formation in situ, which is in agreement with the lack of mottling in the profile and the good drainage of the soil.
Other traits found
- (a)
- Biospheroids
They were biomineralizations composed of radially crystallized calcium carbonate granules that are attributed to the activity of earthworms [17]. They have a rounded shape, and their diameter was from 0.50 to 1 mm (Figure 6d).
- (b)
- Charcoal
These shapes were also variable, and the found modal size was 500 µm. They were found in all thin sections.
4. Discussion
The upper centimeters of the A1 horizon showed a moderately developed crumbly and/or laminar structure, which changed in depth to a moderately developed subangular block structure. However, neither the composition nor the microstructure of the surface horizons depended on the position of the ring zones, so we attributed the development of the laminar structure to grazing cattle trampling [22].
The composition of the soil fungal community at La Bertolina, determined with genetic analysis (meta-barcoding with amplified DNA markers), indicated that the phylum Ascomycota was the most abundant, representing an average of 79.2%, while Basidiomycota accounted for 18.1%. Agaricales, within Basidiomycota, was the order most commonly associated with fairy ring formation, while in the outer zones, the orders Pleosporales and Eurotiales prevailed [6,7].
The echinulate spores taxonomically corresponded to the Basidiomycota phylum, Agaricomycetes class, and Agaricales order [23,24]. According to [6], fungi of the order Agaricales (phylum: Basidiomycota) are those that appear more frequently in areas where rings form. These structures only appeared in the “R13” and “R19” zones, being more abundant in “R13”, which was an older ring. Therefore, the relative abundance of these spores seems to be related to the zones of appearance of the rings.
Orange spores are taxonomically presumed to belong to the Glomeromycota phylum, Glomeromycetes class, and Glomerales order [25,26,27,28]. Due to its symbiotic functionality and its relatively greater abundance in the zones of the “R13” and “R19” rings, it is considered to be related to the greater vegetative growth.
Brown rough spores taxonomically belong to the Ascomycota phylum, Eurotiomycetes class, and Eurotiales order [29,30,31]. The relative abundance of these spores did not seem to be related to the zones of appearance of the rings. Brown smooth spores taxonomically belong to the Ascomycota phylum, Dothideomycetes class, and Pleosporales order, [32,33]. The relative abundance of these spores did not seem to have any relation to the zones of appearance of the rings, since they occurred both inside and outside of the ring appearances. Brown square spores taxonomically belong to the Ascomycota phylum, Eurotiomycetes class, and Eurotiales order [31]. The relative abundance of spores of this order did not seem to be related to the zones of appearance of the rings.
Large sclerotia are persistent, vegetative and resting structures of certain Basidiomycota and Ascomycota fungi [34]. The relative abundance of sclerotia bore no relation to the zones of appearance of the rings, since they occurred in all zones without any attributable pattern. Small sclerotia are persistent, vegetative, and dormant structures of certain Basidiomycota and Ascomycota fungi [34]. Their relative abundance had no relation to the zones where the rings appeared.
Perithecia are a form of ascocarp of the Ascomycota. Taxonomically, they belong to the Ascomycota phylum, Dothideomycetes class, and Pleosporales order [32,33]. Their relative abundance had no relation to the zones where the rings appeared. Hyphae are taxonomically classified as part of the Ascomycota phylum, Dothideomycetes class, and Pleosporales order [32,33]. Their relative abundance had no relation to the zones where the rings appeared.
Fairy ring fungi could function as ecosystem engineers, modulating species coexistence in Mediterranean grasslands, a concept also supported by multikingdom scale observations [9].
Excrement pedofeatures are important micromorphological features that reflect environmental features and certain animal activity, and they often constitute an essential part of the soil structure. Large excrements (from 150 μm to 1 mm in diameter) were more frequent in the “R13” and “R19” zones, which may have responded to a higher availability of nutrients in the soil due to higher fungal activity, resulting in enhanced vegetative development and therefore mesofaunal activity. These droppings could correspond to Diptera or earthworms. Mite droppings (40–150 µm) were located in or around remains of plant tissues. Their abundance and distribution pattern did not relate to the zones where the rings appeared. Enchytraeid droppings (40–100 µm) were very abundant in all thin sections and lacked any distribution pattern associated with the rings. Excrement infillings were also found in all thin sections without any distribution pattern associated with the rings.
Nodules were only observed in three of the studied thin sections, without any distribution pattern associated with the rings. Their anorthic character excludes a formation in situ, which is in agreement with the lack of mottling in the profile and the good drainage of the soil. Only a single biospheroid unit per thin section was found in Ring 1 without any distribution pattern associated with the rings. Charcoal was found in all thin sections, without any distribution pattern associated with the rings. It was inherited from a long history of agricultural use in the area and wildfire until 1998 [6].
Table 1 shows the relative abundance of the fungal structures in each of the analyzed emergent ring zones, which allows us to compare their occurrence between the different positions and rings. Although there was no clear pattern, a certain trend could be observed, according to which there was a lower abundance of fungal spores out of the rings. Echinulate spores and orange spores were the only structures that seemed to have some relationship with the zones of appearance of the fairy rings due to their symbiotic functionality and relatively greater abundance in the internal zones of the rings. Nevertheless, when comparing the relative abundance between rings, it was observed that there was more variability between them than between the areas within them; Ring 3 was the one with more fungal structures, followed by Ring 2 and, finally, Ring 1. On the other hand, it would be very difficult to find a distinct distribution pattern of the features, since the grazing season lasts until November and there has surely been some local redistribution of fungal structures within each ring, masking the original differences. It is possible that a sampling when the rings are visible would allow us to observe a greater quantity of structures clearly related to the rings [22].

Table 1.
Relative abundance of fungal structures in each of the analyzed ring zones in La Bertolina.
5. Conclusions
The micromorphological analysis of topsoils under fairy rings on pastures from the Catalan Pyrenees has proved useful to identify fungal structures (five types of spores, sclerotia, perithecia and hyphae) in agreement with the previous identification of taxa through genomics. Regarding the groundmass of the topsoil, neither the composition nor the microstructure of the surface horizons varied according to the moment of appearance of the ring at the sampled points, so the fungal activity seems to not affect soil porosity. Nevertheless, the more frequent large excrements (mesofauna) in the spots where the rings appear could be related to higher nutrient or water availability due to fungal activity, thus confirming the research hypothesis. The lack of micromorphological differences point to some other mechanisms (chemical, biological) that trigger the appearance and growth of the fairy rings.
Author Contributions
Conceptualization and methodology L.M.S., M.T.S. and R.M.P.; software, validation and resources, M.T.S. and R.M.P.; data curation, investigation, formal analysis, writing—original draft preparation, writing—review and editing, and visualization, L.M.S.; supervision, R.M.P.; project administration and funding acquisition, M.T.S. and R.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out thanks to a collaboration grant from the Department of Horticulture, Botany and Gardening at the University of Lleida through the Catalan Forest Technology Center (CTFC) and the BIOGEI (CGL2013-49142-C21-R) and IMAGINE (CGL2017-85490-R) projects of the National Plan of the Ministry of Science and Innovation (Spain).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
To the Department of Environmental and Soil Sciences and the Department of Horticulture, Botany, and Landscape Architecture of the University of Lleida, and the Forest Technology Center of Catalonia (CTFC).
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
References
- Bonanomi, G.; Incerti, G.; Allegrezza, M. Assessing the impact of land abandonment, nitrogen enrichment and fairy-ring fungi on plant diversity of Mediterranean grasslands. Biodivers. Conserv. 2013, 22, 2285–2304. [Google Scholar] [CrossRef]
- Fidanza, M.; Colbaugh, P.; Davis, S. Fairy Ring Biology and Management in Turfgrass. In TurfGrass Trends; Turfgrass Producers International (TPI): Lombard, IL, USA, 2000. [Google Scholar]
- Gregory, P.H. Fairy rings; free and tethered. Bull. Br. Mycol. Soc. 1982, 16, 161–163. [Google Scholar] [CrossRef]
- Shantz, H.L.; Piemeisel, R.L. Fungus fairy rings in eastern Colorado and their effect on vegetation. J. Agric. Res. 1917, 11, 191–246. [Google Scholar]
- Dowson, C.G.; Rayner, A.D.M.; Boddy, L. Spatial dynamics and interactions of the woodland fairy ring fungus, Clitocybe nebularis. New Phytol. 1989, 111, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Marí, T. Changes in Soil Biodiversity and Activity Along Management and Climatic Gradients. Ph.D. Thesis, Forest and the Environment Management, Universitat de Lleida, Lleida, Spain, 2020. [Google Scholar]
- Marí, T.; Manjón-Cabeza, J.; Rodríguez, A.; San Emeterio, L.; Ibáñez, M.; Sebastià, M.-T. Changes in the Soil Bacterial Community Across Fairy Rings in Grasslands Using Environmental DNA Metabarcoding. Diversity 2025, 17, 322. [Google Scholar] [CrossRef]
- Edwards, P.J. The growth of fairy rings of Agaricus arvensis and their effect upon grassland vegetation and soil. J. Ecol. 1984, 72, 505–513. [Google Scholar] [CrossRef]
- Bonanomi, G.; Mingo, A.; Incerti, G.; Mazzoleni, S.; Allegrezza, M. Fairy rings caused by a killer fungus foster plant diversity in species-rich grassland. J. Veg. Sci. 2012, 23, 236–248. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ibáñez, M.; Bol, R.; Brüggemann, N.; Lobo, A.; Jimenez, J.J.; Ruess, L.; Sebastià, M.-T. Fairy ring-induced soil potassium depletion gradients reshape microbial community composition in a montane grassland. Eur. J. Soil Sci. 2022, 73, e13239. [Google Scholar] [CrossRef]
- Zanella, A.; Jabiol, B.; Ponge, J.F.; Sartori, G.; De Waal, R.; Van Delft, B.; Graefe, U.; Cools, N.; Katzensteiner, K.; Hager, H.; et al. A European morphofunctional classification of humus forms. Geoderma 2011, 164, 138–145. [Google Scholar] [CrossRef]
- Ninyerola, M.; Pons XRoure, J.M. Atlas Climático Digital de la Península Ibérica. In Metodología y Aplicaciones en Bioclimatología y Geobotánica; Universidad Autónoma de Barcelona: Bellaterra, Spain, 2007; ISBN 932860-8-7. [Google Scholar]
- Instituto Cartográfico y Geológico de Cataluña (ICGC). Base de Datos Geológicos. 1:50,000. BG50M_v1r1. 2007. Available online: https://betaportal.icgc.cat/visor/client_utfgrid_geo.html (accessed on 10 May 2025).
- Comisión del Banco de Datos de Suelos y Aguas (CBDSA). SINEDARES Manual Para la Descripción Codificada de los Suelos en el Campo; Ministerio de Agricultura, Pesca y Alimentación de España: Madrid, Spain, 1983; 137p.
- Porta, J.; López-Acevedo, M.; Rodríguez, R. Técnicas y Experimentos en Edafología; Collegi Oficial d’Enginyers Agrònoms de Catalunya: Barcelona, Spain, 1986. [Google Scholar]
- Stoops, G. Guidelines for Analysis and Description of Soil and Regolith Thin Sections; Soil Science Society of America Inc.: Madison, WI, USA, 2003; 184p. [Google Scholar]
- Loaiza, J.C.; Stoops, G.; Poch, R.M.; Casamitjana, M. (Eds.) Manual de Micromorfología de Suelos y Técnicas Complementarias; Fondo Editorial Pascual Bravo: Medellín, Colombia, 2015; 384p. [Google Scholar]
- Nicosia, C.; Stoops, G. (Eds.) Archaeological Soil and Sediment Micromorphology; John Wiley & Sons: Hoboken, NJ, USA, 2017; 476p. [Google Scholar]
- Ismail-Meyer, K.; Stolt, M.H.; Lindbo, D.L. Soil organic matter. In Interpretation of Micromorphological Features of Soils and Regoliths; Stoops, G., Marcelino, V., Mees, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 471–502. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- WRB. Base Referencial Mundial del Recurso Suelo 2014, Actualización 2015. Sistema Internacional de Clasificación de Suelos Para la Nomenclatura de Suelos y la Creación de Leyendas de Mapas de Suelos. Informes Sobre Recursos Mundiales de Suelos; FAO: Roma, Italy, 2015. [Google Scholar]
- Salazar, L.M.; Sebastià, M.T.; Poch, R.M. Effects of Fairy Ring fungi on topsoil micromorphology in Pyrenean grasslands. In Proceedings of the Keep Soil Alive, Protect Soil Biodiversity—Global Symposium on Soil Biodiversity, Rome, Italy, 19–22 April 2021; FAO: Rome, Italy, 2021; pp. 494–502. [Google Scholar] [CrossRef]
- Brandon, M.; Moncalvo, J.M.; Redhead, S.A. Agaricales. Version 09 May 2007. Available online: http://tolweb.org/Agaricales/20551/2007.05.09 (accessed on 10 May 2025).
- Vizzini, A.; Contu, M.; Kalamees, K.; Ercole, E.; Musumeci, E.; Moreno, G.; Manjón JLAlvarado, P. On the variability of spore ornamentation in Laccaria tortilis (Basidiomycota, Agaricales). Mycotaxon 2011, 116, 217–225. [Google Scholar] [CrossRef]
- Almeida, R.T.; Schenck, N.C. A revision of the genus Sclerocystis (Glomaceae, Glomales). Mycologia 1990, 82, 703–714. [Google Scholar] [CrossRef]
- Redecker, D. Glomeromycota. Arbuscular Mycorrhizal Fungi and Their Relative(s). Version 14 January 2008. Available online: http://tolweb.org/Glomeromycota/28715/2008.01.14 (accessed on 10 May 2025).
- Debnath, A.; Karmakar, P.; Debnath, S.; Roy Das, A.; Saha, A.K.; Das, P. Arbuscular mycorrhizal and dark septate endophyte fungal association in some plants of Tripura, North-East India. Curr. Res. Environ. Appl. Mycol. 2015, 5, 398–407. [Google Scholar] [CrossRef]
- Muthumari, V.; Hariram, N. Occurrence of VAM fungi in Kalasalingam University campus. J. Med. Plants Stud. 2017, 5, 101–105. [Google Scholar]
- Castellano, M.A.; Guevara, G.; García, J.; Trappe, J.M. Elaphomyces appalachiensis y E. verruculosus sp. nov. (Ascomycota, Eurotiales, Elaphomycetaceae) del este de Norteamérica. Rev. Mex. Micol. 2012, 35, 17–22. [Google Scholar]
- Castellano, M.A.; Trappe, J.M.; Vernes, K. Australian species of Elaphomyces (Elaphomycetaceae, Eurotiales, Ascomycota). Aust. Syst. Bot. 2011, 24, 32–57. [Google Scholar] [CrossRef]
- Paz, A.; Bellanger, J.M.; Lavoise, C.; Molia, A.; Ławrynowicz, M.; Larsson, E.; Ibarguren, I.O.; Jeppson, M.; Læssøe, T.; Sauve, M.; et al. The genus Elaphomyces (Ascomycota, Eurotiales): A ribosomal DNA-based phylogeny and revised systematics of European ‘deer truffles’. Persoonia 2017, 38, 197–239. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species, 3rd ed.; CRC Press: Boca Ratón, FL, USA, 2010; 397p. [Google Scholar]
- Zhang, Y.; Crous, P.W.; Schoch, C.L.; Hyde, K.D. Pleosporales. Fungal Divers. 2012, 53, 1–221. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.; Henkel, T.W.; Rollins, J.A. How many fungi make sclerotia. Fungal Ecol. 2015, 13, 211–220. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).