Germination of Triticum aestivum L.: Effects of Soil–Seed Interaction on the Growth of Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Wheat Cultivars
2.2. Laboratory Experiment
2.3. Glasshouse Experiment
2.3.1. Seed Disinfection
2.3.2. Sampling and Measurement
Speed and Percentage of Seed Germination
Vigor Index
2.4. Statistical Analysis
3. Results
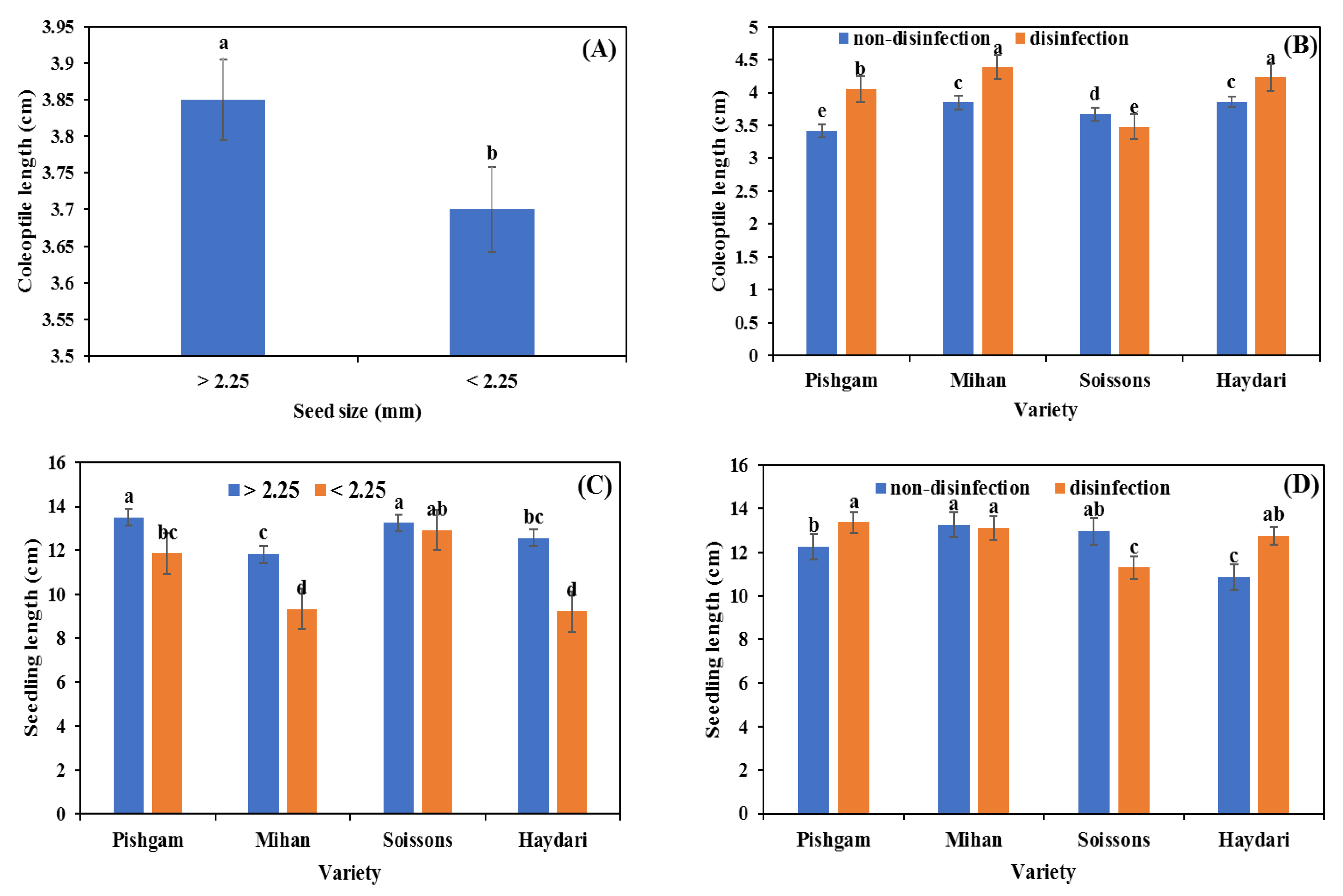
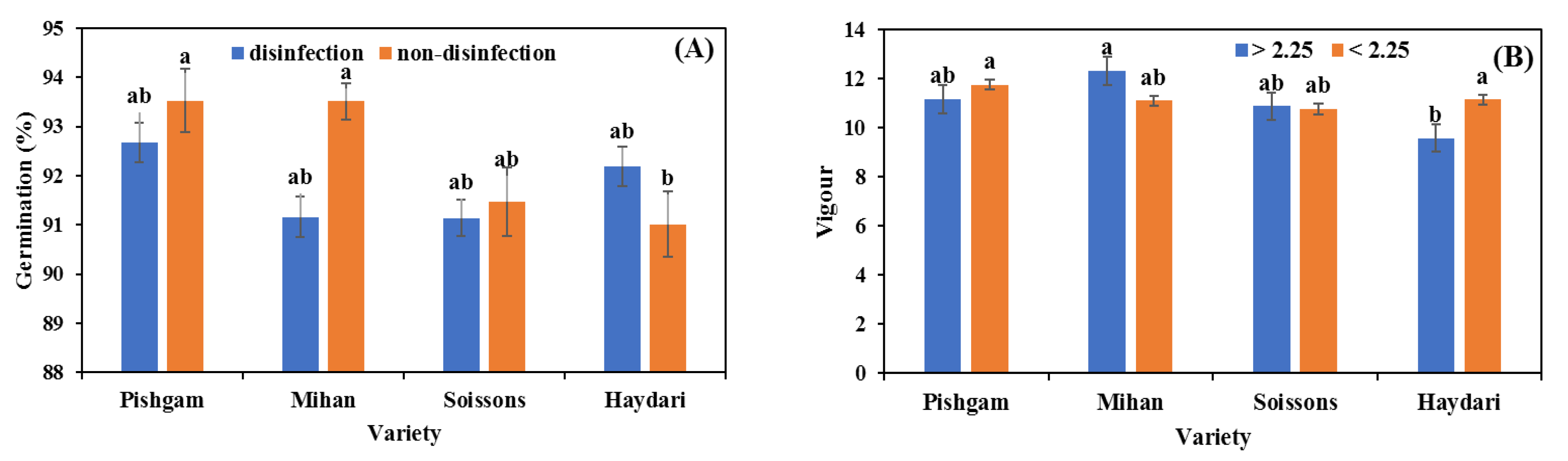
3.1. Seed Germination
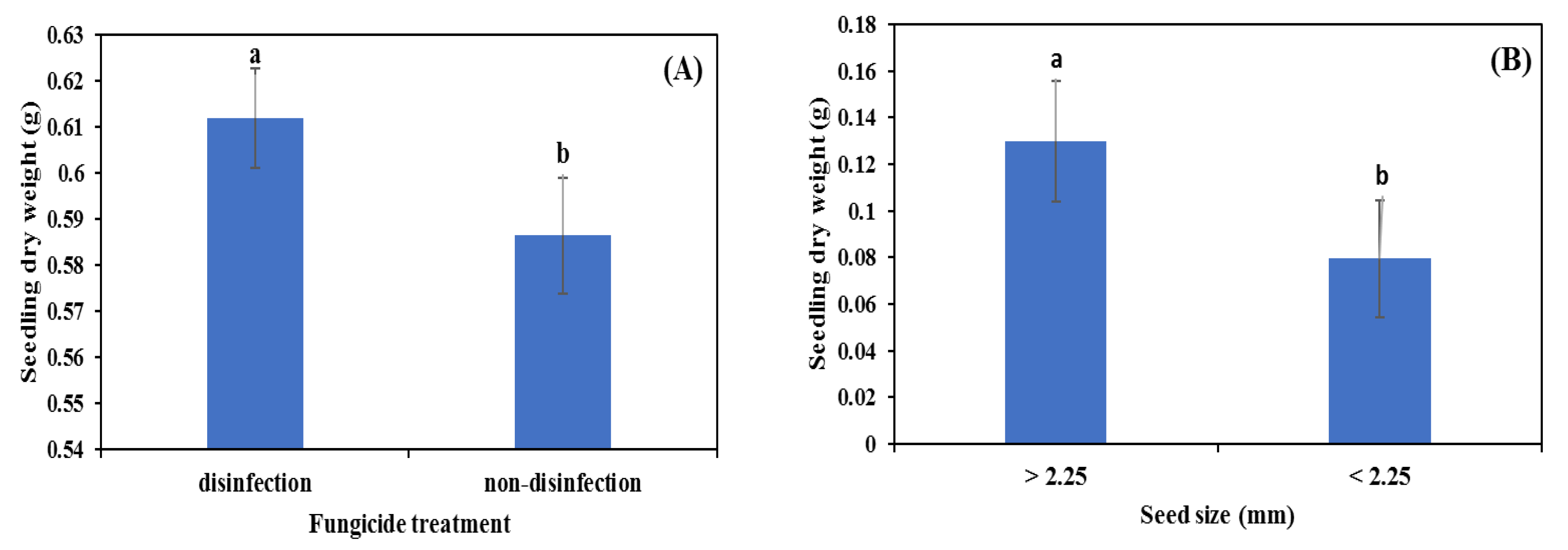
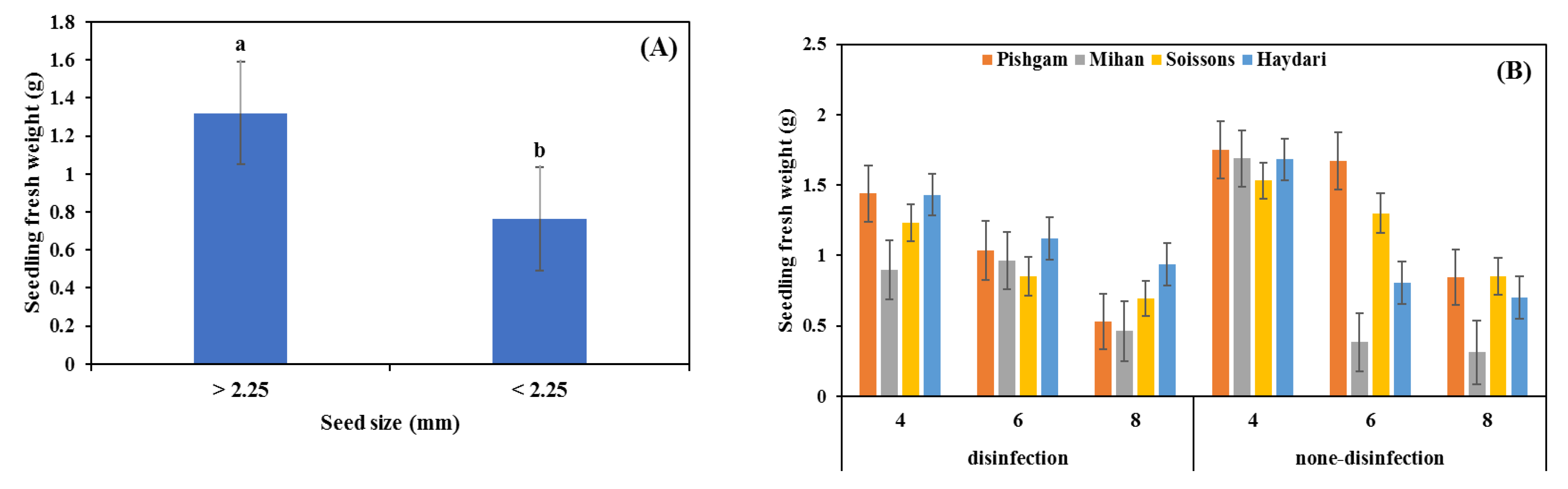
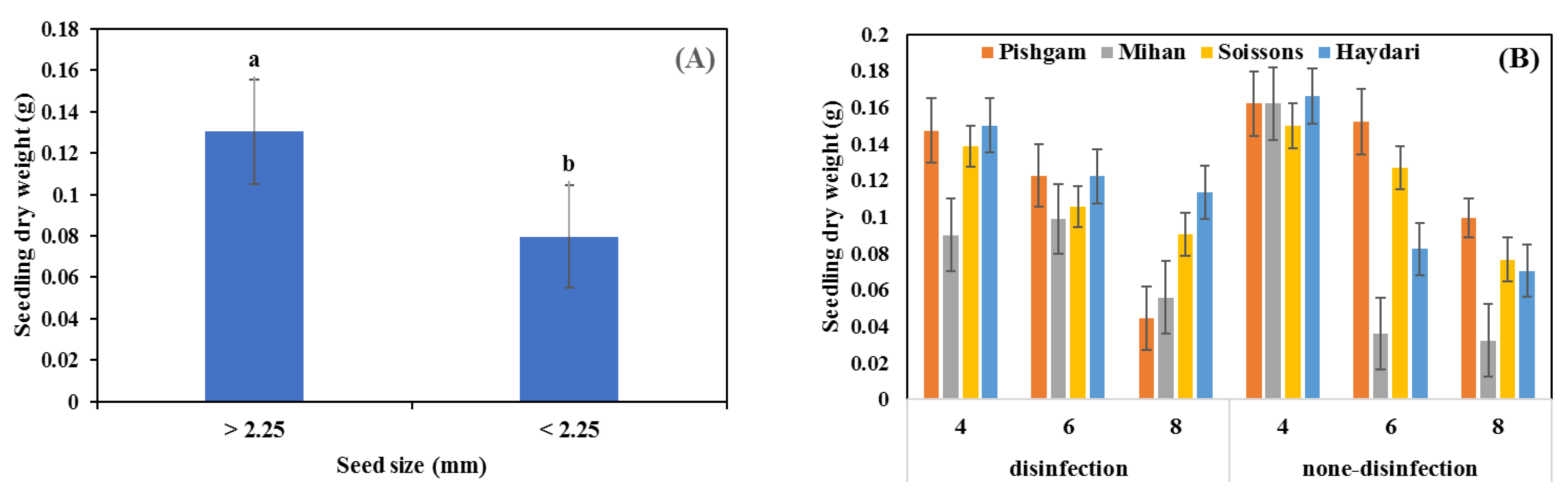
3.2. Glasshouse Experiment
4. Discussion
4.1. Seed Size
4.2. Seed Disinfection
4.3. Sowing Depth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sabouri, H.; Kazerani, B.; Fallahi, H.A.; Dehghan, M.A.; Alegh, S.M.; Dadras, A.R.; Katouzi, M.; Mastinu, A. Association analysis of yellow rust, fusarium head blight, tan spot, powdery mildew, and brown rust horizontal resistance genes in wheat. Physiol. Mol. Plant Pathol. 2022, 118, 101808. [Google Scholar] [CrossRef]
- Kumar, A.; Memo, M.; Mastinu, A. Plant behaviour: An evolutionary response to the environment? Plant Biol. 2020, 22, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.J.; Atkinson, C.J.; Bengough, A.G.; Else, M.A.; Fernandez-Fernandez, F.; Harrison, R.J.; Schmidt, S. Contributions of roots and rootstocks to sustainable, intensified crop production. J. Exp. Bot. 2013, 64, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Seidel, S.J.; Gaiser, T.; Kautz, T.; Bauke, S.L.; Amelung, W.; Barfus, K.; Ewert, F.; Athmann, M. Estimation of the impact of precrops and climate variability on soil depth differentiated spring wheat growth and water, nitrogen and phosphorus uptake. Soil Till. Res. 2019, 195, 104427. [Google Scholar] [CrossRef]
- Ambika, S.; Manonmani, V.; Somasundar, G. Review on Effect of Seed Size on Seedling Vigour and Seed Yield. Res. J. Seed Sci. 2014, 7, 31–38. [Google Scholar] [CrossRef]
- Finch-Savage, W. Influence of Seed Quality on Crop Establishment, Growth, and Yield. In Seed Quality; CRC Press: Boca Raton, FL, USA, 2020; pp. 361–384. [Google Scholar]
- Tabakovic, M.; Simic, M.; Stanisavljevic, R.; Milivojevic, M.; Secanski, M.; Postic, D. Effects of shape and size of hybrid maize seed on germination and vigour of different genotypes. Chil. J. Agric. Res. 2020, 80, 381–392. [Google Scholar] [CrossRef]
- Lehtilä, K.; Ehrlén, J. Seed size as an indicator of seed quality: A case study of Primula veris. Acta Oecol. 2005, 28, 207–212. [Google Scholar] [CrossRef]
- Beyaz, R. The effect of seed size on in vitro seed germination, seedling growth and tissue culture response of callus from mature embryos of wheat (Triticum sp.). Fresenius Environ. Bull. 2019, 28, 502–508. [Google Scholar]
- Beyzi, E.; Gunes, A.; Beyzi, S.B.; Konca, Y. Changes in fatty acid and mineral composition of rapeseed (Brassica napus ssp. oleifera L.) oil with seed sizes. Ind. Crops Prod. 2019, 129, 10–14. [Google Scholar]
- Deb, P.; Sundriyal, R. Effect of seed size on germination and seedling fitness in four tropical rainforest tree species. Indian J. For. 2017, 40, 313–322. [Google Scholar]
- Domic, A.I.; Capriles, J.M.; Camilo, G.R. Evaluating the fitness effects of seed size and maternal tree size on Polylepis tomentella (Rosaceae) seed germination and seedling performance. J. Trop. Ecol. 2020, 36, 115–122. [Google Scholar] [CrossRef]
- Kaydan, D.; Yagmur, M. Germination, seedling growth and relative water content of shoot in different seed sizes of triticale under osmotic stress of water and NaCl. Afr. J. Biotechnol. 2008, 7, 2862–2868. [Google Scholar]
- Clements, D.R.; Jones, V.L. Ten Ways That Weed Evolution Defies Human Management Efforts Amidst a Changing Climate. Agronomy 2021, 11, 284. [Google Scholar] [CrossRef]
- Gharibvandi, A.; Karimmojeni, H.; Ehsanzadeh, P.; Rahimmalek, M.; Mastinu, A. Weed management by allelopathic activity of Foeniculum vulgare essential oil. Plant Biosyst. 2022, 1–9. [Google Scholar] [CrossRef]
- Rad, S.V.; Valadabadi, S.A.R.; Pouryousef, M.; Saifzadeh, S.; Zakrin, H.R.; Mastinu, A. Quantitative and Qualitative Evaluation of Sorghum bicolor L. under Intercropping with Legumes and Different Weed Control Methods. Horticulturae 2020, 6, 78. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Li, L.; Schnable, J.; Gu, R.; Wang, J. QTL identification and epistatic effect analysis of seed size-and weight-related traits in Zea mays L. Mol. Breed. 2019, 39, 1–11. [Google Scholar] [CrossRef]
- Akhter, M.M.; Sabagh, A.E.; Alam, M.N.; Hasan, M.K.; Hafez, E.; Barutçular, C.; Islam, M.S. Determination of seed rate of wheat (Triticum aestivum L.) varieties with varying seed size. Sci. J. Crop Sci. 2017, 6, 161–167. [Google Scholar]
- Zhao, Z.; Wang, E.; Kirkegaard, J.A.; Rebetzke, G.J. Novel wheat varieties facilitate deep sowing to beat the heat of changing climates. Nat. Clim. Change 2022, 12, 291–296. [Google Scholar] [CrossRef]
- Singh, D.K.; Singh, V. Seed size and adventitious (nodal) roots as factors influencing the tolerance of wheat to waterlogging. Aust.J. Agr. Res. 2003, 54, 969–977. [Google Scholar] [CrossRef][Green Version]
- Ghassemi-Golezani, K.; Chadordooz-Jeddi, A.; Zehtab-Salmasi, S. Effects of seed size and aging on field performance of lentil (Lens culinaris Medik.) under different irrigation treatments. Acta Agric. Slov. 2015, 103, 158–166. [Google Scholar] [CrossRef]
- Pandey, R.; Bargali, S.; Bargali, K. Does seed size affect water stress tolerance in Quercus leucotrichophora A. camus at germination and early seedling growth stage. Biodivers. Int. J. 2017, 1, 00005. [Google Scholar]
- Karimmojeni, H.; Rahimian, H.; Alizadeh, H.; Yousefi, A.R.; Gonzalez-Andujar, J.L.; Sweeney, E.M.; Mastinu, A. Competitive Ability Effects of Datura stramonium L. and Xanthium strumarium L. on the Development of Maize (Zea mays) Seeds. Plants 2021, 10, 1922. [Google Scholar] [CrossRef] [PubMed]
- Barej, J.A.M.; Patzold, S.; Perkons, U.; Amelung, W. Phosphorus fractions in bulk subsoil and its biopore systems. Eur.J. Soil Sci. 2014, 65, 553–561. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Lilley, J.M.; Howe, G.N.; Graham, J.M. Impact of subsoil water use on wheat yield. Aust. J. Agr. Res. 2007, 58, 303–315. [Google Scholar] [CrossRef]
- Bauke, S.L.; von Sperber, C.; Tamburini, F.; Gocke, M.I.; Honermeier, B.; Schweitzer, K.; Baumecker, M.; Don, A.; Sandhage-Hofmann, A.; Amelung, W. Subsoil phosphorus is affected by fertilization regime in long-term agricultural experimental trials. Eur. J. Soil Sci. 2018, 69, 103–112. [Google Scholar] [CrossRef]
- Lynch, J.P.; Wojciechowski, T. Opportunities and challenges in the subsoil: Pathways to deeper rooted crops. J. Exp. Bot. 2015, 66, 2199–2210. [Google Scholar] [CrossRef]
- Hobley, E.U.; Honermeier, B.; Don, A.; Gocke, M.I.; Amelung, W.; Kogel-Knabner, I. Decoupling of subsoil carbon and nitrogen dynamics after long-term crop rotation and fertilization. Agr. Ecosyst. Environ. 2018, 265, 363–373. [Google Scholar] [CrossRef]
- Zangani, E.; Afsahi, K.; Shekari, F.; Mac Sweeney, E.; Mastinu, A. Nitrogen and Phosphorus Addition to Soil Improves Seed Yield, Foliar Stomatal Conductance, and the Photosynthetic Response of Rapeseed (Brassica napus L.). Agriculture 2021, 11, 483. [Google Scholar] [CrossRef]
- White, R.G.; Kirkegaard, J.A. The distribution and abundance of wheat roots in a dense, structured subsoil-implications for water uptake. Plant Cell Environ. 2010, 33, 133–148. [Google Scholar] [CrossRef]
- Korucu, T.; Arslan, S. Effects of Direct and Conventional Planting on Soil Properties and Yield Characteristics of Second Crop Maize. J. Agr Sci. Tarim Bili 2009, 15, 157–165. [Google Scholar] [CrossRef]
- Alngiemshy, N.F.; Alkharafi, J.S.; Alharbi, N.S.; Al-Sowayan, N.S. Effect of Seeds Size on Germination of Faba Bean Plant. Agric. Sci. 2020, 11, 457–463. [Google Scholar] [CrossRef]
- Ceseski, A.R.; Al-Khatib, K. Seeding depth effects on elongation, emergence, and early development of California rice cultivars. Crop Sci. 2021, 61, 2012–2022. [Google Scholar] [CrossRef]
- Kimmelshue, C.L.; Goggi, S.; Moore, K.J. Seed Size, Planting Depth, and a Perennial Groundcover System Effect on Corn Emergence and Grain Yield. Agronomy 2022, 12, 437. [Google Scholar] [CrossRef]
- Abbas, A.M.; Rubio-Casal, A.E.; De Cires, A.; Figueroa, E.M.; Pickart, A.J.; Castillo, J.M. Burial effects on seed germination and seedling emergence of two halophytes of contrasting seed size. Plant Ecol. Divers. 2020, 13, 339–349. [Google Scholar] [CrossRef]
- Tsedaley, B. Review on Seed Health Tests and Detection Methods of Seedborne Diseases. J. Biol. Agric. Healthc. 2015, 5, 176–184. [Google Scholar]
- Evrendilek, G.A.; Karatas, B.; Uzuner, S.; Tanasov, I. Design and effectiveness of pulsed electric fields towards seed disinfection. J. Sci. Food Agric. 2019, 99, 3475–3480. [Google Scholar] [CrossRef]
- Hassanisaadi, M.; Bonjar, G.H.S.; Hosseinipour, A.; Abdolshahi, R.; Barka, E.A.; Saadoun, I. Biological Control of Pythium aphanidermatum, the Causal Agent of Tomato Root Rot by Two Streptomyces Root Symbionts. Agronomy 2021, 11, 846. [Google Scholar] [CrossRef]
- Yang, J.; Lovett-Doust, J.; Lovett-Doust, L. Seed germination patterns in green dragon (Arisaema dracontium, Araceae). Am. J. Bot. 1999, 86, 1160–1167. [Google Scholar] [CrossRef]
- Côme, D. Obstacles to germination. Obs. Germination. 1970, 6, 162. [Google Scholar]
- Sidhu, J.S.; Singh, D.; Gill, H.S.; Brar, N.K.; Qiu, Y.Y.; Halder, J.; Al Tameemi, R.; Turnipseed, B.; Sehgal, S.K. Genome-Wide Association Study Uncovers Novel Genomic Regions Associated With Coleoptile Length in Hard Winter Wheat. Front. Genet. 2020, 10, 1345. [Google Scholar] [CrossRef]
- Noryan, M.; Hervan, I.M.; Sabouri, H.; Kojouri, F.D.; Mastinu, A. Drought Resistance Loci in Recombinant Lines of Iranian Oryza sativa L. in Germination Stage. BioTech 2021, 10, 26. [Google Scholar] [CrossRef]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Le, K.A.; Van den Broeck, H.C.; Brouns, F.J.P.H.; De Brier, N.; et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.R.; Rashidi, S.; Moradi, P.; Mastinu, A. Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to PEG-Induced Drought Stress. Environments 2020, 7, 107. [Google Scholar] [CrossRef]
- Karasakal, A. Production of Wheat Seed Through Various Plantation Practices in Maternal Environment. Biosci. Biotechnol. Res. Commun. 2021, 14, 549–552. [Google Scholar] [CrossRef]
- Jorgensen, M.S.; Labouriau, R.; Olesen, B. Seed size and burial depth influence Zostera marina L. (eelgrass) seed survival, seedling emergence and initial seedling biomass development. PLoS ONE 2019, 14, e0215157. [Google Scholar] [CrossRef]
- Gough, R.E.a. Seed Quality: Basic Mechanisms and Agricultural Implications, 1st ed.; CRC Press: New York, NY, USA, 1995. [Google Scholar] [CrossRef]
- Subedi, M.; Willenborg, C.J.; Vandenberg, A. Influence of Harvest Aid Herbicides on Seed Germination, Seedling Vigor and Milling Quality Traits of Red Lentil (Lens culinaris L.). Front. Plant. Sci. 2017, 8, 311. [Google Scholar] [CrossRef]
- Ghiyasi, M.; Moghaddam, S.S.; Amirnia, R.; Damalas, C.A. Chemical Priming with Salt and Urea Improves Germination and Seedling Growth of Black Cumin (Nigella sativa L.) under Osmotic Stress. J. Plant. Growth Regul. 2019, 38, 1170–1178. [Google Scholar] [CrossRef]
- Bochenek, A.; Gielwanowska, I.; Czerminska, M.; Bojarowska, K. The effect of antifungal drugs and fungicides on the viability and vigour of barnyard millet (Echinochloa crus-galli) seeds. Seed Sci. Technol. 2019, 47, 121–130. [Google Scholar] [CrossRef]
- Ambrico, P.F.; Simek, M.; Morano, M.; Angelini, R.M.D.; Minafra, A.; Trotti, P.; Ambrico, M.; Prukner, V.; Faretra, F. Reduction of microbial contamination and improvement of germination of sweet basil (Ocimum basilicum L.) seeds via surface dielectric barrier discharge. J. Phys. D Appl. Phys. 2017, 50, 305401. [Google Scholar] [CrossRef]
- Bernardes, P.M.; Andrade-Vieira, L.F.; Aragao, F.B.; Ferreira, A.; Ferreira, M.F.D. Toxicological effects of comercial formulations of fungicides based on procymidone and iprodione in seedlings and root tip cells of Allium cepa. Environ. Sci. Pollut. Res. 2019, 26, 21013–21021. [Google Scholar] [CrossRef]
- Moshynets, O.V.; Babenko, L.M.; Rogalsky, S.P.; Iungin, O.S.; Foster, J.; Kosakivska, I.V.; Potters, G.; Spiers, A.J. Priming winter wheat seeds with the bacterial quorum sensing signal N-hexanoyl-L-homoserine lactone (C6-HSL) shows potential to improve plant growth and seed yield. PLoS ONE 2019, 14, e0209460. [Google Scholar] [CrossRef] [PubMed]
- Aguado, A.; Savoie, J.M.; Chereau, S.; Ducos, C.; Aguilar, M.; Ferrer, N.; Aguilar, M.; Pinson-Gadais, L.; Richard-Forget, F. Priming to protect maize from Fusarium verticillioides and its fumonisin accumulation. J. Sci. Food Agric. 2019, 99, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Santo Pereira, A.D.; Oliveira, H.C.; Fraceto, L.F.; Santaella, C. Nanotechnology Potential in Seed Priming for Sustainable Agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Okey-Onyesolu, C.F.; Hassanisaadi, M.; Bilal, M.; Barani, M.; Rahdar, A.; Iqbal, J.; Kyzas, G.Z. Nanomaterials as Nanofertilizers and Nanopesticides: An Overview. Chemistryselect 2021, 6, 8645–8663. [Google Scholar] [CrossRef]
- Leslie, J.F.; Moretti, A.; Mesterházy, Á.; Ameye, M.; Audenaert, K.; Singh, P.K.; Richard-Forget, F.; Chulze, S.N.; Ponte, E.M.D.; Chala, A.; et al. Key Global Actions for Mycotoxin Management in Wheat and Other Small Grains. Toxins 2021, 13, 725. [Google Scholar] [CrossRef]
- Rajjou, L.; Debeaujon, I. Seed longevity: Survival and maintenance of high germination ability of dry seeds. Comptes Rendus Biol. 2008, 331, 796–805. [Google Scholar] [CrossRef]
- Ayesha, M.S.; Suryanarayanan, T.S.; Nataraja, K.N.; Prasad, S.R.; Shaanker, R.U. Seed Treatment with Systemic Fungicides: Time for Review. Front. Plant Sci. 2021, 12, 1581. [Google Scholar] [CrossRef]
- Capo, L.; Zappino, A.; Reyneri, A.; Blandino, M. Role of the Fungicide Seed Dressing in Controlling Seed-Borne Fusarium spp. Infection and in Enhancing the Early Development and Grain Yield of Maize. Agronomy 2020, 10, 784. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rezaei, M.; Tseng, T.-M.; Mastinu, A. Effects of Metribuzin Herbicide on Some Morpho-Physiological Characteristics of Two Echinacea Species. Horticulturae 2022, 8, 169. [Google Scholar] [CrossRef]
- Roman, D.L.; Voiculescu, D.I.; Filip, M.; Ostafe, V.; Isvoran, A. Effects of Triazole Fungicides on Soil Microbiota and on the Activities of Enzymes Found in Soil: A Review. Agriculture 2021, 11, 893. [Google Scholar] [CrossRef]
- Abati, J.; Zucareli, C.; Foloni, J.S.S.; Henning, F.A.; Brzezinski, C.R.; Henning, A.A. Treatment with fungicides and insecticides on the physiological quality and health of wheat seeds. J. Seed Sci. 2014, 36, 392–398. [Google Scholar] [CrossRef]
- Radzikowska, D.; Grzanka, M.; Kowalczewski, P.Ł.; Głowicka-Wołoszyn, R.; Blecharczyk, A.; Nowicki, M.; Sawinska, Z. Influence of SDHI Seed Treatment on the Physiological Conditions of Spring Barley Seedlings under Drought Stress. Agronomy 2020, 10, 731. [Google Scholar] [CrossRef]
- Fernando, N.; Humphries, T.; Florentine, S.K.; Chauhan, B.S. Factors Affecting Seed Germination of Feather Fingergrass (Chloris virgata). Weed Sci. 2016, 64, 605–612. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef]
- Carvalho, P.C.D.; Barro, R.S.; Neto, A.B.; Nunes, P.A.D.; de Moraes, A.; Anghinoni, I.; Bredemeier, C.; Bayer, C.; Martins, A.P.; Kunrath, T.R.; et al. Integrating the pastoral component in agricultural systems. Rev. Bras. Zootecn. 2018, 47, e20170001. [Google Scholar] [CrossRef]
- Khaliliaqdam, N.; Soltani, A.; Latifi, N.; Far, F.G. Soybean Seed Aging and Environmental Factors on Seedling Growth. Commun. Soil Sci. Plant Anal. 2013, 44, 1786–1799. [Google Scholar] [CrossRef]
- Niedz, R.P.; Mohan, A.; Schillinger, W.F.; Gill, K.S. Wheat Seedling Emergence from Deep Planting Depths and Its Relationship with Coleoptile Length. PLoS ONE 2013, 8, e73314. [Google Scholar] [CrossRef]
- Flohr, B.M.; Ouzman, J.; McBeath, T.M.; Rebetzke, G.J.; Kirkegaard, J.A.; Llewellyn, R.S. Redefining the link between rainfall and crop establishment in dryland cropping systems. Agric. Syst. 2021, 190, 103105. [Google Scholar] [CrossRef]
- Bazzaz, M.M.; Hossain, A.; Timsina, J.; da Silva, J.A.T.; Nuruzzaman, M. Growth, yield attributes and yield of irrigated spring wheat as influenced by sowing depth. Open Agric. 2018, 3, 72–83. [Google Scholar] [CrossRef]
- Andresen, M.; Wulff, E.G.; Mbega, E.R.; Stokholm, M.S.; Glazowska, S.E.; Zida, P.E.; Mabagala, R.B.; Lund, O.S. Seed treatment with an aqueous extract of Agave sisalana improves seed health and seedling growth of sorghum. Eur. J. Plant Pathol. 2014, 141, 119–132. [Google Scholar] [CrossRef]



| Soil Type | pH | Depth | C Organic | N | Clay | Loam | Sand | EC | P | K |
|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | (%) | (ds/m) | (ppm) | |||||||
| Clay loam | 7/5 | 0–30 | 0/66 | 0.066 | 5/5 | 34 | 60/5 | 0/75 | 27.6 | 400 |
| MS | |||||||
|---|---|---|---|---|---|---|---|
| Treatments | df | CL | SL | DW | PG | SG | VI |
| Cultivars | 3 | 0.77 ** | 5 ** | 0.0042 ns | 0.058 ns | 0.38 ns | 5 ** |
| Seed Size | 1 | 0.27 ** | 0.14 ns | 0.24 ** | 0.75 ns | 0.08 ns | 0.028 ns |
| Disinfection | 1 | 1.41 ** | 0.89 ns | 0.008 * | 0.004 ns | 0.002 ns | 0.82 ns |
| Cultivars × Seed Size | 3 | 0.042 ns | 5.48 ** | 0.00058 ns | 6.13 ns | 0.25 ns | 3.83 ** |
| Cultivars × Disinfection | 3 | 0.39 ** | 7.43 ** | 0.0002 ns | 11.5 * | 0.27 ns | 6.89 ns |
| Seed Size × Disinfection | 1 | 0.076 ns | 0.01 ns | 0.0013 ns | 8.33 ns | 0.083 ns | 1.57 ns |
| Cultivars × Seed Size × Disinfection | 3 | 0.042 ns | 0.46 ns | 0.002 ns | 6.5 ns | 0.58 * | 0.52 ns |
| Error | 32 | 0.021 | 0.49 | 0.018 | 3.25 | 0.18 | 0.46 |
| CV | - | 5.86 | 3.71 | 1.95 | 7.16 | 5.61 | 3.88 |
| MS | |||||||
|---|---|---|---|---|---|---|---|
| Treatments | df | FW | SL | DW | PG | SG | VI |
| Cultivars | 3 | 1.24 ** | 59.24 ** | 0.13 ** | 4001.28 ** | 0.402 ** | 62.31 ** |
| Seed Size | 1 | 10.76 ** | 135.91 ** | 0.01 ** | 5861.81 ** | 0.59 ** | 258.91 ** |
| Disinfection | 1 | 0.95 ** | 2.58 ns | 0.011 ns | 1445.58 ** | 0.14 ** | 35.18 ** |
| Sowingdepth | 2 | 7.76 ** | 229.36 ** | 0.0002 ** | 13480.9 ** | 1.35 ** | 518.7 ** |
| Cultivars × Size | 3 | 0.48 * | 15.06 ** | 0.066 * | 243.32 ns | 0.024 ns | 2.72 ns |
| Cultivars × Disinfection | 3 | 0.53 * | 0.21 ns | 0.0052 * | 74.77 ns | 0.007 ns | 1.15 ns |
| Cultivars × Depth | 6 | 0.21 ns | 25.79 ** | 0.0044 ns | 867.6 ** | 0.085 ** | 9.26 ** |
| Size × Disinfection | 1 | 0.001 ns | 0.05 ns | 0.0025 ns | 13.29 ns | 0.001 ns | 0.26 ns |
| Size × Depth | 2 | 0.19 ns | 4.28 ns | 0.0029 ns | 376.79 * | 0.039 * | 12.76 ** |
| Disinfection × Depth | 2 | 0.57 * | 0.02 ns | 0.0062 ns | 62.12 ns | 0.006 ns | 2.3 ns |
| Cultivars × Size × Disinfection | 3 | 0.19 ns | 0.094 ns | 0.0015 ns | 343.87 * | 0.035 * | 6.43 * |
| Cultivars × Disinfection × Depth | 6 | 0.45 ** | 0.21 ns | 0.0044 * | 129.03 ns | 0.012 ns | 2.28 ns |
| Cultivars × Size × Depth | 6 | 0.10 ns | 2.5 ns | 0.00086 ns | 310.60 * | 0.030 ** | 2.2 ns |
| Size × Disinfection × Depth | 2 | 0.004 ns | 0.19 ns | 0.00085 ns | 19.80 ns | 0.002 ns | 0.42 ns |
| Cultivars×Size×Disinfection× Depth | 12 | 0.15 ns | 1.3 ns | 0.0014 ns | 335.37 ** | 0.033 ** | 3.53 * |
| Error | 96 | 0.13 | 1.68 | 0.0014 | 104.98 | 0.01 | 1.64 |
| CV | - | 14.74 | 10.66 | 15.86 | 16.13 | 16.05 | 15.84 |
| Cultivars | Depth | Disinfection | Size (mm) | PG | SG | VI |
|---|---|---|---|---|---|---|
| (%) | (N/Day) | |||||
| PHM | 4 | Dis | >2.25 | 79.1 ± 2.94 a–g* | 0.79 ± 0.02 a–i | 11.2 ± 0.03 c–f |
| <2.25 | 66.7 ± 2.94 f–k | 0.67 ± 0.02 f–m | 9.5 ± 0.1 f–k | |||
| None-Dis | >2.25 | 93.7 ± 5.1 a | 0.94 ± 0.05 a | 13.7 ± 0.6 a | ||
| <2.25 | 43.7 ± 5.1 m–q | 0.44 ± 0.05 o–q | 5.5 ± 0.62 p–t | |||
| 6 | Dis | >2.25 | 83.3 ± 5.8 a–e | 0.84 ± 0.05 a–e | 12.1 ± 0.64 a–e | |
| <2.25 | 45.8 ± 10.6 i–p | 0.46 ± 0.1 n–q | 5.9 ± 1.5 p–t | |||
| None-Dis | >2.25 | 81.2 ± 10.2 a–f | 0.81 ± 0.1 a–g | 10.5 ± 0.6 e–i | ||
| <2.25 | 52 ± 2.9 k–o | 0.52 ± 0.02 m–p | 6.2 ± 0.1 o–s | |||
| 8 | Dis | >2.25 | 66.6 ± 7.7 h–k | 0.67 ± 0.07 f–m | 8.9 ± 1.2 h–m | |
| <2.25 | 14.5 ± 2.9 s | 0.15 ± 0.02 s | 1.5 ± 0.1 w | |||
| None-Dis | >2.25 | 62.5 ± 10.2 h–k | 0.63 ± 0.1 i–m | 7.6 ± 0.5 j–o | ||
| <2.25 | 35.4 ± 4.7 p–q | 0.35 ± 0.14 q | 4 ± 1.1 t–v | |||
| MHN | 4 | Dis | >2.25 | 70.83 ± 2.9 e–i | 0.71 ± 0.02 d–i | 10 ± 1 f–i |
| <2.25 | 66.7 ± 2.9 h–k | 0.67 ± 0.02 f–m | 9.3 ± 0.6 f–k | |||
| None-Dis | >2.25 | 87.5 ± 5.1 a-c | 0.88 ± 0.05 a–c | 13.3 ± 1.1 a–c | ||
| <2.25 | 16.6 ± 2.8 s | 0.17 ± 0.12 s | 1.3 ±1 w | |||
| 6 | Dis | >2.25 | 68.7 ± 5.1 e–i | 0.69 ± 0.05 e–i | 9.1 ± 0.8 g–m | |
| <2.25 | 37.5 ± 8.8 o–q | 0.38 ± 0.08 pq | 3.2 ± 1 u–w | |||
| None-Dis | >2.25 | 58.3 ± 5.8 j–m | 0.59 ± 0.05 k–o | 7.7 ± 1.2 j–o | ||
| <2.25 | 33.3 ± 2.5 p–r | 0.33 ± 0.23 qr | 4.13 ± 0.9 t-u | |||
| 8 | Dis | >2.25 | 70.8 ± 7.7 d–i | 0.71 ± 0.07 d–k | 9.4 ± 0.2 f–k | |
| <2.25 | 29.1 ± 2.1 q–s | 0.29 ± 0.02 q–s | 1.9 ± 0.1 vw | |||
| None-Dis | >2.25 | 43.7 ± 4.2 m–q | 0.44 ± 0.1 o–q | 5 ± 1.7 s–u | ||
| <2.25 | 18.7 ± 1.5 r–s | 0.19 ± 0.13 rs | 1.3 ± 0.9 w | |||
| SSN | 4 | Dis | >2.25 | 89.5 ± 2.9 ab | 0.9 ± 0.02 ab | 13.1 ± 0.1 a–c |
| <2.25 | 79.1 ± 5.8 a–g | 0.79 ± 0.05 a–h | 11.3 ± 0.9 b–e | |||
| None-Dis | >2.25 | 91.6 ± 5.8 a | 0.92 ± 0.05 ab | 13.7 ± 1.3 a | ||
| <2.25 | 64.5 ± 2.9 g–k | 0.65 ± 0.02 h–m | 7.4 ± 0.2 i–p | |||
| 6 | Dis | >2.25 | 68.7 ± 5.1 g–k | 0.69 ± 0.05 e–i | 9.9 ± 0.8 f–i | |
| <2.25 | 62.5 ± 5.1 h–k | 0.63 ± 0.05 i–m | 7.3 ± 0.8 m–q | |||
| None-Dis | >2.25 | 68.7 ± 5.1 e–i | 0.69 ± 0.05 e–i | 9.8 ± 0.4 f–k | ||
| <2.25 | 54.1 ± 2.9 j–n | 0.54 ± 0.02 l–p | 7.1 ± 0.6 j–o | |||
| 8 | Dis | >2.25 | 78.5 ± 10 abc | 0.87 ± 0.1 a-c | 12.6 ± 1.4 a–d | |
| <2.25 | 41.6 ± 2.9 n–q | 0.42 ± 0.02 pq | 5 ± 0.5 s–u | |||
| None-Dis | >2.25 | 70.82 ± 7.7 d–i | 0.71 ± 0.07 d–j | 9.6 ± 1.4 f–j | ||
| <2.25 | 52 ± 11.7 k–o | 0.52 ± 0.11 m–p | 6.4 ± 1.5 o–s | |||
| HDI | 4 | Dis | >2.25 | 91.6 ± 2.9 ab | 0.92 ± 0.02 ab | 13.4 ± 0.6 ab |
| <2.25 | 70.8 ± 7.7 d–i | 0.71 ± 0.07 d–k | 9 ± 1.1 g–m | |||
| None-Dis | >2.25 | 89.5 ± 5.8 ab | 0.9 ± 0.05 ab | 13.3 ± 1 a–c | ||
| <2.25 | 64.5 ± 5.8 g–k | 0.65 ± 0.05 d–k | 7.2 ± 1.2 m–q | |||
| 6 | Dis | >2.25 | 81.2 ± 8.8 a–f | 0.82 ± 0.08 a–f | 10.8 ± 1.6 d–h | |
| <2.25 | 62.5 ± 6.8 h–k | 0.62 ± 0.08 j–m | 6.8 ± 2 n–s | |||
| None-Dis | >2.25 | 66.6 ± 5.8 f–j | 0.68 ± 0.05 f–i | 8.7 ± 0.7 i–n | ||
| <2.25 | 66.6 ± 5.8 f–j | 0.66 ± 0.05 f–i | 5.5 ± 0.5 p–t | |||
| 8 | Dis | >2.25 | 85.42 ± 7.2 a–d | 0.68 ± 0.04 a–d | 11.07 ± 0.7d–g | |
| <2.25 | 77 ± 2.9 b–h | 0.77 ± 0.02 b–j | 5.5 ± 0.5p–t | |||
| None-Dis | >2.25 | 72.9 ± 7.7 c–i | 0.73 ± 0.07 c–k | 5.1 ± 0.4 q–u | ||
| <2.25 | 60.4 ± 2.9 i–l | 0.61 ± 0.02 j–n | 5.2 ± 0.3 r–u | |||
| LSD (5%) | 16.6 | 0.16 | 2.08 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaichi, M.; Nemati, A.; Dadrasi, A.; Heydari, M.; Hassanisaadi, M.; Yousefi, A.R.; Baldwin, T.C.; Mastinu, A. Germination of Triticum aestivum L.: Effects of Soil–Seed Interaction on the Growth of Seedlings. Soil Syst. 2022, 6, 37. https://doi.org/10.3390/soilsystems6020037
Chaichi M, Nemati A, Dadrasi A, Heydari M, Hassanisaadi M, Yousefi AR, Baldwin TC, Mastinu A. Germination of Triticum aestivum L.: Effects of Soil–Seed Interaction on the Growth of Seedlings. Soil Systems. 2022; 6(2):37. https://doi.org/10.3390/soilsystems6020037
Chicago/Turabian StyleChaichi, Mehrdad, Ahmad Nemati, Amir Dadrasi, Moslem Heydari, Mohadeseh Hassanisaadi, Ali Reza Yousefi, Timothy C. Baldwin, and Andrea Mastinu. 2022. "Germination of Triticum aestivum L.: Effects of Soil–Seed Interaction on the Growth of Seedlings" Soil Systems 6, no. 2: 37. https://doi.org/10.3390/soilsystems6020037
APA StyleChaichi, M., Nemati, A., Dadrasi, A., Heydari, M., Hassanisaadi, M., Yousefi, A. R., Baldwin, T. C., & Mastinu, A. (2022). Germination of Triticum aestivum L.: Effects of Soil–Seed Interaction on the Growth of Seedlings. Soil Systems, 6(2), 37. https://doi.org/10.3390/soilsystems6020037







