Abstract
The availability of P is often insufficient and limited by accumulation in soils. This led to the necessity of solutions for the recovery as well as recycling of secondary P resources. Batch experiments were conducted with CaCl2 and citric acid to characterize P release kinetics from vivianite, hydroxyapatite, and bone char. While the P release during the CaCl2 treatment was so low that only vivianite and hydroxyapatite showed a slightly higher release with increasing CaCl2 concentration, the increase of dissolved P was more pronounced for citric acid. The application of citric acid resulted in a 32,190-fold higher P release for bone char. Fourier-transform infrared spectroscopic data suggested higher instability of hydroxyapatite than for bone char. The kinetic data showed that bone char, especially at a lower particle size, had a higher long-term P release than hydroxyapatite or vivianite. The suitability of hydroxyapatite and bone char as a poorly soluble, but sustainable P source is better than that of vivianite. However, the efficiency as a P fertilizer is also dependent on present soil P mobilization processes. The results underline the importance of the accessibility of fertilized or naturally bound P for plant roots to benefit from the excretion of organic acids.
1. Introduction
Phosphorus (P) is an essential nutrient for physiological processes during plant growth and fertilization in agriculture is necessary to maintain crop productivity. However, the bioavailability of P is often insufficient and limited by rapid precipitation and adsorption processes in soils [1]. Depending on the soil properties, the P use efficiency is as low as about 20 to 10% [2,3]. As a consequence, many soils worldwide have accumulated substantial amounts of fertilized P because of excessive applications over a longer period of time [4,5,6]. These soil P reserves, if they cannot be used efficiently, can cause eutrophication to marine and aquatic environments if lost [7]. Therefore, it has become necessary to find sustainable solutions for an efficient use of the existing soil P reserves as well as secondary P sources (e.g., bone char or sewage sludge to substitute conventional fertilizers).
Apatite is a primary source of P in terrestrial ecosystems and occurs as a lithogenic mineral in soils worldwide. The release of P from the lithosphere into the soil is affected by many physical, chemical, and biological processes [8], where weathering and dissolution of apatite will release secondary precipitated P, differing in stability [9]. The use of solid hydroxyapatites (HA) as direct P source is ineffective, because the large size of the particles, in addition to a high soil pH, limits P mobility and hence, bioavailability to plants. For this reason, modified synthetic nano-HA should promote the efficiency of P fertilizer [2,10]. The insolubility in water should increase the bioavailability of nano-HA due to limited chemical reactions in soil such as precipitation or adsorption on soil colloids [11]. Vivianite (VI) is one of the most common stable Fe phosphates in anthropogenically influenced soils and sediments. Amongst others, it occurs in the sediments of lakes, rivers canals, or water-saturated soils. In addition, it has been found in sewage sludge as a weathering product in hydrothermal deposits [12]. However, for many P recycling products, the value and fertilizer efficiency are unknown and especially for sewage sludge, heavy metals and contaminants can be discharged back into the environment [13]. Bone char (BC) as an alternative P fertilizer in agriculture contains no heavy metals or organic contaminants, but is rich in P and Ca [14,15]. P in BC is mainly bound in a structure similar to HA, which is of low solubility [16,17] Therefore, untreated HA and BC are expected to have a low P fertilizer efficiency.
One aspect of this study was to evaluate the availability of P resources. On one hand, the unguided and passive P mobilization by inorganic constituents of the soil solution such as CaCl2 will take place on a large scale. On the other hand, plants that had developed a range of adaptive strategies to enhance the availability of P in case of demand on a smaller scale. This includes symbiotic root-microbe associations, root-mycorrhiza interactions, and the secretion of root exudates [18]. The exudation of low molecular-weight organic acids [19] such as citric acid (CA), which is often detected at a higher concentration in the rhizosphere, has been described as highly effective in mobilizing inorganic P [20,21]. With regard to an efficient use of present P resources in soils, the aim was to characterize P release kinetics from VI, HA, and BC as well as the detection of rapidly and slowly available P over time. Therefore, the amount of inorganic P that can be released by increasing concentrations of CaCl2 and CA from different P sources has to be determined, and the suitability of low soluble P sources as sustainable P fertilizer with regard to active and passive P mobilization processes will be evaluated.
2. Materials and Methods
The BC used for the P release experiments was produced by Bonechar Carvão Ativado Do Brasil Ltda. (Maringá, Brazil) in 2015. It has been manufactured by pyrolysis of rendered (de-fatted) bovine bones at more than 800 °C. A particle size analysis of BC was carried out, whereby 100 g of BC was divided into three particle size fractions: <200 µm, 200–2000 µm, and >2000 µm. In order to have enough sample material for the P release experiments, coarser material was then ground and sieved. The BC composition was analyzed by determining C, N, and S using a CNS elemental analyzer as well as P, Ca, K, and Mg by pressure digestion with HNO3 (65%) and measured using an inductively coupled plasma atomic emission spectrometer (ICP-AES).
The P bearing minerals used were the commercially available Ca-phosphate HA (Ca5[OH(PO4)3]) (Acros Organics, Geel, Belgium) and the Fe–phosphate VI (Fe32+[PO4]2·8H2O). VI was prepared according to [22], where 250 mL of a 0.035 M H3PO4-solution was added to solid FeSO4. The resulting 0.05 M FeSO4-solution was adjusted to pH 6 with 5 M KOH. The precipitate formed was centrifuged for 5 min at 2090 G and washed with ultrapure water. The prepared VI was dried at 40 °C and ground into powder using mortar and pestle. P, Ca, and Fe were determined using ICP-AES after pressure digestion or aqua regia digestion for VI. The composition of HA and VI was verified using x-ray diffraction (XRD).
Specific surface areas were determined with an Autosorb-1 (Quantachrome, Odelzhausen, Germany) using a multi-point BET-measurement (Brunauer-Emmett-Teller) and N2 as adsorptive medium. An outgas test was performed to verify the completed outgas procedure for each mineral.
The pH of BC, HA, and VI was measured in both 0.01 M CaCl2 and deionized H2O, respectively, with a solid-solution ratio of 1:5. The pH values of BC, HA, and VI were not adjusted prior to the release experiments in order to prevent an undesired P release.
P release experiments were conducted in triplicate by using a batch setup with an initial pH of 6. For this, 2.5 g of HA, VI, and BC in the three particle size fractions of <200 µm (BC200), 200–2000 µm (BC200–2000), and >2000 µm (BC2000), respectively, were weighed into polymethylenebottles. Batch solubilization experiments were performed with CaCl2 (Merck Millipore) and CA with the formula C6H8O7 (99%, Alfa Aesar), adjusted to pH 6 with KOH. These reaction solutions were used in concentrations 0.01 M, 0.05 M, and 0.1 M, respectively. A 50 mL reaction solution was added to the BC samples and 40 mL was added to the HA and VI samples, respectively. The samples were shaken on a horizontal shaker for 24 h at 200 motions min−1, centrifuged for 15 min at 2090 G, and the supernatant was filtrated by using P-poor Whatman 512 1/2 filters. Following that, 50 mL of fresh reaction solution was added to the samples. Samples for P measurement were taken after 2, 6, 24, 48, and 168 h. Additionally, P, Ca, Fe, Mg, and K were determined using ICP-AES as well as Cl using ion chromatography. In the sample solutions using citric acid, only P, Ca, and Mg were measured. K was not determined due to the high KOH additions at the pH adjustment. The pH of the sample solution was measured after 2 and 168 h reaction time. The cumulative P release depending on time was calculated for each mineral and reaction solution. Different kinetic models (Table 1) were fitted to the data.

Table 1.
Selected kinetic equations.
Fourier-transform infrared spectroscopy will be used to estimate the stability of the investigated materials and to draw conclusions about the mechanisms of P release. After the P release experiments, the samples were oven-dried at 40 °C and stored in a desiccator. The FT-IR spectroscopic measurements were performed without further treatment of the samples prior to analyses. Absorbance was measured in the FT-IR DRIFT mode (Tensor 27 HTS-XT, Bruker), with a wavenumber range from 4000 to 400 cm−1. The spectrum of each treatment was created by generating the average of the three replicated samples, where each sample included 40 scans. For analyses, the spectra were baseline corrected with regard to the respective reference spectrum (without P release), truncated in the range 4000 to 550 cm−1, and normalized with respect to the highest absorbance.
3. Results
3.1. Characterization of Bone Char (BC), Hydroxyapatite (HA), and Vivianite (VI)
The particle size analysis of BC revealed that 99% of BC particles were in the size range of >2000 µm and only 0.1% of the particles were smaller than 200 µm. The BET specific surface area was lowest for VI with 39.49 m2·g−1, followed by HA with 68.39 m2·g−1 (Table 2). The specific surface area of BC increased slightly with decreasing particle size and amounted to 94.78 m2·g−1 for BC200.

Table 2.
Brunauer Emmett Teller (BET) specific surface area and elemental composition* of vivianite, hydroxyapatite, and bone char. Given are the mean and standard deviations (SD).
The initial P concentration of BC was in a similar range as for HA, but higher than for VI. The total content of P, C, N, S, Ca, Fe, Mg, and K are shown in Table 2.
3.2. P Release Efficiency
The cumulative P release based on 168 h with CaCl2 was very low for all used materials. While the total P release for BC was close to the detection limit, minor P concentrations were measured for VI and HA, which increased slightly with increasing CaCl2 concentration. The use of CA enabled significantly more P to be solved, whereby an increase of dissolved P with increasing CA concentration was also measured (Table 3). At a low CA concentration of 0.01 M, the lowest amount of P was released from BC, where most P was released from the smallest particle fraction. In total, most P was released from HA after 168 hours.

Table 3.
Efficiency of P release after 168 h reaction time. Given are the mean* and standard deviations (SD).
3.3. Kinetics of P Release
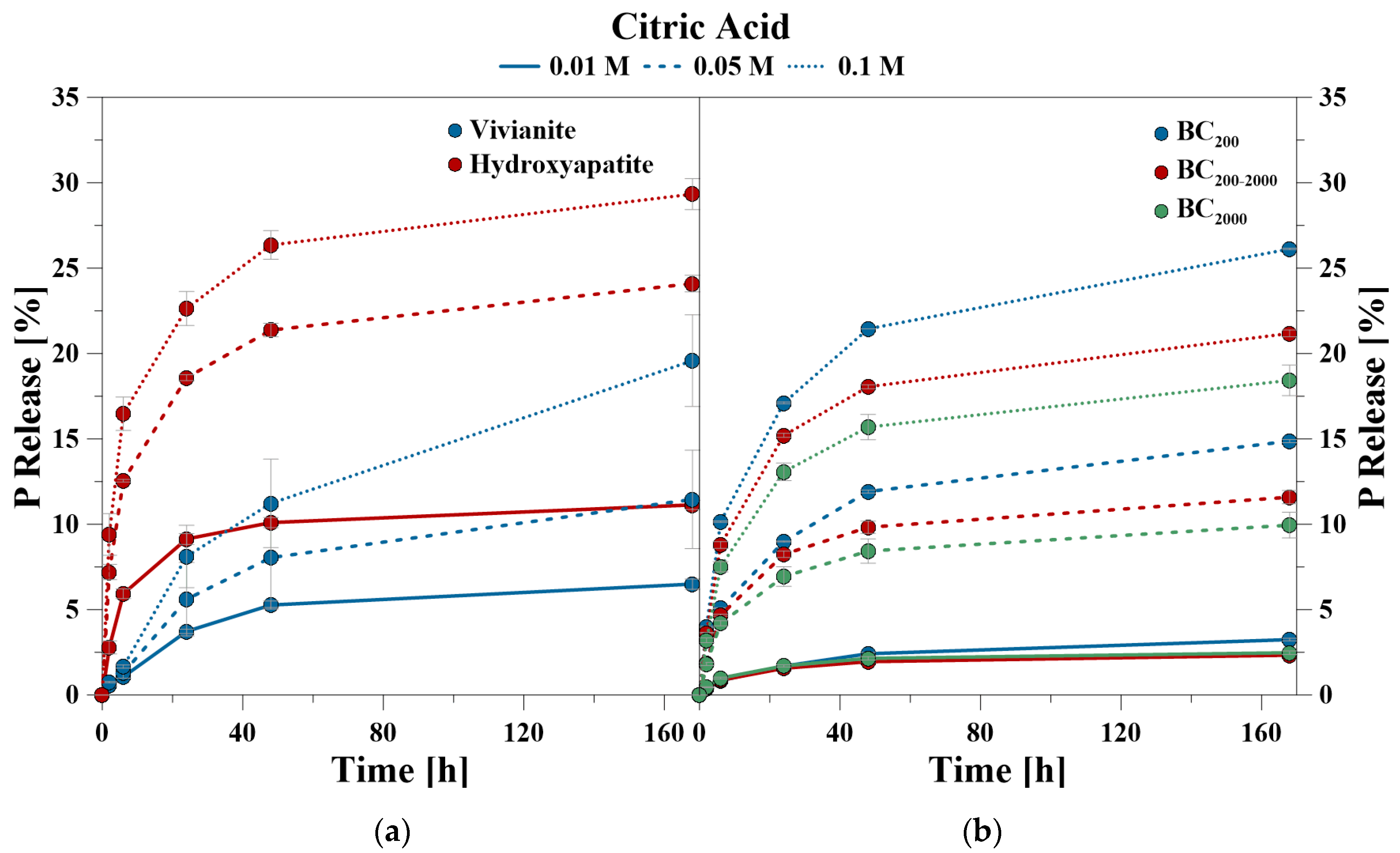
The percentage of P release shown in the kinetic curves refers to the amount of mobilized P related to the amount of total P of the minerals (= 100%), given in Table 2. The P kinetic curves showed a fast release from 0 to 24 h, and a slow release from 24 to 168 h, respectively. The time-dependent data of the CaCl2 treatment showed that for VI between 45 and 52% and for HA between 92 and 99% of the totally mobilized P was solved during the first 24 h. Even at the highest CaCl2 concentration of 0.1 M, HA and VI showed only a very low P release after 24 h (Figure 1).
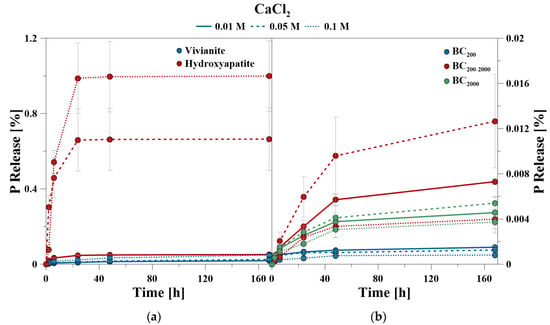
Figure 1.
P release kinetics of (a) hydroxyapatite and vivianite as well as (b) bone char using CaCl2 at pH 6.
In contrast, BC had an ongoing P release after 24 h despite a very low total amount of release P. This was especially observed for BC200–2000. The proportion of mobilized P within the first 24 h ranged between 60% and 82% for BC200, 46% and 60% for BC200–2000, and 49% and 56% for BC2000, respectively.
During the CA treatment, P was also predominantly released in the first 24 h, but lasted notably longer than during the CaCl2 treatment (Figure 2). The time-dependent data of the CA treatment showed that for VI between 41% and 57% and for HA between 77% and 82% of the totally mobilized P was solved during the first 24 h. For the BC samples, the values ranged between 52% and 65% for BC200, 67% and 72% for BC200–2000, and 69% and 71% for BC2000, respectively.
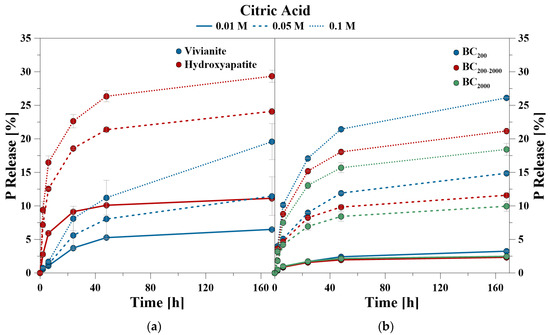
Figure 2.
P release kinetics of (a) hydroxyapatite and vivianite, as well as (b) bone char using citric acid (CA) at pH 6.
The coefficients of determination (R2) for the applied kinetic models indicated that the kinetics of P release for all treatments and materials fitted best with the Elovich equation (mean R2 = 0.95), followed by the Exponential (mean R2 = 0.90) and the Parabolic function (mean R2 = 0.86) (Table 4). The kinetic parameters obtained from Elovich and Exponential functions showed a lower initial P release (α, a) and a higher P release over time (β, b) (Table 5) for the CaCl2 treatment. While α increased and β decreased for VI and HA with increasing CaCl2 concentration, the converse was calculated for the BC samples. For the CA treatment, the kinetic parameters of the Elovich equation showed a higher initial P release and a lower release over time for the investigated materials. The BC samples certainly had a lower initial release than the P release over time for the 0.01 M CA treatment. The kinetic parameters of the Exponential function also had a higher a and a lower b for the used materials, except for HA. Altogether, the initial P release increased while the continuous release decreased with increasing CA concentration. For the Elovich equation, the initial P release was higher for CA and the release over time was higher for CaCl2.

Table 4.
Coefficients of determination (R2) and standard errors (S.E.) for the kinetic equations used to describe the kinetic release of P with CaCl2 and citric acid.

Table 5.
Kinetic parameters of the Elovich, Exponential, and Parabolic function equations.
3.4. Elemental Composition during P Release
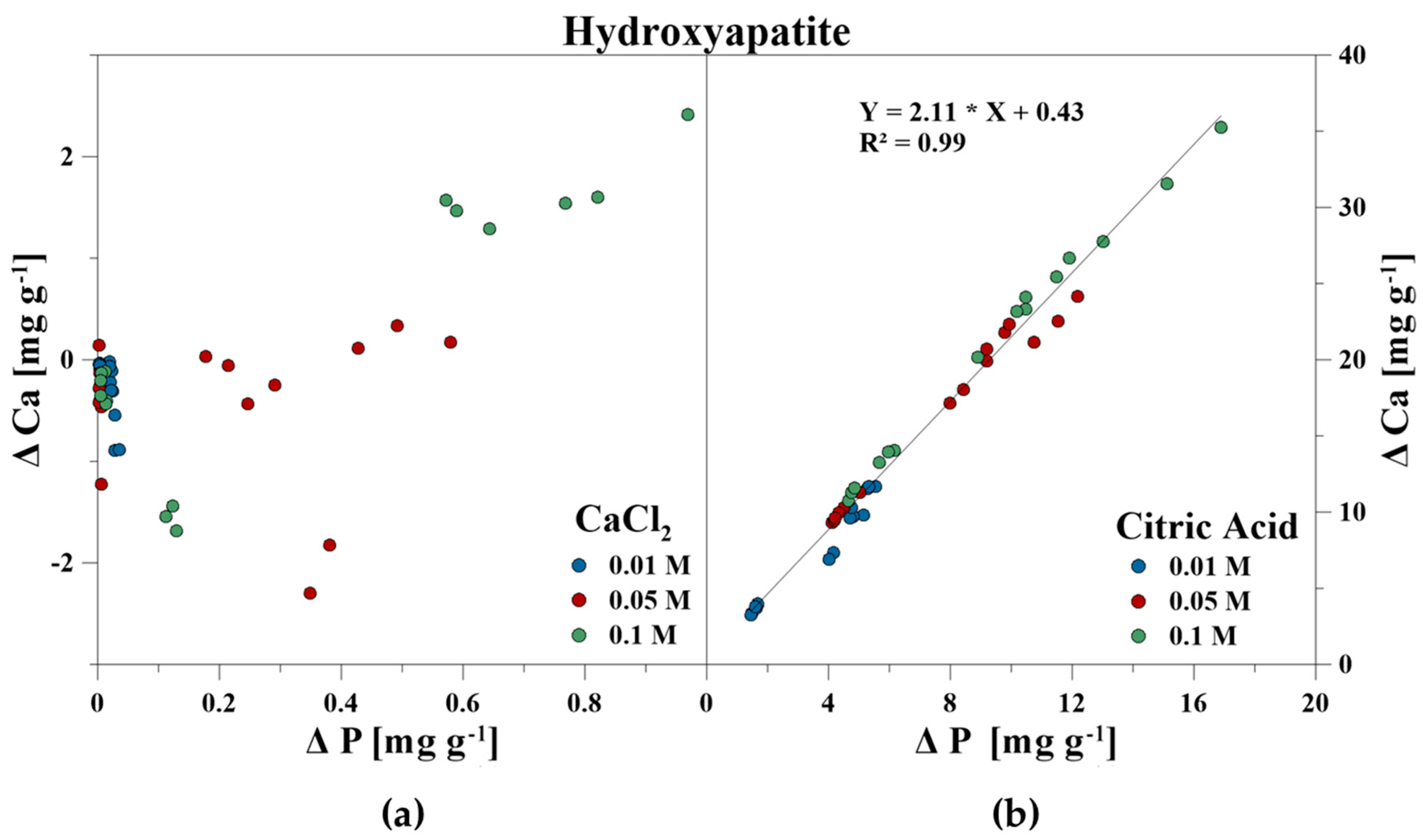
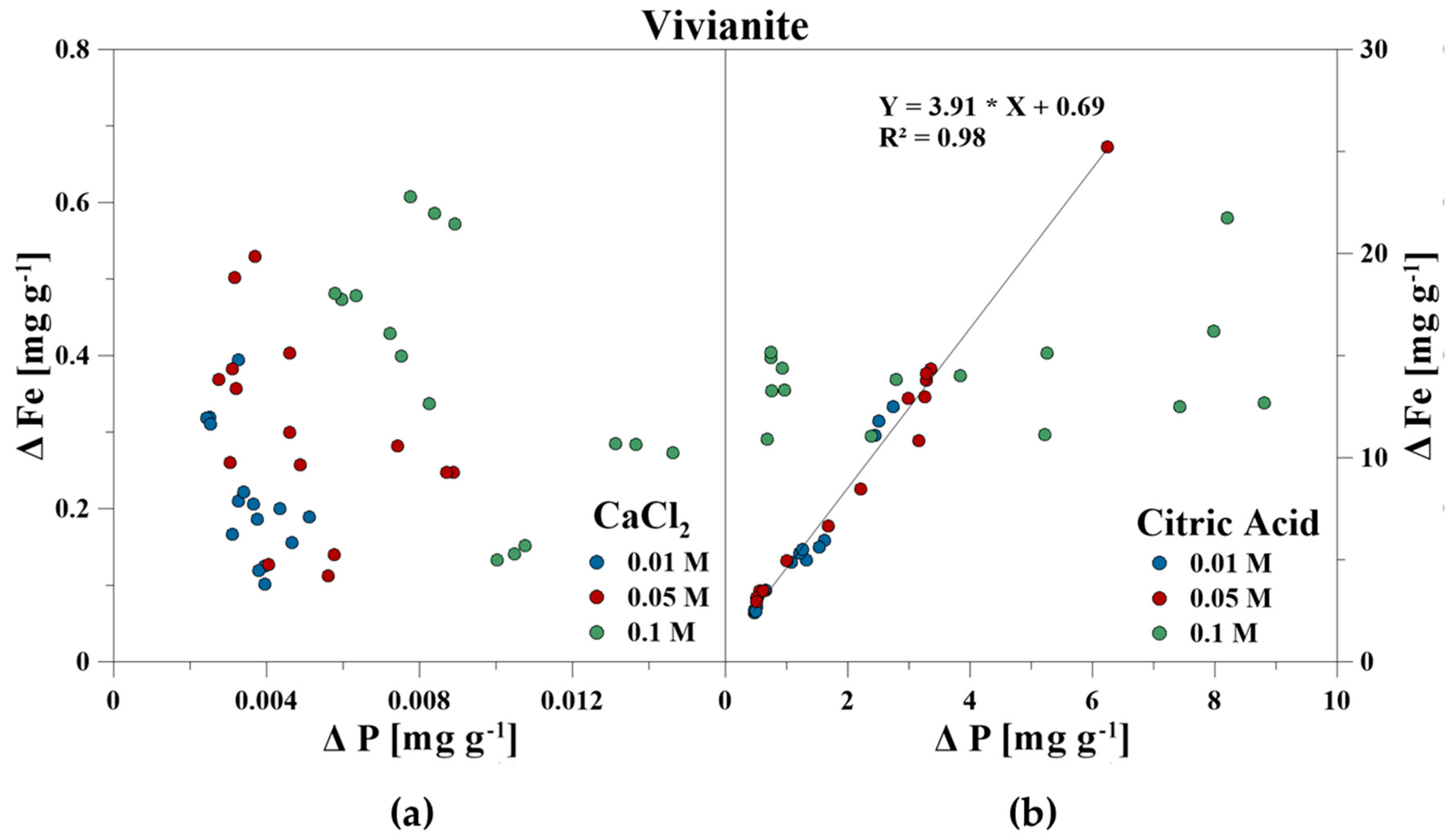
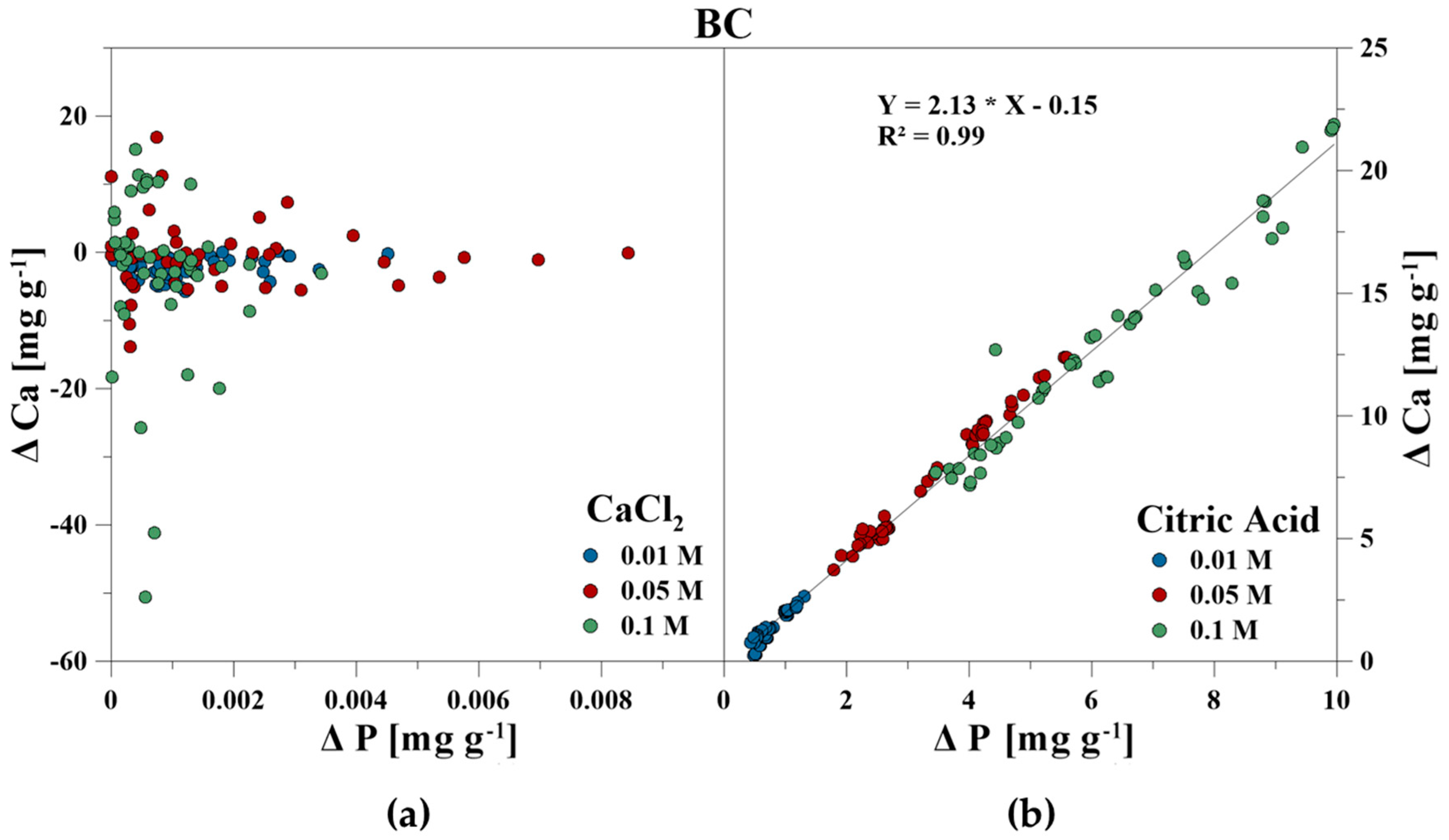
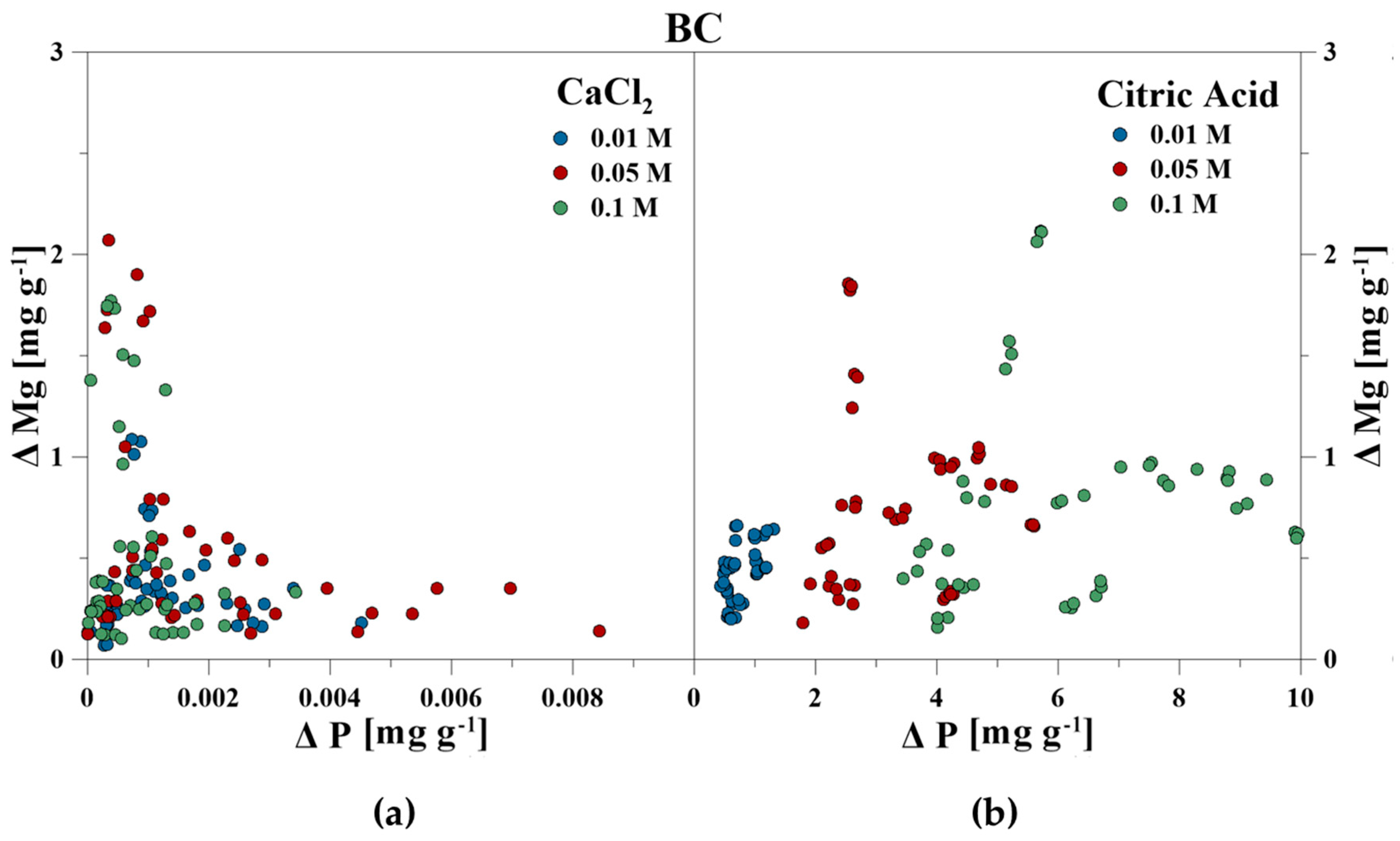
The elemental composition of the reaction solutions and its change in concentration was measured and correlated to the amount of released P in mg g−1 for each time step. For the CaCl2 treatment, no correlations between P–Ca (HA), P–Ca, and P–Fe (VI), and P–K, P–Cl as well as P–Ca (BC) were detected. This also included P–Mg (BC) for the CA treatment. However, a strong linear correlation was revealed for P–Ca at the CA treatment for HA and BC as well as P–Fe for VI (Figure 3, Figure 4 and Figure 5). With increasing P release for both CaCl2 and CA, an enrichment of Ca (HA, BC), Fe (VI), and Mg (BC) in the reaction solution was measurable. However, the Fe concentration in CA for VI remained constant at the highest concentration of 0.1 M and no correlation was found. For BC, the concentration of dissolved Mg initially increased in the CaCl2 solution, but decreased during P release (Figure 6).
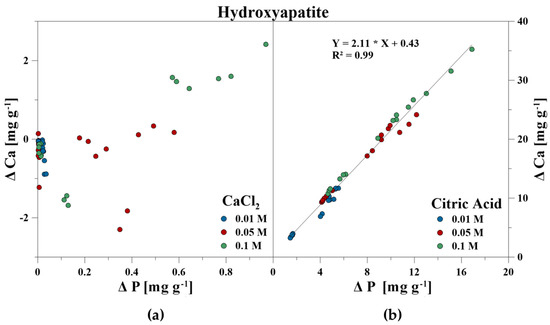
Figure 3.
Correlation between dissolved P and Ca during P release using (a) CaCl2 and (b) CA from hydroxyapatite.
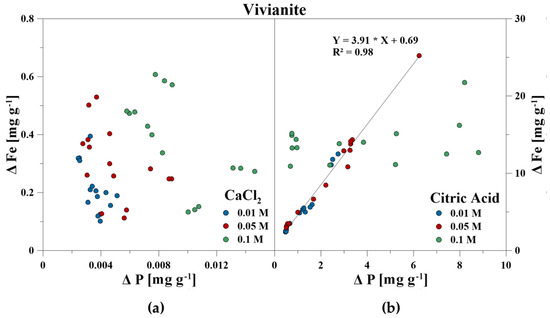
Figure 4.
Correlation between dissolved P and Fe during P release using (a) CaCl2 and (b) CA from vivianite.
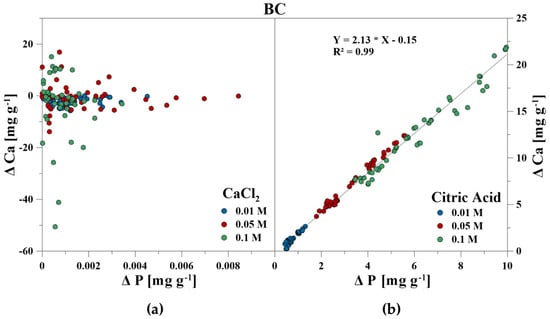
Figure 5.
Correlation between dissolved P and Ca during P release using (a) CaCl2 and (b) CA from bone char.
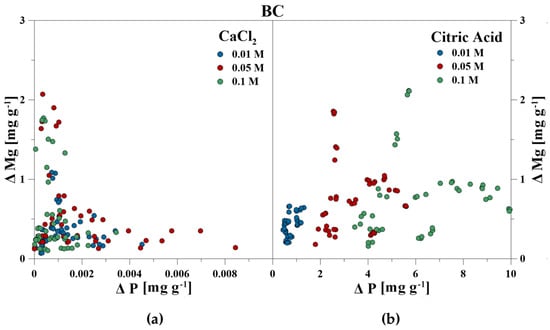
Figure 6.
Correlation between dissolved P and Mg during P release using (a) CaCl2 and (b) CA from bone char.
3.5. pH
The pH of the reaction solution was adjusted prior to the release experiments at a value of 6. Then, the pH was measured again after the 2 h step and the 48 to 168 h step. The pH of the minerals itself was lowest for VI with 4.27 and highest for BC with 9.69 (in H2O), respectively. HA had a pH of 7.28 (Table 6). During P release, the pH of the solutions increased with increasing CaCl2 and CA concentrations for VI. For the CA treatment, the pH of the solution even exceeded the initial solution pH of 6, despite the lower pH of VI itself.

Table 6.
pH value of the raw material and in the reaction solution after 2 h and 48–168 h reaction time.
For HA and BC, the pH decreased with increasing CaCl2 and CA concentrations. During the CaCl2 treatment, the pH increased with increasing particle size of BC, while for the CA treatment, the pH decreased with increasing particle size. No clear correlations were detected between the changing H+ concentration and dissolved P, Fe, Ca, or Mg.
3.6. Fourier-Transform Infrared Spectroscopy
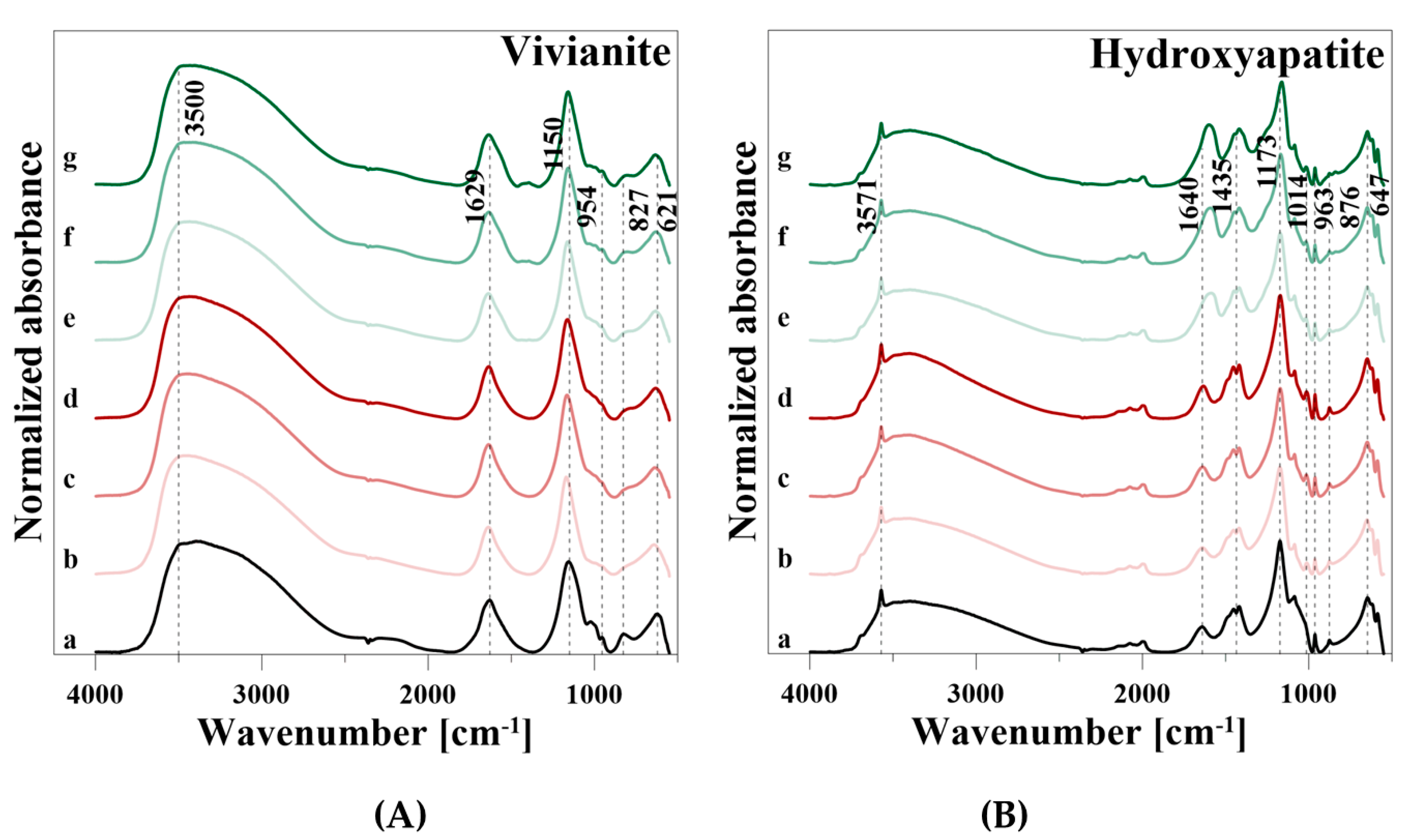
VI showed a broad OH stretching vibration of the Fe–OH groups in the range from 3750 to 2500 cm−1, a H2O (HOH) bending vibration at 1629 cm−1, and an OH libration mode at 827 cm−1, respectively (Table 7). The four PO43− vibrational modes were observed at 1155, 1023, and 993 cm−1 (ν3), 954 cm−1 (ν1), 621 cm−1 (ν4), and very weak at 522 cm−1 (ν1), respectively.

Table 7.
Observed wavenumbers [cm−1] of vivianite, hydroxyapatite, and bone char.
After P release using CaCl2, the intensity of the OH stretching vibration decreased clearly, but increased slightly with increasing CaCl2 concentration and was similar for 0.005 M and 0.1 M CaCl2 (Figure 7A). Similar results were observed for the H2O peak at 1629 cm−1 and the OH libration vibration. In addition, the intensities of the PO43− vibrations in the wavenumber ranged from 1350 to 550 cm−1 decreased during P release. After P release using CA, similar developments of the spectra as for CaCl2 were observed. However, a new band at 1392 cm−1 and a weak shoulder of the degraded H2O vibration at 1629 cm−1 were formed.
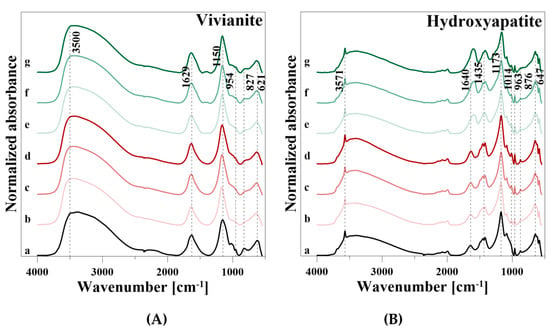
Figure 7.
Fourier-transform infrared spectra of (A) vivianite and (B) hydroxyapatite during P release. (a) reference (b) 0.01 M CaCl2, (c) 0.05 M CaCl2, (d) 0.1 M CaCl2, (e) 0.01 M CA, (f) 0.05 M CA, and (g) 0.1 M CA.
HA showed a broad H2O absorption band from 3745 to 2570 cm−1 with a characteristic OH stretching vibration at 3571 cm−1 (Table 7). Adsorbed H2O also showed a peak at 1640 cm−1, and an OH libration vibration can be observed at 876 cm−1. Phosphate (PO43−) was represented by four infrared active vibrational modes: a ν3 band from 1353 to 982 cm−1 with two peaks at 1173 and 1086 cm−1, a ν1 vibration at 1014 cm−1, three peaks at 647, 619, and 586 cm−1 of the ν4 vibration, and a weak ν2 vibration at 552 cm−1. The vibrational modes of ν1 and ν2 appeared only as shoulders before desorption, and ν2 was particularly very weak. Furthermore, carbonate (CO32−) related vibrations were observed at a broad band with two vibrations at 1452 and 1418 cm−1, and a vibration at 963 cm−1. Vibrations in the range from 1555 to 1360 cm−1 can be attributed to CO32− on the surface of the mineral rather than in the lattice [26], where CO32− can substitute either OH (A–type substitution) or PO43− groups (B-type substitution) of HA [24,33].
After P release using CaCl2, the intensities of the OH, H2O, CO32−, and PO43− vibrations decreased, but increased again with the rise of the CaCl2 concentration near the initial level (Figure 7B). The initial decline and the increase with increasing concentration of the reaction solution were also observed during P release with CA. However, as already observed in VI, a vibration at 1610 cm−1 and a slight shoulder at 1388 cm−1 appeared. In addition, a very weak peak at 841 cm−1 was formed.
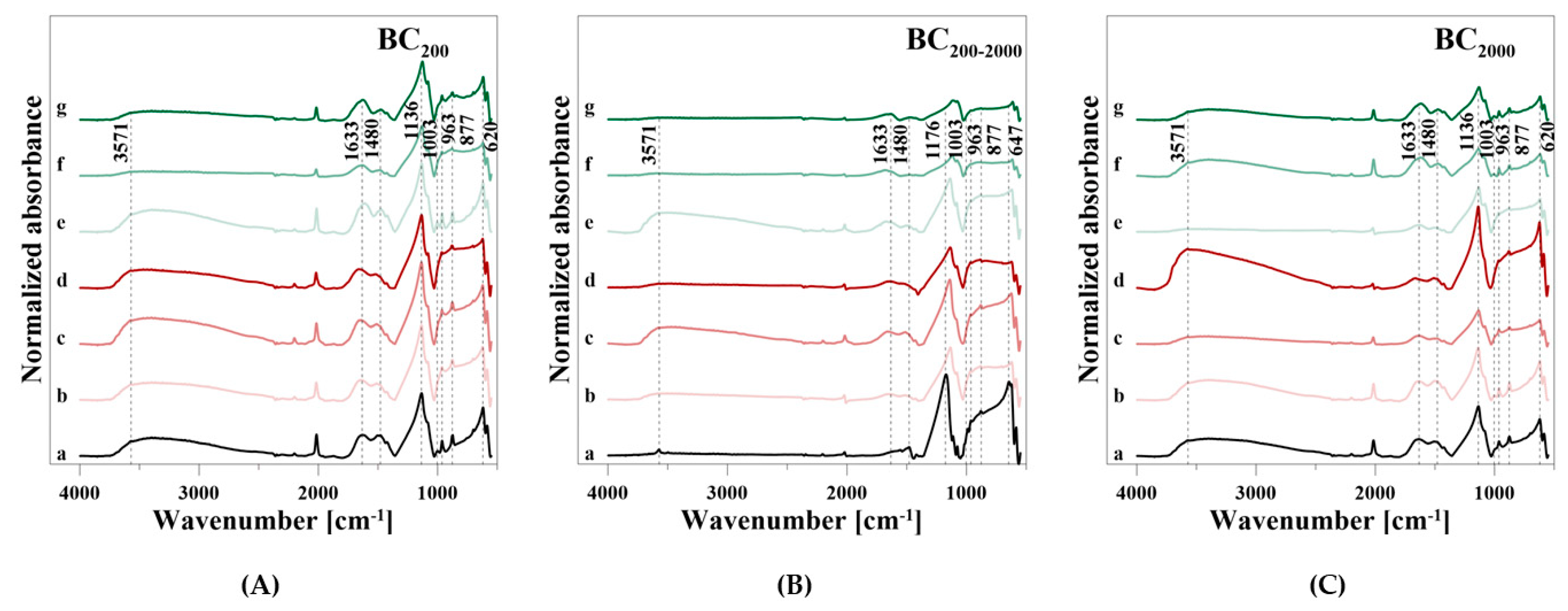
The BC samples showed a weak, but broad adsorbed H2O band in the range from 3737 to 2585 cm−1 and a weak OH stretching vibration at 3571 cm−1 (Table 7). The FT-IR spectrum of BC200–2000 showed no broad H2O adsorption band, though only a more pronounced OH stretching vibration at 3571 cm−1 than the other BC samples. A broader band in the range from 1815 to 1380 cm−1 with three peaks at 1633, 1480, and 1424 cm−1 can be assigned to adsorbed H2O and CO32−. Additionally, in this case, there was only a very weak H2O peak in BC200–2000. This BC sample showed the characteristic PO43− vibrations at 1176 and 1115 cm−1 (ν3), 1077 cm−1 (ν1) as well as 647, 620, and 586 cm−1 (ν4), respectively. BC200 and BC2000 showed PO43− vibrations at 1136 cm−1 (ν3) with a shoulder at 1079 cm−1, at 1003 cm−1 (ν1) as well as at 620 and 586 cm−1 (ν4), respectively. The weak ν2 stretching vibration was not visible for all BC samples. Furthermore, BC200 and BC2000 had, in accordance with the HA spectrum, a CO32− vibration at 963 cm−1 and an OH libration mode at 877 cm−1. Both were very weak for BC200–2000.
During P release using CaCl2, the intensities of the OH and H2O bands between 3737 and 2585 cm−1 fluctuated and increased with increasing particle size at the highest concentration of 0.1 M (Figure 8A–C). The other H2O, OH, CO32−, and PO43− bands also showed an irregular behavior with regard to the used solution concentrations during P release. Merely a new H2O band at 1633 cm−1 was formed in BC200–2000, and the intensity of the P bands increased with increasing particle size and even exceeded the baseline level. When CA was used, a degradation of the H2O, OH, CO32−, and PO43− bands occurred. Again, an additional vibration at 1633 cm−1 was formed, which differed from the H2O band in the broader slope at a higher wavenumber.
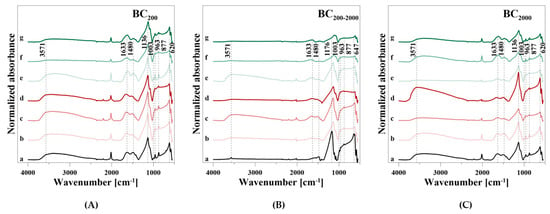
Figure 8.
FT-IR spectra of bone char divided into (A) BC200, (B) BC200–2000, and (C) BC2000 during P release. (a) reference (b) 0.01 M CaCl2, (c) 0.05 M CaCl2, (d) 0.1 M CaCl2, (e) 0.01 M CA, (f) 0.05 M CA, and (g) 0.1 M CA.
4. Discussion
The release of P from primary and secondary P minerals depends on several factors such as mineral stability and solubility properties regarding dissolution by ionic substitution, particle size, or pH [17,34,35,36]. The mobilization of these P resources can take place by different processes, while the cumulative P release using CA was clearly higher than with CaCl2. It is well known that the exchange with inorganic ions plays an inferior role compared to organic components [37,38,39]. Inorganic reaction solutions such as CaCl2 contribute to the mobilization of P from nonspecific adsorbed P from low-affinity sites, while structural bound or specific adsorbed P from high-affinity sites can hardly be affected due to the strong bonds [2,40,41]. In addition, if P was released from the minerals, PO43− had a higher affinity to adsorb again on the mineral surface due to the higher valence and ionic radius compared to Cl− [42]. Hence, it is possible that free P was bound again and therefore not measured as dissolved P in the reaction solution. Another explanation for the very low P mobilization from BC by using CaCl2 could be the point of zero charge, which was described at around pH 7.7 [43]. Above this pH value, BC has a negative charge of the surface, which led to a lower affinity for anion exchange and hence, a lower capacity for PO43− mobilization. In comparison, the pH of the point of zero charge reported for HA was in the range from 6.7 to 8.6 [44,45]. Thus, there was a more positive surface charge, especially for BC, which can lead to even small differences in P mobilization.
Both the CaCl2 and the CA treatment showed a higher P release capacity for HA. Although VI had a higher P release than the BC samples at the lowest CA concentration of 0.01 M, the P release capacity of BC increased more strongly with increasing concentration of the reaction solution. The beneficial effect on P release of further organic acids such as oxalic acid, hydrochloric acid, or sulfuric acid and their increasing concentration were also described for rock phosphates, iron phosphates, and soils [46,47,48,49,50]. While the P release during the CaCl2 treatment was so low that only VI and HA showed a slightly higher release with increasing CaCl2 concentration, this effect was more pronounced for the CA treatment. Although more P was dissolved with increasing CA concentration, this was different for HA, VI, and BC.
BC mainly consists of the elements Ca, P, and Mg, which is similar to other BC obtained by pyrolysis (350 °C–800 °C) of rendered material [15,16,51,52,53,54]. However, the Ca and P concentrations were higher than in other bio and bone chars from nutrient-rich feedstocks such as pig manure or sewage sludge [55,56,57,58]. Since HA is the dominant P compound in BC, and a rising pyrolysis temperature increased the proportion of HA, the specific pre-treatment of BC influenced the P solubility characteristics [14,15,16,52,59]. While higher ion strength of the CA (0.01 M:0.05 M:0.1 M = 1:5:10) increased the P release of HA proportional slightly (1:2.2:2.6), a significantly stronger effect was observed for BC. For BC200–2000, P release was most efficient (1:5.0:9.1), followed by BC200 (1:4.6:8.1) and BC2000 (1:4.0:7.5). Furthermore, a larger P release was observed with a smaller particle size of BC, which is consistent with the results of Ma and Matsunaka [60], Morshedizad and Leinweber [61] as well as Morshedizad et al. [62] during pot and leaching experiments. This can be explained by the larger specific area, where reactants can dissolve P [60,63]. Although the measured specific surface areas of the different BC fractions differed only slightly at first glance, the surface area increased by more than 2.5 m2 g−1 from BC2000 to BC200. For VI, the increase in CA concentration was the least efficient (1:1.8:3). The effectiveness of CA is particularly influenced by the strength of the acid [46], which is why P mobilization also rose with increasing concentration. The critical factor leading to different P release efficiencies with increasing CA concentration could be the higher specific surface area of the BC samples compared to HA. Thus, more exchange sites are available for ionic substitution during the initial phase of P release, whereas the stability of the minerals with regard to dissolution only becomes apparent in a later phase of mobilization. Nevertheless, the chosen CA concentrations exceeded the concentrations commonly measured in the soil or litter solution, which were in the range from 0 to 122 µmol. However, due to the small particle size and envelope density, fine BC particles should be mixed with manure, sludge, or compost prior to field application to reduce a loss of up to 25 mass% by dust formation [64] to maintain a long-term improvement effect in soils.
The best fit of the time-dependent release data was observed by applying the logarithmic Elovich equation. This biphasic kinetic behavior revealed a faster release of easier accessible P from the mineral surface and a slower release phase. This slower second phase can be controlled by diffusion kinetics including the mobilization of more heavily bound P, which can be embedded within the mineral particles or resorption processes [65]. This suggests chemisorption reactions, which were corroborated by previous studies with P desorption from soils [66,67]. The calculated kinetic parameters α and β of the Elovich equation (Table 5) clearly indicated a low initial P release from the mineral phase and a distinct ongoing mobilization over time within the CaCl2 treatment. Regarding the low total P release, it was assumed that a chemical equilibrium between the solid and the liquid phase was desired rather than a significant P release through anion exchange, and hence, the high values of β do not seem realistic.
For the CA treatment, the initial P release increased with increasing CA concentration. The P release rate over time declined with increasing CA concentration, but nevertheless showed a strong ongoing P mobilization process. The presence of a higher concentration of citrate anions can therefore lead to a higher initial P release rate by ligand exchange, followed by dissolution of the mineral particles.
The interpretation of the fit parameters is difficult, since an empirical model like the Elovich equation describes a process in an ideal system. This is therefore not necessarily transferable to a real system such as soils [68]. On one hand, an increase in β or a decrease in α can indicate an increase of the reaction rate [69], while on the other hand, the slope of the equation may also depend more on the conditions of the reaction than to characterize it [70]. Nevertheless, it is possible that the weak curvature of the P release kinetics by using CA can be poorly described by the Elovich equation [68], which is why the release in the range over 48 h can be underestimated, whereas the P release over time can be overestimated for CaCl2.
The release of inorganic bound P by organic acids includes the processes of dissolution of minerals, the direct ligand exchange, and replacement of P by organic acid anions or the formation of metal–organic complexes and blocking of P adsorption sites [18,19,20,21]. It was identified that CA promotes the dissolution of PO43− adsorbing minerals [50,71,72,73], and that the P availability as well as the release of (e.g., Si, Fe, and Al increased with increasing CA concentration) [46,74]. Both the increase of dissolved Fe (VI), Ca (HA, BC), and Mg (BC) with continuing P release showed the dissolution of the minerals by using CA. The same was concluded for VI and the dissolved Fe concentrations. A constant Fe concentration of approximately 15 mg g−1 was measured in the reaction with 0.1 M CA for VI. This could indicate that VI was only soluble to a certain degree which limited P release.
Often P compounds containing Fe are very sensitive to changes in reductive conditions and can be dissolved in anoxic environments. However, Fe-associated P, which is related to VI, remained stable to reductive dissolution in anoxic soils and lake sediments [75]. The extraction of P from VI strongly depends on the crystallinity of the mineral, whereas P was not completely released from highly crystalline VI [12]. HA is also considered to be a poorly soluble mineral, where biological HA is characterized by a smaller crystal size and a higher proportion of PO43− substituted by CO32− compared to geological HA, which enhances the solubility of biological HA [17]. In particular, thermal treatment led to a decreasing CO32− and H2O content and an increasing crystal size, resulting in a higher stability of HA [76,77]. The addition of CO32− through decreased crystallinity enhanced the amenability to dissolution and the potential of the biodegradation of HA [17,78,79,80]. The stability or solubility of carbonated HA was thus defined by the presence of CO32− [17,76,81]. The observed FT-IR spectroscopic data revealed CO32− related vibrations for the HA and BC samples, respectively. The higher intensities of the CO32− bands for HA at 1452 cm−1, 1418 cm−1, and 963 cm−1 than for the BC samples (Figure 8) suggest a lower stability regarding the dissolution of the former and, hence, a higher P release efficiency. In summary, the kinetic data indicated that BC samples, especially BC200, had a lower short-term P release [53], but a higher long-term P release than HA or VI. The fluctuations of Ca, Fe, and Mg concentrations in the CaCl2 reaction solution may be due to low solution or release from the sample material with respect to an equilibrium in the reaction solution, since no correlation to the dissolved P concentrations was detected.
Gypser and Freese [82] determined the concentration of CTotal in CA during desorption experiments with VI and HA. Despite a fluctuation around the initial CTotal concentration, no distinctive change or even decrease during P desorption was measured. Thus, the formation of metal–organic complexes in the reaction solution was excluded. In this case, the possible enrichment of metal–organic complexes in the reaction solution was not considered as there was no reddish-brown coloring of the reaction solution. Further possibilities of differentiation between ligand exchange and metal–organic complexes should be taken into account. Possible evidence of the presence or absence of organic ligands at the surface of HA, VI, and BC could be the formation of C-related vibrations in the FT-IR spectra. For CA, a bond is formed by carboxyl groups (–COOH) of the citrate anion (C6H6O72− at pH 6), which should be visible by characteristic C=O and C–O peaks in the FT-IR spectra. A vibrational analysis of the –COOH groups included vibrations of C=O, C–O, and OH, where the characteristic C=O stretching vibration was usually detected between 1800 cm−1 and 1680 cm−1, and the C–O stretching vibration between 1320 cm−1 and 1210 cm−1 [83,84], depending on the compound. The position of the band can vary accordingly with intermolecular OH…O hydrogen bonding within the material [85]. For the samples VI, HA, BC200, and BC2000, a distinctive H2O vibration ranged in the region of possible C=O peaks. During P release using the CA treatment, the peak of the H2O related vibration shifted to a lower wavenumber or possibly formed a new vibration at 1610 cm−1 for HA and at 1633 cm−1 for BC200 and BC2000. For BC200–2000, a new vibration was formed at 1633 cm−1 where the H2O vibration was missing. The spectrum of VI revealed a shoulder at the degraded H2O vibration at 1629 cm−1. These features can be related to a newly formed C=O peak. Additionally, for VI, a new band was formed at 1392 cm−1, which occurred as a slight shoulder at 1388 cm−1 for HA. This feature can be related to a possible C–O binding. For the BC samples, the range between 1550 cm−1 and 1340 cm−1 was dominated by the CO32− bands. Johnson and Loeppert [21] described the P release from Fe-hydroxides by both ligand exchange and dissolution, because ligand adsorption onto the surface is important for dissolution mechanisms. The accumulation of Fe, Ca, and Mg in the CA reaction solution suggested that dissolution played a major role in P release, while the C-associated changes of the FT-IR spectra, either dried CA residues in the pore space or on the surface of the investigated materials, or indicating ligand exchange took place, played a minor role. Although metal–organic complexes could also form with these dissolved metals, no reddish-brown color change (ferric citrate), or white precipitation (calcium citrate) were observed in this study. Since calcium citrate in particular is poorly soluble in H2O (950 mg l−1 at 25 °C), and 1700 mg l−1 of dissolved Ca was detected in 0.1 M CA after a reaction time of 168 h (in the BC2000 samples), it was suggested that no calcium citrate developed. The development of magnesium citrate could not be excluded due to its high solubility in H2O. Overall, the possibility of the formation of metal–organic complexes should not be discarded, since especially ferric citrate plays an important role in the nutrient uptake of some plants and microorganisms [86].
One of the main aspects of the changes of the solution pH was the attempt to achieve a chemical equilibrium between the solid mineral phase and the reaction solution due to the different pH values. Particularly for the CaCl2 treatment, this chemical equilibrium seemed to be the driving force for pH changes due to the low P mobilization as well as missing correlations to other dissolved elements. For the CA treatment, in addition to the influence of the mineral pH itself, the release of P also changed the pH value of the respective reaction solution. This could also be measured between the individual particle sizes of BC, where the smaller particles of BC200 delivered a higher amount of P than the larger particles of BC2000, thus slightly lowering the pH more. The rise of the pH value above 6 for VI can be possibly caused by the oxidation of dissolved Fe2+ to Fe3+ by atmospheric O2 (Equation (1)).
2 Fe2+ + 0.5 O2 + 2 H+ ↔ 2 Fe3+ + H2O
In acidic to near-neutral soils, the availability of P is mainly limited by processes of adsorption, desorption, and precipitation on soil particle surfaces where the strong binding to Fe- and Al-hydroxides plays a major role in P immobilization [21,40]. However, Roberts and Johnston [3] pointed out that the P fixation and thus unavailability for plants was not supported by field experiments. Rather, they emphasized the presence of an immediately available P pool in the soil solution as well as the further readily available P pool, which are mainly used for the supply of the plants. In addition, a less and much less readily available P pool was formed where the availability for plants was determined on their accessibility to plant roots and its extractability by soil test reagents. If the P use efficiency was balanced according to these P pools, the P recovery is often in the range of 50 to 70% [87]. This means that in addition to the readily available P pools, soil reserves can be used in the long-term, hence, reducing agricultural runoff and eutrophication of natural waters. Depending on the crystallization grade and the Fe/Al-ratio, the P mobilization capacity of these hydroxides can be increased up to 49% by using CA [39]. In particular, the comparison of the efficiency and kinetics of P release from VI and BC showed that VI, despite the influence of CA, had less available P in both the short- and long-term, which can cause an increase in the less available P pool. The very low P release using CaCl2 underlines once again the importance of the accessibility of fertilized or naturally bound P for plant roots to benefit from the excretion of organic acids as a strategy to enhance the P uptake.
5. Conclusions
P release experiments in batch showed a distinctive higher effect of CA on P mobilization than CaCl2. While the P release during the CaCl2 treatment was so low that only VI and HA showed a slightly higher release with increasing CaCl2 concentration, the increase of dissolved P was more pronounced for the CA treatment. At the same concentration, the use of CA resulted in a 32,190-fold higher P release compared to CaCl2 (0.1 M for BC200). The lowest increase was measured for VI with a 29-fold higher release. The observed FT-IR spectroscopic data suggested lower stability of HA than for BC and despite a higher mobilization of P from HA, the kinetic data indicated that BC samples, especially at lower particle size, had a higher long-term P release than HA or VI. The accumulation of Fe, Ca, and Mg in the Ca reaction solution suggested that dissolution played a major role in P release, while ligand exchange seemed to play a subordinate role. In summary, it can be said that the suitability of HA and BC as a poorly soluble, but sustainable P source is better than that of VI. However, the efficiency as a P fertilizer is also dependent on present soil P mobilization processes, since the sole release of P by the soil solution is not remarkable. Accumulated P, precipitated or adsorbed on hydroxides or soil colloids, represents a high potential secondary P source that requires a detailed characterization of easily and heavily releasable fractions for a sustainable recovery.
Author Contributions
Conceptualization, E.S., S.G., and D.F.; Methodology, E.S., S.G., and D.F.; Investigation, E.S., and S.G.; Formal analysis, batch experiments and chemical analysis, E.S.; FT-IR spectroscopic measurement and analysis, S.G.; Kinetic modeling, E.S.; Validation: S.G.; Resources: D.F.; Writing-original draft preparation, E.S.; S.G., Writing-review and editing, D.F. and S.G.; Visualization, S.G.; Supervision, D.F.; Project administration, D.F. and S.G.; Funding acquisition, D.F. and S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Federal Ministry of Education and Research (BMBF) in the BonaRes project InnoSoilPhos (No. 031B509C).
Conflicts of Interest
The authors declare no conflicts of interest. The funding sponsors had no rule in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Taşkin, M.B.; Şahin, Ö.; Taskin, H.; Atakol, O.; Inal, A.; Gunes, A. Effect of synthetic nano-hydroxyapatite as an alternative phosphorus source on growth and phosphorus nutrition of lettuce (Lactuca sativa L.) plant. J. Plant Nutr. 2018, 41, 1148–1154. [Google Scholar] [CrossRef]
- Roberts, T.L.; Johnston, A.E. Phosphorus use efficiency and management in agriculture. Resour. Conserv. Recycl. 2015, 105, 275–281. [Google Scholar] [CrossRef]
- van der Bom, F.J.T.; McLaren, T.I.; Doolette, A.L.; Magid, J.; Frossard, E.; Oberson, A.; Jensen, L.S. Influence of long-term phosphorus fertilisation history on the availability and chemical nature of soil phosphorus. Geoderma 2019, 355, 113909. [Google Scholar] [CrossRef]
- Sattari, S.Z.; Bouwman, A.F.; Giller, K.E.; van Ittersum, M.K. Residual soil phosphorus as the missing piece in the global phosphorus crisis puzzle. Proc. Natl. Acad. Sci. USA 2012, 109, 6348–6353. [Google Scholar] [CrossRef]
- Hooda, P.S.; Truesdale, V.W.; Edwards, A.C.; Withers, P.J.A.; Aitken, M.N.; Miller, A.; Rendell, A.R. Manuring and fertilization effects on phosphorus accumulation in soils and potential environmental implications. Adv. Environ. Res. 2001, 5, 13–21. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Heindel, R.C.; Lyons, W.B.; Welch, S.A.; Spickard, A.M.; Virginia, R.A. Biogeochemical weathering of soil apatite grains in the McMurdo Dry Valleys, Antarctica. Geoderma 2018, 320, 136–145. [Google Scholar] [CrossRef]
- Mehmood, A.; Akhtar, M.S.; Imran, M.; Rukh, S. Soil apatite loss rate across different parent materials. Geoderma 2018, 310, 218–229. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Watanabe, Y.; Yamada, H.; Ikoma, T.; Tanaka, J.; Stevens, G.W.; Komatsu, Y. Preparation of a zeolite NaP1/hydroxyapatite nanocomposite and study of its behavior as inorganic fertilizer. J. Chem. Technol. Biot. 2014, 89, 963–968. [Google Scholar] [CrossRef]
- Rothe, M.; Kleeberg, A.; Hupfer, M. The occurrence, identification and environmental relevance of vivianite in waterlogged soils and aquatic sediments. Earth-Sci. Rev. 2016, 158, 51–64. [Google Scholar] [CrossRef]
- Taylor, K.G.; Hudson-Edwards, K.A.; Bennett, A.J.; Vishnyakov, V. Early diagenetic vivianite [Fe3(PO4)2·8H2O] in a contaminated freshwater sediment and insights into zinc uptake: A μ-EXAFS, μ-XANES and Raman study. Appl. Geochem. 2008, 23, 1623–1633. [Google Scholar] [CrossRef]
- Siebers, N.; Kruse, J.; Leinweber, P. Speciation of Phosphorus and Cadmium in a Contaminated Soil Amended with Bone Char: Sequential Fractionations and XANES Spectroscopy. Water Air Soil Poll. 2013, 224, 1564. [Google Scholar] [CrossRef]
- Zimmer, D.; Panten, K.; Frank, M.; Springer, A.; Leinweber, P. Sulfur-enriched bone char as alternative p fertilizer: Spectroscopic, wet chemical, and yield response evaluation. Agriculture 2019, 9, 21. [Google Scholar] [CrossRef]
- Zwetsloot, M.J.; Lehmann, J.; Solomon, D. Recycling slaughterhouse waste into fertilizer: how do pyrolysis temperature and biomass additions affect phosphorus availability and chemistry? J. Sci. Food Agr. 2015, 95, 281–288. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Chen, C.R.; Condron, L.M.; Davis, M.R.; Sherlock, R.R. Effects of plant species on microbial biomass phosphorus and phosphatase activity in a range of grassland soils. Biol. Fertil. Soils 2004, 40, 313–322. [Google Scholar] [CrossRef]
- Kpomblekou-A, K.; Tabatabai, M.A. Effect of low-molecular weight organic acids on phosphorus release and phytoavailabilty of phosphorus in phosphate rocks added to soils. Agr. Ecosyst. Environ. 2003, 100, 275–284. [Google Scholar] [CrossRef]
- Johnson, S.E.; Loeppert, R.H. Role of organic acids in phosphate mobilization from iron oxide. Soil Sci. Soc. Am. J. 2006, 70, 222. [Google Scholar] [CrossRef]
- Eynard, A.; del Campillo, M.C.; Barrón, V.; Torrent, J. Use of vivianite (Fe3(PO4)2 8H2O) to prevent iron chlorosis in calcareous soils. Fert. Res. 1992, 31, 61–67. [Google Scholar] [CrossRef]
- Frost, R.L.; Martens, W.; Williams, P.A.; Kloprogge, J.T. Raman and infrared spectroscopic study of the vivianite-group phosphates vivianite, baricite and bobierrite. Mineral. Mag. 2002, 66, 1063–1073. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of calcium phosphates using fourier transform infrared spectroscopy. In Introduction to Infrared Spectroscopy; Theophile, T., Ed.; Intech Open: London, UK, 2012; pp. 123–148. [Google Scholar]
- Chang, M.C.; Tanaka, J. FTIR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials 2002, 23, 4811–4818. [Google Scholar] [CrossRef]
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef]
- Ren, F.Z.; Leng, Y. Carbonated apatite, type-A or type-B? Key Eng. Mat. 2011, 493–494, 293–297. [Google Scholar] [CrossRef]
- Vidhya, G.; kumar, G.S.; kattimani, V.S.; Girija, E.K. Comparative study of hydroxyapatite prepared from eggshells and synthetic precursors by microwave irradiation method for medical applications. Mater. Today Proc. 2019, 15, 344–352. [Google Scholar] [CrossRef]
- Wu, C.C.; Huang, S.T.; Tseng, T.W.; Rao, Q.L.; Lin, H.C. FTIR and XRD investigations on sintered fluoridated hydroxyapatite composites. J. Mol. Struct. 2010, 979, 72–76. [Google Scholar] [CrossRef]
- Flores-Cano, J.V.; Leyva-Ramos, R.; Carrasco-Marin, F.; Aragón-Piña, A.; Salazar-Rabago, J.J.; Leyva-Ramos, S. Adsorption mechanism of chromium(III) from water solution on bone char: Effect of operating conditions. Adsorption 2016, 22, 297–308. [Google Scholar] [CrossRef]
- Jia, P.; Tan, H.; Liu, K.; Gao, W. Removal of methylene blue from aqueous solution by bone char. Appl. Sci. 2018, 8, 1903. [Google Scholar] [CrossRef]
- Rojas-Mayorga, C.K.; Bonilla-Petriciolet, A.; Silvestre-Albero, J.; Aguayo-Villarreal, I.A.; Mendoza-Castillo, D.I. Physico-chemical characterization of metal-doped bone chars and their adsorption behavior for water defluoridation. Appl. Surf. Sci. 2015, 355, 748–760. [Google Scholar] [CrossRef]
- Pleshko, N.; Boskey, A.; Mendelsohn, R. Novel infrared spectroscopic method for the determination of crystallinity of hydroxyapatite minerals. Biophys. J. 1991, 60, 786–793. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Oelkers, E.H.; Valsami-Jones, E. Phosphate Mineral Reactivity and Global Sustainability. Elements 2008, 4, 83–87. [Google Scholar] [CrossRef]
- Pierzynski, G.M.; McDowell, R.W.; Sims, J.T. Chemistry, cycling, and potential movement of inorganic phosphorus in soils. In Phosphorus: Agriculture and the Environment; Sims, J.T., Sharpley, A.N., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2005; pp. 53–86. [Google Scholar]
- Dean, L.A.; Rubins, E.J. Anion exchange in soils: I. Exchangeable phosphorus and the anion-exchange capacity. Soil Sci. 1947, 63, 377–388. [Google Scholar] [CrossRef]
- Lazo, D.E.; Dyer, L.G.; Alorro, R.D. Silicate, phosphate and carbonate mineral dissolution behaviour in the presence of organic acids: A review. Miner. Eng. 2017, 100, 115–123. [Google Scholar] [CrossRef]
- Gypser, S.; Schütze, E.; Freese, D. Crystallization of single and binary iron- and aluminum hydroxides affect phosphorus desorption. J. Plant Nutr. Soil Sci. 2019, 741–750. [Google Scholar] [CrossRef]
- Gypser, S.; Hirsch, F.; Schleicher, A.M.; Freese, D. Impact of crystalline and amorphous iron- and aluminum hydroxides on mechanisms of phosphate adsorption and desorption. J. Environ. Sci. 2018, 70, 175–189. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Tan, W.; Li, W.; Feng, X.; Sparks, D.L. Characteristics of phosphate adsorption-desorption onto ferrihydrite. Soil Sci. 2013, 178, 1–11. [Google Scholar] [CrossRef]
- Blume, H.P.; Brümmer, G.W.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.M. Scheffer/Schachtschabel. Lehrbuch der Bodenkunde, 16th ed.; Springer: Heidelberg, Germany, 2010. [Google Scholar]
- Ohtake, H.; Tsuneda, S. Phosphorus Recovery and Recycling; Springer: Singapore, 2019. [Google Scholar]
- Janusz, W.; Skwarek, E. The study of the properties of the hydroxyapatite/electrolyte interface. Ann. UMCS Chem. 2009, 64, 11–22. [Google Scholar] [CrossRef][Green Version]
- Bell, L.C.; Posner, A.M.; Quirk, J.P. The point of zero charge of hydroxyapatite and fluorapatite in aqueous solutions. J. Colloid Interf. Sci. 1973, 42, 250–261. [Google Scholar] [CrossRef]
- Basak, B.B. Phosphorus release by low molecular weight organic acids from low-grade indian rock phosphate. Waste Biomass Valor. 2018, 11, 1. [Google Scholar] [CrossRef]
- Kpomblekou, A.K.; Tabatabai, M.A. Effect of organic acids on release of phosphorus from phosphate rocks. Soil Sci. 1994, 158, 442–453. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Paredes, C.; Zhang, H.; Giles, C.D.; Darch, T.; Stutter, M.; George, T.S.; Shand, C.; Lumsdon, D.; Cooper, P.; et al. Organic Acids Regulation of Chemical-Microbial Phosphorus Transformations in Soils. Environ. Sci. Technol. 2016, 50, 11521–11531. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.K.; Zhu, Y.G.; Chittleborough, D. Phosphorus release from phosphate rock and iron phosphate by low-molecular-weight organic acids. J. Environ. Sci. 2004, 16, 5–8. [Google Scholar]
- Duputel, M.; van Hoye, F.; Toucet, J.; Gérard, F. Citrate adsorption can decrease soluble phosphate concentration in soil: Experimental and modeling evidence. Appl. Geochem. 2013, 39, 85–92. [Google Scholar] [CrossRef]
- Morshedizad, M.; Zimmer, D.; Leinweber, P. Effect of bone chars on phosphorus-cadmium-interactions as evaluated by three extraction procedures. J. Plant Nutr. Soil Sci. 2016, 179, 388–398. [Google Scholar] [CrossRef]
- Robinson, J.S.; Baumann, K.; Hu, Y.; Hagemann, P.; Kebelmann, L.; Leinweber, P. Phosphorus transformations in plant-based and bio-waste materials induced by pyrolysis. Ambio 2018, 47, 73–82. [Google Scholar] [CrossRef]
- Zimmer, D.; Kruse, J.; Siebers, N.; Panten, K.; Oelschläger, C.; Warkentin, M.; Hu, Y.; Zuin, L.; Leinweber, P. Bone char vs. S-enriched bone char: Multi-method characterization of bone chars and their transformation in soil. Sci. Total Environ. 2018, 643, 145–156. [Google Scholar] [CrossRef]
- Iriarte-Velasco, U.; Sierra, I.; Zudaire, L.; Ayastuy, J.L. Preparation of a porous biochar from the acid activation of pork bones. Food Bioprod. Process. 2016, 98, 341–353. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J.; Aguilera, P.G.; Ollero, P. Biogas desulfurization by adsorption on thermally treated sewage-sludge. Separ. Purif. Technol. 2014, 123, 200–213. [Google Scholar] [CrossRef]
- Tsai, W.T.; Liu, S.C.; Chen, H.R.; Chang, Y.M.; Tsai, Y.L. Textural and chemical properties of swine-manure-derived biochar pertinent to its potential use as a soil amendment. Chemosphere 2012, 89, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, X.; Zhao, L.; Sun, T. Comparison of sewage sludge- and pig manure-derived biochars for hydrogen sulfide removal. Chemosphere 2014, 111, 296–303. [Google Scholar] [CrossRef]
- Zielińska, A.; Oleszczuk, P.; Charmas, B.; Skubiszewska-Zięba, J.; Pasieczna-Patkowska, S. Effect of sewage sludge properties on the biochar characteristic. J. Anal. Appl. Pyrol. 2015, 112, 201–213. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Li, W.; Xu, J.; Liang, Q. Behavior of Phosphorus during Co-gasification of Sewage Sludge and Coal. Energy Fuels 2012, 26, 2830–2836. [Google Scholar] [CrossRef]
- Ma, Y.L.; Matsunaka, T. Biochar derived from dairy cattle carcasses as an alternative source of phosphorus and amendment for soil acidity. Soil Sci. Plant Nutr. 2013, 59, 628–641. [Google Scholar] [CrossRef]
- Morshedizad, M.; Leinweber, P. Leaching of phosphorus and cadmium in soils amended with different bone chars. Clean Soil Air Water 2017, 45, 1600635. [Google Scholar] [CrossRef]
- Morshedizad, M.; Panten, K.; Klysubun, W.; Leinweber, P. Bone char effects on soil: sequential fractionations and XANES spectroscopy. SOIL 2018, 4, 23–35. [Google Scholar] [CrossRef]
- Rajan, S.S.S.; Brown, M.W.; Boyes, M.K.; Upsdell, M.P. Extractable phosphorus to predict agronomic effectiveness of ground and unground phosphate rocks. Fert. Res. 1992, 32, 291–302. [Google Scholar] [CrossRef]
- Guo, M. The 3R principles for applying biochar to improve soil health. Soil Syst. 2020, 4, 9. [Google Scholar] [CrossRef]
- McDowell, R.W.; Sharpley, A.N. Phosphorus solubility and release kinetics as a function of soil test P concentration. Geoderma 2003, 112, 143–154. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Whalen, J.K.; Cao, Y.; Quan, Z.; Liu, C.; Shi, Y. Kinetics of inorganic and organic phosphorus release influenced by low molecular weight organic acids in calcareous, neutral and acidic soils. J. Plant Nutr. Soil Sci. 2015, 178, 555–566. [Google Scholar] [CrossRef]
- Shariatmadari, H.; Shirvani, M.; Jafari, A. Phosphorus release kinetics and availability in calcareous soils of selected arid and semiarid toposequences. Geoderma 2006, 132, 261–272. [Google Scholar] [CrossRef]
- Lammers, A. Phosphatformen und Phosphatfreisetzung in Hochgedüngten Böden Europas; Herbert Utz Verlag: München, Germany, 1997. [Google Scholar]
- Chien, S.H.; Clayton, W.R. Application of elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Sparks, D.L. Kinetics of Soil Chemical Processes; Academic Press: Cambridge, MA, USA, 1989. [Google Scholar]
- Goyne, K.W.; Brantley, S.L.; Chorover, J. Effects of organic acids and dissolved oxygen on apatite and chalcopyrite dissolution: Implications for using elements as organomarkers and oxymarkers. Chem. Geol. 2006, 234, 28–45. [Google Scholar] [CrossRef]
- Shi, R.; Jia, Y.; Wang, C.; Yao, S. Mechanism of arsenate mobilization from goethite by aliphatic carboxylic acid. J. Hazard. Mater. 2009, 163, 1129–1133. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Hu, H.; Zhang, T.; Zhou, Y. Dissolution of kaolinite induced by citric, oxalic, and malic acids. J. Colloid Interface Sci. 2005, 290, 481–488. [Google Scholar] [CrossRef]
- Henintsoa, M.; Becquer, T.; Rabeharisoa, L.; Gerard, F. Geochemical and microbial controls of the effect of citrate on phosphorus availability in a ferralsol. Geoderma 2017, 291, 33–39. [Google Scholar] [CrossRef]
- Hyacinthe, C.; van Cappellen, P. An authigenic iron phosphate phase in estuarine sediments: composition, formation and chemical reactivity. Mar. Chem. 2004, 91, 227–251. [Google Scholar] [CrossRef]
- Liu, Q.; Matinlinna, J.P.; Chen, Z.; Ning, C.; Ni, G.; Pan, H.; Darvell, B.W. Effect of thermal treatment on carbonated hydroxyapatite: Morphology, composition, crystal characteristics and solubility. Ceram. Int. 2015, 41, 6149–6157. [Google Scholar] [CrossRef]
- Figueiredo, M.; Fernando, A.; Martins, G.; Freitas, J.; Judas, F.; Figueiredo, H. Effect of the calcination temperature on the composition and microstructure of hydroxyapatite derived from human and animal bone. Ceram. Int. 2010, 36, 2383–2393. [Google Scholar] [CrossRef]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317–332. [Google Scholar]
- Koumoulidis, G.C.; Katsoulidis, A.P.; Ladavos, A.K.; Pomonis, P.J.; Trapalis, C.C.; Sdoukos, A.T.; Vaimakis, T.C. Preparation of hydroxyapatite via microemulsion route. J. Colloid Interface Sci. 2003, 259, 254–260. [Google Scholar] [CrossRef]
- Landi, E.; Celotti, G.; Logroscino, G.; Tampieri, A. Carbonated hydroxyapatite as bone substitute. J. Europ. Ceram. Soc. 2003, 23, 2931–2937. [Google Scholar] [CrossRef]
- Pan, H.; Darvell, B.W. Effect of carbonate on hydroxyapatite solubility. Cryst. Growth Des. 2010, 10, 845–850. [Google Scholar] [CrossRef]
- Gypser, S.; Freese, D. Phosphorus release from vivianite and hydroxyapatite by organic and inorganic compounds. Pedosphere 2020, 30, 190–200. [Google Scholar] [CrossRef]
- Smith, B.C. The C=O bond, part III: Carboxylic acids. Spectroscopy 2018, 33, 14–20. [Google Scholar]
- Issa, T.B.; Sayari, F.; Ghalla, H.; Benhamada, L. Synthesis, crystal structure, DFT calculations and molecular docking of l-pyroglutamic acid. J. Mol. Struct. 2019, 1178, 436–449. [Google Scholar] [CrossRef]
- Shkir, M.; Muhammad, S.; AlFaify, S.; Irfan, A.; Patil, P.S.; Arora, M.; Algarni, H.; Jingping, Z. An investigation on the key features of a D–π–A type novel chalcone derivative for opto-electronic applications. RSC Adv. 2015, 5, 87320–87332. [Google Scholar] [CrossRef]
- Pierre, J.L.; Gautier-Luneau, I. Iron and citric acid: A fuzzy chemistry of ubiquitous biological relevance. Biometals 2000, 13, 91–96. [Google Scholar] [CrossRef]
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of Soil and Fertilizer Phosphorus use. Reconciling Changing Concepts of Soil Phosphorus Behaviour with Agronomic Information; FAO: Roma, Italy, 2008. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).