Abstract
Background and Clinical Significance: Urothelial carcinoma is one of the most commonly diagnosed malignant diseases. However, it has a much more favorable prognosis than other significantly less common malignancies. This statement, however, is true only for conventional urothelial carcinomas, not for those with divergent differentiation or a special type of urothelial carcinoma. Case Presentation: Herein, we present a case report of an 80-year-old female patient with multiple predominantly cardiovascular comorbidities and vascular dementia, who presented to our institution with genital bleeding. Clinical and diagnostic tests were difficult due to patient noncooperation; however, abdominal computer tomography and cystoscopy showed an advanced tumor originating from the ventral bladder wall. Histology of the tumor showed an invasive urothelial malignancy with foci of clear-cell (glycogen-rich) variant and dispersed, pleomorphic cells, which were immunohistochemically positive for beta-human chorionic gonadotropin. Hence, the diagnosis of high-grade urothelial carcinoma with clear-cell (glycogen-rich) morphology and divergent trophoblastic differentiation was established. Patient outcome was poor. Conclusions: While conventionally having a somewhat favorable prognosis, special subtypes and divergent differentiation in urothelial carcinomas, which warrant a high-grade diagnosis are not only rare but also highly aggressive conditions. Further challenges arise in their differential diagnosis with other advanced malignancies, which can develop in adjacent organs in both genders
1. Introduction and Clinical Significance
Nestled between uterine cervix and non-Hodgkin lymphoma malignancies, urothelial carcinoma is the ninth most commonly diagnosed malignancy overall as per the latest GLOBOCAN data [1]. The incidence in males is higher than in females, with a relatively low number of malignancy-related deaths in both genders. Urothelial carcinomas rank 13th overall, falling significantly behind rarer malignancies, such as pancreatic, esophageal, and central nervous system malignancies [1].
The lower mortality-to-incidence ratio can be explained by both the relatively high incidence of clinical symptoms in the early stages of development of urothelial carcinoma, predominantly gross hematuria and the easy and readily available access to urinary bladder endoscopy and biopsy, as well as urinary cytology testing without tissue biopsy [2,3]. Coupled together with the relatively high incidence of in situ papillary non-invasive (pTa) and papillary superficially invasive (pT1) urothelial carcinoma, compared to more advanced stages (pT2-4b) of urinary bladder urothelial carcinoma, the GLOBOCAN data reflects a generalization of this quite mixed and diverse group of malignancies [4].
While predominantly developing in the urinary bladder, urothelial carcinoma can also rarely develop in other parts of the urinary system, such as the renal pelvis, ureters, and urethra, where mortality is also higher [5,6]. Furthermore, other than tumor location and tumor stage, tumor grade is also an important survival factor, with the current classifications recognizing two levels of differentiation grades based off predominantly nuclear pleomorphism features—low-grade (less aggressive and prone to recurrence and progression) and high-grade (more aggressive and prone to recurrence and progression, typically present with higher stage as well) [7,8,9].
While histology of urothelial carcinoma is dominated by conventional urothelial morphology, wherein the above-mentioned characteristics of nuclear pleomorphism are used as grading criteria, there are several subtypes and divergent differentiations observed only in a minority of cases, wherein the designated grade is always high-grade, as these rare cases are associated with a more aggressive clinical behavior [10].
Herein, we present a case report of one such exotic variant of urothelial carcinoma of the urinary bladder, characterized by a unique morphology and aggressive behavior—urothelial carcinoma with trophoblastic differentiation.
2. Case Presentation
A polymorbid 80-year-old female patient presented to our institution with a history of several days of profuse genital bleeding and several days of mild abdominal pain. The patient, as already mentioned, had multiple comorbidities, predominantly cardiovascular, with a history of significant vascular dementia, as reported by her relatives. The patient’s previous gynecologic history included five normal pregnancies and deliveries. Physical and genital exams were difficult to perform as the patient was uncooperative. Both on palpation and ultrasound, the uterus was relatively enlarged (size referring to the first lunar month) and had a heterogeneous appearance on ultrasound. Vaginal exam, which was again difficult due to the patient being uncooperative, revealed a bloody discharge from the cervical canal. Bloodwork revealed significant anemia, with a hemoglobin level of 89 g/L, while other values were within the reference range.
As both the physical and ultrasound exams were limited, abdominopelvic computer tomography was performed under mild sedation. Computer tomography revealed a bladder formation with a density of 36 HU, measuring 43 × 28 mm (sagittal) and 43 × 38 mm (axial), originating from the ventral wall of the bladder. The formation showed post-contrast enhancement to 73 HU, primarily on the periphery. The lumen itself was filled with hyperdense hemorrhagic structures, equivalent to 55–60 Hounsfield units (HU), which did not change their density post-contrast or with gas, resulting in the formation of hydraeric levels.
As the genital bleeding was determined to be secondary to the bladder tumor formation, the patient was transferred to the urology department for bladder endoscopy with biopsy.
Bladder endoscopy under general anesthesia revealed an exophytic and invasive bleeding tumor on the anterior bladder wall. Several biopsies were performed from different areas; however, ablation was not performed due to excessive bleeding from the biopsy sites. Specimens sent for histopathology consisted of three grayish-white, firm fragments, with the largest measuring 18 × 5 mm.
Histopathology of the specimens revealed fragments of urinary bladder wall with fibrovascular structures, characterized by nested submucosal and muscular infiltration, represented by large cellular aggregates with pronounced anisocytosis and anisokaryosis. These aggregates had uneven, hyperchromatic nuclei with multiple ruby-red nucleoli (Figure 1, Figure 2 and Figure 3). The tumor nest exhibited focal, abundant clear-cell transformation, characterized by double eosinophilic contouring of the nuclear outlines and nuclearly dominant cells with hyperchromatic nuclei and uneven borders (Figure 1, Figure 2 and Figure 3). Mitotic figures were abundant, including some pleomorphic ones with hotspots, showing up to three mitotic figures per single high-power field (400× magnification) (Figure 1, Figure 2 and Figure 3). Tumor cell emboli were present in the blood vessels.

Figure 1.
Histopathology of the tumor. (A) Foci of conventional-appearing urothelial papillary carcinoma, H&E stain, original magnification 40×; (B) foci of conventional-appearing papillary urothelial carcinoma admixed with focal clear-cell nests, H&E stain, original magnification 100×.
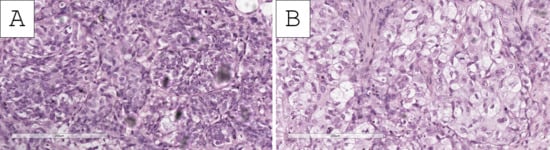
Figure 2.
Histopathology of the tumor. (A Foci of conventional invasive urothelial carcinoma with focal clear cells, H&E stain, original magnification 200×; (B) predominantly clear-cell nests, H&E stain, original magnification 200×.
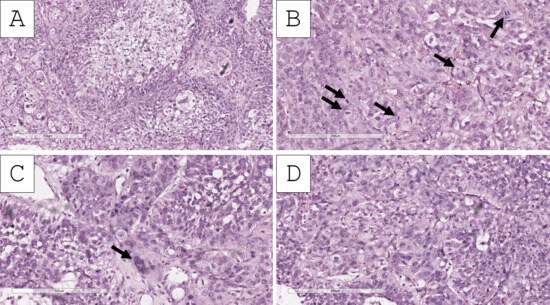
Figure 3.
Histopathology of the tumor. (A) Clear-cell nests, H&E stain, original magnification 100×; (B) clear cells admixed with epithelioid type cells, pronounced mitotic activity (arrows), H&E stain, original magnification 200×; (C) epithelioid cell cords, some of which multinucleated (arrow), H&E stain, original magnification 200×; (D) epithelioid cell nests, H&E stain, original magnification 200×.
Based on the morphological findings, the initial impression of the tumor was of a high-grade urothelial carcinoma with clear-cell (glycogen-rich) morphology and probable divergent differentiation (trophoblastic) or secondary specific histologic type (giant-cell urothelial carcinoma). Immunohistochemistry for beta human chorionic gonadotropin (hCG) showed an intense cytoplasmic reaction in the large, nuclearly dominant cells (Figure 4). Hence, the tumor was interpreted as a high-grade urothelial carcinoma with clear-cell (glycogen-rich) morphology and divergent trophoblastic differentiation. Staging was deemed to be at least pT2 due to invasion within the muscle wall with lymph and blood vessel tumor emboli.
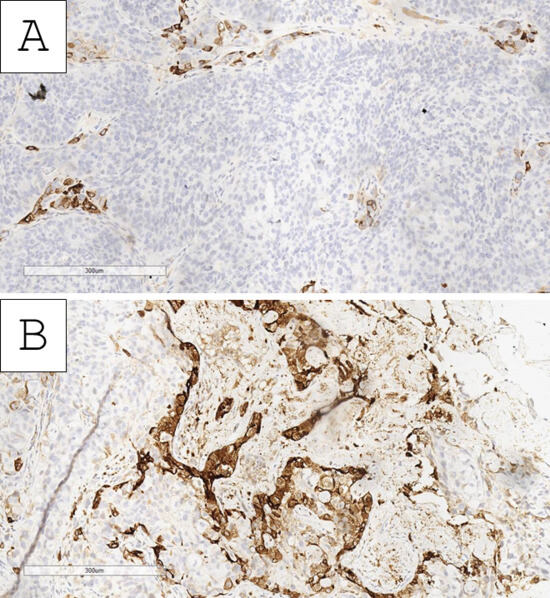
Figure 4.
Immunohistochemistry with beta human chorionic gonadotropin. (A) Clear-cell nests negative and few intersecting zones with positive reaction, original magnification 200×; (B) predominantly positive nests, original magnification 200×.
The patient was referred to the oncology committee, which determined that the disease stage and concomitant conditions were contraindications for treatment, and the patient was referred for end-of-life palliative treatment and expired two months later due to disease progression and severe posthemorrhagic anemia.
3. Discussion
Urothelial carcinomas of special subtypes and divergent differentiation consistently warrant a high-grade designation, based on both their specific morphology and clinical behavior [10]. Specific subtypes are most often micropapillary and variants of nested urothelial carcinoma, with clear-cell (glycogen-rich) being a rare subtype [10]. In the presented case, the main morphological features are dominated by clear-cell (glycogen-rich) morphology. In this subtype, the cells accumulate cytoplasmic glycogen, which, due to the specifics of histological processing, is extracted in the final slide [10,11]. This special subtype is exceedingly rare, predominantly presented in case reports and small case series, and according to published data in the medical literature, it exhibits significantly more aggressive clinical behavior than conventional urothelial carcinoma [10,11,12,13]. This subtype also requires extensive differential diagnosis with one other subtype of urothelial malignancy—clear-cell adenocarcinoma of the urinary tract, which is a special type of Müllerian malignancy (PAX 8-positive, negative for GATA3, p63, ER, PR, and WT1), as well as clear-cell–renal-cell carcinoma (RCC-, PAX8- and CD10-positive) [12,13,14,15,16,17]. In females, this differential diagnosis is expanded to clear-cell carcinoma of the female genital (napsin A, WT1 and PR positive) tract and in males with clear-cell renal-type prostatic acinar adenocarcinoma (AMACR- and NKX3.1-positive) [11,13,14].
The presence of giant cells, especially nuclear-dominated ones, also warrants an extensive differential diagnosis, again with other exotic malignancies [10]. Urothelial carcinomas have both a special subtype with giant cells—giant-cell urothelial carcinoma and a divergent differentiation type—with trophoblastic differentiation, as seen in our case [10].
Giant-cell urothelial carcinoma is an aggressive subtype, also referred to as pleomorphic giant-cell carcinoma of the urinary bladder, represents an extreme spectrum of conventional high-grade urothelial carcinoma, represented by bizarre pleomorphic giant tumor cells in the background of at least minimal foci of conventional urothelial carcinoma, with the pleomorphic at least minimally retaining their urothelial immunohistochemical profile [18,19,20].
Conversely, in urothelial carcinoma with divergent trophoblastic differentiation, as seen in our case, the giant monstrous cells have a trophoblastic immunohistochemical phenotype and, in morphology, can be both cyto- and syncytotrophoblastic in nature to the point of the malignancy being hard to distinguish from choriocarcinoma [21,22,23]. This divergent type of urothelial carcinoma is also extremely rare and only reported in individual case reports and small case series and is often reported to coexist with special urothelial carcinoma subtypes and other forms of divergent differentiation [21,22,23].
Expression of trophoblastic markers in such cases is often not limited to cells of trophoblastic morphology, with hCG, hydroxyl-δ-5-steroid dehydrogenase and sal-like protein 4 being somewhat reliable markers for this divergent differentiation [21]. Of note is the presence of positivity of both hCG- and sal-like protein 4 in the conventional urothelial component of these malignancies, indicating the potential for divergent differentiation in urothelial malignancies [21]. This would also explain the clinical phenomenon of elevated serum hCG in patients with urothelial carcinoma, especially in advanced stages, and its potential to be used as a clinical marker for disease follow-up and progression [24,25,26].
Differential diagnosis, especially in cases with extensive divergent trophoblastic differentiation, is that of choriocarcinoma and other rare pleomorphic malignancies of the urogenital tract, such as giant-cell pleomorphic acinar adenocarcinoma of the prostate in males [21,27].
As seen in the outcome of our case, divergent trophoblastic differentiation in urothelial carcinomas is associated with an aggressive clinical course and poor patient outcome [21].
4. Conclusions
Divergent trophoblastic differentiation is a rare occurrence in urothelial carcinoma and warrants a diagnosis of high-grade malignancy. Although exceedingly rare and often posing a diagnostic challenge, this differentiation should always be kept in mind when diagnosing highly pleomorphic urothelial carcinomas, especially in the presence of other special types of divergent differentiations, as it is associated with a more aggressive clinical course when compared to conventional urothelial carcinoma, as underlined by the poor outcome of the presented case.
Author Contributions
Conceptualization G.S., D.M., P.P., P.G. and H.P.; methodology G.S., D.M., P.P. and H.P.; formal analysis G.S., P.G. and P.P.; investigation G.S., D.M. and P.G.; resources G.S. and H.P.; data curation G.S.; writing—original draft preparation G.S., D.M., P.P. and P.G.; writing—review and editing P.G. and H.P.; visualization G.S.; supervision P.G. and H.P.; project administration G.S.; funding acquisition H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study is financed by the European Union-NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project no BG-RRP-2.004-0009-C02.; The APC was funded by the Medical University—Varna.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study due to the Varna Committee on Scientific Ethics waiving the need for IRB approval for the case report, wherein the patient had signed informed consent.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Satyal, U.; Srivastava, A.; Abbosh, P.H. Urine Biopsy—Liquid Gold for Molecular Detection and Surveillance of Bladder Cancer. Front. Oncol. 2019, 9, 1266. [Google Scholar] [CrossRef] [PubMed]
- Matulewicz, R.S.; DeLancey, J.O.; Meeks, J.J. Cystoscopy. JAMA 2017, 317, 1187. [Google Scholar] [CrossRef]
- Nielsen, M.E.; Smith, A.B.; Meyer, A.M.; Kuo, T.M.; Tyree, S.; Kim, W.Y.; Milowsky, M.I.; Pruthi, R.S.; Millikan, R.C. Trends in Stage-Specific Incidence Rates for Urothelial Carcinoma of the Bladder in the United States: 1988 to 2006. Cancer 2014, 120, 86–95. [Google Scholar] [CrossRef]
- Van Der Poel, H.G.; Antonini, N.; Van Tinteren, H.; Horenblas, S. Upper Urinary Tract Cancer: Location Is Correlated with Prognosis. Eur. Urol. 2005, 48, 438–444. [Google Scholar] [CrossRef]
- Soualhi, A.; Rammant, E.; George, G.; Russell, B.; Enting, D.; Nair, R.; Van Hemelrijck, M.; Bosco, C. The Incidence and Prevalence of Upper Tract Urothelial Carcinoma: A Systematic Review. BMC Urol. 2021, 21, 110. [Google Scholar] [CrossRef]
- Abou Heidar, N.; Mahmood, A.W.; Khan, M.; Harrington, G.; Ahmad, A.; Abdelhaq, D.; Colan, N.; Whitt, J.D.; Sullivan, D.; Howlader, M.; et al. Does Ta Low-Grade Urothelial Carcinoma of the Bladder With Focal High-Grade Features Carry Worse Prognosis? The Roswell Park Comprehensive Cancer Center Experience. Urology 2024, 193, 136–142. [Google Scholar] [CrossRef]
- Deuker, M.; Martin, T.; Stolzenbach, F.; Rosiello, G.; Collà Ruvolo, C.; Nocera, L.; Tian, Z.; Becker, A.; Kluth, L.; Roos, F.C.; et al. Bladder Cancer: A Comparison Between Non-Urothelial Variant Histology and Urothelial Carcinoma Across All Stages and Treatment Modalities. Clin. Genitourin. Cancer 2021, 19, 60–68.e1. [Google Scholar] [CrossRef] [PubMed]
- Boustead, G.B.; Fowler, S.; Swamy, R.; Kocklebergh, R.; Hounsome, L. Stage, Grade and Pathological Characteristics of Bladder Cancer in the UK: British Association of Urological Surgeons (BAUS) Urological Tumour Registry. BJU Int. 2014, 113, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Popov, H.; Kirilova, A.; Naydenova, K.; Softova, E.; Stoyanov, G.S. Urothelial Carcinoma With Divergent Glandular Differentiation. Cureus 2024, 16, e72603. [Google Scholar] [CrossRef]
- Knez, V.M.; Barrow, W.; Lucia, M.S.; Wilson, S.; La Rosa, F.G. Clear Cell Urothelial Carcinoma of the Urinary Bladder: A Case Report and Review of the Literature. J. Med. Case Rep. 2014, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Kotliar, S.; Wood, C.; Schaeffer, A.; Oyasy, R. Transitional Cell Carcinoma Exhibiting Clear Cell Features. A Differential Diagnosis for Clear Cell Adenocarcinoma of the Urinary Tract. Arch. Pathol. Lab. Med. 1995, 119, 79–81. [Google Scholar] [PubMed]
- Mai, K.T.; Bateman, J.; Djordjevic, B.; Flood, T.A.; Belanger, E.C. Clear Cell Urothelial Carcinoma. Int. J. Surg. Pathol. 2017, 25, 18–25. [Google Scholar] [CrossRef]
- Paner, G.P.; Annaiah, C.; Gulmann, C.; Rao, P.; Ro, J.Y.; Hansel, D.E.; Shen, S.S.; Lopez-Beltran, A.; Aron, M.; Luthringer, D.J.; et al. Immunohistochemical Evaluation of Novel and Traditional Markers Associated with Urothelial Differentiation in a Spectrum of Variants of Urothelial Carcinoma of the Urinary Bladder. Hum. Pathol. 2014, 45, 1473–1482. [Google Scholar] [CrossRef]
- Gilcrease, M.Z.; Delgado, R.; Vuitch, F.; Albores-Saavedra, J. Clear Cell Adenocarcinoma and Nephrogenic Adenoma of the Urethra and Urinary Bladder: A Histopathologic and Immunohistochemical Comparison. Hum. Pathol. 1998, 29, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Grosser, D.; Matoso, A.; Epstein, J.I. Clear Cell Adenocarcinoma in Men: A Series of 15 Cases. Am. J. Surg. Pathol. 2021, 45, 270–276. [Google Scholar] [CrossRef]
- Herawi, M.; Drew, P.A.; Pan, C.C.; Epstein, J.I. Clear Cell Adenocarcinoma of the Bladder and Urethra: Cases Diffusely Mimicking Nephrogenic Adenoma. Hum. Pathol. 2010, 41, 594–601. [Google Scholar] [CrossRef]
- Lopez-Beltran, A.; Blanca, A.; Montironi, R.; Cheng, L.; Regueiro, J.C. Pleomorphic Giant Cell Carcinoma of the Urinary Bladder. Hum. Pathol. 2009, 40, 1461–1466. [Google Scholar] [CrossRef]
- Samaratunga, H.; Delahunt, B. Recently Described and Unusual Variants of Urothelial Carcinoma of the Urinary Bladder. Pathology 2012, 44, 407–418. [Google Scholar] [CrossRef]
- Samaratunga, H.; Delahunt, B.; Egevad, L.; Adamson, M.; Hussey, D.; Malone, G.; Hoyle, K.; Nathan, T.; Kerle, D.; Ferguson, P.; et al. Pleomorphic Giant Cell Carcinoma of the Urinary Bladder: An Extreme Form of Tumour de-Differentiation. Histopathology 2016, 68, 533–540. [Google Scholar] [CrossRef]
- Przybycin, C.G.; McKenney, J.K.; Nguyen, J.K.; Shah, R.B.; Umar, S.A.; Harik, L.; Shih, I.M.; Cox, R.M. Urothelial Carcinomas With Trophoblastic Differentiation, Including Choriocarcinoma: Clinicopathologic Series of 16 Cases. Am. J. Surg. Pathol. 2020, 44, 1322–1330. [Google Scholar] [CrossRef]
- Regalado, J.J. Mixed Micropapillary and Trophoblastic Carcinoma of Bladder: Report of a First Case with New Immunohistochemical Evidence of Urothelial Origin. Hum. Pathol. 2004, 35, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Grammatico, D.; Grignon, D.J.; Eberwein, P.; Shepherd, R.R.; Hearn, S.A.; Walton, J.C. Transitional Cell Carcinoma of the Renal Pelvis with Chriocarcinomatous Differentiation. Immunohistochemical and Immunoelectron Microscopic Assessment of Human Chorionic Gonadotropin Production by Transitional Cell Carcinoma of the Urinary Bladder. Cancer 1993, 71, 1835–1841. [Google Scholar] [CrossRef]
- Dexeus, F.; Logothetis, C.; Hossan, E.; Samuels, M.L. Carcinoembryonic Antigen and Beta-Human Chorionic Gonadotropin as Serum Markers for Advanced Urothelial Malignancies. J. Urol. 1986, 136, 403–407. [Google Scholar] [CrossRef]
- Douglas, J.; Sharp, A.; Chau, C.; Head, J.; Drake, T.; Wheater, M.; Geldart, T.; Mead, G.; Crabb, S.J. Serum Total HCGβ Level Is an Independent Prognostic Factor in Transitional Cell Carcinoma of the Urothelial Tract. Br. J. Cancer 2014, 110, 1759–1766. [Google Scholar] [CrossRef]
- Iles, R.K. Ectopic HCGbeta Expression by Epithelial Cancer: Malignant Behaviour, Metastasis and Inhibition of Tumor Cell Apoptosis. Mol. Cell. Endocrinol. 2007, 260–262, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, G.S.; Kirilova, A.; Naydenova, K.; Popov, H. Unusual Type of Acinar Adenocarcinoma of the Prostate With Low PSA: A Histopathological Report of Two Cases of Pleomorphic Giant Cell Adenocarcinoma. Case Rep. Oncol. Med. 2025, 2025, 7658657. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.