Inflammatory Fibroid Gastric Polyps (Vanek’s Tumor): Two Case Reports Highlighting Epidemiological Patterns and Telocyte-Driven Neoplastic Pathogenesis and Diagnosis
Abstract
1. Introduction and Clinical Significance
2. Case Presentations
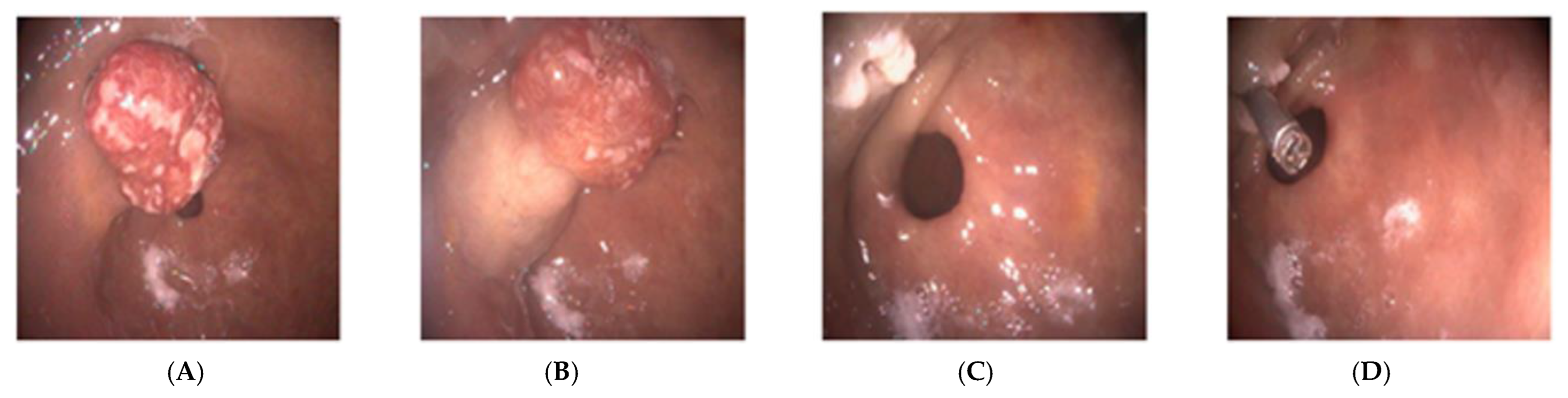
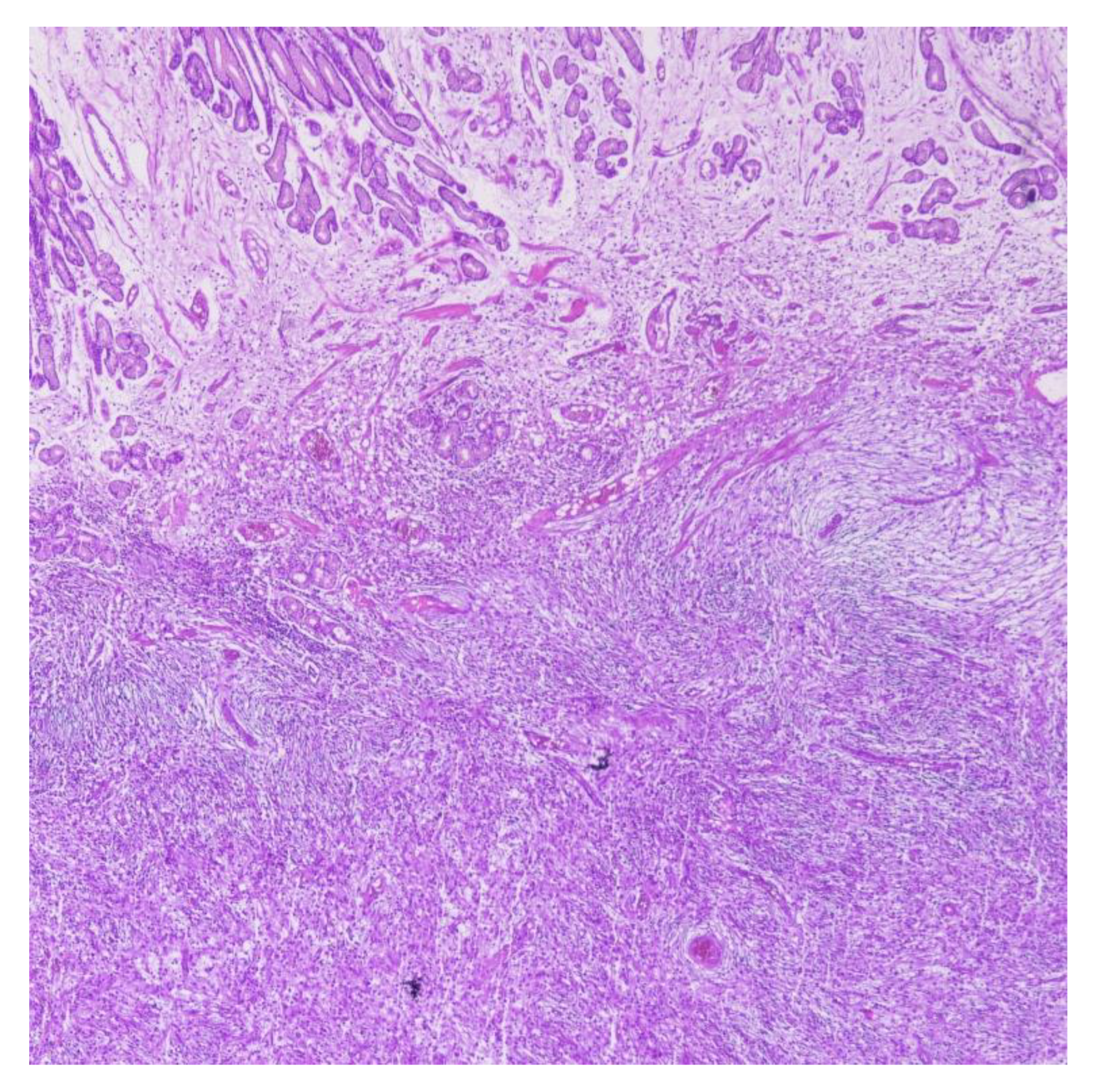
2.1. Case Report 1
2.2. Case Report 2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanek, J. Gastric submucosal granuloma with eosinophilic infiltration. Am. J. Pathol. 1949, 25, 397–411. [Google Scholar]
- Nagao, S.; Tsuji, Y.; Sakaguchi, Y.; Ushiku, T.; Koike, K. Inflammatory fibroid polyp mimicking an early gastric cancer. Gastrointest. Endosc. 2020, 92, 217–218. [Google Scholar] [CrossRef] [PubMed]
- Carmack, S.W.; Genta, R.M.; Schuler, C.M.; Saboorian, M.H. The current spectrum of gastric polyps: A 1-year national study of over 120,000 patients. Am. J. Gastroenterol. 2009, 104, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Dias, E.; Marques, M.; Santos-Antunes, J.; Baldaque-Silva, F.; Moutinho-Ribeiro, P.; Macedo, G. The role of endoscopic submucosal dissection in the management of gastric inflammatory fibroid polyps: A single-center experience. Rev. Esp. Enferm. Dig. 2022, 114, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Nonose, R.; Valenciano, J.S.; da Silva, C.M.; de Souza, C.A.; Martinez, C.A. Ileal Intussusception Caused by Vanek’s Tumor: A Case Report. Case Rep. Gastroenterol. 2011, 5, 110–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paikos, D.; Moschos, J.; Tzilves, D.; Koulaouzidis, A.; Kouklakis, G.; Patakiouta, F.; Kontodimou, K.; Tarpagos, A.; Katsos, I. Inflammatory fibroid polyp or Vanek’s tumour. Dig. Surg. 2007, 24, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cui, M.; Xing, J.; Shi, Y.; Su, X. Massive gastrointestinal bleeding caused by a giant gastric inflammatory fibroid polyp: A case report. Int. J. Surg. Case Rep. 2014, 5, 571–573. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silva, M.; Albuquerque, A.; Cardoso, H.; Costa, J.; Macedo, G. Gastric inflammatory fibroid polyp mimicking a gastrointestinal stromal tumour. Rev. Esp. Enferm. Dig. 2016, 108, 497–498. [Google Scholar] [PubMed]
- Çomut, E.; Arman Karakaya, Y.; Çelik, M.; Çallı Demirkan, N. Histopathologic features of inflammatory fibroid polyps and risk of cancer development: A case series. Pamukkale Med. J. 2023, 16, 476–485. [Google Scholar] [CrossRef]
- Maccagno, A.; Sander, B.; Dintner, S.; Harloff, M.; Fuzesi, L.; Märkl, B. Inflammatory fibroid polyp: A series of 29 cases and a systematic review of the literature. Hum. Pathol. Rep. 2023, 32, 300703. [Google Scholar] [CrossRef]
- Ardeleanu, C.; Bussolati, G. Telocytes are the common cell of origin of both PEComas and GISTs: An evidence-supported hypothesis. J. Cell. Mol. Med. 2011, 15, 2569–2574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buda, C.; Garipoli, C.; Penna, G.; D’Aquino, A.; Galletti, C.; Facciolà, A.; Fedele, F. Endoscopic mucosal resection of a large inflammatory fibroid polyp (Vanek’s tumor): A case report. Acta Biomed. 2021, 92, e2021062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fleres, F.; Mazzeo, C.; Ieni, A.; Rossitto, M.; Cucinotta, E. Gastric inflammatory fibroid polyp tumor with acute intestinal obstruction-Vanek’s tumor can mimick a giant gastrointestinal stromal tumor or a gastric lymphoma. J. Vis. Surg. 2018, 4, 54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnstone, J.M.; Morson, B.C. Inflammatory fibroid polyp of the gastrointestinal tract. Histopathology 1978, 2, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.A. Granuloma (granuloblastoma) gástrico submucoso com eosinófilos [Gastric submucosal granuloma (granuloblastoma) with eosinophilic infiltration]. Gaz. Med. Port. 1950, 3, 751–759. [Google Scholar] [PubMed]
- Garmpis, N.; Damaskos, C.; Garmpi, A.; Georgakopoulou, V.E.; Sakellariou, S.; Liakea, A.; Schizas, D.; Diamantis, E.; Farmaki, P.; Voutyritsa, E.; et al. Inflammatory Fibroid Polyp of the Gastrointestinal Tract: A Systematic Review for a Benign Tumor. In Vivo 2021, 35, 81–93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishiyama, Y.; Koyama, S.; Andoh, A.; Kishi, Y.; Yoshikawa, K.; Ishizuka, I.; Yokono, T.; Fujiyama, Y. Gastric inflammatory fibroid polyp treated with Helicobacter pylori eradication therapy. Intern. Med. 2003, 42, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Hirasaki, S.; Matsubara, M.; Ikeda, F.; Taniguchi, H.; Suzuki, S. Gastric inflammatory fibroid polyp treated with Helicobacter pylori eradication therapy. Intern. Med. 2007, 46, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Mudter, J.; Märkl, B.; Chetty, R. Cytomegalovirus infection presenting as isolated inflammatory polyps of the gastrointestinal tract. Pathology 2011, 43, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.C.; Lin, M.T.; Montgomery, E.A.; Singhi, A.D. Inflammatory fibroid polyps of the gastrointestinal tract: Spectrum of clinical, morphologic, and immunohistochemistry features. Am. J. Surg. Pathol. 2013, 37, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Ozolek, J.A.; Sasatomi, E.; Swalsky, P.A.; Rao, U.; Krasinskas, A.; Finkelstein, S.D. Inflammatory fibroid polyps of the gastrointestinal tract: Clinical, pathologic, and molecular characteristics. Appl. Immunohistochem. Mol. Morphol. 2004, 12, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulos, G.E.; Linardoutsos, D.; Tsamis, D.; Stamopoulos, P.; Giannopoulos, D.; Zagouri, F.; Michalopoulos, N.V. Gastrointestinal stromal tumor causing small bowel intussusception in a patient with Crohn’s disease. World J. Gastroenterol. 2009, 15, 5224–5227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mucientes, P.; Mucientes, F.; Klaassen, R. Inflammatory fibroid polyp associated with early gastric carcinoma: A case report. Ann Diagn Pathol. 2012, 16, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Higgins, J.; Cho, E.Y.; Ko, Y.H.; Oh, Y.L. Expression of CD34, bcl-2, and kit in inflammatory fibroid polyps of the gastrointestinal tract. Appl Immunohistochem Mol Morphol. 2000, 8, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, P.; Yao, T.; Tsuneyoshi, M. Inflammatory fibroid polyp of the stomach. A special reference to an immunohistochemical profile of 42 cases. Am. J. Surg. Pathol. 1993, 17, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, E.A.; Meis, J.M. Nodular fasciitis: Its morphologic spectrum and immunohistochemical profile. Am. J. Surg. Pathol. 1991, 15, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Schildhaus, H.U.; Cavlar, T.; Binot, E.; Büttner, R.; Wardelmann, E.; Merkelbach-Bruse, S. Inflammatory fibroid polyps harbour mutations in the platelet-derived growth factor receptor alpha (PDGFRA) gene. J. Pathol. 2008, 216, 176–182. [Google Scholar] [CrossRef]
- Ricci, R.; Giustiniani, M.C.; Gessi, M.; Lanza, P.; Castri, F.; Biondi, A.; Persiani, R.; Vecchio, F.M.; Risio, M. Telocytes are the physiological counterpart of inflammatory fibroid polyps and PDGFRA-mutant GISTs. J. Cell Mol. Med. 2018, 22, 4856–4862. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Tian, H. Telocytes and inflammation: A review. Medicine 2023, 102, e35983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Xu, Y. Tumor-associated telocytes. Chin. Med. J. 2024, 137, 490–492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vannucchi, M.G. Telocytes and Macrophages in the Gut: From Morphology to Function, Do the Two Cell Types Interact with Each Other? Which Helps Which? Int. J. Mol. Sci. 2022, 23, 8435. [Google Scholar] [CrossRef]
- Sanches, B.D.A.; Teófilo, F.B.S.; Brunet, M.Y.; Villapun, V.M.; Man, K.; Rocha, L.C.; Neto, J.P.; Matsumoto, M.R.; Maldarine, J.S.; Ciena, A.P.; et al. Telocytes: Current methods of research, challenges and future perspectives. Cell Tissue Res. 2024, 396, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Jukic, Z.; Ferencic, Z.; Radulovic, P.; Mijic, A.; Fucic, A. Estrogen and androgen receptors in inflammatory fibroid polyp (Vanek’s tumor): Case report. Anticancer Res. 2014, 34, 7203–7206. [Google Scholar] [PubMed]
- Langlois, M.J.; Servant, R.; Reyes Nicolás, V.; Jones, C.; Roy, S.; Paquet, M.; Perreault, N. Loss of PTEN Signaling in Foxl1+ Mesenchymal Telocytes Initiates Spontaneous Colonic Neoplasia in Mice. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 530–533.e5. [Google Scholar] [CrossRef]
- Daum, O.; Hatlova, J.; Mandys, V.; Grossmann, P.; Mukensnabl, P.; Benes, Z.; Michal, M. Comparison of morphological, immunohistochemical, and molecular genetic features of inflammatory fibroid polyps (Vanek’s tumors). Virchows Arch. 2010, 456, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Palomba, G.; Fernicola, A.; Corte, M.D.; Capuano, M.; De Palma, G.D.; Aprea, G. Artificial intelligence in screening and diagnosis of surgical diseases: A narrative review. AIMS Public Health 2024, 11, 557–576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, K.; Lei, C.; Kan, X.; Ouyang, Y.; Mei, Y.; Guo, Y.; Wang, B.; Zhang, D.; Li, J.; Li, R.; et al. Artificial intelligence for early gastric cancer boundary recognition in NBI and nF-NBI endoscopic images. Scand. J. Gastroenterol. 2025, 60, 624–634. [Google Scholar] [CrossRef] [PubMed]
- El Asmar, N.; Baydoun, M.; Mrad, J.; Barada, K. Role of artificial intelligence in the detection and characterization of gastrointestinal premalignant and early malignant lesions. World J. Gastroenterol. 2025, 31, 111160. [Google Scholar] [CrossRef] [PubMed]
- Venuto, R.; Rizzo, C.E.; Lo Giudice, D.; Fries, W.; Ceccio, C.; Fedele, F.; Squeri, R.; Genovese, C. Vaccination Coverage in Adult Patients with Inflammatory Bowel Disease: Impact of a Tailored Vaccination Pathway Including COVID-19 and Herpes Zoster in a University Hospital Vaccination Center. Vaccines 2025, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.E.; Venuto, R.; Tripodi, P.; Bartucciotto, L.; Ventura Spagnolo, E.; Nirta, A.; Genovese, G.; La Spina, I.; Sortino, S.; Nicita, A.; et al. From Guidelines to Action: Tackling Risk Factors for Surgical Site Infections. Antibiotics 2025, 14, 40. [Google Scholar] [CrossRef]



| Lesion/Tumor Type | Histological Features | Immunohistochemistry Profile | Clinical Notes/ Distinguishing Points |
|---|---|---|---|
| Inflammatory Fibroid Polyp (IFP/Vanek’s tumor) | Bland spindle/stellate cells in onion-skin perivascular pattern; eosinophil-rich stroma | CD34+, PDGFRA+; CD117–, DOG1–, S100–, Desmin– | Benign, submucosal; may mimic GIST; may rarely cause obstruction or bleeding; PDGFRA mutations common |
| Gastrointestinal stromal tumor (GIST) | Spindle or epithelioid cells; variable cellularity | CD117 (c-KIT)+, DOG1+, often CD34+; PDGFRA mutations in subset | Malignant potential; requires risk stratification (size, mitotic index); responds to tyrosine kinase inhibitors |
| Leiomyoma/leiomyosarcoma | Fascicles of spindle cells with cigar-shaped nuclei | SMA+, Desmin+, Caldesmon+; CD117–, DOG1–, CD34– | Leiomyoma benign; leiomyosarcoma malignant; smooth muscle origin |
| Schwannoma | Spindle cells with palisading nuclei; peripheral lymphoid cuff | S100+, GFAP+; CD34–, CD117–, DOG1– | Benign, slow growing; neural crest origin; rare in stomach |
| Solitary fibrous tumor | Pattern-less architecture; collagen bands; staghorn vessels | CD34+, STAT6 nuclear+; CD117–, DOG1– | Rare in GI tract; usually benign but can recur; occasional malignant behavior |
| Nodular fasciitis | Myxoid stroma; tissue culture-like spindle cells; rapid growth | SMA+, Actin+; CD34–, CD117– | Reactive lesion; self-limited; may be misdiagnosed as sarcoma |
| Inflammatory myofibroblastic tumor (IMT) | Myofibroblastic spindle cells with inflammatory infiltrate | ALK+, SMA+, Desmin+; CD34 variable | Intermediate biologic potential; may recur; rare metastasis; uncommon in stomach |
| Lymphoma (gastric involvement) | Diffuse sheets of atypical lymphoid cells | CD20+ (B-cell), CD3+ (T-cell) depending on subtype | Malignant; systemic disease; may mimic submucosal mass; B-cell most common (DLBCL, MALT) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Venuto, R.; Rizzo, C.E.; Loddo, F.; Genovese, G.; Martorana, M.T.; Genovese, C.; Fedele, F. Inflammatory Fibroid Gastric Polyps (Vanek’s Tumor): Two Case Reports Highlighting Epidemiological Patterns and Telocyte-Driven Neoplastic Pathogenesis and Diagnosis. Reports 2026, 9, 2. https://doi.org/10.3390/reports9010002
Venuto R, Rizzo CE, Loddo F, Genovese G, Martorana MT, Genovese C, Fedele F. Inflammatory Fibroid Gastric Polyps (Vanek’s Tumor): Two Case Reports Highlighting Epidemiological Patterns and Telocyte-Driven Neoplastic Pathogenesis and Diagnosis. Reports. 2026; 9(1):2. https://doi.org/10.3390/reports9010002
Chicago/Turabian StyleVenuto, Roberto, Caterina Elisabetta Rizzo, Francesco Loddo, Giovanni Genovese, Maria Teresa Martorana, Cristina Genovese, and Francesco Fedele. 2026. "Inflammatory Fibroid Gastric Polyps (Vanek’s Tumor): Two Case Reports Highlighting Epidemiological Patterns and Telocyte-Driven Neoplastic Pathogenesis and Diagnosis" Reports 9, no. 1: 2. https://doi.org/10.3390/reports9010002
APA StyleVenuto, R., Rizzo, C. E., Loddo, F., Genovese, G., Martorana, M. T., Genovese, C., & Fedele, F. (2026). Inflammatory Fibroid Gastric Polyps (Vanek’s Tumor): Two Case Reports Highlighting Epidemiological Patterns and Telocyte-Driven Neoplastic Pathogenesis and Diagnosis. Reports, 9(1), 2. https://doi.org/10.3390/reports9010002







