1. Introduction and Clinical Significance
Lupus erythematosus is a chronic autoimmune disease with a variety of clinical manifestations that affect both the skin and internal organs with multiple differential diagnoses [
1,
2,
3,
4,
5,
6,
7,
8]. From a dermatological point of view, two main forms are distinguished—systemic lupus erythematosus (SLE) and cutaneous lupus erythematosus (CLE) [
7,
8,
9,
10,
11,
12]. Cutaneous lupus erythematosus encompasses several clinical subtypes—acute, subacute, and chronic forms—among which the most common is discoid lupus erythematosus (DLE), a prototypical manifestation of chronic cutaneous lupus [
7,
8,
9,
10,
11,
12,
13,
14,
15]. DLE is characterized by chronic, well-circumscribed erythematous and hyperkeratotic plaques that most often affect sun-exposed areas such as the face, ears, scalp, and neckline. These lesions can leave permanent scars and cicatricial changes, which emphasize the importance of early diagnosis and appropriate treatment [
10,
11,
12,
13,
14,
15,
16,
17,
18]. In Bulgaria, there is a lack of accurate epidemiological data on the prevalence of DLE; however, clinical practice suggests a number of cases, warranting increased attention among specialists in dermatology and general medicine. In both Europe and the United States, the reported incidence of isolated CLE ranges from 4.0 to 4.3 cases per 100,000 individuals, which is slightly higher than the SLE incidence rate, estimated at approximately 3 cases per 100,000 individuals. DLE accounts for 50% to 85% of all CLE cases. It exhibits a marked female predominance, occurring two to three times more frequently in women compared to men. While DLE can manifest across all age groups, its onset is most commonly observed between the ages of 20 and 40 years [
19,
20,
21]. While DLE is the dominant form of CLE, it remains a rare condition that is difficult to recognize at initial presentation by non-dermatologists [
1,
2,
3,
4,
5,
6]. Initial misdiagnosis of the condition leads to extensive progression and the development of further complications.
In addition to the difficulty in recognition and diagnosis, DLE and other forms of lupus also present a further challenge—treatment and follow-up—as the immunosuppressive treatment can produce complications of its own [
17,
18]. Herein, we present a clinical and morphological case report of DLE with an atypical clinical presentation and a complication against the background of the immunosuppressive treatment—pulmonary tuberculosis.
2. Case Presentation
A 38-year-old woman with no evidence of concomitant chronic diseases and past medical history notable for two spontaneous abortions, followed by a single full-term delivery complicated by postpartum anemia requiring blood transfusion, presented to our department with complaints of skin changes starting two months prior.
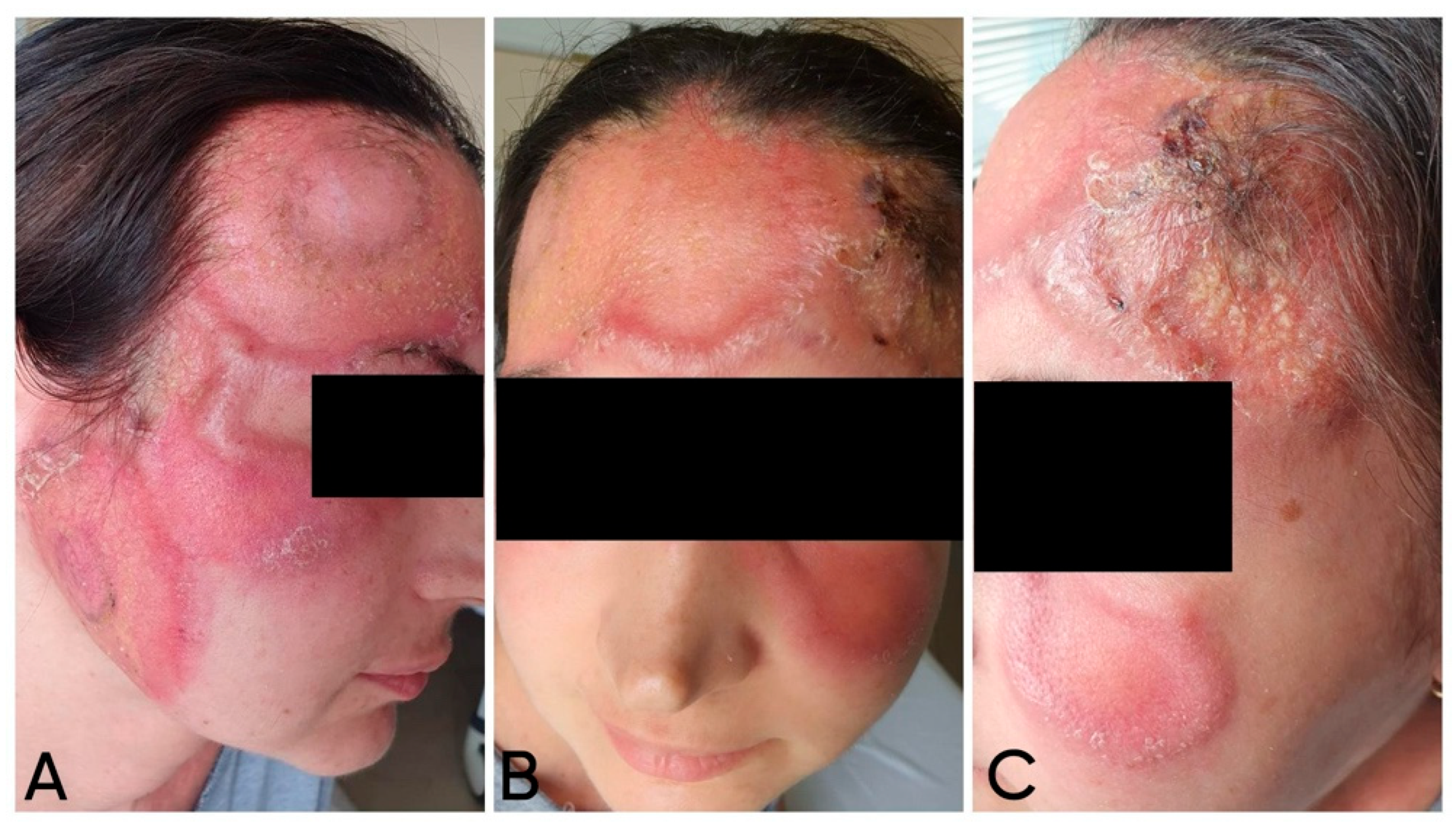
The first skin complaints noted by the patient were two “pustules” (as described and defined by the patient) on her forehead. Despite antibiotic treatment with clindamycin (600 mg orally, three times a day, for 7 days), the lesions progressed—according to the patient, they transformed into “dense blisters”. Later, local treatment was performed by a maxillofacial surgeon, who applied an occlusive dressing twice daily with povidone-iodine solution for 5 days. As a result, the skin changes demonstrated progressive increases in both periphery and height. During a focused interview, the patient reported photosensitivity (
Figure 1).
Given a clinical suspicion of cutaneous tuberculosis, a Mantoux test was performed, yielding a borderline negative result. A skin biopsy revealed findings consistent with “fungal dermatitis” without identification of a specific pathogen. The patient consulted various specialists who prescribed diverse systemic treatments over time, including amoxiclav/clavulonic acid, itraconazole, and betamethasone. Due to a lack of therapeutic response, a parasitological skin examination was performed, in which commensal Demodex folliculorum was identified on a native preparation. Systemic and topical metronidazole treatment was initiated but proved ineffective.
Upon admission to our department for diagnostic clarification, physical examination showed a polymorphic rash involving the central facial area, with near-symmetrical extension to the zygomatic regions. Multiple large erythematous plaques were observed, featuring an active, indurated peripheral border and centrally erythematous skin with scattered pityriasis-like scaling. In some areas, the plaques were confluent, covered with numerous pustules and brownish crusts (
Figure 2).
Complete blood count and differential count revealed erythropenia and lymphocytopenia, decreased monocytes, and leukocytes at the lower limit of the reference range (
Table 1).
Histopathological and immunohistochemical analysis confirmed the suspected diagnosis of discoid lupus erythematosus. Microscopy revealed atrophic stratified squamous keratinizing epithelium, with flattened rete ridges, basal layer vacuolization, focal apoptosis, and a thickened basement membrane (PAS-positive) (
Figure 3 and
Figure 4). The dermis exhibited perivascular and periadnexal lymphoplasmacytic infiltrates, admixed with neutrophils, areas of fibrinoid necrosis of collagen fibers, and basophilic material accumulation (Alcian blue positive; consistent with mucin deposition) (
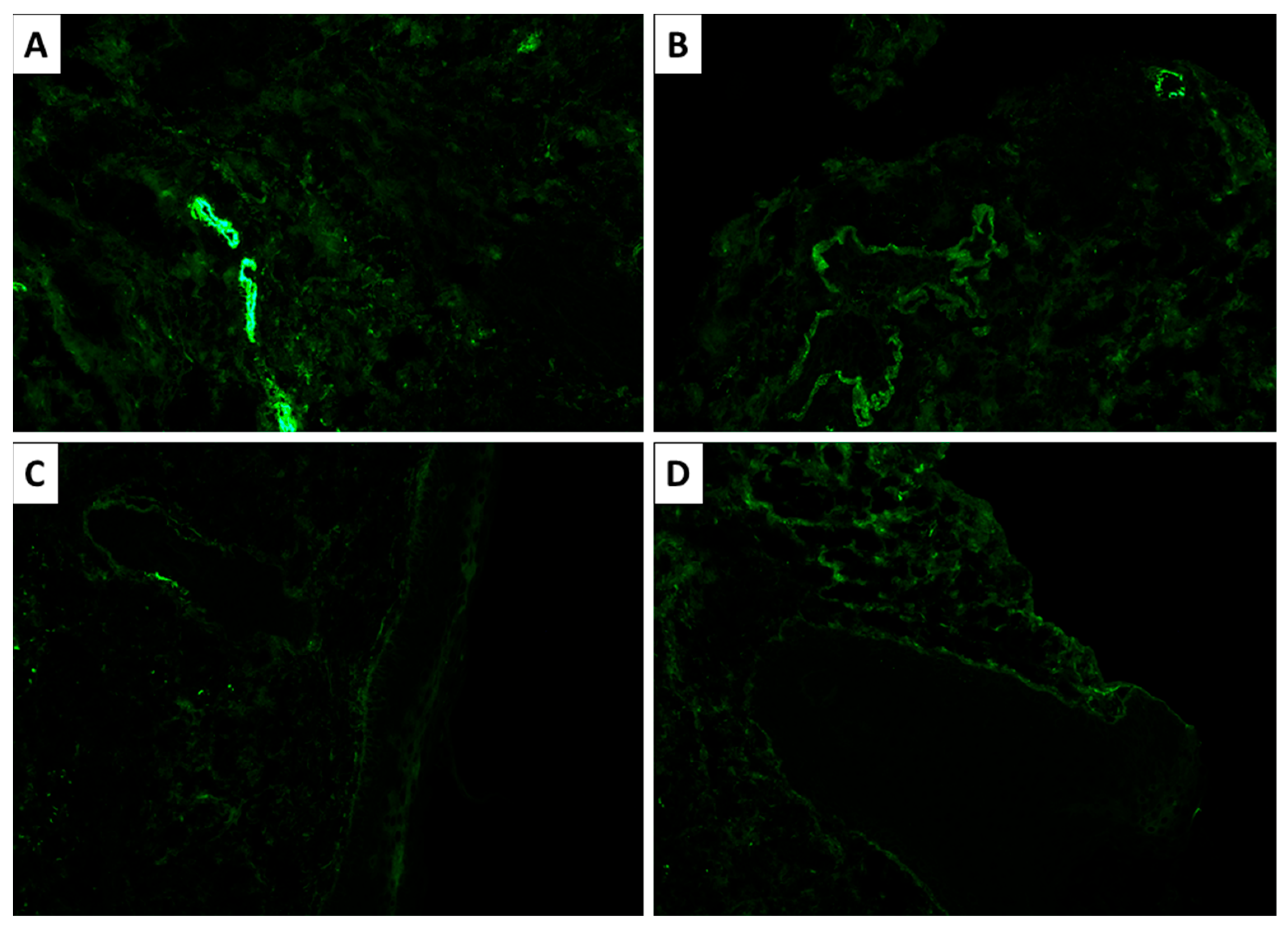
Figure 4). Additional findings included pigment incontinence and follicular plugging. Direct immunofluorescence demonstrated C3 deposition in vascular walls, along with discrete dermoepidermal IgM deposition (
Figure 5).
Due to the pyodermic appearance of the lesions, initial antimicrobial therapy was started with ciprofloxacin, clindamycin, and fluconazole. After receiving the laboratory results, given the severity of the skin lesions and the extremely impaired quality of life of the patient, in accordance with the guideline for the treatment of cutaneous lupus, treatment was initiated with 40 mg of methylprednisolone intravenously, topical corticosteroid cream twice/day, and intensive photoprotection [
18]. During hospitalization, a marked improvement was observed, including the absorption and drying of the purulent lesions, fading of the erythema, and resolution of edema, along with post-lesional hyperpigmentation (
Figure 6). For home treatment, the patient was prescribed 16 mg of methylprednisolone daily, a topical corticosteroid cream, and high-factor sun protection. No further infection workup or antibiotic treatment was prescribed for home care due to the significant improvement in inflammatory parameters upon discharge, as seen in
Table 1.
Given the diagnosed condition, the patient was referred to the Rheumatology Clinic for further monitoring and treatment. Detailed medical history, clinical evaluation, and laboratory tests did not show evidence of SLE and confirmed the diagnosis of a CCLE—DLE. Aberrations were noted in elevated antiphospholipid antibodies, suggesting the presence of antiphospholipid syndrome; however, no specific treatment was initiated, as not all criteria for the syndrome were met (lack of thrombotic events and organ-specific changes such as heart disease, despite her history of two prior miscarriages, as they occurred after the 10th gestational week) (
Table 2). Due to the low antibody titers, lack of other major criteria, and the potential for antibody elevation in the context of inflammation and some treatments, no follow-up titers were tested.
The immunosuppressive therapy was continued with hydroxychloroquine 200 mg (one tablet in the evening); methylprednisolone 4 mg, which was reduced to three tablets in the morning, gradually decreasing by ½ tablet daily over 7 days until reaching a maintenance dose of two tablets daily; and acetylsalicylic acid 75 mg (one tablet in the evening).
One month later, the patient was readmitted to the Rheumatology Clinic due to worsening of her skin condition—redness and edema in the left lacrimal area. Gradually, the edema subsided, and a “red spot” with scaling developed, accompanied by intense itching. She also reported pronounced hair growth in the area of the old lesions. A dermatologic consultation revealed a solitary erythematous-squamous lesion in the left lacrimal area, oval-shaped, approximately 2–3 cm in size. A fungal infection was suspected, possibly triggered by the ongoing treatment with methylprednisolone (two tablets daily) and hydroxychloroquine, or a new flare-up of the underlying disease. The patient was prescribed phenoconazole nitrate cream. Immunosuppressive systemic therapy continued as initially prescribed by the rheumatologists.
During follow-up, with the patient’s general condition deteriorating, she was repeatedly hospitalized in the Rheumatology Clinic and the Internal Medicine Clinic. In her latest hospitalization, she was diagnosed with pulmonary fibrosis in an inflammatory flare, and pulmonary tuberculosis was confirmed via PCR test of sputum. Follow-up Ziehl–Neelsen stain of the original biopsy specimens did not show evidence of acid-fast bacilli. Antituberculosis treatment was initiated, and two years after the initial presentation of the cutaneous lesions, the patient remains under regular follow-up, with only discrete residual hyperpigmentation on the forehead. No further complications suggestive of APS have occurred since the initial workup.
3. Discussion
Cutaneous lupus erythematosus (CLE) is an autoimmune, inflammatory disease of the skin, characterized by a wide range of lesions, including malar rash, discoid plaques, psoriasiform or annular lesions of a polycyclic type, and scarring alopecia [
7,
10]. Based on clinical morphology, histopathology, and lesion duration, CLE is categorized into three major subtypes: acute (ACLE), subacute (SCLE), and chronic (CCLE) [
8].
Discoid lupus erythematosus (DLE) is the most common form of CCLE, comprising 50–80% of chronic cases. Lesions are usually confined to sun-exposed areas such as the face, ears, scalp, and neck. The disease typically begins with erythematous macules or papules that evolve into well-defined, disc-shaped plaques with adherent scales. A hallmark feature is follicular plugging, which may manifest clinically as keratotic spikes revealed upon scale removal—the so-called “carpet tack sign”. Disease progression can lead to permanent follicular destruction and scarring alopecia. Over time, lesions become atrophic, often showing peripheral hyperpigmentation with central depigmentation, leading to irreversible, disfiguring skin changes [
12].
Diagnosis is primarily clinical but is supported by histopathology and direct immunofluorescence in atypical or refractory cases. Histologically, DLE is characterized by hyperkeratosis, interface dermatitis with lymphocytic infiltrates, and basement membrane thickening. Direct immunofluorescence shows deposition of immunoglobulins or complement at the dermoepidermal junction in 50–90% of cases [
13]. Treatment typically involves rigorous photoprotection and topical corticosteroids, with antimalarials and systemic corticosteroids reserved for more severe or treatment-resistant disease [
9].
Although DLE is traditionally considered a skin-limited condition, emerging evidence suggests a capacity for systemic autoimmune activity. One important example is the development of antiphospholipid syndrome (APS).
Several case reports have documented APS arising in patients with longstanding DLE, even in the absence of systemic lupus erythematosus (SLE). A striking case from 2022 described a female patient who developed venous thrombosis and serologic positivity for antiphospholipid antibodies 12 years after the onset of dermatologic symptoms [
15]. This observation aligns with findings from a case–control study showing elevated anticardiolipin antibody levels in patients with DLE compared to healthy controls. In contrast, a cross-sectional study reported a low prevalence of antiphospholipid antibodies, comparable to that in the general population.
These findings support the hypothesis that DLE, although clinically cutaneous, may be immunologically active systemically, potentially predisposing patients to complications like APS [
11]. In the present case, such a complication reinforces the diagnosis of DLE as more than a skin-limited disease.
Due to its ability to mimic a wide range of dermatological and infectious conditions, discoid lupus erythematosus (DLE) is often referred to as “The Great Imitator”, amid many other conditions such as syphilis, tuberculosis, sarcoidosis, and a myriad of tumors, the differential diagnosis of which and the potential for imitation of other conditions is quite broad. This clinical mimicry significantly complicates the diagnostic process and necessitates the consideration of a broad differential diagnosis [
16]. In the present case, several possibilities were considered, including fungal and bacterial infections, demodicosis, and cutaneous tuberculosis—each with its own characteristic yet overlapping clinical features [
17].
Tinea faciei is a superficial dermatophyte infection that typically presents with centrifugally expanding, erythematous, scaly lesions with raised borders. Lesions often involve the cheeks, eyelids, or submandibular area and are usually pruritic. The differential diagnosis should include impetigo, atopic dermatitis, contact dermatitis, discoid lupus erythematosus, and herpes zoster [
1]. In our case, lack of improvement despite antifungal treatment made this diagnosis unlikely [
1].
Another diagnosis that was ruled out was bacterial skin infection (impetigo), which is most commonly caused by Staphylococcus aureus and Streptococcus pyogenes. Impetigo may present with erosions, vesicles, or pustules covered in honey-colored crusts. Bullous forms may exhibit large, thin-walled blisters. Multiple areas may be involved, and a combination of bullous and non-bullous lesions can be present simultaneously [
6]. Negative bacterial cultures and lack of therapeutic response excluded this possibility.
Demodicosis was another suspected disease in the young patient. Caused by Demodex folliculorum, this condition can mimic rosacea, folliculitis, and other facial dermatoses. Three subtypes are recognized: pityriasis folliculorum, rosacea-like demodicosis, and granulomatous demodicosis. Presentation with atypical signs and symptoms that deviate from the classical forms is not uncommon [
2]. Despite positive microscopic detection of Demodex, the lack of improvement following metronidazole therapy suggested it was not the primary cause.
Cutaneous tuberculosis (CTB), though rare (1–1.5% of all extrapulmonary TB cases), remains an important diagnostic consideration in chronic, non-healing facial plaques [
5]. CTB includes entities such as lupus vulgaris, scrofuloderma, and tuberculosis verrucosa cutis. Historically, the term “lupus” originated in the disfiguring ulcerative lesions of facial cutaneous tuberculosis, described by Robert Willan.
In our patient, clinical similarities and a borderline-positive Mantoux test initially raised suspicion for cutaneous tuberculosis. However, histopathological analysis ultimately excluded the diagnosis. Nevertheless, tuberculosis warrants attention not only as part of the differential diagnosis but also as a potential secondary complication in immunosuppressed individuals. In patients with DLE receiving systemic immunomodulatory therapy, the risk of latent tuberculosis reactivation represents a clinically relevant concern. In the present case, this risk manifested as active tuberculosis that emerged during the course of treatment. The presence of radiologically confirmed foci of fibrosis and flares, together with the PCR-proven mycobacterial infection, would indicate that the aggressive immunomodulatory treatment, based on the significance, duration, and flares of DLE in the patient, led to reactivation of latent tuberculosis rather than a new-onset infection.
Immunosuppressive therapy is the cornerstone of treatment for all forms of lupus. While in most cases of cutaneous lupus, patients respond well to topical corticosteroid treatment, in cases of severe, especially long-lasting reactions, systemic administration is required [
22]. As seen in our case, the patient initially responded well to combined systemic and local treatment; however, reducing the systemic corticosteroid dose led to new disease flares, necessitating prolonged, low-dose systemic administration. This treatment is closely linked to the pathogenesis of the condition because discoid lupus, despite being a limited cutaneous form, shares many immunological mechanisms with SLE and, although rare, can progress to SLE [
22].
While broadly following the immunological mechanisms of SLE as an autoimmune reaction, the underlying cause is possibly that of keratinocyte nuclear damage. This mechanism is supported by the association of the condition with photosensitivity, in which UV light damages keratinocyte nuclei, and fragments of these nuclei can, in turn, stimulate an immune response [
23,
24]. These mechanisms would also explain the typical location of DLE, predominantly in the facial area—a topographical zone with significant sunlight exposure [
22]. Alternatively, another nucleus-damaging factor—tobacco smoking—is also closely linked as a triggering factor for DLE. The site-specific immune response of B cells and possibly T cells, as well, based on these triggering factors, can explain some of the aspects that distinguish CCLE from SLE forms, and could underline the nature of DLE as a local reaction to external factors in patients with a genetic predisposition to SLE [
22]. This would also explain the relatively low rate of progression to SLE and the co-occurrence of other immunological conditions, such as APS, as seen in our patient [
15,
22].