Abstract
A paradigm shift is currently taking place in the etiopathogenesis of neuropsychiatric disorders as immunometabolism is replacing the earlier neurotransmitter model. According to the new concept, cellular bioenergetics drives information processing in the central nervous system; therefore, neuropathology is conceptualized as a direct consequence of impaired metabolism. Along the same lines, endoplasmic reticulum stress and gut barrier dysfunction are emerging as novel targets in schizophrenia and affective disorders, linking immune responses to cellular distress. Furthermore, microglia, the brain’s innate immune cells, acquire energy through oxidative phosphorylation, while in the resting state, and glycolysis upon activation, contributing to lactate accumulation and reduced brain pH. The same metabolic signature characterizes neuropsychiatric disorders as the central nervous system derives adenosine triphosphate from aerobic glycolysis, upregulating lactate and generating an acidic environment. Although known for over three decades, the link between dysmetabolism and neuropathology was poorly defined until the discovery of brain-resident innate lymphoid cells, including natural killer cells, and lactylation of histone and nonhistone proteins. In this perspective article, we examine three anti-inflammatory microglial systems relevant for neuropsychiatry: lactate, oxytocin, and the aryl hydrocarbon receptor. We also discuss potential interventions for restoring microglial homeostasis.
1. Introduction
Severe neuropsychiatric illnesses, including schizophrenia and affective disorders, have been associated with brain metabolic disturbances, including excessive aerobic glycolysis and upregulated lactate, emphasizing that in the central nervous system (CNS) bioenergetics and information processing are highly intertwined [1,2,3,4]. Aerobic glycolysis and upregulated lactate, known as the Warburg effect, are the preferred energy sources of cancer cells and likely drive the pathogenesis of neuropsychiatric disorders [5,6]. Although low levels of lactate are neuroprotective, in excess it was demonstrated to induce neuronal apoptosis and decreased glutamatergic and GABAergic neurotransmission, hallmarks of neuropathology [7,8]. Along this line, a novel schizophrenia (SCZ) study has found that oral flora-derived lactate crosses the blood–brain barrier (BBB), generating CNS acidity [9,10].
Microglia are the CNS-resident macrophages known for vigilantly scanning the brain parenchyma, searching for tissue damage, molecular debris, and invading pathogens [10,11]. When changes are detected, microglia become activated and bioenergetically dependent on lactate [10]. Inflamed microglia, a characteristic of several neuropsychiatric disorders, release proinflammatory cytokines and adopt a neurotoxic phenotype [12]. Hence, targeting microglial lactate is an emerging immunometabolic strategy in neuropsychiatric disorders [13,14,15] (see the section on Potential Therapeutic Strategies).
Recent studies have revealed that lactate is an essential CNS metabolite which promotes synaptic plasticity and exerts anti-inflammatory, antipsychotic, and antidepressant properties [16,17,18,19,20]. These are mediated by suppression of nuclear factor kappa B (NF-κB) via lactate signaling with its receptor hydroxycarboxylic acid receptor 1 (HCAR1) (also known as G-protein-coupled receptor 81 (GPR81)) [18]. In addition, lactate promotes axonal myelination as well as the rehabilitation of injured oligodendrocytes (OLs), highlighting a potential treatment modality for multiple sclerosis (MS) [21,22,23].
Lactylation is a post-translational modification (PTM) which utilizes lactate as a substrate; therefore, excessive availability of this metabolite promotes lactylation-mediated pathologies, including malignant transformation, Alzheimer’s disease (AD), and neuropsychiatric disorders [24,25,26,27]. Lysine lactylation (Kla), discovered in 2019 by Yingming Zhao, can be a physiological or pathological process, depending on the lactylation levels, which regulates gene transcription by metabolically reprograming the neurons and glia [28,29] (Figure 1). In addition, the lactylation of nonhistone proteins, such as the high-mobility group box 1 (HMGB1), hypoxia-inducible factor 1 alpha (HIF-1α), and the membrane-organizing extension spike protein (moesin), was associated with tumorigenesis and schizophrenia, further linking dysfunctional lactylation to various pathologies [30,31,32].
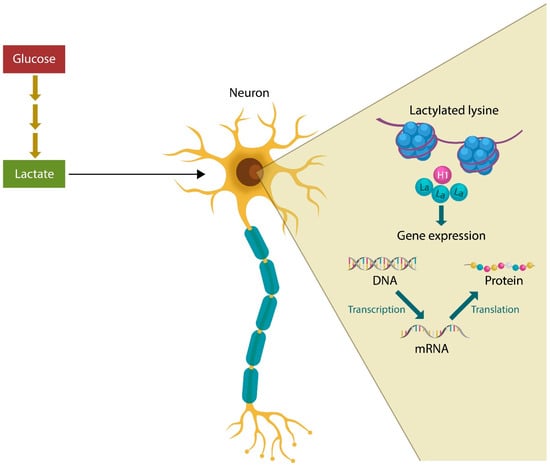
Figure 1.
Excessive lactate derived from glucose metabolism can lead to excessive histone lysine lactylation (Kla), promoting pathologies, such as cancer, AD, schizophrenia, and related neuropsychiatric illnesses. Histone lactylation alters gene expression that in return affects translation and protein synthesis.
Another major discovery took place in 2022, when a subpopulation of oxytocin (OXT)-producing microglia was discovered, revealing a novel anti-inflammatory phenotype of these cells, likely distinct from the resting state [33,34]. Indeed, OXT signaling with OXT receptors (OXTRs), expressed on microglia, astrocytes, and oligodendrocytes, was reported to lower not only neuroinflammation but also depression, suicidal behavior, and psychosis, highlighting new potential pharmacological targets [35,36,37,38,39,40,41,42,43,44]. In addition, OXT promotes myelination and supports the nervous system white matter, likely explaining the reason this hormone was patented as an MS treatment (US patent 3274060A) [38,45,46].
The aryl hydrocarbon receptor (AhR), a cytosolic ligand-activated transcription factor, regulates the expression of numerous genes, including those involved in the response to environmental pollutants, polarization of microglia, myelination, and inflammation [47,48,49]. In the CNS, AhR exerts anti-inflammatory properties on microglia and astrocytes, promoting restorative neurogenesis and post-insult tissue regeneration [50]. However, in ischemic strokes, AhR may activate microglia, triggering inflammation, suggesting that this receptor is not only tissue-specific but also dependent on the pathology type [51]. In addition, AhR modulates meningeal innate lymphoid cells (ILCs), especially the protective CD56bright natural killer cells (NKCs), promoting a tolerant, IL-10-generating phenotype [52,53]. Conversely, dysfunctional NKCs promote neuroinflammation by aberrantly eliminating the anti-inflammatory resting microglia, disrupting immune homeostasis by selectively sparing the activated, inflamed cells [54].
In this perspective article, we take a closer look at three anti-inflammatory microglial systems relevant for neuropsychiatry, lactate, OXT, and AhR, as well as potential interventions for restoring microglial homeostasis.
2. Lactate and Mental Illness
Major neuropsychiatric illnesses, including schizophrenia and affective disorders, have been associated with decreased brain pH, lactic acid accumulation and upregulated glycolysis [1,2,8]. From a bioenergetic perspective, the brain cells of mentally ill individuals resemble cancer cells, as adenosine triphosphate (ATP) is acquired through aerobic glycolysis, or the Warburg effect, decreasing the brain pH, while oxidative phosphorylation (OXPHOS) is downregulated.
Lactate plays a key role in the CNS where it functions as a metabolite as well as a neurotransmitter. Lactate is endowed with anti-inflammatory properties as it inhibits NK-kB, promotes myelination, and regulates microglial function [22,55]. Indeed, microglia possess the molecular machinery to shift metabolism according to phenotype; resting microglia rely on OXPHOS, while activated microglia utilize aerobic glycolysis [23].
Innate lymphoid cells (ILCs) signaling with microglia and the discovery of histone and nonhistone proteins lactylation have contributed to a better understanding of the role of aerobic glycolysis in neuropsychiatric disorders. Although lactate is neuroprotective and mediates synaptic plasticity, excessive lactylation can alter neuronal function, shifting them to an energy state akin to malignant cells [56,57,58] (see section Microglia, lactylation and mental illness). For example, aberrant neuronal cell cycle reentry and aneuploidy have been observed in patients with SCZ, suggesting that lactate triggers “reprimitivization” of brain cells or the acquisition of stem cell properties [30,59,60,61,62,63]. This is further substantiated by the neuronal progenitor cells’ dependency on aerobic glycolysis, suggesting that local acidity promotes undifferentiated cellular phenotypes [64,65]. Indeed, in an acidic environment, somatic mammalian cells could be epigenetically reprogrammed into pluripotent stem cells, while L-lactate has been shown to induce neurogenesis in adult hippocampal neurons [66,67,68]. Moreover, exposed to lactate, cardiomyocytes were demonstrated to reenter the cell cycle in a manner reminiscent of neurons, emphasizing the link between low pH and postmitotic cells’ replication attempts [69].
Moesin is a member of the ezrin-radixin-moesin (ERM) family of proteins which are constituent parts of the cellular cytoskeleton, and thus implicated in mitosis [70]. In AD, moesin activates neuronal cell cycle reentry, indicating that lactylation of this protein may promote neuropsychiatric pathology [71,72,73]. Indeed, moesin was implicated in schizophrenia and plays a major role in the aberrant NKCs activation [74,75,76] (Figure 2).
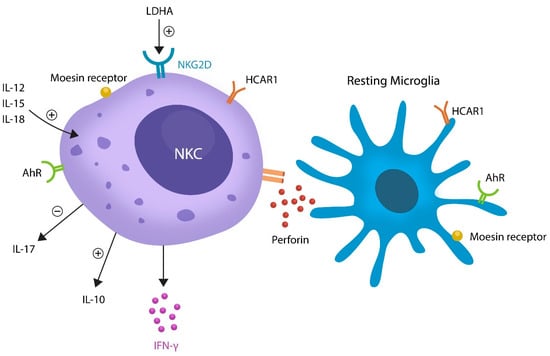
Figure 2.
Aberrant elimination of resting microglia by lactate-activated NKCs. LDHA or LDH-5 can activate NKC via NKG2D receptors. IL-12, IL-15, and IL-18 activate NKCs, facilitating the release of IL-10, IL17, and IFN-γ. NKCs express HCAR1, AhR, and moesin receptors, proteins previously implicated in neuropathology. Activated NKCs release perforin, a toxic molecule known for eliminating resting microglia. Because activated microglia are not killed, this is a likely mechanism of tilting the balance toward inflammation.
Histone Kla, a reversible PTM, is regulated by EP300 and class I histone deacetylases (HDAC1–3), enzymes which add and remove lactate to and from the histone proteins [77,78,79]. Both HDAC1–3 and EP300 have been implicated in SCZ and/or the adverse effects of antipsychotic drugs via the brain-derived neurotrophic factor (BDNF) [80,81,82]. As BDNF gene histone modifications contribute to fear extinction, this system’s malfunction likely triggers Kla-mediated neuropsychiatric symptoms, such as excitation, social defeat, anxiety, and stress [83,84]. Interestingly, the neuroprotective properties of BDNF consist of preventing neuronal cell cycle reentry, further linking attempted mitosis to psychopathology [85].
NKCs are members of ILCs which are activated by lactate and sense pathological changes, migrating to the affected sites, including the brain parenchyma [86]. Lactate-activated NKCs were found to eliminate resting microglia but not the activated phenotype, suggesting that the pathological loss of anti-inflammatory microglia triggers neuroinflammation and the related pathology [54] (Figure 2). Moreover, as activated microglia have been linked to neurotoxicity and demyelination, pathological NKCs may predispose to autoimmune inflammation, including multiple sclerosis (MS) [12,87,88]. This is significant as impaired NKCs were found in the first episode of SCZ in antipsychotic-naïve individuals, suggesting that manipulation of these cells could ameliorate psychotic symptoms [89].
Recent studies have revealed an alternative source of brain lactate, the microbial community residing in various body compartments, including the oral cavity and the GI tract [1,9]. Indeed, the high lactate producers Bifidobacterium and Lactobacillus spp. have been shown to maintain the integrity of intestinal epithelial cells (IECs) and the survival of other commensals [90,91]. However, excessive lactate accumulation in the GI tract can trigger pathology by lowering the production of short-chain fatty acids (SCFA), biomolecules in charge of numerous CNS and peripheral physiological functions [92]. Indeed, the lactate-induced anti-inflammatory and immunosuppressive milieu can predispose to cancer as well as microbial and viral infections [93,94,95]. For example, lactate signaling with microglial HCAR1 promotes tissue regeneration and healing; however, it may also induce pathology by suppressing the phagocytosis of malignant or pathogen-infected cells [94,95,96].
Mental Illness, Lactylation, and Microbes
Histone Kla is not limited to mammals as it was also demonstrated in prokaryotes, linking microbial lactate to host neuropathology [97]. For example, Escherichia coli (E. coli) proteins YiaC and CobB induce histone lactylation, likely explaining the association of this bacterium with schizophrenia [98]. This was exemplified in the 2011 outbreak of E. coli in Germany which was accompanied by psychosis, linking this pathogen to mental illness [99,100]. In another example, urinary tract infection (UTI), caused primarily by E. coli, is known for the potential to trigger new-onset psychosis or the exacerbation of psychotic symptoms in stable patients with schizophrenia, further illustrating the link between this pathogen and mental illness [101,102].
Lactylation of nonhistone proteins, HMGB1, HIF-1α, and moesin, was associated with both schizophrenia and E. coli infection, linking this microbe once more to neuropathology [30,31,103,104,105,106]. Moreover, Toxoplasma gondii, an intracellular protozoan, previously associated with SCZ, is another example of pathogen-mediated lactylation, causing host neuropathology [107]. Indeed, a recent study found that Toxoplasma gondii, containing 523 lactylated proteins, eliminates host inhibitory synapses by aberrantly activating microglia [108,109]. Moreover, Toxoplasma gondii was demonstrated to induce endoplasmic reticulum (ER) stress as well as intestinal barrier disruption, pathologies associated with mental illness [110,111].
Taken together, lactate is a CNS protective metabolite that not only contributes to energy metabolism and signaling but also serves as a substrate for lactylation. Excessive lactate accumulation from local metabolism or the microbiome may trigger lactylation-associated pathology, such as cancer, AD, or neuropsychiatric illness.
3. Oxytocin and Microglia in Mental Illness
Oxytocin (OXT)-oxytocin receptor (OXTR) signaling comprises a microglial anti-inflammatory pathway. OXT is a neuropeptide synthesized in the hypothalamus and stored in the posterior pituitary prior to being released into the systemic circulation. OXT is both a hormone and a neurotransmitter, the former mediating parturition and lactation, while the latter affects social behavior, affiliation, intimacy, and mother–infant bonding [112]. A recent study found that the OXT concentration in the brain can reach levels 1000 times higher than in the systemic circulation, highlighting the importance of this biomolecule for CNS function [113].
Low blood OXT levels were reported in patients with SCZ marked by negative symptoms, while intranasal administration of OXT was shown to ameliorate this condition [114,115]. Indeed, like lactate, OXT modulates synaptic plasticity and upregulates adult hippocampal neurogenesis, enhancing long-term potentiation and memory, properties that likely mediate this hormone’s antipsychotic and antidepressant properties [116,117,118].
OXT decreases microglial inflammation by lowering ER stress via translation initiation factor 2 alpha/transcription factor ATF4 (eIF-2α–ATF4), a common autophagic pathway disrupted in SCZ and major depressive disorder (MDD) [119,120,121]. For example, eIF-2α–ATF4 lowers ER stress by upregulating Sigma-1 receptors (Sig-1Rs), proteins involved in several neuropsychiatric disorders. Indeed, several antidepressant and antipsychotic drugs, including fluvoxamine, sertraline, and haloperidol, lower ER stress, suggesting that the therapeutic properties of these agents could be mediated by eIF-2α–ATF4 [122,123]. Aside from lowering ER stress, Sig-1Rs protect the intestinal barrier and BBB, linking eIF-2α–ATF4 to microbial translocation [124,125]. It stands to reason, therefore, that strengthening the gut barrier and lowering ER stress in intestinal epithelial cells (IECs) are emerging strategies for neuropsychiatric disorders (Table 1) (clinical trials identifier NCT03183609) [126,127,128,129,130,131]).

Table 1.
The action mechanisms of Lactate, OXT, and AhR.
Table 1.
The action mechanisms of Lactate, OXT, and AhR.
| Lactate | OXT | AhR | References |
|---|---|---|---|
| Deactivates microglia by NF-kB inhibition | Deactivates microglia via eIF-2α–ATF4 pathway | Deactivates microglia via NF-kB | [4,15,50] |
| Excess lactate promotes Kla | No known effect on lactylation | Increases LDHA and lactate | [24,132] |
| Lowers ER stress | Lowers ER stress | Lowers ER stress | [16,33,133] |
| Augments gut barrier function | Augments gut barrier | Augments gut barrier | [90,124,134] |
| Upregulated in schizophrenia | Downregulated in schizophrenia | Implicated in schizophrenia | [1,135,136] |
Several studies have connected OXT with the selective serotonin reuptake inhibitors (SSRIs), emphasizing that this hormone may contribute, at least in part, to the action mechanism of antidepressant drugs [137,138]. Moreover, sexual dysfunction, a common SSRI adverse effect, was associated with decreased OXT blood levels, linking this hormone further to the serotonergic system [139,140]. This is significant as OXT exerts antidepressant properties of its own and may be indicated in situations when other antidepressants are to be avoided, such as during pregnancy and the postpartum period as well as in patients with sexual dysfunction.
Aside from the antidepressant action, OXT exerts antipsychotic properties, documented as early as the 1970s and 1980s, followed by clinical trials of intranasal OXT in 2010, studies which have produced encouraging results for schizophrenia patients [35,135,141] (clinical trials identifier NCT01621737).
In 2022, it was discovered that a subpopulation of microglial cells is capable of generating OXT, drawing the attention of researchers and clinicians to the anti-inflammatory properties of these cells [33,34]. Indeed, OXT signaling with OXTR, expressed on microglia, astrocytes, and oligodendrocytes, lowers neuroinflammation, depression, suicidal behavior, and psychosis, indicating that OXT signaling is a significant neuropsychiatric target [35,36,37,38,39,40,41,42,43,44] (see section Microglia, lactylation and mental illness).
Taken together, OXT and lactate exert antipsychotic and antidepressant properties through different mechanisms. Impaired OXT signaling may trigger neuropathology by increasing ER stress in IECs, disrupting the GI tract barrier.
4. Aryl Hydrocarbon Receptor and Mental Illness
ILCs are mucosa-anchored lymphocytes which express transcription factors instead of T- or B-cell receptors; they are activated by specific cytokines and generate their own cytokine output. ILCs, comprised of NKCs, ILCs types 1, 2, 3, and regulatory ILCs (ILCreg), are situated at the biological barriers, including the gut and meninges [142,143]. Human ILCs express AhR which is abundantly represented on CD56bright NKC, emphasizing further the protective role of these lymphocytes [53,144]. Glycolytic enzymes, lactate dehydrogenase A (LDHA) or lactate dehydrogenase-5 (LDH-5), activate NKG2D receptors, regulating CD56bright NKC responses, suggesting that excessive lactate likely disrupts the function of these lymphocytes [144,145].
Aside from AhR and lactate, NKCs, including CD56bright, are activated by IL-12, IL-15, and IL-18, releasing interferon γ (IFN-γ) and IL-10 (Figure 2) [1,2,3,4]. In addition, AhR lowers the proinflammatory IL-17 previously implicated in both SCZ and MS [146,147,148]. Moreover, dysfunctional IL-12, IL-15, IL-18, IFN-γ, and IL-10 have been associated with schizophrenia, highlighting further the key role NKCs in this pathology [149,150]. AhR also suppresses microglial and astrocytic inflammation, switching these cells from inflammatory to the restorative phenotype [51,151,152].
Aberrant elimination of resting microglia by NKCs is likely the root cause of neuroinflammation, documented in many neuropsychiatric disorders; therefore, rescuing these cells should be explored as a therapeutic strategy [53,54]. For example, the expansion of circulating CD56bright NKCs has been associated with improved MS symptoms, suggesting that these cells may also play a beneficial role in SCZ and affective disorders [153,154]. Indeed, a low CD56 lymphocyte count was demonstrated in antipsychotic-naïve patients with schizophrenia, and it was upregulated by treatment, emphasizing the beneficial properties of these cells [134] (for the role of AhR in microglial cells, see the section Microglia, lactylation and mental illness). As CD56bright NKCs express AhR, the clinical symptoms may respond to the pharmacological manipulation of this receptor [155] (discussed in the section on Potential Therapeutic Strategies).
In the gut, AhR protects the intestinal barrier by binding microbiota-derived metabolites as well as the SCFAs, linking dysfunctional AhR signaling to gut barrier disruption and microbial translocation [156,157].
Novel studies have shown that AhR functions in a tissue-specific manner, dependent on the ligand affinity, likely accounting for the variable, often opposite, responses elicited in one organ vs. another [158,159]. For example, endogenous AhR ligands, such as tryptophan and microbial metabolites, may exacerbate breast cancer while exerting salutary effects in colorectal carcinoma [132,160,161,162,163]. Moreover, in the gut, AhR enhances the barrier function while inducing liver toxicity, illustrating the difficulties encountered in trying to develop AhR therapeutics. Having said that, selective modulators, such as flavonoids and isoflavones, may offer a solution [164,165,166,167] (Table 2) (see the section on potential interventions).

Table 2.
Potential interventions for restoring the homeostasis of anti-inflammatory microglia.
AhR promotes myelination and lowers ER stress, suggesting that MS, MDD, schizophrenia, and bipolar disorder may be exacerbated by the dysfunction of this protein [133,186]. Interestingly, carbidopa and the unique antipsychotic drug clozapine are AhR agonists, linking this receptor to the dopaminergic system and probably explaining both the efficacy as well as the side effects of these agents [187,188]. Moreover, dopamine is an AhR ligand, further implicating this receptor in mental illness [136]. Indeed, AhR was implicated in schizophrenia by the earlier studies, emphasizing a novel psychopharmacological target [189]. In a rodent model, clozapine attenuates experimental autoimmune encephalomyelitis (EAE), suggesting that the unique anti-suicide properties of this drug (not shared with other antipsychotic agents) may be mediated by AhR [190]. Yet another study saw that agranulocytosis, an autoimmune-like adverse effect of clozapine, was associated with DRB1*15:01, a rare human leukocyte antigen (HLA), and a major MS risk factor, linking agranulocytosis to this genetic marker [191,192].
Taken together, AhR exerts antidepressant and antipsychotic properties by several mechanisms: 1. microglial deactivation, 2. lowering ER stress, 3. enhancing the gut barrier, 4. upregulating lactate, and 5. myelination. These protective effects may explain the unique clozapine properties (Table 1).
5. Microbial Translocation Outside the GI Tract
In our previous work, we have discussed microbial translocation from the GI tract into the host systemic circulation, suggesting that the pathogenesis of several idiopathic diseases could be explained by the immune responses to extraintestinal microbial proteins [193]. As higher gut and BBB permeability markers, including the soluble form of CD14 (sCD14), were documented in neuropsychiatric illness, this pathology may, at least in part, result from microbial migration through the gut barrier [194,195,196].
Various antigens of GI tract microbes, including Bacteroides, Bifidobacterium, Acinetobacter, and Pseudomonas, have been shown to exhibit molecular mimicry with the host myelin basic protein (MBP), suggesting that autoantibodies against myelin, documented in MS, schizophrenia, and bipolar disorder, may be conventional immunoglobulins directed at the displaced microbes or their components [197,198,199]. In addition, as myelin contains a serotonin binding site, the autoantibodies against serotonin, found in MS and MDD, may be classical antibodies against serotonin-producing microbes, such as Clostridia or staphylococci [200,201]. Furthermore, bacterial N-acetylmuramyl dipeptide was reported to mimic myelin, likely triggering antibodies upon translocation into host tissues [202]. These findings are significant as they emphasize that strengthening the gut barrier to lower microbial migration likely comprises a novel therapeutic strategy in neuropsychiatric disorders. In fact, inflammation in response to translocated microbial proteins was previously associated with suicidal behavior, suggesting that gut barrier restoration should be instituted as a therapy in mental illness [203,204,205]. Indeed, some antidepressant drugs currently utilized in clinical practice were demonstrated to optimize the gut barrier function, suggesting an alternative, noncanonical, mechanism of action [206]. Microbial translocation across the gut barrier has been documented in MDD, suicidal behavior, bipolar disorder, aggression in SCZ, and neurodegeneration, suggesting that the loss of anti-inflammatory microglia plays a crucial role in neuropathology [196,202,203,204,205,207,208].
6. Potential Therapeutic Strategies
Lactate, OXT, and AhR are therapeutic targets rarely considered in the treatment of neuropsychiatric disorders; however, they play a major role in the CNS and immune metabolism.
7. Lactate
Novel studies have shown that peripheral administration of L-lactate exerts antidepressant effects by increasing hippocampal lactic acid which upregulates neurogenesis and inhibits microglial activation [168,209] (Table 2).
- 1.
- Lactylation inhibitors are compounds belonging to several classes of therapeutics, such as LDHA inhibitors, demethylzeylasteral (DML), and phytotherapeutics, including polyphenols. Here, we review only the agents that can cross the BBB, lower ER stress, and strengthen the intestinal barrier [210,211]. These are as follows.
- 2.
- Sirtuins
Studies in oncology have reported that sirtuins (SIRT), especially SIRT2, can remove lactate from the histone lysine, showing that de-lactylation may comprise a novel strategy for microglial depolarization, an approach likely beneficial in MDD and SCZ [170]. The effect of SIRT in these disorders has been documented by earlier research [212,213,214].
Demethylzeylasteral (DML) inhibits lactylation of histone H3, a protein associated with MDD, suggesting that it could exert antidepressant properties [172,215].
- 3.
- LDH inhibitorsLactate-HCAR1 signaling is regulated by LDHA or LDH-5, glycolytic enzymes upregulated by psychological stress, suggesting that inhibiting these enzymes could be therapeutic for neuropsychiatric illness [216]. LDH inhibitors, include the following:
- 3.1.
- Sodium oxamate, a structural analog of pyruvate, is known for lowering ER stress by promoting protective autophagy, properties beneficial for neuropsychiatric disorders [177,217]. To the best of our knowledge this compound has not been evaluated for psychiatric conditions.
- 3.2.
- Chinese Gallnut Extract from Galla chinensis is a traditional Chinese medicine and a potent LDHA inhibitor which was patented for the treatment of MDD and is currently in clinical trials (Chinese patent CN102836354A) (US patent 10098854B2) (clinical trials identifier NCT04080752) [180,218].
- 3.3.
- Galloflavin or NSC 107022 is a polyphenol found in berries which upregulates SIRT6, a key regulator of neuronal mitochondria [183,219]. Dysfunctional SIRT6 was associated with depression-like behavior in mice, suggesting potential benefits for humans with MDD [220]. Galloflavin has been patented as a modulator of protein kinases that can treat a variety of conditions, ranging from cancer to neuropsychiatric disorders (US patent 20030187007A1).
8. OXT
OXT signaling with OXTRs has been shown to inhibit activated microglia and increase the number of NKCs, a phenomenon documented in schizophrenia [221]. Recently, an OXT-generating microglial population was identified, suggesting that anti-inflammatory microglia may regulate many physiological functions that could cause pathology when disrupted [222,223].
8.1. Angiotensin IV (ANG IV) as an OXT Agonist
Several studies demonstrated that microglia express a functional renin-angiotensin system (RAS), including angiotensin II type 1 receptors (AT-1Rs) [224]. Conversely, AT-1R blockers (ARBs), especially the BBB-crossing candesartan, have been found therapeutic in schizophrenia and MDD, linking dysfunctional RAS to inflamed microglia [171]. This is significant as angiotensin II (ANG II) is a negative OXT regulator; thus, ARBs may not only depolarize these cells but also enhance OXT release [33,34,224,225]. Indeed, angiotensin IV was reported to upregulate OXT in vitro and in vivo, probably by inhibiting oxytocinase, the hydrolyzing enzyme of this hormone [226].
OXT inhibits eIF-2α–ATF4 signaling, a pathway implicated in ER stress, suggesting that the beneficial neuropsychiatric effects of OXT can likely be attributed to autophagy [44]. Indeed, the antiretroviral drug nelfinavir has been shown to promote ER stress by eIF2α phosphorylation; in contrast, the integrated stress response inhibitor (ISRIB), a protein with antidepressant and antipsychotic properties, inhibits nelfinavir-induced ER stress via eIF2α dephosphorylation [173]. The related drugs, p21-activated kinase (PAK) inhibitors, were found beneficial in SCZ, emphasizing new potential targets in this disorder [175]. IRIB was patented in Australia as a broad therapeutic agent that may be useful in pathologies involving learning, memory, immunity, intermediary metabolism, insulin production, and resistance to unfolded protein stress (Australian patent 2020229748A1).
8.2. OXT Fragments
OXT, comprised of nine amino acids, was found to retain biological properties even when cleaved in shorter segments. For example, melanocyte-inhibiting factor-1 (MIF-1), the N-terminal segment of oxytocin, possesses antidepressant and antipsychotic properties, suggesting that OXTR can be activated by related small ligands [178,227]. MIF-1 was patented for cancer, autoimmune diseases, fibrotic diseases, inflammation, and neurodegenerative diseases (international patent WO2009040045A2). Several OXT-like molecules have also been studied, including lipo-oxytocin-1 (LOT-1), a synthetic OXTR agonist (also known as TC OT 39), and WAY-267464, an OXTR agonist and vasopressin 1 receptor (V1AR) antagonist [181,184]. The efficacy of these ligands for neuropsychiatric disorders has not been evaluated in clinical trials; however, several patents were obtained for use in neurological and psychiatric disorders (international patent WO2018107216A1) (EP2326341B1).
9. AhR
AhR binds diverse ligands derived from the environment, diet, microbes, and cellular metabolism, enabling cells to adapt to the changing exogenous or endogenous conditions. Drugs, chemicals, and plant-derived compounds, including isoflavones, attach with different affinities to AhR [228]. Nattokinase, a soybean fermented by Bacillus subtilis var. natto, contains AhR-binding flavonoids [169]. Indeed, several isoflavones, including, soybeans, genistein, daidzein, glycitein, formononetin, biochanin, and phytoestrogens, have been found to exert antidepressant and antipsychotic properties [174,229,230].
- -
- Laquinimod, a quinolone-3-carboxamide developed for the treatment of MS, is a selective AhR ligand that demonstrated encouraging results in animal models of MDD and anxiety [176].
- -
- CH223191 is an antagonistic selective AhR ligand with a favorable profile for neuropsychiatric disorders [179]. This compound was patented in the US for cancer treatment but has still to enter clinical trials for neuropsychiatric disorders (US patent 20160175278A1).
- -
- Sumatriptan, a selective 5-hydroxy-triptamine (5-HT1) receptor agonist, activates human AhR, exerting anti-inflammatory properties, suggesting beneficial effects on activated microglia [182,231].
- -
- Dimethyl fumarate, indicated for MS, is not an AhR ligand; however, it upregulates the protective AhR-expressing CD56bright NKCs which eliminate the detrimental autoreactive immune cells to avert autoimmunity [232,233]. For this reason, CD56bright NKCs expansion via AhR or transplantation may comprise a potential therapeutic strategy for neuropsychiatric illness. For example, CD56bright NK cells allogeneic or stem cell transplantation, a treatment used for acute myeloid leukemia, could be therapeutic in both MS and schizophrenia [185,234].
10. Microglia, Lactylation, and Mental Illness
Although microglia comprise less than 10% of the brain cells, it plays a major role in neuropsychiatric pathology [235]. Resting microglia are neuroprotective as evidenced by in vivo two-photon imaging of fluorescent-labeled neurons and microglia, demonstrating that these cells participate in the plasticity of neuronal circuits [236]. Resting microglia actively communicate with other brain cells, including astrocytes, neurons, and ILCs [237,238,239]. Recent studies have shown that microglia express monocarboxylate transporters (MCTs) and HCAR1, suggesting that lactate plays an important role in the reprograming of these cells [240,241]. Numerous studies have found that young and healthy brains rely on glycolysis and lactate, while aging brains lose glycolytic ability [242]. For example, dysfunctional glycolysis was associated with AD pathology as well as schizophrenia [243,244]. Excessive lactate generation contributes to lactylation of histone and nonhistone proteins, engendering pathology [245].
Microglia are brain resident macrophages that express OXT receptors, suggesting that these proteins contribute to anti-inflammatory microglia, a likely protection against mental illness [246,247].
Microglia express AhR, proteins involved in several pathologies, including glioblastoma [248]. AhR is a new receptor of interest in schizophrenia, not only because it binds dopamine but also because schizophrenia-associated exogenous toxins, such as plasticizers, are ligands at this receptor [249,250]. In addition, many protective metabolites synthesized by gut microbes, including indole and tryptophan, are AhR ligands, suggesting that the gut–brain axis likely operates through this receptor [251].
It has been reported that aberrantly activated microglia become neurotoxic and engage in the elimination of healthy neurons or synapses, a pathology involved in schizophrenia [252,253,254].
11. Limitations
Many compounds described above are phytotherapeutics, primarily natural products, such as sirtuins, or traditional foods, including nattokinase and the Chinese Gallnut Extract, that have been used by humans for hundreds of years. The ability of these molecules to act as AhR ligands suggests beneficial effects in neuropsychiatric illness, and clinical trials should be initiated to assess their efficacy. Likewise, the ability of sirtuins to remove histone lysine lactate has reawakened the interest in these compounds, previously studied for neurodegenerative disorders, as potential medical foods or dietary interventions.
Furthermore, several compounds mentioned above have been patented in different countries and some are currently in clinical trials, while others have not reached that stage yet. However, the profile of these agents, estimated by their action mechanism described in the patent application, suggests that they should be evaluated as antidepressant or antipsychotic agents. For example, dimethyl fumarate was patented in Europe for the treatment of MS; however, as this drug exerts neuroprotective as well as anti-inflammatory effects on astrocytes and microglia, it likely possesses antidepressant and antipsychotic properties which should be evaluated in clinical trials.
Additionally, we need to state that most of the pathophysiological mechanisms described in this article are newly elucidated. As the details become increasingly available, it would be much easier to prioritize different potential therapeutic targets. Moreover, understanding these mechanisms will eventually allow for an in-depth stratification of neuropsychiatric patients, and a move toward an improved precision medicine approach.
12. Conclusions
Increasing awareness of the shortcomings of the neurotransmitter paradigm contributed to the development of a new concept, metabolism-driven information processing. This model, illustrated by the previously known association of neuropsychiatric pathology with excessive aerobic glycolysis, was put in perspective by the discovery of ILCs and lactylation. The consequences of excessive brain lactate and increased local acidity include the following:
- The aberrant elimination of anti-inflammatory resting microglia by pathologically activated NKCs.
- The reactivation of the neuronal cell cycle with resultant aneuploidy or apoptosis as these cells lack the molecular machinery to complete mitosis.
- Several interventions may restore the homeostasis of anti-inflammatory microglia, including 1. lowering lactylation, 2. enhancing OXT signaling, 3. modulating AhR, and 4. expanding CD56NKCs. These strategies lower the ER stress and strengthen the gut barrier, limiting microbial migration outside of the GI tract, thus decreasing the risk of microglial activation by translocated bacterial antigens.
More studies are needed to clarify the role of lactylation of histone and nonhistone proteins in mental illnesses and the interaction of lactylation with meningeal ILCs.
Where authors are identified as personnel of the International Agency for Research on Cancer/WHO, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/WHO.
Author Contributions
Conceptualization, A.S., C.K. and Z.K.; methodology, C.V.A.; formal analysis, J.J.A. and C.V.A.; data curation, J.J.A.; writing—original draft preparation, A.S.; writing—review and editing, C.K. and Z.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Where authors are identified as personnel of the International Agency for Research on Cancer/WHO, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/WHO.
Abbreviations
NF-κB = nuclear factor kappa B; HCAR1 = hydroxycarboxylic acid receptor 1; GPR81 = G-protein-coupled receptor 81; Kla = lysine lactylation; OXT = oxytocin; OXTRs = receptors; AhR = aryl hydrocarbon receptor; NKCs = natural killer cells; ERM = ezrin-radixin-moesin; HDAC1 = class I histone deacetylases; ILC = innate lymphoid cells; HMGB1 = high-mobility group box 1; HIF-1α = hypoxia-inducible factor 1α; BDNF = brain-derived neurotrophic factor; IEC = intestinal epithelial cells; SCFA = short-chain fatty acids; ISRIB = integrated stress response inhibitor; MIF-1 = melanocyte-inhibiting factor-1; OXPHOS = oxidative phosphorylation; V1AR = vasopressin 1 receptor; SCZ = schizophrenia.
References
- Rowland, L.M.; Pradhan, S.; Korenic, S.; Wijtenburg, S.A.; Hong, L.E.; Edden, R.A.; Barker, P.B. Elevated brain lactate in schizophrenia: A 7 T magnetic resonance spectroscopy study. Transl. Psychiatry 2016, 6, e967. [Google Scholar] [CrossRef] [PubMed]
- Dogan, A.E.; Yuksel, C.; Du, F.; Chouinard, V.-A.; Öngür, D. Brain lactate and pH in schizophrenia and bipolar disorder: A systematic review of findings from magnetic resonance studies. Neuropsychopharmacology 2018, 43, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.R.; Mielnik, C.A.; Funk, A.; O’Donovan, S.M.; Bentea, E.; Pletnikov, M.; Ramsey, A.J.; Wen, Z.; Rowland, L.M.; McCullumsmith, R.E. Measurement of lactate levels in postmortem brain, iPSCs, and animal models of schizophrenia. Sci. Rep. 2019, 9, 5087. [Google Scholar] [CrossRef]
- Pruett, B.S.; Meador-Woodruff, J.H. Evidence for altered energy metabolism, increased lactate, and decreased pH in schizophrenia brain: A focused review and meta-analysis of human postmortem and magnetic resonance spectroscopy studies. Schizophr. Res. 2020, 223, 29–42. [Google Scholar] [CrossRef]
- Vallée, A.; Vallée, J.-N. Warburg effect hypothesis in autism Spectrum disorders. Mol. Brain 2018, 11, 1. [Google Scholar] [CrossRef]
- Park, H.-J.; Choi, I.; Leem, K.-H. Decreased Brain pH and Pathophysiology in Schizophrenia. Int. J. Mol. Sci. 2021, 22, 8358. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Qin, Q.; Wang, D.; Zhao, J.; Gao, H.; Yuan, X.; Zhang, J.; Zou, Y.; Mao, Z.; et al. Upregulated hexokinase 2 expression induces the apoptosis of dopaminergic neurons by promoting lactate production in Parkinson’s disease. Neurobiol. Dis. 2022, 163, 105605. [Google Scholar] [CrossRef]
- Hollnagel, J.-O.; Cesetti, T.; Schneider, J.; Vazetdinova, A.; Valiullina-Rakhmatullina, F.; Lewen, A.; Rozov, A.; Kann, O. Lactate Attenuates Synaptic Transmission and Affects Brain Rhythms Featuring High Energy Expenditure. iScience 2020, 23, 101316. [Google Scholar] [CrossRef]
- Krzyściak, W.; Karcz, P.; Bystrowska, B.; Szwajca, M.; Bryll, A.; Śmierciak, N.; Ligęzka, A.; Turek, A.; Kozicz, T.; Skalniak, A.E.; et al. The Association of the Oral Microbiota with the Effects of Acid Stress Induced by an Increase of Brain Lactate in Schizophrenia Patients. Biomedicines 2023, 11, 240. [Google Scholar] [CrossRef]
- Ghosh, S.; Castillo, E.; Frias, E.S.; Swanson, R.A. Bioenergetic regulation of microglia. Glia 2017, 66, 1200–1212. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Tauffenberger, A.; Fiumelli, H.; Almustafa, S.; Magistretti, P.J. Lactate and pyruvate promote oxidative stress resistance through hormetic ROS signaling. Cell Death Dis. 2019, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Monsorno, K.; Buckinx, A.; Paolicelli, R.C. Microglial metabolic flexibility: Emerging roles for lactate. Trends Endocrinol. Metab. 2022, 33, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Karnib, N.; El-Ghandour, R.; El Hayek, L.; Nasrallah, P.; Khalifeh, M.; Barmo, N.; Jabre, V.; Ibrahim, P.; Bilen, M.; Stephan, J.S.; et al. Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacology 2019, 44, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Carrard, A.; Elsayed, M.; Margineanu, M.; Boury-Jamot, B.; Fragnière, L.; Meylan, E.M.; Petit, J.-M.; Fiumelli, H.; Magistretti, P.J.; Martin, J.-L. Peripheral administration of lactate produces antidepressant-like effects. Mol. Psychiatry 2016, 23, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Fan, M.; Wang, X.; Xu, J.; Wang, Y.; Tu, F.; Gill, P.S.; Ha, T.; Liu, L.; Williams, D.L.; et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 2021, 29, 133–146. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, H.; Liu, G.; Wu, J.; Yuan, Y.; Shang, A. Tumor Microenvironment: Lactic Acid Promotes Tumor Development. J. Immunol. Res. 2022, 2022, 3119375. [Google Scholar] [CrossRef]
- Ishihara, S.; Hata, K.; Hirose, K.; Okui, T.; Toyosawa, S.; Uzawa, N.; Nishimura, R.; Yoneda, T. The lactate sensor GPR81 regulates glycolysis and tumor growth of breast cancer. Sci. Rep. 2022, 12, 6261. [Google Scholar] [CrossRef]
- Ichihara, Y.; Doi, T.; Ryu, Y.; Nagao, M.; Sawada, Y.; Ogata, T. Oligodendrocyte Progenitor Cells Directly Utilize Lactate for Promoting Cell Cycling and Differentiation. J. Cell. Physiol. 2016, 232, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Cerexhe, L.; Easton, C.; Macdonald, E.; Renfrew, L.; Sculthorpe, N. Blood lactate concentrations during rest and exercise in people with Multiple Sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2021, 57, 103454. [Google Scholar] [CrossRef] [PubMed]
- Keytsman, C.; Hansen, D.; Wens, I.; Eijnde, B.O. Exercise-induced lactate responses in Multiple Sclerosis: A retrospective analysis. Neurorehabilitation 2019, 45, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.-Y.; He, L.; Zhang, J.; Liu, X.; Liao, Y.; Gao, J.; Liao, Y.; Yan, Y.; Li, Q.; Zhou, X.; et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell Metab. 2022, 34, 634–648.e6. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, H.; Liu, M.; Zhou, T.; Cheng, X.; Huang, W.; Cao, L. The role and mechanism of histone lactylation in health and diseases. Front. Genet. 2022, 13, 949252. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218, Erratum in Trends Biochem. Sci. 2016, 41, 287. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, H. Microglial lactate metabolism as a potential therapeutic target for Alzheimer’s disease. Mol. Neurodegener. 2022, 17, 36. [Google Scholar] [CrossRef]
- Sabari, B.R.; Zhang, D.; Allis, C.D.; Zhao, Y. Metabolic regulation of gene expression through histone acylations. Nat. Rev. Mol. Cell Biol. 2017, 18, 90–101. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Okazaki, S.; Boku, S.; Otsuka, I.; Mouri, K.; Aoyama, S.; Shiroiwa, K.; Sora, I.; Fujita, A.; Shirai, Y.; Shirakawa, O.; et al. The cell cycle-related genes as biomarkers for schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 70, 85–91. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Z.; Yu, Y.; Zhang, P. HIF1α lactylation enhances KIAA1199 transcription to promote angiogenesis and vasculogenic mimicry in prostate cancer. Int. J. Biol. Macromol. 2022, 222, 2225–2243. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Yang, H. HMGB1 is a critical molecule in the pathogenesis of Gram-negative sepsis. J. Intensive Med. 2022, 2, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.Y.; Tamir, H.; Ludwig, R.J.; Glickstein, S.B.; Welch, M.G. Colostrum oxytocin modulates cellular stress response, inflammation, and autophagy markers in newborn rat gut villi. Biochem. Biophys. Res. Commun. 2017, 487, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Maejima, Y.; Yokota, S.; Ono, T.; Yu, Z.; Yamachi, M.; Hidema, S.; Nollet, K.E.; Nishimori, K.; Tomita, H.; Yaginuma, H.; et al. Identification of oxytocin expression in human and murine microglia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 119, 110600. [Google Scholar] [CrossRef]
- Pedersen, C.A.; Gibson, C.M.; Rau, S.W.; Salimi, K.; Smedley, K.L.; Casey, R.L.; Leserman, J.; Jarskog, L.F.; Penn, D.L. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr. Res. 2011, 132, 50–53. [Google Scholar] [CrossRef]
- Macdonald, K.; Feifel, D. Oxytocin in schizophrenia: A review of evidence for its therapeutic effects. Acta Neuropsychiatr. 2012, 24, 130–146. [Google Scholar] [CrossRef]
- Caldwell, H.K.; Stephens, S.L.; Young, W.S. Oxytocin as a natural antipsychotic: A study using oxytocin knockout mice. Mol. Psychiatry 2009, 14, 190–196. [Google Scholar] [CrossRef]
- Jahangard, L.; Shayganfard, M.; Ghiasi, F.; Salehi, I.; Haghighi, M.; Ahmadpanah, M.; Bahmani, D.S.; Brand, S. Serum oxytocin concentrations in current and recent suicide survivors are lower than in healthy controls. J. Psychiatr. Res. 2020, 128, 75–82. [Google Scholar] [CrossRef]
- Panaro, M.A.; Benameur, T.; Porro, C. Hypothalamic Neuropeptide Brain Protection: Focus on Oxytocin. J. Clin. Med. 2020, 9, 1534. [Google Scholar] [CrossRef]
- Parris, M.S.; Grunebaum, M.F.; Galfalvy, H.C.; Andronikashvili, A.; Burke, A.K.; Yin, H.; Min, E.; Huang, Y.-Y.; Mann, J.J. Attempted suicide and oxytocin-related gene polymorphisms. J. Affect. Disord. 2018, 238, 62–68. [Google Scholar] [CrossRef]
- Baudon, A.; Creusot, E.C.; Althammer, F.; Schaaf, C.P.; Charlet, A. Emerging role of astrocytes in oxytocin-mediated control of neural circuits and brain functions. Prog. Neurobiol. 2022, 217, 102328. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, J.; Chatzittofis, A.; Hellström, C.; Nordström, P.; Uvnäs-Moberg, K.; Åsberg, M. Low CSF oxytocin reflects high intent in suicide attempters. Psychoneuroendocrinology 2012, 37, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, S.; Bai, X.; Gao, Y.; Liu, G.; Wang, X.; Liu, D.; Li, T.; Hao, A.; Wang, Z. Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J. Neuroinflammation 2016, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.U.; Miwa, H.; Hori, K.; Kaneko, R.; Morimoto, Y.; Koike, E.; Asami, J.; Kamijo, S.; Yamada, M.; Hoshino, M.; et al. Targeting Neurons with Functional Oxytocin Receptors: A Novel Set of Simple Knock-In Mouse Lines for Oxytocin Receptor Visualization and Manipulation. Eneuro 2022, 9, ENEURO.0423-21.2022. [Google Scholar] [CrossRef] [PubMed]
- Mairesse, J.; Zinni, M.; Pansiot, J.; Hassan-Abdi, R.; Demene, C.; Colella, M.; Charriaut-Marlangue, C.; Novais, A.R.B.; Tanter, M.; Maccari, S.; et al. Oxytocin receptor agonist reduces perinatal brain damage by targeting microglia. Glia 2018, 67, 345–359. [Google Scholar] [CrossRef] [PubMed]
- De Cagna, F.; Fusar-Poli, L.; Damiani, S.; Rocchetti, M.; Giovanna, G.; Mori, A.; Politi, P.; Brondino, N. The Role of Intranasal Oxytocin in Anxiety and Depressive Disorders: A Systematic Review of Randomized Controlled Trials. Clin. Psychopharmacol. Neurosci. 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Sorg, O. AhR signalling and dioxin toxicity. Toxicol. Lett. 2014, 230, 225–233. [Google Scholar] [CrossRef]
- Climaco-Arvizu, S.; Domínguez-Acosta, O.; Cabañas-Cortés, M.A.; Rodríguez-Sosa, M.; Gonzalez, F.J.; Vega, L.; Elizondo, G. Aryl hydrocarbon receptor influences nitric oxide and arginine production and alters M1/M2 macrophage polarization. Life Sci. 2016, 155, 76–84. [Google Scholar] [CrossRef]
- Sahebnasagh, A.; Hashemi, J.; Khoshi, A.; Saghafi, F.; Avan, R.; Faramarzi, F.; Azimi, S.; Habtemariam, S.; Sureda, A.; Khayatkashani, M.; et al. Aromatic hydrocarbon receptors in mitochondrial biogenesis and function. Mitochondrion 2021, 61, 85–101. [Google Scholar] [CrossRef]
- Di Giaimo, R.; Durovic, T.; Barquin, P.; Kociaj, A.; Lepko, T.; Aschenbroich, S.; Breunig, C.T.; Irmler, M.; Cernilogar, F.M.; Schotta, G.; et al. The Aryl Hydrocarbon Receptor Pathway Defines the Time Frame for Restorative Neurogenesis. Cell Rep. 2018, 25, 3241–3251.e5. [Google Scholar] [CrossRef]
- Tanaka, M.; Fujikawa, M.; Oguro, A.; Itoh, K.; Vogel, C.F.A.; Ishihara, Y. Involvement of the Microglial Aryl Hydrocarbon Receptor in Neuroinflammation and Vasogenic Edema after Ischemic Stroke. Cells 2021, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, S.; Hu, S.; Yang, B.; Zhang, H. Host-microbiota interactions: The aryl hydrocarbon receptor in the acute and chronic phases of cerebral ischemia. Front. Immunol. 2022, 13, 967300. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Nieves, U.Y.; Mundy, D.C.; Shin, J.H.; Tam, K.; Sunwoo, J.B. The aryl hydrocarbon receptor modulates the function of human CD56bright NK cells. Eur. J. Immunol. 2018, 48, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Lünemann, A.; Lünemann, J.D.; Roberts, S.; Messmer, B.; da Silva, R.B.; Raine, C.S.; Münz, C. Human NK Cells Kill Resting but Not Activated Microglia via NKG2D- and NKp46-Mediated Recognition. J. Immunol. 2008, 181, 6170–6177. [Google Scholar] [CrossRef]
- Peter, K.; Rehli, M.; Singer, K.; Renner-Sattler, K.; Kreutz, M. Lactic acid delays the inflammatory response of human monocytes. Biochem. Biophys. Res. Commun. 2015, 457, 412–418. [Google Scholar] [CrossRef]
- Hagihara, H.; Shoji, H.; Otabi, H.; Toyoda, A.; Katoh, K.; Namihira, M.; Miyakawa, T. Protein lactylation induced by neural excitation. Cell Rep. 2021, 37, 109820. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, D.; Jiang, Y.; Hou, J.; Tian, M.; Li, J.; Sun, L.; Zhang, Y.; Zhang, T.; Li, Z.; et al. Lactate Modulates Cellular Metabolism Through Histone Lactylation-Mediated Gene Expression in Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 647559. [Google Scholar] [CrossRef]
- Mertens, J.; Herdy, J.R.; Traxler, L.; Schafer, S.T.; Schlachetzki, J.C.M.; Böhnke, L.; Reid, D.A.; Lee, H.; Zangwill, D.; Fernandes, D.P.; et al. Age-dependent instability of mature neuronal fate in induced neurons from Alzheimer’s patients. Cell Stem Cell 2021, 28, 1533–1548.e6. [Google Scholar] [CrossRef]
- Yurov, Y.B.; Vorsanova, S.G.; Demidova, I.; Kolotii, A.D.; Soloviev, I.V.; Iourov, I.Y. Mosaic Brain Aneuploidy in Mental Illnesses: An Association of Low-level post-zygotic Aneuploidy with Schizophrenia and Comorbid Psychiatric Disorders. Curr. Genom. 2018, 19, 163–172. [Google Scholar] [CrossRef]
- Shegay, P.V.; Zabolotneva, A.A.; Shatova, O.P.; Shestopalov, A.V.; Kaprin, A.D. Evolutionary View on Lactate-Dependent Mechanisms of Maintaining Cancer Cell Stemness and Reprimitivization. Cancers 2022, 14, 4552. [Google Scholar] [CrossRef]
- Fan, Y.; Abrahamsen, G.; McGrath, J.J.; Mackay-Sim, A. Altered Cell Cycle Dynamics in Schizophrenia. Biol. Psychiatry 2012, 71, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Katsel, P.; Davis, K.L.; Li, C.; Tan, W.; Greenstein, E.; Hoffman, L.B.K.; Haroutunian, V. Abnormal Indices of Cell Cycle Activity in Schizophrenia and their Potential Association with Oligodendrocytes. Neuropsychopharmacology 2008, 33, 2993–3009. [Google Scholar] [CrossRef] [PubMed]
- Lineweaver, C.H.; Bussey, K.J.; Blackburn, A.C.; Davies, P.C.W. Cancer progression as a sequence of atavistic reversions. Bioessays 2021, 43, e2000305. [Google Scholar] [CrossRef]
- Alvarez, Z.; Hyroššová, P.; Perales, J.C.; Alcantara, S.; Ova, P.H. Neuronal Progenitor Maintenance Requires Lactate Metabolism and PEPCK-M-Directed Cataplerosis. Cereb. Cortex 2014, 26, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Odenwelder, D.C.; Lu, X.; Harcum, S.W. Induced pluripotent stem cells can utilize lactate as a metabolic substrate to support proliferation. Biotechnol. Prog. 2020, 37, e3090. [Google Scholar] [CrossRef]
- Goldman, S.; Pulsinelli, W.A.; Clarke, W.Y.; Kraig, R.P.; Plum, F. The Effects of Extracellular Acidosis on Neurons and Glia in vitro. J. Cereb. Blood Flow Metab. 1989, 9, 471–477. [Google Scholar] [CrossRef]
- Tan, Z.; Chu, D.Z.V.; Chan, Y.J.A.; Lu, Y.E.; Rancati, G. Mammalian Cells Undergo Endoreduplication in Response to Lactic Acidosis. Sci. Rep. 2018, 8, 2890. [Google Scholar] [CrossRef]
- Lev-Vachnish, Y.; Cadury, S.; Rotter-Maskowitz, A.; Feldman, N.; Roichman, A.; Illouz, T.; Varvak, A.; Nicola, R.; Madar, R.; Okun, E. L-Lactate Promotes Adult Hippocampal Neurogenesis. Front. Neurosci. 2019, 13, 403. [Google Scholar] [CrossRef]
- Ordoño, J.; Pérez-Amodio, S.; Ball, K.; Aguirre, A.; Engel, E. The generation of a lactate-rich environment stimulates cell cycle progression and modulates gene expression on neonatal and hiPSC-derived cardiomyocytes. Biomater. Adv. 2022, 139, 213035. [Google Scholar] [CrossRef]
- Rosenblatt, J. Mitosis: Moesin and the Importance of Being Round. Curr. Biol. 2008, 18, R292–R293. [Google Scholar] [CrossRef]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, A.; Ramirez, P.; Gamez, M.; Gonzalez, E.; De Mange, J.; Bieniek, K.F.; Ray, W.J.; Frost, B. Moesin is an effector of tau-induced actin overstabilization, cell cycle activation, and neurotoxicity in Alzheimer’s disease. iScience 2023, 26, 106152. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhou, J.; Chen, Q.; Xu, X.; Gao, J.; Li, X.; Shao, Q.; Zhou, B.; Zhou, H.; Wei, S.; et al. Tumor metabolite lactate promotes tumorigenesis by modulating MOESIN lactylation and enhancing TGF-β signaling in regulatory T cells. Cell Rep. 2022, 40, 111122. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Matsui, M.; Ichioka, S.; Nakamura, Y.; Hirata, T. The ERM protein moesin regulates natural killer cell homeostasis in vivo. Cell. Immunol. 2022, 371, 104456. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shmuel, A.; Sabag, B.; Biber, G.; Barda-Saad, M. The Role of the Cytoskeleton in Regulating the Natural Killer Cell Immune Response in Health and Disease: From Signaling Dynamics to Function. Front. Cell Dev. Biol. 2021, 9, 609532. [Google Scholar] [CrossRef] [PubMed]
- Bowden, N.A.; Weidenhofer, J.; Scott, R.J.; Schall, U.; Todd, J.; Michie, P.T.; Tooney, P.A. Preliminary investigation of gene expression profiles in peripheral blood lymphocytes in schizophrenia. Schizophr. Res. 2006, 82, 175–183. [Google Scholar] [CrossRef]
- Moreno-Yruela, C.; Zhang, D.; Wei, W.; Bæk, M.; Liu, W.; Gao, J.; Danková, D.; Nielsen, A.L.; Bolding, J.E.; Yang, L.; et al. Class I histone deacetylases (HDAC1–3) are histone lysine delactylases. Sci. Adv. 2022, 8, eabi6696. [Google Scholar] [CrossRef]
- Yu, J.; de Belle, I.; Liang, H.; Adamson, E.D. Coactivating Factors p300 and CBP Are Transcriptionally Crossregulated by Egr1 in Prostate Cells, Leading to Divergent Responses. Mol. Cell 2004, 15, 83–94. [Google Scholar] [CrossRef]
- Gilbert, T.; Zürcher, N.R.; Wu, C.J.; Bhanot, A.; Hightower, B.G.; Kim, M.; Albrecht, D.S.; Wey, H.-Y.; Schroeder, F.A.; Rodriguez-Thompson, A.; et al. PET neuroimaging reveals histone deacetylase dysregulation in schizophrenia. J. Clin. Investig. 2018, 129, 364–372. [Google Scholar] [CrossRef]
- Martínez-Pinteño, A.; Gassó, P.; Prohens, L.; Segura, A.G.; Parellada, M.; Saiz-Ruiz, J.; Cuesta, M.J.; Bernardo, M.; Lafuente, A.; Mas, S.; et al. Identification of EP300 as a Key Gene Involved in Antipsychotic-Induced Metabolic Dysregulation Based on Integrative Bioinformatics Analysis of Multi-Tissue Gene Expression Data. Front. Pharmacol. 2021, 12, 729474. [Google Scholar] [CrossRef]
- Tan, M.; Shen, L.; Hou, Y. Epigenetic modification of BDNF mediates neuropathic pain via miR-30a-3p/EP300 axis in CCI rats. Biosci. Rep. 2020, 40, BSR20194442. [Google Scholar] [CrossRef] [PubMed]
- Gören, J.L. Brain-derived neurotrophic factor and schizophrenia. Ment. Health Clin. 2016, 6, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Bredy, T.W.; Wu, H.; Crego, C.; Zellhoefer, J.; Sun, Y.E.; Barad, M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn. Mem. 2007, 14, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Yang, F.; Xiao, Z.; Luo, H.; Chen, H.; Chen, Z.; Liu, Q.; Xiao, Y. Lactylation: Novel epigenetic regulatory and therapeutic opportunities. Am J Physiol Endocrinol Metab. 2023, 324, E330–E338. [Google Scholar] [CrossRef] [PubMed]
- Boutahar, N.; Reynaud, E.; Lassabliere, F.; Borg, J. Brain-derived neurotrophic factor inhibits cell cycle reentry but not endoplasmic reticulum stress in cultured neurons following oxidative or excitotoxic stress. J. Neurosci. Res. 2010, 88, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.J.; Ghazanfari, N.; Constantinescu, P.; Mantamadiotis, T.; Barrow, A.D. The Role of NK Cells and Innate Lymphoid Cells in Brain Cancer. Front. Immunol. 2020, 11, 1549. [Google Scholar] [CrossRef]
- Marzan, D.E.; Brügger-Verdon, V.; West, B.L.; Liddelow, S.; Samanta, J.; Salzer, J.L. Activated microglia drive demyelination via CSF1R signaling. Glia 2021, 69, 1583–1604. [Google Scholar] [CrossRef]
- Gaffney, D.O.; Jennings, E.Q.; Anderson, C.C.; Marentette, J.O.; Shi, T.; Oxvig, A.-M.S.; Streeter, M.D.; Johannsen, M.; Spiegel, D.A.; Chapman, E.; et al. Non-enzymatic Lysine Lactoylation of Glycolytic Enzymes. Cell Chem. Biol. 2020, 27, 206–213.e6. [Google Scholar] [CrossRef]
- Tarantino, N.; Leboyer, M.; Bouleau, A.; Hamdani, N.; Richard, J.R.; Boukouaci, W.; Ching-Lien, W.; Godin, O.; Bengoufa, D.; Le Corvoisier, P.; et al. Natural killer cells in first-episode psychosis: An innate immune signature? Mol. Psychiatry 2021, 26, 5297–5306. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, T.-Y.; Kim, Y.; Lee, S.-H.; Kim, S.; Kang, S.W.; Yang, J.-Y.; Baek, I.-J.; Sung, Y.H.; Park, Y.-Y.; et al. Microbiota-Derived Lactate Accelerates Intestinal Stem-Cell-Mediated Epithelial Development. Cell Host Microbe 2018, 24, 833–846.e6. [Google Scholar] [CrossRef]
- Wang, S.P.; Rubio, L.A.; Duncan, S.; Donachie, G.E.; Holtrop, G.; Lo, G.; Farquharson, F.M.; Wagner, J.; Parkhill, J.; Louis, P.; et al. Pivotal Roles for pH, Lactate, and Lactate-Utilizing Bacteria in the Stability of a Human Colonic Microbial Ecosystem. Msystems 2020, 5, e00645-20. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-F.; Wang, C.-Y.; Wang, J.-H.; Wang, Q.-N.; Li, S.-J.; Wang, H.-O.; Zhou, F.; Li, J.-M. Short-Chain Fatty Acids Ameliorate Depressive-like Behaviors of High Fructose-Fed Mice by Rescuing Hippocampal Neurogenesis Decline and Blood–Brain Barrier Damage. Nutrients 2022, 14, 1882. [Google Scholar] [CrossRef] [PubMed]
- Caslin, H.L.; Abebayehu, D.; Pinette, J.A.; Ryan, J.J. Lactate Is a Metabolic Mediator That Shapes Immune Cell Fate and Function. Front. Physiol. 2021, 12, 688485. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Glesaaen, E.R.; Palibrk, V.; Pannone, M.; Wang, W.; Al-Jabri, A.; Suganthan, R.; Meyer, N.; Austbø, M.L.; Lin, X.; et al. Lactate receptor HCAR1 regulates neurogenesis and microglia activation after neonatal hypoxia-ischemia. Elife 2022, 11, e76451. [Google Scholar] [CrossRef] [PubMed]
- Errea, A.; Cayet, D.; Marchetti, P.; Tang, C.; Kluza, J.; Offermanns, S.; Sirard, J.-C.; Rumbo, M. Lactate Inhibits the Pro-Inflammatory Response and Metabolic Reprogramming in Murine Macrophages in a GPR81-Independent Manner. PLoS ONE 2016, 11, e0163694. [Google Scholar] [CrossRef]
- Nicola, R.; Madar, R.; Okun, E. HCAR1-Mediated l-Lactate Signaling Suppresses Microglial Phagocytosis. NeuroMol. Med. 2022, 24, 399–404. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, J.; Zhang, H.; Han, Y.; Lu, C.; Chen, C.; Tan, X.; Wang, S.; Bai, X.; Zhai, G.; et al. YiaC and CobB regulate lysine lactylation in Escherichia coli. Nat. Commun. 2022, 13, 6628. [Google Scholar] [CrossRef]
- Wiwanitkit, V. Psychosis and E. coli Infection: A Forgotten Issue. Indian J. Psychol. Med. 2012, 34, 407–408. [Google Scholar] [CrossRef]
- Kleimann, A.; Toto, S.; Eberlein, C.K.; Kielstein, J.T.; Bleich, S.; Frieling, H.; Sieberer, M. Psychiatric Symptoms in Patients with Shiga Toxin-Producing E. coli O104:H4 Induced Haemolytic-Uraemic Syndrome. PLoS ONE 2014, 9, e101839. [Google Scholar] [CrossRef]
- Fouladkhah, A.; Geornaras, I.; Yang, H.; Sofos, J.N. Lactic acid resistance of Shiga toxin-producing Escherichia coli and multidrug-resistant and susceptible Salmonella Typhimurium and Salmonella Newport in meat homogenate. Food Microbiol. 2013, 36, 260–266. [Google Scholar] [CrossRef]
- Graham, K.L.; Carson, C.M.; Ezeoke, A.; Buckley, P.F.; Miller, B.J. Urinary Tract Infections in Acute Psychosis. J. Clin. Psychiatry 2014, 75, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; McEvoy, J.P.; Miller, B.J. Urinary tract infection, inflammation, and cognition in phase 1 of the Clinical Antipsychotic Trials of Intervention Effectiveness Study. Ann. Clin. Psychiatry 2019, 31, 242–248. [Google Scholar] [PubMed]
- Tadié, J.-M.; Bae, H.-B.; Banerjee, S.; Zmijewski, J.W.; Abraham, E. Differential activation of RAGE by HMGB1 modulates neutrophil-associated NADPH oxidase activity and bacterial killing. Am. J. Physiol. -Cell Physiol. 2012, 302, C249–C256. [Google Scholar] [CrossRef]
- Lin, A.E.; Beasley, F.C.; Olson, J.; Keller, N.; Shalwitz, R.A.; Hannan, T.; Hultgren, S.J.; Nizet, V. Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Escherichia coli Infection. PLoS Pathog. 2015, 11, e1004818. [Google Scholar] [CrossRef]
- Manosalva, C.; Quiroga, J.; Hidalgo, A.I.; Alarcón, P.; Anseoleaga, N.; Hidalgo, M.A.; Burgos, R.A. Role of Lactate in Inflammatory Processes: Friend or Foe. Front Immunol. 2022, 12, 808799. [Google Scholar] [CrossRef]
- Al-Dujaili, A.H.; Mousa, R.F.; Al-Hakeim, H.K.; Maes, M. High Mobility Group Protein 1 and Dickkopf-Related Protein 1 in Schizophrenia and Treatment-Resistant Schizophrenia: Associations With Interleukin-6, Symptom Domains, and Neurocognitive Impairments. Schizophr. Bull. 2020, 47, 530–541. [Google Scholar] [CrossRef]
- Torrey, E.F.; Yolken, R.H. Toxoplasma gondii and Schizophrenia. Emerg. Infect. Dis. 2003, 9, 1375–1380. [Google Scholar] [CrossRef]
- Zhao, W.; Yu, H.; Liu, X.; Wang, T.; Yao, Y.; Zhou, Q.; Zheng, X.; Tan, F. Systematic identification of the lysine lactylation in the protozoan parasite Toxoplasma gondii. Parasites Vectors 2022, 15, 180. [Google Scholar] [CrossRef]
- Carrillo, G.L.; Ballard, V.A.; Glausen, T.; Boone, Z.; Teamer, J.; Hinkson, C.L.; Wohlfert, E.A.; Blader, I.J.; Fox, M.A. Toxoplasma infection induces microglia-neuron contact and the loss of perisomatic inhibitory synapses. Glia 2020, 68, 1968–1986. [Google Scholar] [CrossRef]
- Obed, C.; Wu, M.; Chen, Y.; An, R.; Cai, H.; Luo, Q.; Yu, L.; Wang, J.; Liu, F.; Shen, J.; et al. Toxoplasma gondii dense granule protein 3 promotes endoplasmic reticulum stress-induced apoptosis by activating the PERK pathway. Parasites Vectors 2022, 15, 276. [Google Scholar] [CrossRef]
- Briceño, M.P.; Nascimento, L.A.C.; Nogueira, N.P.; Barenco, P.V.C.; Ferro, E.A.V.; Rezende-Oliveira, K.; Goulart, L.R.; Alves, P.T.; Barbosa, B.D.F.; Lima, W.R.; et al. Toxoplasma gondii Infection Promotes Epithelial Barrier Dysfunction of Caco-2 Cells. J. Histochem. Cytochem. 2016, 64, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.H.; Abele, H.; Plappert, C.F. The Role of Oxytocin and the Effect of Stress During Childbirth: Neurobiological Basics and Implications for Mother and Child. Front. Endocrinol. 2021, 12, 742236. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.; Leng, G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 2006, 7, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Sabe, M.; Zhao, N.; Crippa, A.; Strauss, G.P.; Kaiser, S. Intranasal Oxytocin for Negative Symptoms of Schizophrenia: Systematic Review, Meta-Analysis, and Dose-Response Meta-Analysis of Randomized Controlled Trials. Int. J. Neuropsychopharmacol. 2021, 24, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Kéri, S.; Kiss, I.; Kelemen, O. Sharing secrets: Oxytocin and trust in schizophrenia. Soc. Neurosci. 2009, 4, 287–293. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Matsushita, H.; Tomizawa, K.; Matsui, H. Oxytocin: A therapeutic target for mental disorders. J. Physiol. Sci. 2012, 62, 441–444. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Chen, C.-C.; Huang, C.-C.; Nishimori, K.; Hsu, K.-S. Oxytocin stimulates hippocampal neurogenesis via oxytocin receptor expressed in CA3 pyramidal neurons. Nat. Commun. 2017, 8, 537. [Google Scholar] [CrossRef]
- Marlin, B.J.; Froemke, R.C. Oxytocin modulation of neural circuits for social behavior. Dev. Neurobiol. 2016, 77, 169–189. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Gobert, D.; Stern, E.; Gamache, K.; Colina, R.; Cuello, C.; Sossin, W.; Kaufman, R.; Pelletier, J.; Rosenblum, K.; et al. eIF2α Phosphorylation Bidirectionally Regulates the Switch from Short- to Long-Term Synaptic Plasticity and Memory. Cell 2007, 129, 195–206. [Google Scholar] [CrossRef]
- Trinh, M.A.; Kaphzan, H.; Wek, R.C.; Pierre, P.; Cavener, D.R.; Klann, E. Brain-Specific Disruption of the eIF2α Kinase PERK Decreases ATF4 Expression and Impairs Behavioral Flexibility. Cell Rep. 2012, 1, 676–688. [Google Scholar] [CrossRef]
- Timberlake, M., II; Dwivedi, Y. Linking unfolded protein response to inflammation and depression: Potential pathologic and therapeutic implications. Mol. Psychiatry 2019, 24, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Mitsuda, T.; Omi, T.; Tanimukai, H.; Sakagami, Y.; Tagami, S.; Okochi, M.; Kudo, T.; Takeda, M. Sigma-1Rs are upregulated via PERK/eIF2α/ATF4 pathway and execute protective function in ER stress. Biochem. Biophys. Res. Commun. 2011, 415, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Cobos, E.J.; Entrena, J.M.; Nieto, F.R.; Cendán, C.M.; Del Pozo, E. Pharmacology and Therapeutic Potential of Sigma1 Receptor Ligands. Curr. Neuropharmacol. 2008, 6, 344–366. [Google Scholar] [CrossRef]
- Almási, N.; Török, S.; Dvorácskó, S.; Tömböly, C.; Csonka, Á.; Baráth, Z.; Murlasits, Z.; Valkusz, Z.; Pósa, A.; Varga, C.; et al. Lessons on the Sigma-1 Receptor in TNBS-Induced Rat Colitis: Modulation of the UCHL-1, IL-6 Pathway. Int. J. Mol. Sci. 2020, 21, 4046. [Google Scholar] [CrossRef]
- Liu, D.; Yang, L.; Liu, P.; Ji, X.; Qi, X.; Wang, Z.; Chi, T.; Zou, L. Sigma–1 receptor activation alleviates blood–brain barrier disruption post cerebral ischemia stroke by stimulating the GDNF–GFRα1–RET pathway. Exp. Neurol. 2021, 347, 113867. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, M.; Kowalczyk, E.; Kwiatkowski, P.; Łopusiewicz, Ł.; Talarowska, M.; Sienkiewicz, M. Cellular Response to Unfolded Proteins in Depression. Life 2021, 11, 1376. [Google Scholar] [CrossRef]
- Kamarudin, M.N.A.; Parhar, I. Emerging therapeutic potential of anti-psychotic drugs in the management of human glioma: A comprehensive review. Oncotarget 2019, 10, 3952–3977. [Google Scholar] [CrossRef]
- Liśkiewicz, P.; Kaczmarczyk, M.; Misiak, B.; Wroński, M.; Bąba-Kubiś, A.; Skonieczna-Żydecka, K.; Marlicz, W.; Bieńkowski, P.; Misera, A.; Pełka-Wysiecka, J.; et al. Analysis of gut microbiota and intestinal integrity markers of inpatients with major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 106, 110076. [Google Scholar] [CrossRef]
- Mao, J.; Hu, Y.; Ruan, L.; Ji, Y.; Lou, Z. Role of endoplasmic reticulum stress in depression (Review). Mol. Med. Rep. 2019, 20, 4774–4780. [Google Scholar] [CrossRef]
- Patel, S.; Sharma, D.; Kalia, K.; Tiwari, V. Crosstalk between endoplasmic reticulum stress and oxidative stress in schizophrenia: The dawn of new therapeutic approaches. Neurosci. Biobehav. Rev. 2017, 83, 589–603. [Google Scholar] [CrossRef]
- Hayashi, T. Conversion of psychological stress into cellular stress response: Roles of the sigma-1 receptor in the process. Psychiatry Clin. Neurosci. 2015, 69, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Jin, U.-H.; Park, H.; Chapkin, R.S.; Jayaraman, A. Aryl Hydrocarbon Receptor (AHR) Ligands as Selective AHR Modulators (SAhRMs). Int. J. Mol. Sci. 2020, 21, 6654. [Google Scholar] [CrossRef] [PubMed]
- Shackleford, G.; Sampathkumar, N.K.; Hichor, M.; Weill, L.; Meffre, D.; Juricek, L.; Laurendeau, I.; Chevallier, A.; Ortonne, N.; Larousserie, F.; et al. Involvement of Aryl hydrocarbon receptor in myelination and in human nerve sheath tumorigenesis. Proc. Natl. Acad. Sci. USA 2018, 115, E1319–E1328. [Google Scholar] [CrossRef] [PubMed]
- Saraste, M.; Irjala, H.; Airas, L. Expansion of CD56Bright natural killer cells in the peripheral blood of multiple sclerosis patients treated with interferon-beta. Neurol. Sci. 2007, 28, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Feifel, D.; Macdonald, K.; Nguyen, A.; Cobb, P.; Warlan, H.; Galangue, B.; Minassian, A.; Becker, O.; Cooper, J.; Perry, W.; et al. Adjunctive Intranasal Oxytocin Reduces Symptoms in Schizophrenia Patients. Biol. Psychiatry 2010, 68, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Schubert, K.O.; Föcking, M.; Cotter, D.R. Proteomic pathway analysis of the hippocampus in schizophrenia and bipolar affective disorder implicates 14-3-3 signaling, aryl hydrocarbon receptor signaling, and glucose metabolism: Potential roles in GABAergic interneuron pathology. Schizophr. Res. 2015, 167, 64–72. [Google Scholar] [CrossRef]
- Uvnäs-Moberg, K.; Björkstrand, E.; Hillegaart, V.; Ahlenius, S. Oxytocin as a possible mediator of SSRI-induced antidepressant effects. Psychopharmacology 1999, 142, 95–101. [Google Scholar] [CrossRef]
- Galbally, M.; Watson, S.J.; Keelan, J.A.; Spigset, O.; Lewis, A. The relationship between oxytocin blood concentrations and antidepressants over pregnancy and the postpartum. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 109, 110218. [Google Scholar] [CrossRef]
- Humble, M.B.; Bejerot, S. Orgasm, Serotonin Reuptake Inhibition, and Plasma Oxytocin in Obsessive-Compulsive Disorder. Gleaning From a Distant Randomized Clinical Trial. Sex Med. 2016, 4, e145–e155. [Google Scholar] [CrossRef]
- Abbasinazari, M.; Heidari-Kord, M.; Mazaheri-Meybodi, A.; Eshraghi, A.; Bayati, N. Plasma Oxytocin Level and Sexual Dysfunction in Depressed Women Treated by Either Fluoxetine or Citalopram: A Pilot Clinical Trial. Iran. J. Pharm. Res. 2018, 17, 408–414. [Google Scholar]
- Bujanow, W. Is Oxytocin an Anti-Schizophrenic Hormone? Can. Psychiatr. Assoc. J. 1974, 19, 323. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhu, J. Innate Lymphoid Cells and Intestinal Inflammatory Disorders. Int. J. Mol. Sci. 2022, 23, 1856. [Google Scholar] [CrossRef] [PubMed]
- Robinette, M.L.; Fuchs, A.; Cortez, V.S.; Lee, J.S.; Wang, Y.; Durum, S.K.; Gilfillan, S.; Colonna, M.; Immunological Genome Consortium. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat. Immunol. 2015, 16, 306–317. [Google Scholar] [CrossRef]
- Crane, C.A.; Austgen, K.; Haberthur, K.; Hofmann, C.; Moyes, K.W.; Avanesyan, L.; Fong, L.; Campbell, M.J.; Cooper, S.; Oakes, S.A.; et al. Immune evasion mediated by tumor-derived lactate dehydrogenase induction of NKG2D ligands on myeloid cells in glioblastoma patients. Proc. Natl. Acad. Sci. USA 2014, 111, 12823–12828. [Google Scholar] [CrossRef]
- Wensveen, F.M.; Jelenčić, V.; Polić, B. NKG2D: A Master Regulator of Immune Cell Responsiveness. Front. Immunol. 2018, 9, 441. [Google Scholar] [CrossRef] [PubMed]
- Borovcanin, M.M.; Minic Janicijevic, S.; Jovanovic, I.P.; Gajovic, N.M.; Jurisevic, M.M.; Arsenijevic, N.N. Type 17 Immune Response Facilitates Progression of Inflammation and Correlates with Cognition in Stable Schizophrenia. Diagnostics 2020, 10, 926. [Google Scholar] [CrossRef] [PubMed]
- Matusevicius, D.; Kivisäkk, P.; He, B.; Kostulas, N.; Özenci, V.; Fredrikson, S.; Link, H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult. Scler. J. 1999, 5, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Dawidowski, B.; Górniak, A.; Podwalski, P.; Lebiecka, Z.; Misiak, B.; Samochowiec, J. The Role of Cytokines in the Pathogenesis of Schizophrenia. J. Clin. Med. 2021, 10, 3849. [Google Scholar] [CrossRef]
- Al-Asmary, S.M.; Kadasah, S.; Arfin, M.; Tariq, M.; Al-Asmari, A. Genetic variants of interleukin-10 gene promoter are associated with schizophrenia in Saudi patients: A case-control study. North Am. J. Med. Sci. 2014, 6, 558–565. [Google Scholar] [CrossRef]
- Warre-Cornish, K.; Perfect, L.; Nagy, R.; Duarte, R.R.R.; Reid, M.J.; Raval, P.; Mueller, A.; Evans, A.L.; Couch, A.; Ghevaert, C.; et al. Interferon-γ signaling in human iPSC–derived neurons recapitulates neurodevelopmental disorder phenotypes. Sci. Adv. 2020, 6, eaay9506. [Google Scholar] [CrossRef]
- Ramirez, J.-M.; Brembilla, N.C.; Sorg, O.; Chicheportiche, R.; Matthes, T.; Dayer, J.-M.; Saurat, J.-H.; Roosnek, E.; Chizzolini, C. Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells. Eur. J. Immunol. 2010, 40, 2450–2459. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Chang, L.H.; Huang, S.S.; Huang, Y.J.; Chih, C.L.; Kuo, H.C.; Lee, Y.H.; Lee, I.H. Aryl hydrocarbon receptor modulates stroke-induced astrogliosis and neurogenesis in the adult mouse brain. J. Neuroinflam. 2019, 16, 187. [Google Scholar] [CrossRef]
- van Venrooij, J.A.E.M.; Fluitman, S.B.A.H.A.; Lijmer, J.G.; Kavelaars, A.; Heijnen, C.J.; Westenberg, H.G.M.; Kahn, R.S.; Wied, C.C.G.-D. Impaired Neuroendocrine and Immune Response to Acute Stress in Medication-Naive Patients With a First Episode of Psychosis. Schizophr. Bull. 2012, 38, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.C.; Ahmetspahic, D.; Ruck, T.; Schulte-Mecklenbeck, A.; Schwarte, K.; Jörgens, S.; Scheu, S.; Windhagen, S.; Graefe, B.; Melzer, N.; et al. Alemtuzumab treatment alters circulating innate immune cells in multiple sclerosis. Neurol. -Neuroimmunol. Neuroinflamm. 2016, 3, e289. [Google Scholar] [CrossRef]
- Chu, C.-S.; Li, D.-J.; Chu, C.-L.; Wu, C.-C.; Lu, T. Decreased IL-1ra and NCAM-1/CD56 Serum Levels in Unmedicated Patients with Schizophrenia Before and After Antipsychotic Treatment. Psychiatry Investig. 2018, 15, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Postal, B.G.; Ghezzal, S.; Aguanno, D.; André, S.; Garbin, K.; Genser, L.; Brot-Laroche, E.; Poitou, C.; Soula, H.; Leturque, A.; et al. AhR activation defends gut barrier integrity against damage occurring in obesity. Mol. Metab. 2020, 39, 101007. [Google Scholar] [CrossRef]
- Ghiboub, M.; Verburgt, C.M.; Sovran, B.; Benninga, M.A.; de Jonge, W.J.; Van Limbergen, J.E. Nutritional Therapy to Modulate Tryptophan Metabolism and Aryl Hydrocarbon-Receptor Signaling Activation in Human Diseases. Nutrients 2020, 12, 2846. [Google Scholar] [CrossRef]
- Marinelli, L.; Martin-Gallausiaux, C.; Bourhis, J.-M.; Béguet-Crespel, F.; Blottière, H.M.; Lapaque, N. Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Sci. Rep. 2019, 9, 643. [Google Scholar] [CrossRef]
- Tagliabue, S.G.; Faber, S.C.; Motta, S.; Denison, M.S.; Bonati, L. Modeling the binding of diverse ligands within the Ah receptor ligand binding domain. Sci. Rep. 2019, 9, 10693. [Google Scholar] [CrossRef]
- Wu, K.K. Cytoguardin: A Tryptophan Metabolite against Cancer Growth and Metastasis. Int. J. Mol. Sci. 2021, 22, 4490. [Google Scholar] [CrossRef]
- Peyraud, F.; Guegan, J.-P.; Bodet, D.; Cousin, S.; Bessede, A.; Italiano, A. Targeting Tryptophan Catabolism in Cancer Immunotherapy Era: Challenges and Perspectives. Front. Immunol. 2022, 13, 807271. [Google Scholar] [CrossRef] [PubMed]
- Juhász, C.; Nahleh, Z.; Zitron, I.; Chugani, D.C.; Janabi, M.Z.; Bandyopadhyay, S.; Ali-Fehmi, R.; Mangner, T.J.; Chakraborty, P.K.; Mittal, S.; et al. Tryptophan metabolism in breast cancers: Molecular imaging and immunohistochemistry studies. Nucl. Med. Biol. 2012, 39, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, N.; Gunter, M.J.; Murphy, N.; Gicquiau, A.; Achaintre, D.; Brezina, S.; Gumpenberger, T.; Baierl, A.; Ose, J.; Geijsen, A.J.M.R.; et al. Circulating tryptophan metabolites and risk of colon cancer: Results from case-control and prospective cohort studies. Int. J. Cancer 2021, 149, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front Cell Infect Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef]
- Takeda, T.; Komiya, Y.; Koga, T.; Ishida, T.; Ishii, Y.; Kikuta, Y.; Nakaya, M.; Kurose, H.; Yokomizo, T.; Shimizu, T.; et al. Dioxin-induced increase in leukotriene B4 biosynthesis through the aryl hydrocarbon receptor and its relevance to hepatotoxicity owing to neutrophil infiltration. J. Biol. Chem. 2017, 292, 10586–10599. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Q.; Ma, Y.; Li, L.; Yu, K.; Zhang, Z.; Chen, G.; Li, X.; Xiao, W.; Xu, P.; et al. Aryl Hydrocarbon Receptor Activation Modulates Intestinal Epithelial Barrier Function by Maintaining Tight Junction Integrity. Int. J. Biol. Sci. 2018, 14, 69–77. [Google Scholar] [CrossRef]
- Goya-Jorge, E.; Gonza, I.; Bondue, P.; Douny, C.; Taminiau, B.; Daube, G.; Scippo, M.-L.; Delcenserie, V. Human Adult Microbiota in a Static Colon Model: AhR Transcriptional Activity at the Crossroads of Host–Microbe Interaction. Foods 2022, 11, 1946. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Wang, H.; Liu, L.; Li, W.; Xie, P. The regulatory effects of lactic acid on neuropsychiatric disorders. Discov. Ment. Health 2022, 2, 8. [Google Scholar] [CrossRef]
- Guo, N.; Jiang, Y.W.; Song, X.R.; Li, Y.Y.; Liu, Z.M.; Fu, Y.J. Effect of Bacillus natto solid-state fermentation on the functional constituents and properties of Ginkgo seeds. J. Food Biochem. 2019, 43, e12820. [Google Scholar] [CrossRef]
- Zhang, Y.; Anoopkumar-Dukie, S.; Davey, A.K. SIRT1 and SIRT2 Modulators: Potential Anti-Inflammatory Treatment for Depression? Biomolecules 2021, 11, 353. [Google Scholar] [CrossRef]
- Vasconcelos, G.S.; Dos Santos Júnior, M.A.; Monte, A.S.; da Silva, F.E.R.; Lima, C.N.C.; Moreira Lima Neto, A.B.; Medeiros, I.D.S.; Teixeira, A.L.; de Lucena, D.F.; Vasconcelos, S.M.M.; et al. Low-dose candesartan prevents schizophrenia-like behavioral alterations in a neurodevelopmental two-hit model of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110348. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Feng, F.; Wu, J.; Fan, S.; Han, J.; Wang, S.; Yang, L.; Liu, W.; Wang, C.; Xu, K. Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol. Res. 2022, 181, 106270. [Google Scholar] [CrossRef] [PubMed]
- Sidrauski, C.; McGeachy, A.M.; Ingolia, N.T.; Walter, P. The small molecule ISRIB reverses the effects of eIF2α phosphorylation on translation and stress granule assembly. Elife 2015, 4, e05033. [Google Scholar] [CrossRef] [PubMed]
- Hritcu, L.; Ionita, R.; Postu, P.A.; Gupta, G.K.; Turkez, H.; Lima, T.C.; Carvalho, C.U.S.; de Sousa, D.P. Antidepressant Flavonoids and Their Relationship with Oxidative Stress. Oxidative Med. Cell. Longev. 2017, 2017, 5762172. [Google Scholar] [CrossRef] [PubMed]
- Hayashi-Takagi, A.; Araki, Y.; Nakamura, M.; Vollrath, B.; Duron, S.G.; Yan, Z.; Kasai, H.; Huganir, R.L.; Campbell, D.A.; Sawa, A. PAKs inhibitors ameliorate schizophrenia-associated dendritic spine deterioration in vitro and in vivo during late adolescence. Proc. Natl. Acad. Sci. USA 2014, 111, 6461–6466. [Google Scholar] [CrossRef]
- Gil-Ad, I.; Amit, B.H.; Hayardeni, L.; Tarasenko, I.; Taler, M.; Gueta, R.U.; Weizman, A. Effects of the Anti-Multiple Sclerosis Immunomodulator Laquinimod on Anxiety and Depression in Rodent Behavioral Models. J. Mol. Neurosci. 2014, 55, 552–560. [Google Scholar] [CrossRef]
- Mao, N.; Fan, Y.; Liu, W.; Yang, H.; Yang, Y.; Li, Y.; Jin, F.; Li, T.; Yang, X.; Gao, X.; et al. Oxamate Attenuates Glycolysis and ER Stress in Silicotic Mice. Int. J. Mol. Sci. 2022, 23, 3013. [Google Scholar] [CrossRef]
- Khan, R.S.; Yu, C.; Kastin, A.J.; He, Y.; Ehrensing, R.H.; Hsuchou, H.; Stone, K.; Pan, W. Brain Activation by Peptide Pro-Leu-Gly-NH2 (MIF-1). Int. J. Pept. 2010, 2010, 537639. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Lee, H.; Dingle, R.W.C.; Kim, K.B.; Swanson, H.I. Development of Novel CH223191-Based Antagonists of the Aryl Hydrocarbon Receptor. Mol. Pharmacol. 2011, 81, 3–11. [Google Scholar] [CrossRef]
- Deiab, S.; Mazzio, E.; Eyunni, S.; McTier, O.; Mateeva, N.; Elshami, F.; Soliman, K.F.A. 1,2,3,4,6-Penta-O-galloylglucose within Galla Chinensis Inhibits Human LDH-A and Attenuates Cell Proliferation in MDA-MB-231 Breast Cancer Cells. Evid. -Based Complement. Altern. Med. 2015, 2015, 276946. [Google Scholar] [CrossRef]
- Cid-Jofré, V.; Moreno, M.; Reyes-Parada, M.; Renard, G.M. Role of Oxytocin and Vasopressin in Neuropsychiatric Disorders: Therapeutic Potential of Agonists and Antagonists. Int. J. Mol. Sci. 2021, 22, 12077. [Google Scholar] [CrossRef] [PubMed]
- Ala, M.; Ghasemi, M.; Mohammad Jafari, R.; Dehpour, A.R. Beyond its anti-migraine properties, sumatriptan is an anti-inflammatory agent: A systematic review. Drug Dev. Res. 2021, 82, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Manerba, M.; Vettraino, M.; Fiume, L.; Di Stefano, G.; Sartini, A.; Giacomini, E.; Buonfiglio, R.; Roberti, M.; Recanatini, M. Galloflavin (CAS 568-80-9): A Novel Inhibitor of Lactate Dehydrogenase. Chemmedchem 2011, 7, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Uba, A.I.; Radicella, C.; Readmond, C.; Scorese, N.; Liao, S.; Liu, H.; Wu, C. Binding of agonist WAY-267,464 and antagonist WAY-methylated to oxytocin receptor probed by all-atom molecular dynamics simulations. Life Sci. 2020, 252, 117643. [Google Scholar] [CrossRef] [PubMed]
- Vukicevic, M.; Chalandon, Y.; Helg, C.; Matthes, T.; Dantin, C.; Huard, B.; Chizzolini, C.; Passweg, J.; Roosnek, E. CD56bright NK cells after hematopoietic stem cell transplantation are activated mature NK cells that expand in patients with low numbers of T cells. Eur. J. Immunol. 2010, 40, 3246–3254. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Zhu, K.; Wang, D.; Zhao, X.; Zhang, H.; Wu, S.; Wang, Y.; Wang, J. Microglial aryl hydrocarbon receptor enhances phagocytic function via SYK and promotes remyelination in the cuprizone mouse model of demyelination. J. Neuroinflamm. 2023, 20, 83. [Google Scholar] [CrossRef]
- Fehsel, K.; Schwanke, K.; Kappel, B.; Fahimi, E.; Meisenzahl-Lechner, E.; Esser, C.; Hemmrich, K.; Haarmann-Stemmann, T.; Kojda, G.; Lange-Asschenfeldt, C. Activation of the aryl hydrocarbon receptor by clozapine induces preadipocyte differentiation and contributes to endothelial dysfunction. J. Psychopharmacol. 2022, 36, 191–201. [Google Scholar] [CrossRef]
- Korac, K.; Rajasekaran, D.; Sniegowski, T.; Schniers, B.; Ibrahim, A.; Bhutia, Y.D. Carbidopa, an activator of aryl hydrocarbon receptor, suppresses IDO1 expression in pancreatic cancer and decreases tumor growth. Biochem. J. 2022, 479, 1807–1824. [Google Scholar] [CrossRef]
- Juricek, L.; Coumoul, X. The Aryl Hydrocarbon Receptor and the Nervous System. Int. J. Mol. Sci. 2018, 19, 2504. [Google Scholar] [CrossRef]
- Robichon, K.; Sondhauss, S.; Jordan, T.W.; Keyzers, R.A.; Connor, B.; La Flamme, A.C. Localisation of clozapine during experimental autoimmune encephalomyelitis and its impact on dopamine and its receptors. Sci. Rep. 2021, 11, 2966. [Google Scholar] [CrossRef]
- Mosca, L.; Mantero, V.; Penco, S.; La Mantia, L.; De Benedetti, S.; Marazzi, M.R.; Spreafico, C.; Erminio, C.; Grassi, L.; Lando, G.; et al. HLA-DRB1*15 association with multiple sclerosis is confirmed in a multigenerational Italian family. Funct. Neurol. 2017, 32, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.I.; Jarskog, L.F.; Hilliard, C.; Alfirevic, A.; Duncan, L.; Fourches, D.; Huang, H.; Lek, M.; Neale, B.M.; Ripke, S.; et al. Clozapine-induced agranulocytosis is associated with rare HLA-DQB1 and HLA-B alleles. Nat. Commun. 2014, 5, 4757. [Google Scholar] [CrossRef] [PubMed]
- Sfera, A.; Hazan, S.; Klein, C.; del Campo, C.M.Z.-M.; Sasannia, S.; Anton, J.J.; Rahman, L.; Andronescu, C.V.; Sfera, D.O.; Kozlakidis, Z.; et al. Microbial Translocation Disorders: Assigning an Etiology to Idiopathic Illnesses. Appl. Microbiol. 2023, 3, 212–240. [Google Scholar] [CrossRef]
- Johansson, V.; Jakobsson, J.; Fortgang, R.G.; Zetterberg, H.; Blennow, K.; Cannon, T.D.; Hultman, C.M.; Wetterberg, L.; Landén, M. Cerebrospinal fluid microglia and neurodegenerative markers in twins concordant and discordant for psychotic disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 267, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.S.; Gressitt, K.L.; Cowan, D.N.; Niebuhr, D.W.; Yolken, R.H.; Severance, E.G. Monocyte activation detected prior to a diagnosis of schizophrenia in the US Military New Onset Psychosis Project (MNOPP). Schizophr. Res. 2018, 197, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Severance, E.G.; Gressitt, K.L.; Stallings, C.R.; Origoni, A.E.; Khushalani, S.; Leweke, F.M.; Dickerson, F.B.; Yolken, R.H. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr. Res. 2013, 148, 130–137. [Google Scholar] [CrossRef]
- Kamaeva, D.A.; Smirnova, L.P.; Vasilieva, S.N.; Kazantseva, D.V.; Vasilieva, A.R.; Ivanova, S.A. Catalytic Antibodies in Bipolar Disorder: Serum IgGs Hydrolyze Myelin Basic Protein. Int. J. Mol. Sci. 2022, 23, 7397. [Google Scholar] [CrossRef]
- von Zedtwitz, K.; Matteit, I.; Michel, M.; Feige, B.; Runge, K.; Denzel, D.; Schlump, A.; Nickel, K.; Schiele, M.A.; Berger, B.; et al. Anti-MOG autoantibody-associated schizophreniform psychosis. Acta Neuropsychiatr. 2021, 34, 47–54. [Google Scholar] [CrossRef]
- Ponomarenko, N.; Durova, O.M.; Vorobiev, I.I.; Belogurov, A.A.; Telegin, G.B.; Suchkov, S.V.; Misikov, V.K.; Morse, H.C., 3rd; Gabibov, A.G. Catalytic activity of autoantibodies toward myelin basic protein correlates with the scores on the multiple sclerosis expanded disability status scale. Immunol. Lett. 2006, 103, 45–50. [Google Scholar] [CrossRef]
- Schott, K.; Schaefer, J.-E.; Richartz, E.; Batra, A.; Eusterschulte, B.; Klein, R.; Berg, P.A.; Bartels, M.; Mann, K.; Buchkremer, G. Autoantibodies to serotonin in serum of patients with psychiatric disorders. Psychiatry Res. 2003, 121, 51–57. [Google Scholar] [CrossRef]
- Zong, S.; Hoffmann, C.; Mané-Damas, M.; Molenaar, P.; Losen, M.; Martinez-Martinez, P. Neuronal Surface Autoantibodies in Neuropsychiatric Disorders: Are There Implications for Depression? Front. Immunol. 2017, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Westall, F.C. Molecular Mimicry Revisited: Gut Bacteria and Multiple Sclerosis. J. Clin. Microbiol. 2006, 44, 2099–2104. [Google Scholar] [CrossRef] [PubMed]
- Brundin, L.; Erhardt, S.; Bryleva, E.Y.; Achtyes, E.D.; Postolache, T.T. The role of inflammation in suicidal behaviour. Acta Psychiatr. Scand. 2015, 132, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, L.; Gustafsson, A.; Lavant, E.; Suneson, K.; Brundin, L.; Westrin, Å.; Ljunggren, L.; Lindqvist, D. Leaky gut biomarkers in depression and suicidal behavior. Acta Psychiatr. Scand. 2018, 139, 185–193, Erratum in Acta Psychiatr. Scand. 2020, 142, 423. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, A.P.; Sanchez-Padilla, D.E.; Drew, J.C.; Oli, M.W.; Roesch, L.F.W.; Triplett, E.W. Saliva microbiome, dietary, and genetic markers are associated with suicidal ideation in university students. Sci. Rep. 2022, 12, 14306. [Google Scholar] [CrossRef]
- Eyzaguirre-Velásquez, J.; González-Toro, M.P.; González-Arancibia, C.; Escobar-Luna, J.; Beltrán, C.J.; Bravo, J.A.; Julio-Pieper, M. Sertraline and Citalopram Actions on Gut Barrier Function. Dig. Dis. Sci. 2020, 66, 3792–3802. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, T.; He, L.; Fu, J.-Y.; Deng, H.-X.; Xue, X.-L.; Chen, B.-T. Bacterial Translocation Associates With Aggression in Schizophrenia Inpatients. Front. Syst. Neurosci. 2021, 15, 704069. [Google Scholar] [CrossRef]
- Bulgart, H.R.; Neczypor, E.W.; Wold, L.E.; Mackos, A.R. Microbial involvement in Alzheimer disease development and progression. Mol. Neurodegener. 2020, 15, 42. [Google Scholar] [CrossRef]
- Liang, L.; Liu, P.; Deng, Y.; Li, J.; Zhao, S. L-lactate inhibits lipopolysaccharide-induced inflammation of microglia in the hippocampus. Int. J. Neurosci. 2022, 23, 1–8. [Google Scholar] [CrossRef]
- Guglielmetti, S.; Bernardi, S.; Del Bo’, C.; Cherubini, A.; Porrini, M.; Gargari, G.; Hidalgo-Liberona, N.; Gonzalez-Dominguez, R.; Peron, G.; Zamora-Ros, R.; et al. Effect of a polyphenol-rich dietary pattern on intestinal permeability and gut and blood microbiomics in older subjects: Study protocol of the MaPLE randomised controlled trial. BMC Geriatr. 2020, 20, 77. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tang, W.; Zhao, J.; Fan, W.; Zhang, Y.; Zhang, C. A Comprehensive Analysis of the Effect of SIRT1 Variation on the Risk of Schizophrenia and Depressive Symptoms. Front. Genet. 2020, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- Iacono, L.L.; Visco-Comandini, F.; Valzania, A.; Viscomi, M.T.; Coviello, M.; Giampà, A.; Roscini, L.; Bisicchia, E.; Siracusano, A.; Troisi, A.; et al. Adversity in childhood and depression: Linked through SIRT1. Transl. Psychiatry 2015, 5, e629. [Google Scholar] [CrossRef] [PubMed]
- Zu, H.; Li, C.; Dai, C.; Pan, Y.; Ding, C.; Sun, H.; Zhang, X.; Yao, X.; Zang, J.; Mo, X. SIRT2 functions as a histone delactylase and inhibits the proliferation and migration of neuroblastoma cells. Cell Discov. 2022, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Peña, C.J.; Nestler, E.J. Progress in Epigenetics of Depression. Prog. Mol. Biol. Transl. Sci. 2018, 157, 41–66. [Google Scholar] [CrossRef]
- Cui, B.; Luo, Y.; Tian, P.; Peng, F.; Lu, J.; Yang, Y.; Su, Q.; Liu, B.; Yu, J.; Luo, X.; et al. Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J. Clin. Investig. 2019, 129, 1030–1046. [Google Scholar] [CrossRef]
- Zhao, Z.; Han, F.; Yang, S.; Wu, J.; Zhan, W. Oxamate-mediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: Involvement of the Akt–mTOR signaling pathway. Cancer Lett. 2015, 358, 17–26. [Google Scholar] [CrossRef]
- Ren, Y.-Y.; Zhang, X.-R.; Li, T.-N.; Zeng, Y.-J.; Wang, J.; Huang, Q.-W. Galla Chinensis, a Traditional Chinese Medicine: Comprehensive review of botany, traditional uses, chemical composition, pharmacology and toxicology. J. Ethnopharmacol. 2021, 278, 114247. [Google Scholar] [CrossRef]
- Rahnasto-Rilla, M.; Järvenpää, J.; Huovinen, M.; Schroderus, A.-M.; Ihantola, E.-L.; Küblbeck, J.; Khadeer, M.; Moaddel, R.; Lahtela-Kakkonen, M. Effects of galloflavin and ellagic acid on sirtuin 6 and its anti-tumorigenic activities. Biomed. Pharmacother. 2020, 131, 110701. [Google Scholar] [CrossRef]
- Mao, Q.; Gong, X.; Zhou, C.; Tu, Z.; Zhao, L.; Wang, L.; Wang, X.; Sun, L.; Xia, J.; Lian, B.; et al. Up-regulation of SIRT6 in the hippocampus induced rats with depression-like behavior via the block Akt/GSK3β signaling pathway. Behav. Brain Res. 2017, 323, 38–46. [Google Scholar] [CrossRef]
- Sever, I.H.; Ozkul, B.; Erisik Tanriover, D.; Ozkul, O.; Elgormus, C.S.; Gur, S.G.; Sogut, I.; Uyanikgil, Y.; Cetin, E.O.; Erbas, O. Protective effect of oxytocin through its anti-inflammatory and antioxidant role in a model of sepsis-induced acute lung injury: Demonstrated by CT and histological findings. Exp. Lung Res. 2021, 47, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Erbaş, O.; Altuntaş, İ. Oxytocin and Neuroprotective Effects; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Gonzalez, A.; Hammock, E.A.D. Oxytocin and microglia in the development of social behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20210059. [Google Scholar] [CrossRef] [PubMed]
- Labandeira-García, J.L.; Garrido-Gil, P.; Rodriguez-Pallares, J.; Valenzuela, R.; Borrajo, A.; Rodríguez-Perez, A.I. Brain renin-angiotensin system and dopaminergic cell vulnerability. Front. Neuroanat. 2014, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, S.; Aulinas, A.; O’malley, T.; Maehler, P.; Adler, G.K.; Grinspoon, S.K.; Lawson, E.A. Oxytocin response to controlled dietary sodium and angiotensin II among healthy individuals. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E671–E675. [Google Scholar] [CrossRef] [PubMed]
- Gard, P.R.; Daw, P.; Mashhour, Z.S.; Tran, P. Interactions of angiotensin IV and oxytocin on behaviour in mice. J. Renin-Angiotensin-Aldosterone Syst. 2007, 8, 133–138. [Google Scholar] [CrossRef]
- Kokare, D.M.; Singru, P.S.; Dandekar, M.P.; Chopde, C.T.; Subhedar, N.K. Involvement of alpha-melanocyte stimulating hormone (α-MSH) in differential ethanol exposure and withdrawal related depression in rat: Neuroanatomical–behavioral correlates. Brain Res. 2008, 1216, 53–67. [Google Scholar] [CrossRef]
- Bialesova, L.; Novotna, A.; Macejova, D.; Brtko, J.; Dvorak, Z. Agonistic effect of selected isoflavones on arylhydrocarbon receptor in a novel AZ-AhR transgenic gene reporter human cell line. Gen. Physiol. Biophys. 2015, 34, 331–334. [Google Scholar] [CrossRef]
- Venkataramaiah, C.; Payani, S.; Priya, B.L.; Pradeepkiran, J.A. Therapeutic potentiality of a new flavonoid against ketamine induced glutamatergic dysregulation in schizophrenia: In vivo and in silico approach. Biomed. Pharmacother. 2021, 138, 111453. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Long, T.; He, W.; Pan, Q.; Zhang, S.; Zhang, D.; Qin, G.; Chen, L.; Zhou, J. Microglia P2X4R-BDNF signalling contributes to central sensitization in a recurrent nitroglycerin-induced chronic migraine model. J. Headache Pain 2020, 21, 4. [Google Scholar] [CrossRef]
- Smith, M.; Calabresi, P.; Bhargava, P. Dimethyl fumarate treatment alters NK cell function in multiple sclerosis. Eur. J. Immunol. 2017, 48, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.; Ødum, N.; Ursø, B.; Lanier, L.L.; Spee, P. Cytotoxicity of CD56bright NK Cells towards Autologous Activated CD4+ T Cells Is Mediated through NKG2D, LFA-1 and TRAIL and Dampened via CD94/NKG2A. PLoS ONE 2012, 7, e31959. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of Donor Natural Killer Cell Alloreactivity in Mismatched Hematopoietic Transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef] [PubMed]
- Monji, A.; Kato, T.; Kanba, S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin. Neurosci. 2009, 63, 257–265. [Google Scholar] [CrossRef]
- Wake, H.; Moorhouse, A.J.; Jinno, S.; Kohsaka, S.; Nabekura, J. Resting Microglia Directly Monitor the Functional State of Synapses In Vivo and Determine the Fate of Ischemic Terminals. J. Neurosci. 2009, 29, 3974–3980. [Google Scholar] [CrossRef]
- Jha, M.K.; Jo, M.; Kim, J.-H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neurosci. 2018, 25, 227–240. [Google Scholar] [CrossRef]
- Correa, F.G.; Hernangómez, M.; Guaza, C. Understanding Microglia–Neuron Cross Talk: Relevance of the Microglia–Neuron Cocultures. Methods Mol. Biol. 2013, 1041, 215–229. [Google Scholar] [CrossRef]
- Mills, J.; Ladner, L.; Soliman, E.; Leonard, J.; Morton, P.D.; Theus, M.H. Cross-Talk and Subset Control of Microglia and Associated Myeloid Cells in Neurological Disorders. Cells 2022, 11, 3364. [Google Scholar] [CrossRef]
- Yang, S.; Qin, C.; Hu, Z.W.; Zhou, L.Q.; Yu, H.H.; Chen, M.; Bosco, D.B.; Wang, W.; Wu, L.J.; Tian, D.S. Microglia reprogram metabolic profiles for phenotype and function changes in central nervous system. Neurobiol. Dis. 2021, 152, 105290. [Google Scholar] [CrossRef]
- Nijland, P.G.; Michailidou, I.; Witte, M.E.; Mizee, M.R.; van der Pol, S.M.; van Het Hof, B.; Reijerkerk, A.; Pellerin, L.; van der Valk, P.; de Vries, H.E.; et al. Cellular distribution of glucose and monocarboxylate transporters in human brain white matter and multiple sclerosis lesions. Glia 2014, 62, 1125–1141. [Google Scholar] [CrossRef]
- Goyal, M.S.; Vlassenko, A.G.; Blazey, T.M.; Su, Y.; Couture, L.E.; Durbin, T.J.; Bateman, R.J.; Benzinger, T.L.-S.; Morris, J.C.; Raichle, M.E. Loss of Brain Aerobic Glycolysis in Normal Human Aging. Cell Metab. 2017, 26, 353–360.e3. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.R. Aging, Alzheimer’s Disease and Dysfunctional Glycolysis; Similar Effects of Too Much and Too Little. Aging Dis. 2019, 10, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Zuccoli, G.S.; Guest, P.C.; Martins-De-Souza, D. Effects on Glial Cell Glycolysis in Schizophrenia: An Advanced Aging Phenotype? Adv. Exp. Med. Biol. 2019, 1178, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Lv, X.; Thompson, E.W.; Ostrikov, K.K. Histone lactylation: Epigenetic mark of glycolytic switch. Trends Genet. 2021, 38, 124–127. [Google Scholar] [CrossRef]
- Szeto, A.; Sun-Suslow, N.; Mendez, A.J.; Hernandez, R.I.; Wagner, K.V.; McCabe, P.M. Regulation of the macrophage oxytocin receptor in response to inflammation. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E183–E189. [Google Scholar] [CrossRef]
- Kaneko, Y.; Pappas, C.; Tajiri, N.; Borlongan, C.V. Oxytocin modulates GABAAR subunits to confer neuroprotection in stroke in vitro. Sci. Rep. 2016, 6, 35659. [Google Scholar] [CrossRef]
- Buonfiglioli, A.; Hambardzumyan, D. Macrophages and microglia: The cerberus of glioblastoma. Acta Neuropathol. Commun. 2021, 9, 54. [Google Scholar] [CrossRef]
- Genuis, S.J. Toxic causes of mental illness are overlooked. Neurotoxicology 2008, 29, 1147–1149. [Google Scholar] [CrossRef]
- Krüger, T.; Long, M.; Bonefeld-Jørgensen, E.C. Plastic components affect the activation of the aryl hydrocarbon and the androgen receptor. Toxicology 2008, 246, 112–123. [Google Scholar] [CrossRef]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387. [Google Scholar] [CrossRef]
- Zhang, J.; Velmeshev, D.; Hashimoto, K.; Huang, Y.; Hofmann, J.W.; Shi, X.; Chen, J.; Leidal, A.M.; Dishart, J.G.; Cahill, M.K.; et al. Neurotoxic microglia promote TDP-43 proteinopathy in progranulin deficiency. Nature 2020, 588, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, G.; Mango, D.; Saidi, A.; Corbo, M.; Nisticò, R. Targeting Microglia-Synapse Interactions in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2342. [Google Scholar] [CrossRef] [PubMed]
- Gober, R.; Ardalan, M.; Shiadeh, S.M.J.; Duque, L.; Garamszegi, S.P.; Ascona, M.; Barreda, A.; Sun, X.; Mallard, C.; Vontell, R.T. Microglia activation in postmortem brains with schizophrenia demonstrates distinct morphological changes between brain regions. Brain Pathol. 2021, 32, e13003. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).