Focused Ultrasound-Mediated Blood–Brain Barrier Opening Best Promotes Neuroimmunomodulation through Brain Macrophage Redistribution
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.1.1. Focused Ultrasound Treatment
2.1.2. Magnetic Resonance Imaging
2.2. Tissue Processing
2.2.1. Microglia Isolation
Imaging Flow Cytometry
Single-Cell Sequencing
2.2.2. Immunohistochemistry
2.3. Data Analysis
2.3.1. Cavitation Processing
2.3.2. Flow Cytometry
2.3.3. Single-Cell Sequencing Analysis
Data Alignment
Hashtag Demultiplexing
Quality Control and Integration
Group Defining Gene Sets
2.3.4. Spatial Transcriptomics
Alignment
Seurat Processing
Cell2Location
3. Results
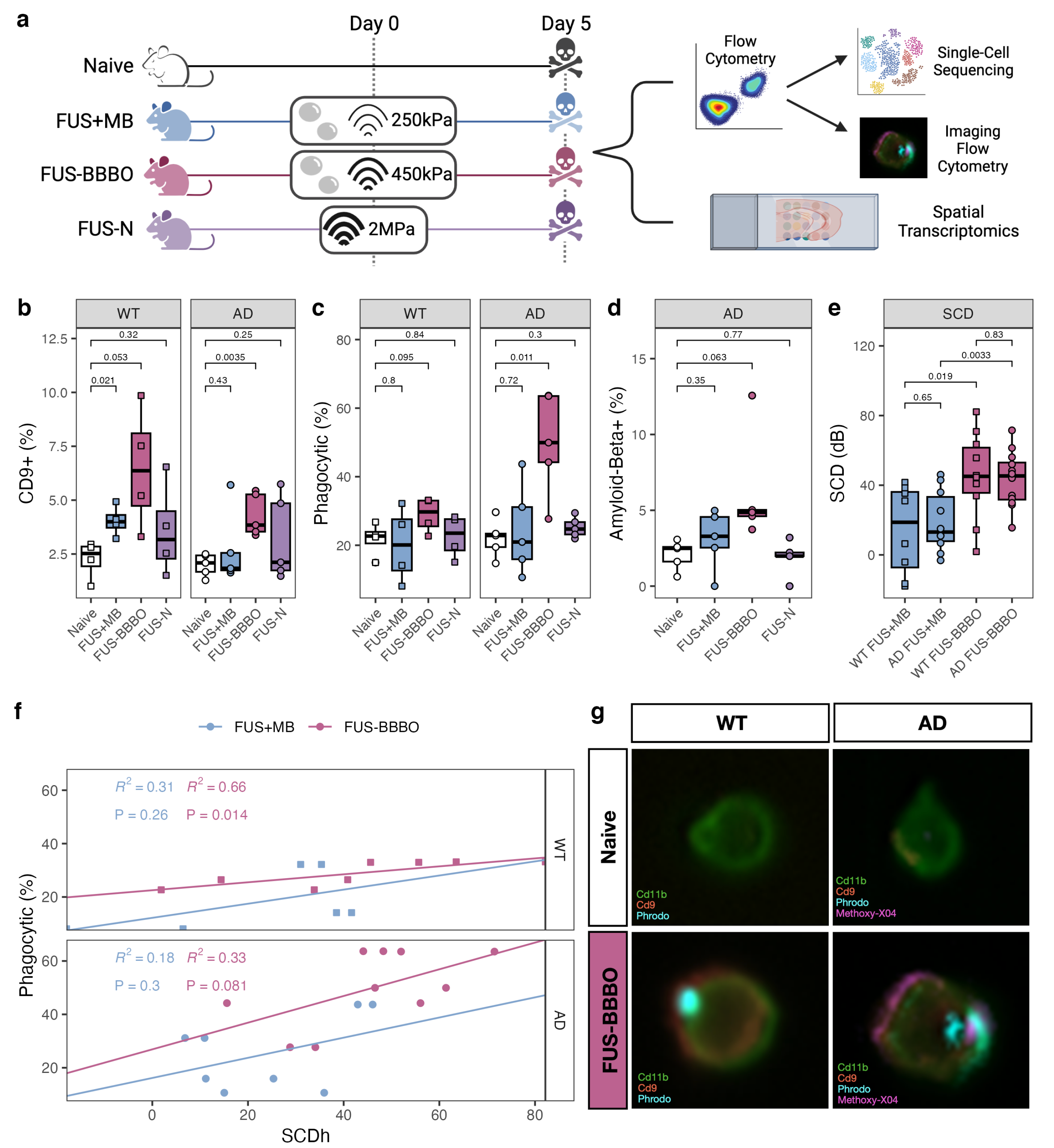
3.1. FUS-BBBO Maximizes Brain Macrophage Response
3.2. FUS-BBBO Alters Brain Macrophage Cluster Distribution
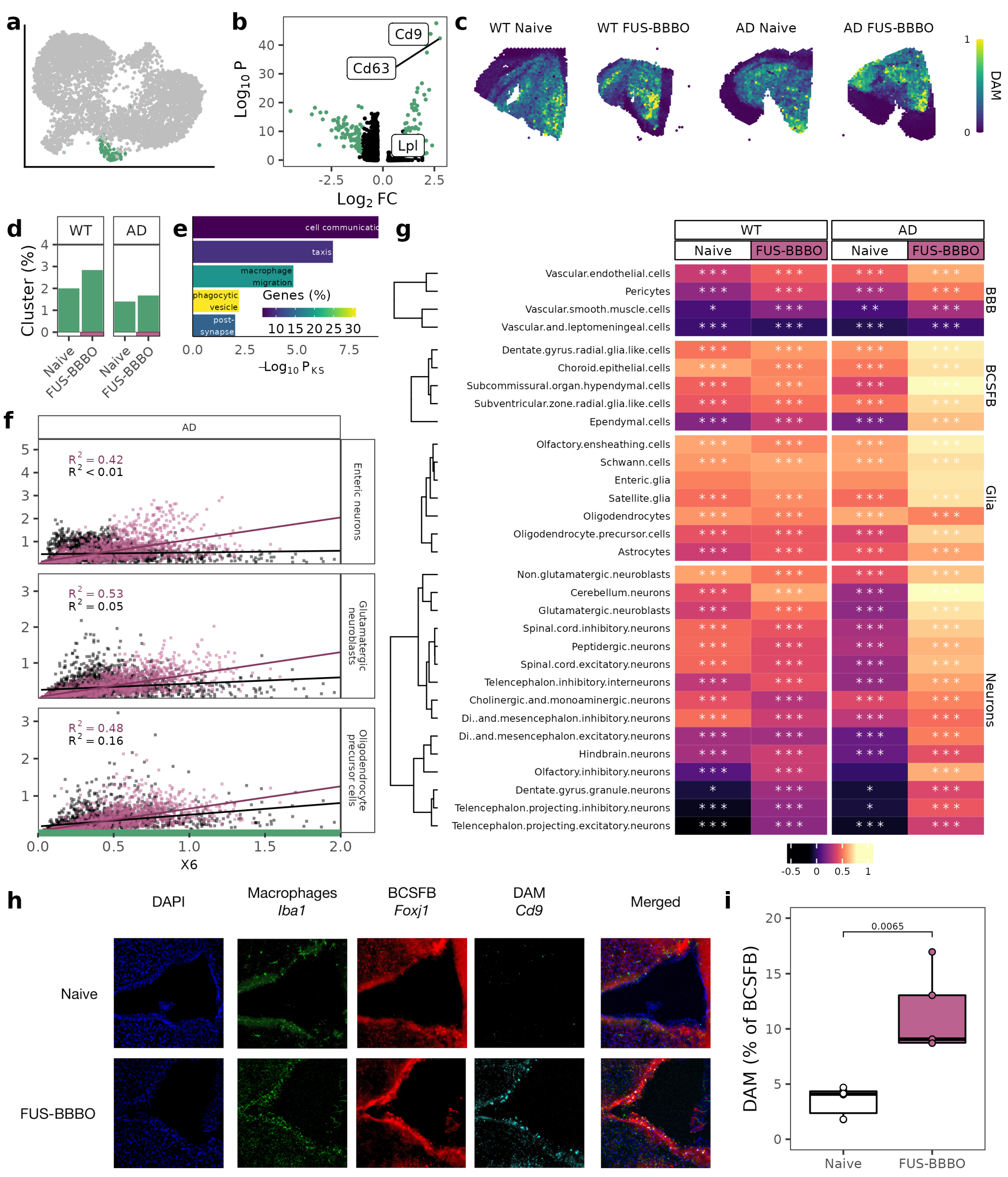
3.3. FUS-BBBO Alters DAM Cellular Associations
3.4. FUS-BBBO Alters IAM Cellular Associations
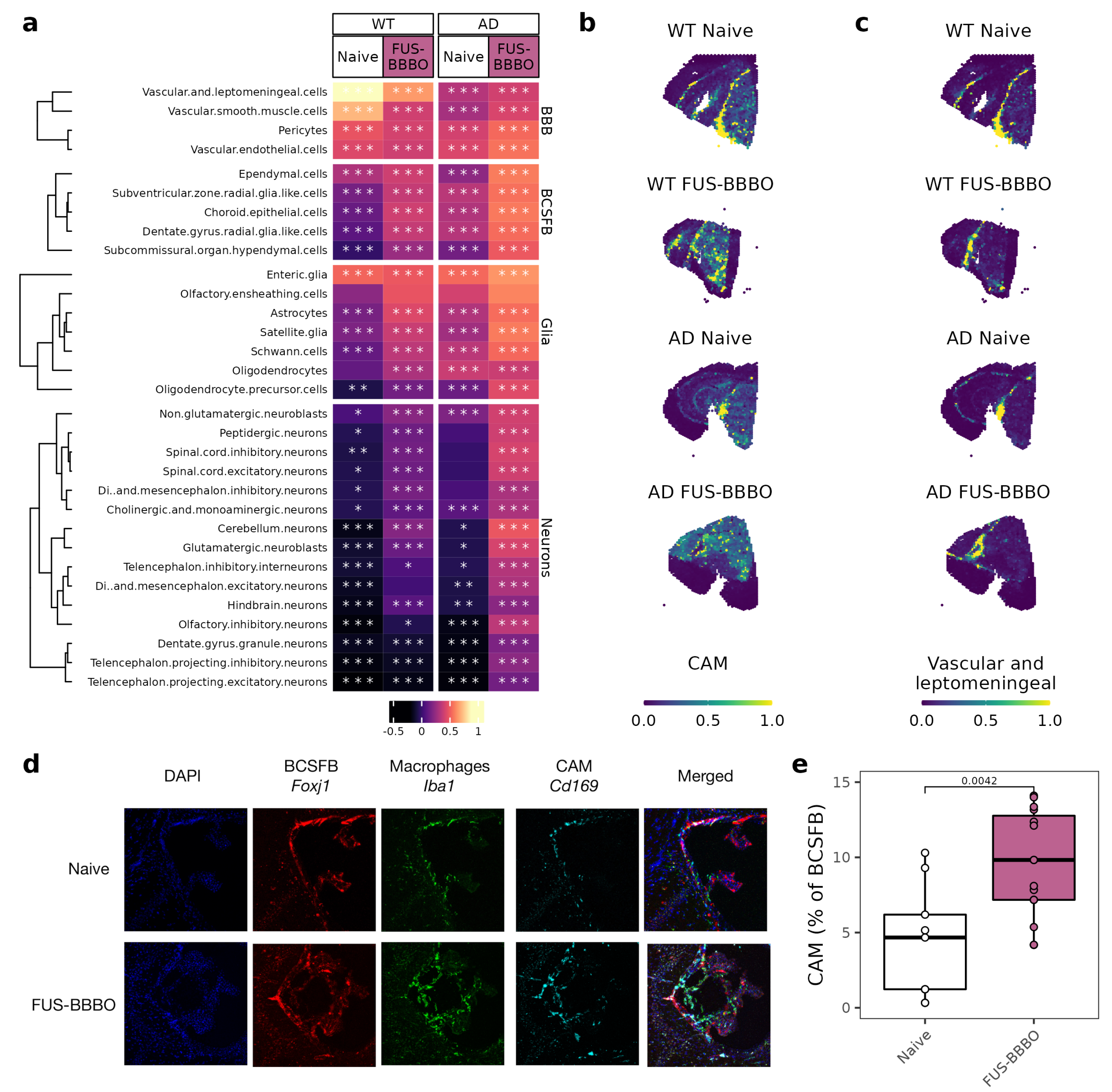
3.5. FUS-BBBO Alters CAM Cellular Associations
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBB | Blood–brain barrier |
| FUS | Focused ultrasound |
| MB | Microbubbles |
| FUS-BBBO | Focused ultrasound blood–brain barrier opening |
| FUS-N | Focused ultrasound neuromodulation |
| FUS + MB | Focused ultrasound and microbubbles |
| AD | Alzheimer’s disease |
| WT | Wild-type |
| BCSFB | Brain–cerebrospinal fluid barrier |
| SCD | Stable cavitation dose |
| DAM | Disease-associated microglia |
| IAM | Interferon-associated microglia |
| CAM | Central-nervous-system-associated macrophages |
| UMAP | Uniform Manifold Approximation and Projection |
Appendix A

References
- Konofagou, E.E.; Tung, Y.S.; Choi, J.; Deffieux, T.; Baseri, B.; Vlachosa, F. Ultrasound-induced blood–brain barrier opening. Curr. Pharm. Biotechnol. 2012, 13, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Hua, L.; Yeh, C.K.; Shen, L.; Ying, M.; Zhang, Z.; Liu, G.; Li, S.; Chen, S.; Chen, X.; et al. Ultrasound with microbubbles improves memory, ameliorates pathology and modulates hippocampal proteomic changes in a triple transgenic mouse model of Alzheimer’s disease. Theranostics 2020, 10, 11794–11819. [Google Scholar] [CrossRef] [PubMed]
- Karakatsani, M.E.; Kugelman, T.; Ji, R.; Murillo, M.; Wang, S.; Niimi, Y.; Small, S.A.; Duff, K.E.; Konofagou, E.E. Unilateral Focused Ultrasound-Induced blood–brain Barrier Opening Reduces Phosphorylated Tau from The rTg4510 Mouse Model. Theranostics 2019, 9, 5396–5411. [Google Scholar] [CrossRef] [PubMed]
- Leinenga, G.; Gotz, J. Scanning ultrasound removes amyloid-beta and restores memory in an Alzheimer’s disease mouse model. Sci. Transl. Med. 2015, 7, 278–293. [Google Scholar] [CrossRef]
- Burgess, A.; Dubey, S.; Yeung, S.; Hough, O.; Eterman, N.; Aubert, I.; Hynynen, K. Alzheimer Disease in a Mouse Model: MR Imaging–guided Focused Ultrasound Targeted to the Hippocampus Opens the blood–brain Barrier and Improves Pathologic Abnormalities and Behavior. Radiology 2014, 273, 736–745. [Google Scholar] [CrossRef]
- Mooney, S.J.; Nobrega, J.N.; Levitt, A.J.; Hynynen, K. Antidepressant effects of focused ultrasound induced blood–brain-barrier opening. Behav. Brain Res. 2018, 16, 57–61. [Google Scholar] [CrossRef]
- Choi, J.J.; Wang, S.; Brown, T.R.; Small, S.A.; Duff, K.E.K.; Konofagou, E.E. Noninvasive and transient blood–brain barrier opening in the hippocampus of Alzheimer’s double transgenic mice using focused ultrasound. Ultrason. Imaging 2008, 30, 189–200. [Google Scholar] [CrossRef]
- Mooney, S.J.; Shah, K.; Yeung, S.; Burgess, A.; Aubert, I.; Hynynen, K. Focused ultrasound-induced neurogenesis requires an increase in blood–brain barrier permeability. PLoS ONE 2016, 11, e0159892. [Google Scholar] [CrossRef]
- Shin, J.; Kong, C.; Lee, J.; Choi, B.Y.; Sim, J.; Koh, C.S.; Park, M.; Na, Y.C.; Suh, S.W.; Chang, W.S.; et al. Focused ultrasound-induced blood–brain barrier opening improves adult hippocampal neurogenesis and cognitive function in a cholinergic degeneration dementia rat model. Alzheimer’S Res. Ther. 2019, 11, 110. [Google Scholar] [CrossRef]
- Karakatsani, M.E. Quantitative Analysis of the Focused Ultrasound-Induced Blood–Brain Barrier Opening with Applications in Neurodegenerative Disorders; Columbia Library: New York, NY, USA, 2020. [Google Scholar]
- Joiner, J.B.; Kren, N.P.; Durham, P.G.; McRee, A.J.; Dayton, P.A.; Pylayeva-Gupta, Y. Low-Intensity Focused Ultrasound Produces Immune Response in Pancreatic Cancer. Ultrasound Med. Biol. 2022, 48, 2344–2353. [Google Scholar] [CrossRef]
- Burks, S.R.; Lorsung, R.M.; Nagle, M.E.; Tu, T.W.; Frank, J.A. Focused ultrasound activates voltage-gated calcium channels through depolarizing TRPC1 sodium currents in kidney and skeletal muscle. Theranostics 2019, 9, 5517–5531. [Google Scholar] [CrossRef]
- Sun, T.; Samiotaki, G.; Wang, S.; Acosta, C.; Chen, C.C.; Konofagou, E.E. Acoustic Cavitation-Based Monitoring of the Reversibility and Permeability of Ultrasound-Induced blood–brain Barrier Opening. Phys. Med. Biol. 2015, 60, 9079–9094. [Google Scholar] [CrossRef]
- Tung, Y.S.; Vlachos, F.; Choi, J.J.; Deffieux, T.; Selert, K.; Konofagou, E.E. In vivo transcranial cavitation threshold detection during ultrasound-induced blood–brain barrier opening in mice. Phys. Med. Biol. 2010, 55, 6141–6155. [Google Scholar] [CrossRef]
- Ji, R.; Karakatsani, M.E.; Burgess, M.; Smith, M.; Murillo, M.F.; Konofagou, E.E. Cavitation modulated inflammatory response following focused ultrasound blood–brain barrier opening. J. Control. Release 2021, 337, 458–471. [Google Scholar] [CrossRef]
- Oh, S.J.; Lee, J.M.; Kim, H.B.; Lee, J.; Han, S.; Bae, J.Y.; Hong, G.S.; Koh, W.; Kwon, J.; Hwang, E.S.; et al. Ultrasonic Neuromodulation via Astrocytic TRPA1. Curr. Biol. 2019, 29, 3386–3401. [Google Scholar] [CrossRef]
- Bobola, M.; Chen, L.; Ezeokeke, C.; Olmstead, T.; Nguyen, C.; Sahota, A.; Williams, R.G.; Mourad, P. Transcranial focused ultrasound, pulsed at 40 Hz, activates microglia acutely and reduces Aβ load chronically, as demonstrated in vivo. Brain Stimul. 2020, 13, 1014–1023. [Google Scholar] [CrossRef]
- Jeong, H.; Im, J.J.; Park, J.S.; Na, S.H.; Lee, W.; Yoo, S.S.; Song, I.U.; Chung, Y.A. A pilot clinical study of low-intensity transcranial focused ultrasound in Alzheimer’s disease. Ultrasonography 2021, 40, 512–519. [Google Scholar] [CrossRef]
- Eguchi, K.; Shindo, T.; Ito, K.; Ogata, T.; Kurosawa, R.; Kagaya, Y.; Monma, Y.; Ichijo, S.; Kasukabe, S.; Miyata, S.; et al. Whole-brain low-intensity pulsed ultrasound therapy markedly improves cognitive dysfunctions in mouse models of dementia—Crucial roles of endothelial nitric oxide synthase. Brain Stimul. 2018, 11, 959–973. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s disease. Cell 2017, 169, 1276–1290. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Z.; Zhou, L.; Darmanis, S.; Neff, N.F.; Okamoto, J.; Gulati, G.; Bennett, M.L.; Sun, L.O.; Clarke, L.E.; et al. Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron 2019, 101, 207–223. [Google Scholar] [CrossRef]
- Hove, H.V.; Martens, L.; Scheyltjens, I.; Vlaminck, K.D.; Antunes, A.R.P.; Prijck, S.D.; Vandamme, N.; Schepper, S.D.; Isterdael, G.V.; Scott, C.L.; et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 2019, 22, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Bottcher, C.; Amann, L.; Sagar; Scheiwe, C.; Nessler, S.; Kunz, P.; van Loo, G.; et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 2019, 566, 388–392. [Google Scholar] [CrossRef]
- Tan, Y.L.; Yuan, Y.; Tian, L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 2020, 25, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Matcovitch-Natan, O.; Winter, D.R.; Giladi, A.; Aguilar, S.V.; Spinrad, A.; Sarrazin, S.; Ben-Yehuda, H.; David, E.; González, F.Z.; Perrin, P.; et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016, 353, 6301. [Google Scholar] [CrossRef] [PubMed]
- Sevenich, L. Brain-Resident Microglia and Blood-Borne Macrophages Orchestrate Central Nervous System Inflammation in Neurodegenerative Disorders and Brain Cancer. Front. Immunol. 2018, 9, 697. [Google Scholar] [CrossRef]
- Zelco, A.; Börjesson, V.; de Kanter, J.K.; Lebrero-Fernandez, C.; Lauschke, V.M.; Rocha-Ferreira, E.; Nilsson, G.; Nair, S.; Svedin, P.; Bemark, M.; et al. Single-cell atlas reveals meningeal leukocyte heterogeneity in the developing mouse brain. Genes Dev. 2021, 10, 1190–1207. [Google Scholar] [CrossRef]
- Deczkowska, A.; Keren-Shaul, H.; Weiner, A.; Colonna, M.; Schwartz, M.; Amit, I. Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell 2018, 173, 1073–1081. [Google Scholar] [CrossRef]
- Dorman, L.C.; Nguyen, P.T.; Escoubas, C.C.; Vainchtein, I.D.; Xiao, Y.; Lidsky, P.V.; Nakajo, H.; Silva, N.J.; Lagares-Linares, C.; Wang, E.Y.; et al. A type I interferon response defines a conserved microglial state required for effective neuronal phagocytosis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kleshchevnikov, V.; Shmatko, A.; Dann, E.; Aivazidis, A.; King, H.W.; Li, T.; Elmentaite, R.; Lomakin, A.; Kedlian, V.; Gayoso, A.; et al. Cell2location maps fine-grained cell types in spatial transcriptomics. Nat. Biotechnol. 2022, 40, 661–671. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Guo, C.; Xu, H.; Li, L.; Fang, M.; Hu, Y.; Zhang, X.; Yao, X.; Tang, M.; et al. Benchmarking spatial and single-cell transcriptomics integration methods for transcript distribution prediction and cell type deconvolution. Nat. Methods 2022, 19, 662–670. [Google Scholar] [CrossRef]
- Solar, P.; Zamani, A.; Kubickova, L.; Dubovy, P.; Joukal, M. Choroid plexus and the blood–cerebrospinal fluid barrier in disease. Fluids Barriers CNS 2020, 17, 35. [Google Scholar] [CrossRef]
- Ayub, M.; Jin, H.K.; Bae, J.S. The blood cerebrospinal fluid barrier orchestrates immunosurveillance, immunoprotection, and immunopathology in the central nervous system. BMB Rep. 2021, 54, 196–202. [Google Scholar] [CrossRef]
- Gonzalez-Marrero, I.; Gimenez-Llort, L.; Johanson, C.E.; Carmona-Calero, E.M.; Castaneyra-Ruiz, L.; Brito-Armas, J.M.; Castaneyra-Perdomo, A.; Castro-Fuentes, R. Choroid plexus dysfunction impairs beta-amyloid clearance in a triple transgenic mouse model of Alzheimer’s disease. Front. Cell. Neurosci. 2015, 9, 17. [Google Scholar] [CrossRef]
- Capozza, M.A.; Triarico, S.; Mastrangelo, S.; Attinà, G.; Maurizi, P.; Ruggiero, A. Narrative review of intrathecal drug delivery (IDD): Indications, devices and potential complications. Ann. Transl. Med. 2019, 9, 186. [Google Scholar] [CrossRef]
- Aryal, M.; Azadian, M.M.; Hart, A.R.; Macedo, N.; Zhou, Q.; Rosenthal, E.L.; Airan, R.D. Noninvasive ultrasonic induction of cerebrospinal fluid flow enhances intrathecal drug delivery. J. Control. Release 2022, 349, 434–442. [Google Scholar] [CrossRef]
- Focused-ultrasound mediated blood brain barrier opening remodels the immune landscape via microglia proliferation and central-nervous-system-associated macrophage recruitment. Nat. Biomed. Eng. 2023; in press.
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Choi, J.J.; Pernot, M.; Small, S.A.; Konofagou, E.E. Noninvasive, transcranial and localized opening of the blood–brain barrier using focused ultrasound in mice. Ultrasound Med. Biol. 2007, 33, 95–104. [Google Scholar] [CrossRef]
- Grubman, A.; Choo, X.Y.; Chew, G.; Ouyang, J.F.; Sun, G.; Croft, N.P.; Rossello, F.J.; Simmons, R.; Buckberry, S.; Landin, D.V.; et al. Transcriptional signature in microglia associated with Amyloid-Beta plaque phagocytosis. Nat. Commun. 2019, 12, 3015. [Google Scholar] [CrossRef]
- Lau, S.F.; Wu, W.; Seo, H.; Fu, A.K.; Ip, N.Y. Quantitative in vivo assessment of amyloid-beta phagocytic capacity in an Alzheimer’s disease mouse model. STAR Protoc. 2021, 2, 100265. [Google Scholar] [CrossRef]
- Zheng, G.X.; Terry, J.M.; Belgrader, P.; Ryvkin, P.; Bent, Z.W.; Wilson, R.; Ziraldo, S.B.; Wheeler, T.D.; McDermott, G.P.; Zhu, J.; et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 2017, 8, 14049. [Google Scholar] [CrossRef] [PubMed]
- Menon, V. Clustering single cells: A review of approaches on high-and low-depth single-cell RNA-seq data. Brief. Funct. Genom. 2019, 17, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, A.; Hochgerner, H.; Lönnerberg, P.; Johnsson, A.; Memic, F.; van der Zwan, J.; Häring, M.; Braun, E.; Borm, L.E.; Manno, G.L.; et al. Molecular Architecture of the Mouse Nervous System. Cell 2018, 174, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Fairn, G.D.; Grinstein, S. How nascent phagosomes mature to become phagolysosomes. Cell 2012, 33, 397–405. [Google Scholar] [CrossRef]
- Hafemeister, C.; Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019, 20, 296. [Google Scholar] [CrossRef]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional Microglia–Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018, 12, 323. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Rosin, J.M.; Marsters, C.M.; Malik, F.; Far, R.; Adnani, L.; Schuurmans, C.; Pittman, Q.J.; Kurrasch, D.M. Embryonic Microglia Interact with Hypothalamic Radial Glia during Development and Upregulate the TAM Receptors MERTK and AXL following an Insult. Cell Rep. 2021, 34, 108587. [Google Scholar] [CrossRef]
- Fernández-Arjona, M.D.M.; León-Rodríguez, A.; López-Ávalos, M.D.; Grondona, J.M. Microglia activated by microbial neuraminidase contributes to ependymal cell death. Fluids Barriers CNS 2021, 18, 15. [Google Scholar] [CrossRef]
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s disease: A matter of blood–brain barrier dysfunction. J. Exp. Med. 2017, 214, 3151–3169. [Google Scholar] [CrossRef]
- Gotz, J.; Richter-Stretton, G.; Cruz, E. Therapeutic Ultrasound as a Treatment Modality for Physiological and Pathological Ageing Including Alzheimer’s Disease. Pharmaceutics 2021, 13, 1002. [Google Scholar] [CrossRef]
- Jordao, J.F.; Thevenot, E.; Markham-Coultes, K.; Scarcelli, T.; Weng, Y.Q.; Xhima, K.; O’Reilly, M.; Huang, Y.; McLaurin, J.; Hynynen, K.; et al. Amyloid-Beta plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp. Neurol. 2013, 248, 16–29. [Google Scholar] [CrossRef]
- Stavarache, M.A.; Petersen, N.; Jurgens, E.M.; Milstein, E.R.; Rosenfeld, Z.B.; Ballon, D.J.; Kaplitt, M.G. Safe and stable noninvasive focal gene delivery to the mammalian brain following focused ultrasound. J. Neurosurg. 2018, 130, 989–998. [Google Scholar] [CrossRef]
- Scarcelli, T.; Jordão, J.F.; O’Reilly, M.A.; Ellens, N.; Hynynen, K.; Aubert, I. Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul. 2014, 7, 304–307. [Google Scholar] [CrossRef]
- Kovacs, Z.I.; Tu, T.W.; Sundby, M.; Qureshi, F.; Lewis, B.K.; Jikaria, N.; Burks, S.R.; Frank, J.A. MRI and histological evaluation of pulsed focused ultrasound and microbubbles treatment effects in the brain. Theranostics 2018, 8, 4837–4855. [Google Scholar] [CrossRef]
- Huang, Y.; Meng, Y.; Pople, C.B.; Bethune, A.; Jones, R.M.; Abrahao, A.; Hamani, C.; Kalia, S.K.; Kalia, L.V.; Lipsman, N.; et al. Cavitation Feedback Control of Focused Ultrasound blood–brain Barrier Opening for Drug Delivery in Patients with Parkinson’s Disease. Pharmaceutics 2022, 14, 2607. [Google Scholar] [CrossRef]
- Rezai, A.R.; Ranjan, M.; Haut, M.W.; Carpenter, J.; D’Haese, P.F.; Mehta, R.I.; Najib, U.; Wang, P.; Claassen, D.O.; Chazen, J.L.; et al. Focused ultrasound-mediated blood–brain barrier opening in Alzheimer’s disease: Long-term safety, imaging, and cognitive outcomes. J. Neurosurg. 2022, 1, 1–9. [Google Scholar] [CrossRef]


| Use | Target | Concentration | Company | Reference |
|---|---|---|---|---|
| FC | CD11B-PE | 1:100 | Invitrogen | 12-0112-82 |
| FC | CD45-APC | 1:100 | Invitrogen | 17-0451-82 |
| IHC/FC | CD9-PE | 1:1000/1:100 | Biolegend | 124806 |
| IHC | CD169-PE | 1:200 | Biolegend | 142404 |
| IHC | NEUN-Cy3 | 1:500 | MilliporeSigma | MAB377C3 |
| IHC | IBA1-AF488 | 1:200 | MilliporeSigma | MABN92AF488 |
| IHC | Gt IBA1 | 1:500 | abcam | ab5076 |
| IHC | Rb IFITM3 | 1:1000 | Proteintech | 117141AP |
| IHC | Ms FOXJ1 | 1:1000 | Invitrogen | 14996582 |
| IHC | Dk anti-Goat FITC | 1:1000 | Invitrogen | A-11055 |
| IHC | Dk anti-Rb DY647 | 1:1000 | abcam | ab150079 |
| IHC | Dk anti-Ms TxRed | 1:1000 | Invitrogen | ab6818 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kline-Schoder, A.R.; Noel, R.L.; Phatnani, H.; Menon, V.; Konofagou, E.E. Focused Ultrasound-Mediated Blood–Brain Barrier Opening Best Promotes Neuroimmunomodulation through Brain Macrophage Redistribution. Neuroglia 2023, 4, 141-157. https://doi.org/10.3390/neuroglia4020010
Kline-Schoder AR, Noel RL, Phatnani H, Menon V, Konofagou EE. Focused Ultrasound-Mediated Blood–Brain Barrier Opening Best Promotes Neuroimmunomodulation through Brain Macrophage Redistribution. Neuroglia. 2023; 4(2):141-157. https://doi.org/10.3390/neuroglia4020010
Chicago/Turabian StyleKline-Schoder, Alina R., Rebecca L. Noel, Hemali Phatnani, Vilas Menon, and Elisa E. Konofagou. 2023. "Focused Ultrasound-Mediated Blood–Brain Barrier Opening Best Promotes Neuroimmunomodulation through Brain Macrophage Redistribution" Neuroglia 4, no. 2: 141-157. https://doi.org/10.3390/neuroglia4020010
APA StyleKline-Schoder, A. R., Noel, R. L., Phatnani, H., Menon, V., & Konofagou, E. E. (2023). Focused Ultrasound-Mediated Blood–Brain Barrier Opening Best Promotes Neuroimmunomodulation through Brain Macrophage Redistribution. Neuroglia, 4(2), 141-157. https://doi.org/10.3390/neuroglia4020010





