Abstract
Glioblastoma (GBM) is the most commonly occurring type of primary tumor of the central nervous system (CNS) and is considered the worst type of glioma. Despite the current standard treatment for newly diagnosed GBM, which involves surgery followed by chemotherapy with temozolomide (TMZ) and radiation therapy, the average survival time for patients with GBM is only about 15 months. This is due to GBM’s tendency to recur, its high proliferative rates, its ability to evade apoptosis, and its ability to invade healthy tissue. Therefore, it is crucial to explore new treatment options for GBM. This study investigated the potential anticancer activities of a new series of synthetic chalcones, which are natural compounds found in the biosynthesis of flavonoids in plants. Primary cell culture of glioblastoma (GBM1) from surgical resection was used to evaluate the effects of synthetic chalcones on viability, cell death, reactive oxygen species (ROS), mitochondrial membrane potential (ΔΨm), cell cycle, and invasion. One chalcone, Q1VA (at concentrations of 10, 50, and 100 μM for 24 h) induced cytotoxicity by increasing apoptosis levels and depolarizing the mitochondrial membrane, as evidenced by a TMRE assay. Further analysis using the molecular fluorescent probe H2DCFDA indicated that the increased levels of reactive oxygen species (ROS) might be linked to altered mitochondrial membrane potential and cell death. Furthermore, viable cells were observed to be delayed in the cell cycle, primarily in the M phase, and the invasion process was reduced. The findings of this study indicate that Q1VA is a potential adjuvant therapeutic agent for GBM due to its significant antitumor effects. If its safety and efficacy can be confirmed in animal models, Q1VA may be considered for clinical trials in humans. However, additional research is required to determine the optimal dosage, treatment schedule, and potential side effects of Q1VA.
1. Introduction
Gliomas are the most common type of central nervous system (CNS) neoplasm, arising from glial cells that grow uncontrollably and affect both the brain and spinal cord. According to their histopathological characteristics, gliomas are classified by the WHO into different grades (I–IV). Glioblastoma (GBM) is the most aggressive form (IV) and the most common malignant primary brain tumor.
The standard treatment for patients with newly diagnosed GBM is surgery, followed by chemotherapy with temozolomide (TMZ) plus radiotherapy. However, neither treatment nor surgery has helped increase the life expectancy of the patients. Although aggressive treatments are used, the average survival time for patients with GBM remains around 14 months, mainly due to tumor recurrence as surgery fails to remove all tumor cells [1].
GBM includes a high degree of intratumorally cellular heterogeneity and a high rate of recurrence [2]. Within the tumor are subpopulations of cells that express markers associated with tumor-initiating cells, such as CD133, CD15, and ABCG2. These cells exhibit longstanding proliferation, self-renewal potential, and resistance to therapy, which contribute to the therapeutic resistance of GBM. This is a significant challenge because even a single tumor-initiating cell can repopulate the entire tumor [3].
The resistance to treatment can also be attributed to the cancer stem cell (CSC) hypothesis [4]. CSCs can promote radio and chemoresistance, as well as tumor recurrence by repopulating the tumor [5]. For instance, CSCs present in GBM are known to contribute to the resistance to chemotherapy because they have efficient drug efflux transport systems, especially the ATP Binding Cassette (ABC) transporters [6]. These ABC transporters are a family of transmembrane proteins that function as drug efflux pumps, transporting a wide range of endogenous and exogenous molecules out of cells by harnessing the energy from ATP hydrolysis. ABC transporters play a crucial role in the development of multidrug resistance (MDR) in cancer cells, which can lead to chemotherapy failure and poor prognosis. They are expressed at high levels in various types of cancer, including leukemia, breast, and colon cancer, and contribute to the efflux of chemotherapeutic agents from cancer cells, reducing their intracellular concentration and efficacy [7]. ABC transporter inhibitors are currently being investigated as potential therapeutic agents to overcome MDR and improve cancer treatment outcomes.
The biology of GBM cells is characterized by their invasive and proliferative nature, as well as their ability to reprogram cell functions. They can evade apoptosis by reducing the expression of pro-apoptotic proteins (BAX) and increasing the levels of anti-apoptotic proteins (BCL-2). This allows GBM cells to invade the healthy tissue surrounding the tumor, making complete surgical resection difficult. The high proliferation rates of GBM cells lead to an increased tumor mass, which in turn results in the release of protumor and angiogenic factors [8,9].
Despite the widespread use of TMZ, its effects are limited due to the presence of the blood–brain barrier (BBB). The BBB controls the distribution of chemotherapy and reduces the availability of treatment options. Multiple factors are involved in the drug concentration in the CNS such as the permeability of the BBB and the drug concentration and delivery method. As a result, numerous studies have been conducted to find better treatments, including studies of epigenetics, immunotherapy, biochemistry, and the development of new cytotoxic molecules [10,11,12,13,14]. However, effective alternative options are still not available.
Chalcones are a type of natural compound that have shown potential as adjuvant antitumoral agents. These compounds are precursors in the biosynthesis of flavonoids in plants and can be synthesized by condensing aryl ketones with aromatic aldehydes in the presence of a condensing agent. Chalcones have been used to synthesize a variety of synthetic heterocyclic compounds and have been demonstrated to have biological effects such as antimicrobial, anti-inflammatory, analgesic, and antileukemic properties [15].
Chalcones (1,3-diaryl-2-propen-1-ones) can be modified with different functional groups, such as aryls, hydroxyls, carboxyls, etc., to change their target molecules and produce distinct biological activities. This versatility makes chalcones a useful tool in medicinal chemistry for drug discovery. Both natural and synthetic chalcone compounds have shown various interesting biological activities such as anti-inflammatory, antimalarial, antiviral, and anticancer [10,16,17,18]. For instance, Sinha and collaborators investigate the antimalarial potential of three synthetic chalcones and demonstrate that chalcones can reduce the levels of Plasmodium berghei infection after 8 days post-infection when compared to the control [19]. Furthermore, the effect of another synthetic chalcone was observed on bacterial Gram-positive (both non-resistant and resistant) and Gram-negative bacteria, showing a capacity to inhibit efflux pumps and prevent biofilm formation [20]. In terms of anti-inflammatory properties, several chalcones were found to modulate transcript factors, and it was also demonstrated that chalcones have the ability to inhibit Cyclooxygenase-2 (COX-2) [21].
Studies on the use of chalcones as an antitumoral agent have demonstrated the ability of various types of chalcones to inhibit cell proliferation and block cells in the M phase of the cell cycle. This is due to their activity as a microtubule depolymerizing agent [22,23,24,25,26]. The proper segregation of chromosomes during cell division is essential for the maintenance of genomic stability, and defects in this process can lead to various diseases, including cancer. The mitotic spindle, a complex and dynamic macromolecular machine, plays a crucial role in the accurate segregation of chromosomes during mitosis. It is primarily composed of microtubules, which are dynamic filaments that undergo polymerization and depolymerization to form a highly organized structure that interacts with chromosomes [27]. In glioblastomas, aberrant spindle assembly and defects in chromosome segregation have been reported, leading to genomic instability. These defects are often associated with alterations in key spindle regulators and checkpoint proteins, such as Aurora kinases and Polo-like kinases, which are frequently overexpressed or mutated in glioblastoma [28,29,30].
Previous studies from our laboratory evaluated the effects of three synthetic chalcones (A23, C31, and J11) on the glioma cell line A-172 (ATCC CRL-1620) and surgery-obtained GBM cells. The treatment resulted in an increase in apoptosis with no further increase in necrosis. This effect was associated with oxidative stress caused by the increased presence of reactive oxygen species and nitric oxide production. The effects of chalcones on cell cycle regulation showed an arrest at G0/G1 and S phases, suggesting that chalcone C31 interferes with cell cycle control [31]. Chalcones are a class of compounds with diverse chemical structures that have shown promising anticancer activity in various studies. The aim of this study was to evaluate the effects of a new series of synthetic chalcones on GBM cells with the goal to discover new adjuvant drugs that can improve the efficacy of current treatments and ultimately improve the prognosis of patients with brain cancer.
2. Materials and Methods
2.1. Synthesis and Characterization of Chalcones
The chalcones Q1VA, Q2VA, and Q18VA were prepared using an aldol condensation. Aldol condensation refers to condensation of ketones and aldehydes with a catalyst followed with a dehydration reaction to form C-C bonds [32]. The aldol condensation was between 4-carboxybenzaldehyde (1 mmol) and corresponding acetophenones (1 mmol) using p-toluene sulfonic acid (PTSA) as a catalyst and methanol as a solvent. The reaction was maintained under reflux for 24 h, and when finished, the precipitation was performed in distilled water for faster and interference-free precipitation of the compounds, because regular water contains minerals that can cause slow precipitation [33].
All compounds were identified with melting point determination, 1H, and 13C Nuclear Magnetic Resonance (NMR) spectroscopy, and High-Resolution Mass Spectra (HRMS), according to our previous studies and supporting data [34].
NMR is a characterization technique used to identify the carbons and hydrogens present in the studied structure. HRMS, a mass detector, enables the analysis of the molecular fragments from each component of the sample, providing a powerful tool for identifying unknown compounds and determining purity together with the NMR.
In summary, melting points were determined using a Microquimica MGAPF-301 apparatus. The 1H and 13C NMR spectra were recorded using a Bruker Avance DRX 400 spectrometer (operating at 400 MHz for 1H and 100 MHz for 13C) using tetramethylsilane as the internal standard. HRMS were recorded using a micrOTOF-QII mass spectrometer (Bruker Daltonics) equipped with an automatic syringe pump (KD Scientific) for sample injection (constant flow of 3 μL min−1) in the positive mode of Atmospheric Pressure Photoionization (APPI) (1.0 kV) using acetone as solvent. The instrument was calibrated in the m/z range of 50 to 3000 using a low-concentration tuning mix solution (Agilent Technologies). Data were processed using Bruker Data Analysis software version 4.0. When the calculated and experimental masses were compared, the error was within 2 ppm.
Data on the characterization of chalcones Q1VA, Q2VA, and Q18VA are presented below.
Q1VA: (E)-methyl 4-(3-oxo-3-phenylprop-1-en-1-yl) benzoate. Yield: 53%. Yellow solid. mp 118.3–118.9 °C; 1H NMR (400 MHz, CDCl3) δ (ppm): 8.09 (2H, d, J = 8.0 Hz); 8.04 (2H, d, J = 8.0 Hz); 7.82 (1H, d, J = 15.6 Hz); 7.71 (2H, d, J = 8.0 Hz); 7.62 (m, 1H); 7.61 (2H, d, J = 15.6 Hz); 7.52 (2H, d, J = 8.0 Hz); 3.94 (3H, s); 13C NMR (100 MHz, CDCl3) δ (ppm): 189.9; 166.1; 142.8; 138.7; 137.5; 132.7; 131.2; 129.8 (2); 127.9 (2); 128.4 (2); 128.2 (2); 123.7; 52.0. HRMS (APPI -TOF) m/z: 267.1022 [M + H]+, calculated for C17H14O3, 267.1016.
Q2VA: (E)-methyl 4-(3-oxo-3-(p-tolyl)prop-1-en-1-yl)benzoate. Yield: 51%. Light yellow solid. mp 154.1–154.9 °C; 1H NMR (400 MHz, CDCl3) δ (ppm): 8.07 (2H, d, J = 8.0 Hz); 7.94 (2H, d, J = 8.0 Hz); 7.80 (1H, d, J = 16.0 Hz); 7.69 (2H, d, J = 8.0 Hz); 7.60 (1H, d, J = 16.0 Hz); 7.31 (2H, d, J = 8.0 Hz); 3.93 (3H, s); 2.43 (3H, s); 13C NMR (100 MHz, CDCl3) δ (ppm): 189.5; 166.4; 144.0; 142.7; 139.2; 135.3; 131.4; 130.1 (2); 129.4 (2); 128.7 (2); 128.2 (2); 124.1; 52.3; 21.7. HRMS (APPI -TOF) m/z: 281.1174 [M + H]+, calculated for C18H16O3, 281.1172.
Q18VA:(E)-methyl4-(3-(4-hydroxy-3,5-dimethoxyphenyl)-3-oxoprop-1-en-1-yl)benzoate. Yield 12%. Yellow solid. mp 149.0–149.8; 1H NMR (400 MHz, CDCl3) δ (ppm): 8.08 (2H, d, J = 8.0 Hz); 7.81 (1H, d, J = 15.6 Hz); 7.70 (2H, d, J = 8.0 Hz); 7.57 (1H, d, J = 15.6 Hz); 7.33 (2H, d, J = 8.0 Hz); 6.01 (1H, s, OH); 3.99 (6H, s); 3.94 (3H, s); 13C NMR (100 MHz, CDCl3) δ (ppm): 188.2; 166.5; 147.0 (2); 142.8; 140.0; 139.3; 130.2 (2); 131.5; 129.5; 128.3 (2); 123.7; 106.1 (2); 56.7 (2); 52.4. HRMS (APPI -TOF) m/z: 343.1174 [M + H]+, calculated for C19H18O6, 343.1176.
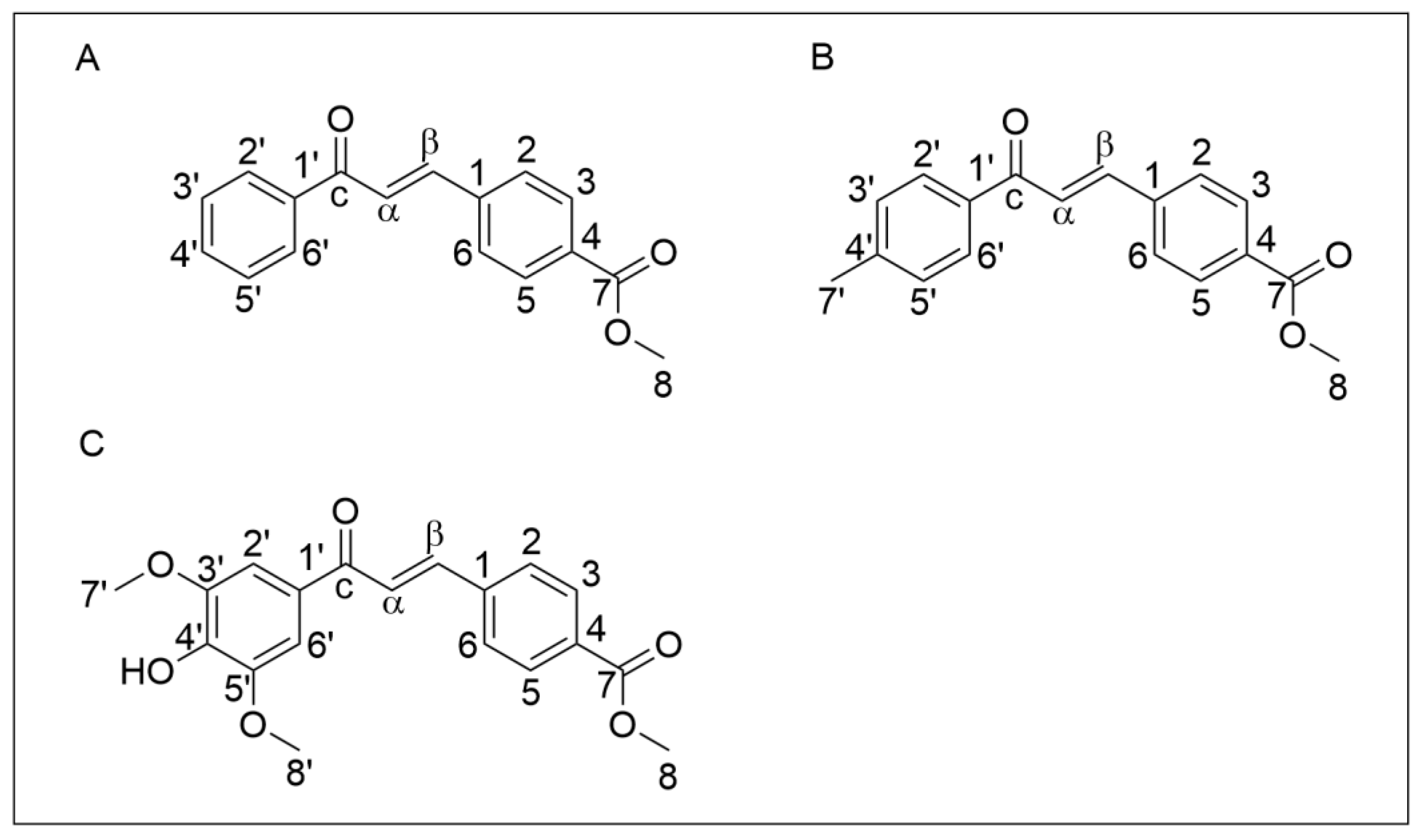
The chemical structure of chalcones Q1VA, Q2VA, and Q18VA is shown in Figure 1.
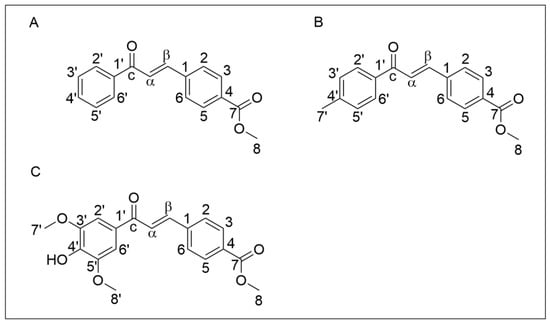
Figure 1.
Chemical structure of chalcones. (A) Chalcone Q1VA, (B) Chalcone Q2VA, and (C) Chalcone Q18VA.
2.2. Primary GBM Cell Culture
The sample was obtained from a resection surgery for GBM on a patient at the Celso Ramos Hospital, in Florianopolis, Santa Catarina, Brazil. The obtained sample was collected in a 15 mL conical tube containing Dulbecco’s Modified Eagle’s medium and nutrient mixture F12 (DMEM-F12; Invitrogen) with 10% fetal bovine serum (FBS; Cultilab). Then, in a laminar flow cabinet, the blood vessels and cerebral membranes were removed. The sample was trypsinized (Trypsin/EDTA, 0.05%; Gibco) and homogenized to be plated in 25 cm2 culture flasks, containing DMEM F-12 with 10% FBS, at 37 °C in culture conditions (atmosphere of 5% CO2). To assure the cell culture did not contain unspecific cell types, we started the experiments after at least 10 passages. The primary human glioblastoma cell, obtained and referred to as GBM1, was used in subsequent experiments.
When 80% of confluence was reached, the cells were trypsinized and plated in 96-well plates (104 cells/well) for the experiments. All the procedures were approved by the local ethics committee for human research (CEPHS 108.286).
2.3. Cell Treatment
First, the chalcones were diluted in dimethylsulfoxide (DMSO, Neon) and initial screening was performed with 50 µM of chalcone with DMSO to determine the potential cytotoxicity on GBM1 cells. After the chalcones were identified, the GBM cells were treated for 24 h at 50 µM, 100 µM, 150 µM, and 200 µM to find the concentration to perform subsequent assays. Vehicle controls were assayed with the same volume of DMSO (max of 1%).
2.4. Cell Viability Assay
The cell viability was determined with an MTT (3-(4,5-Diamethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) reduction assay. Briefly, the MTT is a tetrazolium salt that is up taken up by viable cells and reduced into a purple color; the MTT reduction occurs due to mitochondrial succinate dehydrogenase [35]. After 24 h treatment with the chalcones Q1VA, Q2VA, and Q18VA, the medium was removed, and the cells were incubated with an MTT solution (0.2 mg/mL in PBS: Sigma®) for 2 h at 37 °C. Then, the MTT solution was removed, the formazan crystals were dissolved in DMSO, and the viability was quantified spectrophotometrically at a wavelength of 540 nm, using the multimode reader Infinite M200 TECAN.
2.5. Cell Cycle
The cell cycle was identified with PI incorporation in flow cytometer analysis. In brief, after the 24 h of treatment, the cells were aspirated and washed in cold PBS, centrifuged, and fixed in PFA 4% in 70% ethanol (Synth) at 4 °C for 30 min. Then, the cells were resuspended in PBS containing 20 mg/mL RNAse A (ThermoFisher), Triton 0.001% (Usb), and 50 mg/mL PI (Millipore) and incubated at 4 °C for 30 min in the dark. The results were obtained using a FACS Canto II flow cytometer.
2.6. Measurement of ROS Production
To measure cellular ROS production, the molecular fluorescent probe H2DCFDA (Sigma) was used. After chalcone treatment for 3, 6, 12, and 24 h, 104 cells were loaded with 10 mM H2DCFDA for 20 min. H2DCFDA diffuses through the cell membrane and intracellular esterases hydrolyze it into the nonfluorescent form dichlorofluorescein (DCFH). DCFH reacts with intracellular H2O2 to form dichlorofluorescein (DCF), a green, fluorescent dye. The fluorescence was measured with a multimode reader Infinite M200 TECAN with wavelengths of excitation and emission of 485 and 520 nm, respectively.
2.7. Measurement of Mitochondrial Membrane Potential
Tetramethylrhodamine ethyl ester (TMRE), a fluorescent dye, was used to measure the mitochondrial membrane potential after Q1VA treatment. GBM1 cells were plated in a 96-well plate and treated with Q1VA for 24 h after reaching confluence. TMRE (100 nM; Sigma-Aldrich) was then added and allowed to incubate for 30 min at 37 °C. Fluorescence was measured at 550 nm. As a positive control, a mitochondrial uncoupler, carbonyl cyanide p-trifluoromethoxy phenylhydrazone (FCCP 20 μM; Sigma-Aldrich), was used.
2.8. Apoptosis and Necrosis Detection
The Annexin-V FITC assay kit (Millipore) was used to determine the levels of apoptosis and necrosis in flow cytometry. After 24 h of treatment with chalcones or vehicle, GBM1 cells were trypsinized, centrifuged, and resuspended in cold PBS, followed by one more centrifugation, and then resuspended in binding buffer 1 X. Therefore, the GBM1 cells were separated: Annexin positive, PI (propidium iodide) positive, PI/Annexin negative, and PI/Annexin positive. The concentrations and periods of incubation were performed following the protocol. The results were obtained using a FACS Canto II flow cytometer.
2.9. In Vitro Invasion Assay
To measure the migration and invasive potential of GBM1 cells following treatment, we used a Transwell® insert coated with Matrigel®. In order to migrate and invade, GBM1 cells undergo biochemical changes, and Matrigel® is a basement membrane matrix that can be used to evaluate this ability. First, 50 µL of Matrigel® (5 mg/mL, Corning) were placed on top of the Transwell® insert and incubated for 30 min for polymerization. Then, cells that were suspended in serum-free and treated with Q1VA and then added to the upper part of the chamber. Next, the chemoattractant solution containing DMEM-F12 and 20% FBS was added to the lower chamber, and the plate was placed for 24 h at 37 °C in a humidified incubator with 5% CO2. The cells located on the membrane’s upper surface (non-invading cells), were detached with a cotton-tipped applicator. The invasive cells attached to the insert’s lower membrane surface were stained with Hoechst (5 μg/mL) for 10 min and counted using a fluorescence microscope (Olympus IX83). The invaded cells were counted in 10 randomly chosen fields from each membrane.
2.10. Statistical Analysis
Data were analyzed using GraphPad Prism 9 software with a one-way or two-way ANOVA and post-tests Student–Newman–Keuls and Bonferroni’s, respectively. The results were considered significant when p < 0.05. All the experiments were performed three independent times in triplicate.
3. Results
3.1. Effects of Chalcone on Cell Viability
3.1.1. Reaction to Obtain the Chalcones
The chalcones were synthesized with the Claisen–Schmidt condensation using an acid catalyst and methanol. With this method, the carboxyl group of 4-carboxybenzaldehyde was esterified as a parallel reaction, since the esterification can happen between a carboxylic acid and an alcohol in the presence of acid. All compounds were characterized unequivocally, and in addition, the formation of the E isomer of chalcones was verified, which is known to be the most stable in most cases. After the synthesis and characterization of the compounds, biological tests were performed.
3.1.2. Initial Screening of Synthetic Chalcones
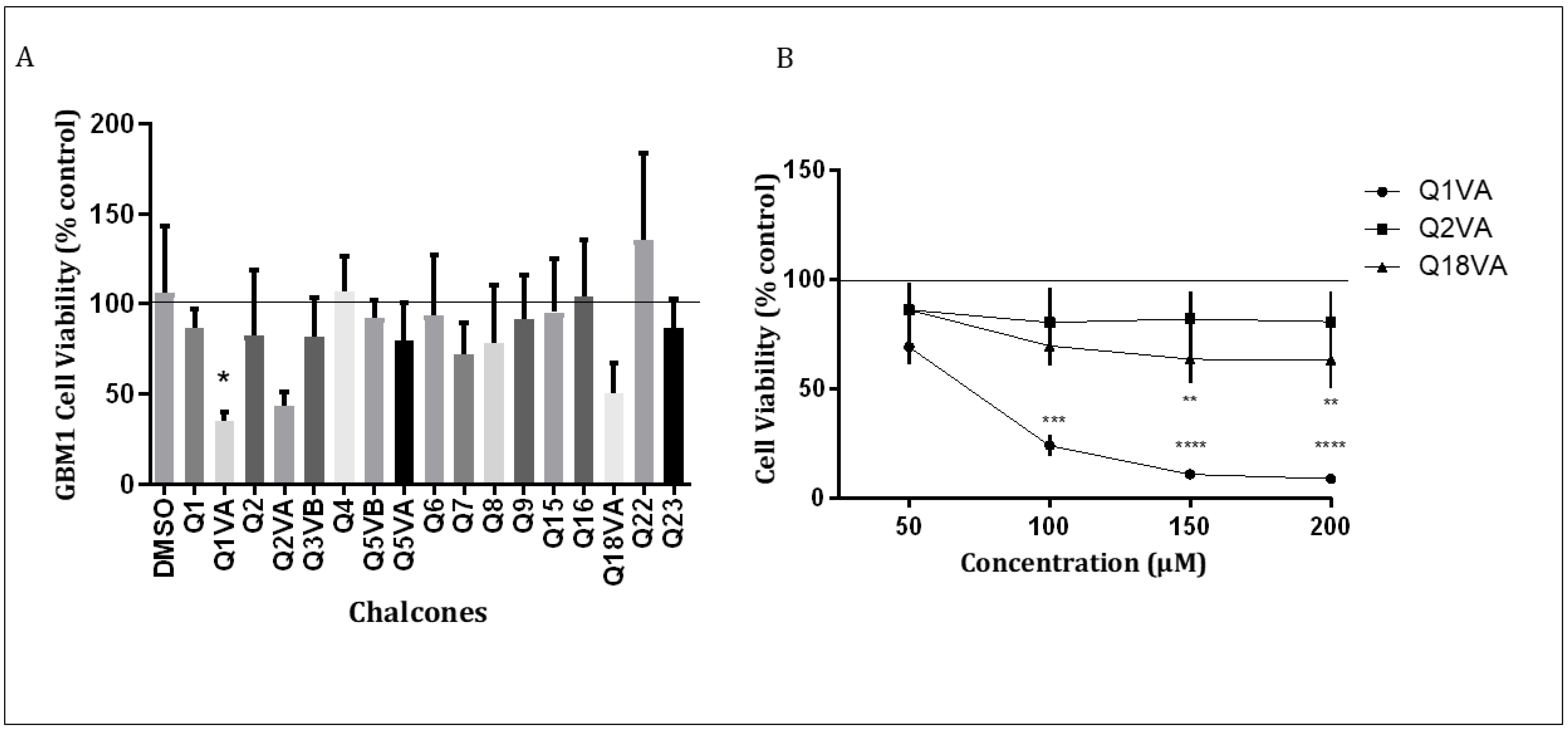
The primary goal of this study was to assess the efficacy of a new series of synthetic chalcones. To this end, we conducted an initial screening of 17 chalcones at a concentration of 50 µM for 24 h and evaluated their effect on GBM1 cells (Figure 2A). Our results showed that among the 17 chalcones, Q1VA, Q2VA, and Q18VA exhibited some reduction in GBM1 cell viability.
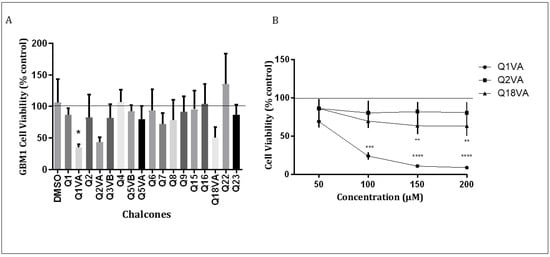
Figure 2.
Cytotoxic effect of chalcone derivatives on GBM1 cells. (A) Initial screening of cell viability after treating with chalcones (50 μM). The x-axis represents the different chalcones, and the y-axis represents the viability as a percentage of the control. The line represents the average result from the control. (B) GBM1 cells treated with chalcones Q1VA, Q2VA, and Q18VA for 24 h. The line represents the average result from the control. Data are represented as mean ± SEM (n 3 per group). Significant differences are compared to the control group (CT), * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001.
We further tested the three chalcones at four different concentrations (50 µM, 100 µM, 150 µM, and 200 µM) for 24 h (Figure 2B). Our results revealed that chalcone Q1VA significantly reduced GBM1 cell viability at all four concentrations (69%, 24%, 10%, and 8%) compared to the control. On the other hand, chalcone Q2VA demonstrated a reduction in viability but no significant difference was observed (85%, 80%, 81%, and 80%). Chalcone Q18VA exhibited a significant reduction in viability only at 150 µM and 200 µM (63% in both), but there was no significant difference at 50 µM and 100 µM (85% and 69%).
We chose Q1VA for further assays based on our findings, as it demonstrated significant effects at lower concentrations compared to the other chalcones. Additionally, we obtained previous results from primary cultures of murine astrocytes, which indicated that higher concentrations of Q1VA can reduce the viability of normal cells (data not shown).
3.2. Q1VA Interferes with the Cell Cycle
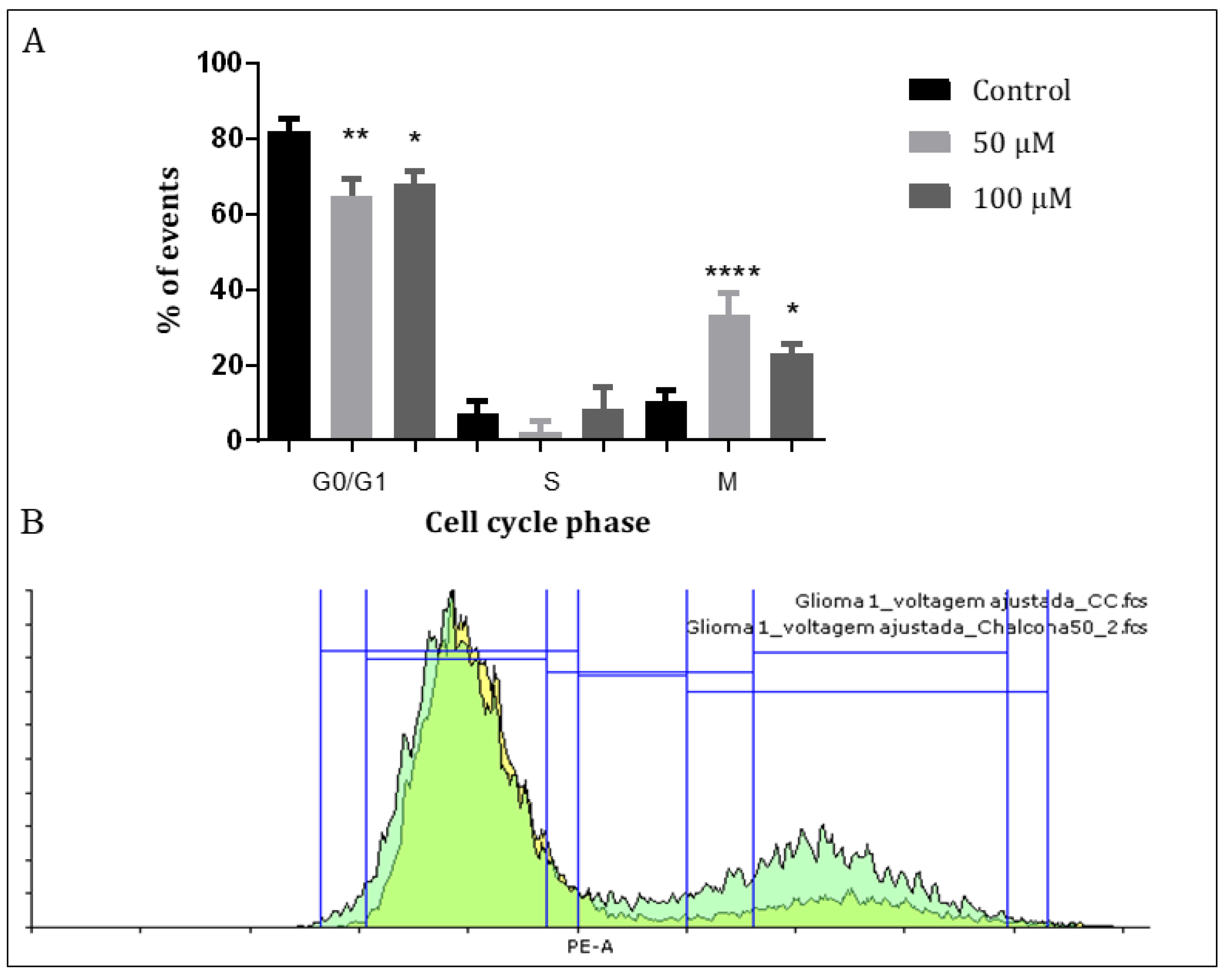
Previous studies showed that chalcones have the ability to alter microtubule polymerization which, in turn, could affect the cell cycle proliferative status of the GBM cells [26,31]. To assess the interference of Q1VA with the cell cycle, a Propidium Iodide (PI) assay was performed. The results showed that after treating GBM1 cells with 50 µM and 100 µM of Q1VA for 24 h, there was a decrease of 20% and 14%, respectively, in the G0/G1 phase and an increase of 23% and 13%, respectively, in the M phase, showing significant differences in both phases. There was no significant difference in the S phase. This suggests that the influence of Q1VA allows a cell to pass through the S phase, but the cell cannot finish a division in the M phase. Furthermore, in phase G0/G1, the treated cells are probably dealing with the cytotoxic effects of Q1VA. (Figure 3).
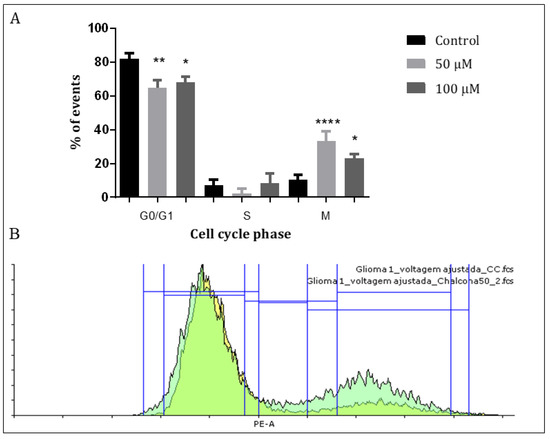
Figure 3.
Cell cycle analysis. After 24 h of treatment, GBM1 cells were treated with Iodate Propidium, and the incorporation was measured with fluorescence. (A) The 24 h treatment showed a cell cycle arrest with an increased number of cells trapped in the M phase and a decreased number of cells in the G0/G1 phase. (B) Graphic representation of the flow cytometry results with the treatment of chalcone Q1VA on GBM1 cells. Values are means ± SEM (n 3 per group). Significant differences compared to the control group, * p < 0.05, ** p < 0.01, and **** p < 0.0001.
3.3. Q1VA Triggers ROS Production and Mitochondria Depolarization
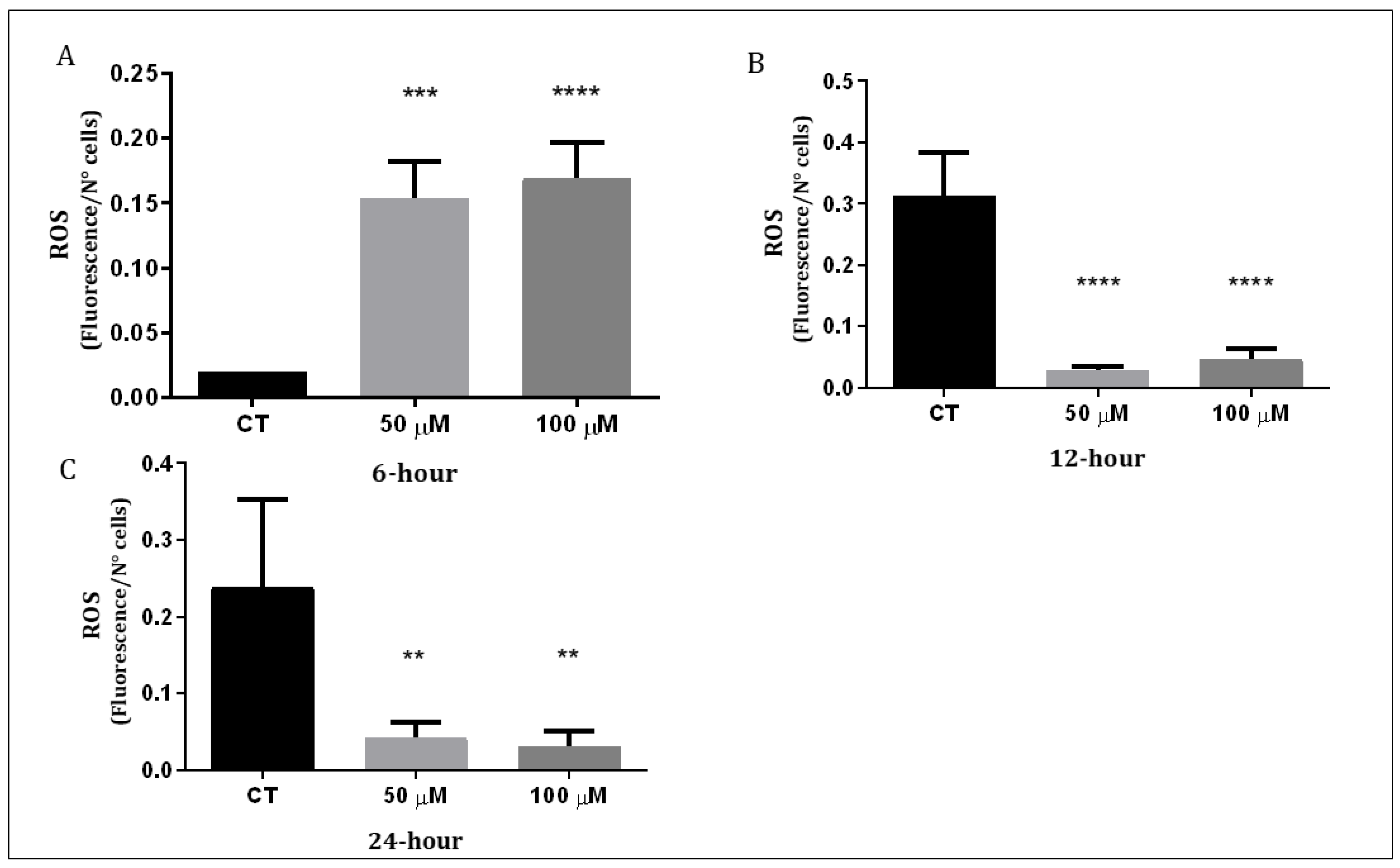
The Q1VA molecule was found to trigger the generation of ROS. The levels of ROS were measured 6 h, 12 h, and 24 h after treatment with 50 µM and 100 µM of Q1VA. The results showed that 6 h after treatment with 50 µM of Q1VA, the levels of ROS increased by 744% compared to the control and by 819% when treated with 100 µM. After 12 h, the levels of ROS decreased compared to the control by 8% with 50 µM and by 14% with 100 µM. After 24 h, the levels of ROS decreased further by 17% with 50 µM and by 13% with 100 µM (Figure 4).
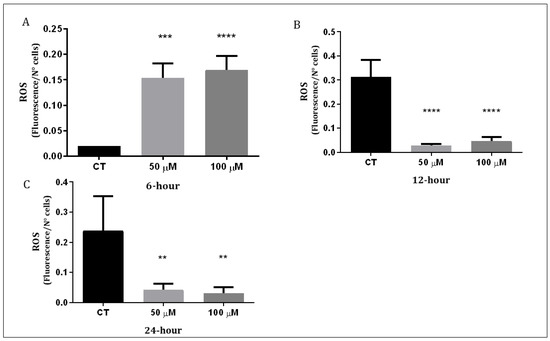
Figure 4.
Measurement of intracellular ROS production. (A) GBM1 cells were treated for 6 h, (B) 12 h, and (C) 24 h with QVA1 followed with 20 min incubation of 10 mM H2DCFDA to measure fluorescence intensity. Data are represented as mean ± SEM (n 3 per group). Significant differences were compared to the control group (CT), ** p < 0.01, *** p < 0.001 and **** p < 0.0001.
When ROS levels are high, this can indicate that there is an imbalance in the production and elimination of these species, which can have damaging effects on cellular components such as mitochondrial membranes [36].
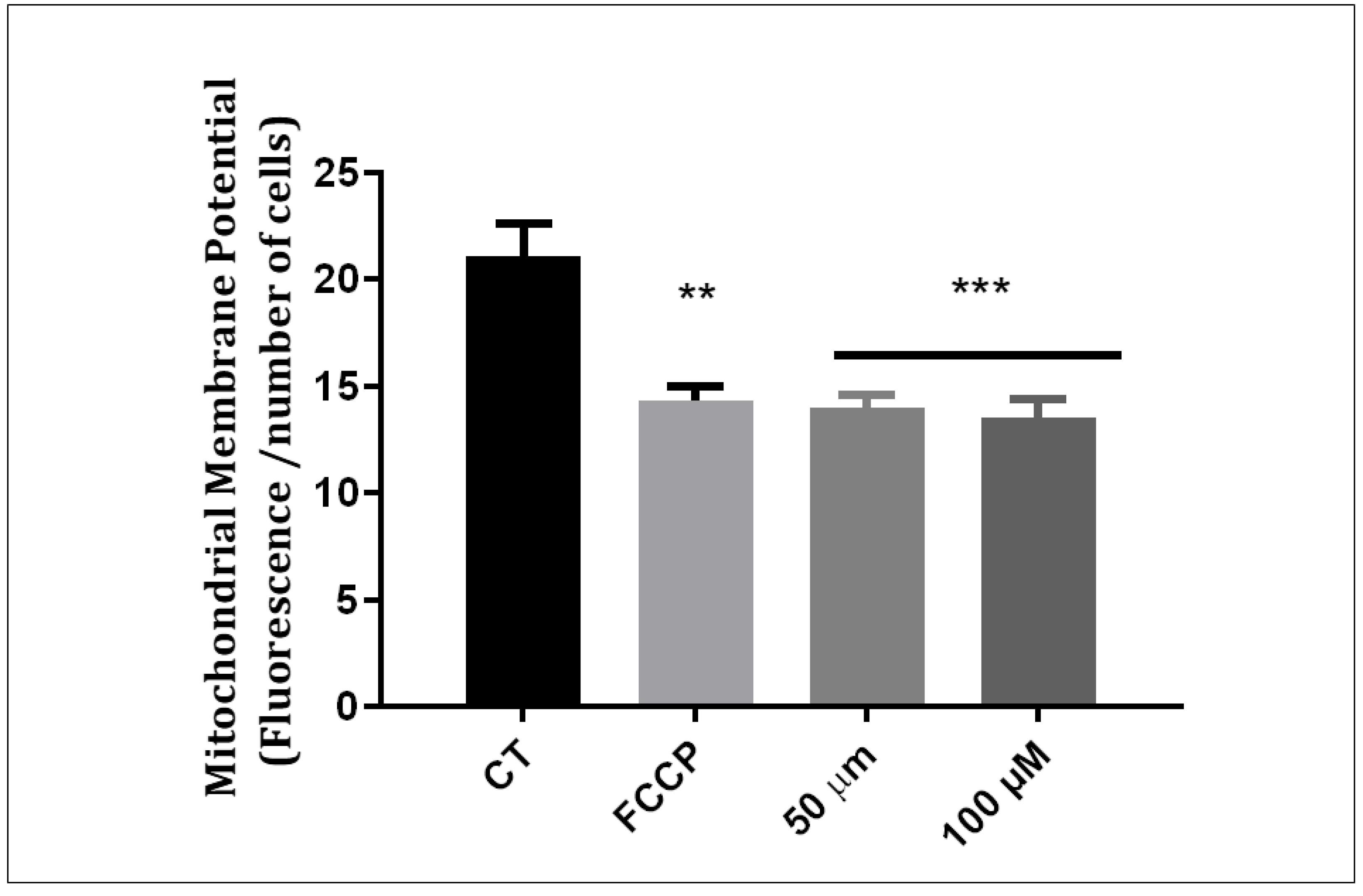
The results of the test using a TMRE probe showed that Q1VA chalcone can depolarize the mitochondrial membrane in both concentrations of 50 µM and 100 µM. The control had an average of 21, and a positive depolarizer (FCCP) was used as the positive control (Figure 5).

Figure 5.
Mitochondrial Membrane Potential (Δψm) measured using a fluorochrome TMRE assay system. The ratio (Fluorescence/number of cells) represents the coupling efficiency (Δψm) of the mitochondria expressed as a percentage of the control. The positive control used was FCCP, an uncoupling agent that transports H+ ions through the mitochondrial membrane causing depolarization. After 24 h of treatment, chalcone Q1VA causes loss of potential mitochondrial membrane. Data are represented as mean ± SEM (n 3 per group). Significant differences compared to the control group (CT), ** p < 0.01, and *** p < 0.001.
3.4. Identification of Cell Death
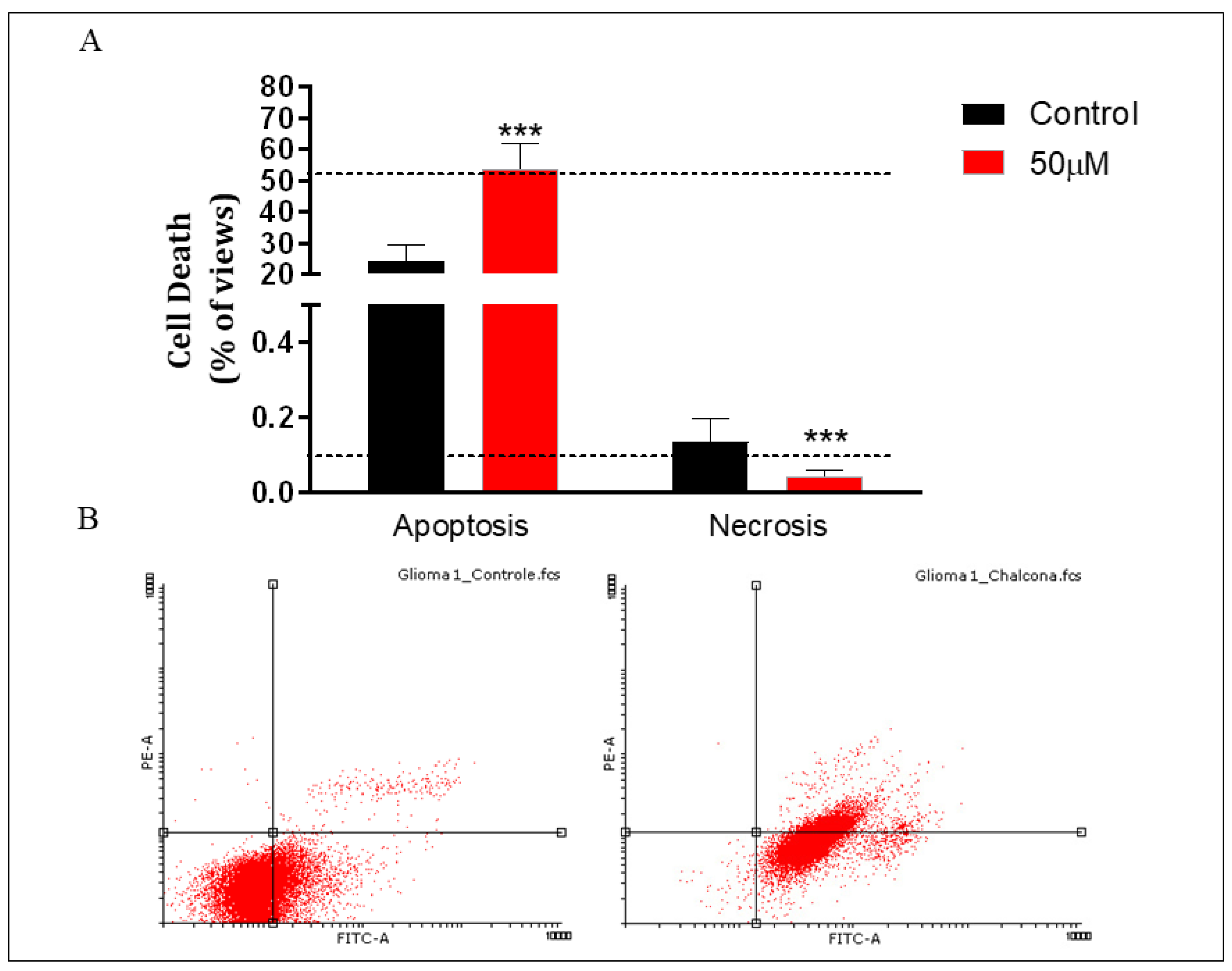
To identify the type of cell death, the Annexin-V assay was used. The results showed that after 24 h of treatment with Q1VA, there was an increase of 30% in the events of apoptosis death compared to the control, while the levels of necrosis were lower than the control (Figure 6).

Figure 6.
Cell apoptosis/necrosis detection using Annexin V/FITC flow cytometry. (A) Graphical representation of the percentage from (B) flow cytometer results with treatment of 50 µM of Q1VA on GBM cells. The upper left quadrant (PI+/Annexin V−) represents necrotic cells, the left lower quadrant (PI−/Annexin V−) represents healthy cells, the upper right quadrant (PI+/Annexin V+) represents early apoptotic cells, and the lower right quadrant (PI−/Annexin V+) represents late apoptotic cells. FITC, fluorescein isothiocyanate; PI, propidium iodide; ISO, isoproterenol. Data are represented as mean ± SEM (n 3 per group). Significant differences compared to the control group, *** p < 0.001.
3.5. Suppression of the Invasive Potential of GBM Cells with Q1VA
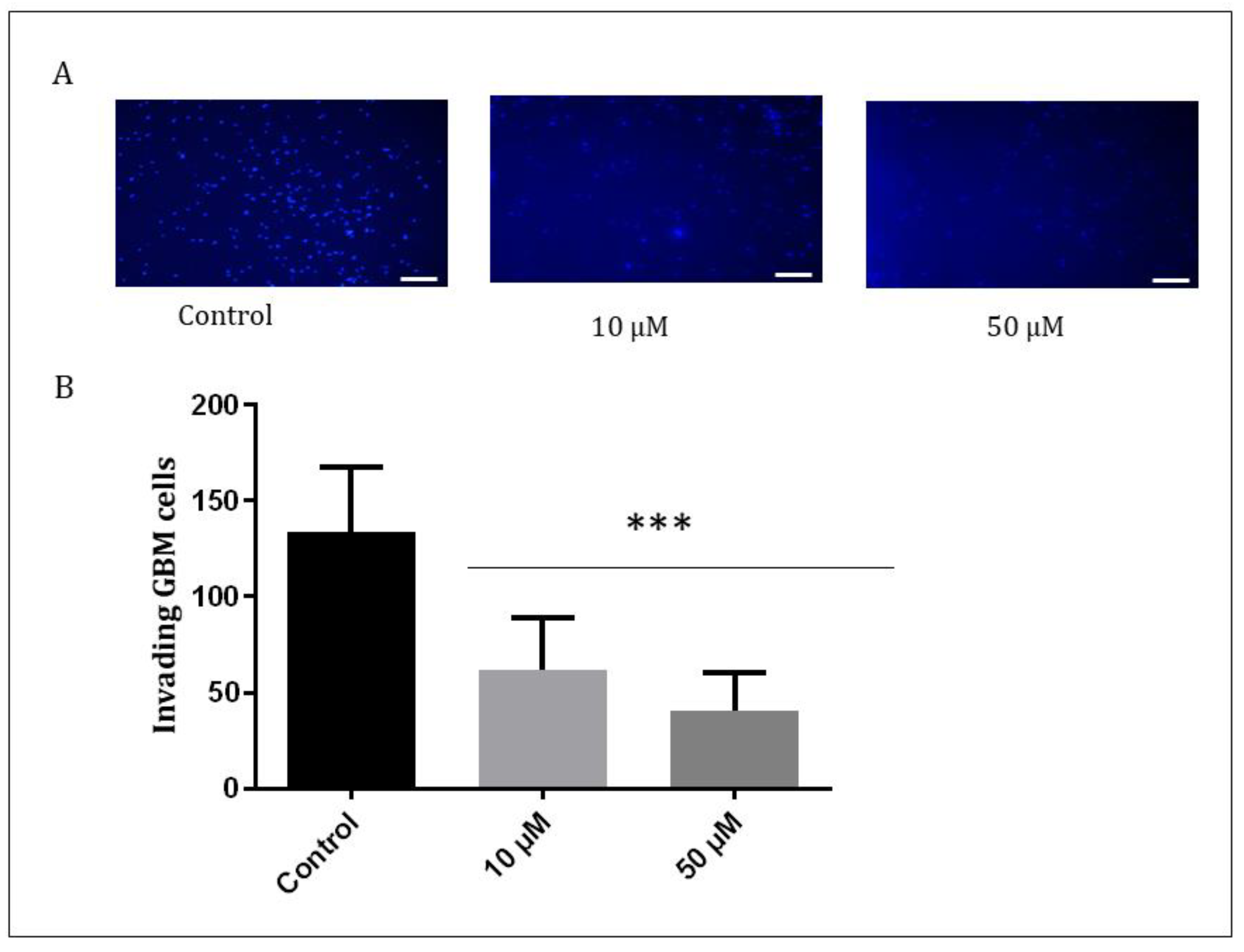
The GBM1 cells exhibit a high capacity for invasiveness. In order to assess the behavior of the cells after treatment, the Matrigel assay was conducted. Matrigel is a basement membrane matrix used to mimic the cell matrix to measure both migration and invasion [37]. The results showed that Q1VA decreased the potential for migration and invasion, with positive cells to HO being three times fewer compared to the control. Even treatment with a lower concentration of 10 µM reduced the positive cells by half (Figure 7).
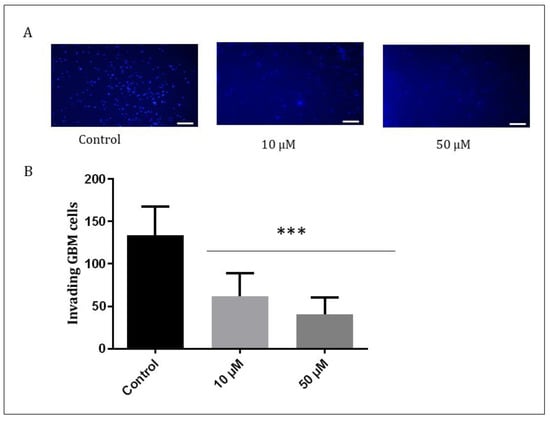
Figure 7.
Invasive behavior of GBM1 cell. A Matrigel-precoated membrane filter insert was used to measure in vitro invasiveness. The GBM cells were treated with Q1VA 50 µM, and after 24 h of incubation, the cells that migrated through the membrane were stained, and representative fields were photographed. Original magnification: 200×. Scale bars represent 50 μm in all images. (A) Representative images showing the Matrigel assay containing GBM cells stained with Hoechst. (B) Graphical representation showing invading cell quantification. Data are represented as mean ± SEM (n 3 per group). Significant differences compared to the control group, *** p < 0.001.
4. Discussion
GBM is one of the deadliest and most untreatable human tumors, representing a significant health challenge. Despite numerous studies that have been conducted using various approaches, only a few have demonstrated potential anti-tumor activity. Among these, chalcones have shown promise as a potential treatment due to their significant anti-tumor effect, ability to inhibit angiogenesis, and ability to modulate ATP-binding cassette (ABC) proteins involved in multidrug resistance [22].
Chalcones are compounds with a common structure of 1,3-diphenylpropenone, which are widely expressed by vegetables and can be easily synthesized in a laboratory with various structures. These compounds have also been found to play a role in cell regulation and inducing apoptosis [10,16].
This study evaluated the effect of a novel synthesized chalcone. After prescreening 17 different chalcones (Figure 2A), the chalcones Q1VA, Q2VA, and Q18VA showed potent cytotoxic activity. Q1VA (Figure 2B) reduced GBM cell viability in all doses tested, and the 50 µM and 100 µM concentrations were chosen for subsequent tests. The chalcones Q2VA (Figure 2B) and Q18VA (Figure 2B) only had an effect at high concentrations. The chemical structure of Q2VA and Q118VA may be the reason why they are not efficient.
With the reduced viability of GBM1 cells observed after treatment with Q1VA, we aimed to study the effects on the remaining alive cells. Previous studies have demonstrated that chalcones can block the cell cycle by depolymerizing microtubules, thereby preventing cell proliferation [10,22,31]. Our results showed an increase in the M phase after treating GBM1 cells with Q1VA, suggesting that Q1VA could interfere with cell division and some fundamental cell functions, such as migration or invasion since the rates of proliferation and invasion are linked.
Treatment with Q1VA decreased the invasion capacity of GBM1 cells, as observed in the Matrigel assay. This result suggests that Q1VA can alter microtubules and potentially interfere with the modulation of metalloproteases [38]. This is a noteworthy finding because a decrease in the invasiveness of GBM1 cells can potentially extend the time until recurrence. The invasive behavior of these cells is associated with the epithelial–mesenchymal transition, which allows cells to gain the ability to migrate and invade [39].
While chalcones show promising activity as potential adjuvant drugs for the treatment of brain cancer, caution should be exercised in interpreting the results of the assays used to measure cellular viability and invasiveness. The assays used to measure cellular viability (MTT assay) and invasiveness (Matrigel assay) have limitations. The MTT assay measures the reduction of a tetrazolium salt by mitochondrial dehydrogenases as an indicator of cellular viability, but this assay is susceptible to interference from compounds that may alter mitochondrial function, such as chalcones [40]. The Matrigel assay is a commonly used technique for measuring the invasive potential of cells. However, one limitation of this assay is the potential lack of communication between the tumor microenvironment and the extracellular matrix (ECM) within the assay. This is a particular concern for glioblastoma (GBM) cells, which actively interact with the ECM to remodel it for migration and infiltration. Furthermore, the ECM contains several transcription factors, which can also influence cellular behavior and may not be adequately represented in the Matrigel assay [41].
Exposure to toxins can have detrimental effects on cell survival as it can disrupt the function of mitochondria. The mitochondria play a crucial role in cellular energy production, and if its potential is lost, it can trigger apoptosis. Cancer cells have the ability to regulate the production of ROS, and an imbalance in this system can also trigger apoptosis. We have observed from our previous studies that fucoxanthin, a carotenoid, has the capability to depolarize the mitochondria in GBM cells, leading to apoptosis, similar to some chalcones [31,42].
The levels of reactive ROS showed an increase after treatment with Q1VA during the initial 12 h. This spike in ROS production has the potential to oxidize the lipids present in the mitochondrial membrane, which could result in the release of cytochrome C. Cytochrome C is an essential component of the respiratory chain and is normally present within the inner membrane of the mitochondria. However, when it is released into the cytosol, it can trigger apoptosis. This highlights the importance of the balance between ROS production and cellular defenses, as an imbalance in this system caused by agents such as Q1VA can have negative effects on cell survival [43]. As a result, the Q1VA-induced increase in ROS levels may be associated with the loss of Δψm, which triggers apoptosis. This is supported by similar findings in other studies that investigated the effect of different chalcones [31,36,44,45,46,47,48].
Q1VA was shown to induce a loss of mitochondrial potential in GBM1 cells, leading to an increase in cell death by apoptosis, as confirmed with the Annexin-V assay. GBM cells are known to have intense proliferative activity and can cause necrosis in tumor tissue due to a lack of oxygen. However, the decrease in necrotic death of the cells, as observed with Q1VA treatment, is positive since necrosis can promote tumor progression. On the other hand, the increase in death from apoptosis is desirable as it helps prevent the release of pro-tumor factors [49,50]. This, in fact, is an interesting fact about chalcones as they have the ability to induce cellular death by either apoptosis or autophagy. In human gastric carcinoma, a chalcone flavokawain B induced autophagic-cell death by reactive oxygen species and suppressed tumor growth in nude mice [51].
In recent years, several studies have highlighted the crucial role of microRNAs (miRNAs) in glioblastoma. For example, miR-21, a type of miRNA, has been found to be upregulated in glioblastoma cells, leading to increased proliferation, invasion, and angiogenesis [52]. Moreover, a recent study revealed that a chalcone derivative can suppress tumor growth through the NOX4-IRE1α sulfonation-RIDD-miR-23b axis. This finding is significant as it highlights the need of targeting specific miRNAs as a new and effective approach to treating aggressive tumors [53].
Chalcones have shown promising anticancer activity in our study, together with various studies, but there is a possibility of off-target effects of chalcone treatment on brain cells. Chalcones have been reported to exhibit cytotoxicity and can induce apoptosis in normal cells as well [54]. Therefore, careful consideration should be given to the dose and duration of chalcone treatment to minimize any potential adverse effects.
5. Conclusions
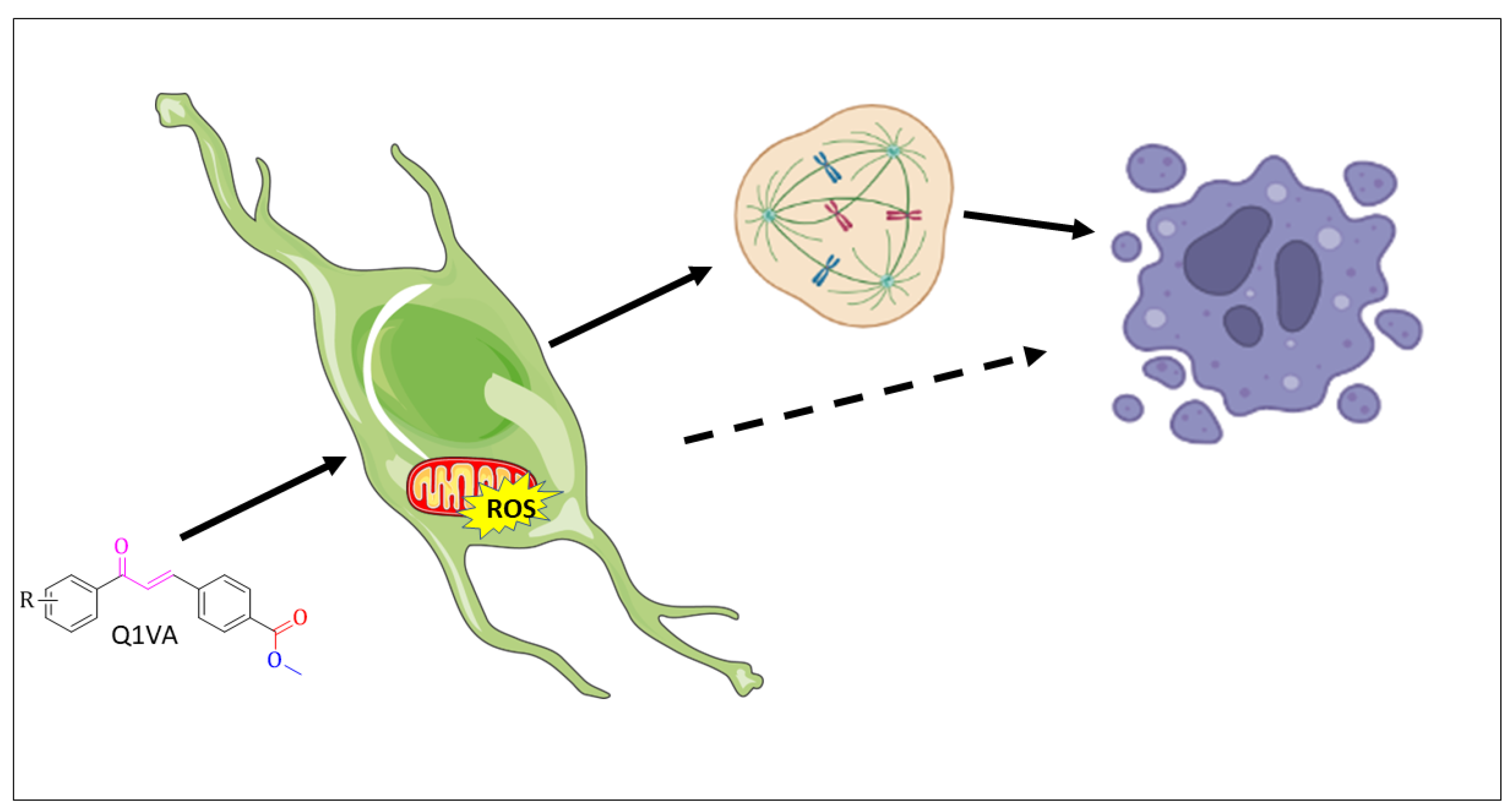
In conclusion, our findings provide evidence for the toxicity of the chalcone, Q1VA, with a hydrogen atom as its R group. This chalcone stimulates an increase in the production of reactive oxygen species, which is associated with a decrease in mitochondrial potential and initiation of apoptotic cell death. Moreover, the surviving cells have a disrupted cell cycle and decreased invasiveness (Figure 8). These results highlight the potential of Q1VA to impact multiple biological processes in tumor cells, and it is a start for further research to fully understand the molecular mechanisms involved in response to Q1VA treatment.
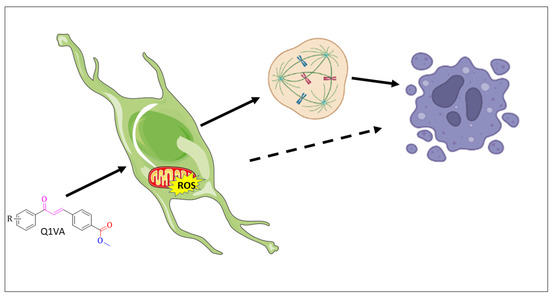
Figure 8.
Schematic illustration showing the effects of chalcone treatment and proposed mechanisms in GBM cells. After 24 h of treatment, the chalcone induces an increase in reactive oxygen species and an imbalance in the mitochondrial membrane potential, ultimately leading to apoptosis. Additionally, the chalcone treatment results in a delay in the cell cycle, which can also contribute to apoptosis over time.
Author Contributions
C.B.N.: conceptualization, funding acquisition, project administration, supervision, and writing. A.T.: conceptualization, data curation, formal analysis, investigation, methodology, and writing. T.P.: conceptualization, data curation, formal analysis, investigation, methodology, and reviewing. L.F.S.d.S.: investigation and methodology. L.D.C.D.: investigation and methodology. R.J.N.: funding acquisition, project administration, and supervision. C.I.T.: funding acquisition, project administration, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)—Instituto Nacional de Ciência e Tecnologia (INCT) and Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES) (CAPES-PVE 052/2012). The financial support agencies had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Institutional Review Board Statement
All procedures were approved by the Institutional Ethics Committee (CEPSH-UFSC-108.286).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declared no conflict of interest.
References
- Serafim, R.B.; da Silva, P.; Cardoso, C.; Di Cristofaro, L.F.M.; Netto, R.P.; de Almeida, R.; Navegante, G.; Storti, C.B.; de Sousa, J.F.; de Souza, F.C.; et al. Expression Profiling of Glioblastoma Cell Lines Reveals Novel Extracellular Matrix-Receptor Genes Correlated With the Responsiveness of Glioma Patients to Ionizing Radiation. Front. Oncol. 2021, 11, 668090. [Google Scholar] [CrossRef]
- Llaguno, S.R.A.; Wang, Z.; Sun, D.; Chen, J.; Xu, J.; Kim, E.; Hatanpaa, K.J.; Raisanen, J.M.; Burns, D.K.; Johnson, J.E.; et al. Adult Lineage-Restricted CNS Progenitors Specify Distinct Glioblastoma Subtypes. Cancer Cell 2015, 28, 429–440. [Google Scholar] [CrossRef]
- Deleyrolle, L.P.; Harding, A.; Cato, K.; Siebzehnrubl, F.A.; Rahman, M.; Azari, H.; Olson, S.; Gabrielli, B.; Osborne, G.; Vescovi, A.; et al. Evidence for label-retaining tumour-initiating cells in human glioblastoma. Brain 2011, 134, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Gimple, R.C.; Bhargava, S.; Dixit, D.; Rich, J.N. Glioblastoma stem cells: Lessons from the tumor hierarchy in a lethal cancer. Genes Dev. 2019, 33, 591–609. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Mortezaee, K.; Majidpoor, J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019, 234, 116781. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.L.V.; Gomes, I.N.F.; Carloni, A.C.; Rosa, M.N.; da Silva, L.S.; Evangelista, A.F.; Reis, R.M.; Silva, V.A.O. Role of glioblastoma stem cells in cancer therapeutic resistance: A perspective on antineoplastic agents from natural sources and chemical derivatives. Stem Cell Res. Ther. 2021, 12, 1–22. [Google Scholar] [CrossRef]
- Wang, J.Q.; Wu, Z.X.; Yang, Y.; Teng, Q.X.; Li, Y.D.; Lei, Z.N.; Jani, K.A.; Kaushal, N.; Chen, Z.S. ATP-binding cassette (ABC) transporters in cancer: A review of recent updates. J. Evid.-Based Med. 2021, 14, 232–256. [Google Scholar] [CrossRef]
- Chen, G.; Di Zhou, D.; Li, X.-Z.; Jiang, Z.; Tan, C.; Wei, X.-Y.; Ling, J.; Jing, J.; Liu, F.; Li, N. A natural chalcone induces apoptosis in lung cancer cells: 3D-QSAR, docking and an in vivo/vitro assay. Sci. Rep. 2017, 7, 10729. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Leon-Gonzalez, A.; Acero, N.; Munoz-Mingarro, D.; Navarro, I.; Martín-Cordero, C. Chalcones as Promising Lead Compounds on Cancer Therapy. Curr. Med. Chem. 2015, 22, 3407–3425. [Google Scholar] [CrossRef]
- Morandi, L.; Franceschi, E.; de Biase, D.; Marucci, G.; Tosoni, A.; Ermani, M.; Pession, A.; Tallini, G.; Brandes, A. Promoter methylation analysis of O6-methylguanine-DNA methyltransferase in glioblastoma: Detection by locked nucleic acid based quantitative PCR using an imprinted gene (SNURF) as a reference. BMC Cancer 2010, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Long, Y.; Yang, C.; Jin, L.; Tao, H.; Ge, H.; Chang, Y.E.; Karachi, A.; Kubilis, P.S.; De Leon, G.; et al. The IDH1 Mutation-Induced Oncometabolite, 2-Hydroxyglutarate, May Affect DNA Methylation and Expression of PD-L1 in Gliomas. Front. Mol. Neurosci. 2018, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Turkowski, K.; Brandenburg, S.; Mueller, A.; Kremenetskaia, I.; Bungert, A.D.; Blank, A.; Felsenstein, M.; Vajkoczy, P. VEGF as a modulator of the innate immune response in glioblastoma. Glia 2017, 66, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, N.D.; Muldoon, L.L.; Culp, A.Y.; Neuwelt, E.A. Delivery of Chemotherapeutics Across the Blood–Brain Barrier. Adv. Pharmacol. 2014, 71, 203–243. [Google Scholar] [CrossRef]
- WalyEldeen, A.A.; Sabet, S.; El-Shorbagy, H.M.; Abdelhamid, I.A.; Ibrahim, S.A. Chalcones: Promising therapeutic agents targeting key players and signaling pathways regulating the hallmarks of cancer. Chem.-Biol. Interact. 2022, 369, 110297. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Ducki, S. The development of chalcones as promising anticancer agents. IDrugs Investig. Drugs J. 2007, 10, 42. [Google Scholar]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone synthesis, properties and medicinal applications: A review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar] [CrossRef]
- Sinha, S.; Medhi, B.; Radotra, B.D.; Batovska, D.I.; Markova, N.; Bhalla, A.; Sehgal, R. Antimalarial and immunomodulatory potential of chalcone derivatives in experimental model of malaria. BMC Complement. Med. Ther. 2022, 22, 1–14. [Google Scholar] [CrossRef]
- Pereira, D.; Durães, F.; Szemerédi, N.; Freitas-Da-Silva, J.; Pinto, E.; Martins-Da-Costa, P.; Pinto, M.; Correia-Da-Silva, M.; Spengler, G.; Sousa, E.; et al. New Chalcone–Triazole Hybrids with Promising Antimicrobial Activity in Multidrug Resistance Strains. Int. J. Mol. Sci. 2022, 23, 14291. [Google Scholar] [CrossRef]
- Pereira, R.; Silva, A.M.; Ribeiro, D.; Silva, V.L.; Fernandes, E. Bis-chalcones: A review of synthetic methodologies and anti-inflammatory effects. Eur. J. Med. Chem. 2023, 252, 115280. [Google Scholar] [CrossRef] [PubMed]
- Champelovier, P.; Chauchet, X.; Puch, F.; Vergnaud, S.; Garrel, C.; Laporte, F.; Boutonnat, J.; Boumendjel, A. Cellular and molecular mechanisms activating the cell death processes by chalcones: Critical structural effects. Toxicol. Vitr. 2013, 27, 2305–2315. [Google Scholar] [CrossRef]
- Mantzanidou, M.; Hadjipavlou-Litina, D. Chalcones and their Potential Role in Inflammation. Mini-Rev. Med. Chem. 2008, 8, 1224–1242. [Google Scholar] [CrossRef]
- Daraei, B.; Karimi, G.; Makhdoumi, P.; Zarghi, A. Evaluation of Cytotoxicity Effects of Chalcone Epoxide Analogues as a Selective COX-II Inhibitor in the Human Liver Carcinoma Cell Line. J. Pharmacopunct. 2017, 20, 207–212. [Google Scholar] [CrossRef]
- Nowakowska, Z. A review of anti-infective and anti-inflammatory chalcones. Eur. J. Med. Chem. 2007, 42, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Boumendjel, A.; McLeer-Florin, A.; Champelovier, P.; Allegro, D.; Muhammad, D.; Souard, F.; Derouazi, M.; Peyrot, V.; Toussaint, B.; Boutonnat, J. A novel chalcone derivative which acts as a microtubule depolymerising agent and an inhibitor of P-gp and BCRP in in-vitro and in-vivoglioblastoma models. BMC Cancer 2009, 9, 242. [Google Scholar] [CrossRef]
- Bunning, A.R.; Gupta, M.L., Jr. The importance of microtubule-dependent tension in accurate chromosome segregation. Front. Cell Dev. Biol. 2023, 11, 1096333. [Google Scholar] [CrossRef]
- Ryu, J.; Pyo, J.; Lee, C.-W.; Kim, J.-E. An Aurora kinase inhibitor, AMG900, inhibits glioblastoma cell proliferation by disrupting mitotic progression. Cancer Med. 2018, 7, 5589–5603. [Google Scholar] [CrossRef]
- Metselaar, D.S.; du Chatinier, A.; Meel, M.H.; ter Huizen, G.; Waranecki, P.; Goulding, J.R.; Bugiani, M.; Koster, J.; Kaspers, G.J.; Hulleman, E. AURKA and PLK1 inhibition selectively and synergistically block cell cycle progression in diffuse midline glioma. iScience 2022, 25, 104398. [Google Scholar] [CrossRef]
- Mesic, A.; Rogar, M.; Hudler, P.; Bilalovic, N.; Eminovic, I.; Komel, R. Genetic variations in AURORA cell cycle kinases are associated with glioblastoma multiforme. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Bittencourt, L.F.F.; de Oliveira, K.A.; Cardoso, C.B.; Lopes, F.G.; Dal-Cim, T.; Chiaradia-Delatorre, L.D.; Mascarello, A.; Maluf, S.W.; Yunes, R.A.; Garcez, R.C.; et al. Novel synthetic chalcones induces apoptosis in human glioblastoma cells. Chem. Interact. 2016, 252, 74–81. [Google Scholar] [CrossRef]
- Septianingtyas, D.; Zafira, N.; Zulhipri; Kurniadewi, F.; Dianhar, H. Green synthesis of chalcones derivatives. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2021; Volume 2331, p. 040020. [Google Scholar] [CrossRef]
- Perrin, C.L.; Chang, K.-L. The Complete Mechanism of an Aldol Condensation. J. Org. Chem. 2016, 81, 5631–5635. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.C.; Mori, M.; Sens, L.; Rocha, R.F.; Tizziani, T.; de Souza, L.F.; Chiaradia-Delatorre, L.D.; Botta, M.; Nunes, R.J.; Terenzi, H.; et al. A chalcone derivative binds a putative allosteric site of YopH: Inhibition of a virulence factor of Yersinia. Bioorganic Med. Chem. Lett. 2020, 30, 127350. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-induced ROS release: An update and review. Biochim. Biophys. Acta (BBA)—Bioenerg. 2006, 1757, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.; Hsu, E.-C.; Liu, S.; Stoyanova, T. Quantifying the invasion and migration ability of cancer cells with a 3D Matrigel drop invasion assay. Biol. Methods Protoc. 2021, 6, bpab014. [Google Scholar] [CrossRef]
- Koh, I.; Cha, J.; Park, J.; Choi, J.; Kang, S.-G.; Kim, P. The mode and dynamics of glioblastoma cell invasion into a decellularized tissue-derived extracellular matrix-based three-dimensional tumor model. Sci. Rep. 2018, 8, 4608. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- So, J.-S.; Kim, H.; Han, K.-S. Mechanisms of Invasion in Glioblastoma: Extracellular Matrix, Ca2+ Signaling, and Glutamate. Front. Cell. Neurosci. 2021, 15, 663092. [Google Scholar] [CrossRef]
- Lopes, F.G.; Oliveira, K.A.; Lopes, R.G.; Poluceno, G.G.; Simioni, C.; Gabriel, D.S.P.; Bauer, C.M.; Maraschin, M.; Derner, R.B.; Garcez, R.C.; et al. Anti-cancer Effects of Fucoxanthin on Human Glioblastoma Cell Line. Anticancer Res. 2020, 40, 6799–6815. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Geng, F.; Pan, M.; Wu, X.; Zhong, Y.; Wang, C.; Tian, Z.; Cheng, C.; Zhang, R.; Puduvalli, V.; et al. Targeting DGAT1 Ameliorates Glioblastoma by Increasing Fat Catabolism and Oxidative Stress. Cell Metab. 2020, 32, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Tabata, K.; Arakawa, M.; Ito, Y.; Kimura, Y.; Akihisa, T.; Nagai, H.; Sakuma, A.; Kohno, H.; Suzuki, T. Isobavachalcone, a Chalcone Constituent of Angelica keiskei, Induces Apoptosis in Neuroblastoma. Biol. Pharm. Bull. 2007, 30, 1878–1883. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-B.; Wang, D.-G.; Guo, F.-F.; Xuan, C. Mitochondrial membrane potential and reactive oxygen species in cancer stem cells. Fam. Cancer 2015, 14, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.; Prasad, S.; Yadav, V.; Park, B.; Kim, J.; Gupta, S.; Yoon, S.; Lavasanifar, A.; Sung, B. Targeting inflammatory pathways by dietary agents for prevention and therapy of cancer. J. Food Drug Anal. 2012, 20, 57. [Google Scholar] [CrossRef]
- Ismail, S.; Haris, K.; Ghani, A.R.I.A.; Abdullah, J.M.; Johan, M.F.; Yusoff, A.A.M. Enhanced induction of cell cycle arrest and apoptosis via the mitochondrial membrane potential disruption in human U87 malignant glioma cells by aloe emodin. J. Asian Nat. Prod. Res. 2013, 15, 1003–1012. [Google Scholar] [CrossRef]
- Aldape, K.; Zadeh, G.; Mansouri, S.; Reifenberger, G.; von Deimling, A. Glioblastoma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015, 129, 829–848. [Google Scholar] [CrossRef]
- Yee, P.P.; Wei, Y.; Kim, S.-Y.; Lu, T.; Chih, S.Y.; Lawson, C.; Tang, M.; Liu, Z.; Anderson, B.; Thamburaj, K.; et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat. Commun. 2020, 11, 1–22. [Google Scholar] [CrossRef]
- Chang, C.-T.; Hseu, Y.-C.; Thiyagarajan, V.; Lin, K.-Y.; Way, T.-D.; Korivi, M.; Liao, J.-W.; Yang, H.-L. Chalcone flavokawain B induces autophagic-cell death via reactive oxygen species-mediated signaling pathways in human gastric carcinoma and suppresses tumor growth in nude mice. Arch. Toxicol. 2017, 91, 3341–3364. [Google Scholar] [CrossRef]
- Masoudi, M.S.; Mehrabian, E.; Mirzaei, H. MiR-21: A key player in glioblastoma pathogenesis. J. Cell. Biochem. 2017, 119, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Lee, H.-Y.; Alam Riaz, T.; Bhattarai, K.R.; Chaudhary, M.; Ahn, J.H.; Jeong, J.; Kim, H.-R.; Chae, H.-J. Chalcone suppresses tumor growth through NOX4-IRE1α sulfonation-RIDD-miR-23b axis. Redox Biol. 2021, 40, 101853. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).