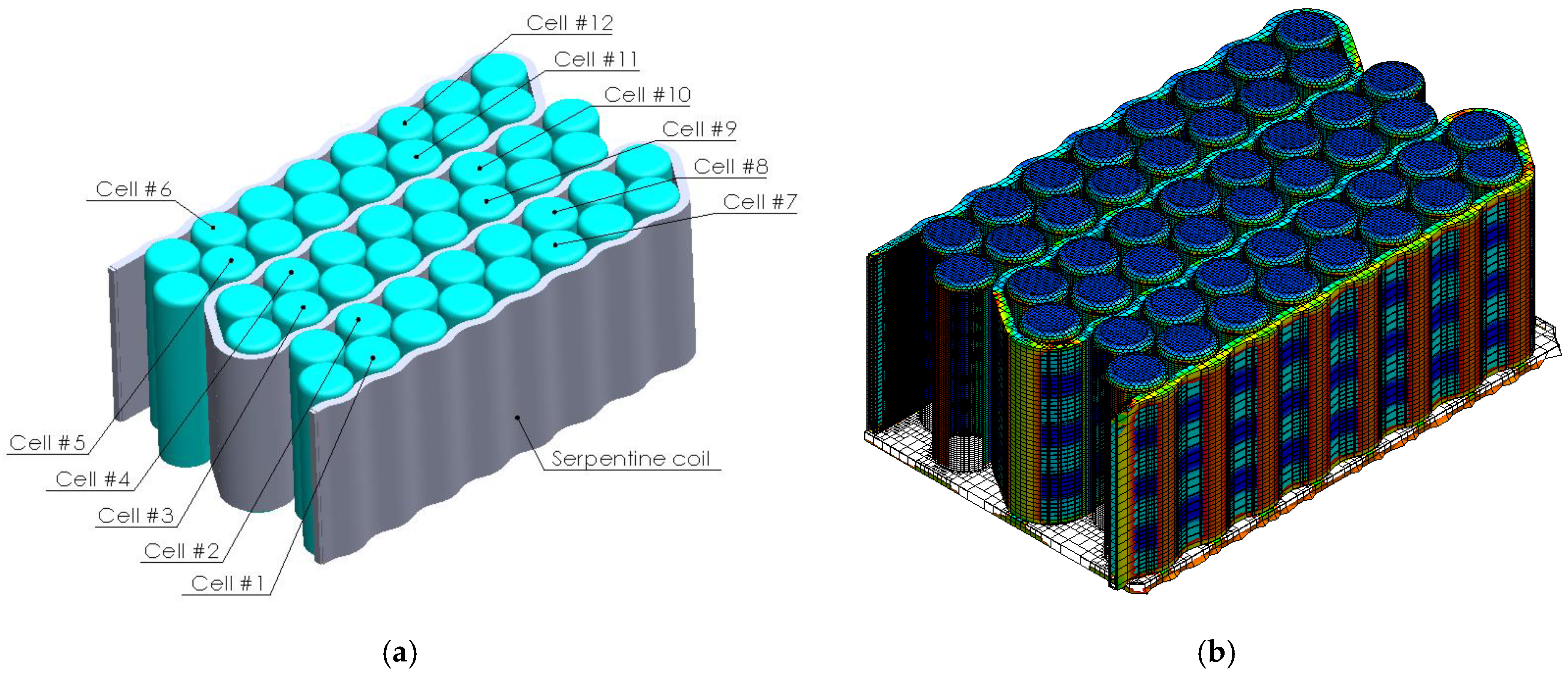
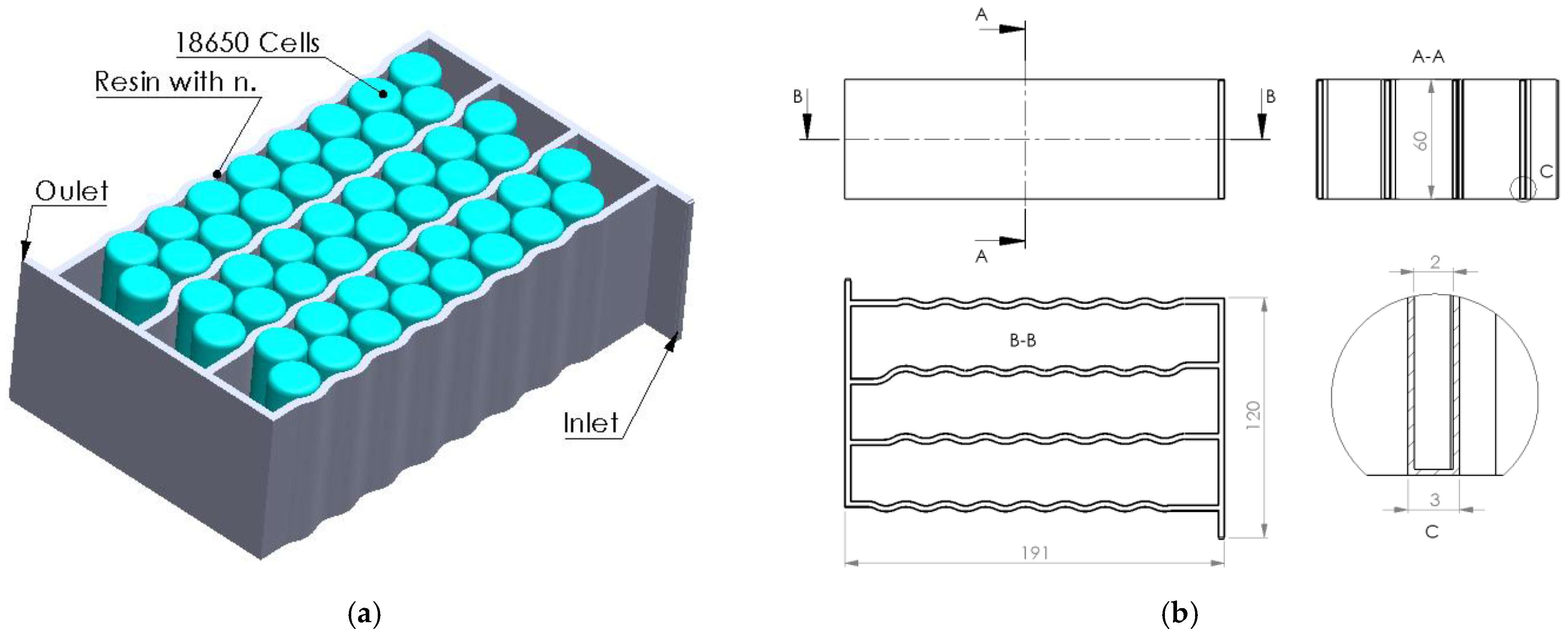
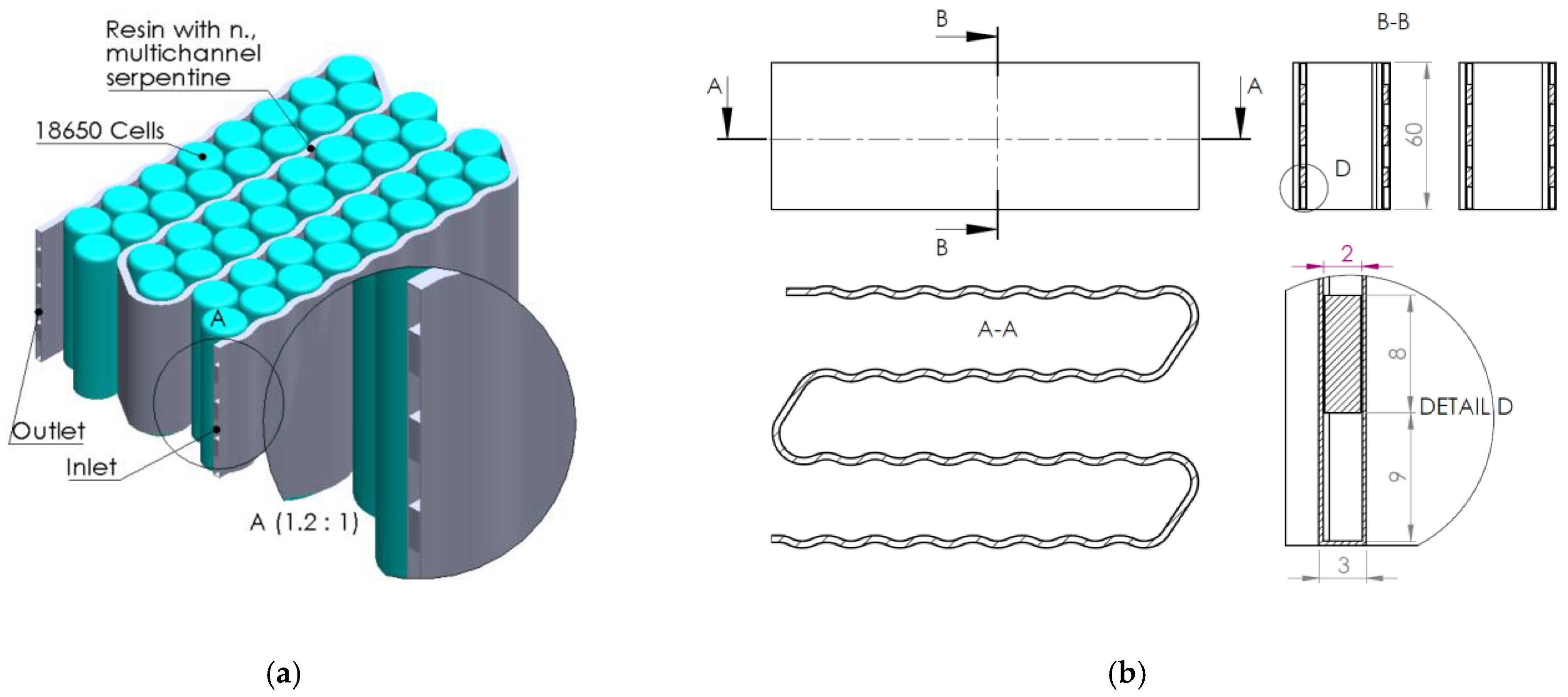
1. Introduction
It is now widely accepted that electric vehicles (EVs) are the solution to decarbonization and pollution reduction in the transport sector. In addition to the environmental benefits of their use (zero local emissions), energy efficiency considerations (higher than vehicles equipped with internal combustion engines that use fossil fuels), dynamic performance (achieving instant torque that provides rapid vehicle acceleration) and lower operating costs due to the price of electricity and fewer maintenance and repair operations, all of these make electric vehicles have a constant increase in the penetration rate of the automotive market [
1].
However, it must be taken into account that electric vehicles base their operation on the energy sources (battery and/or fuel cell) with which they are equipped, which also have a direct influence on the selling price. Currently, battery electric vehicles (BEVs) mainly use Li-ion technologies to build the electrochemical cells that form the battery. Li-ion energy cell technology offers several immediate advantages highlighted by [
2,
3]:
High energy density: storing a large amount of energy in a small volume;
Relatively long life: can be charged and discharged many times (cycle life) before their capacity significantly decreases;
Fast charging: ready for use in a short time;
Maintenance-free: does not require regular maintenance in operation.
However, the disadvantages of using a Li-ion battery as an energy source must also be taken into account, such as high costs, aging and degradation, environmental impact, safety concerns and temperature sensitivity; and in this context, the dangers due to fire and fire risks must be taken into account [
4]. Electric vehicles (EVs) present fire risks, mainly due to the particularities and characteristics of the energy sources (lithium-ion batteries). In the event of improper operation or manufacturing defects, there is the possibility that a battery cell will overheat and ignite (which leads to a chain reaction due to the adjacent location of other cells that form the battery). The phenomenon is known as battery thermal runaway.
There is extensive research worldwide that tries to minimize or eliminate this effect by designing, developing and building high-performance battery thermal management systems (BTMS) [
5]. It should be emphasized that there is still no single solution regarding a standard topology for the construction of a thermal management system and, even more so, solutions effectively implemented in the construction of energy sources to reduce/eliminate the risk of fire. Research into the possibilities of reducing/eliminating the risk of fire in the battery (due to the phenomenon of thermal runaway or due to road accidents that can lead to the perforation of the electrochemical cell casing) has developed in several directions from reinforcing the battery case (box) to applying fireproof solutions with more or less convincing results.
The development of flame-retardant polymers with high standards has always been a challenge due to traditional time-consuming methods based on empirical intuition and trial and error screenings, and their application in BTMS systems is even more so. Research studies addressing BTMS in relation to fire resistance are relatively few, most of them considering the possibility of using phase change materials (PCM) both for efficient heat transfer and as a barrier against fire spread. Yang et al. [
6] propose the use of a flame-retardant multifunctional phase change composite material based on paraffin (PA), styrene-butadiene-styrene (SBS), expanded graphite (EG), methylphenyl silicone resin (MPS) and triphenyl phosphate (TPP) in the BTMS of 26,650 ternary power battery modules. In addition to maintaining the optimal internal temperature of the battery module for 2C discharges, the developed composite material exhibits excellent suppression of thermal runaway triggered by multiple heat sources and the occurrence time of thermal runaway. Zhao et al. [
7] analyze that research on phase change materials (PCM) for BTMS often focuses on only one major characteristic: either flame resistance or flexibility (because it is difficult to obtain solutions that combine both characteristics). To solve this, a flexible flame retardant PCM created from aluminum hydroxide (ATH)/magnesium hydroxide (MTH)/ammonium polyphosphate (APP) was developed and experimented. The optimal ratio of ATH/MTH/APP was determined to be 9:3:8, successfully combining the characteristics of flame retardancy and flexibility (LOI 28.3, delayed the time triggered by thermal escape by 84 s and reduced the maximum temperature by 92.3 °C). Zhang et al. [
8] added two flame retardant materials (ammonium polyphosphate (APP) and red phosphorus (RP)) to a PCM composed of paraffin (PA)/expanded graphite (EG)/(ER). It was found that for an optimal ratio of APP to RP of 23/10, the LOI index has a value of 27.6, and by using it in BTMS, the internal temperature can be effectively controlled at a 3C discharge rate of the battery. The efficient control of the battery thermal management was also successfully achieved by adopting a flexible and flame-retardant PCM proposed by Liu et al. [
9]. The composition was based on the use of a mixture of paraffin, expandable graphite, carbon fiber powder, ammonium polyphosphate (APP) and α,ω-Dihydroxy-poly-siloxane (RTV 107). The battery surface temperature was 7 °C lower at 2 °C charge and discharge cycles (compared to the considered basic flexible flame retardant).
One direction of research is to use fireproof solutions based on composite resins (epoxy). Epoxy resins are a class of organic polymers containing two or more epoxy groups in their molecules, and due to their excellent adhesion, insulation, heat resistance and corrosion properties, they are widely used in various industries, including the automotive industry [
10]. However, the use of epoxy resins presents a high risk of ignition and combustion due in principle to the LOI (Limiting Oxygen Index) characteristic of approx. 19.8%, a combustion process characterized by the release of large amounts of heat and toxic smoke [
11]. The latest research advances on reactive flame-retardant epoxy resins are comprehensively presented in laborious works that analyze the flame-retardant effects of epoxy resins in combination with different chemical compounds (with the advantages and disadvantages of their use) [
11,
12].
Based on their chemistry and mode of action, phosphorus-based flame retardants are one of the most researched classes due to their good compatibility with epoxy resins and low toxicity compared to other classes, such as halogenated compounds [
13].
SiO
2 nanoparticles and the phenethyl bridged DOPO derivative (DiDOPO) were used to prepare epoxy composite resins in order to increase their flame retardant performance and thermal stability [
14]. It was observed that by increasing the SiO
2 content from 2 to 15 wt%, the UL-94 index changed from N.R. to V-0 rating, and the LOI value increased from 21.8 to 30.2% (compared to pure epoxy resin), which provides future premises to develop and use these types of composite epoxy resins in fireproof applications. The development of a bio-based epoxy resin based on phosphorus-nitrogen was also carried out. The product obtained and subjected to analysis shows superior flame resistance and fire safety compared to epoxy resin (epoxy value 0.51). The LOI value of new epoxy resins increases from 25.8% to 42.3%, and the UL-94 index from N.R. to V-0 rating (flame self-extinguishes in 3 s) [
15]. Based on the same premises, research was also carried out in which the flame retardant contribution of only phosphorus compounds (phosphorus content: 0.22%) was investigated [
16]. The LOI value of the new epoxy compound increased from 21.0% to 40.0%, the UL-94 test rating was improved from N.R. to V-0, while the yield measured in carbon residues of the new epoxy compound (28.6%) was more than double compared to pure epoxy resins (11.6%).
A universal strategy was researched and proposed by introducing suitable rigid-flexible groups (Si–O segment) into the flame-retardant epoxy resin, which can simultaneously improve its transparency, flame retardancy and mechanical properties [
17]. The optimized resin compound exhibits an increase in toughness (impact strength from 6.8 kJ/m
2 to 25.2 kJ/m
2), an LOI value of 32.3%, a V-0 rating in UL-94 test, a high transmittance of about 92%, and excellent smoke and heat suppression properties. The possibilities of incorporating organic materials into the structure of epoxy resins to increase their flame-retardant properties have also been investigated [
18]. The flame retardant mechanism of epoxy composites with the combination of organic (CF-PO(OPh)
2) and inorganic compounds (SiO
2) showed an excellent thermal barrier effect, flame retardant and mechanical properties, as well as a high smoke suppression effect. The addition of synergistic compounds based on SiO
2/MOF (silica, polyaniline and zeolitic imidazolate) in the epoxy resin (6%) led to an LOI value of 28% under conditions in which the mechanical properties of the new composite have a slight decrease compared to a pure epoxy resin, due to the formation of a carbon layer that prevents combustion in the gaseous and solid phases [
19]. Research on a new halogen-free flame retardant (DTB) containing phosphorus, nitrogen and boron introduced into an epoxy resin indicated that the flame retardant and smoke inhibition performance of the developed thermosets were significantly improved with the incorporation of DTB [
20]. The possibility of incorporating fire-retardant nanofillers into epoxy resins was studied and analyzed in [
21]. The epoxy nanocomposite with only 2.5 wt% MgAl@NiCo passed the UL-94 test with a V-0 rating, while the sample containing the same amount of MgAl did not show any rating. The LOI value of the epoxy nanocomposite increased from 23.5 to 26.0% compared to that of the pure epoxy.
Among the organophosphorus flame retardants, phosphazene compounds can significantly improve the flame retardancy of epoxy resins due to the phosphorous and nitrogen flame-retardant synergy [
13]. The primary factors limiting flame propagation are the growth of closed pore size and the thickening of coke walls formed during combustion, both of which are enhanced by an increased phosphorus–nitrogen content [
22]. Research demonstrated that cured epoxy compositions with 75 wt% of phosphazene I and phosphazene II fall into the category of self-extinguishing materials [
22]. Epoxy resins cured with arylaminocyclotriphosphazenes demonstrate fire resistance to the UL-94 standard of V-0 rating [
23].
From the presentation of current research related to the use of fire-retardant epoxy resins, it can be seen that they are predominantly oriented towards establishing the exclusive properties related to fire resistance (UL-94 test, LOI) without emphasizing the thermal transfer characteristics. In the field of developing solutions that aim to reduce/eliminate fire risks for the energy sources that equip EVs, it is necessary for these characteristics to be considered. It is well known that both the operating efficiency and the service life of a battery depend on the temperature developed (reached) by the electrochemical cells (due to repetitive charging/discharging processes at various intensities) [
24,
25,
26].
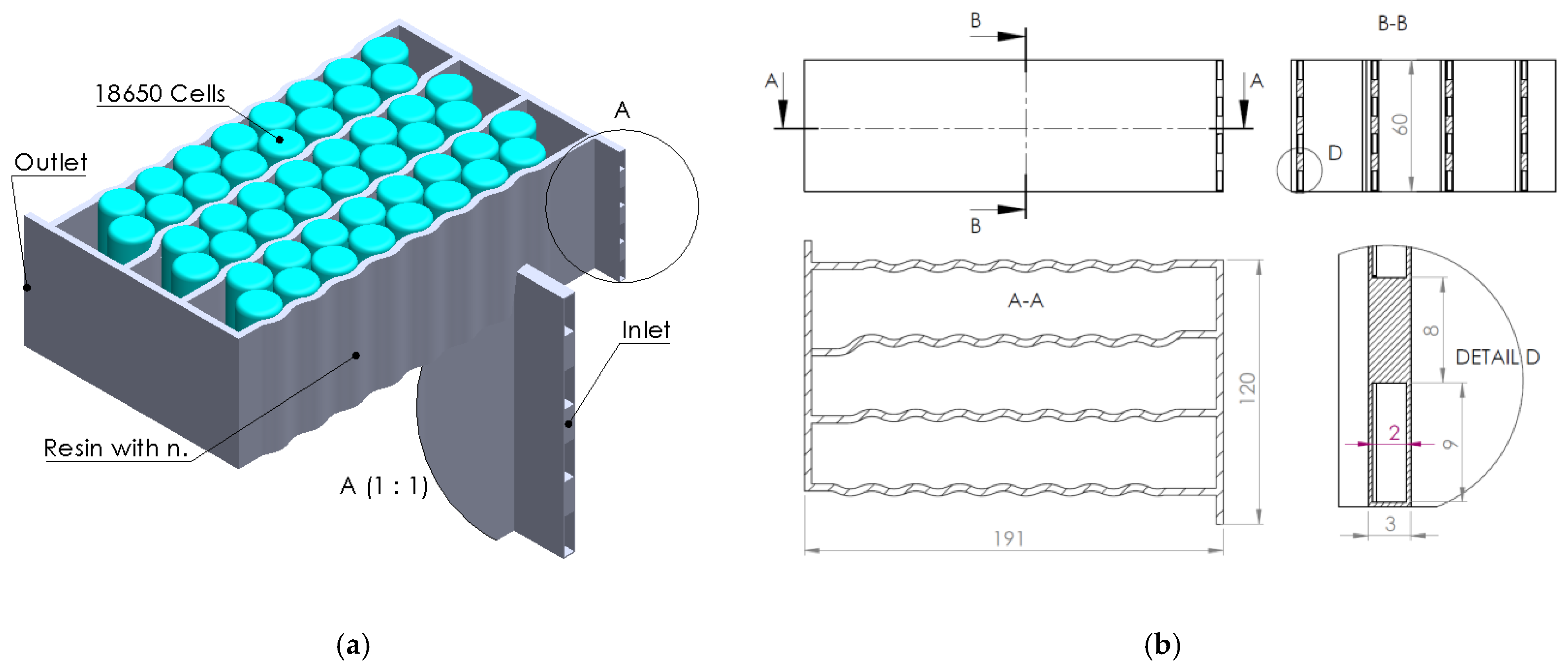
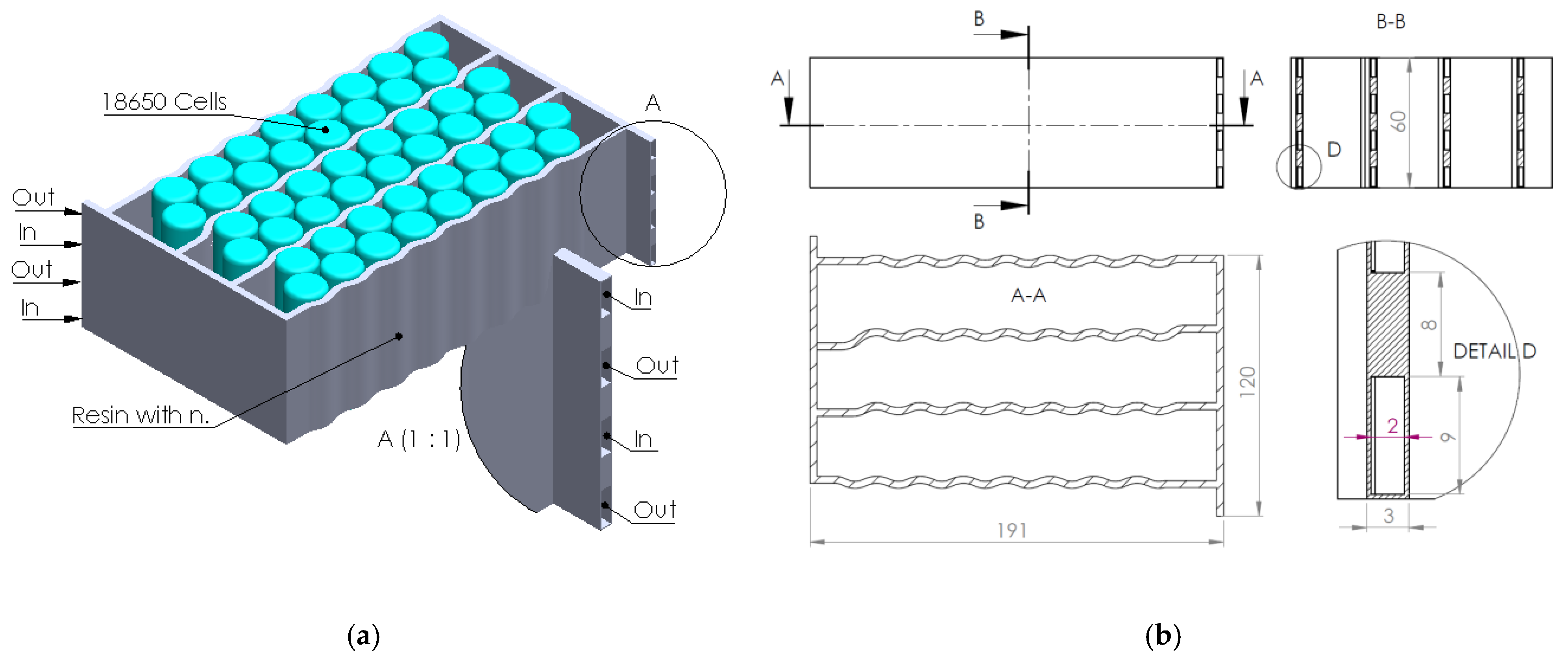
The present article’s authors’ research was directed towards the creation and application of an epoxy composite resin containing boron-based nanomaterials in a BTMS design (cooling serpentine). This ceramic filler has excellent applicability in components surrounding electronic components and, therefore, also in BTMSs, especially due to its high thermal conductivity and electrical insulation properties [
27,
28]. This type of nanomaterial was used due to the good results obtained and experimentally validated by the authors’ previous works [
29,
30].
In the context of the research presented above, the present article aims to analyze the effect and efficiency of using a composite resin as a thermal transfer element generated by electrochemical cells integrated into a liquid thermal management system (water-cooling serpentine-based, single and multichannel, and single and multiple inputs/outputs). Several serpentine designs made of composite resin were developed and analyzed (through numerical analysis methods) to establish the thermal transfer efficiency of the heat generated by the cells while providing fireproof protection. The study regarding the possibility of replacing aluminum components in the design of a BTMS with fire-retardant components is due to the exothermic and violent combustion character of aluminum under fire conditions.
Section 2 presents the characteristics of the material used for the study and the way in which the BTMS constructive cases were chosen for performing the numerical analyses of the heat transfer field.
Section 3 presents the results obtained through simulation, and these results are interpreted and discussed in
Section 4.
Section 5 makes a summative assessment of the study’s needs, objectives, and results, and it especially identifies future research directions in the field.
4. Discussion
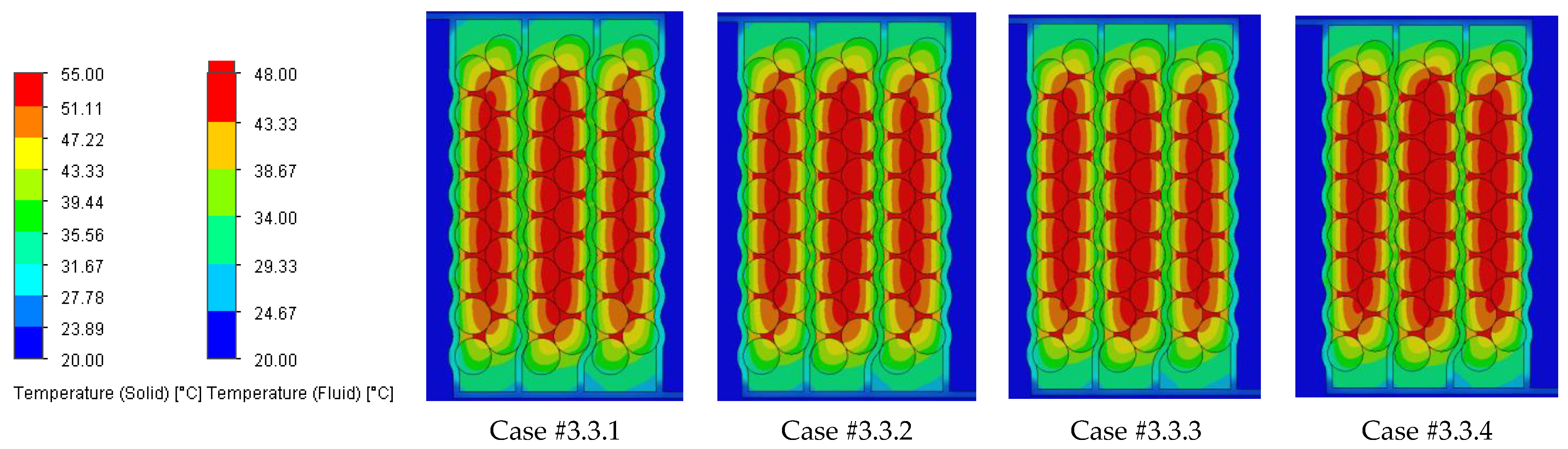
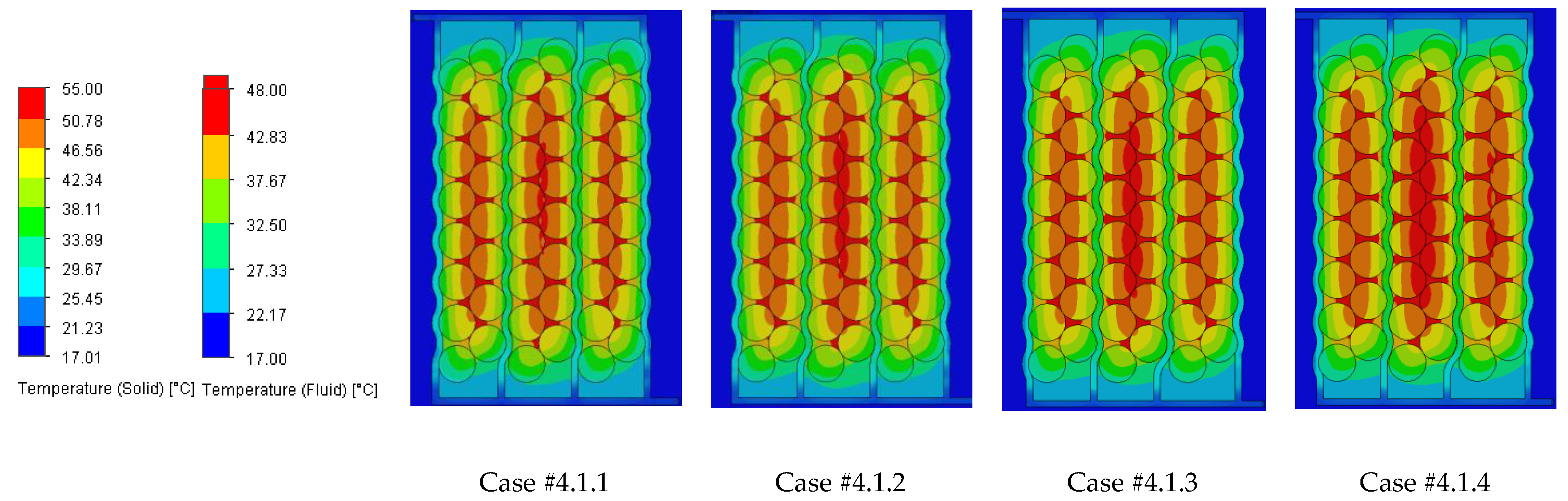
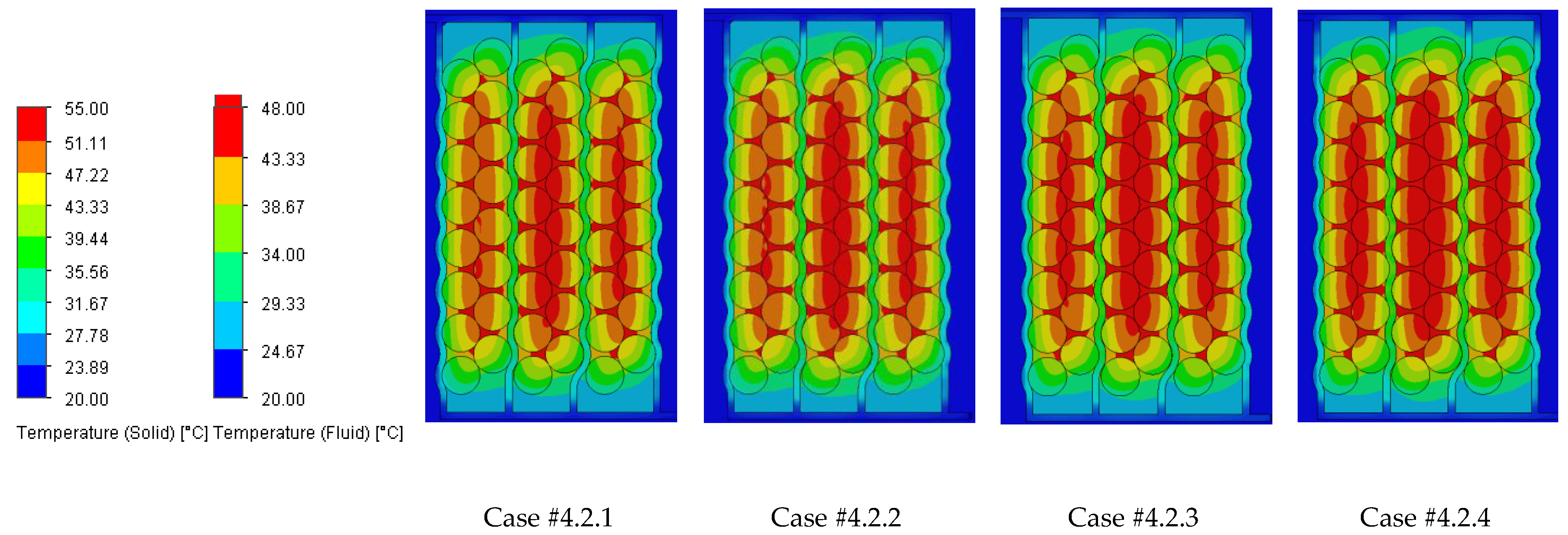
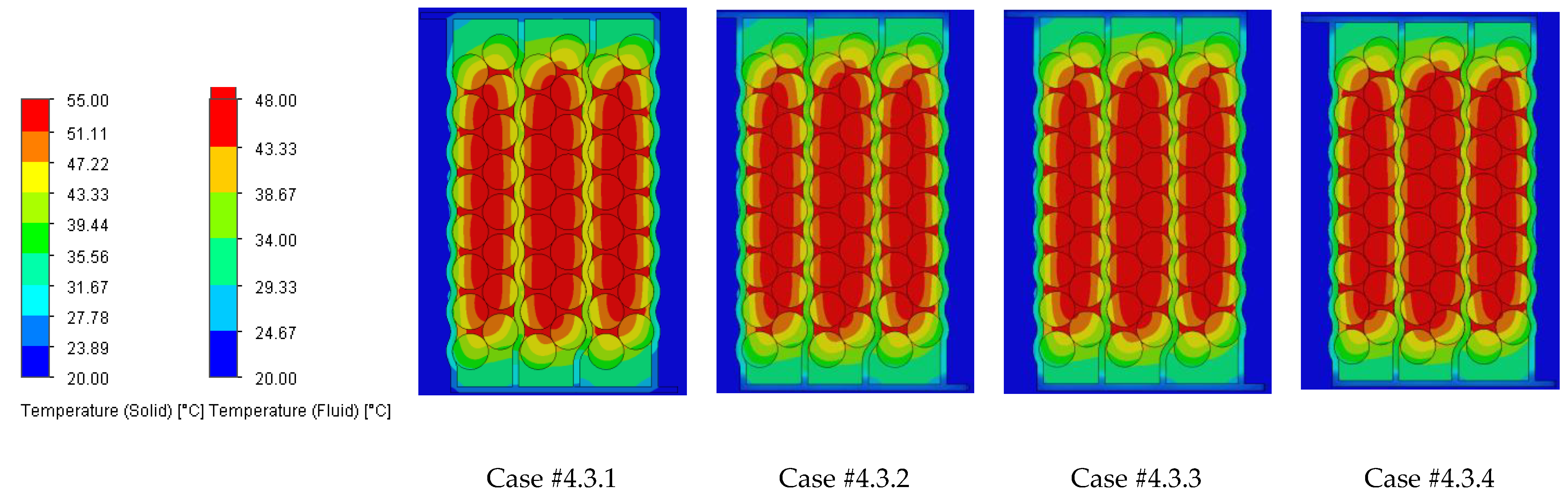
The temperature results from the SolidWorks simulations of the battery pack cooling systems are presented in
Figure 3,
Figure 4,
Figure 5,
Figure 7,
Figure 8,
Figure 9,
Figure 11,
Figure 12,
Figure 13,
Figure 15,
Figure 16 and
Figure 17 and
Table 1,
Table 2,
Table 3,
Table 4,
Table 5,
Table 6,
Table 7,
Table 8,
Table 9,
Table 10,
Table 11 and
Table 12 considering different flow rates (18, 15, 12 and 9 L/min) of the cooling fluid (water) at different inlet temperatures of 17, 20 and 25 °C. The analysis is completed with the heatmap from
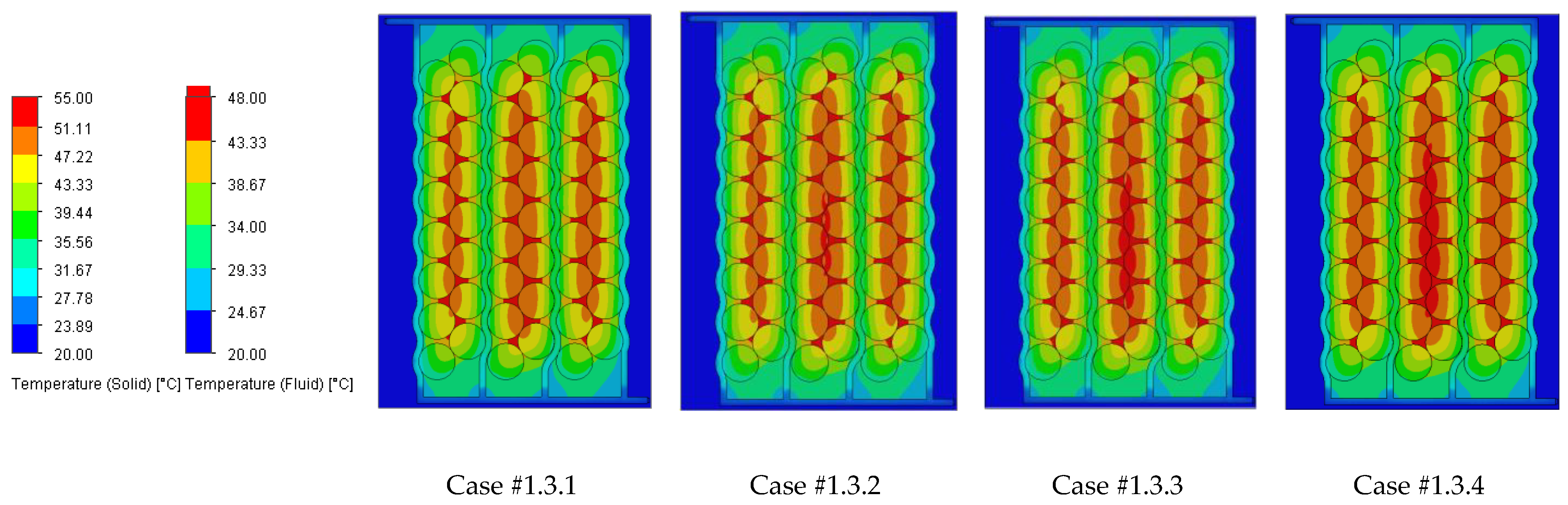
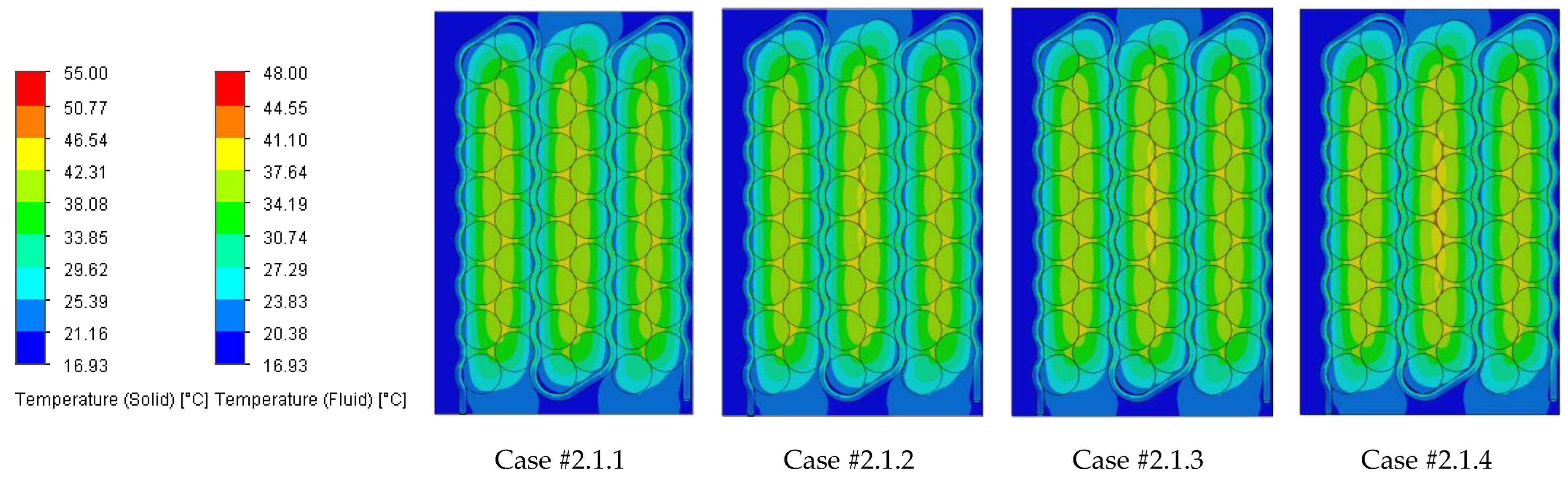
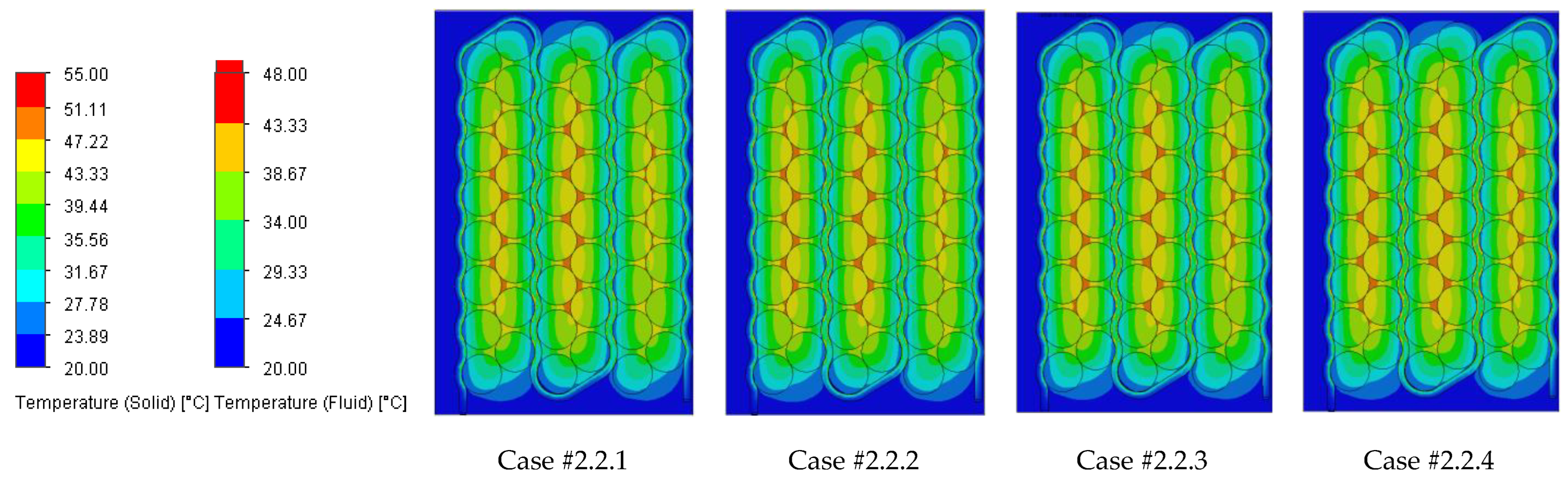
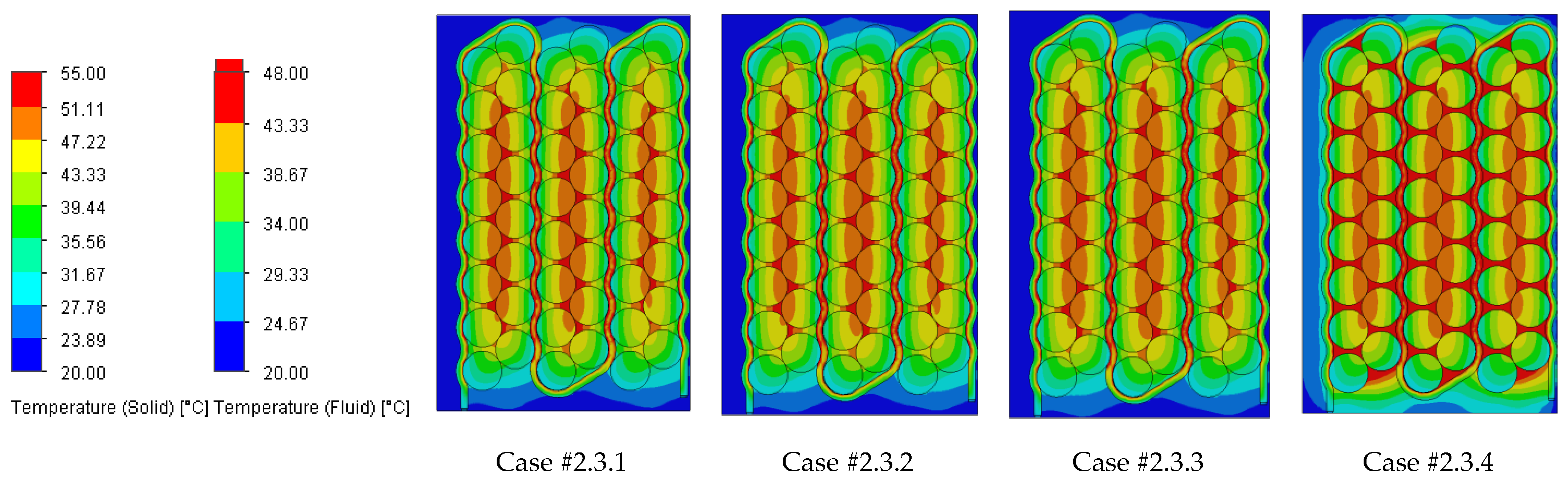
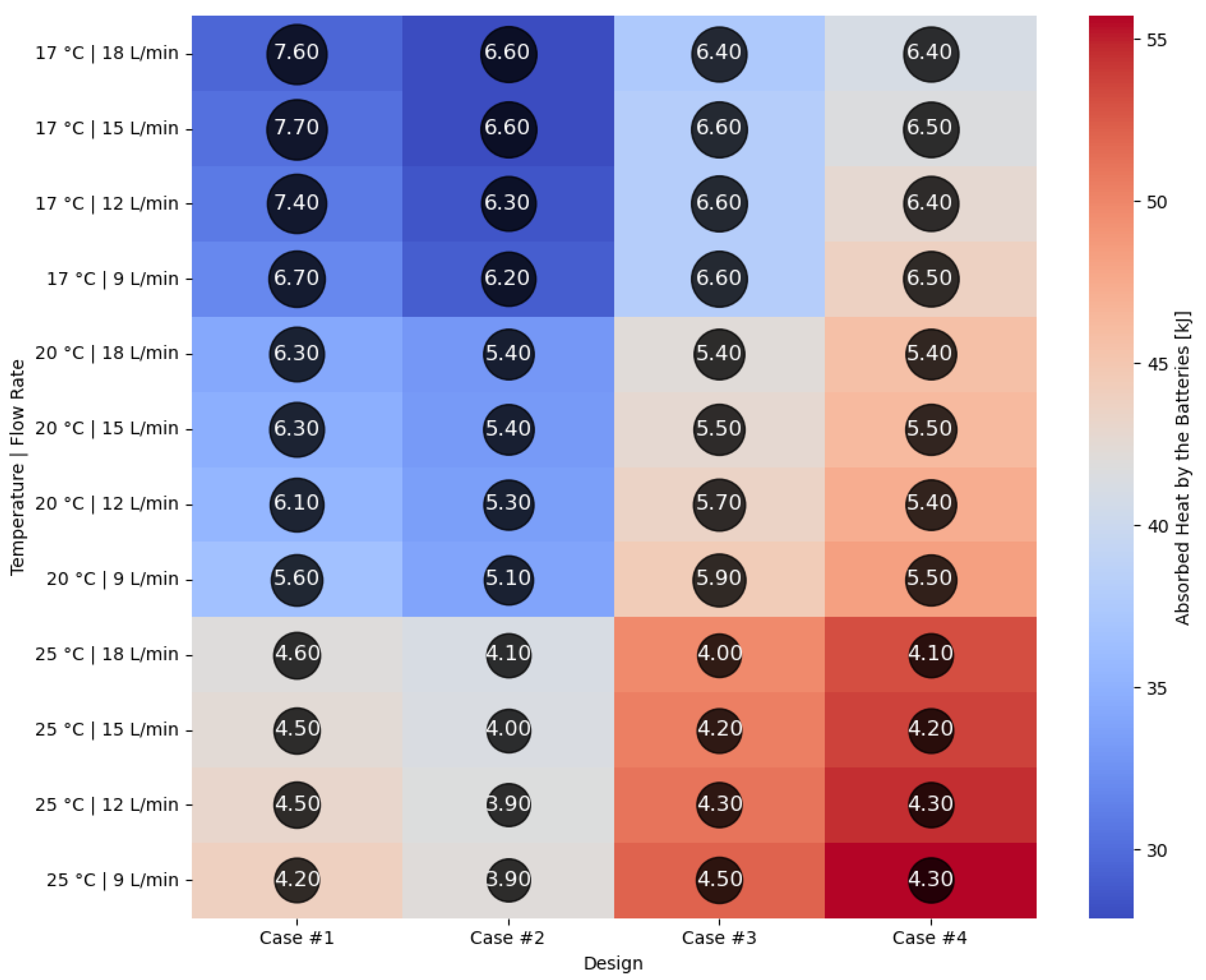
Figure 18 illustrating the heat absorbed by the cells and the additional markers that highlight the temperature gradient created in the system, represented by the difference in the cells’ internal resistance during operation.
Case #2 features a multichannel serpentine structure, where the serpentine channels are designed to facilitate the circulation of cooling fluid through a network of parallel and curved pathways. This design allows for greater surface contact between the cooling medium and the battery cells, potentially improving heat transfer. The average temperature of all cells for every testing condition combination (flow rate and coolant inlet temperature) is 36.7 °C, slightly lower than that of Case #1 (37.4 °C) and significantly lower than that of Case #3 (41.3 °C) and Case #4 (43.2 °C). This superior heat dissipation can also be observed in
Figure 18 by the colors of the second column from the heatmap, which tend more towards the blue specter compared to the other columns. This is supported by calculating the average value of the heat absorbed by the batteries for every testing combination, which is 36.25 kJ for Case #1, 34.44 kJ for Case #2, 43.98 kJ for Case #3 and 47.82 kJ for Case #4, respectively. This means a difference of 38.8% between the best (Case #2) and the worst (Case #4) design, highlighting the importance of the cooling channels’ structure and configuration for these applications. Despite the overall better cooling performance, the curved paths may introduce additional pressure losses at the end of the rows, added to the ones created by the small flow section due to the presence of four channels, which can affect the system’s power consumption.
It is also noticed that even though not in all cases, in most of them (9 out of 12 rows), Case #2 offers the smallest temperature gradient and internal resistance difference between the cells. The average difference for every testing combination is 5.96% for Case #1, 5.23% for Case #2, 5.48% for Case #3 and 5.38% for Case #4, respectively. For this criterion, the differences between cases are much smaller, but one should notice that removing a greater quantity of heat does not automatically mean also good thermal uniformity (see changes in order between Case #2 and #4), which highlights the importance of a good balance between these requirements and of a complete thermal analysis when evaluating BTMSs. Despite the single-inlet configuration and, therefore, longer continuous channel, this ensures the same flow rate in all conditions, while flow rate uniformity is difficult to achieve in the various branches of the multi-inlet configurations.
Thus, it can be concluded that to maximize the thermal management of the battery cells, designs incorporating single-inlet configurations (in the present study with four channels) are recommended. These designs can help achieve more efficient cooling by evenly distributing the coolant throughout the battery volume.
The use of nanomaterials as fillers in flame-retardant epoxy resins further improves its thermal conductivity, making it superior to air-based BTMS designs. Its performance is reliable and efficient, making it ideal for advanced battery cooling applications that must additionally involve high flame-retardant protection and a reduction/elimination of fire risks. This highlights the nanocomposite resin model’s suitability for high-performance battery thermal management systems, particularly in electric vehicle applications where weight, thermal efficiency, and uniform cooling are critical.
5. Conclusions
With the increase in the number of electric vehicles in traffic, it is necessary to take measures (constructive and functional) to minimize and/or eliminate potential dangers and accidents related to their operation. The main energy source of electric vehicles is batteries based on Li-ion technologies; there are premises that due to the functional characteristics and improper operation (high temperatures of electrochemical cells) or possible manufacturing defects (unbalanced electrochemical cells, imperfect electrical contacts, under sizing of electrical circuits a.s.o.) there are risks of self-ignition and fire. A method of reducing and/or eliminating these risks is to use components of BTMSs with flame-retardant properties, and this article proposes the use of cooling serpentines made of a flame-retardant nanocomposite resin (replacing the current aluminum serpentines, which in case of fire can be a factor in accelerating combustion).
By means of computer numerical analysis methods (modeling and simulation), the use of flame-retardant nanocomposite resin cooling serpentines under different constructive and functional forms of the inlet and outlet of the cooling liquid (water) was analyzed. The major conclusion is that based on the results obtained, the design consisting of nanocomposite resin cooling serpentine four-channel configuration offers the lowest heat absorbed by the battery cells, of 27.9 kJ, at a water inlet temperature of 17 °C and an 18 L/min flow rate. This design’s average heat absorption value for all analyzed combinations is 34.44 kJ, which is 38.88% lower than in the case of the design consisting of multi-inlet and multiflow capability channels. Regarding the smallest temperature gradient between the cells, the best design is the same and offers an average internal resistance difference of 5.23%, with immediate beneficial effects on battery performance. The conducted analysis also highlighted that lowering the coolant’s temperature from 25 °C to 17 °C has a much greater impact on the system’s cooling performance than doubling the flow rate to 18 L/min.
Future research is needed in the direction of the study approached by the authors, both in terms of the use of real operating data and especially in terms of improving the mechanical resistance properties (to vibrations) of flame-retardant resins that have applicability potential in BTMSs of electric vehicles, due to the nature of their specific operating conditions.