Abstract
This study examines the impact of a dual cure activator (DCA) when applied in combination with self-adhesive resin cements on the zirconia. Sixty zirconia were prepared in compliance with the manufacturer’s directions. The specimens were randomly assigned to each group under the dark condition, following DCA and self-adhesive resin cements [RelyX universal resin cement (RXS); Maxcem elite chroma (MAC); Panavia SA cement multi (PSM)]; group 1, RXS; group 2, MAC; group 3, PSM; group 4, DCA + RXS; group 5, DCA + MAC; and group 6, DCA + PSM. The resin composite was fixed to the zirconia, surface-treated, and maintained in a dark container for 30 min. The specimens were kept in an incubator at a temperature of 37 degrees Celsius for 24 h. The universal testing device was employed to compute the shear bond strength (SBS). A stereomicroscope was used to analyze the fractured types. The data were analyzed employing the one-way ANOVA and Tukey’s test. Group 2 had the lowest SBS (4.93 ± 0.53 MPa). Group 1 (11.17 ± 0.86 MPa) and group 3 (11.48 ± 1.17 MPa) were not significantly different in SBS. Group 6 (15.61 ± 0.68 MPa) had the highest SBS but was not significantly different from group 4 (15.45 ± 1.20 MPa). The findings show that treating the zirconia surface with DCA before using the self-curing mode of self-adhesive resin cements is the best way to improve the bond between the zirconia and resin cement.
1. Introduction
In dentistry, esthetic restorations such as veneers, crowns, bridges, and other prosthetic appliances are made with dental ceramic materials. Their ability to match shade and translucency makes them esthetically pleasing and replicates the appearance of teeth []. The crucial attributes of all ceramic structures, including the ratio of the glassy part and the level of porosity, impact both the mechanical and optical qualities []. These ceramics provide high durability, biocompatibility, and wear resistance, resulting in their widespread acceptance in the fields of restorative dentistry. Zirconia is a polycrystalline product composed mostly of zirconium dioxide, which is often used as a restorative ceramic material []. The application of high-strength zirconia in esthetic and restorative dentistry is growing quickly because of their exceptional mechanical qualities, biocompatibility, and pleasing esthetic []. Moreover, the zirconia crown has the best performance in terms of marginal gap when compared to the lithium disilicate crown and composite crown [].
Zirconia ceramics have difficulties in bonding because of their chemical composition and no silica content []. Zirconia’s surfaces undergo surface modifications, such as air abrasion using aluminum oxide particles, to improve mechanical interlocking and increase the bonding capacity. There are zirconia coupling agents or primers that can attach chemicals to the surface of zirconia. One example is 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP), and this is made from phosphate ester molecules. These agents have demonstrated the ability to improve the effectiveness of the bond to zirconia surfaces [,,]. The process of bonding zirconia with resin cement is an important one in contemporary esthetic restorative dentistry. A phosphate-containing adhesive resin cement is more advantageous than other resin cements when bonding with zirconia. This is because it enhances the chemical interaction with the zirconia surface, resulting in improved long-term stability and strength of the bonding layer [].
Self-adhesive resin cements are a type of luting system that contains an acidic functional monomer. Acidic functional monomers have the ability to remove minerals and alter the tooth structure’s surface. They may then enter partly decalcified dentin and form chemical bonds with hydroxyapatite. Moreover, acidic functional monomers have the ability to chemically bond with zirconia []. On the other hand, an acidic residual monomer provides a negative impact since it might interfere with the amine-based self-adhesive resin cement polymerization process. To prevent this phenomenon, a dual cure activator (DCA) was created to start the polymerization reaction by generating the free radical. The addition of DCA enhanced both the promoter of the polymerization process and resolved the chemical incompatibility issue caused by chemical curing components and acidic monomers. On the contrary, the free amine-based self-adhesive resin cement is not affected by an acidic residual monomer. Nevertheless, there is little research on the DCA and the polymerization features of the free amine-based self-adhesive resin cement when used in dark-curing mode [].
Therefore, with the increasing use of zirconia and self-adhesive resin cement, we are exploring ways to enhance their bonding ability through DCA for use in clinical dental practice. In this study, we intentionally examine the impact of DCA when applied in combination with various self-adhesive resin cements on the zirconia surface by evaluating their shear bond strengths (SBSs). The null hypothesis holds that using DCA with various self-adhesive resin cements has no effect on zirconia’s shear bonding capacity.
2. Materials and Methods
2.1. Zirconia Preparation
The G*Power 3.1 system was implemented to estimate the sample size, considering a significance level of 0.05 and a power of 0.95. Sixty fully sintered zirconia specimens were created using a sixty-zirconia block (Argen™ Z HT+ Multilayer Zirconia Disc, San Diego, CA, USA). Each specimen had a 6 × 6 mm in dimension and 4 mm thickness. The sintering procedure was carried out on the zirconia specimens in compliance with the manufacturer’s directions. The samples were put inside polyvinyl chloride tubes and then filled with epoxy resin (Figure 1).
Figure 1.
Zirconia put inside polyvinyl chloride tubes and filled with epoxy resin.
The specimens were polished using an automated sanding machine (Leco, St. Joseph, MI, USA) with a 3M Wetordry abrasive sheet 600-grit (3M, St. Paul, MN, USA). The polishing was performed at a force of 2 kg/cm2, 100 rounds per minute, for two minutes while water was flowing.
Subsequently, the specimens underwent sandblasting using 50 µm of alumina for 15 s. The sandblasting device (Nicchu, Bangkok, Thailand) carried out the sandblasting at a 3.8 bar pressure and a 10 mm distance. The samples underwent a cleaning process for a duration of fifteen minutes employing an ultrasonic cleaner (GT sonic, NT, Hongkong) in distilled water, followed by a rinse with running water.
2.2. Zirconia Surface Alteration
Table 1 summarizes the materials used in this research. In order to verify the bonding location, circular apertures of 2.0 mm in diameter and about 80 microns in thickness were affixed to the surfaces of all specimens using adhesive scotch tape (3M, MN, USA). Thirty specimens’ surfaces were randomly divided into three subgroups (n = 10 each), untreated with DCA (Clearfil DC activator, Kuraray Noritake Dental Inc., Okayama, Japan), and bonded with three self-adhesive resin cements [RelyX universal resin cement (RXS), 3M, Seefeld, Germany; Maxcem elite chroma (MAC), Kerr corporation, Orange, CA, USA; Panavia SA cement multi (PSM), Kuraray Noritake Dental Inc., Okayama, Japan]. And another thirty specimens’ surfaces were randomly divided into three subgroups (n = 10 each), treated with DCA, and cementated with three self-adhesive resin cements. Table 2 displays the grouping of zirconia surface treatments.

Table 1.
Summarizes the materials used in this research.

Table 2.
Displays the grouping of zirconia surface treatments.
2.2.1. Zirconia Surface Treatment without DCA
Under dark conditions, the specimens were treated with three types of self-adhesive resin cement (RXS, MAC, and PSM). The self-adhesive resin cement was mixed and then applied into a hole that was made in the region covered by one-sided adhesive tape. Afterwards, a microbrush was applied to remove any residual self-adhesive resin cement.
2.2.2. Zirconia Surface Treatment with DCA
The specimens were conducted in light-restricted conditions using a microbrush saturated with a DCA solution. The microbrush was utilized to the zirconia surface once, and any excess DCA was eliminated using a fresh microbrush. A dry triple syringe was forcefully blasted at a 10 mm distance with a pressure of 40–50 pounds per centimeter until the solvent had fully evaporated. Afterward, the specimens were treated with three types of self-adhesive resin cement (RXS, MAC, and PSM). The self-adhesive resin cement was mixed and then applied into a hole that was made in the region covered by one-sided adhesive tape. Afterwards, a microbrush was applied to remove any residual self-adhesive resin cement.
2.3. Bonded Specimen
A silicone model with a diameter of 3 mm and a height of 2 mm, made of Honigum putty (DMG, Hamburg, Germany), was used to form the resin composite rods. The resin composite rods were made using Harmonize Shade A4E (Kerr Corporation, Orange, CA, USA). The resin composite rods were activated by a light curing system (Coltene Inc., Cuyahoga Falls, OH, USA) positioned as near and perpendicularly to the sample as feasible for a duration of 40 s. Following the removal of the silicone mold, a further 40 s of light curing were performed.
Afterwards, a self-adhesive resin cement was loaded into the bonding location, which was controlled by a polyethylene adhesive tape hole. The resin composite rod was fixed on the self-adhesive resin cement using a modified durometer, applying a force of 50 N []. Any remaining cement was then removed (Figure 2). The specimens were maintained in a dark container for a duration of 30 min. Subsequently, the specimens were put in distilled water and kept in an incubator (Vevor lab, Rch Cucamonga, CA, USA) at a temperature of 37 degrees Celsius for a duration of 24 h.

Figure 2.
Bonded specimen.
2.4. Assessment of SBS and Analysis of Failure Characteristics
The AGS-X 500N universal metering device, manufactured by Shimadzu Corporation in Kyoto, Japan, was employed to compute the bond strength data. The measurements were performed at a controlled speed of 0.5 mm/minute by a knife-edge blade. The size of the bonding domain and the strength of the broken adhesion were divided to compute the bond strength score.
The fracture mode profiles of zirconia and self-adhesive resin cements were studied employing a stereomicroscope with a magnification of ×40. Three configurations were devised to determine the fracture mechanisms [,,,]: (a) an adhesive pattern (separation at the interface between the zirconia and the self-adhesive resin cement), (b) a cohesive pattern (fracture occurring within either the zirconia or the self-adhesive resin cement), and (c) a mixed pattern (fractures involving both the self-adhesive resin cement and the zirconia).
2.5. The Statistical Determination of the Data
The level of normality of the SBS data distribution was determined employing the Kolmogorov–Smirnov analysis. A variance analysis (one-way ANOVA) was utilized to assess the differences in the shear bonding ability of zirconia while utilizing untreated or treated DCA with different self-adhesive resin cements. Tukey HSD comparisons were used to evaluate the SBS data in megapascals (MPa). All analyses were conducted using a significance level of 0.05 and a confidence level of 95%.
3. Results
3.1. Data of SBS
In the untreated DCA group, the mean shear bond strengths and standard deviation (SD) of zirconia and self-adhesive resin cement interfaces varied between 4.93 ± 0.53 (mean ± SD) MPa and 11.48 ± 1.17 MPa. Group 2 had the lowest SBS (4.93 ± 0.53 MPa) compared to all groups. However, group 1 (11.17 ± 0.86 MPa) and group 3 (11.48 ± 1.17 MPa), which were untreated DCA groups, were not significantly different in SBS. The SBSs were much stronger in all groups after being treated with a DCA (p < 0.05), ranging from 9.94 ± 0.67 MPa to 15.61 ± 0.68 MPa. The DCA also made the shear bond capacity much stronger compared to the unprimed group (p < 0.05). Nevertheless, group 6 (15.61 ± 0.68 MPa) had the highest SBS but was not significantly different from group 4 (15.45 ± 1.20 MPa). Group 5 (9.94 ± 0.67 MPa) had the lowest SBS compared to all DCA-treated groups.
3.2. Failure-Type Patterns
None of the groups that were evaluated had a cohesive pattern mode. Groups 2 and 5 show a 100 percent adhesive fracture pattern with a lower SBS. However, groups 1, 3, 4, and 6 had increased mixed fracture patterns with greater SBS, while group 6 had the highest percentage of mixed fracture patterns at 30 percent. Table 3 displays the mean SBS and SD together with the patterns of fracture failure. Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8 provide visual representations of different fracture forms within groups 1–6.

Table 3.
Mean shear bond strength ± standard deviation (Megapascal) and failure type (%).
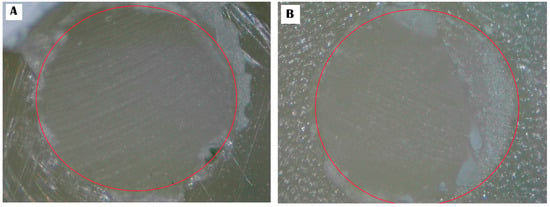
Figure 3.
Provides a visual representation of the fracture pattern for RXS: (A) adhesive pattern; (B) mixed pattern.
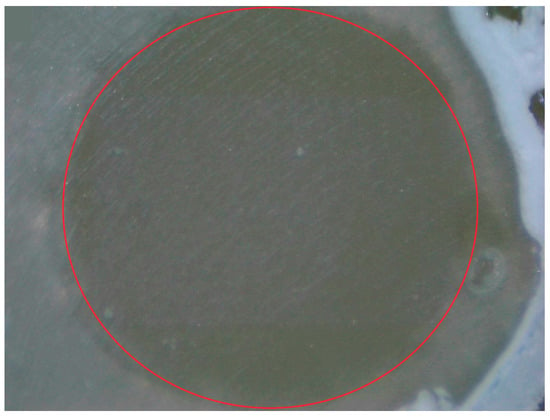
Figure 4.
Provides a visual representation of the adhesive fracture pattern for MAC.

Figure 5.
Provides a visual representation of the fracture pattern for PSM: (A) adhesive pattern; (B) mixed pattern.

Figure 6.
Provides a visual representation of the fracture pattern for DCA + RXS: (A) adhesive pattern; (B) mixed pattern.

Figure 7.
Provides a visual representation of the adhesive fracture pattern for DCA + MAC.
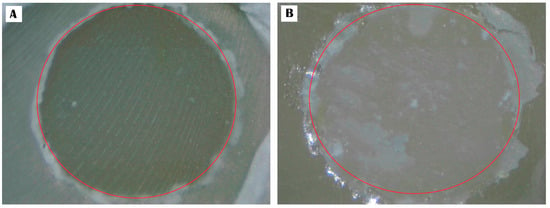
Figure 8.
Provides a visual representation of the fracture pattern for DCA + PSM: (A), adhesive pattern; (B), mixed pattern.
4. Discussion
In this research, we specifically investigate the impact of DCA when combined with different self-adhesive resin cements on the surface of zirconia by examining their SBS. These findings show that the use of DCA, when combined with different self-adhesive resin cements, significantly affects the shear bonding performance of zirconia. Therefore, this result disproves the null hypothesis.
The concept of adhesion is primarily based on the interplay between micro-mechanical preparation and chemical adhesion. In order to examine the performance of the adhesion between the zirconia and self-adhesive resin cement, it is essential to recognize the influence of different surface alterations on their interaction. A prior study discovered that the sandblasting approach enhanced the SBS and was the most successful method of creating micro-mechanical adhesion in zirconia [,,]. The explanation is that zirconia’s surface energy and roughness are greatly increased by sandblasting [,,]. Based on chemical surface modifications, the 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP) molecule is specifically formulated to strengthen the chemical interaction between zirconia and resin materials, ensuring a durable and long-lasting adhesive interaction [,,,]. Moreover, the bond-promoting action of 10-MDP was proven by Nagaoka et al., who also demonstrated that commercial primers and resin cements incorporating 10-MDP often attain better bond qualities with dental zirconia []. However, pretreating zirconia with universal adhesive, which enhances the bonding interaction between zirconia and resin materials, was shown in earlier investigations to be the most successful alternative surface modification strategy [,]. These reasons led to the use of the sandblast technique for micro-mechanical preparation and self-adhesive resin cement-contained phosphate functional molecules bonded to zirconia in the present investigation.
The commercialization of self-adhesive resin cements is one of the best recent developments in restorative and esthetic dentistry. The formulation is an essential issue to take into consideration since the majority of self-adhesive resin cements are made of specific acidic phosphate and/or carboxylate functional molecules. In several self-adhesive resin cements, for example, the acidic functional monomer 10-MDP is contained. This hydrophilic, functional monomer has a slight potential to self-etch and has shown adhesion to titanium, zirconia, base metal alloys, and teeth [,,,]. Regarding the untreated DCA groups, the SBS of RXS and PSM (11.17 ± 0.86 MPa and 11.48 ± 1.17 MPa, respectively) was significantly greater than that of MAC (4.93 ± 0.53 MPa). The RXS and PSM were self-adhesive resin cements containing 10-MDP, while the MAC was a self-adhesive resin cement containing glycerol phosphate dimethacrylate (GPDM). This investigation found these two acidic functional monomers among the 10-MDP and the GPDM self-adhesive resin cements, despite their relatively significant variances in SBS, which corresponds to Sriamporn et al., who suggested the possibility that the difference may be caused by the acidic functional monomer present in each self-adhesive resin cement []. The strong shear bond ability of zirconia might be caused by the 10-MDP chemical interaction with the zirconium oxide layer. The acidic phosphate functional monomer, like 10-MDP, has a longer chain spacer compared to GPDM []. The long chain spacer may enhance the hydrophobicity of the 10-MDP self-adhesive resin cement; its adhesive strength surpasses that of other SACs containing other acidic phosphate functional monomers, like GPDM, which has more hydrophilicity than 10-MDP []. Moreover, GPDM contains two polymerizable groups [], whereas 10-MDP only has one. The high polymerization shrinkage stress caused by these two polymerizable groups may have led to a decrease in the SBS. For these explanations, the bond ability of 10-MDP self-adhesive resin cements (RXS and PSM) was greater than that of the GPDM self-adhesive resin cement (MAC).
In terms of treated DCA groups, the shear bond ability was significantly greater than that of untreated DCA groups. The DCA might induce the self-adhesive resin cement polymerization reaction, resulting in enhanced SBS. Self-adhesive resin cements used in this research are RXS, MAC, and PSM, which are amine-free resin cements with mild self-etch acidic pH values. The DCA is composed of sodium sulfonate salt, which is in contact with the mildly acidic pH values of self-adhesive resin cements, hence triggering the generation of free radicals to initiate the self-adhesive resin cement polymerization process. Based on a few studies, the aromatic sodium sulfonate salts might help make free radicals, which then improve the ability of self-adhesive resin cement polymerization [,]. The process of DCA involves the initial instability of aryl sulfinic acid, leading to its decomposition into aryl sulfonic acid and thiolsulfonate during the early stage. The last organic configuration formed was an arylsulfonyl radical, which has the ability to initiate polymerization processes. The self-curing or redox polymerization process was aided by the aryl radical, which increased the rate of polymerization []. Due to these factors, the SBS of treated DCA groups increased when compared to untreated DCA groups in the self-curing modes of all self-adhesive resin cements in current research.
To ascertain the fracture processes, three designs were established [,,,]: (a) an adhesive pattern (separation at the interface between the zirconia and self-adhesive resin cement), (b) a cohesive pattern (fracture occurring within either the zirconia or the self-adhesive resin cement), and (c) a mixed pattern (fractures involving both the zirconia and the self-adhesive resin cement). In these findings, the SBS testing corroborated the conclusions derived from the examination of the breakdown processes seen in the debonded samples. There was no cohesive failure mode seen in any of the tested groups. Groups 2 and 5 exhibited a 100% adhesive fracture pattern, accompanied by a lower SBS. However, increasing mixed fracture patterns accompanied by greater SBS were seen in groups 1, 3, 4, and 6. Group 6 experienced the greatest SBS and the highest percentage of mixed fracture patterns at thirty percent. The higher SBS is associated with an increased mixed fracture pattern [,,].
The design of this research study was limited; the zirconia and self-adhesive resin cements’ SBSs could only be ascertained by the incubated specimen 24 h after bonding. Short-term 24 h data without accelerated aging may not fully represent clinical conditions. Future studies may use thermocycling and fatigue loading to evaluate the durability and longevity of bond strength over time in clinical practice.
5. Conclusions
The outcomes of the current in vitro research indicate that the most successful strategy for enhancing the bond ability between zirconia and self-adhesive resin cements is to pretreat the zirconia surface with DCA before cementation using the self-curing mode of self-adhesive resin cements.
Author Contributions
T.S., N.T. and A.K. conceived and designed this study; P.A., S.V. and A.K. performed the experiments and interpreted the results; P.A., T.S., S.V., N.T. and A.K. drafted this manuscript; P.A., T.S. and A.K. revised this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Faculty of Dentistry Thammasat University Research Fund, Contract No. 1/2567.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Conrad, H.J.; Seong, W.J.; Pesun, I.J. Current ceramic materials and systems with clinical recommendations: A systematic review. J. Prosthet. Dent. 2007, 98, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.H.D.; Lima, E.; Miranda, R.B.P.; Favero, S.S.; Lohbauer, U.; Cesar, P.F. Dental ceramics: A review of new materials and processing methods. Braz. Oral Res. 2017, 31 (Suppl. S1), e58. [Google Scholar] [CrossRef]
- Benalcázar-Jalkh, E.B.; Bergamo, E.T.P.; Campos, T.M.B.; Coelho, P.G.; Sailer, I.; Yamaguchi, S.; Alves, L.M.; Witek, L.; Tebcherani, S.M.; Bonfante, E.A. A narrative review on polycrystalline ceramics for dental applications and proposed update of a classification system. Materials 2023, 16, 7541. [Google Scholar] [CrossRef]
- Kongkiatkamon, S.; Rokaya, D.; Kengtanyakich, S.; Peampring, C. Current classification of zirconia in dentistry: An updated review. PeerJ 2023, 11, e15669. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, F.; Paolone, G.; Di Domenico, G.L.; Pagani, N.; Gherlone, E.F. SEM Evaluation of the Marginal Accuracy of Zirconia, Lithium Disilicate, and Composite Single Crowns Created by CAD/CAM Method: Comparative Analysis of Different Materials. Materials 2023, 16, 2413. [Google Scholar] [CrossRef]
- Scaminaci Russo, D.; Cinelli, F.; Sarti, C.; Giachetti, L. Adhesion to zirconia: A systematic review of current conditioning methods and bonding materials. Dent. J. 2019, 7, 74. [Google Scholar] [CrossRef]
- Gracis, S.; Thompson, V.P.; Ferencz, J.L.; Silva, N.R.; Bonfante, E.A. A new classification system for all-ceramic and ceramic-like restorative materials. Int. J. Prosthodont. 2015, 28, 227–235. [Google Scholar] [CrossRef]
- Klaisiri, A.; Maneenacarith, A.; Jirathawornkul, N.; Suthamprajak, P.; Sriamporn, T.; Thamrongananskul, N. The Effect of multiple applications of phosphate-containing primer on shear bond strength between zirconia and resin composite. Polymers 2022, 14, 4174. [Google Scholar] [CrossRef] [PubMed]
- Klaisiri, A.; Krajangta, N.; Thamrongananskul, N. The Durability of zirconia/resin composite shear bond strength using different functional monomer of universal adhesives. Eur. J. Dent. 2022, 16, 756–760. [Google Scholar] [CrossRef]
- Sriamporn, T.; Thamrongananskul, N.; Klaisiri, A. The effectiveness of various functional monomers in self-adhesive resin cements on prosthetic materials. J. Int. Soc. Prev. Community Dent. 2022, 12, 332–335. [Google Scholar] [CrossRef]
- Leeprakobboon, W.; Sriamporn, T.; Klaisiri, A.; Thamrongananskul, N. The effect of a dual cure activator composed of aromatic sulfinate amide derivatives on the microhardness of self-adhesive resin cements without light activation. J. Int. Dent. Med. Res. 2021, 14, 39–45. [Google Scholar]
- Maneenacarith, A.; Rakmanee, T.; Klaisiri, A. The influence of resin cement thicknesses on shear bond strength of the cement-zirconia. J. Stomatol. 2022, 75, 7–12. [Google Scholar] [CrossRef]
- Lopes, G.R.S.; Ramos, N.C.; Grangeiro, M.T.V.; Matos, J.; Bottino, M.; Özcan, M.; Valandro, L.; Melo, R. Adhesion between zirconia and resin cement: A critical evaluation of testing methodologies. J. Mech. Behav. Biomed. Mater. 2021, 120, 104547. [Google Scholar] [CrossRef] [PubMed]
- Reslan, M.R.; Sayed, M.; Rayyan, M.M.; Farouk, H. Effect of cement type on fracture resistance and mode of failure of monolith vs bilayered zirconia single crowns. J. Contemp. Dent. Pract. 2023, 24, 576–581. [Google Scholar] [PubMed]
- Karthigeyan, S.; Ravindran, A.J.; Bhat, R.T.R.; Nageshwarao, M.N.; Murugesan, S.V.; Angamuthu, V. Surface modification techniques for zirconia-based bioceramics: A review. J. Pharm. Bioallied Sci. 2019, 11 (Suppl. S2), S131–S134. [Google Scholar]
- Rigos, A.E.; Sarafidou, K.; Kontonasaki, E. Zirconia bond strength durability following artificial aging: A systematic review and meta-analysis of in vitro studies. Jpn. Dent. Sci. Rev. 2023, 59, 138–159. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, M.D.; Sharma, V.; Madaan, R.; Sareen, K.; Gurjar, B.; Saini, A.K. Effect of surface treatment of zirconia on the shear bond strength of resin cement: A systematic review and meta-analysis. Cureus 2023, 15, e45045. [Google Scholar] [CrossRef]
- Nagaoka, N.; Yoshihara, K.; Feitosa, V.P.; Tamada, Y.; Irie, M.; Yoshida, Y.; Van Meerbeek, B.; Hayakawa, S. Chemical interaction mechanism of 10-MDP with zirconia. Sci. Rep. 2017, 7, 45563. [Google Scholar] [CrossRef]
- Comino-Garayoa, R.; Peláez, J.; Tobar, C.; Rodríguez, V.; Suárez, M.J. Adhesion to zirconia: A systematic review of surface pretreatments and resin cements. Materials 2021, 14, 2751. [Google Scholar] [CrossRef]
- Calamita, R.S.; Oliveira, A.A.D.; Pizzanelli, G.G.; Salvador, M.V.O.; Mesquita, A.M.M.; Pecorari, V.G.A.; Lima, A.F. Interaction of different concentrations of 10-MDP and GPDM on the zirconia bonding. Dent. Mater. 2023, 39, 665–668. [Google Scholar] [CrossRef]
- Dos Santos, R.A.; de Lima, E.A.; Mendonça, L.S.; de Oliveira, J.E.; Rizuto, A.V.; Tavares, F.d.A.S.; da Silva, R.B. Can universal adhesive systems bond to zirconia? J. Esthet. Restor. Dent. 2019, 31, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Ikemura, K.; Kojima, K.; Endo, T.; Kadoma, Y. Effect of the combination of dithiooctanoate monomers and acidic adhesive monomers on adhesion to precious metals, precious metal alloys and non-precious metal alloys. Dent. Mater. J. 2011, 30, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.Y.; Stoner, B.R.; Piascik, J.R.; Smith, R. Adhesion/cementation to zirconia and other non-silicate ceramics: Where are we now? Dent. Mater. 2011, 27, 71–82. [Google Scholar] [CrossRef]
- Jayasheel, A.; Niranjan, N.; Pamidi, H.; Suryakanth, M.B. Comparative evaluation of shear bond strength of universal dental adhesives -an in vitro study. J. Clin. Exp. Dent. 2017, 9, e892–e896. [Google Scholar] [CrossRef]
- Llerena-Icochea, A.E.; Costa, R.M.; Borges, A.; Bombonatti, J.; Furuse, A.Y. Bonding polycrystalline zirconia with 10-MDP containing adhesives. Oper. Dent. 2017, 42, 335–341. [Google Scholar] [CrossRef]
- Senyilmaz, D.P.; Palin, W.M.; Shortall, A.C.; Burke, F.J. The effect of surface preparation and luting agent on bond strength to a zirconium-based ceramic. Oper. Dent. 2007, 32, 623–630. [Google Scholar] [CrossRef]
- Yoshihara, K.; Nagaoka, N.; Hayakawa, S.; Okihara, T.; Yoshida, Y.; Van Meerbeek, B. Chemical interaction of glycero-phosphate dimethacrylate (GPDM) with hydroxyapatite and dentin. Dent. Mater. 2018, 34, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.Y.; Bagheri, R.; Kim, Y.K.; Kim, K.H.; Burrow, M.F. Cure mechanisms in materials for use in esthetic dentistry. J. Investig. Clin. Dent. 2012, 3, 3–16. [Google Scholar] [CrossRef]
- Arrais, C.A.G.; Giannini, M.; Rueggeberg, F.A. Effect of sodium sulfinate salts on the polymerization characteristics of dual cured resin cement systems exposed to attenuated light activation. J. Dent. 2009, 37, 219–227. [Google Scholar] [CrossRef]
- Cura, C.; Özcan, M.; Isik, G.; Saracoglu, A. Comparison of alternative adhesive cementation concepts for zirconia ceramic: Glaze layer vs. zirconia primer. J. Adhes. Dent. 2012, 14, 75–82. [Google Scholar]
- Stefani, A.; Brito, R.B., Jr.; Kina, S.; Andrade, O.S.; Ambrosano, G.M.; Carvalho, A.A.; Giannini, M. Bond strength of resin cements to zirconia ceramic using adhesive primers. J. Prosthodont. 2016, 25, 380–385. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).