Abstract
Analyzing the effect of anions on the structure of geopolymers is crucial because anions can significantly influence the material’s chemical stability, mechanical properties, and long-term durability. Understanding these effects helps optimize geopolymer compositions for various applications, such as construction materials and waste encapsulation. This research report describes the effects of nitrate, sulfate, and phosphate anions on alkali-activated blast furnace slag’s structural integrity and properties. Advanced techniques like XRD, FT-IR, Raman spectroscopy, and XPS have been employed to analyze structural modifications caused by anions, providing insights into their interactions and effects. These anions generally decrease compressive strength by disrupting geopolymerization and altering microstructure. For example, sulfate ions lead to the formation of ettringite, while phosphate ions bind calcium into separate phases. We can also observe microstructural changes, such as increased porosity with phosphate, which significantly reduces strength. Nitrate’s effect is less detrimental but still influences the overall structural dynamics.
1. Introduction
Alkali-activated binders are a class of inorganic binding materials obtained by reacting a material rich in aluminum oxide and silica with an alkaline activator. Metakaolin can be utilized as a precursor for their production, as can waste materials such as fly ash, blast furnace slag, ash from the combustion of municipal solid waste, red sludge, and other mine wastes [1,2]. The alkaline activator commonly consists of sodium or potassium hydroxides and carbonates, as well as potassium or sodium water glass. The selection of waste raw material and activator used in binder production affects the reactions’ mechanisms and the final product’s characteristics. However, the precise reaction process governing the setting and curing of alkali-activated binders remains incompletely defined [3].
Alkaline-activated composites are considered as promising alternatives to traditional construction materials due to their unique properties and environmental advantages. So far, they have been applied in various industries, including construction, refractory manufacturing, geotechnical engineering, waste management, and high-performance materials [4,5,6,7]. Geopolymers exhibit potential in the immobilization and stabilization of hazardous wastes. The alkaline activation process can encapsulate contaminants within the matrix, reducing their leachability and potential environmental impact. Particularly, alkali-activated blast furnace slag (AABFS) can be used in the immobilization or stabilization of hazardous wastes [8,9]. AABFS-based materials can be used in the construction of containment barriers or as solidification agents for hazardous waste disposal. Continued research and development are crucial for refining the application of AABFS in waste management and encouraging their broader use as a sustainable approach to tackling waste-related issues.
Heavy-metal-containing compounds are currently widely used in rapidly developing industrial sectors such as mining, nuclear power plants, and plastics [10]. These industries generate a growing amount of hazardous solid waste that pollutes air, water, and soil. Therefore, it is essential to develop an effective method for immobilizing heavy metals to prevent their negative environmental impact. According to the United States Environmental Protection Agency (USEPA), solidification/stabilization is the best technology for hazardous waste disposal because it is simple, relatively fast, cost-effective, and convenient for cleaning up contaminated sediments or soil. It has been found that construction and building materials are the best solutions for immobilizing heavy metals [11]. Literature results [12,13,14] show that metals such as Cu, Ni, Cd, Pb, Zn, and Cr can be efficiently stabilized in a three-dimensional geopolymeric matrix. However, hazardous waste may contain significant amounts of other chemical compounds, also in anionic form [15,16]. For example, municipal solid waste incinerator fly ash may contain significant amounts of nitrates and sulphates [17] as well as phosphates [18].
In contrast to the numerous adsorbents designed for cationic metals, only a few geopolymer-based materials have been developed for anion removal. Halogen anions, including F− [19], Cl− [20], and I− [21], were analyzed. Among oxyanions, attention was paid to selenate [22,23,24,25], arsenate [25,26,27], chromate [28,29], antimoniate [15,30], phosphate [31], and sulfate [32] ions. In most cases, the sorption mechanisms considered are ion exchange and precipitation. However, it is worth noting that substantial leaching of anions has been consistently observed across most studies, primarily attributed to the electrostatic repulsion stemming from the negative charge of the framework.
Listed literature data indicate that inorganic anions may significantly impact the properties of geopolymer composites. Lee and Deventer [33] found that the precise incorporation of inorganic salts can be employed to adjust the setting and rheological properties of alkali-activated fly-ash-based cement. Findings regarding how various anions affect the mechanical properties of geopolymers also suggest differing trends for each specific anion studied. It was discovered that the compressive strength of geopolymers increases as the nitrate content rises [34,35]. In contrast, in systems based on slag, a decrease in compressive strength was recorded due to the presence of nitrate and sulfate anions [36]. According to this work, both anions utilize a significant portion of the alkali activator molecules available, impeding geopolymerization reactions and consequently restricting the amount of gel produced. Several studies have noted the rapid development of nitrate-containing crystalline phases such as sodalite and cancrinite in metakaolin-based geopolymers [34]. Similarly, the presence of sulfates during the alkaline activation of fly ash tends to slow down the formation of N-A-S-H gel and reduces the time required for zeolite precipitation [37]. Conversely, other research indicates that sodium sulfate solutions have minimal or negligible impact on the structure of alkali-activated fly ash [38].
The reaction mechanism in the presence of PO43− ions has not fully been explained so far. Lee and Deventer [39] proposed a hypothesis regarding the hydration kinetics of PO43− in alkali-activated materials derived from fly ash. Their proposition suggests a strong affinity between phosphate anions and Ca2+ cations, leading to the precipitation of Ca10(PO4)6(OH)2 (hydroxyapatite) instead of Ca(OH)2. On the other hand, Shi and Day [40] propose an alternate reaction mechanism in AABFS, implicating the formation of Ca3(PO4)2, which slows the activation of slag, reminiscent of observations during Portland cement hydration. Yet the precise role of phosphate in alkali-activated systems remains ambiguous.
Examining the impact of anions on the structure of geopolymers is vital because they affect the chemical bonds and microstructure, which in turn influence the material’s strength and durability. Various anions can modify the polymerization process, impacting both the setting time and mechanical properties of the geopolymer. Understanding these effects is key to refining formulations for improved performance in areas like construction, where long-term stability is essential. It also allows for the customization of geopolymers for specialized uses, such as the safe containment of hazardous waste, ensuring both material resilience and environmental protection. Despite the strides made in elucidating the adsorption characteristics of anions, there is a notable dearth of literature of their impact on the structural properties of geopolymers. Previous investigations have predominantly focused on elucidating the effect of anions on fly-ash- or metakaolin-based geopolymers. Consequently, this study aims to bridge this gap by investigating the influence of select oxyanions—specifically sulfate, phosphate, and nitrate—on the structure of alkali-activated blast furnace slag (AABFS). Advanced characterization techniques such as X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), Raman spectroscopy, and X-ray photoelectron spectroscopy (XPS) were utilized to analyze the structural modifications induced by these anions.
2. Materials and Methods
2.1. Specimen Preparation
The granulated blast furnace slag (BFS) was subjected to alkali activation. Its chemical composition was as follows: 32.26% SiO2, 0.55% TiO2, 5.86% Al2O3, 1.25% Fe2O3, 54.77% CaO, 2.46% MgO, 0.33% MnO, 0.45% K2O, and 1.80% SO3 (measured by X-ray fluorescence (XRF) spectrometer). Four series of samples were produced, and their detailed composition is given in Table 1. The first was a reference series, obtained by activating the BFS with an aqueous solution of sodium hydroxide (8 M solution) and sodium silicate (water glass with silicate modulus 2.5). A constant solution/solid ratio of 0.5 was maintained. The next three series differed in the addition of inorganic salts: NaNO3, Na2SO4, and Na3PO4 (Chempur, Piekary Śląskie, Poland). The amounts of salts were 0.25%, 0.50%, 0.75%, 1.00%, and 1.50% by weight relative to the BFS. Individual series of samples were named N, S, and P, for NaNO3, Na2SO4, and Na3PO4 respectively, and numbered from 1 to 5 according to the increasing salt addition. The pH of the binders prepared in this way was in the range of 11–12. After mechanical mixing, the slurries were poured into cubic silicon molds measuring 15 × 15 × 15 mm while vibrating to remove air bubbles. Since the polycondensation process does not occur spontaneously but requires energy [41], the samples were covered with plastic film and placed in a laboratory dryer at 80 °C for 24 h for activation. Previous work has shown that this is the optimal temperature from the point of view of the alkaline activation process [41]. Subsequently, the solidified samples were removed from the molds, placed in plastic bags, and stored at room temperature. Compressive strength tests and structural analyses were conducted on the samples after this 28-day hardening period.

Table 1.
Composition of the AABFS samples.
2.2. Instrumentation
The chemical composition of BFS was confirmed by X-ray fluorescence spectrometry (XRF). For this purpose, a wavelength dispersive X-ray fluorescence (WD-XRF) Axios Max spectrometer with a Rh 4 kW PANalytical lamp was used (PANalytical, Malvern, UK).
Phase analysis of AABFS was conducted employing the X-ray powder diffraction technique (XRD), utilizing an X-ray X’Pert system (PANalytical, Malvern, UK) equipped with CuKα radiation. Measurements were executed within the 2θ angle range of 5–90° over 2 h, with a step size of 0.007. The X’Pert HighScore Plus (PANalytical, Malvern, UK) application based on the International Centre for Diffraction Data facilitated the identification of phases.
The absorption spectra in the mid-infrared range (4000–400 cm−1) were acquired using a Bruker VERTEX 70v vacuum Fourier-transform infrared (FT-IR) spectrometer (Bruker, Billerica, MA, USA), employing the standard KBr pallet technique. Spectra were captured in absorbance mode, using 128 scans and a resolution of 4 cm−1.
Raman measurements were carried out on the LabRAM HR apparatus (Horiba, Kioto, Japan). The 1800 gr/mm grating, 532 nm (green) laser, and Olympus BX-41 50× long working objective were used. The result shows the average of three measurements with an integration time per pixel of 2 × 120 s.
The X-ray photoelectron spectroscopy (XPS) measurements were conducted on a PHI VersaProbeII Scanning XPS system (ULVAC-PHI, Hagisono, Japan). This system utilized monochromatic Al Kα X-rays with an energy of 1486.6 eV, which were focused into a 100 µm spot and scanned across a 400 × 400 µm area. The photoelectron take-off angle was set at 45°, and the analyzer pass energy was configured to 117.50 eV for broad survey scans and 46.95 eV to achieve high energy resolution. To stabilize the sample’s surface potential irrespective of its conductivity, a dual beam charge compensation was employed using 7 eV Ar+ ions and 1 eV electrons. For all XPS spectra, charge referencing was aligned to the unfunctionalized, saturated carbon (C–C) C 1s peak at 285.0 eV. The analysis chamber was maintained at a pressure below 2 × 10−9 mbar during operation. The spectra were deconvoluted using PHI MultiPak software (version 9.9.3), and the background was subtracted using the Shirley method.
The pore structure of all specimens was characterized by a mercury intrusion porosimetry (MIP) method on the apparatus Poremaster 33 Quantachrome Instruments (Boynton Beach, FL, USA) within the pressure range 0.1–200 MPa.
Scanning electron microscopic (SEM) observations were performed using a PhenomXL tabletop scanning electron microscope (ThermoFisher Scientific, Waltham, MA, USA). The accelerating voltage was 10 eV. The samples were sputtered with a layer of gold.
3. Results and Discussion
Figure 1 shows a cumulative plot illustrating how chemical composition influences the compressive strength of the samples. In the case of a series of samples containing nitrate(V) anions, it can be seen that the general trend is for strength to rise with an increase in the content of these anions. The highest strength of 57 MPa was obtained for the sample containing 1% by weight of nitrate(V), which represents a 46% increase compared to the reference sample. In the case of sulfate ions, it can be concluded that small amounts (up to 0.5%) have no significant effect on the mechanical parameters of the samples. However, a subsequent increase in the content of sulfate(VI) anions led to an increase in the strength of the samples. For samples containing above 0.75% by weight of sulfate(VI), the strength is around 50 MPa and remains relatively constant. This phenomenon may be attributed to the sealing effect of sulfate on the mineral matrix, a concept well-known in cement-based materials technology [42,43]. The first conclusions drawn from these results indicate the positive effect of a small amount of oxyanions on the compressive strength of the produced geopolymers. The analysis of the available literature shows that the behavior of these anions can vary based on the type of alkali activator used. For instance, sulfate ions tend to increase strength in sodium-based geopolymers but decrease it in potassium-based ones, while nitrate ions reduce strength in sodium-based systems and have mixed effects in potassium-based systems, with long-term strengths similar to reference materials after 28 days [35]. On the other hand, the studies on geopolymers based on precursors with low CaO content showed that even low quantities of NO3− and SO42− anions generally inhibit gel hardening and the development of sufficient compressive strength [36]. These observations indicate the possibility of different interactions of anions in the presence of different cations and suggest a need for further analysis of the synergetic effect of anions and cations on the physical properties of geopolymer to understand these effects. Analyzed anions might stabilize the gel phases differently in sodium-activated systems, potentially leading to a more compact and less porous structure, which can increase compressive strength.
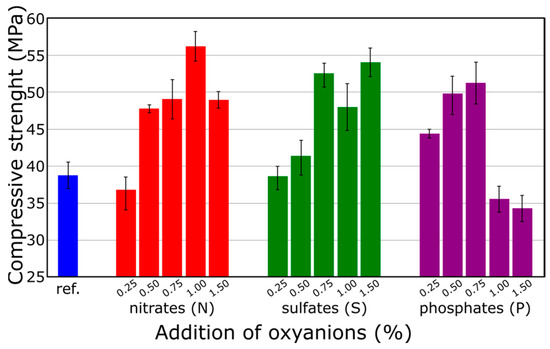
Figure 1.
Compressive strength of alkali-activated BSF depending on chemical composition.
In the case of the third series of samples containing phosphate ions, it is observed that up to an anion content of 0.75% by weight, the strength steadily increases, reaching its maximum at 52 MPa. Similarly, the bulk density exhibits a similar trend (results not presented here), with its maximum value at 0.5% by weight of phosphate(V), measuring 2015 kg/m3. However, at 1% and 1.5% by weight of sodium phosphate, both density and compressive strength significantly decrease compared to those of the reference sample. Literature analysis suggests that the addition of phosphate(V) influences the strength of cement-based materials due to the reaction between phosphate(V) anions and calcium cations, resulting in the precipitation of scarcely soluble calcium phosphate on the surface of cement grains, thereby impeding hydration [44]. Cement with a high concentration of soluble phosphorus compounds typically exhibits a slower setting time and lower compressive strength after two days of hardening. It can be hypothesized that alkali-activated binders containing phosphate(V) may also experience the precipitation of calcium phosphate, a notion that will be further investigated in this paper.
In summary, the highest strength of 57 MPa was achieved by the sample containing 1 wt. % nitrate(V), while the lowest strength of 34 MPa was achieved by the sample containing 1.5 wt. % phosphate(V). The results obtained cannot be compared with literature data, since there is a lack of research on the effect of inorganic anions on the strength properties of alkali-activated binders. Considering the strength of the samples, it can be concluded that the geopolymer matrix is suitable for the immobilization of wastes containing nitrate(V) and sulfate(VI) anions, since the content of these ions can improves the mechanical properties of the binder. Caution should be exercised in the case of the high content of phosphate ions in waste.
Mercury porosimetry (MIP) was conducted to determine the porosity of the samples, and the results are presented in Figure 2. Figure 2a presents the cumulative pore volume as a function of the pore dimensions of the exemplary specimens. The presented curves lack a sigmoidal shape but instead exhibit distinct steps. This characteristic suggests the presence of various pore types, classified as micropores (<0.1 μm), mesopores (0.1–1 μm), and macropores (>1 μm) [45]. Upon comparison of the curves, despite their similar shapes indicating the presence of similar pore size classes, it is evident that the presence of oxyanions significantly increases the overall porosity. Specifically, the P5 specimen displays a total porosity approximately 70% higher than the reference sample.
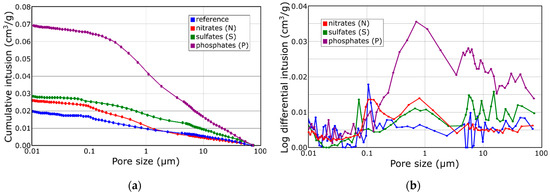
Figure 2.
Cumulative (a) and differential (b) pore size distributions obtained for BFS-based composites.
Figure 2b shows the pore size distribution curves for the reference specimen and the samples with the highest analyzed oxyanion additions. It can be observed that the porosity of the sample containing nitrate(V) is comparable to the reference sample porosity regardless of concentration. The sample containing the highest concentration of sulfates(V) had the highest number of the smallest pores with a diameter of about 0.01 µm, which may confirm the sealing effect of these ions. The samples with phosphates(V) have significantly higher mesoporosity compared to the other samples. The pore volume increases significantly in the range of 0.1–10 µm with the increase in the amount of phosphate salt added at the sample preparation stage. The results of mercury porosimetry indicate that there is a strong influence of sample porosity on the compressive strength results obtained.
After strength tests, the samples were also subjected to microscopic observations. Examples of SEM images of samples with the highest share of oxyanions are presented in Figure 3. The microstructure of the reference sample (Figure 3a) shows a very uniform and compact structure with small pores, numerous cracks, and some unreacted particles. This suggests that significant amounts of C–(A)–S–H and N–A–S–H gels were produced. The microstructures of the samples with nitrate (Figure 3b) and sulfate (Figure 3c) appear to be similar to the reference sample, while the microstructure of samples with phosphorus shows a significant number of particles with different morphology (Figure 3d). Lower magnifications also show the presence of macropores, which was consistent with the MIP analysis (Figure 2). By comparing the compressive strength and microstructure of the AABFS, a more homogeneous structure and superior mechanical strength can be observed.

Figure 3.
SEM images of BFS-based composites: reference sample (a), and the samples with nitrate (b), sulfate (c), and phosphate ions (d).
The X-ray diffraction (XRD) patterns of the initial BFS source and its resultant products are depicted in Figure 4. Analysis reveals that the principal crystalline constituents present in the BFS are merwinite (Ca3Mg[SiO4]2; ICDD: 00-025-0161), akermanite (Ca2Mg[Si2O7]; ICDD: 00-010-0391), gehlenite (Ca2Al[AlSiO7]; ICDD: 01-079-1726), larnite (Ca2[SiO4]; ICDD: 04-007-9746), and calcite (CaCO3; ICDD: 01-086-2339), with a minor presence of quartz (SiO2; ICDD: 01-085-0798). This composition is typical for granulated BFS [46]. In contrast, the XRD patterns of the composites obtained exhibit predominantly weak and broad peaks, indicating the amorphous nature of the structure of alkali-activated composites. Notably, the BFS-derived products exhibit the presence of merwinite, while the peaks attributed to the other phases diminish, suggesting that akermanite and larnite derived from BFS could undergo dissociation in highly alkaline conditions, contributing to the formation of an amorphous structure. A broad peak at about 7° can be attributed to the C-A-S-H phase [47,48]—the main reaction product in AABFS. The XRD patterns did not show the precipitation of any additional crystalline phases—the anions were incorporated into the amorphous aluminosilicate phase.
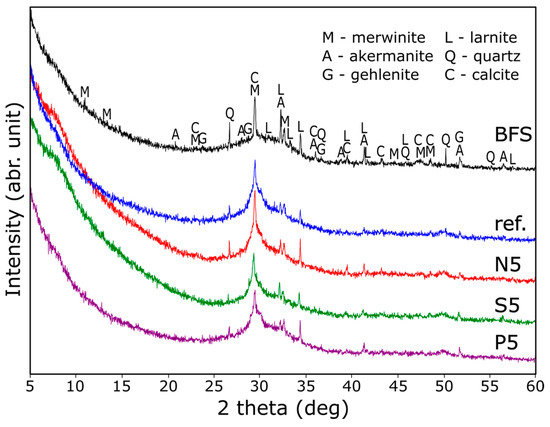
Figure 4.
XRD patterns of initial blast furnace slag (BFS) and BFS-based composites.
To further investigate the structure of the resulting hydration products, Fourier-transform infrared spectroscopy (FT-IR) measurements were carried out, as shown in Figure 5a. For all obtained AABFS composites, strong absorption bands of approximately 980–960 cm−1 are identified as the typical adsorption of Si–O (Q2 units) asymmetric stretching vibrations [49]. The adsorption bands at about 455 cm−1 are likely due to the Si–O–Si deformation vibrations inside [SiO4] tetrahedra. No peaks appear within the range of 1200–1000 cm−1, suggesting the lack of Q3 and Q4 units within the Si–O bands in the resulting structure. This indicates a low degree of polymerization of the structure. Essentially, this implies that the structure of hydrates obtained in the analyzed process consists of short Si–O chains [50]. The bands around 3440 and 1650 cm−1 are assigned to the stretching vibrations generated by the O–H bonds, and bending vibrations of H–O–H, which may be attributed to the adsorbed water or hydrates [51]. However, no typical bands from hydroxides are observed. Additionally, the bands at 1430–1420 cm−1 that are typical of C–O stretching vibrations might be due to the presence of calcium carbonate due to atmospheric carbonation [52].
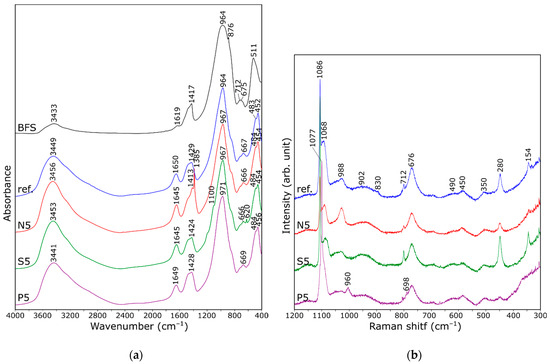
Figure 5.
FT-IR (a) and Raman (b) spectra of BFS-based composites.
The IR spectra also show the presence of bands associated with vibrations occurring within the introduced oxyanions. The bands at 1385 cm−1 are associated with the vibrations of the nitrate groups [53], while the band at 1100 cm−1 is connected to sulfates [49]. Also, the small bands at around 620 cm−1 in the spectra of specimens with sulfur are due to the presence of sulfate ions [54].
Figure 5b shows the Raman spectra of the samples analyzed. According to the relevant literature [55], Raman spectra of C-S-H gels display bands corresponding to Si–O symmetric stretching vibrations. The most intense band, located around 676 cm−1, is linked to Si–O–Si symmetric bending vibrations in linear structures (Q2 units). The band at 988 cm−1 can be attributed to the Al–OH bending vibration, Si–OH in-plane deformation, and asymmetric stretching of Si–O(Ca) in Q1 units [56,57]. This wavenumber range is also characteristic of sulfates [58]. The band at 1068 cm−1 is associated with T–O stretching in Q3 units, where T represents Si, Al, and also P [56,57]. Bands at lower wavenumbers correspond to Si–O–Si bridge vibrations, with the band near 490 and 450 cm−1 attributed to highly polymerized Q4 and Q2 units, respectively [59]. The band at 350 cm−1 is related to Ca–O lattice vibrations specific to the C-S-H structure. Bands around 1086, 712, and 280 cm−1 are linked to vibrations of C–O and Ca–O bonds in carbonate phases [60].
Comparing the spectra, an increase in the intensity of the band at 988 cm−1 is observed in the sample containing nitrate ions, compared to the reference sample. At the same time, the intensity of the 1068 cm−1 band decreases (both in N5 and S5 spectra). This suggests an increased presence of units with a lower degree of polymerization, which may explain the inhibitory effect of nitrate ions on the tetrahedral condensation process, as reported in the literature. For samples with sulfur, the spectral differences are less pronounced, except for the increased intensity of the band at 280 cm−1, which could be due to calcium binding by sulfate ions. In samples with phosphate ions, bands associated with isolated phosphate tetrahedra become prominent, particularly the band at 960 cm−1, which may indicate the phosphate formation as a separate phase. There is also a shift of the band at 1068 cm−1 towards higher wavenumbers, which can be related to an increase in the polymerization degree of the aluminosilicate structure.
From the XPS survey spectra (Figure 6) elements of O, C, Si, Na, Ca, and Mg were observed in all samples. For the modified composites bearing additional ions, peaks assigned to corresponding elements were also observed. To examine the binding states of surface heavy metal elements, a high-resolution XPS scan analysis on the samples was conducted (Figure 7). Table 2 details the surface concentrations of the chemical bonds, as determined by fitting the XPS data for each sample.
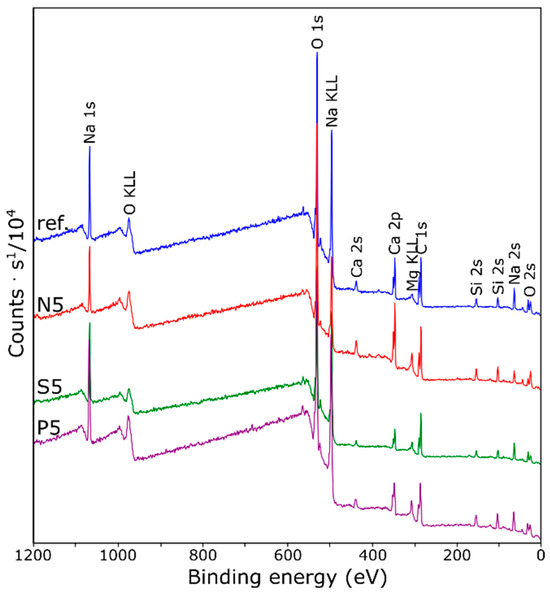
Figure 6.
XPS survey scans of BFS-based composites.
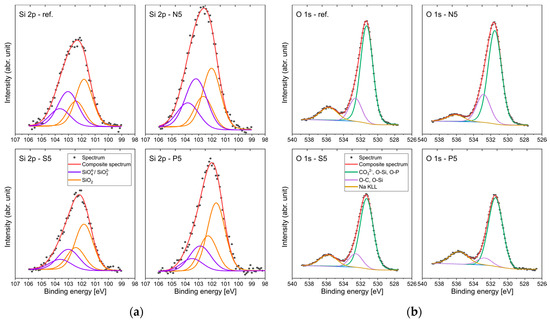
Figure 7.
High-resolution XPS Si 2p (a) and XPS O 1s spectra (b).

Table 2.
Surface composition (atomic %) determined by fitting XPS spectra for analyzed samples.
The Si 2p binding energies in all samples are similar (Figure 7a), which indicates that the silicon-containing phases in these samples are comparable. All observed spectra displayed two prominent peaks: one at 103.1 eV and the other at 101.9 eV. These binding energies are consistent with those reported for materials based on cement [61]. Typically, a higher degree of polymerization of silicate anions correlates with an increase in Si 2p binding energy [62]. The binding energy of 103.1 eV is indicative of a highly polymerized silicate structure, such as silica, whereas the peak at 101.9 eV suggests the presence of silicate compounds with a lower polymerization degree [63]. While in the case of sulfate ions, the intensity of the band at 103.1 decreases, in the case of phosphate ions, the share of the band associated with higher polymerized units increases compared to the reference sample, a conclusion also supported by Raman spectroscopy. A reason for this state of affairs may be the binding of calcium ions into phases co-occurring with the C-S-H gel. Interestingly, the influence of nitrate ions on the composite structure is not significant, which is confirmed by the strength results.
This variation in silicate polymerization can also be seen when examining the O 1s spectra [64] (Figure 7b). Two components were identified, the peak associated with siloxane (Si–O–Si) bonds (so-called bridging oxygen) at 532.7 eV and the peak attributed to non-bridging oxygen signals situated at 531.4 eV. The course of the spectra in this range is not clear. This phenomenon can be explained by the diversity in binding energy among Si–O−, S–O−, and P–O− due to the different electronegativity of the element bound to oxygen. The more the electron–electron repulsion at the oxygen atom decreases, the more the binding energy of the electrons increases [65]. Therefore, for our results, the analysis of XPS spectra in this range will not provide information about the degree of polymerization of the silicate structure.
Studies using XRD, FTIR, Raman, and XPS techniques have provided some insights into the structural modifications of AABSF composites caused by the addition of oxyanions. However, each of these methods has certain limitations, necessitating further research to fully elucidate the impact of anions on the structure of geopolymers. Future research should focus on the detailed investigation of geopolymerization reactions in the presence of oxyanions. To address the encapsulation of hazardous waste within the geopolymer structure, comprehensive leaching tests to evaluate the immobilization efficiency of various contaminants were planned to be conducted. Future research will also include modeling studies to analyze the synergistic effects of cations and anions on the structure of alkaline-activated binders. Additionally, the impact of incorporating actual waste on the geopolymer’s structure and stability will be assessed. Future research will also involve long-term stability assessments under different environmental conditions to simulate real-world scenarios.
4. Conclusions
To sum up, based on the presented research results, the following conclusions can be drawn:
- Anions commonly found in waste, such as nitrates, sulfates, and phosphates, modify the structure and properties of geopolymers.
- The compressive strength of geopolymers is positively impacted by the presence of nitrates or sulfates in the starting mixture but negatively affected by the presence of phosphates.
- Oxyanions likely consume the available alkali activator, thereby hindering the geopolymerization reactions and limiting the quantity of gel produced. Specifically, sulfate ions lead to the precipitation of ettringite, while phosphate ions bind calcium into a separate phase.
- In the case of phosphate ions, a significant decrease in strength may result from microstructural changes and high porosity.
- The effect of nitrates on the structure of AABSF composites is rather neutral.
Author Contributions
Conceptualization, M.K.; methodology, M.K.; software, M.K.; validation, M.K.; formal analysis, D.Ś. and M.K.; investigation, D.Ś., M.M. and M.K.; data curation, M.M. and M.K.; writing—original draft preparation, D.Ś. and M.K.; writing—review and editing, W.M. and M.K.; visualization, M.K.; supervision, W.M. and M.K.; project administration, W.M. and M.K.; funding acquisition, W.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by The National Science Centre Poland under grant no. 2018/31/B/ST8/03109.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting this study are openly available from RODBUK Cracow Open Research Data Repository at https://doi.org/10.58032/AGH/A0OORS.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kozhukhova, N.; Kozhukhova, M.; Zhernovskaya, I.; Promakhov, V. The correlation of temperature-mineral phase transformation as a controlling factor of thermal and mechanical performance of fly ash-based alkali-activated binders. Materials 2020, 13, 5181. [Google Scholar] [CrossRef] [PubMed]
- Tarique, O.; Kovtun, M. Novel one-part fly ash alkali-activated cements for ambient applications. Adv. Cem. Res. 2022, 34, 458–471. [Google Scholar] [CrossRef]
- Pacheco Torgal, F.; Castro Gomes, J.; Jalali, S. Alkali activated binders: A review. Part 1. Historical background, terminology, reaction mechanisms and hydration products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar]
- Ettahiri, Y.; Bouargane, B.; Fritah, K.; Akhsassi, B.; Pérez-Villarejo, L.; Aziz, A.; Bouna, L.; Benlhachemi, A.; Novais, R.M. A state-of-the-art review of recent advances in porous geopolymer: Applications in adsorption of inorganic and organic contaminants in water. Constr. Build. Mater. 2023, 395, 132269. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Ding, B.; Gu, J.; Ukrainczyk, N.; Cai, J. Development of geopolymer-based composites for geothermal energy applications. J. Clean. Prod. 2023, 419, 138202. [Google Scholar] [CrossRef]
- Tang, J.; Liu, P.; Shang, J.; Fei, Y. Application of CO2-loaded geopolymer in Zn removal from water: A multi-win strategy for coal fly ash disposal, CO2 emission reduction, and heavy metal-contaminated water treatment. Environ. Res. 2023, 237, 117012. [Google Scholar] [CrossRef] [PubMed]
- Paiva, H.; Yliniemi, J.; Illikainen, M.; Rocha, F.; Ferreira, V.M. Mine tailings geopolymers as a waste management solution for a more sustainable habitat. Sustainability 2019, 11, 995. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Knapik, A.; Mozgawa, W. Disposal of bottom ash from the incineration of hazardous waste in two different mineral matrixes. Environ. Prog. Sustain. Energy. 2017, 36, 1074–1082. [Google Scholar] [CrossRef]
- Komljenović, M.; Tanasijević, G.; Džunuzović, N.; Provis, J.L. Immobilization of cesium with alkali-activated blast furnace slag. J. Hazard. Mater. 2020, 388, 121765. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology. Experientia Supplementum; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 101. [Google Scholar]
- Ramadan, M.; Habib, A.O.; Hazem, M.M.; Amin, M.S.; Mohsen, A. Synergetic effects of hydrothermal treatment on the behavior of toxic sludge-modified geopolymer: Immobilization of cerium and lead, textural characteristics, and mechanical efficiency. Constr. Build. Mater. 2023, 367, 130249. [Google Scholar] [CrossRef]
- Rasaki, S.A.; Bingxue, Z.; Guarecuco, R.; Thomas, T.; Minghui, Y. Geopolymer for use in heavy metals adsorption, and advanced oxidative processes: A critical review. J. Clean. Prod. 2019, 213, 42–58. [Google Scholar] [CrossRef]
- Arokiasamy, P.; Al Bakri Abdullah, M.M.; Abd Rahim, S.Z.; Sadique, M.; Ming, L.Y.; Salleh, M.A.A.M.; Zainol, M.R.R.M.A.; Ghazali, C.M.R. Diverse material based geopolymer towards heavy metals removal: A review. J. Mater. Res. Technol. 2023, 22, 126–156. [Google Scholar] [CrossRef]
- Bouzar, B.; Mamindy-Pajany, Y. Immobilization study of As, Cr, Mo, Pb, Sb, Se and Zn in geopolymer matrix: Application to shooting range soil and biomass fly ash. Int. J. Environ. Sci. Technol. 2023, 20, 11891–11912. [Google Scholar] [CrossRef]
- Qing-Guo Dong, Q.G.; Li, J.; Kang, Z.Q.; Anwar, M.I.; Asad, M.; Miao, B.; Wang, S.; Younas, A. Unlocking the potential: A comprehensive review on blast furnace slag and silica analog adsorbents for sustainable industrial and pharmaceutical pollution control and resource utilization. Emerg. Contam. 2024, 10, 100387. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, Z.; Chen, W.; Pei, Y. Codisposal of landfill leachate concentrate and antimony mine soils using a one-part geopolymer system for cationic and anionic heavy metals immobilization. J. Hazard. Mater. 2024, 464, 132909. [Google Scholar] [CrossRef] [PubMed]
- Fermo, P.; Cariati, F.; Pozzi, A.; Demartin, F.; Tettamanti, M.; Collina, E.; Lasagni, M.; Pitea, D.; Puglisi, O.; Russo, U. The analytical characterization of municipal solid waste incinerator fly ash: Methods and preliminary results. Fresenius J. Anal. Chem. 1999, 365, 666–673. [Google Scholar] [CrossRef]
- Kalmykova, Y.; Fedje, K.K. Phosphorus recovery from municipal solid waste incineration fly ash. Waste Manag. 2013, 33, 1403–1410. [Google Scholar] [CrossRef]
- Chen, F.; Wang, K.; Shao, L.; Muhammad, Y.; Wei, Y.; Gao, F.; Wang, X.; Cui, X. Synthesis of Fe2O3-modified porous geopolymer microspheres for highly selective adsorption and solidification of F– from waste-water. Compos. Part B Eng. 2019, 178, 107497. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. The effects of inorganic salt contamination on the strength and durability of geopolymers. Colloids Surf. A 2002, 211, 115–126. [Google Scholar] [CrossRef]
- Chen, S.; Qi, Y.; Cossa, J.J.; Deocleciano Salomao Dos, S.I. Efficient removal of radioactive iodide anions from simulated wastewater by HDTMA-geopolymer. Prog. Nucl. Energy 2019, 117, 103112. [Google Scholar] [CrossRef]
- Niu, X.; Elakneswaran, Y.; Islam, C.R.; Provis, J.L.; Sato, T. Adsorption behaviour of simulant radionuclide cations and anions in metakaolin-based geopolymer. J. Hazard. Mater. 2022, 429, 128373. [Google Scholar] [CrossRef]
- Tian, Q.; Sasaki, K. A novel composite of layered double hydroxide/geopolymer for co-immobilization of Cs+ and SeO42– from aqueous solution. Sci. Total Environ. 2019, 695, 133799. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Elakneswaran, Y.; Chaerun, R.I.; Fang, C.; Hiroyoshi, N.; Provis, J.L.; Sato, T. Development of metakaolin-based geopolymer for selenium oxyanions uptake through in-situ ettringite formation. Sep. Purif. Technol. 2023, 324, 124530. [Google Scholar] [CrossRef]
- Tian, Q.; Chen, C.; Wang, M.; Guo, B.; Zhang, H.; Sasaki, K. Effect of Si/Al molar ratio on the immobilization of selenium and arsenic oxyanions in geopolymer. Environ. Pollut. 2021, 274, 116509. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jiménez, A.; Palomo, A. Fixing arsenic in alkali-activated cementitious matrices. J. Am. Ceram. Soc. 2005, 88, 1122–1126. [Google Scholar] [CrossRef]
- Tian, Q.; Sasaki, K. Application of fly-ash-based geopolymer for removal of cesium, strontium and arsenate from aqueous solutions: Kinetic, equilibrium and mechanism analysis. Water Sci. Technol. 2019, 79, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Al-Mashqbeh, A.; Abuali, S.; El-Eswed, B.; Khalili, F.I. Immobilization of toxic inorganic anions (Cr2O72−, MnO4− and Fe(CN)63−) in metakaolin based geopolymers: A preliminary study. Ceram. Int. 2018, 44, 5613–5620. [Google Scholar] [CrossRef]
- Luukkonen, T.; Runtti, H.; Niskanen, M.; Tolonen, E.T.; Sarkkinen, M.; Kemppainen, K.; Rämö, J.; Lassi, U. Simultaneous removal of Ni(II), As(III), and Sb(III) from spiked mine effluent with metakaolin and blast-furnace-slag geopolymers. J. Environ. Manag. 2016, 166, 579–588. [Google Scholar] [CrossRef]
- Arif, M.A.; Abdel-Gawwad, H.A.; Elshimy, A.S.; Seliem, M.K.; Ali, M.A.; Maodaa, S.N.; Federowicz, K.; Mobarak, M.; Bendary, H.I.; Salama, Y.F.; et al. Facile synthesis and characterization of metakaolin/carbonate waste-based geopolymer for Cr(VI) remediation: Experimental and theoretical studies. Inorg. Chim. Acta 2024, 564, 121939. [Google Scholar] [CrossRef]
- Salam, M.A.; Mokhtar, M.; Albukhari, S.M.; Baamer, D.F.; Palmisano, L.; AlHammadi, A.A.; Abukhadra, M.R. Synthesis of zeolite/geopolymer composite for enhanced sequestration of phosphate (PO43−) and ammonium (NH4+) ions; equilibrium properties and realistic study. J. Environ. Manag. 2021, 300, 113723. [Google Scholar] [CrossRef]
- Runtti, H.; Luukkonen, T.; Niskanen, M.; Tuomikoski, S.; Kangas, T.; Tynjälä, P.; Tolonen, E.T.; Sarkkinen, M.; Kemppainen, K.; Rämö, J. Sulphate removal over barium-modified blast-furnace-slag geopolymer. J. Hazard. Mater. 2016, 317, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.W.; van Deventer, J.S.J. The effect of ionic contaminants on the early-age properties of alkali-activated fly ash-based cements. Cem. Concr. Res. 2002, 32, 577–584. [Google Scholar] [CrossRef]
- Ofer-Rozovsky, E.; Arbel Haddad, M.; Bar-Nes, G.; Katz, A. The formation of crystalline phases in metakaolin-based geopolymers in the presence of sodium nitrate. J. Mater. Sci. 2016, 51, 4795–4814. [Google Scholar] [CrossRef]
- Desbats-Le Chequer, C.; Frizon, F. Impact of sulfate and nitrate incorporation on potassium- and sodium-based geopolymers: Geopolymerization and materials properties. J. Mater. Sci. 2011, 46, 5657–5664. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G. Effect of sulphate and nitrate anions on heavy metal immobilisation in ferronickel slag geopolymers. Appl. Clay Sci. 2013, 73, 103–109. [Google Scholar] [CrossRef]
- Criado, M.; Fernandez, J.A.; Palomo, A. Effect of sodium sulfate on the alkali activation of fly ash. Cem. Concr. Compos. 2010, 32, 589–594. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Hamdan, S.; van Deventer, J.S.J. Microstructural changes in alkali activated fly ash/slag geopolymers with sulfate exposure. Mater. Struct. 2013, 46, 361–373. [Google Scholar] [CrossRef]
- Lee, W.; van Deventer, J.J. Effects of anions on the formation of aluminosilicate gel in geopolymers. Ind. Eng. Chem. Res. 2002, 41, 4550–4558. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. A calorimetric study of early hydration of alkali-slag cements. Cem. Concr. Res. 1995, 25, 1333–1346. [Google Scholar] [CrossRef]
- Xu, H.; Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Liu, P.; Chen, Y.; Yu, Z.; Lu, Z. Effect of sulfate solution concentration on the deterioration mechanism and physical properties of concrete. Constr. Build. Mater. 2019, 227, 116641. [Google Scholar] [CrossRef]
- Neto, J.A.; De la Torre, A.G.; Kirchheim, A.P. Effects of sulfates on the hydration of Portland cement—A review. Constr. Build. Mater. 2021, 279, 122428. [Google Scholar] [CrossRef]
- Tkaczewska, E.; Kłosek-Wawrzyn, E. Effect of phosphate PO43- ions on cement hydration. CWB 2012, 6, 401–408. [Google Scholar]
- Corinaldesi, V. Environmentally-friendly bedding mortars for repair of historical buildings. Constr. Build. Mater. 2012, 35, 778–784. [Google Scholar] [CrossRef]
- Fredericci, C.; Zanotto, E.D.; Ziemath, E.C. Crystallization mechanism and properties of a blast furnace slag glass. J. Non-Cryst. Solids 2000, 273, 64–75. [Google Scholar] [CrossRef]
- Taylor, H.F. Cement Chemistry; Thomas Telford: London, UK, 1997. [Google Scholar]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodríguez, J. A model for the C-A-S-H gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Blanco, M.T.; Palomo, A. FTIR study of the sol–gel synthesis of cementitious gels: C–S–H and N–A–S–H. J. Sol-Gel Sci. Technol. 2008, 45, 63–72. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of calcium silicate hydrate (C-S-H): Near-, mid-, and far-infrared spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Libnau, F.O.; Kvalheim, O.M.; Christy, A.A.; Toft, J. Spectra of water in the near- and mid-infrared region. Vib. Spectrosc. 1994, 7, 243–254. [Google Scholar] [CrossRef]
- Huang, C.K.; Kerr, P.F. Infrared study of the carbonate minerals. Am. Miner. 1960, 45, 311–324. [Google Scholar]
- Mihaylov, M.Y.; Zdravkova, V.R.; Ivanova, E.Z.; Aleksandrov, H.A.; Petkov, P.S.; Vayssilov, G.N.; Hadjiivanov, K.I. Infrared spectra of surface nitrates: Revision of the current opinions based on the case study of ceria. J. Catal. 2021, 394, 245–258. [Google Scholar] [CrossRef]
- Tai, H.; Underwood, A.L. Infrared spectrophotometry of sulfate ion. Anal. Chem. 1957, 29, 1430–1433. [Google Scholar] [CrossRef]
- McMillan, P. Structural studies of silicate glasses and melts—Applications and limitations of Raman spectroscopy. Am. Mineral. 1984, 69, 622–644. [Google Scholar]
- Kirkpatrick, R.J.; Yarger, J.L.; McMillan, P.F.; Yu, P.; Cong, X. Raman spectroscopy of C-S-H, tobermorite, and jennite. Adv. Cem. Based Mater. 1997, 5, 93–99. [Google Scholar] [CrossRef]
- Ortaboy, S.; Li, J.; Geng, G.; Myers, R.J.; Monteiro, P.J.M.; Maboudian, R.; Carraro, C. Effects of CO2 and temperature on the structure and chemistry of C–(A–)S–H investigated by Raman spectroscopy. RSC Adv. 2017, 7, 48925–48933. [Google Scholar] [CrossRef]
- Renaudin, G.; Segni, R.; Mentel, D.; Nedelec, J.-M.; Leroux, F.; Taviot-Gueho, C. A Raman study of the sulfated cement hydrates: Ettringite and monosulfoaluminate. J. Adv. Concr. Technol. 2007, 5, 299–312. [Google Scholar] [CrossRef]
- Losq, C.L.; Neuville, D.R.; Chen, W.; Florian, P.; Massiot, D.; Zhou, Z.; Greaves, G.N. Percolation channels: A universalidea to describe the atomic structure and dynamics of glasses and melts. Sci. Rep. 2017, 7, 16490. [Google Scholar] [CrossRef]
- Stoch, P.; Goj, P.; Ciecińska, M.; Stoch, A. Structural features of 19Al2O3-19Fe2O3-62P2O5 glass from a theoretical and experimental point of view. J. Non-Cryst. Solids 2019, 521, 119499. [Google Scholar] [CrossRef]
- Donnelly, F.C.; Purcell-Milton, F.; Framont, V.; Cleary, O.; Dunne, P.W.; Gun’ko, Y.K. Synthesis of CaCO3 nano- and micro-particles by dry ice carbonation. Chem. Commun. 2017, 53, 6657–6660. [Google Scholar] [CrossRef]
- Okada, K.; Kameshima, Y.; Yasumori, A. Chemical shifts of silicon X-ray photoelectron spectra by polymerization structures of silicates. J. Am. Ceram. Soc. 1998, 81, 1970–1972. [Google Scholar] [CrossRef]
- Wagner, C.D.; Passoja, D.E.; Hillery, H.F.; Kinsky, T.G.; Six, H.A.; Jansen, W.T.; Taylor, J.A. Auger and photoelectron line energy relationships in aluminum–oxygen and silicon–oxygen compounds. J. Vac. Sci. Technol. 1982, 21, 933–944. [Google Scholar] [CrossRef]
- Pintori, G.; Cattaruzza, E. XPS/ESCA on glass surfaces: A useful tool for ancient and modern materials. Opt. Mater. 2022, 13, 100108. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Sønderby, C.; Li, Z.; Søgaard, E.G. XPS and FT-IR investigation of silicate polymers. J. Mater. Sci. 2009, 44, 2079–2088. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).