Study on Microwave Dielectric Materials an Adjustable Temperature Drift Coefficient and a High Dielectric Constant
Abstract
1. Introduction
2. Experimental Procedure
3. Results and Discussion
| Samples | εr | Q × f (GHz) | τf (°C) | Sintering Temperature (°C) | Reference |
|---|---|---|---|---|---|
| Ca0.8Sr0.2TiO3 | 181 | 1000 | +991 | 1350 | [1] |
| Ca0.6La0.2667TiO3 | 117.4 | 13000 | +217 | 1450 | [30] |
| SrTiO3 | 290 | 4200 | +1700 | 1400 | [31] |
| (Ca0.95Sr0.05)(Ti1−xSnx)O3 | 92 | 10,000 | +810 | 1350 | This work |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, S.H.; Chen, Y.B. Structure and characterization of B2O3 modifed yNd(Mg1/2Ti1/2)O3-(1-y)Ca0.8Sr0.2TiO3 ceramics with a near-zero temperature coeffcient at microwave frequency. Ceram. Int. 2017, 43, 2368–2371. [Google Scholar] [CrossRef]
- Zhang, P.; Hao, M.; Xiao, M. Microwave dielectric properties of Li3Mg2NbO6-based ceramics with (MxW1−x)5+(M =Li, Mg, Al, Ti) substitutions at Nb sites. J. Alloys Compd. 2021, 853, 157386. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, H.; Luan, X.; Hu, S.; Zhou, X.; He, S.; Wang, X.; Zhou, S.; Chen, X. NaTaO3 microwave dielectric ceramic a with high relative permittivity and as an excellent compensator for the temperature coefficient of resonant frequency. Ceram. Int. 2021, 47, 121–129. [Google Scholar] [CrossRef]
- Chen, C.; Peng, Z.; Xie, L.; Bi, K.; Fu, X. Microwave dielectric properties of novel (1 − x)MgTiO3−xCa0.5Sr0.5TiO3 ceramics. J. Mater. Sci. Mater. Electron. 2020, 31, 13696–13703. [Google Scholar] [CrossRef]
- Hameed, X.; Liu, Q.; Li, L.; Liu, M.Y.; Chen, X.M. Structure evolution and improved microwave dielectric characteristics in CaTi1−x(Al0.5Nb0.5)xO3 ceramics. J. Alloys Compd. 2020, 845, 155435. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Xiang, M. Synthesis, characterization and dielectric properties of a novel temperature stable (1 − x)CoTiNb2O8−xZnNb2O6 ceramic. J. Adv. Ceram. 2019, 8, 228–237. [Google Scholar] [CrossRef]
- Shi, F.; Fu, G.; Xiao, E.; Li, J. Lattice vibrational characteristics and dielectric properties of pure phase CaTiO3 ceramic. J. Mater. Sci. Mater. Electron. 2020, 31, 18070–18076. [Google Scholar] [CrossRef]
- Yang, S.; Liang, B.; Liu, C.; Liu, J.; Fang, C.; Ai, Y. Microwave sintering and microwave dielectric properties of (1 − x)Ca0.61La0.26TiO3−xNd(Mg0.5Ti0.5)O3 ceramics. Materials 2021, 14, 438. [Google Scholar] [CrossRef]
- Takahashi, H.; Baba, Y.; Ezaki, K.; Okamoto, Y.; Shibaba, K.; Kuroki, K.; Nakano, S. Dielectric characteristics of (A1+ 0.5A3+ 0.5)TiO3 ceramics at microwave frequencies. Jpn. J. Appl. Phys. 1991, 30, 2339–2342. [Google Scholar] [CrossRef]
- Mercurio, J.P.; Manier, M.; Frit, B. Dielectric properties of ceramics within the BaOLn2O3-TiO2 system. Ferroelectrics 1992, 127, 35–40. [Google Scholar] [CrossRef]
- Ezaki, K.; Baba, Y.; Takahashi, H.; Shibata, K.; Nakano, S. Microwave dielectric properties of CaO-Li2O-Ln2O3-TiO2 ceramics. Jpn. J. Appl. Phys. 1993, 1, 4319–4322. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Z.; Zhang, M.; Sun, P.; Xu, Y. Preparation and microwave dielectric properties of Ca0.6La0.8/3(SnxTi1−x)O3 ceramics. Ceram. Int. 2017, 43, 8534–8537. [Google Scholar] [CrossRef]
- Wise, P.L.; Reaney, I.M.; Lee, W.E.; Price, T.J.; Iddles, D.M.; Cannell, D.S. Structure–microwave property relations in (SrxCa(1−x))n+1TinO3n+1. J. Eur. Ceram. Soc. 2001, 21, 1723–1726. [Google Scholar] [CrossRef]
- Jancar, B.; Suvorov, D.; Valant, M.; Drazic, G. Characterization of CaTiO3-NdAlO3 dielectric ceramics. J. Eur. Ceram. Soc. 2003, 23, 1391–1400. [Google Scholar] [CrossRef]
- Moon, J.H.; Jang, H.M.; Park, H.S.; Shin, J.Y.; Kim, H.S. Sintering behavior and microwave dielectric properties of (Ca, La)(Ti, Al)O3 ceramics. Jpn. J. Appl. Phys. 1999, 38, 6821–6826. [Google Scholar] [CrossRef]
- Chen, G.H.; Chen, J.S.; Kang, X.L.; Yang, T.; Luo, Y.; Yuan, C.L.; Zhou, C.R. Structural characteristics and microwave dielectric properties of In3+ and Nb5+ co-doped CaTiO3 ceramics. J. Mater. Sci. Mater. Electron. 2017, 28, 6301–6307. [Google Scholar] [CrossRef]
- Huang, C.-L.; Tsai, J.-T.; Chen, Y.-B. Dielectric properties of (1−y)Ca1−xLa2x/3TiO3-y(Li,Nd)1/2TiO3 ceramic system at microwave frequency. Mater. Res. Bull. 2001, 36, 547–556. [Google Scholar] [CrossRef]
- Mahanty, S.; Roy, S.; Sen, S. Effect of Sn doping on the structural and optical properties of sol–gel TiO2 thin films. J. Cryst. Growth 2004, 261, 77–81. [Google Scholar] [CrossRef][Green Version]
- Hakki, B.W.; Coleman, P.D. A dielectric resonator method of measuring inductive capacities in the millimeter range. IEEE Trans. Microw. Theory Technol. 1960, 8, 402–410. [Google Scholar] [CrossRef]
- Courtney, W.E. Analysis, and evaluation of a method of measuring the complex permittivity and permeability microwave insulators. IEEE Trans. Microw. Theory Technol. 1970, 18, 476–485. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, D.; Liu, Q. Tolerance factor and phase stability of the garnet structure. Acta Cryst. 2019, C75, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, B.Q. Synthesis and optical absorption property of the Zn2TixSn1−xO4 (0 <= x <= 1) solid solutions. Int. J. Quantum Chem. 2004, 177, 3448–3453. [Google Scholar]
- Akbas, M.A.; Davies, P.K. Cation Ordering Transformations in the Ba(Zn1/3Nb2/3)O3–La(Zn2/3Nb1/3)O3 System. J. Am. Ceram. Soc. 1998, 81, 1061–1064. [Google Scholar] [CrossRef]
- Shannon, R.D. Dielectric polarizabilities of ions in oxides and fluorides. J. Appl. Phys. 1993, 73, 348–366. [Google Scholar] [CrossRef]
- Huang, C.-L.; Chen, Y.-B.; Lo, C.W. New Dielectric Material System of La(Mg1/2Ti1/2)O3-CaTiO3 at Microwave Frequencies. Jpn. J. Appl. Phys. 2005, 44, 3147–3150. [Google Scholar] [CrossRef]
- Young-Il, K.; Patrick, M. Woodward, Syntheses and characterizations of complex perovskite oxynitrides LaMg1/3Ta2/3O2N, LaMg1/2Ta1/2O5/2N1/2, and BaSc0.05Ta0.95O2.1N0.9. J. Solid State Chem. 2007, 180, 2798–2807. [Google Scholar] [CrossRef]
- Kreller, C.R.; Valdez, J.A.; Holesinger, T.G.; Mukundan, R.; Brosha, E.L.; Williamson, T.; Wang, Y.Q.; Uberuaga, B.P. Synthesis and characterization of dense Gd2Ti2O7 pyrochlore thin films deposited using RF magnetron sputtering. Solid State Ion. 2018, 341, 36–40. [Google Scholar] [CrossRef]
- Reaney, I.M.; Colla, E.L.; Setter, N. Dielectric and Structural Characteristics of Ba and Sr-Based Complex Perovskites as a Function of Tolerance Factor. Jpn. J. Appl. Phys. 1994, 33 Pt 1, 3984–3990. [Google Scholar] [CrossRef]
- Zhang, S.; Sahin, H.; Torun, E.; Peeters, F.M.; Martien, D.; Dapron, T.; Dilley, N.; NEWMAN, N. Fundamental mechanisms responsible for the temperature coefficient of resonant frequency in microwave dielectric ceramics. J. Am. Ceram. Soc. 2017, 100, 1508–1516. [Google Scholar] [CrossRef]
- Li, J.M.; Qiu, T. Microwave sintering of Ca0.6La0.2667TiO3 microwave dielectric ceramics. Int. J. Miner. Metall. Mater. 2012, 3, 63–69. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Yu, S.; Zheng, H.; Sun, Z.; Luo, W. The effects of inequivalent La3+ introduction on the structure and dielectric properties of SrTiO3 ceramic at microwave range. Mater. Chem. Phys. 2018, 216, 339–344. [Google Scholar] [CrossRef]
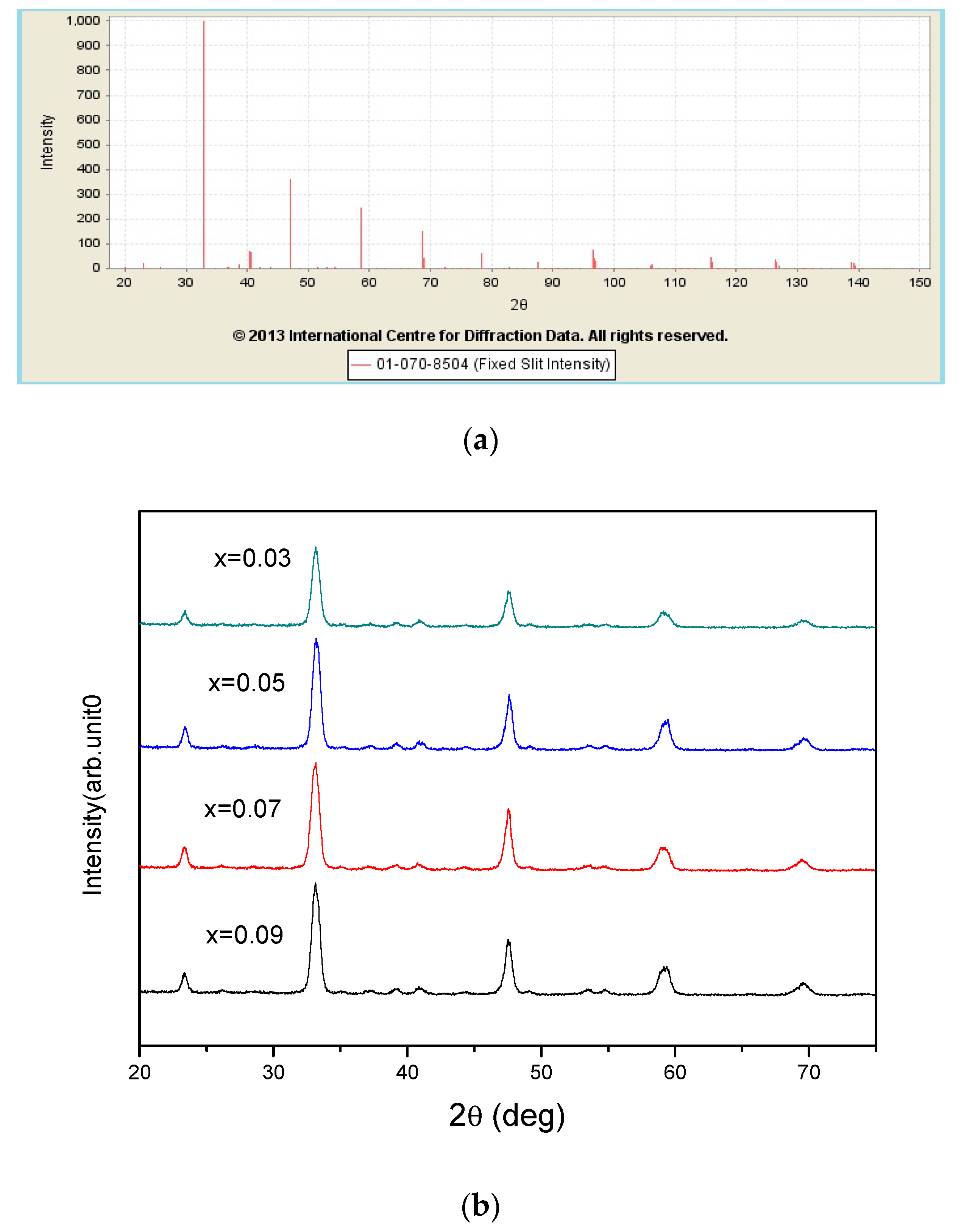
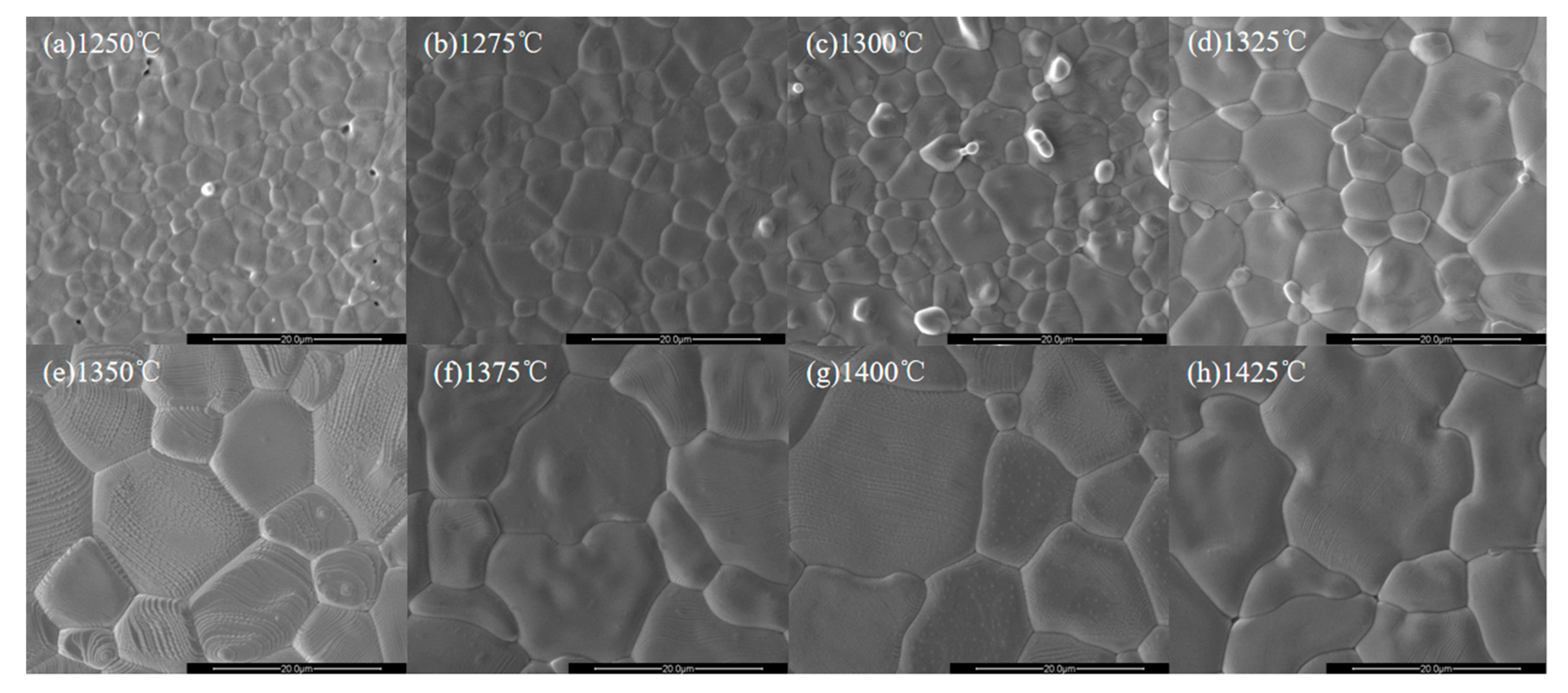
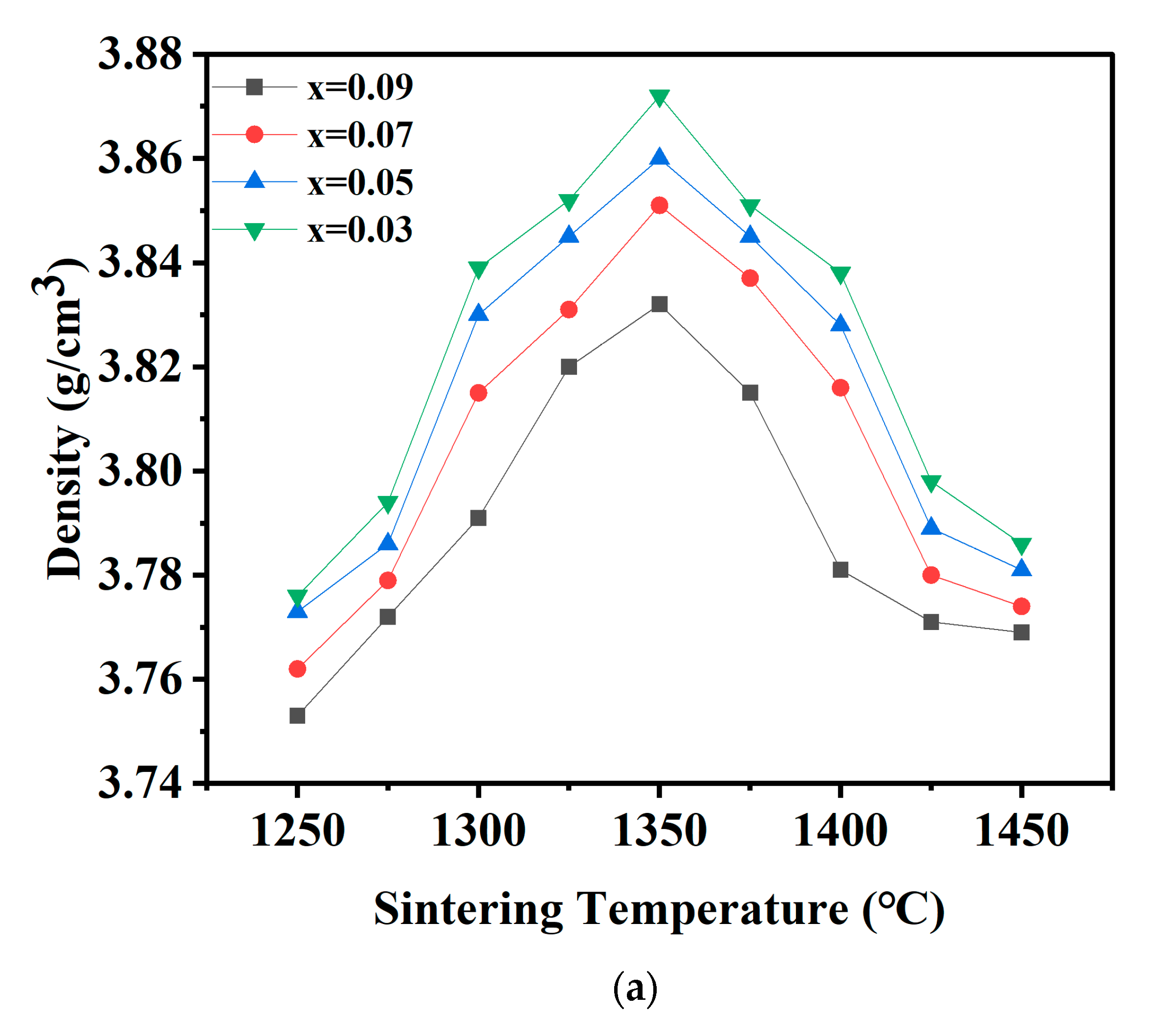
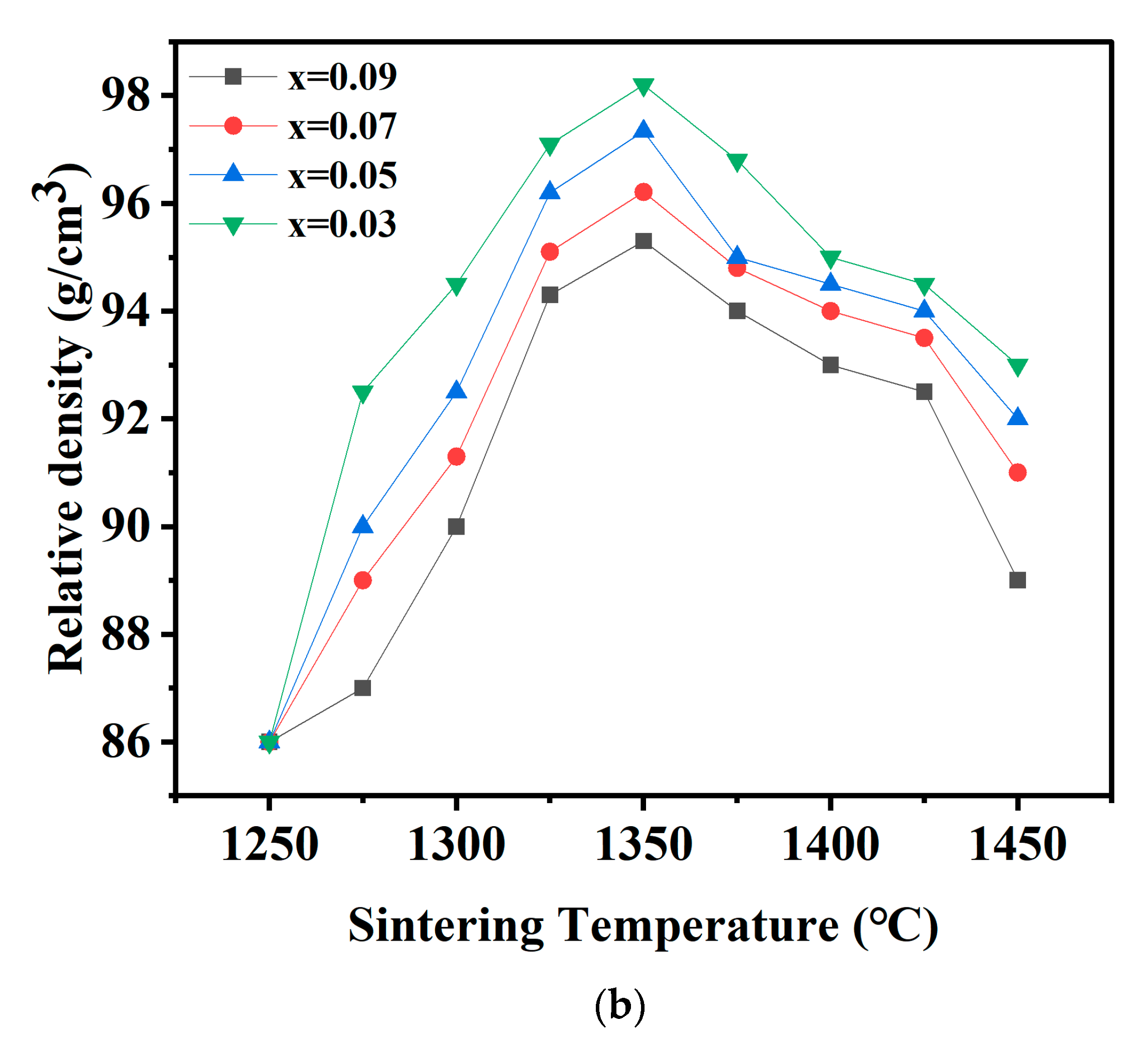
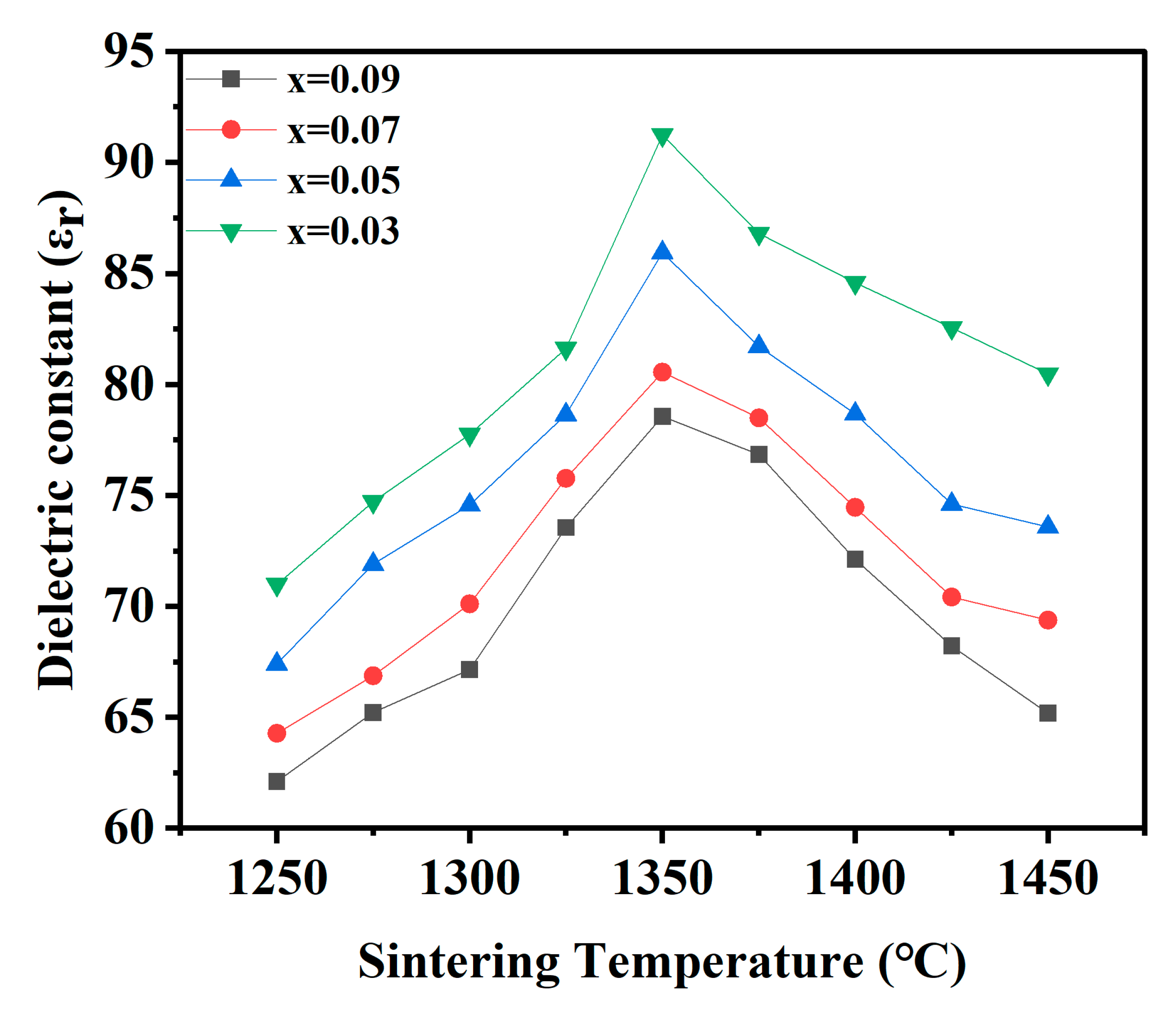
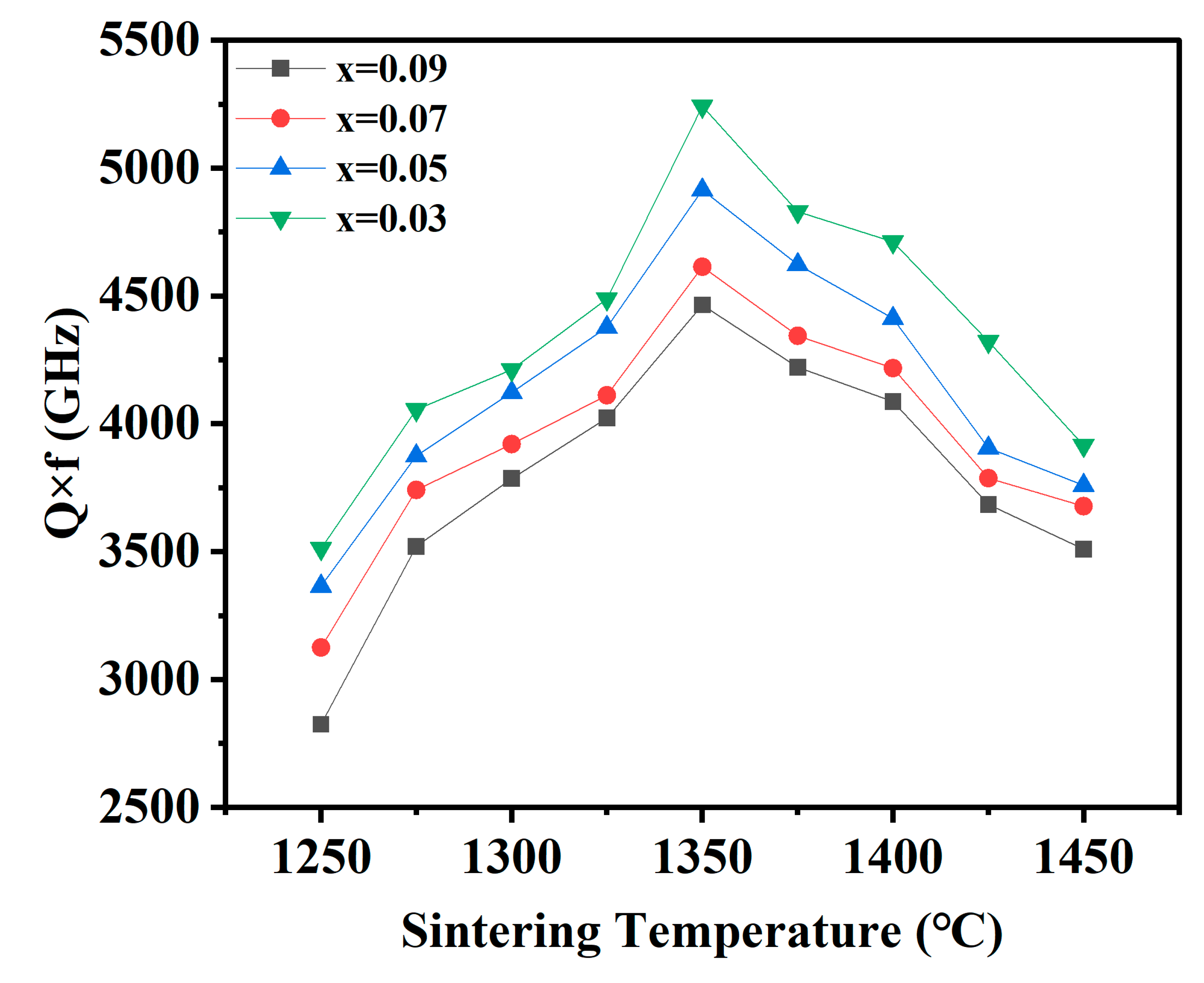
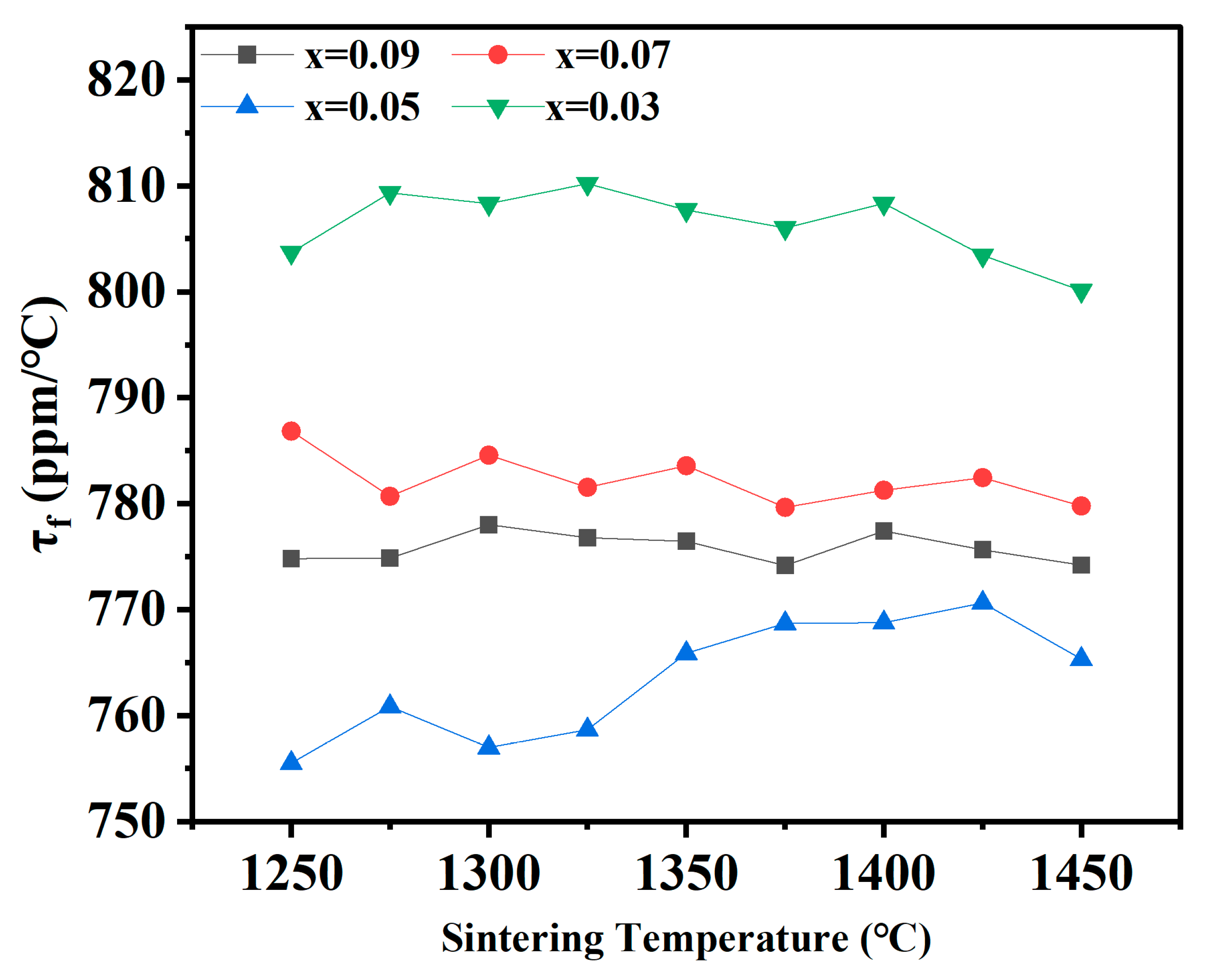
| x = | 0.03 | 0.05 | 0.07 | 0.09 |
| a = b = c (Å) | 6.2046 | 6.2055 | 6.2066 | 6.2081 |
| 2.6224 | 2.6238 | 2.6248 | 2.6265 | |
| Vm (Å3) | 238.8589 | 238.9628 | 239.09 | 239.263 |
| αm(theory) | 12.171 | 12.169 | 12.165 | 12.167 |
| αm(exp) | 11.98 | 11.961 | 11.952 | 11.934 |
| Dielectric (cal.) | 92.35 | 86.43 | 92.12 | 79.89 |
| Dielectric (measured) | 91.24 | 85.94 | 80.55 | 78.56 |
| Tolerance factor | 0.94731 | 0.94621 | 0.94538 | 0.94501 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-B.; Fan, Y.; Chang, S.-H.; Shen, S. Study on Microwave Dielectric Materials an Adjustable Temperature Drift Coefficient and a High Dielectric Constant. Ceramics 2024, 7, 1227-1236. https://doi.org/10.3390/ceramics7030081
Chen Y-B, Fan Y, Chang S-H, Shen S. Study on Microwave Dielectric Materials an Adjustable Temperature Drift Coefficient and a High Dielectric Constant. Ceramics. 2024; 7(3):1227-1236. https://doi.org/10.3390/ceramics7030081
Chicago/Turabian StyleChen, Yuan-Bin, Yu Fan, Shiuan-Ho Chang, and Shaobing Shen. 2024. "Study on Microwave Dielectric Materials an Adjustable Temperature Drift Coefficient and a High Dielectric Constant" Ceramics 7, no. 3: 1227-1236. https://doi.org/10.3390/ceramics7030081
APA StyleChen, Y.-B., Fan, Y., Chang, S.-H., & Shen, S. (2024). Study on Microwave Dielectric Materials an Adjustable Temperature Drift Coefficient and a High Dielectric Constant. Ceramics, 7(3), 1227-1236. https://doi.org/10.3390/ceramics7030081





