Abstract
Tailoring electrical and mechanical properties in the fluorite oxides family is of great interest for technological applications. Other than doping and substitution, entropy-driven stabilization is an emerging technique for new solid solutions formation and enhancing or exploring new functionalities. However, there is a high number of possible combinations for higher-order diagram investigations, and the current state of the art shows limited possibilities in predicting phase formation and related properties. In this paper, we expand the compositional space of fluorite oxides in ZrO2-HfO2-CeO2-Nb2O5-RE2O3 systems. X-ray diffractometry and scanning electron microscopy measurements showed the formation of cubic fluorite-type structures when processing compositions at 1600 °C.
1. Introduction
Fluorites are one of the most important family of oxides with a wide range of prominent members in the field of energy materials. Their chemical formula can be expressed as AO2, where A is a cation in a 4+ oxidation state. The most common representatives are ZrO2 and CeO2 and their solid solutions [1,2]. Compounds structured with fluorite-type cubic symmetry may be tailored to possess a wide range of properties, such as electrical properties, and to withstand high temperatures, high temperature gradients and mechanical stresses, which make them suitable for various technological applications not limited to ion conductors [3,4], thermal barrier coatings [5,6,7], ferroelectrics [8,9] or sensing [10].
Recently, a new strategy to enhance or discover new properties was reported and consisted of entropy-driven stabilization of fluorite-type structures [11,12,13,14,15]. To achieve this, a complex multi-component solid solution is designed by mixing at least five precursor oxides in an equimolar ratio.
The purpose of the present study is to further expand the compositional space in such systems and to explore the influence of pentavalent cation oxide (Nb2O5) and trivalent rare earth oxide’s introduction. The phase formation and microstructure of four compositions are reported in ZrO2-HfO2-CeO2-Nb2O5-RE2O3 type systems (RE = Y, Yb, Nd, Gd). To the best of our knowledge, phase formation in the mentions systems has not been reported yet. However, binary and ternary systems based on ZrO2 and CeO2 were extensively studied. The ZrO2-HfO2 system shows complete solubility over the whole composition range with three regions of solid solutions: monoclinic, tetragonal and cubic [16]. In the case of ZrO2-CeO2 and HfO2-CeO2 systems, there is evidence of limited mutual solubility in the solid-state [17,18]. Phase equilibria in ternary ZrO2-HfO2-CeO2 were reported by Andrievskaya et al. [17,19] and showed the formation of a mixture of the three polymorphs near the equimolar region of the diagram for processing temperatures of 1250 and 1500 °C. Gild et al. [13] reported successful preparation via high energy ball milling and spark plasma sintering of eight compositions with five cations in equimolar amounts designed by the addition of a four-principal-cation Hf0.25Zr0.25Ce0.25Y0.25O2-δ. The obtained materials showed low thermal conductivities, which the authors state is a result of multiple different cation species. In this context, the addition of Nb2O5 to Hf0.25Zr0.25Ce0.25Y0.25O2-δ or other compositions with hafnium, zirconium and cerium oxide core might contribute to enhancing the properties of the materials by balancing the charge and thus avoiding oxygen vacancies formation.
2. Materials and Methods
2.1. Materials Processing
Ceramic samples belonging to equimolar region of ZrO2-HfO2-CeO2-Nb2O5-RE2O3 type (RE = Y, Yb, Nd, Gd) systems were synthetized by solid-state route. The compositions will be referred to as follows: ZHCNY for 0.2ZrO2–0.2HfO2-0.2CeO2-0.2Nb2O3-0.2Y2O3, ZHCNYb for 0.2ZrO2-0.2HfO2-0.2CeO2-0.2Nb2O3-0.2Yb2O3, ZHCNNd for 0.2ZrO2-0.2HfO2-0.2CeO2-0.2Nb2O3-0.2Nd2O3 and ZHCNGd for 0.2ZrO2-0.2HfO2-0.2CeO2-0.2Nb2O3-0.2Gd2O3.
First, for all compositions, a 20 g mixture was prepared by hand milling for 60 min in the presence of isopropanol (Sigma-Aldrich, St. Louis, MO, USA ACS reagent ≥99.5%) using an agate mortar and pestle, starting from high purity oxides: ZrO2 (Sigma-Aldrich, St. Louis, MO, USA 99% trace metals basis), HfO2 (Sigma-Aldrich, St. Louis, MO, USA 98%), CeO2 (Sigma-Aldrich, St. Louis, MO, USA ≥ 99%), Nb2O5 (Sigma-Aldrich, St. Louis, MO, USA 99.9% trace metals basis), Y2O3 (Sigma-Aldrich, St. Louis, MO, USA 99.99% trace metals basis), Yb2O3 (Sigma-Aldrich, St. Louis, MO, USA 99.9% trace metals basis), Nd2O3 (Sigma-Aldrich, St. Louis, MO, USA 99.9% trace metals basis) and Gd2O3 (Sigma-Aldrich, St. Louis, MO, USA 99.9% trace metals basis). The precursor composition for each mixture is summarized in Table 1.

Table 1.
Precursor amount for heterovalent equimolar quinary oxide system of ZrO2-HfO2-CeO2-Nb2O5-RE2O3 type (RE = Y, Yb, Nd, Gd).
The powder mixtures were then pressed into 13 mm pellets using a 10-ton force 4555 Manual Bench Top Pellet Press Equipment (Carver, Inc., Wabash, IN, USA). The green bodies were then subjected to several thermal treatments performed in the range of 1300–1600 °C in an HT 18 High Temperature Furnace (Nabertherm, Lilienthal, Germany). The presintering heat treatment stage was performed at 1300 °C in air, with a heating rate of 5 °C/min, a dwell time of 6 h and a cooling rate at the normal speed of the oven. After the presintering stage, the samples were ground in an agate mortar and reshaped into 13 mm pellets under 400 MPa uniaxial pressure. The sintering stage was performed at 1400, 1500 or 1600 °C in air and with a heating rate of 5 °C/min, a dwell time of 6 h and a cooling rate at the normal speed of the oven.
2.2. Materials Characterization
Room-temperature X-ray diffraction (XRD) measurements were performed on the heat-treated sample for phase composition determination. The analyses were carried out on Empyrean equipment (PANalytical, Almelo, The Netherlands), using Ni-filtered Cu-Kα radiation (λ = 1.5418 Å) with a step size of 0.0263° and counting time per step of 510 s in the 2θ range of 20–80°. Phase search and match, as well as Rietveld refinement of structures, were performed in HighScore Plus 3.0.e software (PANalytical, Almelo, The Netherlands) coupled with the ICDD PDF4+ 2021 database (Newtown Square, PA, USA).
The microstructure and elemental distribution were investigated by scanning electron microscopy—SEM—operated at 30 kV coupled with energy dispersive spectrometer—EDS (Inspect F50, FEI, Hillsboro, OR, USA). The average grain size distribution was determined using OriginPro 9.0 software (OriginLab, Northampton, MA, USA) by considering size measurements on ≈500 grains performed by means of image processing software (ImageJ 1.50b, National Institutes of Health and the Laboratory for Optical and Computational Instrumentation, Madison, WI, USA).
3. Results and Discussion
3.1. Phase Composition
Phase composition was studied by XRD measurements and subsequent Rietveld refinement of patterns. The obtained and matched XRD patterns, as well as angular range from 27 to 31° 2θ, are presented in Figure 1 and the corresponding phase content for different heat treatment conditions are summarized in Table 2.
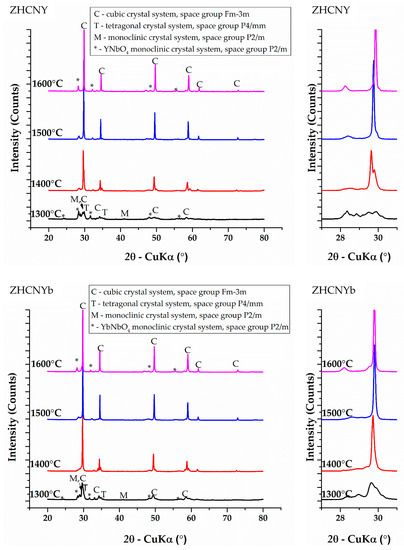
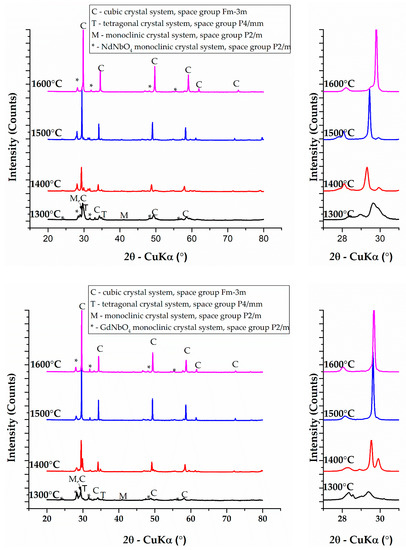
Figure 1.
XRD patterns corresponding to samples heat-treated at 1300, 1400, 1500 and 1600 °C belonging to equimolar ZrO2-HfO2-CeO2-Nb2O5-RE2O3 systems (RE = Y, Yb, Nd, Gd).

Table 2.
Phase content for samples treated at 1300, 1400, 1500 and 1600 °C belonging to equimolar ZrO2-HfO2-CeO2-Nb2O5-RE2O3 systems (RE = Y, Yb, Nd, Gd).
After thermal treatment at 1300 °C, the composition is complex for all studied samples, and it consists of three solid solutions of cubic [20], tetragonal [21] and monoclinic [22] symmetries and RENbO4 of monoclinic symmetry, where RE is the rare earth ion in the 3+ oxidation state. The peak profiles are broad and show a low intensity, which suggests a limited mutual solubility of the precursor oxides in this temperature condition (Figure 1). The increase of the heat treatment temperature to 1400 °C shows two kinds of effects on the studied compositions: ZHCNNd forms a higher content of lower symmetry P2/m solid solutions at this temperature approximated at 33.70%, whereas in the case of ZHCNY, ZHCNYb and ZHCNGd, an increase in the content of tetragonal P4/mmm and cubic Fm-3m solid solutions was evidenced. A further increase in the temperature to 1500 °C is beneficial in stabilizing higher-ordered solid solutions of tetragonal symmetry in the case of ZHCNY and cubic symmetry in the case of ZHCNYb and ZHCNGd. The ZHCNNd composition shows a remanent P2/m solid solution at 1500 °C, but with a decreased content. XRD results after processing at 1600 °C show a binary phase composition consisting of fluorite-type cubic Fm-3m solid solution and RENbO4 (RE = Y, Yb, Nd, Gd).
3.2. Microstructure
Figure 2 depicts SEM images and corresponding EDS maps for the equimolar quinary compositions after thermal treatment at 1600 °C.
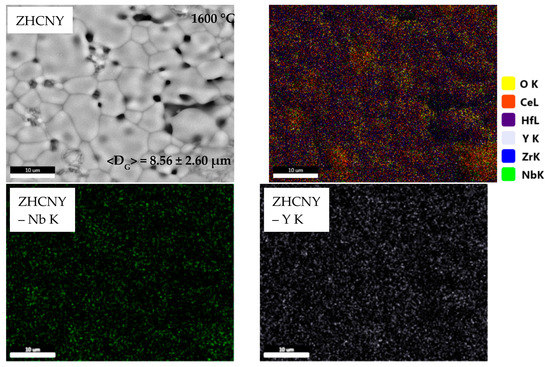
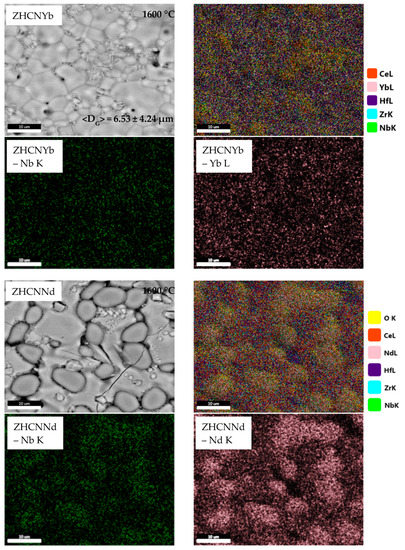
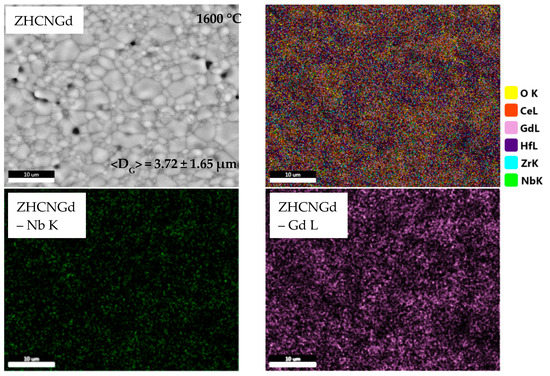
Figure 2.
Microstructure and elemental distribution of samples treated at 1600 °C belonging to ZrO2-HfO2-CeO2-Nb2O5-RE2O3 equimolar systems (RE = Y, Yb, Nd, Gd).
The microstructure of the ZHCNY sample is heterogeneous and shows grains with an average size of 8.56 ± 2.60 μm and macropores, which are placed intra- and intergranular. The corresponding EDS maps show niobium and yttrium aggregation, which is in good agreement with XRD results, where the formation of YNbO4 was evidenced. Moreover, in these areas, a melting and recrystallization process is evidenced by plate-like grains and low-angle junctions. This effect is most probably caused by the lower melting temperature of Nb2O5 of 1512 °C [23]. The ZHCNYb sample also shows a heterogeneous microstructure, with grains of an average size of 6.53 ± 4.24 μm and intergranular pores. In this case, the ceramic has pronounced recrystallization plate-like grains, probably caused by a lower melting temperature of Yb2O3 (2355 °C [24]) compared to Y2O3 (2425 °C [25]) and, as a result, a lower porosity.
The ZHCNNd sample shows a typical particulate composite microstructure, where NdNbO4 is placed in a (Zr,Hf,Ce)O2 matrix. In these processing conditions, the SEM image evidences also intragranular and intergranular cracks formation.
In the case of the ZHCNGd sample, the average grain size is the lowest in the studied series (3.72 ± 1.65 μm). The grains are well defined and are of polyhedral shape with round edges.
4. Conclusions
Four compositions in ZrO2-HfO2-CeO2-Nb2O5-RE2O3 type systems (RE = Y, Yb, Nd, Gd) were studied over the temperature range of 1300–1600 °C. XRD results showed a complex composition at lower temperatures and a binary composition at 1600 °C, consisting of cubic fluorite-type oxide and RENbO4. Phase formation in ZrO2-HfO2-CeO2-Nb2O5-RE2O3 type systems (RE = Y, Yb, Nd, Gd) shows the obtaining of single fluorite-type polymorphs when RENbO4 is present in the composition when compared to a mixture of three polymorphs obtained at 1250 and 1500 °C in a ZrO2-HfO2-CeO2 system. Therefore, RENbO4 might reduce the temperature of higher symmetry cubic phase formation in the temperature range of 1400–1600 °C. The microstructure of the ceramics processed at the highest temperature is heterogeneous and shows evidence of melting and recrystallization due to the partial volatilization of Nb2O5. Moreover, measurements on the SEM images showed coarse-grain ceramics formation.
Author Contributions
Conceptualization, V.-A.S.; methodology, V.-A.S.; formal analysis, V.-A.S.; investigation, V.-A.S.; resources, E.A.; data curation, V.-A.S.; writing—original draft preparation, V.-A.S.; writing—review and editing, E.A.; supervision, E.A. Both authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project ANTREPRENORDOC, grant number 36355/23.05.2019 HRD OP/380/6/13-SMIS Code: 123847.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article.
Acknowledgments
Vasile-Adrian Surdu acknowledges the support of this work by the project ANTREPRENORDOC, in the framework of Human Resources Development Operational Programme 2014-2020, financed from the European Social Fund under the contract number 36355/23.05.2019 HRD OP/380/6/13-SMIS Code: 123847.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parras, J.P.; De Souza, R.A. Grain-boundary diffusion of cations in fluorite-type oxides is faster but not always easier. Acta Mater. 2020, 195, 383–391. [Google Scholar] [CrossRef]
- Solomon, J.M.; Shamblin, J.; Lang, M.; Navrotsky, A.; Asta, M. Chemical ordering in substituted fluorite oxides: A computational investigation of Ho2Zr2O7 and RE2Th2O7 (RE = Ho, Y, Gd, Nd, La). Sci. Rep. 2016, 6, 38772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Liu, Z.; Pang, L.; Han, Y.; Yao, S.; Wang, X. Preparation of Y2O3–ZrO2–CeO2 solid solution by co-precipitation and its electrical property. Phys. B Condens. Matter 2021, 612, 412972. [Google Scholar] [CrossRef]
- Wen, T.; Yuan, L.; Liu, T.; Sun, Q.; Jin, E.; Tian, C.; Yu, J. Enhanced ionic conductivity and thermal shock resistance of MgO stabilized ZrO2 doped with Y2O3. Ceram. Int. 2020, 46, 19835–19842. [Google Scholar] [CrossRef]
- Guo, L.; Li, G.; Zhilin, G. Effects of surface roughness on CMAS corrosion behavior for thermal barrier coating applications. J. Adv. Ceram. 2021, 10, 472–481. [Google Scholar] [CrossRef]
- Fan, W.; Bai, Y.; Wang, Y.; He, T.; Gao, Y.; Zhang, Y.; Zhong, X.; Li, B.; Chang, Z.; Ma, Y. Microstructural design and thermal cycling performance of a novel layer-gradient nanostructured Sc2O3–Y2O3 co-stabilized ZrO2 thermal barrier coating. J. Alloys Compd. 2020, 829, 154525. [Google Scholar] [CrossRef]
- Fang, H.; Wang, W.; Huang, J.; Li, Y.; Ye, D. Corrosion behavior and thermos-physical properties of a promising Yb2O3 and Y2O3 co-stabilized ZrO2 ceramic for thermal barrier coatings subject to calcium-magnesium-aluminum-silicate (CMAS) deposition: Experiments and first-principles calculation. Corros. Sci. 2021, 182, 109230. [Google Scholar] [CrossRef]
- Park, M.H.; Hwang, C.S. Fluorite-structure antiferroelectrics. Rep. Prog. Phys. 2019, 82, 124502. [Google Scholar] [CrossRef]
- Ali, F.; Zhou, D.; Sun, N.; Ali, H.W.; Abbas, A.; Iqbal, F.; Dong, F.; Kim, K.-H. Fluorite-structured ferroelectric-/antiferroelectric-based electrostatic nanocapacitors for energy storage applications. ACS Appl. Energy Mater. 2020, 3, 6036–6055. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Qin, C.; Han, C.; Sun, L.; Wang, Y. Band-gap-tunable CeO2 nanoparticles for room-temperature NH3 gas sensors. Ceram. Int. 2020, 46, 19232–19240. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.; Huang, C.; Nieto, A.; Chen, R.; Luo, J. From high-entropy ceramics to compositionally-complex ceramics: A case study of fluorite oxides. J. Eur. Ceram. Soc. 2020, 40, 2120–2129. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowa, J.; Szymczak, M.; Zajusz, M.; Mikuła, A.; Moździerz, M.; Berent, K.; Wytrwal-Sarna, M.; Bernasik, A.; Stygar, M.; Świerczek, K. Stabilizing fluorite structure in ceria-based high-entropy oxides: Influence of Mo addition on crystal structure and transport properties. J. Eur. Ceram. Soc. 2020, 40, 5870–5881. [Google Scholar] [CrossRef]
- Gild, J.; Samiee, M.; Braun, J.L.; Harrington, T.; Vega, H.; Hopkins, P.E.; Vecchio, K.; Luo, J. High-entropy fluorite oxides. J. Eur. Ceram. Soc. 2018, 38, 3578–3584. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Z.; Liu, J.; Do-Thanh, C.-L.; Chen, H.; Xu, S.; Lin, Q.; Jiao, Y.; Wang, J.; Wang, Y. Entropy-stabilized single-atom Pd catalysts via high-entropy fluorite oxide supports. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Spiridigliozzi, L.; Ferone, C.; Cioffi, R.; Dell’Agli, G. A simple and effective predictor to design novel fluorite-structured High Entropy Oxides (HEOs). Acta Mater. 2021, 202, 181–189. [Google Scholar] [CrossRef]
- Ruh, R.; Garrett, H.J.; Domagala, R.F.; Tallan, N.M. The System Zirconia-Hafnia. J. Am. Ceram. Soc. 1968, 51, 23–27. [Google Scholar] [CrossRef]
- Andrievskaya, E.R.; Gerasimyuk, G.I.; Kornienko, O.A.; Samelyuk, A.V.; Lopato, L.M. Phase equilibria in the HfO2-ZrO2-CeO2 system at 1250 °C. Inorg. Mater. 2006, 42, 1352–1359. [Google Scholar] [CrossRef]
- Andrievskaya, E.R.; Samelyuk, A.V.; Lopato, L.M. Reactions of Ceria with Zirconia and Yttria at 1250 °C. Poroshk. Met. 2002, 41, 1–2. [Google Scholar] [CrossRef]
- Andrievskaya, E.R.; Gerasimyuk, G.I.; Kornienko, O.A.; Samelyuk, A.V.; Lopato, L.M.; Red’ko, V.P. Phase equilibria in the system HfO2-ZrO2-CeO2 at 1500 °C. Powder Metall. Met. Ceram. 2006, 45, 448–456. [Google Scholar] [CrossRef]
- Wang, Z.; Saxena, S.K.; Pischedda, V.; Liermann, H.P.; Zha, C.S. In situ X-ray diffraction study of the pressure-induced phase transformation in nanocrystalline CeO2. Phys. Rev. B 2001, 64, 12102. [Google Scholar] [CrossRef]
- Xia, X.; Oldman, R.; Catlow, R. Computational modeling study of bulk and surface of yttria-stabilized cubic zirconia. Chem. Mater. 2009, 21, 3576–3585. [Google Scholar] [CrossRef]
- Adams, D.M.; Leonard, S.; Russell, D.R.; Cernik, R.J. X-ray diffraction study of hafnia under high pressure using synchrotron radiation. J. Phys. Chem. Solids 1991, 52, 1181–1186. [Google Scholar] [CrossRef]
- Emeka, N.C.; Imoisili, P.E.; Jen, T.-C. Preparation and Characterization of NbxOy Thin Films: A Review. Coatings 2020, 10, 1246. [Google Scholar] [CrossRef]
- Henriques, M.S.; Ferreira, A.C.; Cruz, A.; Ferreira, L.M.; Branco, J.B.; Brazda, P.; Jurek, K.; Stora, T.; Goncalves, A.P. Preparation of Yb2O3 submicron-and nano-materials via electrospinning. Ceram. Int. 2015, 41, 10795–10802. [Google Scholar] [CrossRef]
- Delice, S.; Isik, M.; Gasanly, N.M. Effect of heating rate on thermoluminescence characteristics of Y2O3 nanoparticles. J. Lumin. 2019, 212, 233–237. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).