Image-Based Formalization of Tabular Data for Threshold-Based Prediction of Hospital Stay Using Convolutional Neural Networks: An Intelligent Decision Support System Applied in COPD
Abstract
1. Introduction
- Proposing an innovative image-based formalization of tabular clinical and social data, enabling the use of Convolutional Neural Networks for hospital stay prediction.
- Introducing a conceptual architecture for a novel intelligent decision support system aimed at predicting short- or long-term hospital stays.
- Selecting a reduced subset of relevant variables from a large dataset, enhancing the prediction of hospitalization duration.
- Implementing the system into a user-friendly interface and exemplifying its applicability through a practical case study.
2. Materials and Methods
2.1. Database
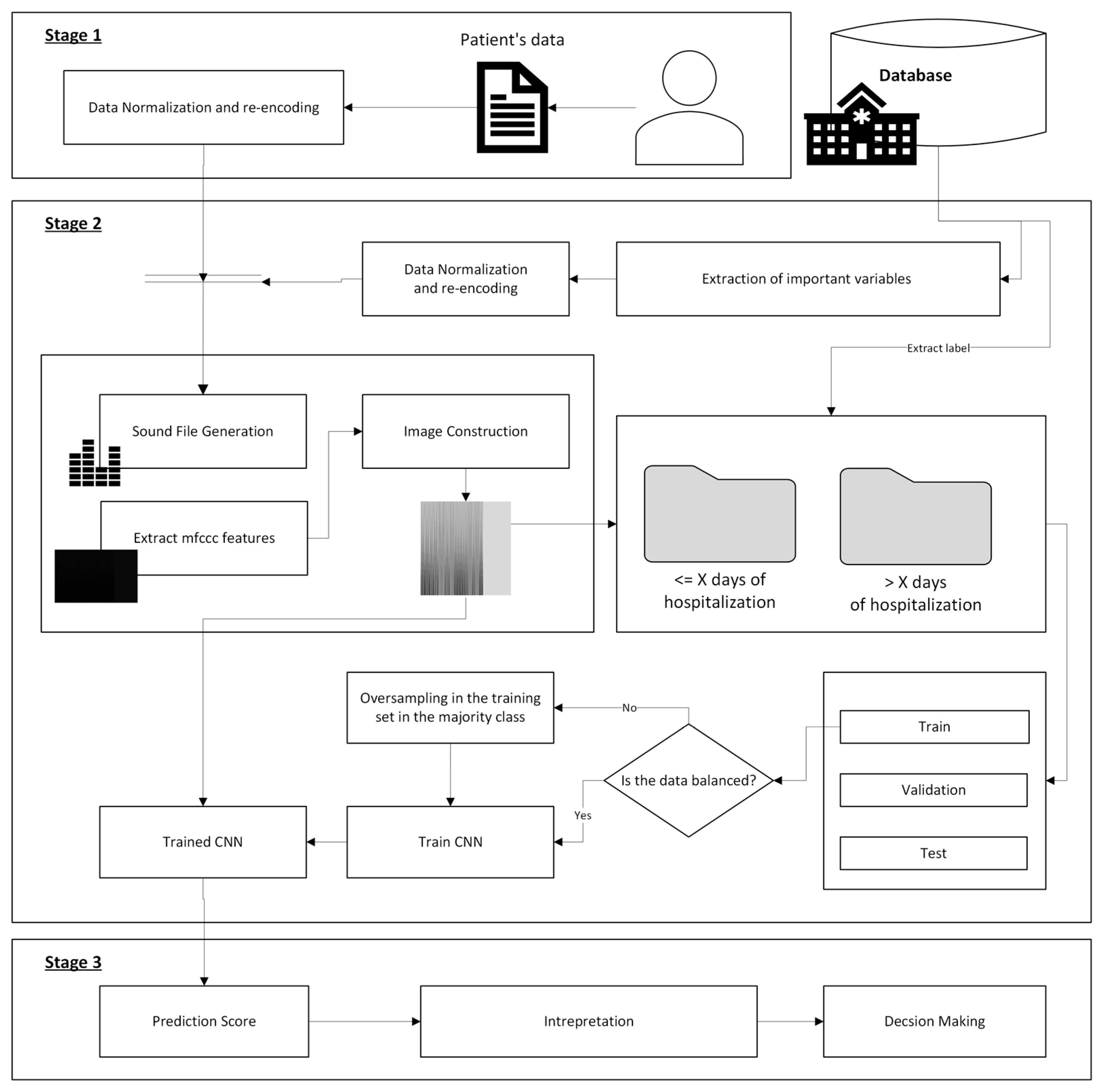
2.2. Conceptual Design
2.2.1. Stage 1: Patient’s Information Compilation
2.2.2. Stage 2: Data Processing
- Feature selection and preprocessing: First, the variables are preprocessed by rescaling the numerical variables using the Min-Max Scaler, transforming their values into a [0–1] range. Categorical variables are encoded as binary values; for example, diabetes is represented as 1 for ‘Yes’ and 0 for ‘No’. After preprocessing, a feature selection process is carried out to determine the optimal subset of variables that enables an accurate estimation of the average hospital stay and facilitates its future application in clinical practice. To achieve this, the XGBoost algorithm is employed, allowing the identification of the most relevant features in the database by leveraging feature rankings derived from fast and scalable tree-boosting models [27]. Although other feature selection techniques could be applied, such as filter-based methods (e.g., Chi-square, ANOVA) or wrapper-based approaches (e.g., Recursive Feature Elimination) [28], XGBoost was chosen due to its ability to efficiently perform feature selection during the model’s training phase. This embedded characteristic optimally balances computational cost and predictive accuracy, making it particularly suitable for large datasets and complex clinical variables.
- Data transformation: After preprocessing and feature selection, the dataset—initially in tabular format and containing the selected and rescaled variables—is subjected to a new formalization process by transforming it into an image representation. This transformation allows for a more compact representation of patient data, optimizing its structure for processing. Furthermore, this image-based formalization enables the application of more complex inference models and advanced architectures, which are highly effective in capturing intricate patterns and dependencies within multidimensional data. The image generation process is performed in two phases: first, the tabular data is converted into a sinusoidal sound wave. Next, once the sound wave is generated, the Mel-frequency cepstral coefficients (MFCCs) are extracted, which are then used to create the final image representation.
- Inference: Once the image has been determined, it is processed by the system using a Convolutional Neural Network. The dataset used to train the CNN was previously introduced in Section 2.1. However, prior to this step, the construction of the image associated with each patient is performed. Based on these images and their corresponding labels, the CNN is trained to operate as a binary classifier for each predefined threshold. In this study, two specific thresholds were established for calculating the LOHS: 6 and 10 days. Nevertheless, these cut-off values are flexible and can be adjusted according to medical team recommendations. At the output of the CNN, a score is generated for each case, referred to as the Risk of Extended Stay (RES), providing a probability estimate for exceeding each threshold.
2.2.3. Stage 3: Alert Generation and Decision Making
- Fewer than 6 days of hospital stay;
- Between 6 and 10 days of hospital stay;
- More than 10 days of hospital stay.
2.3. System Implementation
- Stage 1—Data Collection Panel—where patient information is gathered;
- Stage 2—Data Processing Panel—where the collected data is transformed into images and the RES (Risk of Extended Stay) is computed for each threshold;
- Stage 3—Alert Generation and Decision-Making Panel—where risk alerts are generated and appropriate decisions can be made.
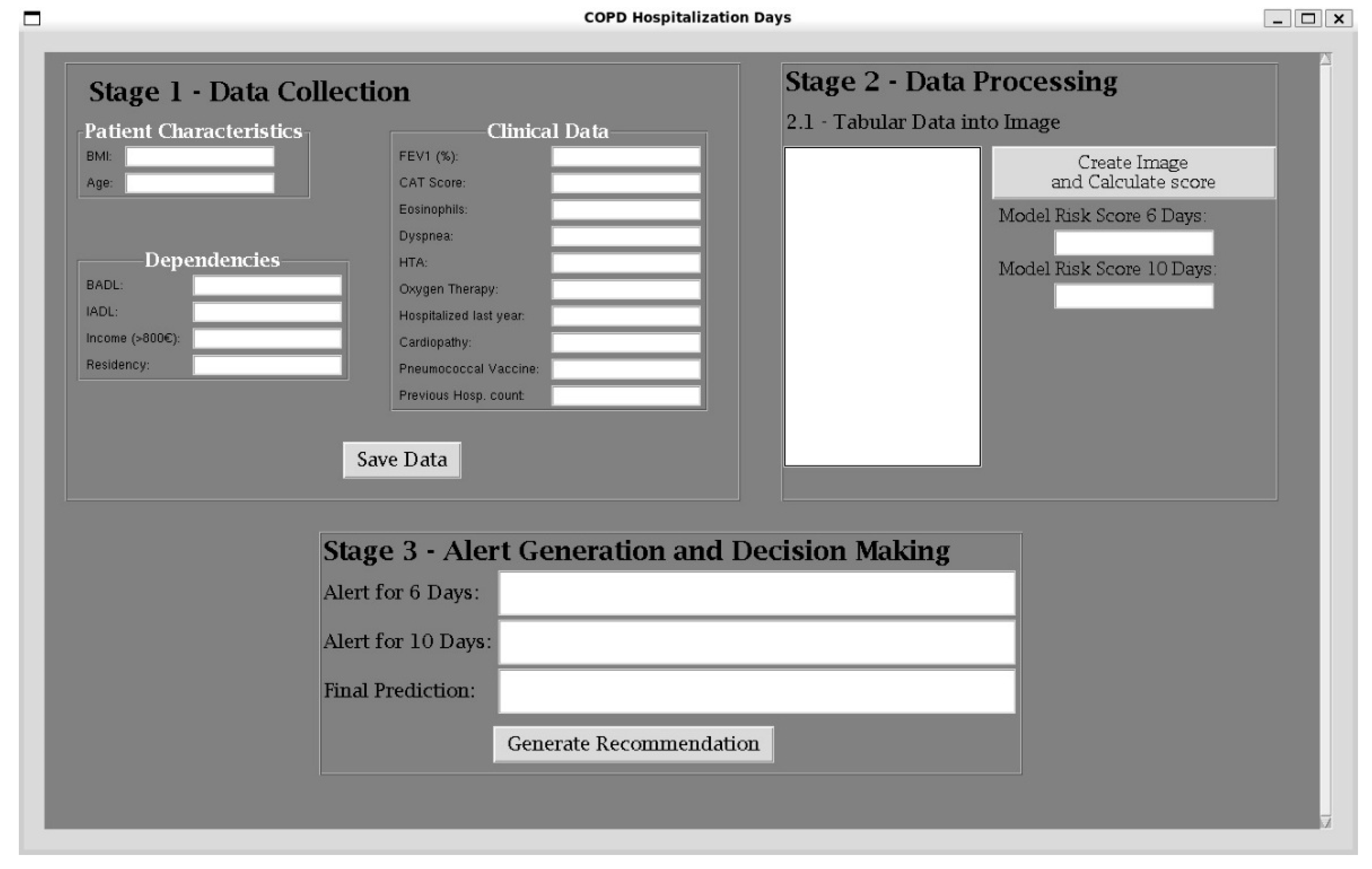
2.3.1. Data Collection
2.3.2. Data Processing
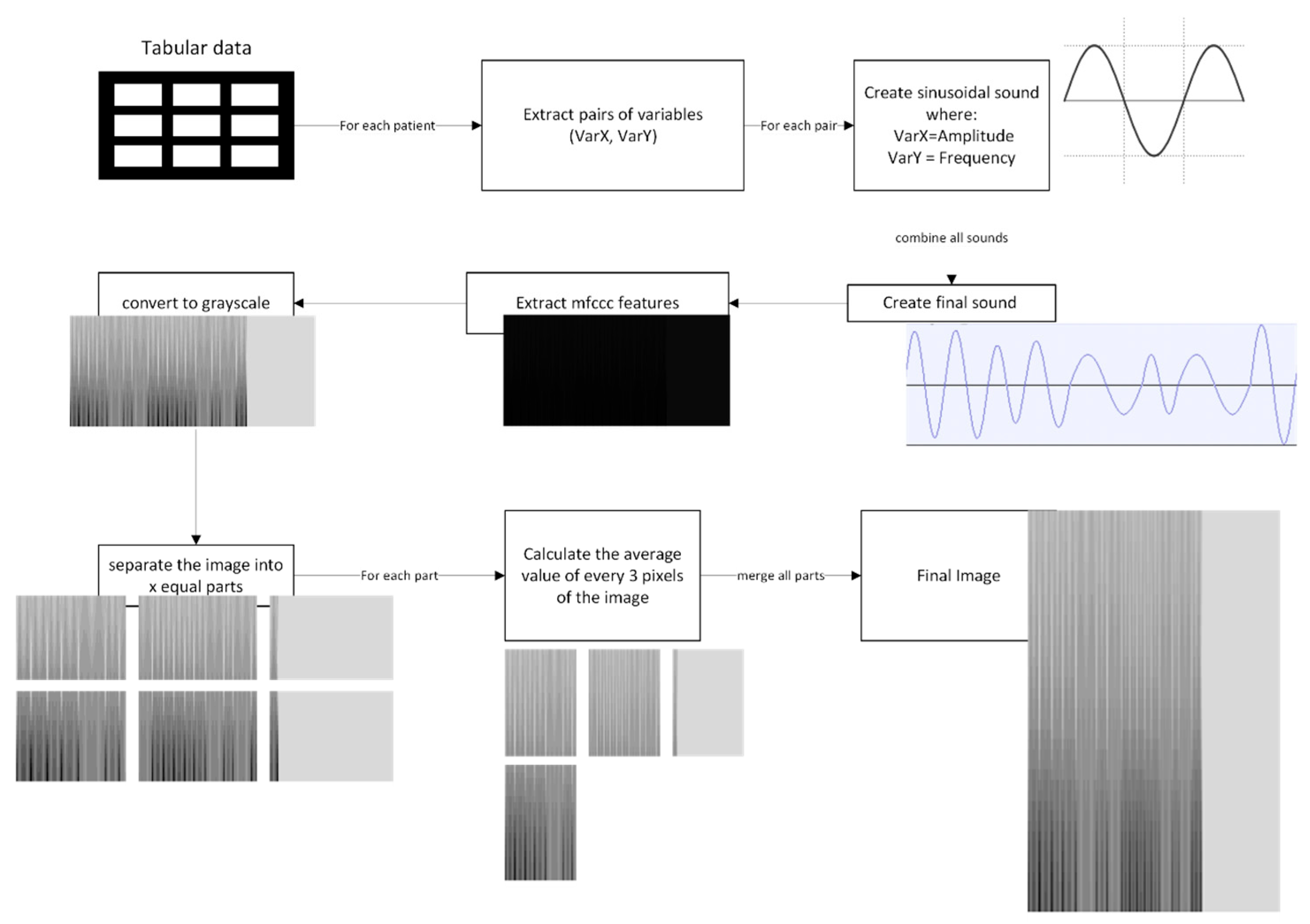
Image Construction
- Stage 1—Sinusoidal Wave Generation: The preprocessed data, already normalized to the [0, 1] interval, is structured into pairs of variables (e.g., BMI, FEV1 (%)). For each pair, a sinusoidal wave is generated, where one variable defines the amplitude (A) and the other controls the frequency (f). This is mathematically represented as shown in Equation (1), where t is the time vector, generated using the line-space function from NumPy [32]. We defined t as follows: creating a sample rate/f samples between 0 and the period (p) of the wave. The wave is sampled at 440 Hz to maintain consistency across all variables. This step transforms each pair of numerical patient data into structured waveform signals. Once all sinusoidal waves are created, they are merged into a single continuous signal per patient, appending each wave in sequence. To standardize input lengths across the dataset, the audio sample with the longest duration was first identified. All remaining samples were then zero-padded at the end with silence to match this maximum duration, ensuring uniform temporal dimensions for subsequent processing.
- Stage 2—MFCC Extraction and Image Generation: Once the combined signal for each patient is generated, it is treated as a sound representation. A custom function was implemented to extract and plot the Mel-frequency cepstral coefficients (MFCCs). For each input audio file, the signal was loaded using the librosa library [31] with the default sampling rate. The choice of MFCCs is motivated by their ability to capture both temporal and spectral characteristics of audio signals, making them highly effective for feature extraction. A total of 40 MFCC coefficients are selected, encapsulating the main features of the sinusoidal wave. Each MFCC matrix was visualized as a spectrogram with a reversed grayscale colormap to enhance contrast. For a cleaner image output, the axes figures were removed, and each plot was saved as a PNG file.
- Stage 3—Image Structuring and Final Preparation: To enhance the perceptual contrast of MFCC spectrogram images, a post-processing step was implemented using a custom tone curve transformation. Each image was processed using a piecewise quadratic interpolation curve defined by three control points: (0, 0), (14, 180), and (255, 255). This transformation significantly increases the brightness of darker regions while preserving highlights, thereby improving the visibility of low-intensity features that may carry relevant information for classification tasks. The transformation was implemented by generating a lookup table (LUT) based on the interpolated curve and applying it to all pixel values in the image, saving the processed images into transparent PNG files. To extract spatially localized features and reduce input dimensionality, a multistage image preprocessing pipeline was implemented. The first stage segmented each spectrogram image into six equal parts by dividing it into a 2 × 3 grid of tiles. This was achieved using a custom function that cropped each input image into six rectangular regions based on the specified dimensions (900 × 400 pixels total). Subsequently, each tile underwent horizontal resolution reduction through a custom local averaging function. This function processed each row of RGBA pixel data, excluding fully transparent pixels (i.e., alpha channel = 0), and grouped the remaining visible pixels into non-overlapping sets of three. The average of the red, green, blue, and alpha values for each group was computed, producing a smooth, horizontally compressed image representation that preserved essential spectral features while minimizing noise. The reduced tiles were saved in a dedicated output directory, with filenames encoding their original row and column positions to maintain spatial context. To facilitate visual inspection and provide CNN-compatible input, a final step recombined the reduced tiles into a composite grid image. This reconstruction preserved the original 2 × 3 tile arrangement, resulting in a single, spatially structured image per spectrogram. These final images were used as input for the model.
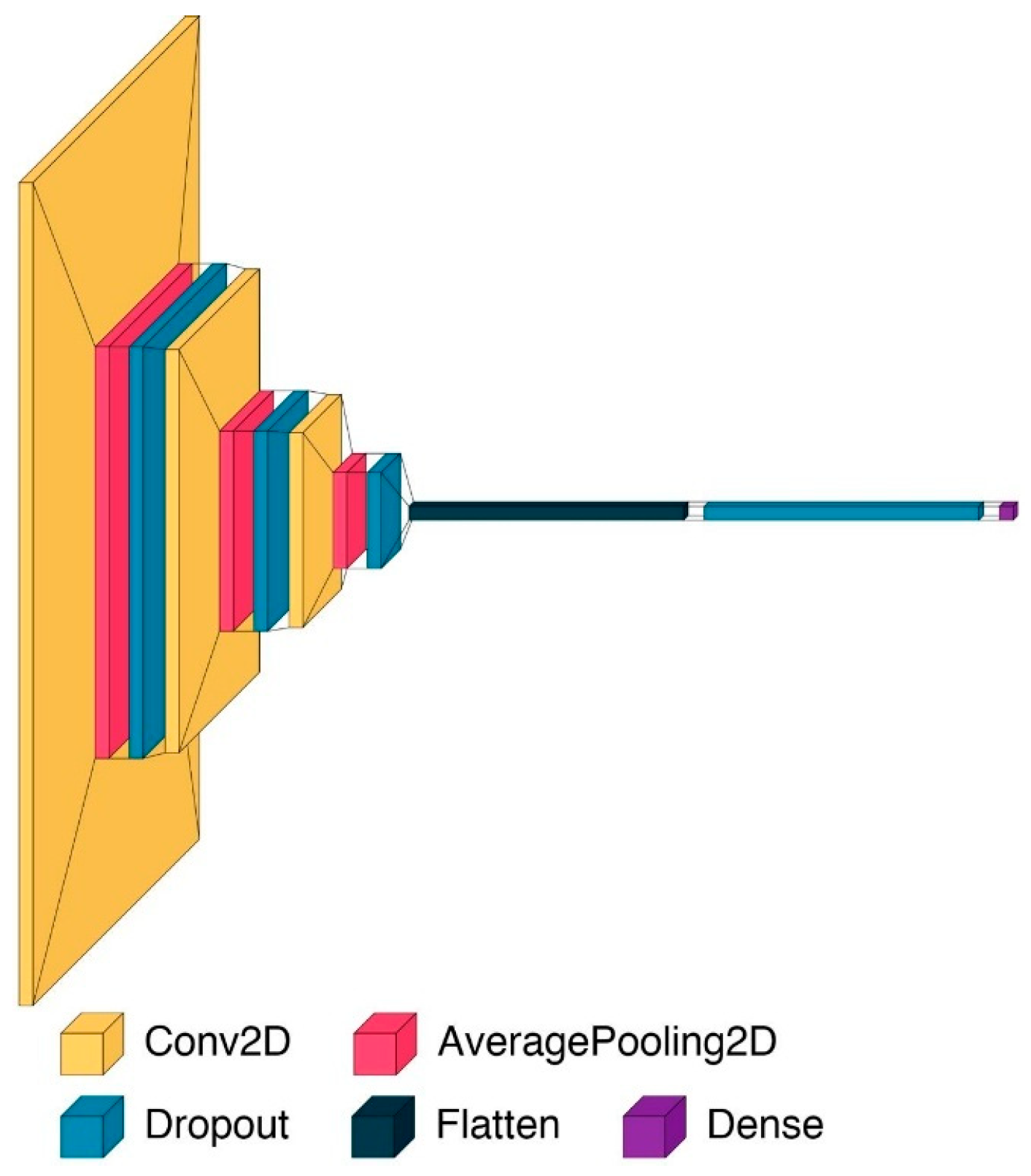
Inference with Convolutional Neural Network
- The model includes three convolutional layers, each followed by average pooling and dropout. All convolutional layers use the ReLU (Rectified Linear Unit) activation function to introduce non-linearity. The first convolutional layer applies 16 filters of size 5 × 5, the second uses 32 filters of size 4 × 4, and the third uses 64 filters of size 3 × 3. This progressive reduction in filter size is designed to allow initial layers to capture broad spectral features, while deeper layers extract increasingly fine-grained local patterns.
- Each convolutional layer is followed by an AveragePooling2D layer with a 2 × 2 pooling window. Average pooling is selected over max pooling to retain smooth transitions and preserve the overall energy distribution in the data.
- After the final convolutional block, the feature maps are flattened into a one-dimensional vector. This is followed by a fully connected layer. A dropout layer with a rate of 0.3 is applied prior to this output neuron to further mitigate overfitting by randomly deactivating features during training.
- The output layer contains a single neuron with a sigmoid activation function. This setup is appropriate for binary classification tasks, as it outputs a probability score between 0 and 1, representing the model’s confidence in the predicted class.
- Dropout is used throughout the architecture to improve generalization—specifically, 0.2 dropout rates follow each of the first two convolutional blocks, and a 0.3 rate precedes the final dense layer. The model is trained using the Stochastic Gradient Descent (SGD) optimizer, with a learning rate of 0.001 applied consistently across both the 6-day and 10-day thresholds and momentum set to 0.9, providing controlled and stable convergence during training. This optimization technique allows the model to converge efficiently by iteratively updating the weights in small batches.
Comparison with Other Conventional Machine Learning Models
2.3.3. Alert Generation and Decision Making
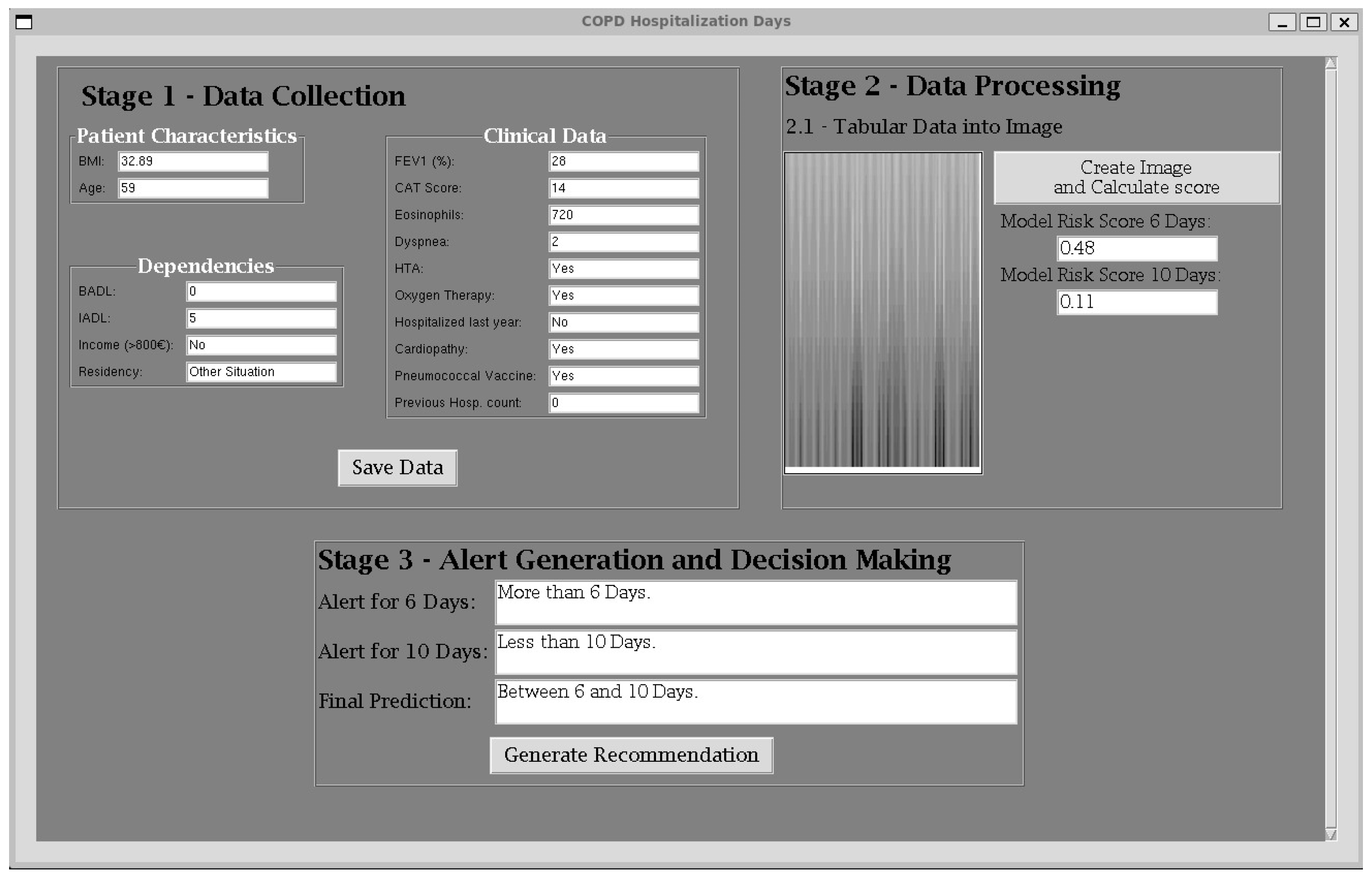
3. Case Study
3.1. Initial Data Collection
3.2. Data Processing
3.3. Alert Generation and Decision Making
4. Discussion
4.1. Model Performance and Clinical Utility
4.2. Ethical Considerations and Future Clinical Use
4.3. Comparison with Existing Models
4.4. Added Value of Image-Based Representation and Feature Selection
4.5. Limitations and Future Directions
- Data Expansion and Multicenter Validation: Collect larger and more diverse datasets to improve model generalizability and support external validation efforts.
- Overfitting Mitigation Strategies: Employ regularization techniques, data augmentation, or transfer learning to reduce the risk of overfitting in low-data scenarios.
- Optimization of the Image Generation Pipeline: Replace manual design steps with automated optimization methods (e.g., neural architecture search, differentiable signal-to-image encoding).
- Exploration of Advanced Architectures: Evaluate deeper or pre-trained CNNs and hybrid models to enhance feature extraction without compromising interpretability.
- Explainability Tools Integration: Incorporate visual explanation frameworks to identify which image regions contribute most to each prediction and relate them back to specific clinical variables.
- Clinical Usability Studies: Conduct user-centered evaluations with healthcare professionals to assess real-world applicability, interpretability, and integration into clinical workflows.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; de Oca, M.M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Arch. Bronconeumol. 2023, 59, 232–248. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Román-Rodríguez, M.; Singh, D.; Han, M.L.K.; Rodríguez-Roisin, R.; Ferguson, G.T. Goals of COPD treatment: Focus on symptoms and exacerbations. Respir. Med. 2020, 166, 105938. [Google Scholar] [CrossRef]
- Wang, Y.; Stavem, K.; Dahl, F.A.; Humerfelt, S.; Haugen, T. Factors associated with a prolonged length of stay after acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Int. J. COPD 2014, 9, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Inabnit, L.S.; Blanchette, C.; Ruban, C. Comorbidities and length of stay in chronic obstructive pulmonary disease patients. COPD J. Chronic Obstr. Pulm. Dis. 2018, 15, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, E.; Ielpo, A.; Barbeta, E.; Ceccato, A.; Huerta, A.; Gabarrús, A.; Soler, N.; Chetta, A.; Torres, A. Clinical variables predicting the risk of a hospital stay for longer than 7 days in patients with severe acute exacerbations of chronic obstructive pulmonary disease: A prospective study. Respir. Res. 2018, 19, 261. [Google Scholar] [CrossRef]
- Agboado, G.; Peters, J.; Donkin, L. Factors influencing the length of hospital stay among patients resident in Blackpool admitted with COPD: A cross-sectional study. BMJ Open 2012, 2, e000869. [Google Scholar] [CrossRef]
- Wong, A.W.M.; Gan, W.Q.; Burns, J.; Sin, D.D.; van Eeden, S.F. Exacerbation aiguë de la bronchopneumopathie obstructive chronique: Influence des facteurs sociaux sur la durée de l’hospitalisation et les taux de réadmission. Can. Respir. J. 2008, 15, 361–364. [Google Scholar] [CrossRef]
- Ko, F.W.S.; Chan, K.P.; Ngai, J.; Ng, S.S.; Yip, W.H.; Ip, A.; Chan, T.; Hui, D.S. Blood eosinophil count as a predictor of hospital length of stay in COPD exacerbations. Respirology 2020, 25, 259–266. [Google Scholar] [CrossRef]
- Pokharel, P.; Lamichhane, P.; Pant, P.; Shrestha, A.B. Factors affecting length of hospital stay in chronic obstructive pulmonary disease patients in a tertiary hospital of Nepal: A retrospective cross-sectional study. Ann. Med. Surg. 2022, 80, 104246. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, S.; Represas-Represas, C.; Ruano-Raviña, A.; Botana-Rial, M.; Mouronte-Roibas, C.; Ramos-Hernández, C.; Fernández-Villar, A. Predictores sociales y clínicos asociados con estancia hospitalaria prolongada en pacientes con agudización grave de EPOC. Rev. Clin. Esp. 2020, 220, 79–85. [Google Scholar] [CrossRef]
- Turgeman, L.; May, J.H.; Sciulli, R. Insights from a machine learning model for predicting the hospital Length of Stay (LOS) at the time of admission. Expert. Syst. Appl. 2017, 78, 376–385. [Google Scholar] [CrossRef]
- Lüthi-Corridori, G.; Boesing, M.; Ottensarendt, N.; Leuppi-Taegtmeyer, A.B.; Schuetz, P.; Leuppi, J.D. Predictors of Length of Stay, Mortality and Rehospitalization in COPD Patients: A Retrospective Cohort Study. J. Clin. Med. 2023, 12, 5322. [Google Scholar] [CrossRef] [PubMed]
- Rajula, H.S.R.; Verlato, G.; Manchia, M.; Antonucci, N.; Fanos, V. Comparison of conventional statistical methods with machine learning in medicine: Diagnosis, drug development, and treatment. Medicina 2020, 56, 455. [Google Scholar] [CrossRef] [PubMed]
- Casal-Guisande, M.; Represas-Represas, C.; Golpe, R.; Fernández-García, A.; González-Montaos, A.; Comesaña-Campos, A.; Ruano-Raviña, A.; Fernández-Villar, A. Clinical and Social Characterization of Patients Hospitalized for COPD Exacerbation Using Machine Learning Tools. Arch. Bronconeumol. 2025, 61, 264–273. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Zhang, L.; Li, L.; Huang, Y.; Sun, Y.; Yuan, X. Artificial intelligence in clinical decision support systems for oncology. Int. J. Med. Sci. 2023, 20, 79–86. [Google Scholar] [CrossRef]
- Niraula, D.; Sun, W.; Jin, J.; Dinov, I.D.; Cuneo, K.; Jamaluddin, J.; Matuszak, M.M.; Luo, Y.; Lawrence, T.S.; Jolly, S.; et al. A clinical decision support system for AI-assisted decision-making in response-adaptive radiotherapy (ARCliDS). Sci. Rep. 2023, 13, 5279. [Google Scholar] [CrossRef]
- Naiseh, M.; Al-Thani, D.; Jiang, N.; Ali, R. How the different explanation classes impact trust calibration: The case of clinical decision support systems. Int. J. Hum. Comput. Stud. 2023, 169, 102941. [Google Scholar] [CrossRef]
- Casal-Guisande, M.; Fernández-Villar, A.; Mosteiro-Añón, M.; Comesaña-Campos, A.; Cerqueiro-Pequeño, J.; Torres-Durán, M. Integrating tabular data through image conversion for enhanced diagnosis: A novel intelligent decision support system for stratifying obstructive sleep apnoea patients using convolutional neural networks. Digit. Health 2024, 10, 20552076241272630. [Google Scholar] [CrossRef]
- Ferreira, P.; Dutra, I.; Salvini, R.; Burnside, E. Interpretable models to predict Breast Cancer. In Proceedings of the 2016 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Shenzhen, China, 15–18 December 2016; pp. 1507–1511. [Google Scholar] [CrossRef]
- Zheng, L.; Eniola, E.; Wang, J. Machine Learning for Colorectal Cancer Risk Prediction. In Proceedings of the 2021 International Conference on Cyber-Physical Social Intelligence (ICCSI), Beijing, China, 18–20 December 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Ghazal, S.; Sauthier, M.; Brossier, D.; Bouachir, W.; Jouvet, P.A.; Noumeir, R. Using machine learning models to predict oxygen saturation following ventilator support adjustment in critically ill children: A single center pilot study. PLoS ONE 2019, 14, e0198921. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Martínez, M.-J.; Casal-Guisande, M.; Torres-Durán, M.; Sopeña, B.; Fernández-Villar, A. Clinical Characterization of Patients with Syncope of Unclear Cause Using Unsupervised Machine-Learning Tools: A Pilot Study. Appl. Sci. 2025, 15, 7176. [Google Scholar] [CrossRef]
- Miotto, R.; Wang, F.; Wang, S.; Jiang, X.; Dudley, J.T. Deep learning for healthcare: Review, opportunities and challenges. Brief. Bioinform. 2018, 19, 1236–1246. [Google Scholar] [CrossRef]
- Dogu, E.; Albayrak, Y.E.; Tuncay, E. Length of hospital stay prediction with an integrated approach of statistical-based fuzzy cognitive maps and artificial neural networks. Med. Biol. Eng. Comput. 2021, 59, 483–496. [Google Scholar] [CrossRef]
- Zolbanin, H.M.; Davazdahemami, B.; Delen, D.; Zadeh, A.H. Data analytics for the sustainable use of resources in hospitals: Predicting the length of stay for patients with chronic diseases. Inf. Manag. 2022, 59, 103282. [Google Scholar] [CrossRef]
- López-Canay, J.; Casal-Guisande, M.; Pinheira, A.; Golpe, R.; Comesaña-Campos, A.; Fernández-García, A.; Represas-Represas, C.; Fernández-Villar, A. Predicting COPD Readmission: An Intelligent Clinical Decision Support System. Diagnostics 2025, 15, 318. [Google Scholar] [CrossRef]
- Alsahaf, A.; Petkov, N.; Shenoy, V.; Azzopardi, G. A framework for feature selection through boosting. Expert. Syst. Appl. 2022, 187, 115895. [Google Scholar] [CrossRef]
- Galli, S. Feature Selection in Machine Learning with Python; Leanpub: Victoria, BC, Canada, 2022. [Google Scholar]
- RAPIDS Development Team. RAPIDS: Libraries for End-to-End GPU Data Science. 2023. Available online: https://rapids.ai (accessed on 20 May 2025).
- Yu, L.; Li, B.; Jiao, B. Research and Implementation of CNN Based on TensorFlow. IOP Conf. Ser. Mater. Sci. Eng. 2019, 490, 042022. [Google Scholar] [CrossRef]
- McFee, B.; Raffel, C.; Liang, D.; Ellis, D.; McVicar, M.; Battenberg, E.; Nieto, O. librosa: Audio and Music Signal Analysis in Python. In Proceedings of the 14th Python in Science Conference, Austin, TX, USA, 6–12 July 2015; pp. 18–24. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Pedregosa, F.; Michel, V.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Varoquaux, G.; Gramfort, A.; Thirion, B.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Symum, H.; Zayas-Castro, J.L. Prediction of chronic disease-related inpatient prolonged length of stay using machine learning algorithms. Healthc. Inform. Res. 2020, 26, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Lian, S.; Feng, C.; Huang, D.; Zhang, W. Data mining-based detection of rapid growth in length of stay on COPD patients. In Proceedings of the 2017 IEEE 2nd International Conference on Big Data Analysis (ICBDA), Beijing, China, 10–12 March 2017; pp. 254–258. [Google Scholar] [CrossRef]
- Mohd Zulkifli, S.W.H.; Samsudin, H.B.; Majid, N. Generalized Linear Model to Estimate Length of Stay in The Hospital due to Respiratory Diseases. Univers. J. Public Health 2023, 11, 177–184. [Google Scholar] [CrossRef]
- Zhang, X.; Han, N.; Zhang, J. Comparative analysis of VGG, ResNet, and GoogLeNet architectures evaluating performance, computational efficiency, and convergence rates. Appl. Comput. Eng. 2024, 44, 172–181. [Google Scholar] [CrossRef]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-CAM: Visual Explanations from Deep Networks via Gradient-Based Localization. In Proceedings of the 2017 IEEE International Conference on Computer Vision (ICCV), Venice, Italy, 22–29 October 2017; pp. 618–626. [Google Scholar] [CrossRef]
| Variable | Type | Observation |
|---|---|---|
| Sex | Categorical | Male (1)/Female (0) |
| Age | Numerical | - |
| BMI (kg/m2) | Numerical | - |
| Active smoker | Categorical | Yes (1)/No (0) |
| High ingestion of alcohol | Categorical | Yes (1)/No (0) |
| Other drugs | Categorical | Yes (1)/No (0) |
| Previous year hospitalizations | Categorical | Yes (1)/No (0) |
| Number of hospitalizations in the previous year | Numerical | - |
| Number of positive sputum cultures | Numerical | - |
| Pneumococcal vaccination | Categorical | Yes (1)/No (0) |
| Eosinophils (total/uL) | Numerical | - |
| FEV1 (%) | Numerical | - |
| Dyspnea mMRC score | Numerical | - |
| CAT score | Numerical | - |
| Anemia | Categorical | Yes (1)/No (0) |
| Cardiopathy | Categorical | Yes (1)/No (0) |
| Obstructive sleep apnea | Categorical | Yes (1)/No (0) |
| Depression/Anxiety | Categorical | Yes (1)/No (0) |
| Arterial Hypertension | Categorical | Yes (1)/No (0) |
| Arteriopathy | Categorical | Yes (1)/No (0) |
| Diabetes | Categorical | Yes (1)/No (0) |
| Cancer | Categorical | Yes (1)/No (0) |
| Continuous home oxygen therapy | Categorical | Yes (1)/No (0) |
| Home non-invasive ventilation | Categorical | Yes (1)/No (0) |
| Type of residence | Categorical | Owns Property (0)/Other situation (1) |
| Monthly income EUR > 800 | Categorical | Yes (1)/No (0) |
| Working | Categorical | Active (0)/Pensioner (1) |
| Living alone | Categorical | Yes (1)/No (0) |
| Caretaker | Categorical | Yes (1)/No (0) |
| Uses social services | Categorical | Yes (1)/No (0) |
| Social Relationships | Categorical | Nobody or Family (0)/Friends and Neighbors (1) |
| Number of basic activities of daily living with dependency (BADL) | Numerical | The number of basic activities for which the individual is dependent is recorded. There are 5 (eating, dressing, taking a bath, going to the toilet, moving), so they vary in a range from 0 to 5. |
| Number of instrumental activities of daily living with dependency (IADL) | Numerical | The number of instrumental activities for which the individual is dependent is recorded. There are 8 (help with food preparation, house cleaning, laundry, telephone use, shopping, financial management, transport, and medication), so they vary in range from 0 to 8. |
| Precision | Recall | F1-Score | Accuracy | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
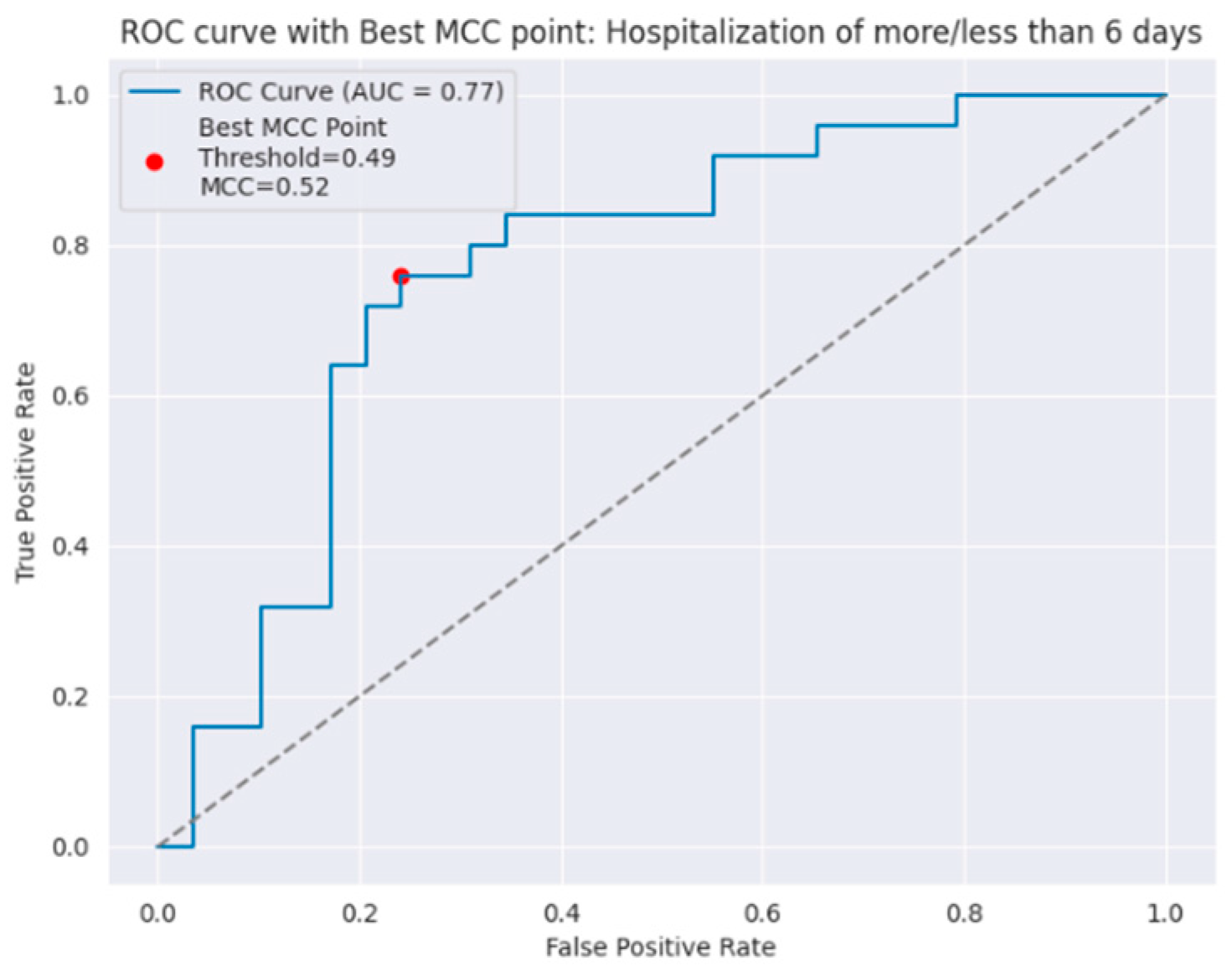
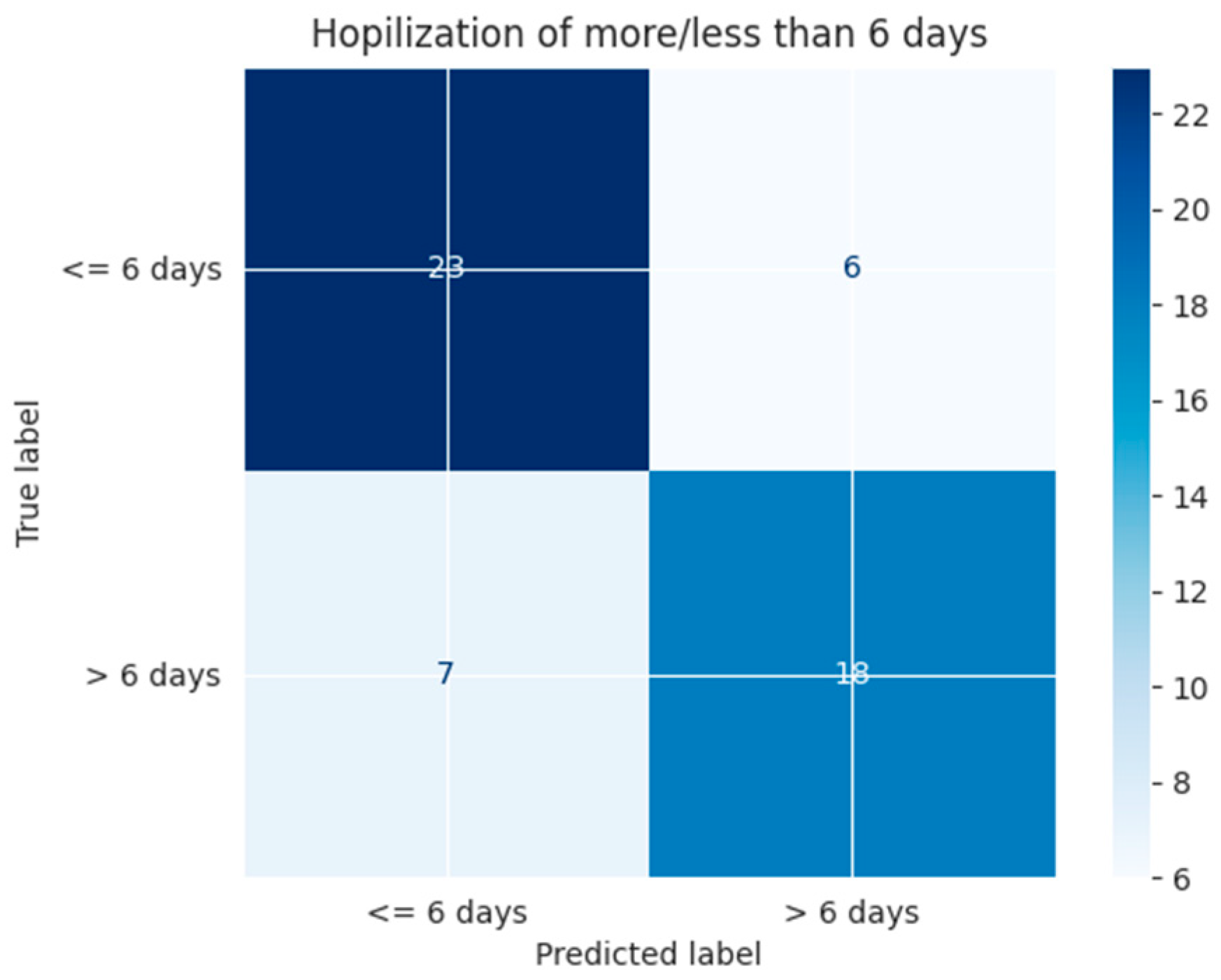
| ≤6 days | 0.77 | 0.79 | 0.78 | 0.76 | 0.79 | 0.72 |
| >6 days | 0.75 | 0.72 | 0.73 |
| Precision | Recall | F1-Score | Accuracy | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
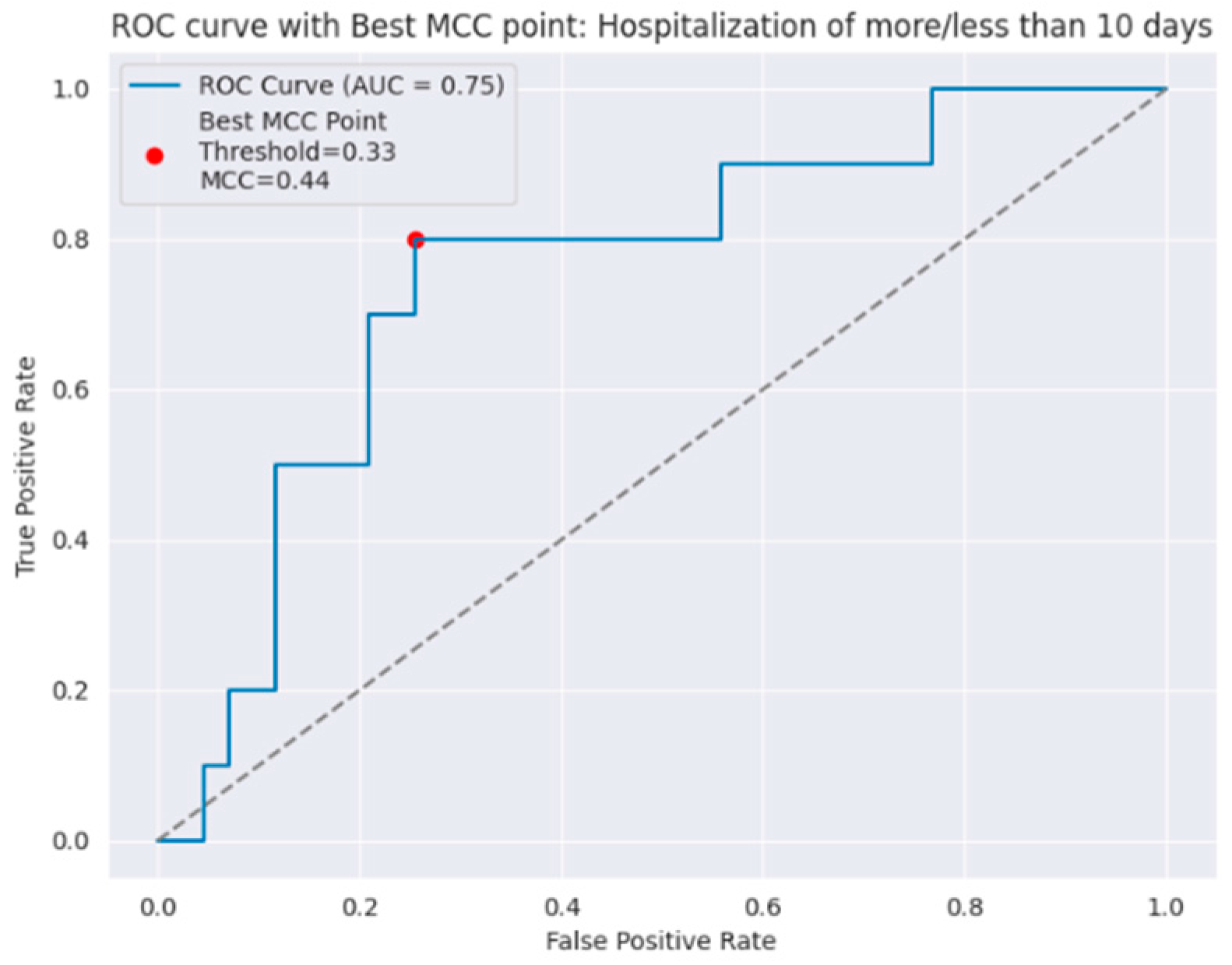
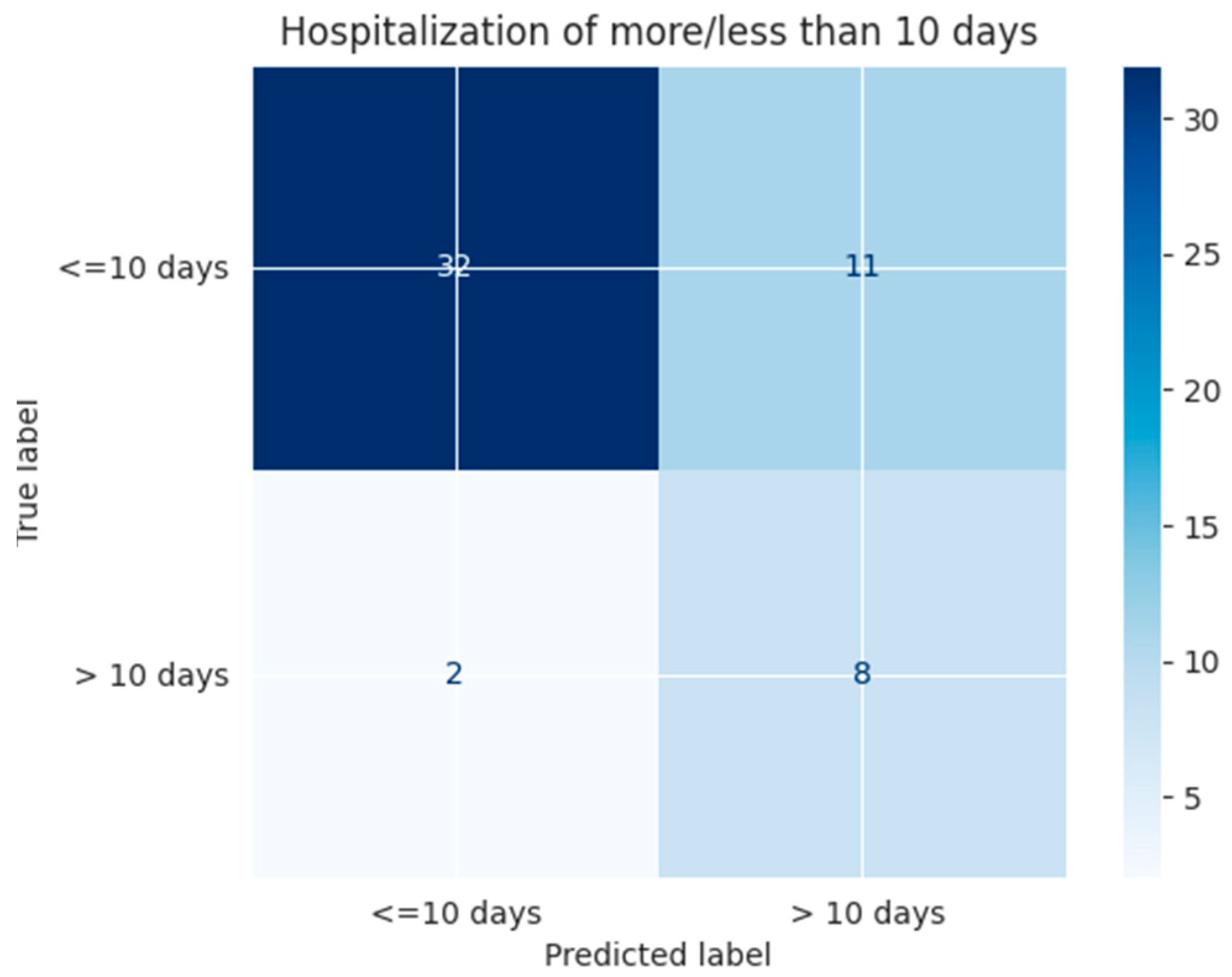
| ≤10 days | 0.94 | 0.74 | 0.83 | 0.75 | 0.74 | 0.80 |
| >10 days | 0.42 | 0.80 | 0.55 |
| Model | Comment | AUC Value |
|---|---|---|
| Decision Trees | - | 0.63 |
| Random Forest | Number of estimators = 100 | 0.65 |
| Random Forest | Number of estimators = 200 | 0.65 |
| SVM | C parameter (box constraint level) = 1; kernel scale mode = automatic; kernel = gaussian | 0.61 |
| SVM | C parameter (box constraint level) = 1; kernel scale mode = automatic; kernel = linear | 0.58 |
| SVM | C parameter (box constraint level) = 1; kernel scale mode = automatic; kernel = quadratic | 0.61 |
| SVM | C parameter (box constraint level) = 1; kernel scale mode = automatic; kernel = cubic | 0.62 |
| K-Nearest Neighbors | Number of neighbors = 5; distance = Euclidean | 0.58 |
| K-Nearest Neighbors | Number of neighbors = 100; distance = Euclidean | 0.62 |
| Naïve Bayes | -- | 0.57 |
| Multilayer Perceptron | Number of fully connected layers = 2; number of neurons = 100 for the 1st layer and 50 for the 2nd; activation function = ReLU; iteration limit = 300 | 0.6 |
| Model | Comment | AUC Value |
|---|---|---|
| Decision Trees | - | 0.62 |
| Random Forest | Number of estimators = 100 | 0.55 |
| Random Forest | Number of estimators = 200 | 0.53 |
| SVM | C parameter (box constraint level) = 1; kernel scale mode = automatic; kernel = gaussian | 0.38 |
| SVM | C parameter (box constraint level) = 1; kernel scale mode = automatic; kernel = linear | 0.39 |
| SVM | C parameter (box constraint level) = 1; kernel scale mode = automatic; kernel = quadratic | 0.42 |
| SVM | C parameter (box constraint level) = 1; kernel scale mode = automatic; kernel = cubic | 0.50 |
| K-Nearest Neighbors | Number of neighbors = 5; distance = Euclidean | 0.43 |
| K-Nearest Neighbors | Number of neighbors = 100; distance = Euclidean | 0.47 |
| Naïve Bayes | -- | 0.54 |
| Multilayer Perceptron | Number of fully connected layers = 2; number of neurons = 100 for the 1st layer and 50 for the 2nd; activation function = ReLU; iteration limit = 300 | 0.43 |
| Variable | Patient |
|---|---|
| BMI | 32.89 |
| FEV1 (%) | 28 |
| Age | 59 |
| CAT score | 14 |
| Eosinophils (total) | 720 |
| IADL modified | 5 |
| BADL modified | 0 |
| Dyspnea mMRC score | 2 |
| Arterial Hypertension | Yes |
| Chronic home oxygen therapy | Yes |
| Income (EUR > 800) | No |
| Type of residency | Other situation |
| Previous year hospitalized | No |
| Cardiopathy | Yes |
| Pneumococcal vaccination | Yes |
| Number of previous hospitalizations in the previous year | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Institute of Knowledge Innovation and Invention. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheira, A.; Casal-Guisande, M.; López-Canay, J.; Fernández-García, A.; Golpe, R.; Represas-Represas, C.; Torres-Durán, M.; Cerqueiro-Pequeño, J.; Comesaña-Campos, A.; Fernández-Villar, A. Image-Based Formalization of Tabular Data for Threshold-Based Prediction of Hospital Stay Using Convolutional Neural Networks: An Intelligent Decision Support System Applied in COPD. Appl. Syst. Innov. 2025, 8, 128. https://doi.org/10.3390/asi8050128
Pinheira A, Casal-Guisande M, López-Canay J, Fernández-García A, Golpe R, Represas-Represas C, Torres-Durán M, Cerqueiro-Pequeño J, Comesaña-Campos A, Fernández-Villar A. Image-Based Formalization of Tabular Data for Threshold-Based Prediction of Hospital Stay Using Convolutional Neural Networks: An Intelligent Decision Support System Applied in COPD. Applied System Innovation. 2025; 8(5):128. https://doi.org/10.3390/asi8050128
Chicago/Turabian StylePinheira, Alberto, Manuel Casal-Guisande, Julia López-Canay, Alberto Fernández-García, Rafael Golpe, Cristina Represas-Represas, María Torres-Durán, Jorge Cerqueiro-Pequeño, Alberto Comesaña-Campos, and Alberto Fernández-Villar. 2025. "Image-Based Formalization of Tabular Data for Threshold-Based Prediction of Hospital Stay Using Convolutional Neural Networks: An Intelligent Decision Support System Applied in COPD" Applied System Innovation 8, no. 5: 128. https://doi.org/10.3390/asi8050128
APA StylePinheira, A., Casal-Guisande, M., López-Canay, J., Fernández-García, A., Golpe, R., Represas-Represas, C., Torres-Durán, M., Cerqueiro-Pequeño, J., Comesaña-Campos, A., & Fernández-Villar, A. (2025). Image-Based Formalization of Tabular Data for Threshold-Based Prediction of Hospital Stay Using Convolutional Neural Networks: An Intelligent Decision Support System Applied in COPD. Applied System Innovation, 8(5), 128. https://doi.org/10.3390/asi8050128






