New Records of Panthera gombaszoegensis (Kretzoi, 1938) from Europe
Abstract
1. Introduction
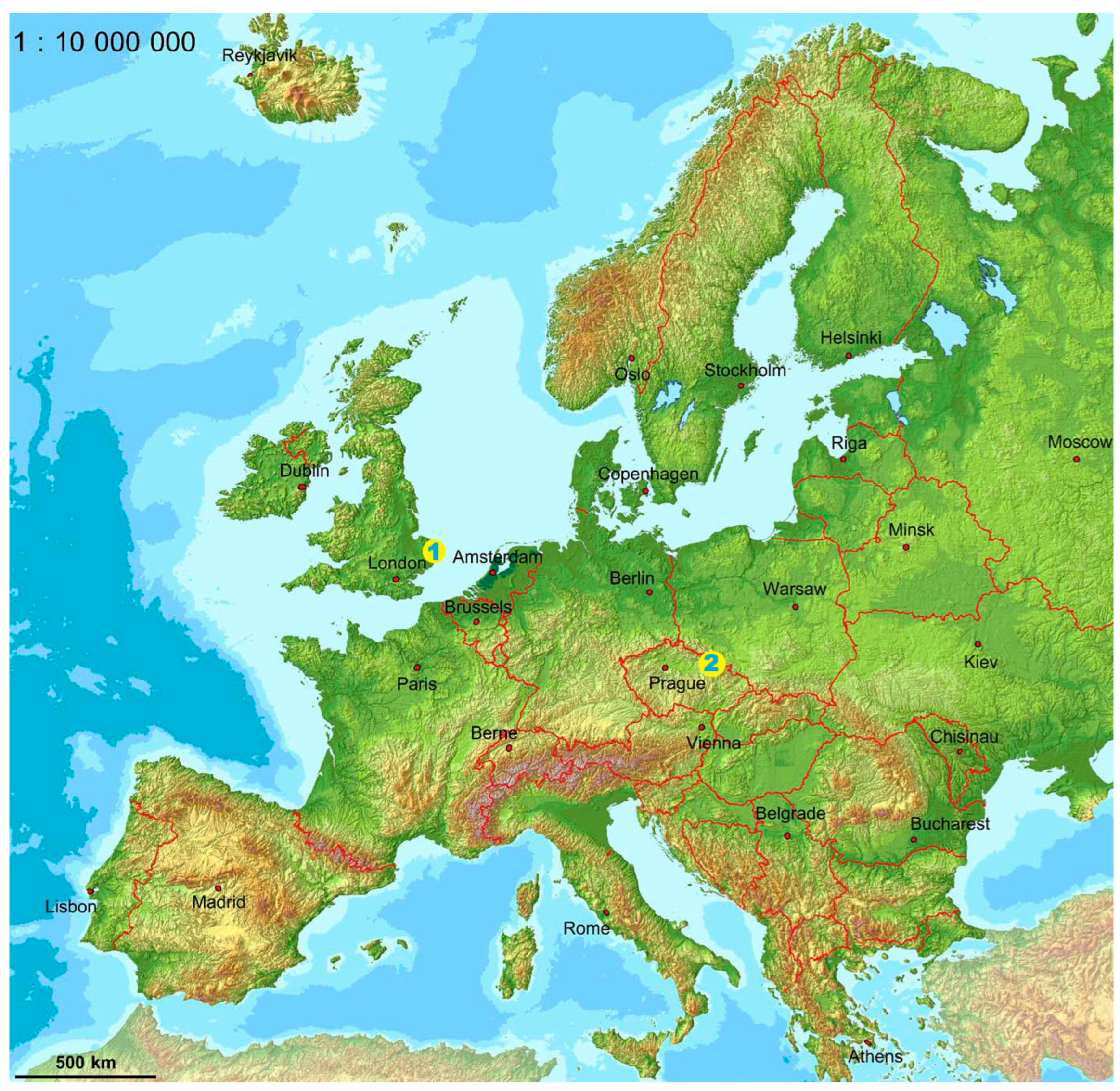
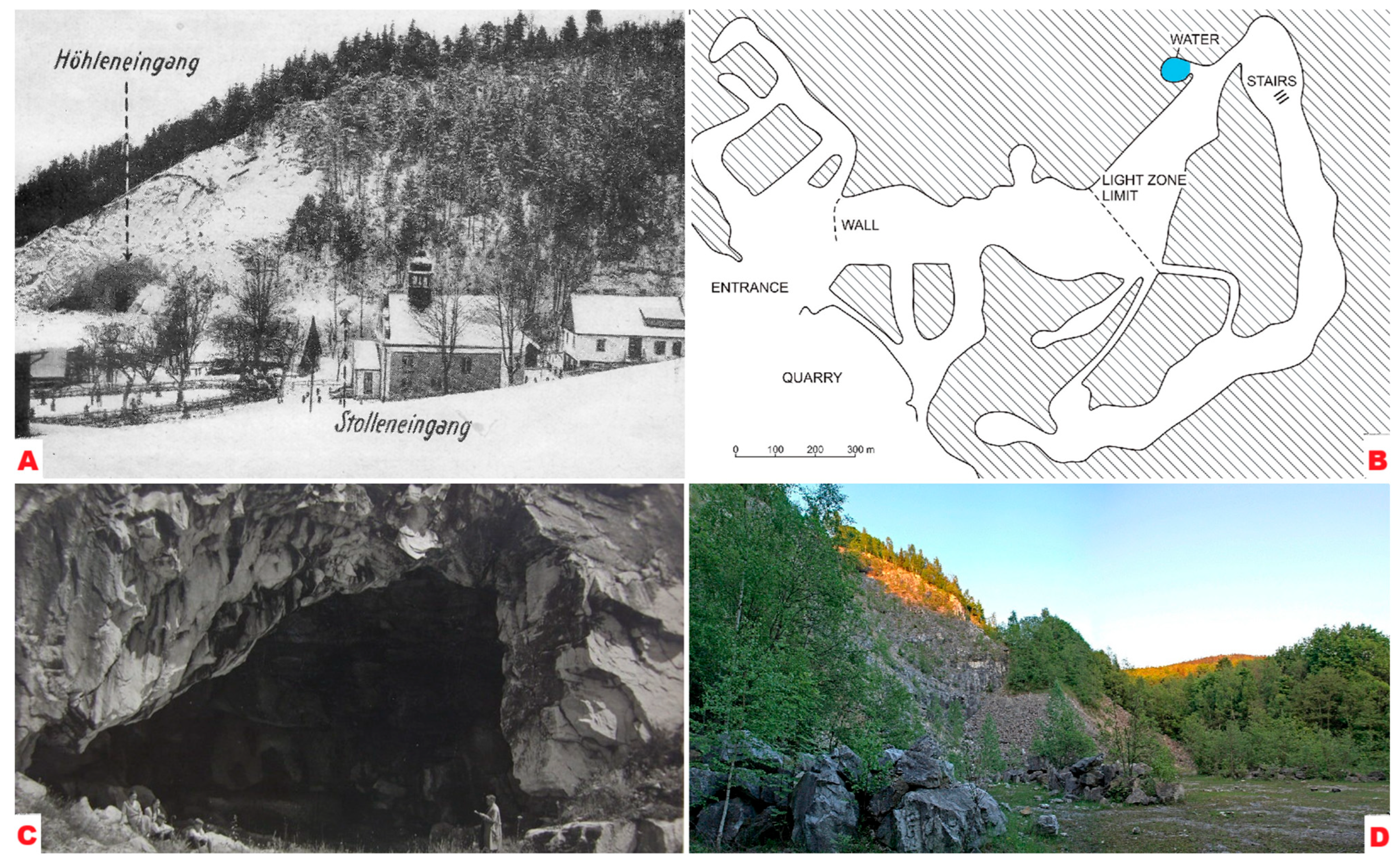
2. Geographical and Geological Setting
3. Materials and Methods
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hemmer, H. Pleistozäne Katzen Europas—eine Übersicht. Cranium 2003, 20, 6–22. [Google Scholar]
- Hemmer, H. Notes on the ecological role of European cats (Mammalia: Felidae) of the last two million years. In Zona Arqueológica 4. Miscelánea en Homenaje a Emiliano Aguirre, Vol. 2; Baquedano, E., Rubio Jara, S., Eds.; Museo Arqueológico Regional: Madrid, Spain, 2004; Volume 4, pp. 214–232. [Google Scholar]
- O’Regan, H.J.; Turner, A. Biostratigraphic and palaeoecological implications of new fossil material from the Plio-Pleistocene site of Tegelen, The Netherlands. Palaeontology 2004, 47, 1181–1193. [Google Scholar] [CrossRef]
- Madurell-Malapeira, J.; Barrasa-Morondo, I.; Bartolini-Lucenti, S.; Prat-Vericat, M.; Badiola, A.; Rodríguez-Hidalgo, A.; Gómez-Olivencia, A.; Rook, L. A review on Iberian and Italian occurrences of Quaternary lions. Earth Hist. Biodiv. 2025, 3, 100016. [Google Scholar] [CrossRef]
- Hemmer, H. Die Feliden aus dem Epivillafranchium von Untermaßfeld bei Meiningen (Thüringen). Monog. Röm.-German. Zentralmus. Mainz. 2001, 40, 699–782. [Google Scholar] [CrossRef]
- Argant, A.; Argant, J.; Jeannet, M.; Erbajeva, M. The big cats of the fossil site Château Breccia Northern Section (Saône-et-Loire, Burgundy, France): Stratigraphy, palaeoenvironment, ethology and biochronological dating, in: Kahlke, R.-D., Maul, L.C., Mazza, P.P.A., (Eds.), Late Neogene and Quaternary biodiversity and evolution: Regional developments and interregional correlations. Proceedings of the 18th International Senckenberg Conference (VI International Palaeontological Colloquium in Weimar). Volume II. Cour. Forsch. Senckenberg 2007, 259, 121–140. [Google Scholar]
- Argant, A.; Argant, J. The Panthera gombaszogensis story: The contribution of the Château Breccia (Saône-et-Loire, Burgundy, France). Quaternaire 2011, 4, 247–269. [Google Scholar]
- Marciszak, A.; Lipecki, G. Panthera gombaszoegensis (Kretzoi, 1938) from Poland in the scope of the species evolution. Quat. Int. 2022, 633, 36–51. [Google Scholar] [CrossRef]
- Hemmer, H.; Kahlke, R.-D.; Vekua, A.K. Panthera onca georgica ssp. nov. from the Early Pleistocene of Dmanisi (Republic of Georgia) and the phylogeography of jaguars (Mammalia, Carnivora, Felidae). Neues Jahrb. Geol. Paläontol. Abh. 2010, 257, 115–127. [Google Scholar] [CrossRef]
- Jiangzuo, Q.; Liu, J. First record of the Eurasian jaguar in southern Asia and a review of dental differences between pantherine cats. J. Quat. Sci. 2020, 35, 817–830. [Google Scholar] [CrossRef]
- Werdelin, L.; Peigné, S. Carnivora. In Cenozoic Mammals of Africa; Werdelin, L., Sanders, W.J., Eds.; University of California Press: Berkeley, CA, USA, 2010; pp. 609–663. [Google Scholar]
- Barry, J.C. Large carnivores (Canidae, Hyaenidae, Felidae) from Laetoli. In Laetoli: A Pliocene Site in Northern Tanzania; Leakey, M.D., Harris, J.M., Eds.; Clarendon Press: Oxford, UK, 1987; pp. 235–258. [Google Scholar]
- Turner, A. Late Neogene/Lower Pleistocene Felidae of Africa: Evolution and dispersal. Quartärpaläontologie 1990, 8, 247–256. [Google Scholar]
- Hemmer, H. The story of the cave lion—Panthera leo spelaea (Goldfuss, 1810)—A review. Quaternaire 2011, 4, 201–208. [Google Scholar]
- Hemmer, H. The identity of the “lion”, Panthera principialis sp. nov., from the Pliocene Tanzanian site of Laetoli and its significance for molecular dating the pantherine phylogeny, with remarks on Panthera shawi (Broom, 1948), and a revision of Puma incurve (Ewer, 1956), the Early Pleistocene Swartkrans “leopard” (Carnivora, Felidae). Paleobiodivers. Paleoenviron. 2023, 103, 465–487. [Google Scholar] [CrossRef]
- Madurell-Malapeira, J. A critical review of the Pliocene and Pleistocene European Felidae fossil record. Boll. Soc. Paleontol. Ital. 2025, 64, 133–163. [Google Scholar] [CrossRef]
- Davis, B.W.; Li, G.; Murphy, W.J. Supermatrix and species tree methods resolve phylogenetic relationships among the big cats, Panthera (Carnivora: Felidae). Mol. Phylogenetics Evol. 2010, 56, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Argant, A.; Argant, J.; Barriquand, J.; Barriquand, L.; Bonnefoy, M.; Guillot, L.; Jeannet, M. Pleistocene carnivores in the Mâconnais. Field trip of the 16th International Cave Bear and Lion Symposium (Azé, 2010). Quaternaire 2011, 4, 27–37. [Google Scholar]
- Argant, A.; Argant, J. La brèche à carnivores du Pléistocène moyen de Château (Saône-et-Loire, France). Quaternaire 2018, 29, 271–285. [Google Scholar] [CrossRef]
- Hemmer, H.; Schütt, G. Ein Unterkiefer von Panthera gombaszoegensis (Kretzoi, 1938) aus den Mosbacher Sanden. Mainz. Naturwiss. Archiv. 1969, 8, 90–101. [Google Scholar]
- Hemmer, H.; Schütt, G. Körpergröße und Extremitätenmasse latest-und altpleistozäner europäischer Pantherkatzen (Genus Panthera). Mainz. Naturwiss. Archiv. 1970, 9, 132–146. [Google Scholar]
- Hemmer, H. Zur Charakterisierung und stratigraphischen Bedeutung von Panthera gombaszoegensis (Kretzoi, 1938). Neues Jahrb. Geol. Paläontol. Monatsh. 1971, 12, 701–711. [Google Scholar]
- Marciszak, A. Presence of Panthera gombaszoegensis (Kretzoi, 1938) in the late Middle Pleistocene of Biśnik Cave, Poland, with an overview of Eurasian jaguar size variability. Quat. Int. 2014, 326–327, 104–113. [Google Scholar] [CrossRef]
- Chatar, N.; Michaud, M.; Fischer, V. Not a jaguar after all? Phylogenetic affinities and morphology of the Pleistocene felid Panthera gombaszoegensis. Pap. Palaeontol. 2022, 8, e1464. [Google Scholar] [CrossRef]
- Hemmer, H.; Kahlke, R.-D.; Keller, T. Panthera onca gombaszoegensis (Kretzoi, 1938) aus den frühmittelpleistozänen Mosbach-Sanden (Wiesbaden, Hessen, Deutschland)—Ein Beitrag zur Kenntnis der Variabilität und Verbreitungsgeschichte des Jaguars. Neues Jahrb. Geol. Paläontol. Abhan. 2003, 229, 31–60. [Google Scholar] [CrossRef]
- Hemmer, H.; Kahlke, R.-D.; Vekua, A.K. The jaguar—Panthera onca gombaszoegensis (Kretzoi, 1938) (Carnivora: Felidae) in the late Lower Pleistocene of Akhalkalaki (South Georgia; Transcaucasia) and its evolutionary and ecological significance. Geobios 2001, 34, 475–486. [Google Scholar] [CrossRef]
- Hankó, E.P. A revision of three Pleistocene subspecies of Panthera, based on mandible and teeth remains stored in Hungarian collections. Fragm. Palaeont. Hung. 2007, 30, 14–25. [Google Scholar]
- Hemmer, H.; Kahlke, R.-D. New results on felids from the Early Pleistocene site of Untermassfeld. Monog. Römich-German. Zentralm. 2022, 40, 1465–1566. [Google Scholar]
- Koufos, G.D. New material and revision of the Carnivora, Mammalia from the Lower Pleistocene locality Apollonia 1, Greece. Quaternary 2018, 1, 6. [Google Scholar] [CrossRef]
- Pax, F. Die Höhlenfauna des Glatzer Schneeberges. 10. Wandlungen des Tierlebens in der Wolmsdorfer Tropfsteinhöhle. Beit. Biol. Glatzer Schneeberges 1937, 3, 289–293. [Google Scholar]
- Pax, F.; Maschke, K. Höhlenfauna des Glatzer Schneeberges. Die rezente Metazoenfauna. Beit. Biol. Glatzer Schneeberges 1935, 1, 4–72. [Google Scholar]
- Bieroński, J.; Socha, P.; Stefaniak, K.; Hercman, H.; Gąsiorowski, M. Caves in Rogóżka—Origin, sediments and fauna. Stud. Fac. Earth Scien. Univ. Silesia. 2009, 56, 477–489. [Google Scholar]
- Demidziuk, K. Archiwalia sprzed 1845 roku po archeologii polskich Sudetów—Góry Kaczawskie w Sudetach Zachodnich. Silesia Antiq. 2012, 48, 275–342. [Google Scholar]
- Marciszak, A.; Sobczyk, A.; Kasprzak, M.; Gornig, W.; Ratajczak, U.; Wiśniewski, A.; Stefaniak, K. Taphonomic and paleoecological aspects of large mammals from Sudety Mts (Silesia, SW Poland), with particular interest to the carnivores. Quat. Int. 2020, 546, 42–63. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Penny, L.F.; Shotton, F.W.; West, R.G. A revisited correlation of Quaternary deposits in the British Isles. Geol. Soc. London Spec. Rep. 1999, 23, 1–174. [Google Scholar]
- Gibbard, P.L.; Van Der Vegt, P. The genesis and significance of the Middle Pleistocene glacial meltwater and associated deposits in East Anglia. In A Celebration of Suffolk Geology; GeoSuffolk 10th Anniversary Volume; Geoscience Suffolk: Ipswich, UK, 2012; pp. 303–326. [Google Scholar]
- Stuart, A.J.; Lister, A.M. The mammalian faunas of Pakefield/Kessingland and Corton, Suffolk, UK: Evidence for a new temperate episode in the British early Middle Pleistocene. Quat. Sci. Rev. 2001, 20, 1677–1692. [Google Scholar] [CrossRef]
- West, R.G. The Pre-Glacial Pleistocene of the Norfolk and Suffolk Coasts; Cambridge University Press: Cambridge, UK, 1980. [Google Scholar]
- Iannucci, A.; Mecozzi, B.; Pineda, A.; Sardella, R.; Carpentieri, M.; Rabinovich, R.; Moncel, M.H. Early occurrence of lion (Panthera spelaea) at the Middle Pleistocene Acheulean site of Notarchirico (MIS 16, Italy). J. Quat. Sci. 2024, 39, 683–690. [Google Scholar] [CrossRef]
- Schmid, E. Variationstatistische Untersuchungen am Gebiss pleistozäner und rezenter Leoparden und anderer Feliden. Zeit. Säuget. 1940, 15, 1–179. [Google Scholar]
- Argant, A. Carnivores (Canidae, Felidae et Ursidae) de Romain-la-Roche (Doubs, France). Rev. Paléob. 2010, 29, 495–601. [Google Scholar]
- International Committee on Veterinary Gross Anatomical Nomenclature (I.C.V.G.A.N.). Nomina Anatomica Veterinaria, 6th ed.; World Association of Veterinary Anatomists: Hanover, Germany; Ghent, Belgium; Colombia, MO, USA; Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Lisiecki, L.E.; Raymo, M.E. A Pliocene-Pleistocene stack of 57 globally distributed benthic d18O records. Paleoceanography 2005, 20, PA1003. [Google Scholar] [CrossRef]
- Ruiz-Ramoni, D.; Montellano-Ballesteros, M.; Arroyo-Cabrales, J.; Caso, A.; Carvajal-Villarreal, S. The large jaguar that lived in the past of México: A forgotten fossil. Therya 2020, 11, 33–40. [Google Scholar] [CrossRef]
- Seymour, K. Panthera onca. Mamm. Spec. 1989, 340, 1–9. [Google Scholar] [CrossRef]
- Sotnikova, M.V.; Foronova, I. First Asian record of Panthera (Leo) fossilis (Mammalia, Carnivora, Felidae) in the Early Pleistocene of Western Siberia, Russia. Integ. Zool. 2014, 9, 517–530. [Google Scholar] [CrossRef]
- Marciszak, A.; Gornig, W.; Matyaszczyk, L.; Demidziuk, K.; Kasprzak, M. Remains of Canidae and Felidae from Południowa Cave (Sudety Mts, Silesia, SW Poland). Geol. Quart. 2024, 68, 23. [Google Scholar]
- García, N.; Virgós, E. Evolution of community composition in several carnivore palaeoguilds from the European Pleistocene: The role of interspecific competition. Lethaia 2007, 40, 33–44. [Google Scholar] [CrossRef]
- Nowell, K.; Jackson, P. Wild Cats-Status Survey and Conservation Action Plan; IUCN: Gland, Switzerland, 1996.

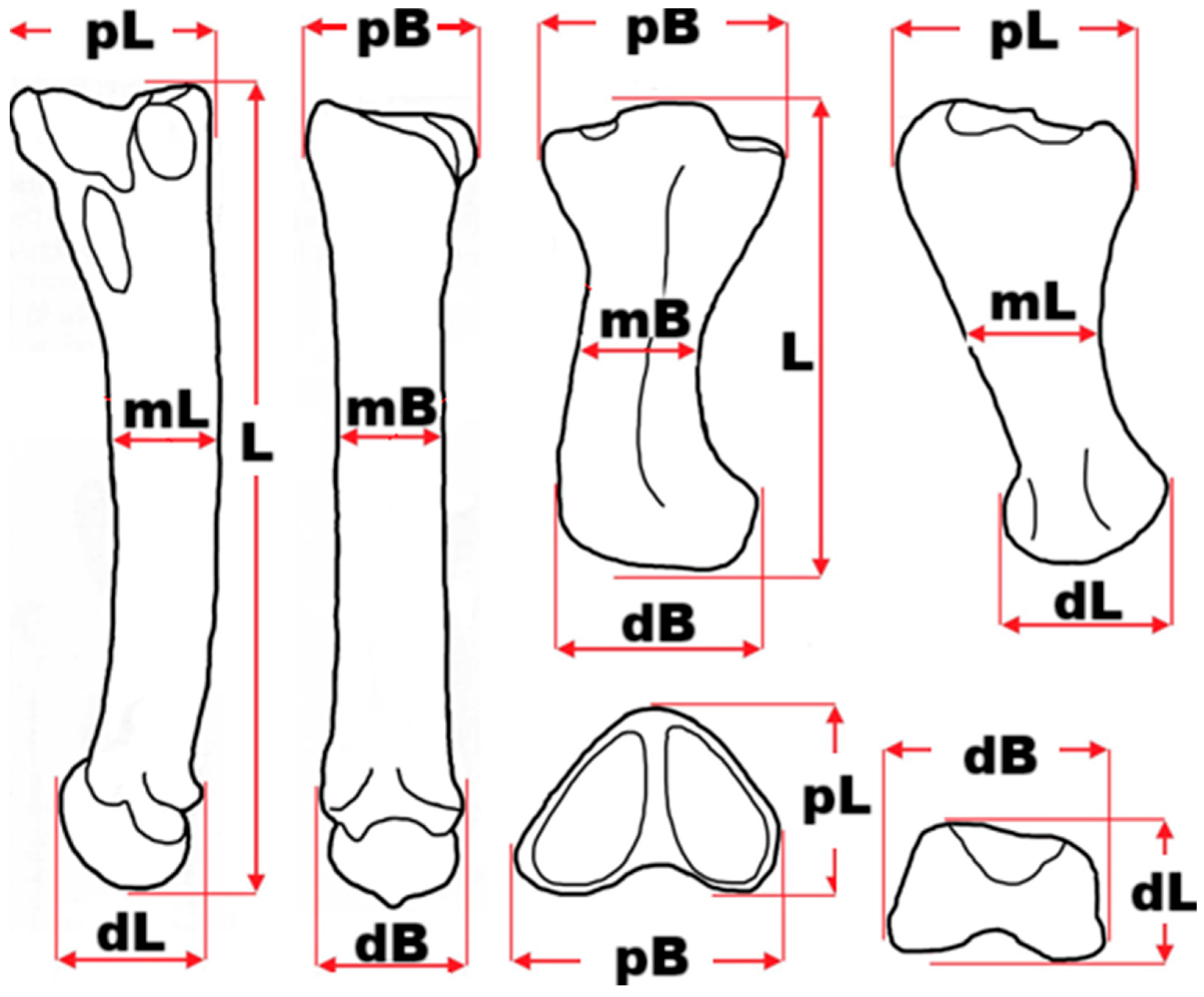

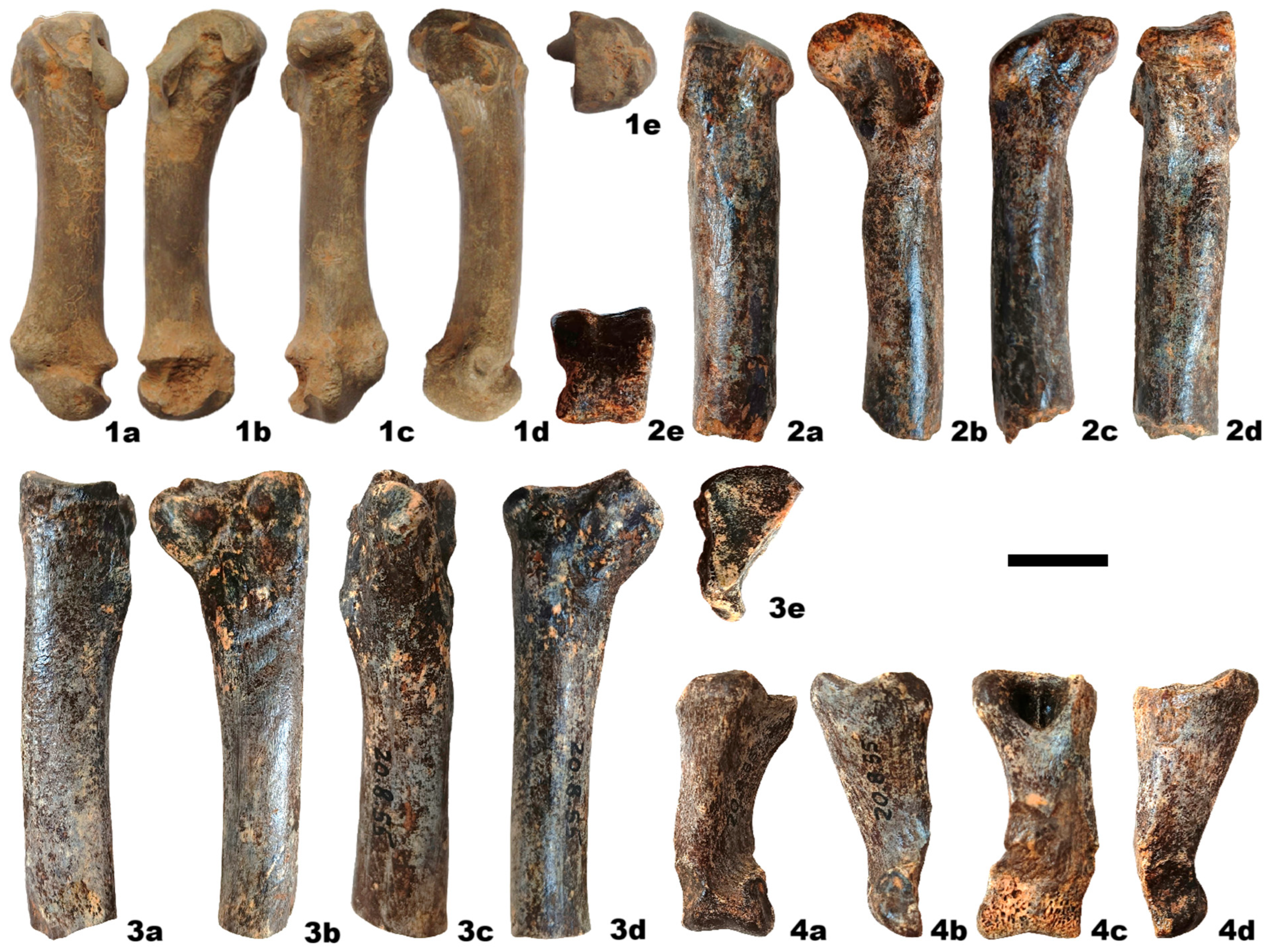
| Radius | mc 5 | mt 2 | mt 4 | ph II-3-p | |
|---|---|---|---|---|---|
| MS 22 | MS 14 | Rog. 21.1 | Rog. 21.2 | Rog. 21.3 | |
| L | + 200 | 69.55 | 41.46 | ||
| pL | 18.66 | 26.74 | 22.64 | 16.74 | |
| pB | 16.87 | 17.15 | 18.24 | 17.88 | |
| mL | 25.34 | 9.51 | 13.93 | 12.94 | 10.92 |
| mB | 19.38 | 11.69 | 14.97 | 14.15 | 11.97 |
| dL | 15.76 | ||||
| dB | 15.84 | 18.02 |
| Metacarpal 5 | ||||||
|---|---|---|---|---|---|---|
| Corton | P. s. fossilis | P. leo ♂ | P. leo ♀ | P. gombaszoegensis | P. pardus | |
| n = 1 | n = 17 | n = 23 | n = 21 | n = 13 | n = 22 | |
| L | 69.6 | 119.6 100.0–134.5 | 94.9 89.5–101.5 | 86.6 80.0–90.0 | 71.1 66.0–74.6 | 60.2 52.2–64.5 |
| mB | 11.7 | 21.4 16.0–28.1 | 14.0 12.0–16.0 | 11.6 10.5–13.5 | 11.9 11.0–12.6 | 9.5 7.4–11.4 |
| dB | 15.8 | 29.8 18.0–26.5 | 21.5 19.5–25.5 | 18.6 17.5–20.0 | 18.5 17.3–19.5 | 13.3 12.0–15.0 |
| Metatarsal 2 | ||||||
| Rogóżka | P. s. fossilis | P. leo ♂ | P. leo ♀ | P. gombaszoegensis | P. pardus | |
| n = 1 | n = 12 | n = 21 | n = 19 | n = 6 | n = 16 | |
| pB | 17.2 | 22.1 18.9–26.4 | 16.7 13.4–22.3 | 12.4 11.9–16.6 | 15.8 13.1–17.8 | 10.4 8.4–13.6 |
| mB | 15.0 | 26.7 18.1–32.9 | 16.6 13.8–22.1 | 12.9 11.2–15.9 | 13.9 11.4–15.9 | 9.8 7.7–12.1 |
| Metatarsal 4 | ||||||
| Rogóżka | P. s. fossilis | P. leo ♂ | P. leo ♀ | P. gombaszoegensis | P. pardus | |
| n = 1 | n = 23 | n = 23 | n = 22 | n = 7 | n = 17 | |
| pB | 18.2 | 31.2 23.3–44.9 | 23.3 21.1–27.8 | 19.1 14.9–22.2 | 18.4 15.9–23.7 | 15.3 13.6–17.8 |
| mB | 14.2 | 25.6 21.7–39.8 | 15.3 13.7–17.7 | 13.4 12.8–15.4 | 14.7 12.9–16.8 | 10.9 9.7–12.3 |
| Third medial phalanx of the pes (phalanx II-2-p) | ||||||
| Rogóżka | P. s. fossilis | P. leo ♂ | P. leo ♀ | P. gombaszoegensis | P. pardus | |
| n = 1 | n = 27 | n = 16 | n = 18 | n = 7 | n = 16 | |
| L | 41.5 | 53.9 44.6–62.7 | 45.7 39.8–50.7 | 38.4 33.9–44.2 | 37.8 34.1–47.8 | 29.4 25.4–34.2 |
| mB | 12.0 | 17.5 13.3–22.9 | 13.7 10.9–16.7 | 11.9 9.9–13.4 | 13.1 10.4–15.9 | 6.9 5.8–7.9 |
| dB | 18.0 | 23.8 19.7–27.4 | 18.6 15.6–21.4 | 15.7 13.2–18.7 | 16.9 13.4–20.1 | 10.6 8.7–11.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marciszak, A.; Bower, A. New Records of Panthera gombaszoegensis (Kretzoi, 1938) from Europe. Quaternary 2025, 8, 65. https://doi.org/10.3390/quat8040065
Marciszak A, Bower A. New Records of Panthera gombaszoegensis (Kretzoi, 1938) from Europe. Quaternary. 2025; 8(4):65. https://doi.org/10.3390/quat8040065
Chicago/Turabian StyleMarciszak, Adrian, and Alfie Bower. 2025. "New Records of Panthera gombaszoegensis (Kretzoi, 1938) from Europe" Quaternary 8, no. 4: 65. https://doi.org/10.3390/quat8040065
APA StyleMarciszak, A., & Bower, A. (2025). New Records of Panthera gombaszoegensis (Kretzoi, 1938) from Europe. Quaternary, 8(4), 65. https://doi.org/10.3390/quat8040065






