Abstract
Background: A number of assessment methods for the pelvic floor have been described. Male pelvic floor ultrasound is an accessible, noninvasive assessment tool. Objective: To evaluate current published literature on anatomical parameters on pre- and postoperative ultrasound imaging of the male pelvic floor and correlation with continence status following radical prostatectomy (RP). Methods: A comprehensive literature search was conducted using the PRISMA guidelines to identify publications up to November 2022. Exclusion criteria consisted of animal studies, non-English articles, case reports, reviews and abstracts or reports from conferences. A full-text review was performed on 12 papers using ultrasound to assess pelvic floor anatomy and correlation with continence status following RP. Results: A total of 18 anatomical parameters were evaluated using US. Membranous urethral length (MUL), striated urethral sphincter (SUS) morphology and activation were most commonly studied. Shorter pre- and postoperative MUL, decreased preoperative SUS thickness and vascularity, postoperative discontinuity of SUS muscle fibres and decreased SUS activation are associated with post-prostatectomy incontinence (PPI). There is a paucity of data comparing anatomical changes in men prior to and following RP. The benefits of transperineal ultrasound are that it is minimally invasive, accessible, provides dynamic imaging of all three striated muscle complexes simultaneously and includes a bony landmark to reference measures of pelvic floor muscle displacement. Conclusions: Ultrasound evaluation of the male pelvic floor is an evolving field as there is development in technology and understanding of pelvic floor anatomy. It is an accessible and dynamic imaging modality, which allows both morphological and functional assessment of pelvic floor anatomy and its role in PPI. MUL and SUS morphology and activation are associated with continence status following RP. Several other anatomical parameters that may predict PPI were identified. Current literature is limited by small, single-centre studies with heterogeneous cohorts and methodologies.
1. Introduction
Prostate cancer is the second-most commonly diagnosed cancer in men globally [1]. Clinically localised intermediate and high-risk prostate cancer is treated surgically by radical prostatectomy (RP), which involves the removal of the prostate and seminal vesicles [2]. Post-prostatectomy incontinence (PPI) is a common side effect affecting 6–52% of men one year following RP [3,4]. PPI has a major impact on patient quality of life [5,6].
Despite the significant burden of PPI, its aetiology is not fully understood. Patient-related factors, including age [7,8], body mass index [8], Charlson comorbidity index [7] and pre-existing lower urinary tract symptoms [8] are negatively correlated with PPI. Operative factors, including damage to neurovascular bundles and postoperative fibrosis, have a negative impact on continence status following RP [8]. Anatomical factors may also have predictive value for PPI, with studies largely focusing on MRI-based anatomical parameters [7,8,9,10,11,12]. A number of pelvic floor assessment methods have been described, including clinical observation, digital palpation, electromyography, dynamometry and MRI [13]. Their clinical use has been limited by accessibility, invasiveness and cost. Male pelvic floor ultrasound has gained popularity as an accessible, noninvasive assessment tool in the last two decades. As a dynamic imaging modality, ultrasound allows both morphological and functional assessment of pelvic floor anatomy. However, there is a paucity in the literature on ultrasound imaging in men undergoing RP, particularly ultrasound-based anatomical parameters and their relationship with PPI.
The aim of our study was to systematically summarise data for ultrasound-based anatomical parameters and their prognostic value for recovery of PPI. The prognostic value of anatomical parameters on ultrasound could ultimately be incorporated into risk stratification models for PPI to guide treatment decisions.
2. Methods
2.1. Search Strategy
A systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) Statement [14]. A comprehensive literature search of Medline, Embase, Scopus and PubMed databases was conducted to identify publications up to 30 November 2022 regarding ultrasound imaging to evaluate anatomical parameters and correlation with continence status in men following RP. The search strategy is summarised in Table 1. Keywords and medical subject heading (MeSH) terms were used including [prostatectomy OR radical prostatectomy OR open prostatectomy OR robotic prostatectomy] AND [urinary incontinence] AND [imaging OR ultrasound] AND [pelvic floor OR pelvic floor muscle OR muscle contraction OR urethra OR striated urethral sphincter OR rhabdosphincter OR bulbocavernosus OR puborectalis OR levator ani].

Table 1.
Summary of search strategy.
2.2. Study Selection
Two reviewers (CTP/JEC) independently screened all titles and abstracts to identify potentially relevant articles for eligibility. Full-text articles were retrieved where there was insufficient information in the title or abstract to determine eligibility. Inclusion and exclusion criteria are summarised in Table 2. The inclusion of papers was discussed and agreed upon by consensus.

Table 2.
Inclusion and exclusion criteria.
2.3. Data Extraction
Data extraction was performed by both reviewers (CTP/JEC). Data were extracted into a standardised data extraction form. Data regarding the patient characteristics, study design (surgical technique, ultrasound assessment timepoints, anatomical prognostic factor, continence definition) and study findings were extracted.
2.4. Quality Appraisal
Two reviewers (CTP/JEC) independently assessed the risk of bias of included studies using the Quality in Prognosis Studies (QUIPS) tool as recommended by the Cochrane Prognosis Methods Group [15]. Any disagreements were discussed and agreed upon by consensus.
3. Results
The search yielded 284 articles (Figure 1). Following the exclusion of duplicates using EndNoteTM 20.4 (ClarivateTM, London, UK), 225 articles remained. After the screening of titles and abstracts, 14 articles remained. A full-text review revealed 13 articles that presented relevant data [16,17,18,19,20,21,22,23,24,25,26,27,28]. Two of the remaining articles contained overlapping data (confirmed by the authors) leading to one additional exclusion [28]. Finally, 12 studies were included in the review.
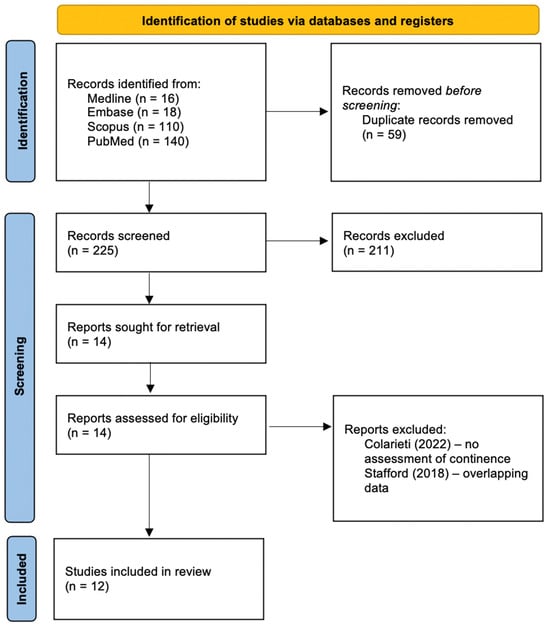
Figure 1.
Flow diagram for study inclusion from a systematic literature search.
3.1. Risk of Bias Assessment
The risk of bias assessment is summarised in Figure 2. The majority of the studies gave incomplete data on the characteristics of the study population [16,18,20,21,24,25,26,27] and did not provide data on the recruitment period [16,18,20,24,25,26,27], yielding potential bias. There was a significant risk of attrition bias in all of the studies, with inadequate reporting of response rate and no attempts to collect data on patients lost to follow-up or to consider the impact of attrition. Some studies did not provide a clear definition of the outcome measurements [20,26,27], had varying outcome assessment timepoints between patients [25] or selectively reported on continence status at a specific timepoint despite collecting continence data at multiple timepoints [22,23]. Most of the studies provided a sufficient description of the prognostic factor measurement, statistical analysis and results.
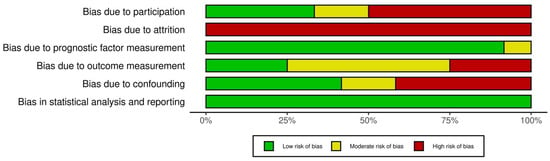
Figure 2.
Risk of bias assessment using the Quality in Prognosis Studies (QUIPS) tool.
3.2. Characteristics of the Included Studies
Patient and study characteristics are outlined in Table 3. The majority of the studies had a prospective design (n = 11). There was variation in surgical technique used, including open [16,17,22,24,26,27], laparoscopic [17,19,23] and robotic-assisted [21,24], both within and between studies. There were varying continence definitions used, including one study using 24 h pad weight of 0 g [16], four studies using 0–1 pads daily [17,19,22,23], and four studies using validated questionnaires such as International Consultation on Incontinence Questionnaire—Urinary Incontinence Short Form (ICIQ-SF) [18], Expanded Prostate Cancer Index Composite Short Form (EPIC-26) [21] and International Continence Society Male Short Form (ICSmale-SF) [24,25]. The pre- and postoperative anatomical features assessed for correlation with continence status are summarised in Table 4.

Table 3.
Summary of study characteristics.

Table 4.
Pre- and postoperative anatomical features assessed for correlation with continence.
3.3. Urethral Sphincter Parameters
Both morphology and function of the striated urethral sphincter (SUS) are important for continence recovery. PPI is associated with shorter pre- [23] and postoperative [19,22,23] membranous urethral length (MUL), decreased preoperative SUS thickness and vascularity [17] and postoperative discontinuity of SUS muscle fibres due to scar tissue or muscle atrophy [26,27]. Decreased displacement, and thereby activation, of SUS postoperatively is also associated with PPI [24,25,26,27].
Strasser et al. (1998) analysed transurethral ultrasound images with histological sections to validate the use of endoluminal transurethral ultrasound with catheter-based transducers [27]. They subsequently correlated transurethral ultrasound findings with continence status in eight incontinent men following RP (n = 5) and transurethral resection of the prostate (TURP) (n = 3) and 40 continent men following RP. Men with stress urinary incontinence (SUI) were found to have discontinuity of muscle fibres due to scar tissue or muscle atrophy. SUS function was measured by dorsal displacement towards the perineal body, and thereby the transducer, during muscle contraction. The mean SUS displacement was less for incontinent men and the degree of displacement correlated with the degree of SUI. Strasser et al. (2004) subsequently evaluated the feasibility of transrectal ultrasound in 37 incontinent men following RP (n = 35) and TURP (n = 2) and 40 continent men following RP [26]. Men with SUI were again found to have distinct morphological SUS defects, including discontinuity of muscle fibres by scar tissue or atrophy. Furthermore, incontinent men had less SUS displacement towards the perineal body during contraction and this correlated with the degree of SUI. Dell’Atti et al. (2015) correlated preoperative anatomy of the SUS on transrectal ultrasound and continence status in 23 incontinent men and 188 continent men following laparoscopic and open RP [17]. PPI was associated with decreased preoperative SUS thickness and decreased preoperative vascularity, including flow velocity, area of the vessels, resistance index and pulsatility index as measured using colour Doppler ultrasound. Amongst continent men, there was an inverse relationship between SUS thickness and time to reach stable continence. Stafford et al. (2019) demonstrated greater SUS displacement in continent men than incontinent men at a single postoperative timepoint using transperineal ultrasound [25]. There was no difference between continent men and controls without prostate cancer. Men with PPI had significantly less SUS displacement than controls, with a threshold displacement of SUS ≥ 4.1 mm discriminating between continent and incontinent men. Stafford et al. (2022) subsequently compared pre- and postoperative SUS displacement [24]. They demonstrated that continent men had greater SUS displacement at two weeks postoperatively.
Okihara et al. (2009) performed transrectal ultrasound immediately before and after open RP [22]. They compared pre- and postoperative MUL in 32 continent men and 38 men incontinent men one month following open RP. There was no difference in preoperative MUL, however, continent men had greater postoperative MUL than incontinent men. Men with postoperative MUL > 12 mm had greater recovery of continence. Mizutani et al. (2011) similarly performed transrectal ultrasound immediately before and after laparoscopic RP in 53 men and also found that greater MUL correlated with continence at 1, 3 and 6 months postoperatively [19]. Piotr et al. (2021) compared pre- and postoperative MUL on transperineal ultrasound and continence status in 84 men following laparoscopic RP [23]. They showed that both pre- and postoperative MUL was significantly longer in men who were continent 1 and 12 months postoperatively. A greater percentage change in MUL is also associated with early continence recovery. Meanwhile, Stafford et al. (2019) did not demonstrate a significant difference in MUL between continent and incontinent men [25].
3.4. Pelvic Floor Muscle Parameters
PFM function is important for continence recovery. PPI is associated with decreased displacement resulting from reduced activation of the bulbocavernosus (BC), puborectalis (PR) and levator ani (LA) postoperatively.
Stafford et al. (2019) demonstrated greater BC and PR displacement in continent men than incontinent men at a single postoperative timepoint [25]. There was no difference between continent men and controls without prostate cancer. Men with PPI had significantly less PR displacement than controls but not BC displacement. They proposed that a threshold displacement of PR ≥ 2.4 mm can discriminate between continent and incontinent men. Stafford et al. (2022) subsequently compared pre- and postoperative BC and PR displacement [24]. There was less PR displacement postoperatively for both continent and incontinent men but no difference in BC displacement.
Bladder displacement can be used as a marker for levator plate movement. Levator plate movement by the LA moves the bladder neck anteriorly while elevating the levator plate, thereby closing the urethra [29]. Nahon et al. (2011) assessed the male pelvic floor using transabdominal ultrasound in 10 incontinent men and 18 continent men with prostate cancer who were under active surveillance and underwent RP or radiation therapy [20]. Incontinent men had less bladder neck displacement in a cephalad direction than continent men. Nahon et al. (2011) were the first to demonstrate that reduced LA contraction may correlate with PPI [20]. Furthermore, Costa Cruz et al. (2014) compared dynamic transperineal ultrasound evaluation of pelvic floor anatomy at rest, during contraction and during Valsalva manoeuvre in 27 men before RP, 31 men with PPI and 34 men without PPI ≥ 1 year following RP [16]. Similarly, they showed that men with PPI, particularly severe PPI (24 h pad weight > 400 g), had less anterior bladder neck displacement during pelvic floor contraction than continent men. Meanwhile, Neumann et al. (2018) did not find an association between preoperative LA activation (bladder neck displacement) and postoperative continence status [21].
3.5. Bladder Neck and Proximal Urethra Parameters
PPI is associated with funnelling of the bladder neck and proximal urethra hypermobility.
Kirschner-Hermanns et al. (2011) used transperineal ultrasound to evaluate a cohort of 21 incontinent men and 12 continent men ≥ 1 year following RP [18]. They showed that bladder neck funnelling (opening) during cough and Valsalva was seen more often in men with PPI. However, Mizutani et al. (2011) did not find any association between continence status and bladder-urethra angle between the anterior bladder wall and membranous urethra [19].
Furthermore, Kirschner-Hermanns et al. (2011) found that proximal urethral hypermobility (vertical movement >1.5 cm during Valsalva) was only seen in men with PPI [18].
Costa Cruz et al. (2014) measured the urethral angle between the penile and bulbar urethra and found that there was no difference in urethral angle between continent and incontinent men during pelvic floor contraction or Valsalva manoeuvre [16].
4. Discussion
Male pelvic floor ultrasound is an evolving field as there is development in technology and understanding of pelvic floor anatomy. There is a paucity of literature on ultrasound evaluation of men with PPI, with a previous systematic review of three transperineal ultrasound studies [11]. We present a more exhaustive review of studies using various ultrasound approaches to evaluate men with PPI. To date, most studies imaging the male pelvic floor have utilised MRI [7,8,9,10,11,12]. Ultrasound may be an accessible substitute for pelvic floor imaging following RP. This systematic review demonstrates the potential predictive value for earlier return to continence of several anatomical parameters measured on pelvic floor ultrasound. In agreement with MRI studies, pelvic floor ultrasound demonstrates a relationship between MUL and continence following RP [19,22,23]. The benefit of ultrasound over MRI is that it is a dynamic imaging modality that can be used to study PFM function. The role of PFMs in continence recovery following RP has been demonstrated in several ultrasound studies. The majority of studies focus on SUS morphology [17,26] and activation [24,25,27]. Continence has also been shown to correlate with activation of other PFM, including BC [24,25], PR [25] and LA [16,20]. Given ultrasound is an accessible, noninvasive and affordable imaging modality, urologists and pelvic floor physiotherapists can use ultrasound for serial clinical assessment of PFM function prior to and following RP. Milios et al. (2019) have shown that PFM training can improve the speed and duration of PFM contractions following surgery [30]. Ultrasound may help to identify risk factors for PPI that may be modifiable with PFM training and develop individualised PFM training programs to target specific PFMs.
There is a lack of data comparing anatomical changes in men prior to and following RP. Most studies assessed anatomical differences between continent and incontinent men at a single timepoint postoperatively [16,18,20,25,26,27]. However, they were limited by a lack of baseline preoperative ultrasound precluding comparison of pre- and postoperative PFM morphology and function. Meanwhile, Dell’Atti et al. (2015) and Neumann et al. (2018) compared preoperative anatomical features between continent and incontinent men [17,21]. Whilst preoperative assessment is important to determine baseline PFM function, the lack of postoperative ultrasound data makes it difficult to draw a direct correlation with PPI in these studies. Future studies with longitudinal pre- and postoperative assessment of PFM are needed to better understand the pattern of perioperative PFM function and to determine which pelvic floor anatomical features are important in continence recovery following RP.
A number of studies have heterogeneous cohorts, which preclude correlation with continence status. Strasser et al. (1998, 2004) reported on a mixed cohort of incontinent men following RP and TURP [26,27]. A TURP does not breach the prostatic capsule and is significantly less disruptive to pelvic floor anatomy than an open RP. Hence, these cohorts of patients are not comparable. Furthermore, Nahon et al. (2011) presented a heterogeneous cohort of patients with prostate cancer receiving different treatments, including open or laparoscopic prostatectomy, radiation or active surveillance [20]. These treatment modalities have differing effects on pelvic floor anatomy and are not comparable. All (bar one [23]) of the aforementioned studies have not specified the number or expertise of surgeons or surgical approach. There is emerging evidence that greater surgeon experience with an annual surgical caseload of greater than 50 cases results in improved PPI recovery time [31]. Furthermore, the increasing use of robotic surgery and improvements in surgical technique have accelerated continence recovery following RP [32]. Hence, confounding variables of surgical approach and expertise must be considered when conducting a comparative analysis of factors influencing PPI rates, and efforts should be made to decrease the heterogeneity of the study cohort.
There is no universally accepted definition of continence, hence there is variability in PPI assessment methods and timepoints across the aforementioned studies. Strasser et al. (1998, 2004) and Nahon et al. (2011) did not report their definition of continence [20,26,27]. Some studies have used objective definitions of continence, such as daily pad number [17,19,22,23] or 24 h pad weight [16], whereas some have used subjective patient-reported outcome measures (PROM) such as the ICIQ-SF [18], EPIC-26 [21] or ICSmale-SF [24,25] questionnaires. Pad number is commonly used in clinical practice, but some studies have suggested that pad number may be limited by patient recall and has a poor correlation with incontinence severity [33,34]. The 24 h pad weight test is considered the gold standard for objective measurement of urinary incontinence [35,36]. However, it can be burdensome and result in poor compliance. This has led to the development of accessible and validated PROMs for PPI assessment. There are limited data on whether PROMs for PPI assessment correlate with objective measures, and future studies should be developed to evaluate the correlation between 24 h pad weight, daily pad number and PROMs.
We reviewed studies using various ultrasound approaches. Transurethral, transrectal and transabdominal ultrasound do not have a static anatomical landmark from which to measure muscle displacement. Early studies attempted to objectively define PFM function by determining muscle displacement during voluntary contraction, using the ultrasound transducer probe as the point of reference [20,21,26,27]. However, the probe is not truly a static point. It is particularly difficult to calculate the movement of the transducer probe in transabdominal ultrasound, as there is movement of the abdominal wall during pelvic floor contraction and thereby movement of the transducer probe away from the target tissue. Whilst transurethral and transrectal ultrasound has been shown to be highly specific for the SUS [26], they are invasive approaches, with intraurethral or rectal placement of a stiff probe likely to mechanically affect PFM contraction [37]. Although transabdominal ultrasound is a noninvasive approach, it only allows visualisation of bladder base elevation and not the striated PFM complex. It may be technically difficult to visualise the bladder using transabdominal ultrasound in patients with large body habitus, scar tissue from lower midline incision for open RP and inability to retain an adequate amount of urine in men with severe PPI. The benefits of transperineal ultrasound are that it is minimally invasive, accessible, provides dynamic imaging of all three striated muscle complexes simultaneously and includes a bony landmark to reference measures of PFM movement [24,25]. Transperineal ultrasound has become an increasingly popular and reliable imaging modality for the male pelvic floor. To our knowledge, only six studies of men following RP have been performed using transperineal ultrasound [16,18,21,23,24,25]. Kirschner-Hermanns et al. (2011) used a grading system to quantify voluntary pelvic floor contractions as “excellent”, “good” or “hardly any or none,” which provided visual biofeedback to teach correct PFM contraction [18]. However, they did not objectively measure muscle contraction. Furthermore, one patient had undergone transobturator tape for PPI and should have been excluded to achieve a homogenous cohort. Stafford et al. (2019, 2022) showed that PFM function, particularly SUS activation, was related to continence recovery following RP [24,25].
Our review has one main strength, in that it is the first systematic review purely focusing on the correlation of ultrasound measurements of pelvic floor anatomical parameters with continence following RP. Our review has several limitations. There was heterogeneity between the studies. Interstudy comparisons are limited by differences in ultrasound approach and techniques, operative characteristics, small sample sizes and a lack of consensus on PPI definition and the timeframe for PPI assessment.
5. Conclusions
Ultrasound evaluation of the male pelvic floor is an evolving field as there is development in technology and understanding of pelvic floor anatomy. It is an accessible and dynamic imaging modality that allows both morphological and functional assessment of pelvic floor anatomy and its role in PPI. Ultrasound measurements of MUL and SUS morphology and activation are associated with earlier return to continence following RP. Several other anatomical parameters that may predict PPI were identified. Current literature is limited by small, single-centre studies with heterogeneous cohorts and methodologies.
Author Contributions
(I) Conception and design: C.T.P. and M.I.P. (II) Administrative support: C.T.P. (III) Provision of study materials: C.T.P. (IV) Collection and assembly of data: C.T.P. and J.E.C. (V) Data analysis and interpretation: C.T.P. and J.E.C. (VI) Manuscript writing: C.T.P., J.E.C. and M.I.P. (VII) Final approval of manuscript: C.T.P., J.E.C. and M.I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. Eau-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Neal, D.E.; Metcalfe, C.; Donovan, J.L.; Lane, J.A.; Davis, M.; Young, G.J.; Dutton, S.J.; Walsh, E.I.; Martin, R.M.; Peters, T.J.; et al. Ten-year Mortality, Disease Progression, and Treatment-related Side Effects in Men with Localised Prostate Cancer from the ProtecT Randomised Controlled Trial According to Treatment Received. Eur. Urol. 2020, 77, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Novara, G.; Rosen, R.C.; Artibani, W.; Carroll, P.R.; Costello, A.; Menon, M.; Montorsi, F.; Patel, V.R.; Stolzenburg, J.-U.; et al. Systematic Review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur. Urol. 2012, 62, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Punnen, S.; Cowan, J.E.; Chan, J.M.; Carroll, P.R.; Cooperberg, M.R. Long-term health-related quality of life after primary treatment for localized prostate cancer: Results from the CAPSURE Registry. Eur. Urol. 2015, 68, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Sanda, M.G.; Dunn, R.L.; Michalski, J.; Sandler, H.M.; Northouse, L.; Hembroff, L.; Lin, X.; Greenfield, T.K.; Litwin, M.S.; Saigal, C.S.; et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N. Engl. J. Med. 2008, 358, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Lardas, M.; Grivas, N.; Debray, T.P.; Zattoni, F.; Berridge, C.; Cumberbatch, M.; Broeck, T.V.D.; Briers, E.; De Santis, M.; Farolfi, A.; et al. Patient- and tumour-related prognostic factors for urinary incontinence after radical prostatectomy for nonmetastatic prostate cancer: A systematic review and meta-analysis. Eur. Urol. Focus 2022, 8, 674–689. [Google Scholar] [CrossRef]
- Heesakkers, J.; Farag, F.; Bauer, R.M.; Sandhu, J.; De Ridder, D.; Stenzl, A. Pathophysiology and contributing factors in postprostatectomy incontinence: A Review. Eur. Urol. 2017, 71, 936–944. [Google Scholar] [CrossRef]
- van Dijk-de Haan, M.C.; Boellaard, T.N.; Tissier, R.; Heijmink, S.W.; van Leeuwen, P.J.; van der Poel, H.G.; Schoots, I.G. Value of different magnetic resonance imaging-based measurements of anatomical structures on preoperative prostate imaging in predicting urinary continence after radical prostatectomy in men with prostate cancer: A systematic review and meta-analysis. Eur. Urol. Focus 2022, 8, 1211–1225. [Google Scholar] [CrossRef]
- Muñoz-Calahorro, C.; García-Sánchez, C.; Barrero-Candau, R.; García-Ramos, J.B.; Rodríguez-Pérez, A.J.; Medina-López, R.A. Anatomical predictors of long-term urinary incontinence after robot-assisted laparoscopic prostatectomy: A systematic review. Neurourol. Urodyn. 2021, 40, 1089–1097. [Google Scholar] [CrossRef]
- Colarieti, A.; Thiruchelvam, N.; Barrett, T. Evaluation of image-based prognostic parameters of post-prostatectomy urinary incontinence: A literature review. Int. J. Urol. 2021, 28, 890–897. [Google Scholar] [CrossRef]
- Dubbelman, Y.D.; Bosch, J.L.H.R. Urethral sphincter function before and after radical prostatectomy: Systematic review of the prognostic value of various assessment techniques. Neurourol. Urodyn. 2013, 32, 957–963. [Google Scholar] [CrossRef]
- Bø, K.; Sherburn, M. Evaluation of female pelvic-floor muscle function and strength. Phys. Ther. 2005, 85, 269–282. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280. [Google Scholar] [CrossRef] [PubMed]
- Costa Cruz, D.; D’Ancona, C.; Baracat, J.; Alves, M.; Cartapatti, M.; Damião, R. Parameters of two-dimensional perineal ultrasonography for evaluation of urinary incontinence after Radical Prostatectomy. Int. Braz. J. Urol. 2014, 40, 596–604. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dell’Atti, L. Ultrasound evaluation of the striated urethral sphincter as a predictive parameter of urinary continence after radical prostatectomy. Arch. Ital. Urol. Androl. 2015, 87, 317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kirschner-Hermanns, R.; Najjari, L.; Brehmer, B.; Blum, R.; Zeuch, V.; Maass, N.; Heidenreich, A. Two- and three-/four dimensional perineal ultrasonography in men with urinary incontinence after radical prostatectomy. BJU Int. 2011, 109, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, Y.; Uehara, H.; Fujisue, Y.; Takagi, S.; Nishida, T.; Inamoto, T.; Ubai, T.; Nomi, H.; Katsuoka, Y.; Azuma, H. Urinary continence following laparoscopic radical prostatectomy: Association with postoperative membranous urethral length measured using real-time intraoperative transrectal ultrasonography. Oncol. Lett. 2011, 3, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Nahon, I.; Waddington, G.; Adams, R.; Dorey, G. Assessing muscle function of the male pelvic floor using real time ultrasound. Neurourol. Urodyn. 2011, 30, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; O’Callaghan, M. The Role of Preoperative Puborectal Muscle Function Assessed by Transperineal Ultrasound in Urinary Continence Outcomes at 3, 6, and 12 Months After Robotic-Assisted Radical Prostatectomy. Int. Neurourol. J. 2018, 22, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Okihara, K.; Kamoi, K.; Kanazawa, M.; Yamada, T.; Ukimura, O.; Kawauchi, A.; Miki, T. Transrectal ultrasound navigation during minilaparotomy retropubic radical prostatectomy: Impact on positive margin rates and prediction of earlier return to urinary continence. Int. J. Urol. 2009, 16, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Piotr, K.; Rafał, M.; Marcin, K.; Karol, D.; Monika, K.; Jakub, B.; Bartosz, Z.; Tadeusz, D.; Maciej, S. Transperineal ultrasound as a reliable tool in the assessment of membranous urethra length in radical prostatectomy patients. Sci. Rep. 2021, 11, 1759. [Google Scholar] [CrossRef] [PubMed]
- Stafford, R.E.; Doorbar-Baptist, S.; Hodges, P.W. The relationship between pre- and postprostatectomy measures of pelvic floor muscle function and development of early incontinence after surgery. Neurourol. Urodyn. 2022, 41, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Stafford, R.E.; Coughlin, G.; Hodges, P.W. Comparison of dynamic features of pelvic floor muscle contraction between men with and without incontinence after prostatectomy and men with no history of prostate cancer. Neurourol. Urodyn. 2019, 39, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Strasser, H.; Pinggera, G.M.; Gozzi, C.; Horninger, W.; Mitterberger, M.; Frauscher, F.; Bartsch, G. Three-dimensional transrectal ultrasound of the male urethral rhabdosphincter. World J. Urol. 2004, 22, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Strasser, H.; Frauscher, F.; Helweg, G.; Colleselli, K.; Reissigl, A.; Bartsch, G. Transurethral ultrasound: Evaluation of anatomy and function of the rhabdosphincter of the male urethra. J. Urol. 1998, 159, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Stafford, R.; van den Hoorn, W.; Coughlin, G.; Hodges, P. Postprostatectomy incontinence is related to pelvic floor displacements observed with trans-perineal ultrasound imaging. Neurourol. Urodyn. 2017, 37, 658–665. [Google Scholar] [CrossRef]
- Thompson, J.A.; O’Sullivan, P.B. Levator plate movement during voluntary pelvic floor muscle contraction in subjects with incontinence and prolapse: A crosssectional study and review. Int. Urogynecol. J. 2003, 14, 84–88. [Google Scholar] [CrossRef]
- Milios, J.E.; Ackland, T.R.; Green, D.J. Pelvic floor muscle training in radical prostatectomy: A randomized controlled trial of the impacts on pelvic floor muscle function and urinary incontinence. BMC Urol. 2019, 19, 116. [Google Scholar] [CrossRef]
- Trieu, D.; Ju, I.; Chang, S.; Mungovan, S.; Patel, M. Surgeon case volume and continence recovery following radical prostatectomy: A systematic review. ANZ J. Surg. 2020, 91, 521–529. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Jia, Z.; Wang, Y.; Song, Y.; Liao, L.; Zhang, X. Urinary continence outcomes of four years of follow-up and predictors of early and late urinary continence in patients undergoing robot-assisted radical prostatectomy. BMC Urol. 2020, 20, 29. [Google Scholar] [CrossRef]
- Dylewski, D.; Jamison, M.; Borawski, K.; Sherman, N.; Amundsen, C.; Webster, G. A statistical comparison of pad numbers versus pad weights in the quantification of urinary incontinence. Neurourol. Urodyn. 2006, 26, 3–7. [Google Scholar] [CrossRef]
- Tsui, J.F.; Shah, M.B.; Weinberger, J.M.; Ghanaat, M.; Weiss, J.P.; Purohit, R.S.; Blaivas, J.G. Pad Count is a Poor Measure of the Severity of Urinary Incontinence. J. Urol. 2013, 190, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Thind, P.; Gerstenberg, T.C. One-hour Ward Test vs. 24-hour home pad weighing test in the diagnosis of urinary incontinence. Neurourol. Urodyn. 1991, 10, 241–245. [Google Scholar] [CrossRef]
- Rasmussen, A.; Mouritsen, L.; Dalgaard, A.; Frimodt-Møller, C. Twenty-four hour pad weighing test: Reproducibility and dependency of activity level and fluid intake. Neurourol. Urodyn. 1994, 13, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Stafford, R.; Ashton-Miller, J.; Constantinou, C.; Hodges, P. Novel Insight into the Dynamics of Male Pelvic Floor Contractions Through Transperineal Ultrasound Imaging. J. Urol. 2012, 188, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).