Abstract
Coal combustion generates significant volumes of ash, a technogenic by-product that poses a serious threat to regional environmental sustainability (environmental chemical contamination and air pollution). This study aims to assess the feasibility of utilizing this type of ash as a raw material component in the fabrication of refractory bricks and to investigate the fundamental properties of the resulting experimental products. Ash was incorporated into the batch composition at concentrations ranging from 10% to 40% by weight, blended with clay and water, then shaped through pressing and subjected to firing at 1000 °C and 1100 °C in an air atmosphere for 2 h. After complete cooling, the samples were subjected to compressive strength testing. Samples containing 40 wt% coal ash exhibited insufficient compressive strength and were therefore excluded from subsequent investigations. For the remaining samples, apparent density, open porosity and slag resistance were determined. The microstructural characterization was performed, and the phase composition of the samples was analyzed. The results revealed that the phase composition of the experimental samples differs significantly from that of the reference sample (ShA-grade chamotte brick in accordance with GOST 390-96, currently used as lining in metallurgical furnaces across the country), exhibiting a higher mullite content and the absence of muscovite. A small amount of kaolinite was detected in the experimental samples even after a 2-h firing process. This observation may be attributed to the effect of kaolinite crystallinity on the transformation process from kaolinite to metakaolinite. The mechanical strength of the experimental samples meets the relevant standards, while slag resistance demonstrated an improvement of approximately 15%. Open porosity was found to decrease in the experimental samples. In addition, a change in the pore size distribution was observed. Notably, the proportion of pores larger than 10,000 nm was significantly reduced. These findings confirm the feasibility of incorporating coal ash as a viable raw material component in the formulation of refractory materials.
1. Introduction
One of the major challenges in the operation of metallurgical furnaces is the durability of the refractory lining, which, in most cases, consists of chamotte bricks. The service life of the lining is primarily determined by its mechanical strength and its resistance to thermal and slag erosion [1]. The lining is continuously subjected to substantial stress from molten metal pressure and temperature fluctuations. Slag infiltrates the open pores of the refractory material, causing degradation due to the chemical interaction between the slag components and the refractory. Therefore, studies focused on enhancing the service life of refractory materials are of significant practical importance.
In recent years, a considerable body of research has emerged focused on the development of refractory materials incorporating various industrial by-products as raw material components. The impact of different waste materials, such as rice husk ash, blast furnace slag, volcanic ash, and others, on the properties of the resulting refractory materials has been extensively investigated.
It is also important to note that a wide range of technologies for the utilization of anthropogenic waste has been developed. For instance, the study presented in [2] demonstrated that the use of coal-generated fly ash (CGFA) for road base stabilization is not only effective but also environmentally sustainable and economically viable. These advantages are particularly evident under conditions of alkaline activation. Furthermore, the findings in [3] revealed that ash derived from Integrated Gasification Combined Cycle (IGCC) power plants, when incorporated into medium plasticity clay, enhances the performance of fired ceramic materials. However, it does not effectively mitigate shrinkage when high-plasticity clay is used.
In a broader context, various types of industrial by-products, such as ash, aluminum slags, and other secondary materials, are under active investigation as functional additives in ceramic and composite systems. These materials have been shown to improve the physicochemical properties of the final products.
Upon heating a mixture of kaolin and aluminum slag, mullitization occurs, with the extent of mullitization increasing with firing temperature. This process leads to the formation of elongated primary mullite crystals from metakaolin and needle-like secondary mullite grains originating from the glassy phase [4]. The addition of magnesium oxide (MgO) to cordierite, derived from rice husk silica and sintered at 1230 °C, results in the formation of forsterite and spinel. This, in turn, enhances the material’s density, hardness, compressive strength and thermal expansion coefficient, while simultaneously reducing its porosity [5]. The addition of magnesium oxide (MgO) to coal fly ash during sintering at 1600 °C promotes an increase in the content of mullite and corundum, while simultaneously reducing the amount of cristobalite [6]. The use of volcanic ash forms aluminosilicate geopolymers with compressive strengths up to 55 MPa. These geopolymers retain approximately 60% of their strength when heated to 900 °C, making them suitable for both construction and refractory applications [7].
The study [8] investigated the effect of fly ash content, an aluminosilicate by-product of coal combustion, on the properties of refractory fibers. The results demonstrated that the obtained product exhibited properties comparable to those of materials derived from high-quality raw materials. However, it was found to have a significantly lower production cost. This study highlights the potential of using fly ash as a raw material in the manufacturing of refractory materials.
The study [9] researched the feasibility of using brown coal ash in production of refractory chamotte bricks. The results indicated that brown coal ash can be utilized as a potential raw material for production of chamotte refractory bricks that meet ASTM standards.
The study [10] focuses on improving the refractory properties of aluminosilicate clay by blending it with coal ash for production of refractory bricks. The coal ash content in the batch varied from 5 to 25 wt%. Refractory properties, such as linear shrinkage, apparent porosity, bulk density, cold crushing strength and thermal shock resistance, were tested. The results were compared with the standard refractory properties of chamotte bricks. All the parameters obtained for the mixtures fall within the recommended values for medium-sized chamotte bricks.
The study [11] explored the possibility of using fly ash, generated during the combustion of coal and petroleum coke, in production of environmentally friendly bricks. The effect of varying fly ash content (ranging from 0 to 80% clay replacement) and firing temperature (800–1000 °C) on the properties of the bricks was investigated. The results indicated that bricks with a high content of fly ash exhibited high strength and low water absorption. These bricks posed no environmental hazard. However, the strength decreased as the clay replacement ratio increased.
The study [12] focuses on investigating the potential use of fly ash from the Barsingsar Thermal Power Station in India for the production of thermal insulating refractory bricks. The analysis revealed that the fly ash is rich in alumina and silica, contains mullite and quartz crystals, and exhibits a needle-like and fibrous structure. This makes it a promising and cost-effective raw material for brick production.
The study [13] explores the use of fly ash in mine backfilling applications, where it partially replaces cement and other components. The influence of fly ash particle size, its proportion in the mixture, and slurry concentration on key properties, such as setting time, porosity and compressive strength, was analyzed. The study identified an optimal composition consisting of 50% fly ash and 60% slurry concentration, which provided the best performance characteristics for mine void filling.
In the Karaganda region of Kazakhstan, coal remains the primary source of energy. Local thermal power plants generate vast quantities of ash annually as a by-product of coal combustion. The situation is further complicated by the declining quality of coal currently being used due to the depletion of high-grade reserves. As a result, combustion produces not fine fly ash, but rather a conglomerate of ash and slag with low mechanical strength. During transportation, this material easily disintegrates, contributing to atmospheric pollution. Ash disposal requires vast land areas and additional investments to prevent wind erosion. The increasing volume of ash necessitates urgent and comprehensive solutions for its utilization. One of the most promising approaches is the incorporation of ash as a raw material for large-scale material production.
The review study [14] provides a detailed analysis of the environmental impact of various industrial wastes, including ash, and outlines potential strategies for their utilization aimed at the development of eco-friendly products.
The chemical composition of ash typically includes oxides of silicon and aluminum, along with other compounds. These substances possess relatively high melting points and are key constituents in refractory materials.
The study [15] investigated the formation of mullite through a single-step firing process using aluminum oxide and silica. The research focused on the effects of temperature (ranging from 1200 to 1600 °C) and the addition of magnesium oxide (MgO) in concentrations from 1 to 6 wt% on mullite formation and densification. It was established that mullite formation begins at 1200 °C. However, the reaction reaches completion at 1600 °C with the addition of 4 wt% MgO.
The study [16] examines the sol-gel synthesis method for mullite, with a particular focus on the characterization of gels at various temperatures and the factors influencing the synthesis process. The study also discusses research related to mullite precursors obtained using this technique.
The study [17] demonstrates the feasibility of producing low-cost mullite glass-ceramics from coal ash and alumina using high-temperature plasma technology. An optimal mixture ratio of 50% coal ash and 50% alumina enables complete mullitization within 5 min at 1200 °C. This results in the formation of single-phase mullite with a characteristic needle-like microstructure. The study [18] describes the fabrication of refractory ceramics based on mullite and alumina, utilizing both pure and recycled alumina sources. The samples were fired at a high temperature of 1600 °C, and their properties were subsequently analyzed. The results demonstrated that this method enables the production of promising refractory materials.
Study [19] focuses on the phase transformation of α-Al2O3 into mullite (2:1) within the SiO2-Al2O3 system. The samples were synthesized by impregnating ultraporous alumina monoliths with silica vapor. These vapors were derived from organosilicon precursors such as TMES and TEOS, and the process was conducted at room temperature. When the SiO2 content exceeded 20 mol.%, a complete transformation of α-Al2O3 into mullite was observed. Notably, no secondary phases were detected under these conditions.
The study [20] investigates the influence of temperature and fly ash particle size on mullite formation in porcelain stoneware compositions. The findings revealed that the fly ash with finer particle sizes promotes faster formation of mullite and glassy phases. Additionally, it initially contains a higher amount of mullite. The maximum mullite content was achieved at a firing temperature of 1600 °C.
Thus, the conducted literature analysis highlights the potential and promise of utilizing ash as one of the components in refractory raw material formulations. As is well known, ash is a technogenic by-product generated during coal combustion. Depending on the type, grade and quality of coal, the combustion of 1 ton of coal can produce up to 400 kg of ash. The use of ash as a component in raw material formulations addresses two key issues: it results in a product with properties comparable to those of the existing standard product, while simultaneously enabling the disposal of harmful technogenic waste. This also frees up land areas and improves the environmental situation in the region. Therefore, it is crucial to conduct research to better understand the role of ash in the properties of fireclay refractory products.
This study was conducted with the aim of determining the acceptable limits for the use of this type of ash. The research also aimed to investigate the key properties of the experimental product for production of fireclay refractories, ensuring compliance with industrial requirements. Successful implementation of this approach would lead to the disposal of coal combustion waste, which demands special attention. Moreover, it would facilitate the efficient utilization of ash as a refractory raw material.
The main distinction of this study from previous research lies in the use of not fine-dispersed fly ash, but rather a conglomerate of ash and slag generated during the combustion of low-quality coal. This paper presents our preliminary experimental results on the microstructure of the experimental refractories, which were characterized using X-ray diffraction (XRD) and scanning electron microscopy (SEM).
2. Materials and Methods
The object of the study was refractory samples made from a mixture of coal ash and red clay in varying proportions (Figure 1). Table 1 presents the chemical composition of the initial materials, as well as that of the ShA-grade chamotte. ShA-grade chamotte (GOST 390-96) [21] was subsequently used as the reference sample.

Figure 1.
(a) Initial sample; (b) raw material mixture; (c) general view of the briquettes; and (d) sintered samples.

Table 1.
Chemical composition of the materials used (averaged).
The ash used was a conglomerate of coal ash and slag sourced from the Karaganda region, Kazakhstan. For convenience, it will be referred to as “ash” hereafter. Since the coal and, consequently, the ash, are heterogeneous in composition, samples were collected from several points across the storage site. Red clay (Karaganda region, Kazakhstan) was used as the clay component. The averaged sample was obtained from samples collected at different points of the site.
The chemical analysis was performed using an ARL QUANT’X spectrometer. Additionally, X-ray phase analysis was conducted on the averaged samples. The X-ray diffractograms and the quantitative composition of the ash and clay used in this study are presented in Figure 2, Figure 3 and Figure 4 and Table 2, Table 3 and Table 4. X-ray diffraction (XRD) analysis was conducted on a D2Phaser Bruker X-ray diffractometer with a Siemens KFL-CU-2K Cu-2K X-ray tube (copper anode). Phase identification and quantitative phase content calculations were performed using the DIFFRAC.EVA (Version V7) and DIFFRAC.TOPAS software (Version 3.7.0.). The instrument error, according to the technical specifications, is 0.3%.
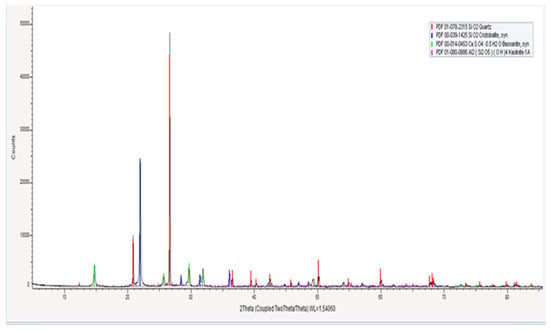
Figure 2.
X-ray diffraction pattern of the ash.
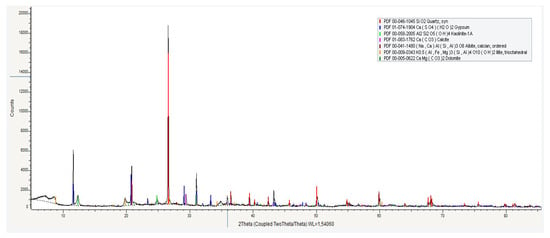
Figure 3.
X-ray diffraction pattern of a clay sample.
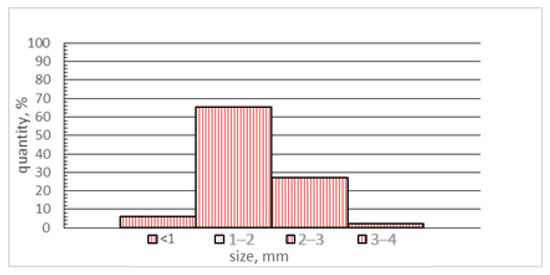
Figure 4.
Particle size distribution.

Table 2.
The ash mineral composition.

Table 3.
Mineral composition of the clay.

Table 4.
Batch composition of the samples used in this study.
As seen from Table 2, the majority of the ash consists of SiO2, which is present in the composition of quartz and cristobalite. Unlike quartz, the particles of cristobalite have a more rounded shape. This characteristic will influence the formation of the structure during the pressing process. The presence of SiO2 ensures the refractory properties of the material.
The mineral bassanite loses its hydration water upon heating, transforming into anhydrite, which has relatively low hardness.
Red clay from the Karaganda region of Kazakhstan was used as the raw material. An average sample was taken from samples collected at various points across the site (Figure 3).
The distinctive feature of this clay is the presence of a significant amount of quartz and kaolinite, which contribute to its high refractoriness. According to studies on the transformation of kaolinite upon heating [22,23,24,25,26], kaolinite first transforms into metakaolinite (an amorphous phase), and subsequently into mullite and cristobalite.
2.1. Sample Preparation
All components of the charge were pre-ground in an Emax ball mill for 10 min. The volume of the grinding jar was 50 mL, with six 12 mm grinding balls made of tungsten carbide. The jar material was stainless steel, and the rotation speed was 1000 rpm. The dispersion of all components was 90% represented by a fraction of 2–3 mm. The particle size distribution of the materials was determined using an AS 200 control analytical sieving machine (Retsch, Haan, Germany).
Subsequently, the components were homogenized using a laboratory-scale mixer, with the addition of water at a dosage of 10% by weight relative to the total dry mass (i.e., 10% over 100%).
The prepared mixture was shaped into disc-shaped samples with a diameter of 70 mm and a height of 5 mm using a manually operated RP-50 press under a uniaxial load of 50 kN. These samples were subsequently sintered in a Nabertherm furnace at target temperatures of 1000 °C and 1100 °C in ambient air for 2 h.
After complete furnace cooling, the sintered samples were subjected to characterization, including compressive strength, apparent density and open porosity measurements.
2.2. Study of Sample Properties
Compressive strength testing was performed using an INSTRON-100 universal testing machine in accordance with GOST 4071.1-2021 (Republic of Kazakhstan) [27].
Apparent density was determined in accordance with GOST 2409-14 and was calculated using the following equation:
where:
- —apparent density, g/cm3
- m—mass of the sample, g
- v—total volume of the sample, including open and closed pores, cm3
The mass of the samples was measured using a CAS CAUW-220D (CAS Corporation, Yangju-si, South Korea) analytical balance.
Microstructural analysis was carried out using a Tescan Vega scanning electron microscope (SEM). To enhance surface conductivity, the samples were coated with a thin layer of carbon using a Q150T ES Plus (Quorum Technologies Ltd., Lewes, United Kingdom) sputter coater.
Open porosity was determined using a PoreMaster 60 (Anton Paar GmbH, Graz, Austria) mercury intrusion porosimeter, which enables the quantification of pore volume and the pore size distribution for open pores with diameters as small as 2 nm.
To obtain a general understanding of the open porosity, this parameter was additionally determined using the water absorption method, in accordance with GOST 2409-2014 [28]. Open porosity was calculated using the following equation:
where:
- mdry is the mass of the dried sample (g);
- msat.air is the mass of the water-saturated sample measured in air (g);
- msubm is the mass of the saturated sample measured while submerged in liquid (g).
Mercury intrusion porosimetry provides detailed information on pore size distribution. This is essential for understanding both the sintering behavior and the subsequent infiltration of pores by molten phases. In contrast, the water absorption method yields only the total value of open porosity, expressed as a percentage.
The slag resistance of the refractory materials was evaluated using the following procedure. Cylindrical cavities with a diameter of 15 mm and a depth of 35 mm were drilled into the samples. These cavities were then filled with a synthetic mixture of steelmaking slags up to the level of the sample surface. The prepared samples were placed in a high-temperature furnace and exposed to a temperature of 1550 °C for 3 h, during which the slag remained in a molten state. Following complete cooling to room temperature, the samples were sectioned along the (010) plane. The maximum penetration depth of the slag was subsequently measured.
3. Results and Discussion
The results of the slag resistance testing are summarized in Table 5.

Table 5.
Presents the results of the tests carried out.
As shown in Table 4, all experimental samples met the density requirements specified by the applicable GOST standard for this class of refractory materials.
The compressive strength of samples 1 through 3 also complied with the specifications for ShA-grade refractory materials. Although it was slightly lower than that of the reference material. An increase in fly ash content in the batch composition led to a progressive decline in compressive strength. In sample 4, the compressive strength dropped to just 19.4 MPa, which falls below the minimum value [21]. As a result, sample 4 was excluded from further investigation. A comparison of the data obtained at firing temperatures of 1000 °C and 1100 °C indicates that increasing the firing temperature has a positive effect on key performance indicators such as compressive strength and slag resistance. Nevertheless, a firing temperature of 1000 °C was also sufficient to ensure compressive strength and acceptable open porosity for materials of this class, as specified by GOST 390-96.
3.1. Pore Structure and Microstructure
The data in Table 4 show that the total open porosity decreases with increasing fly ash content in the batch composition. This phenomenon can be attributed to the formation of a more densely packed structure. Fly ash has relatively low strength [29], so during the pressing process, it breaks down into even finer particles, which fill the gaps between the clay particles.
It should be noted that, in addition to the total open porosity, the nature of the pore size distribution also changes. The volume of pores with a size of 10,000 nm significantly decreases, while the proportion of smaller pores increases (Figure 5). This pore distribution is more favorable in terms of slag resistance, as the narrow pore entrance inhibits the infiltration of molten slag.
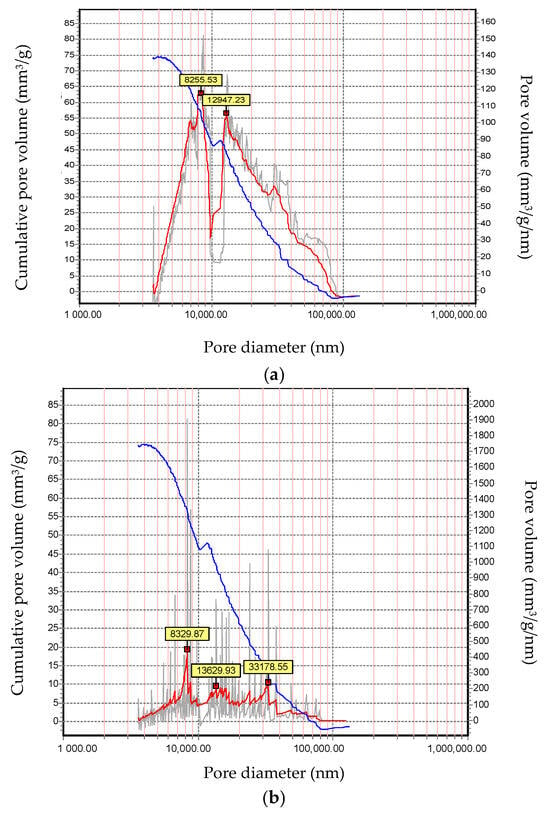
Figure 5.
Total open porosity and pore size distribution: (a) sample 0; (b) sample 3.
After firing, the microstructures of the resulting samples were examined (Figure 6).
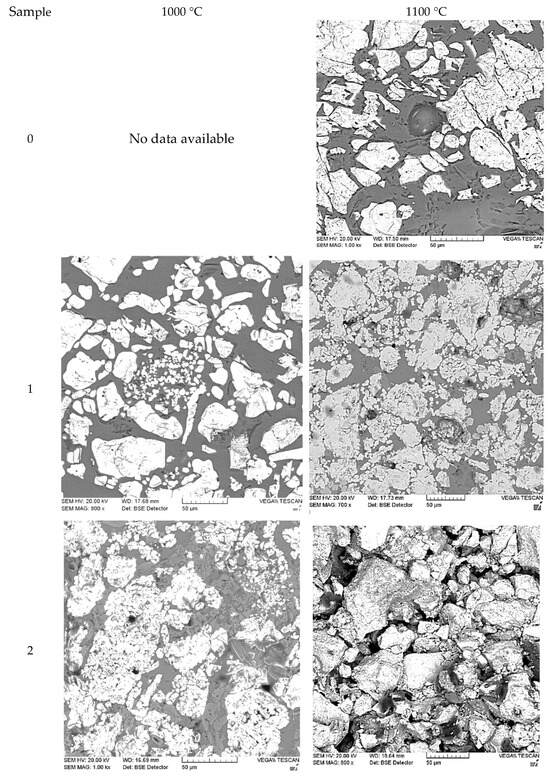
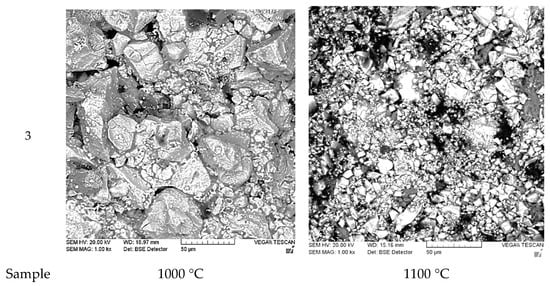
Figure 6.
Microstructures of the experimental samples at 1000 °C and 1100 °C. The number indicates the sample number.
In all the investigated samples, the structure can be approximately characterized as biphasic, consisting of dark and light regions. As the fly ash content in the sample increases, the light particles become more closely spaced, and the gaps between them decrease. Increasing the firing temperature results in the formation of a more dispersed and homogeneous structure. Compared to the reference material, all experimental samples exhibit a denser and more uniform structure. It should be noted that the structural characteristics of the experimental samples differ significantly from those presented in other studies focused on the use of fly ash [5,9,10,11]. In the referenced papers, the particles exhibit a more rounded shape, and the overall structure is more layered. In contrast, the structure of the experimental samples shows distinct boundaries between the particles, with irregular particle edges and a significant variation in particle sizes. This is despite the fact that the initial batch primarily consisted of a 2–3 mm fraction. The structure of Sample 0 (ShA-grade refractory brick) is provided for comparison. It consists of larger particles that are clearly separated from each other. The structure of the experimental samples is primarily determined by the shape of the fly ash particles used in the study. In contrast to the studies [5,9,10,11], where fine, volatile “soft” fly ash was utilized, the present work employed a dense ash-slag conglomerate. This material is formed during the combustion of low-grade bituminous coals with a high content of impurities, including various metallic inclusions.
3.2. Phase Composition
To determine the phase composition of the experimental samples after firing, X-ray diffraction (XRD) analysis was performed. The results of the XRD analysis are presented in Figure 7 and Table 6.
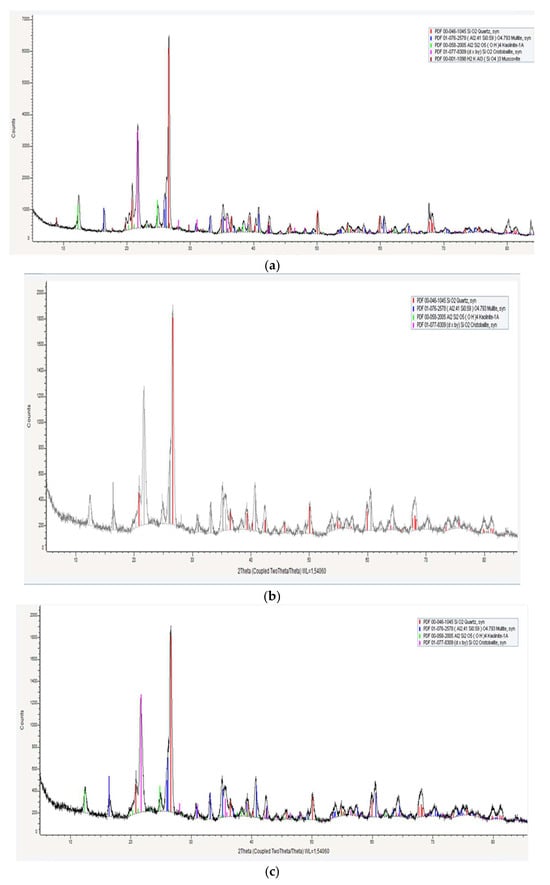
Figure 7.
Phase composition of the samples: (a) sample 0; (b) sample 3 after firing at 1000 °C; (c) sample 3 after firing at 1100 °C.

Table 6.
Phase composition of the samples.
A comparison of the data presented in Table 6 reveals no significant qualitative differences in the phase composition of the samples sintered at 1000 °C and 1100 °C. However, notable quantitative differences are observed. Specifically, the amount of mullite increases substantially in the samples fired at 1100 °C, while the content of kaolinite decreases accordingly. Mullite is a high-temperature phase with a melting point of 1810 °C, which contributes to the enhanced refractoriness of the material. An interesting observation was made that kaolinite was still present in the experimental samples even after sintering at 1100 °C. According to previous studies [22,23,24,25,26], kaolinite typically undergoes a phase transformation upon heating: it first converts to metakaolinite, and then, at temperatures exceeding 1100 °C, further transforms into mullite, which is responsible for the refractory properties of the material. However, in this study, kaolinite remained detectable as a distinct crystalline phase even after sintering at 1100 °C for 2 h. This indicates an incomplete kaolinite-to-mullite transformation process. To complete the kaolinite-to-mullite transformation, it is likely necessary to increase either the sintering temperature or the holding time.
The incomplete kaolinite-to-mullite transformation is likely due to the slow transition from kaolinite to metakaolinite, from which mullite is subsequently formed. The completeness of the kaolinite-to-metakaolinite transition is influenced by the degree of crystallinity of the initial kaolinite (Hinkley index), as well as the presence of carbon and the nature of the sintering atmosphere [25,26].
The completion of the kaolinite-to-mullite transformation would further enhance refractoriness, as mullite is the most high-temperature stable compound of Al2O3 with SiO2. However, increasing the temperature and duration of sintering imposes additional strain on the equipment, which must be considered when selecting the optimal technological parameters.
3.3. Slag Resistance
The final stage of this study involved testing the slag resistance (Figure 8).

Figure 8.
Samples after slag resistance testing: (a) Sample 0; (b) Sample 3 after sintering at 1100 °C.
Slag resistance was measured on Samples 0 and 3 according to the methodology described above. Currently, there is no standardized method for determining this parameter. However, the aforementioned approach is widely employed in industrial settings and has proven to be sufficiently reliable.
As seen in Table 5, the slag resistance of Sample 3 is higher than that of the reference sample. With the increase in ash content and the reduction in open porosity, slag resistance was indeed improved, the maximum depth of slag penetration decreased by 15%. This suggests a longer service life for the experimental samples compared to the reference.
This phenomenon can be explained by two main factors. First, the higher content of mullite that is less susceptible to slag attack. Second, the nature of the open porosity: a smaller volume of open pores and a lower average pore radius that hinder the infiltration of slag into the channels.
Figure 9 presents a comparative diagram of the characteristics of the samples as a function of ash content in the mixture. For clarity, all data have been normalized to a common scale: apparent density is multiplied by 100; open porosity values are multiplied by 10; and slag resistance is multiplied by 100.
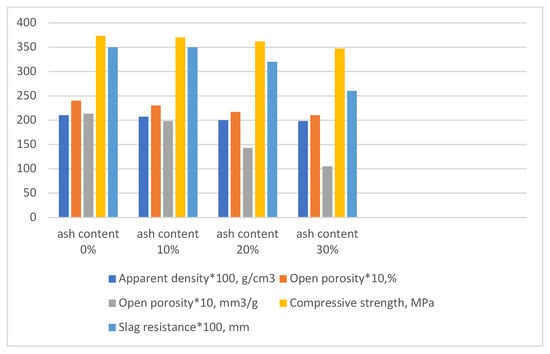
Figure 9.
Comparative diagram of the properties of the experimental samples.
Figure 9 presents a comparative diagram of the properties of the experimental samples and the reference sample. As shown in the diagram, all experimental samples exhibit comparable properties to the reference sample. The experimental samples slightly lag behind the reference in terms of compressive strength (approximately 7%). However, there is a significant improvement in slag resistance, which increases by about 25%. As noted earlier, this phenomenon is associated with a slight decrease in overall open porosity, primarily due to the reduction in the proportion of larger pores (over 10,000 nm). This, in turn, hinders the infiltration of molten slag and the degradation of the material.
4. Conclusions
The conducted studies demonstrated the feasibility of using ash from low-quality coal as a component of the batch for refractory production. Incorporating coal ash in quantities of 10–30% by mass, mixed with clay, for manufacturing refractories allows for the retention of compressive strength and sample density at levels comparable to existing ShA-grade refractory materials (reference sample). Simultaneously, it leads to a reduction in open porosity and a decrease in the proportion of larger pores (greater than 10,000 nm), which results in enhanced slag resistance.
In contrast to the reference sample (ShA-grade refractory materials), the phase composition of the experimental samples is characterized by a higher content of mullite, quartz and cristobalite. However, the composition lacks muscovite.
It is noteworthy that kaolinite is present in all the examined samples after firing, indicating the incomplete progression of the kaolinite-meta-kaolinite-mullite transformation. With an increase in sintering temperature, the content of kaolinite decreases, while the amount of mullite increases. This positively impacts the slag resistance.
Thus, the incorporation of coal ash into the batch in quantities ranging from 10 to 30% enables the production of refractories with properties that meet, and even slightly exceed, the standards of existing ShA-grade refractory materials. Moreover, this approach contributes to the disposal of harmful technogenic waste.
Author Contributions
Conceptualization, S.K.A. and S.S.K.; data curation V.Y.K.; methodology V.Y.K. and A.Z.I., formal analysis, A.E.A.; investigation, S.K.A. and S.S.K.; writing—original draft preparation, S.K.A.; writing—review and editing, S.S.K.; visualization, A.E.A. All authors participated in writing the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP23487471).
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Horckmans, L.; Nielsen, P.; Dierckx, P.; Ducastel, A. Recycling of refractory bricks used in basic steelmaking: A review. Resour. Conserv. Recycl. 2019, 140, 297–304. [Google Scholar] [CrossRef]
- Xiao, R.; Nie, Q.; He, J.; Lu, H.; Shen, Z.; Huang, B. Utilizing lowly-reactive coal gasification fly ash (CGFA) to stabilize aggregate bases. J. Clean. Prod. 2022, 10, 133320. [Google Scholar] [CrossRef]
- Aineto, M.; Acosta, A.; Iglesias, I. The role of a coal gasification fly ash as clay additive in building ceramic. J. Eur. Ceram. Soc. 2006, 26, 3783–3787. [Google Scholar]
- Chargui, F.; Hamidouche, M.; Belhouchet, H.; Jorand, Y.; Doufnoune, R.; Fantozzi, G. Mullite Fabrication from Natural Kaolin and Aluminium Slag. Bol. Soc. Esp. Cerám. Vidr. 2018, 57, 169–177. [Google Scholar]
- Sembiring, S.; Simanjuntak, W.; Situmeang, R.; Riyanto, A.; Junaidi, J. Structural and Physical Properties of Refractory Cordierite Precursors Prepared from Rice Husk Silica with different MgO Addition. Ceram.-Silik. 2018, 62, 163–172. [Google Scholar] [CrossRef]
- Sultana, P.; Das, S.; Bhattacharya, A.; Basu, R.; Nandy, P. Mullite Formation in Coal Fly Ash is Facilitated by the Incorporation of Magnesium Oxide. Rev. Adv. Mater. Sci. 2011, 27, 69–74. [Google Scholar]
- Lemougna, P.N.; MacKenzie, K.J.; Melo, U.C. Synthesis and thermal Properties of Inorganic Polymers (Geopolymers) for Structural and Refractory Applications from Volcanic Ash. Ceram. Int. 2011, 37, 3011–3018. [Google Scholar]
- Kim, M.; Ko, H.; Kwon, T.; Bae, H.-C.; Jang, H.; Heo, B.-U.; Park, S.-M. Development of novel refractory ceramic continuous fibers of fly ash and comparison of mechanical properties with those of E-glass fibers using the Weibull distribution. Ceram. Int. 2020, 46, 13255–13262. [Google Scholar]
- Debnath, N.K.; Boga, S.; Singh, A.; Majhi, M.R.; Singh, V.K. Fabrication of low to high duty fireclay refractory bricks from lignite fly ash. Ceram. Int. 2022, 48, 12152–12160. [Google Scholar]
- Hassan, S.B.; Aigbodion, V.S. Effect coal ash on some refractory properties of alumino-silicate (Kankara) clay for furnace lining. Egypt. J. Basic Appl. Sci. 2014, 1, 107–114. [Google Scholar]
- Leiva, C.; Arenas, C.; Alonso-Farina, B.; Vilches, L.F.; Peceño, B.; Rodriguez-Galan, M.; Baena, F. Characteristics of fired bricks with co-combustion fly ashes. J. Build. Eng. 2016, 5, 114–118. [Google Scholar]
- Debnath, N.; Acharya, V.; Jangu, S.; Singh, P.; Majhi, M.R.; Singh, V.K. Characterization of fly ash solid-waste for low-cost insulation refractory bricks. Mater. Proc. 2021, 47, 1598–1600. [Google Scholar]
- Zhang, C.; Fu, J.; Song, W. Mechanical model and strength development evolution of high content fly ash–cement grouting material. Constr. Build. Mater. 2023, 398, 132492. [Google Scholar]
- Kurda, R.; Silvestre, J.D.; Brito, J. Toxicity and environmental and economic performance of fly ash and recycled concrete aggregates use in concrete: A review. Helyion 2018, 4, 4. [Google Scholar]
- Sarkar, R.; Mallick, M. Formation and Densification of Mullite through Solid-Oxide Reaction Technique Using Commercial-Grade Raw Materials. Bull. Mater. Sci. 2018, 41, 31. [Google Scholar]
- Cividanes, L.S.; Campos, T.M.; Rodrigues, L.A.; Brunelli, D.D.; Thim, G.P. Review of Mullite Synthesis Routes by Sol–Gel Method. J. Sol-Gel Sci. Technol. 2010, 55, 111–125. [Google Scholar]
- Suriyanarayanan, N.; Nithin, K.K.; Bernardo, E. Mullite Glass Ceramics Production from Coal Ash and Alumina by High Temperature Plasma. J. Non-Oxide Glas. 2009, 1, 247–260. [Google Scholar]
- Sadik, C.; Amrani, I.-E.E.; Albizane, A. Processing and Characterization of Alumina–Mullite Ceramics. J. Asian Ceram. Soc. 2014, 2, 310–316. [Google Scholar]
- Khatim, O.; Nguyen, T.; Amamra, M.; Museur, L.; Khodan, A.; Kanaev, A. Synthesis and Photoluminescence Properties of Nanostructured Mullite/α-Al2O3. Acta Mater. 2014, 71, 108–116. [Google Scholar] [CrossRef]
- Sultana, P.; Das, S.; Bagchi, B.; Bhattacharya, A.; Basu, R.; Nandy, P. Effect of Size of Fly Ash Particle on Enhancement of Mullite Content and Glass Formation. Bull. Mater. Sci. 2011, 34, 1663–1670. [Google Scholar]
- GOST 390-96; Fireclay and Semiacidic Refractory Products of General-Purpose and Mass Production. Publishing House of Standards: Minsk, Belarus, 1997; Semi-Acid Products.
- The National Encyclopedia; Kazakh Encyclopedias Limited Liability Partnership: Nur-Sultan, Kazakhstan, 2005; Volume 3, p. 530.
- Sánchez-Soto, P.J.; García-Garzón, V.; Martínez-Martínez, S.; Pérez-Villarejo, L.; Sánchez-Garrido, J.A.; Garzón, E. Influence of features and firing temperature on the ceramic properties and phase evolution of raw kaolins. Constr. Build. Mater. 2025, 466, 140215. [Google Scholar]
- Platov, Y.T.; Platova, R.A.; Molodkina, P.G. The use of IR spectrum decomposition in the analysis of structural and phase transformations of kaolinite. J. Appl. Spectrosc. 2020, 6, 897–904. [Google Scholar]
- Lamberov, A.; Sitnikova, E.; Abdulganeeva, A. Kinetic features of phase transformation of kaolinite into metakaolinite for kaolin clays from different deposits. Russ. J. Appl. Chem. 2012, 85, 892–897. [Google Scholar]
- Xue, H.; Dong, X.; Fan, Y.; Ma, X.; Yao, S. Study of Structural Transformation and Chemical Reactivity of Kaolinite-Based High Ash Slime during Calcination. Minerals 2023, 13, 466. [Google Scholar] [CrossRef]
- GOST 4071.1-2021; Refractory Products with Less Than 45% True Porosity. Method for Determination of Compressive Strength at Room Temperature. Federal Agency for Technical Regulation and Metrology: Moscow, Russia, 2021.
- GOST 2409-2014; Refractories. Method for Determination of Bulk Density, Apparent and True Porosity, Water Absorption. Federal Agency for Technical Regulation and Metrology: Moscow, Russia, 2014.
- Chousidis, N.; Ioannou, I.; Rakanta, E.; Koutsodontis, C.; Batis, G. Effect of fly ash chemical composition on the reinforcement corrosion, thermal diffusion and strength of blended cement concretes. Constr. Build. Mater. 2016, 126, 86–97. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).