Electrical Properties of Semiconductor/Conductor Composites: Polypyrrole-Coated Tungsten Microparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Characterisation
3. Results and Discussion
3.1. Preparation of Composites
3.2. Morphology
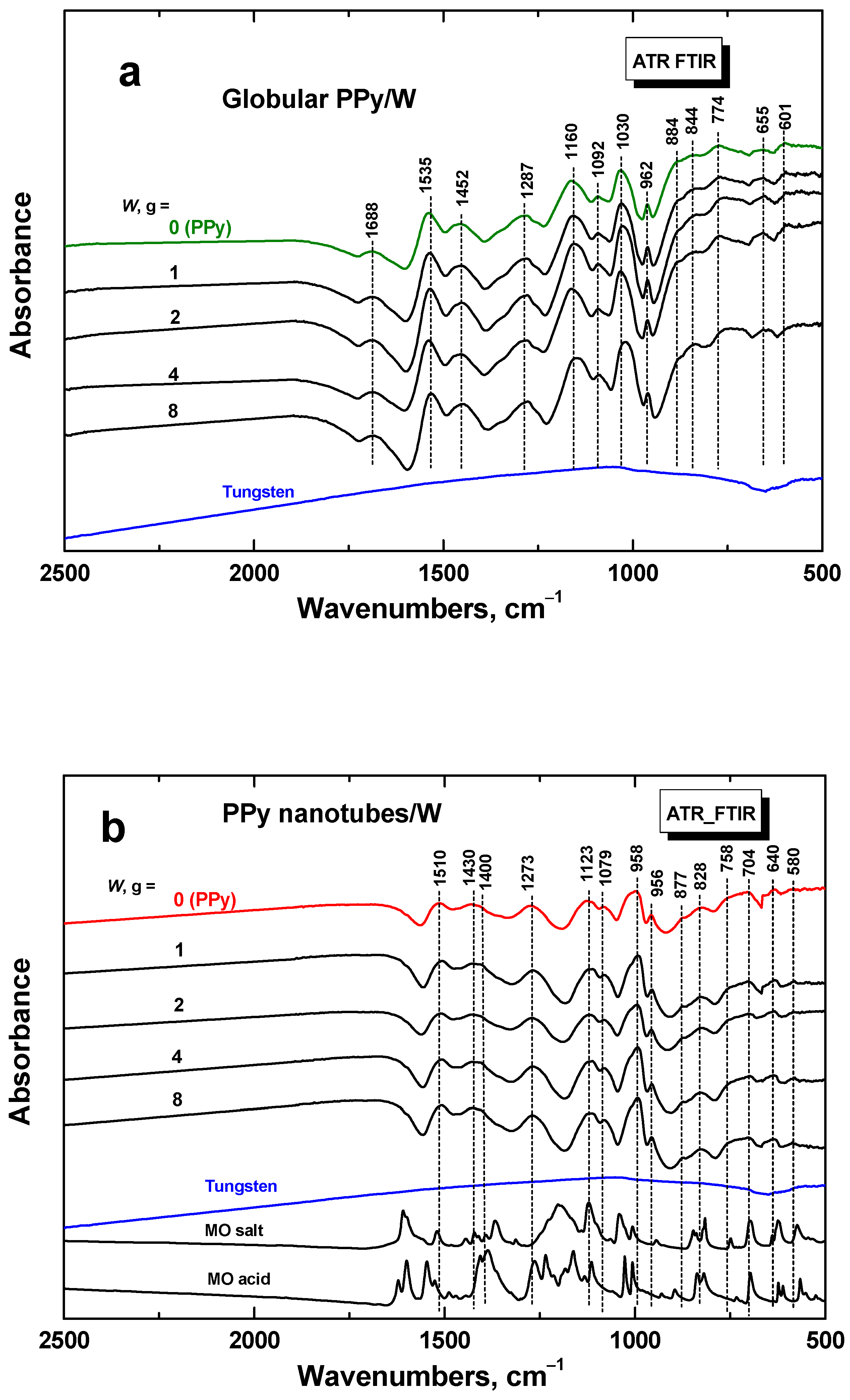
3.3. FTIR Spectra
3.4. Raman Spectra
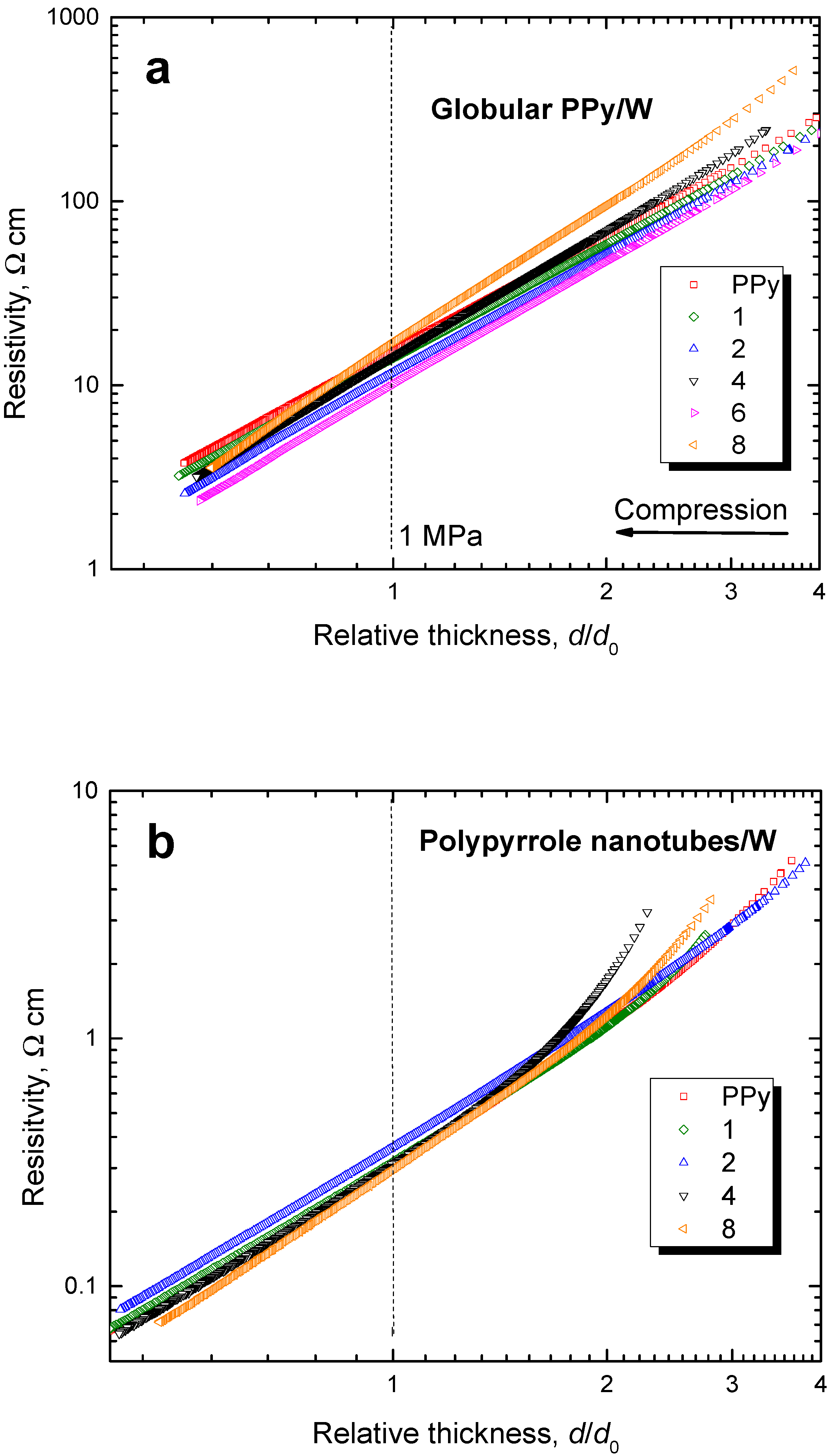
3.5. Resistivity
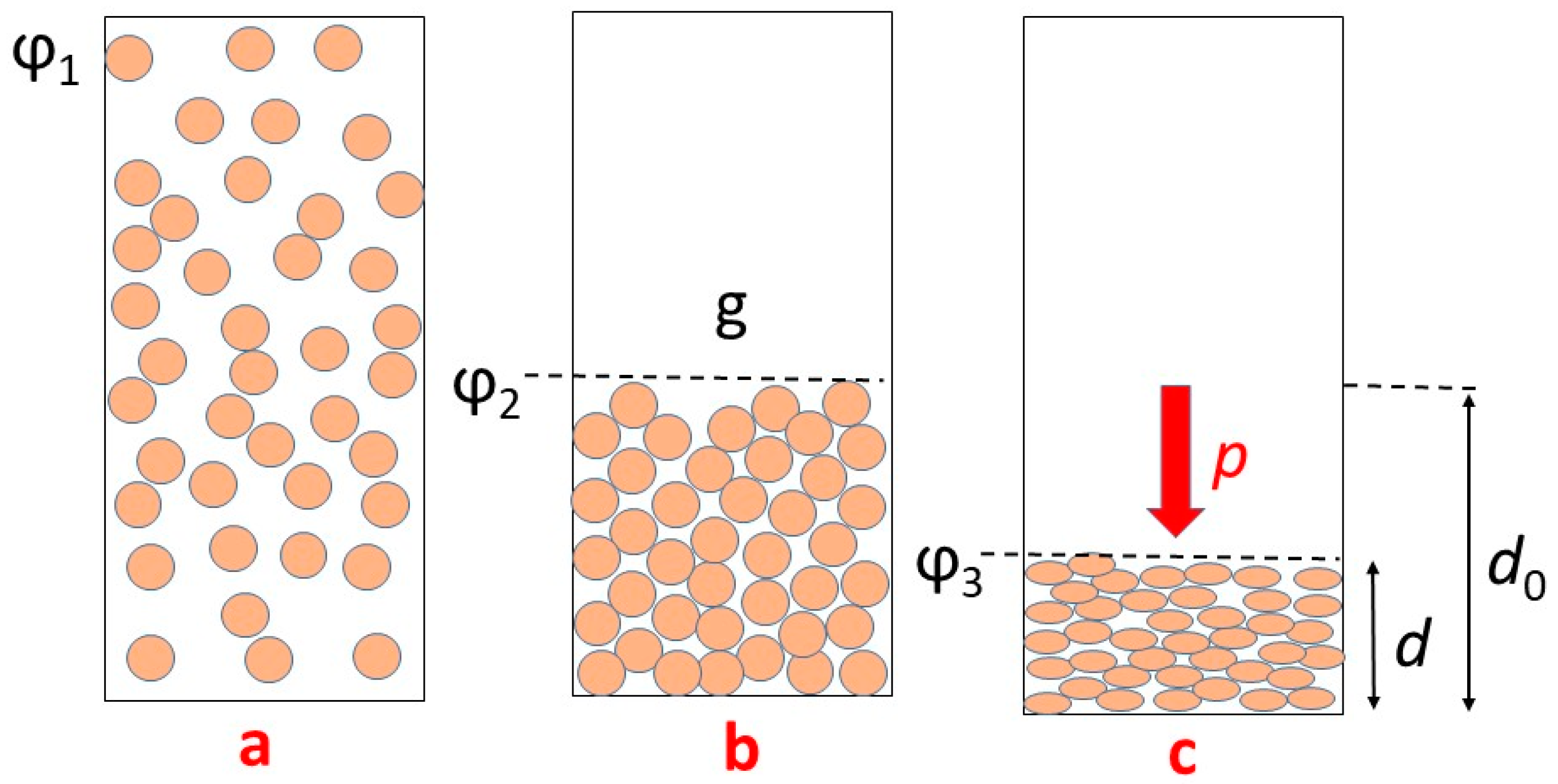
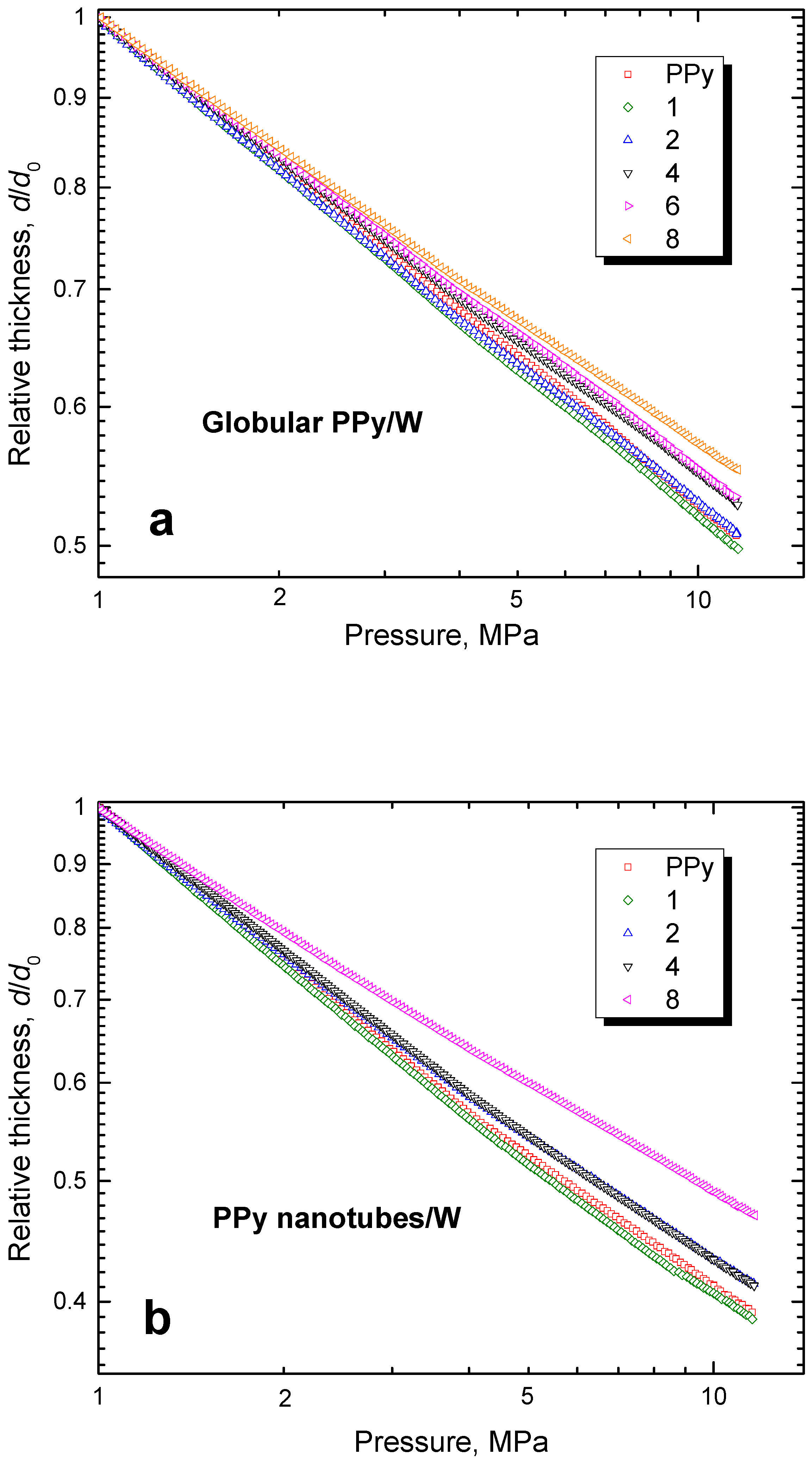
3.6. Mechanical Properties
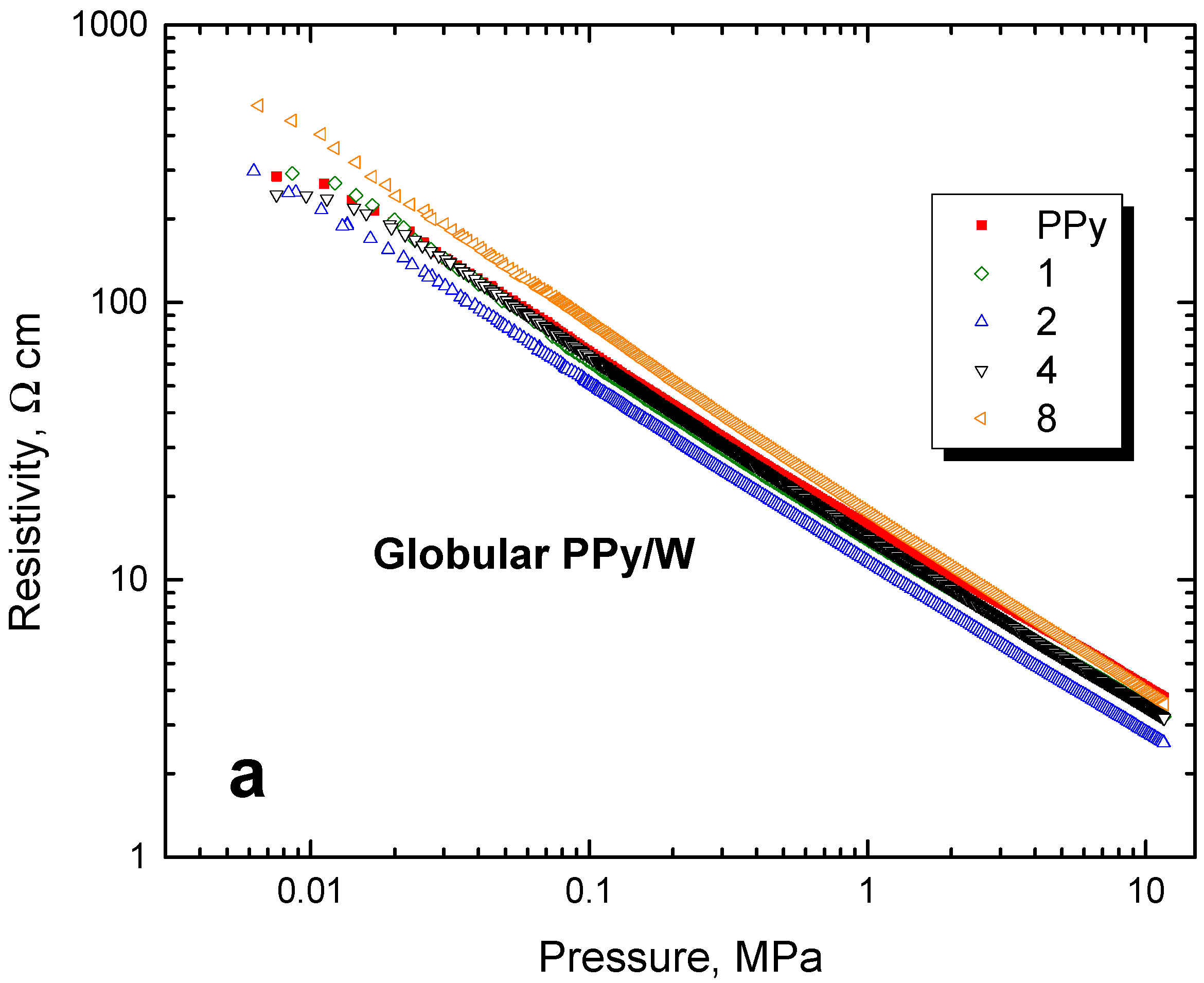
3.7. Pressure Dependences of Resistivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liao, Z.W.; Zoumhani, O.; Boutry, C.M. Recent advances in magnetic polymer composites for bioMEMS: A review. Materials 2023, 16, 3802. [Google Scholar] [CrossRef]
- Macdonald, M.; Zhitomirsky, I. Pseudocapacitive and magnetic properties of SrFe12O19-polypyrrole composites. J. Compos. Sci. 2024, 8, 351. [Google Scholar] [CrossRef]
- Wang, G.P.; Zhang, L.; Zhang, J.J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Soares, B.G.; Barra, G.M.O.; Indrusiak, T.J. Conducting polymeric composites based on intrinsically conducting polymers as electromagnetic interference shielding/microwave absorbing materials—A review. J. Compos. Sci. 2021, 5, 173. [Google Scholar] [CrossRef]
- Ul Hoque, M.I.; Holze, R. Intrinsically conducting polymer composites as active masses in supercapacitors. Polymers 2023, 15, 730. [Google Scholar] [CrossRef] [PubMed]
- Nedjar, L.; Mekki, A.; Sayah, Z.B.D.; Cherfa, M.C.; Lounes, R.A.; Manseri, A.; Durastanti, J.F.; Mekhalif, Z. Towards an efficient p-n heterojunction in ternary composite material based polypyrrole, metal oxide and surface functionalized graphene for thermoelectric properties enhancement. Synth. Met. 2023, 298, 117427. [Google Scholar] [CrossRef]
- Filipescu, M.; Dobrescu, S.; Bercea, A.I.; Bonciu, A.F.; Marascu, V.; Brajnicov, S.; Palla-Papavlu, A. Polypyrrole-tungsten oxide nanocomposite fabrication through laser-based techniques for an ammonia sensor: Achieving room temperature operation. Polymers 2024, 16, 79. [Google Scholar] [CrossRef]
- Rabia, M.; Aldosari, E.; Elsayed, A.M.; Sanna, A.; Farid, O. Highly porous network of tungsten (VI) oxide-iodide/polypyrrole nanocomposite photocathode for the green hydrogen generation. Opt. Quant. Electron. 2024, 56, 1068. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.; Zhang, J.W.; Chen, Z.; Liu, X.; Wang, K.L.; Sobolev, A.V.; Savilov, S.V.; Chen, M.H. High pseudocapacitance electrode enabled by heterovalent doping and surface coating for rapid charge storage. Surf. Coat. Technol. 2024, 476, 130169. [Google Scholar] [CrossRef]
- Cogal, S.; Cogal, G.C.; Mičušík, M.; Kotlár, M.; Omastová, M. Cobalt-doped WSe2@conducting polymer nanostructures as bifunctional electrocatalysts for overall water splitting. Int. J. Hydrogen Energy 2024, 49, 689–700. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, H.J.; Wang, C.; Wang, F.; Wang, H.C.; Zhang, W.H.; Wang, X.F.; Yan, C.L.; Kim, B.H.; Ren, F.Z. Nitrogen-doped carbon coated WS2 nanosheets as anode for high-performance sodium-ion batteries. Front. Chem. 2018, 6, 236. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, J.; Acharya, U.; Bober, P.; Hajná, M.; Trchová, M.; Mičušík, M.; Omastová, M.; Pašti, I.; Gavrilov, N. Surface modification of tungsten disulfide with polypyrrole for enhancement of the conductivity and its impact on hydrogen evolution reaction. Appl. Surf. Sci. 2019, 492, 497–503. [Google Scholar] [CrossRef]
- Sunilkumar, A.; Manjunatha, S.; Machappa, T.; Chethan, B.; Ravikiran, Y.T. A tungsten disulphide-polypyrrole composite-based humidity sensor at room temperature. Bull. Mater. Sci. 2019, 42, 271. [Google Scholar] [CrossRef]
- Hsiao, P.F.; Anbazhagan, R.; Tsai, H.C.; Krishnarnoorthi, R.; Lin, S.J.; Lin, S.Y.; Lee, K.Y.; Kao, C.Y.; Chen, R.S.; Lai, J.Y. Fabrication of electroactive polypyrrole-tungsten disulfide nanocomposite for enhanced in vivo drug release in mice skin. Mater. Sci. Eng. C 2020, 107, 110330. [Google Scholar] [CrossRef] [PubMed]
- Sunilkumar, A.; Manjunatha, S.; Ravikiran, Y.T.; Revanasiddappa, M.; Prashantkumar, M.; Machappa, T. AC conductivity and dielectric studies in polypyrrole wrapped tungsten disulphide composites. Polym. Bull. 2021, 79, 1391–1407. [Google Scholar] [CrossRef]
- Selvam, S.; Yim, J.H. Multifunctional supercapacitor integrated sensor from Oyster and Cicada derived bio-ternary composite: Vanillin/caffeine detections in beverages. J. Energy Storage 2022, 45, 103791. [Google Scholar] [CrossRef]
- Kozma, M.; Bissessur, R.; Gao, B.W.; Dahn, D.C. Exfoliated tungsten disulfide-polypyrrole nanocomposites. J. Inorg. Organomet. Polym. Mater. 2024; early access. [Google Scholar] [CrossRef]
- Selvam, S.; Jo, Y.H.; Chan, A.D.; Cumming, M.; Jordan, M.; Khadka, R.; Yim, J.H. Biocompatible supercapacitor engineered from marine collagen impregnated with polypyrrole and tungsten disulfide. J. Energy Storage 2024, 96, 112735. [Google Scholar] [CrossRef]
- Mohan, V.V.; Rakhi, R.B. WS2/conducting polymer nanocomposite-based flexible and binder-free electrodes for high-performance supercapacitors. Elecrochim. Acta 2024, 498, 144657. [Google Scholar] [CrossRef]
- Arslantürk, G.; Ugraskan, V. Thermoelectric properties investigation of tungsten carbide-filled poly(vinyl alcohol)/polypyrrole ternary composites. Polym. Plast. Technol. Mater. 2024, 63, 151–160. [Google Scholar] [CrossRef]
- Minisy, I.M.; Gupta, S.; Taboubi, O.; Acharya, U.; Bober, P. Polypyrrole/tungsten carbide nanocomposites for electrochemical applications. ACS Appl. Polym. Mater. 2024, 6, 8244–8253. [Google Scholar] [CrossRef]
- Turek, W.; Lapkowski, M.; Stolarczyk, A.; Debiec, J. EPR and XPS measurements of polymeric catalysts doped with heteropolyacids in oxygen adsorption studies. Appl. Surf. Sci. 2005, 252, 801–806. [Google Scholar] [CrossRef]
- Yasami, S.; Mazinani, S.; Abdouss, M. Developed composites materials for flexible supercapacitors electrode: “Recent progress & future aspects”. J. Energy Storage 2023, 72, 108807. [Google Scholar] [CrossRef]
- Stejskal, J.; Jurča; Vilčáková, J.; Trchová, M.; Kolská, Z.; Prokeš, J. Conducting polypyrrole silicotungstate deposited on macroporous melamine sponge for electromagnetic interference shielding. Mater. Chem. Phys. 2023, 293, 126707. [Google Scholar] [CrossRef]
- Tyagi, N.; Singh, M.K.; Khanuja, M. Synergistic effect of polypyrrole modified WS2 nanosheets on visible light assisted catalysis for the removal of chromium (VI) and humic acid. Mater. Res. Bull. 2023, 163, 112216. [Google Scholar] [CrossRef]
- Tyagi, N.; Ashraf, W.; Mittal, H.; Fatima, T.; Khanuja, M.; Singh, M.K. A facile synthesis of ternary hybrid nanocomposite of WS2/ZnO/PPy: An efficient photocatalyst for the degradation of chromium hexavalent. Dye. Pigment. 2023, 210, 110998. [Google Scholar] [CrossRef]
- Muthumeenal, A.; Rethinam, A.J.; Nagendran, A. Sulfonated polyethersulfone based composite membranes containing heteropolyacids laminated with polypyrrole for electrochemical energy conversion devices. Solid State Ion. 2016, 296, 106–113. [Google Scholar] [CrossRef]
- Jurča, M.; Munteanu, L.; Vilčáková, J.; Stejskal, J.; Trchová, M.; Prokeš, J.; Křivka, I. Core–shell inorganic/organic composites composed of polypyrrole nanoglobules or nanotubes deposited on MnZn ferrite microparticles: Electrical and magnetic properties. J. Compos. Sci. 2024, 8, 373. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M. Conducting polypyrrole nanotubes: A review. Chem. Pap. 2018, 72, 1563–1595. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Resonance Raman spectroscopy of conducting polypyrrole nanotubes: Disordered surface versus ordered body. J. Phys. Chem. A 2018, 122, 9298–9306. [Google Scholar] [CrossRef]
- Zallen, R.; Scher, H. Percolation on a continuum and localization–delocalization transition in amorphous semiconductors. Phys. Rev. B-Solid State 1971, 4, 4471–4476. [Google Scholar] [CrossRef]
- Křivka, I.; Prokeš, J.; Tobolková, E.; Stejskal, J. Application of percolation concepts to electrical conductivity of polyaniline-inorganic salt composites. J. Mater. Chem. 1991, 9, 2425–2428. [Google Scholar] [CrossRef]
- Oskouyi, A.B.; Sundararaj, U.; Mertiny, P. Tunneling conductivity and piezoresistivity of composites containing randomly dispersed conductive nano-platelets. Materials 2014, 7, 2501–2521. [Google Scholar] [CrossRef]









| W, g | Yield, g | w, wt% W | φ, vol% W |
|---|---|---|---|
| 0 (PPy) | 1.40 | 1 | 1 |
| 2 | 3.40 | 0.21 | 0.019 |
| 4 | 5.40 | 0.74 | 0.174 |
| 6 | 7.40 | 0.81 | 0.240 |
| 8 | 9.40 | 0.85 | 0.295 |
| W Content a | Globular PPy | PPy Nanotubes | ||
|---|---|---|---|---|
| 10 MPa | Pellet (527 MPa) | 10 MPa | Pellet (527 MPa) | |
| 0 | 0.244 | 0.288 | 14.4 | 28.4 |
| 1 | 0.283 | 0.285 | 14.5 | 34.2 |
| 2 | 0.353 | 0.418 | 11.5 | 19.6 |
| 4 | 0.256 | 0.347 | 15.0 | 36.1 |
| 8 | 0.254 | 0.335 | 12.8 | 27.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stejskal, J.; Jurča, M.; Trchová, M.; Prokeš, J. Electrical Properties of Semiconductor/Conductor Composites: Polypyrrole-Coated Tungsten Microparticles. J. Compos. Sci. 2025, 9, 98. https://doi.org/10.3390/jcs9030098
Stejskal J, Jurča M, Trchová M, Prokeš J. Electrical Properties of Semiconductor/Conductor Composites: Polypyrrole-Coated Tungsten Microparticles. Journal of Composites Science. 2025; 9(3):98. https://doi.org/10.3390/jcs9030098
Chicago/Turabian StyleStejskal, Jaroslav, Marek Jurča, Miroslava Trchová, and Jan Prokeš. 2025. "Electrical Properties of Semiconductor/Conductor Composites: Polypyrrole-Coated Tungsten Microparticles" Journal of Composites Science 9, no. 3: 98. https://doi.org/10.3390/jcs9030098
APA StyleStejskal, J., Jurča, M., Trchová, M., & Prokeš, J. (2025). Electrical Properties of Semiconductor/Conductor Composites: Polypyrrole-Coated Tungsten Microparticles. Journal of Composites Science, 9(3), 98. https://doi.org/10.3390/jcs9030098






