Abstract
There is a significant drive towards the development of edible biocompatible films for food packaging application due to the environmental and health impacts of synthetic packaging materials. This has inspired the exploration of biodegradable natural polymers as packaging materials. To address the instant water disintegration of most natural polymers, polymers with conditional water solubility, such as chitosan (needing acidic conditions for dissolution in water), have gained significant research attention. To this end, chitosan has been blended with different natural proteins, including whey protein isolates, to prepare edible food films. However, consumption of whey protein isolates in their natural form has been proposed in the literature to prolong processing (digestion) time upon consumption. To circumvent this limitation, here we report the development of chitosan/whey protein hydrolysate-based edible films with additional antioxidant properties. The developed films revealed that the inclusion of whey protein hydrolysate improved physicochemical properties and mechanical strength of the films with tensile strength of 26.3 MPa at 1 wt% WPH loading compared to 10.9 MPa in control neat chitosan films (0 wt% WPH). Furthermore, chitosan/whey protein hydrolysate exhibited a significant (whey protein hydrolysate) dose-dependent antioxidant response with a maximum value of 83% DPPH in chitosan/WPH (1 wt%) films assessed using two different antioxidant assays. Based on the results from this study, we envisage the exploration of whey protein hydrolysate-based films for commercial food packaging application in future.
1. Introduction
Food spoilage of perishable food products (fruits and vegetables) remains a significant commercial problem which has been one of the key challenges in the global food supply chain of fresh produce [1]. Synthetic polymers, while effective in fresh food packaging, cause significant environmental pollution. In recent years, there has been a drive to explore biopolymer-based fruit coatings as a safe, edible and environmentally friendly packaging material [2,3,4,5,6,7,8]. It is believed that biopolymer coatings can even extend the shelf-life of fruits and vegetables.
To this end, chitosan remains the most explored biopolymer showing promising results as food packaging material [2]. Chitosan is a naturally occurring polysaccharide composed of d–glucosamine and N–acetyl–d–glucosamine, which is obtained by the deacetylation of chitin (β-N-acetyl-d-glucosamine polymer) found in the exoskeleton of crustaceans [9,10,11]. Chitosan is a biodegradable, biocompatible biopolymer with significant antioxidant and antimicrobial properties which drove the interest to explore it for food packaging application [12,13,14,15]. However, neat chitosan suffers from low mechanical and thermal stability and high sensitivity to humidity with prolonged exposure. These factors remain the limiting factors restricting its industrial application [3]. Different strategies have been developed over the years to improve the intrinsic properties of chitosan, including blending it with other biopolymers and inclusion of fillers and additives (such as essential oils) [2,4]. One of the strategies includes blending chitosan with natural proteins such as protein isolates, including whey, rice and rapeseed protein isolates, to prepare edible food packaging coatings [7,16,17]. Whey protein isolates have many advantages due to their intrinsic properties such as oxygen barrier properties, being edible, biodegradable and wide availability [18,19]. Neat whey protein isolates are moisture sensitive, akin to neat chitosan, limiting their use as a food coating. However, when blended together, they form a stable coating which has shown promising results as fruit packaging coatings [20,21]. However, it is to be noted that whey protein isolates can require a relatively longer time to digest and process in the gut.
To take advantage of the food packaging properties of whey protein isolates and overcome the potential limitation towards slower digestive degradation, in this study, we have used whey protein hydrolysates (WPH) as a replacement to whey protein isolates. The inspiration was drawn from a previous study reporting that in acidic conditions (potentially emulating digestive environment), rapeseed protein isolate becomes hydrolyzed to form rapeseed protein hydrolysate, which considerably improves the solubility of rapeseed protein [22]. From that study [22], it was deduced that hydrolyzed rapeseed protein isolate has higher solubility in an acidic environment compared to unhydrolyzed rapeseed protein isolate. Traditionally, WPH are produced by treating whey protein isolates with acids, enzymes, or heat to cleave peptide bonds in whey protein to form smaller peptides and amino acids (known as WPH) [23]. Such a pre-digested form of whey protein (i.e., WPH) is effectively absorbed in the gut. Furthermore, WPH, produced by protease enzyme-mediated hydrolysis, exhibit a similar amino acid profile as whey protein isolate but with better digestion. Thus, this causes rapid absorption of different individual amino acids in the bloodstream compared to when ingested in the intact form (i.e., whey protein isolate) [23]. Some of the additional advantages of using WPH-based coatings include (i) significant antimicrobial and antioxidant activity, (ii) relatively improved mechanical properties compared to their whey protein isolates-based films, and (iii) oxygen permeability similar to whey protein isolates-based films [23,24]. To the best of our knowledge, chitosan has not been blended with WPH to prepare edible coatings for food packaging application.
In this study, we produced chitosan/WPH blends with different concentrations of WPH as potential food packaging films. While whey protein isolates have been explored as edible food packaging films, WPH remains greatly unexplored. WPH used in this work were produced using the method previously developed by us [25]. The obtained chitosan/WPH blend films were evaluated for their physical, mechanical, thermal and antioxidant properties. We observed the WPH concentration-dependent response in the physical, mechanical, and antioxidant properties of the developed films. However, no significant change in thermal properties was observed. The potential significance of this work is in the usage of WPH in edible chitosan-based food packaging film application.
2. Material and Methods
2.1. Chemicals
Chitosan (91.3% degree of deacetylation) was purchased from Sigma-Aldrich (Merck, Germany), and whey protein concentrate (WPC) of 85% was obtained from the cheese making process of Iraqi buffalo milk in the dairy factory located at the College of Agriculture, University of Basrah using our previously optimized method [25]. Alcalase, with the activity of 5 U/g, 1,1–diphenyl–2–picrylhydrazyl (DPPH), and ascorbic acid were purchased from Sigma-Aldrich (Steinheim, Germany), and trichloroacetic acid, chloroform, and acetic acid were purchased from Merck Chemicals Co. (Darmstadt, Germany).
2.2. Preparation of Whey Protein Concentrates
The whey proteins were separated using an ultrafiltration method previously developed by our group with some modifications [25]. Briefly, whey was passed through ultrafiltration membranes with pore size MWCO 10 kDa under the pressure of 5 bar followed by drying under a rotary evaporator (Franklin electric, Birmingham UK) at 40 °C. The protein concentrate was subsequently lyophilized to obtain the dried whey protein concentrate.
2.3. Preparation of Whey Protein Hydrolysate (WPH)
The hydrolysates were produced by solubilizing whey protein concentrate (5 g) in distilled water (10 mL) and heating the dispersion at 80 °C for 2 min. Subsequently, enzyme Alcalase (1 wt% relative to the protein) was added after dissolving it with a small amount of distilled water and allowed to react for 4 h. The solution’s pH was adjusted using hydrochloric acid and sodium hydroxide solutions, and the temperature was maintained at 50 °C by using a vibrating water bath. Next, the enzyme was inhibited by heating the solution to 90 °C for 10 min before freeze drying to obtain the dried hydrolysate. The method was adopted from a previously studied approach [26].
2.4. Amino Acid Composition
The amino acid composition of the hydrolysate was determined using an amino acids analyzer (SW company, Hongkong) equipped with an S4300 column and operating and two wavelengths (440 and 570 nm). The sample was prepared by adding the lyophilized hydrolysate (10–20 mg) to a HCl solution (5 mL, 6 N) in a close vacuum tube and heating the mixture to 110 °C for 24 h. Following this, the solution was centrifuged at 4000× g for 10 min to remove any unreacted hydrolysate, then 375 μL filtrate was taken and mixed with 0.12 N buffer solution of lithium citrate pH 2.9, and the solution was ready for analysis.
2.5. Preparation of Film-Forming Solution
To prepare chitosan films, first chitosan (1 g) was dissolved in 80 mL of aqueous acetic acid solution (1% v/v) under constant stirring at 800 rpm for 4 h. Next, whey protein hydrolysate (WPH) dissolved in 20 mL of distilled water at concentrations of 0.25%, 0.50%, 0.75%, and 1.0% (w/v) were added to the chitosan solution. The mixtures were drop cast in Petri dishes (diameter of 6 cm) and allowed to form the film for 48 h at 25 °C. Neat chitosan (1% w/v) film was used as a control.
2.6. Characterization of Films
2.6.1. Thickness
Film thickness was measured using a micrometer with an accuracy of 0.001 mm. Three separate measurements were taken at random locations along the length of each film sample. Data are presented as an average ± standard deviation of three measurements.
2.6.2. Film Solubility
Water solubility of films was investigated using the previously reported method by Nafchi et al. [27] with some minor modifications. The films were cut into squares (1 × 1 cm2) and were approximately 0.8 g. The individual film was placed in a plastic container with 15 mL distilled water and kept on a shaker at a speed of 200 rpm at 25 °C for 24 h to enable dissolution, followed by filtration using a Whatman filter paper to recover the undissolved film, which was then dried at 105 °C until the weight was stable. The solubility of a film was calculated according to the following equation:
Film solubility = [(initial weight − final weight)/initial weight] × 100%
2.6.3. Mechanical Testing
Mechanical testing of films in terms of tensile strength and elongation at break was carried out using a Texture Analyzer (BTI-FR, Zwick Roell, Ulm, Germany) fitted with a 50 N load cell and an initial separation of 80 mm between the two ends of sample clamps. Samples were prepared by cutting chitosan/WPH films with dimensions of 80 mm × 20 mm. The measurements were conducted with a crosshead speed of 50 mm/min until failure. The cutting speed was 200 mm/min, and the ramp speed of the device was 5 mm/min.
2.7. Microstructure of Films
2.7.1. Thermogravimetric Analysis (TGA)
TGA analysis was conducted using a TA Instruments TGA Q5000. A sample of 2 to 5 mg was loaded in a platinum sample holder heated at a temperature ramp of 25 to 800 °C with a heating rate of 10 °C/min under air atmosphere with a flow rate of 25 mL/min.
2.7.2. X-ray Diffraction
X-ray diffraction (XRD) pattern of films was analyzed using an Empyrean XRD diffractometer fitted with a cobalt source using Bragg-Brentano geometry operating at 45 kV and 40 mA. Samples were scanned at 2θ = 5–90° with a step size of 0.04°, time per step of 190 s and a scan speed of 0.052 degrees/s.
2.7.3. Scanning Electron Microscopy (SEM)
The surface morphology of films was characterized using a FEI Nova NanoSEM 450 FE-SEM at an accelerating voltage of 5 kV. The samples were sputter coated with a 10 nm platinum prior to imaging.
2.8. Antioxidant Properties
2.8.1. DPPH Radical Scavenging Activity Assay
DPPH (1,1–diphenyl–2-picryl–hydrazil) radical scavenging activity of the films were determined using the method reported previously with minor changes [28]. Briefly, 2 mL of the sample or ascorbic acid (0.25 mg/mL) as a positive control was mixed with 1 mL of 0.1 mM DPPH dissolved in 95% ethanol. The mixture was mixed vigorously and then kept for 30 min in the dark at room temperature. The absorbance was recorded at 517 nm (Spectrophotometer, Sunny, Germany). A blank was prepared in the same manner, except that 95% ethanol was used instead of the sample. The inhibitory activity of DPPH was calculated as:
2.8.2. Ferric Reducing Antioxidant Power (FRAP) Assay
FRAP analysis of the chitosan/WPH samples was conducted using the previously established method with minor modifications [29]. Briefly, 0.5 mL of chitosan/WPH solution was added to 0.5 mL of phosphate buffer (0.2 M, pH 6.6) and 0.5 mL of 1% K3[Fe(CN)6] mixture. The mixture was kept in an incubator at 60 °C for 30 min, followed by centrifugation with 0.5 mL of 10 % trichloroacetic acid. The supernatant was then added to 1 mL of distilled water containing 0.2 mL of 0.1 % ferric chloride. The mixture was allowed to react for 10 min, followed by absorbance measurement of the color change at 700 nm.
2.9. Statistics
The results for film thickness, solubility, tensile strength and elongation at break, DPPH and FRAP are expressed as mean ± standard deviation and analyzed using one-way analysis of variance (ANOVA). Significance was evaluated using a Bonferroni post-hoc analysis and set at 95% confidence (p < 0.05).
3. Results and Discussion
3.1. Amino Acid Composition
We first characterized amino acid composition in our whey protein hydrolysate (WPH) prepared from buffalo milk. It is important to characterize the main types of amino acids and their respective relevance, as they are highly dependent on the protein source, and amino acid composition can have a considerable influence on film properties. Table 1 lists the type and amount of different amino acids in our WPH. The amino acid analysis revealed a total of 17 amino acids in our WPH. Out of 17 amino acids, 31.23% were hydrophobic, 34.03% were essential amino acids (total eight), and 12.43% amino acids had an acidic functional group. The amino acid cysteine was the most abundant. The obtained composition and relative amounts of different amino acids are in partial agreement with previously published report by Bassan et al. (2015) [30]. However, the main difference between the work of Bassan et al. [30] and this work is that they obtained 14 amino acids, including six essential amino acids, compared to 17 and eight, respectively, in this work. The reason for the variation in the concentrations of amino acids may be due to the hydrolysis conditions in terms of the specificity of the action of the enzyme used and the time of hydrolysis. The type of amino acids and their position in the peptide chain depends on the mechanism of action of the enzyme [31].

Table 1.
Amino acid composition of WPH.
3.2. Characterization of Nanocomposite Films
3.2.1. Physical Properties
Thickness
The chitosan/WPH composite films with different WPH loading were prepared by a dropcast method. The obtained films were characterized for thickness (Figure 1). Composite film thickness is an important parameter in food packaging application due to its need to function as a barrier to restrict moisture from the outside environment interacting with packaged food. We obtained a strong positive correlation showing a significant increase in composite film thickness with an increasing amount of WPH (p < 0.05) with values of 19.3 ± 0.40 mm at 0 wt% WPH (control neat chitosan film), 22.5 ± 0.61 mm at 0.25 wt% WPH, 25.6 ± 0.64 mm at 0.5 wt% WPH, 28.3 ± 0.93 mm at 0.7 wt% WPH, and 31.95 ± 1.08 mm at 1 wt% WPH. The maximum thickness of 31.95 mm was obtained at 1% WPH, which was significantly greater than control (neat chitosan—0 wt% WPH), 0.25 wt%, 0.50 wt% and 0.75 wt% WPH loaded films (p < 0.05). The obtained film thicknesses were significantly greater than whey protein isolate/glycerol/pullulan films [32] and marginally lower than edible films made from calcium caseinate/whey protein isolates reported previously [33]. The observed increase in film thickness in this work may be attributed to whey peptides of WPH due to their covalent and non-covalent interactions with amine groups of chitosan [34].
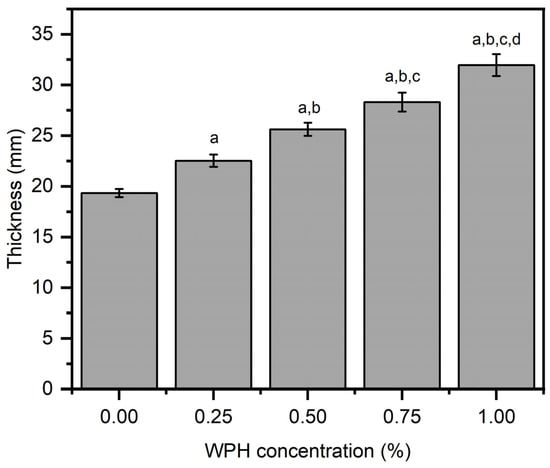
Figure 1.
The thickness properties of chitosan/WPH films containing different concentrations of WPH. Data are presented as an average of three measurements ± standard deviation. Values with different letters on the top of bars are significantly different (p < 0.05) and determined by using a Bonferroni post hoc test in a one way ANOVA analysis—a, b, c, d are relative to films comprising 0 wt%, 0.25 wt%, 0.5 wt% and 0.75 wt% WPH, respectively.
Film Solubility
Water solubility is an important factor in the application of films for food packaging to preserve the product and to reduce the environmental problems caused by non-biodegradable films [35]. Figure 2 showed that the addition of WPH considerably affected the solubility of films, i.e., the solubility increased with increasing concentration of WPH, although not reaching significance (Figure 2). The film solubility increased significantly (p < 0.05) from 21.65 ± 1.39% at 0 wt% WPH (neat chitosan film) to 28.31 ± 0.62% at 1 wt% WPH. The highest film solubility was observed for 1 wt% WPH loaded composite film (~28.31%), which was only significantly higher than control (near chitosan—0 wt% WPH) and 0.25 wt% WPH films. The marginally increased solubility of composite films with increasing WPH loading can be explained by relatively higher aqueous solubility of WPH compared chitosan (requires acidic conditions to induce aqueous solubility). We envisage that the observed marginal increase in film solubility with increasing WPH loading will not be sufficient to completely disintegrate the film. It is hypothesized that water-insoluble chitosan is a predominant component in protecting the developed films from completely disintegrating in the presence of water. The obtained film solubility is significantly lower than previously reported for whey protein isolate-based edible films [32].
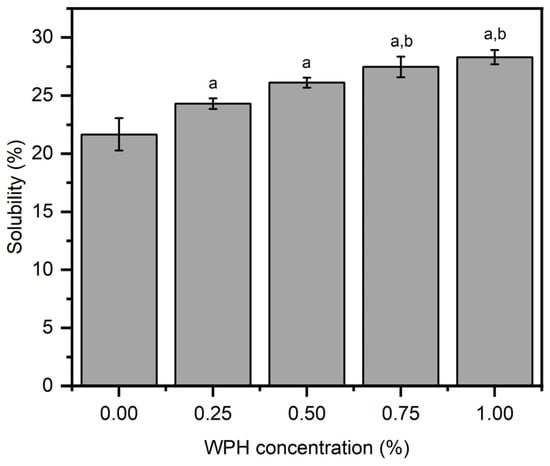
Figure 2.
The solubility properties of chitosan/WPH films containing different concentrations of WPH. Data are presented as an average of three measurements ± standard deviation. Values with different letters on the top of bars are significantly different (p < 0.05) and determined by using a Bonferroni post hoc test in a one-way ANOVA analysis—a and b are relative to films comprising 0 wt% and 0.25 wt% WPH, respectively.
3.2.2. Mechanical Testing
Next, we assessed the mechanical properties of composite films. Tensile strength is a critical mechanical parameter for a packaging film attributed to preventing potential damages incurred during post-production storage and transportation. Tensile testing was used to determine the strength of the developed films, which entails elongating the film under increasing applied stress until failure. At failure, the maximum measured stress equates to the tensile strength of the films, whereas the elongation corresponds to the percentage change in the elongation from the initial starting length of the film [36]. As shown in Figure 3a, the addition of WPH significantly improves the tensile strength of chitosan/WPH composite films (p < 0.05). As compared to control (neat chitosan film—10.90 ± 1.08 MPa), the addition of WPH increased the tensile strength of composite films regardless of the WPH loading concentration (p < 0.05), except between 0.75 wt% to 1 wt% WPH loaded films. The highest tensile strength was obtained for chitosan/WPH (1 wt%) (26.27 ± 0.89 MPa), which was significantly higher than control (neat chitosan), 0.25 wt% and 0.5 wt% WPH loaded films (p < 0.05). The observed increase in tensile strength may be attributed to covalent interactions (crosslinking) of chitosan by WPH mediated by a condensation reaction between amine (from chitosan) and carboxylic acid (WPH amino acids). It has been recently shown that carboxylic acid groups can react with primary amines to form amide-like linages resulting in crosslinking of substrates [37]. Intermolecular crosslinking, as hypothesized here, has been widely accepted to cause a considerable increase in mechanical properties [38,39,40]. Contrary to tensile strength, we observed a reduction in elongation at break with increasing amounts of WPH in composite films. As shown in Figure 3b, the highest elongation in the control sample was 16.14%, then it decreased with the increase in peptide concentration (0.25, 0.50, 0.75, 1 wt%) to 14.17, 13.21, 11.22 and 9.57%, respectively. However, the reduction in elongation at break was found significant only in films with >0.25 wt% WPH loading. The obtained elongation at break results are consistent with a previous study suggesting that the increase in tensile strength usually leads to a decrease in flexibility of composite films (elongation at break) with the inclusion of a filler [41].
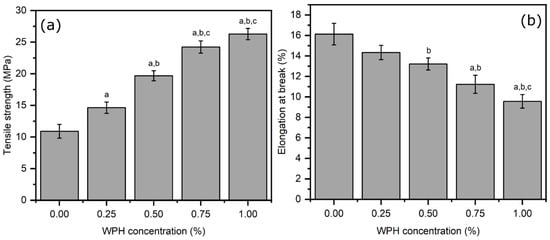
Figure 3.
Mechanical properties of chitosan/WPH films containing different concentrations of WPH showing (a) tensile strength and (b) elongation at break. Data are presented as an average of three measurements ± standard deviation. Values with different letters on the top of bars are significantly different (p < 0.05) determined using a Bonferroni post hoc test in a one-way ANOVA analysis—a, b, c are relative to films comprising 0 wt%, 0.25 wt% and 0.5 wt% WPH, respectively.
3.2.3. Antioxidant Properties
Antioxidant Capacity DPPH Radical Scavenging Activity Assay
One of the key roles of food packaging material is to preserve food leading to longer shelf-life. One of the ways this can be achieved is if the packaging material exhibits antioxidant properties necessary to prevent oxidation-mediated fouling of packaged food. To this end, we conducted the well-established DPPH assay to determine the antioxidant capacity of our chitosan/WPH films. DPPH (2,2′-diphenyl-1-picrylhydrazyl radical) assay is a simple colorimetric method to determine the radical scavenging potential of a material. DPPH, when exposed to an antioxidant compound, loses its free radical-generating ability leading to the change in color from violet to yellow, which is used to quantify the antioxidant efficiency of a testing material. Figure 4 shows the DPPH activity against different films developed in this work. We observed a significant increase in antioxidant activity (adjudged with increasing %DPPH) with an increasing amount of WPH in composite films (p < 0.05) reaching the maximum value of ~83% at 1 wt% WPH loading (Figure 4). The control film made from neat chitosan exhibited antioxidant activity of ~39%. The antioxidant activity was 38.9 ± 0.47 mm at 0 wt% WPH (control neat chitosan film), 44.3 ± 1.14 mm at 0.25 wt% WPH, 66.7 ± 2.40 mm at 0.5 wt% WPH, 74.4 ± 1.65 mm at 0.75 wt% WPH, 82.8 ± 1.95 mm at 1 wt% WPH. This noticeable antioxidant activity of chitosan can be attributed to its intrinsic free-radical scavenging property [3,12]. The observed antioxidant activity of chitosan/WPH composite films is in line with previous reports on neat WPH [42]. It has been reported that peptides (fragments from β-lactoglobulin, α-lactoalbumin, and β-casein) released during the hydrolysis of whey protein exhibit antioxidative, antimicrobial, antihypertensive, antithrombotic activity [42,43,44,45]. For example, β-lactoglobulin is the source of γ–glutamylcysteine dipeptide, which is a precursor of a strong antioxidant, glutathione [23]. The amino acid residues responsible for the antioxidant activity that is released during the hydrolysis of whey protein include tyrosine, methionine, lysine, histidine and tryptophan [46]. These antioxidant amino acids and their peptide moieties function by reducing peroxidation, scavenging free radicals and reactive oxygen species (ROS) neutralization in general [47].
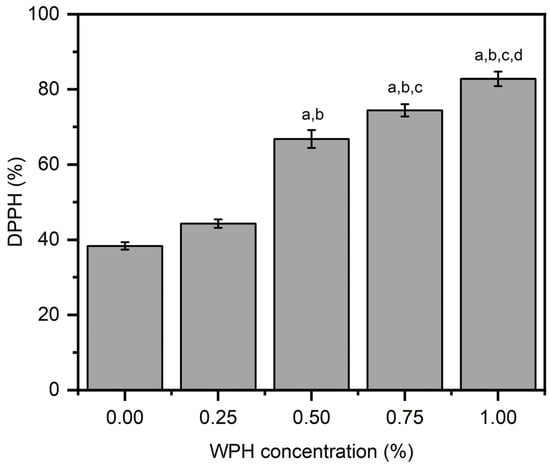
Figure 4.
DPPH radical of chitosan/WPH films containing different concentrations of WPH. Data are presented as an average of three measurements ± standard deviation. Values with different letters on the top of bars are significantly different (p < 0.05) and determined by using a Bonferroni post hoc test in a one-way ANOVA analysis—a, b, c, d are relative to films comprising 0 wt%, 0.25 wt%, 0.5 wt% and 0.75 wt% WPH, respectively.
Ferric Reducing Antioxidant Power (FRAP) Assay
To corroborate DPPH data, we conducted FRAP analysis which is used to determine the total antioxidant content in developed chitosan/WPH composite films. FRAP utilizes ferric (Fe+3)-TPTZ (iron [III]-2,4,6-tripyridyl-S-triazine), which reduces to an intense blue color ferrous (Fe+2)-TPTZ compound in the presence of antioxidant material. The intensity of the blue color leads to the quantification of the total antioxidant activity of the tested material. Similar to the DPPH assay, we observed an almost linear increase in the total antioxidant amount with increasing concentrations of WPH in chitosan/WPH composite films (Figure 5). The measured values were 0.20 ± 0.01 mm at 0 wt% WPH (control neat chitosan film), 0.25 ± 0.05 mm at 0.25 wt% WPH, 0.35 ± 0.06 mm at 0.5 wt% WPH, 0.5 ± 0.01 mm at 0.75 wt% WPH, 0.57 ± 0.06 mm at 1 wt% WPH. The local antioxidant values measured using FRAP were significantly higher (p < 0.05) for films comprising 0.75 wt% and 1 wt% WPH relative to control (neat chitosan), 0.25 wt% and 0.5 wt% WPH loaded films (Figure 5). The trend observed in FRAP analysis is similar to the DPPH analysis which further corroborates the antioxidant properties of WPH used in this study. As per the discussion above, the significantly higher antioxidant response in films loaded with considerably high WPH loading (0.75 wt% and 1 wt%) can be attributed to the amino acids in WPH and the products released during the in situ hydrolysis of WPH [23,46,47].
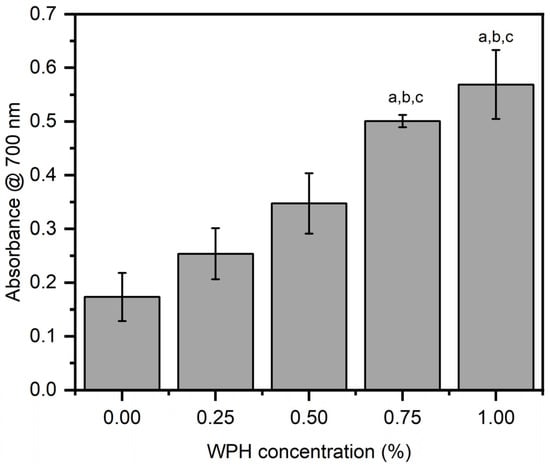
Figure 5.
FRAP analysis of chitosan/WPH films containing different concentrations of WPH. Data are presented as an average of three measurements ± standard deviation. Values with different letters on the top of bars are significantly different (p < 0.05) and determined by using a Bonferroni post hoc test in a one-way ANOVA analysis—a, b, c are relative to films comprising 0 wt%, 0.25 wt% and 0.5 wt% WPH, respectively.
3.3. Films Surface Microstructure Characterization
The surface morphology of the chitosan/WPH films was analyzed using SEM. SEM images of composite films are shown in Figure 6. SEM imaging revealed unevenness on the surface of the neat chitosan film, which has been attributed to the preparation step. The inclusion of WPH at lower concentrations (0.25 wt% and 0.5 wt%) resulted in smoother films indicating efficient blending of WPH in chitosan solution, similar to a previous report on chitosan/rice protein hydrolysates [17]. Further increase in WPH (0.75 wt% and 1 wt%) induced (WPH) concentration-dependent increase in surface roughness in composite films. However, the surface roughness at these concentrations (0.75 wt% and 1 wt% WPH) is quite uniform, indicating a homogeneous distribution of WPH in the chitosan matrix and corresponding films. The obtained surface morphology of chitosan/WPH films and (WPH) concentration-dependent increase in surface roughness is similar to previous reports on chitosan/rapeseed protein hydrolysate and chitosan/whey protein composite films [22,48].
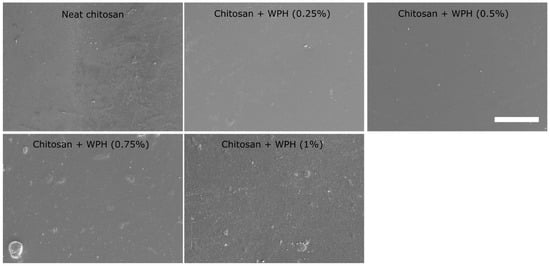
Figure 6.
SEM images of chitosan/WPH films containing different concentrations of WPH. Scale bar = 100 µm.
3.4. X-ray Diffraction (XRD) Patterns
The XRD analysis of the chitosan/WPH nanocomposite films exhibited a characteristic broad peak centered around 2θ = 23° (Figure 7). The broadness of the peak indicates the amorphous nature of nanocomposite films. We observed no considerable difference in the XRD patterns with the inclusion of WPH in the chitosan matrix regardless of the concentration of WPH. The observed broad peak is consistent with previous reports on proteins and similar matrices [49,50,51,52]. It was concluded that the inclusion of WPH, regardless of the concentration, caused no structural or chemical change in the chitosan matrix, which could be considered an advantage. It is to be noted that some previous reports have shown two peaks for the next chitosan [53,54], which is inconsistent with our results. The anomalous observation here could be attributed to the nature of our samples being too thin as opposed to a powdered analysis conducted in those studies using bulk chitosan.
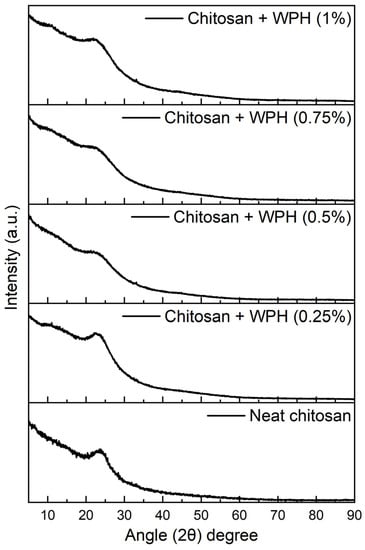
Figure 7.
XRD analysis of chitosan/WPH films containing different concentrations of WPH showing amorphous nature of nanocomposites.
3.5. Thermal Properties
The thermal property of the chitosan/WPH nanocomposite films was studied using thermal gravimetric analysis (TGA). All the samples exhibited three main decomposition events—(i) 25–100 °C, (ii) 100–350 °C, and (c) 400–600 °C (Figure 8). The first decomposition (25–100 °C) can be attributed to the loss of water, the second step (100–350 °C) to a loss of functional groups such as amines, hydroxyl and carboxyl from chitosan and WPH, and the final decomposition (400–600 °C) can be ascribed to the decomposition of the carbon backbone. All the samples exhibited ~10% weight loss to 100 °C due to loss of water in the samples. From 100 to 350 °C, chitosan/WPH films exhibited marginally higher weight loss (lower residual mass) compared to neat chitosan film (Figure 8). This difference could be attributed to the higher number of functional groups in the chitosan/WPH films compared to the neat chitosan film. Finally, between 400 and 600 °C, neat chitosan film exhibited marginally lower weight loss compared to chitosan/WPH films which could be attributed to the carbon backbone of chitosan (with relatively slower decomposition). Chitosan/WPH films exhibited lower weight loss due to a lower amount of chitosan in samples (due to supplementation of WPH) and thermal vulnerability of WPH compared to chitosan. There were no significant differences between the WPH samples, and no specific trend was observed at different WPH loading in nanocomposite films. Almost complete degradation was observed at 800 °C for all samples, which is indicative of the organic nature of the samples.
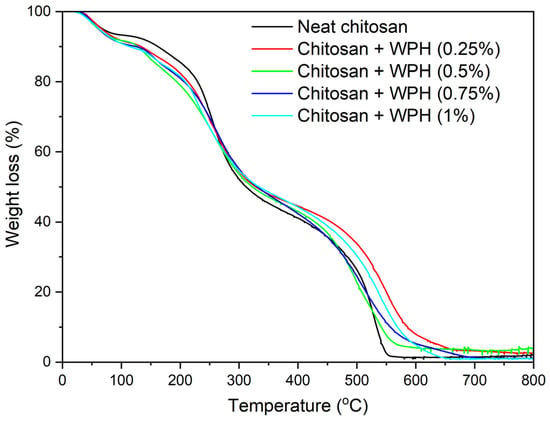
Figure 8.
TGA analysis of chitosan/WPH films containing different concentrations of WPH showing loss in mass with increasing annealing temperature.
4. Conclusions
The globalization of the food industry has seen the transportation of perishable food products such as fruits and vegetables across the globe. This global transportation of goods has drawn commercial interest toward the use of edible films to prolong the shelf-life of perishable food products. To this end, we have developed chitosan/whey protein hydrolysate (WPH)-based films for food packaging application. By varying the amount of WPH, we observed a considerable increase in chitosan/WPH films, which in turn significantly improved the mechanical properties (tensile strength) of these composite films. Although, an increase in WPH compromised the stretchability of the developed films (elongation at break) to some extent. The inclusion of WPH further induced antioxidant properties in the developed films. Composite chitosan/WPH films exhibited a WPH dose-dependent antioxidant response in DPPH and FRAP assays. SEM imaging revealed some increase in surface roughness at high concentrations of WPH (0.75 and 1 wt%). However, no change in intrinsic chitosan behavior (in XRD) or thermal properties of composite films (in TGA) was observed with the inclusion of WPH, regardless of the concentration. These taken together, this study presents commercially viable edible food films with intrinsic antioxidant properties and ease of digestibility when ingested for food packaging application.
Author Contributions
Conceptualization, S.A.A.-H.; methodology, S.A.A.-H., O.T.A.-I., R.R.A.-H., R.M.A.-A., N.M. and Y.Y.; software, S.A.A.-H., O.T.A.-I., R.R.A.-H., R.M.A.-A., N.M. and Y.Y.; validation, S.A.A.-H., O.T.A.-I., R.R.A.-H. and R.M.A.-A.; formal analysis, O.T.A.-I., R.R.A.-H., N.M. and V.A.; investigation, S.A.A.-H., O.T.A.-I., R.R.A.-H., R.M.A.-A., N.M. and Y.Y.; resources, V.A.; data curation, S.A.A.-H., O.T.A.-I., R.R.A.-H., R.M.A.-A., N.M., Y.Y., N.M. and Y.Y.; writing—original draft preparation, S.A.A.-H., O.T.A.-I. and V.A.; writing—review and editing, V.A.; visualization, S.A.A.-H., O.T.A.-I., R.R.A.-H., R.M.A.-A., N.M. and Y.Y.; supervision, S.A.A.-H.; project administration, S.A.A.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data available on reasonable request from the authors.
Acknowledgments
The authors acknowledge the College of Agriculture at the University of Basrah for infrastructural support. V.A. acknowledges the University of New South Wales (UNSW), Australia, for a Safety Net Fellowship. The authors acknowledge the facilities and the scientific and technical assistance of Microscopy Australia at the Electron Microscope Unit (EMU), and Solid State & Elemental Analysis Unit within the Mark Wainwright Analytical Centre (MWAC) at UNSW Sydney.
Conflicts of Interest
Authors declare no conflict of interest.
References
- Sung, S.-Y.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Rahmat, A.R.; Rahman, W.A.W.A.; Tan, A.-C.; Vikhraman, M. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation—A review of advances and applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef] [PubMed]
- Basumatary, I.B.; Mukherjee, A.; Katiyar, V.; Kumar, S. Biopolymer-based nanocomposite films and coatings: Recent advances in shelf-life improvement of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2022, 62, 1912–1935. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, C.V.; Sarkar, P. Composite edible films and coatings from food-grade biopolymers. J. Food Sci. Technol. 2018, 55, 4369–4383. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Al-Hilifi, S.A.; Al-Ali, R.M.; Al-Ibresam, O.T.; Kumar, N.; Paidari, S.; Trajkovska Petkoska, A.; Agarwal, V. Physicochemical, Morphological, and Functional Characterization of Edible Anthocyanin-Enriched Aloevera Coatings on Fresh Figs (Ficus carica L.). Gels 2022, 8, 645. [Google Scholar] [CrossRef]
- Eroglu, E.; Agarwal, V.; Bradshaw, M.; Chen, X.; Smith, S.M.; Raston, C.L.; Swaminathan Iyer, K. Nitrate removal from liquid effluents using microalgae immobilized on chitosan nanofiber mats. Green Chemistry 2012, 14, 2682–2685. [Google Scholar] [CrossRef]
- Kou, S.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Eroglu, E.; Chen, X.; Bradshaw, M.; Agarwal, V.; Zou, J.; Stewart, S.G.; Duan, X.; Lamb, R.N.; Smith, S.M.; Raston, C.L.; et al. Biogenic production of palladium nanocrystals using microalgae and their immobilization on chitosan nanofibers for catalytic applications. RSC Adv. 2013, 3, 1009–1012. [Google Scholar] [CrossRef]
- Rizwana, N.; Agarwal, V.; Nune, M. Antioxidant for Neurological Diseases and Neurotrauma and Bioengineering Approaches. Antioxidants 2022, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, Y.; Duan, S.; Li, C.; Hu, B.; Liu, A.; Wu, D.; Cui, H.; Lin, L.; He, J.; et al. Preparation and characterization of chitosan films with three kinds of molecular weight for food packaging. Int. J. Biol. Macromol. 2020, 155, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.K.T.; Frias, R.R.; Alvarez, L.V.; Bigol, U.G.; Guzman, J.P.M.D. Comparative antibacterial activity of commercial chitosan and chitosan extracted from Auricularia sp. Biocatal. Agric. Biotechnol. 2019, 17, 189–195. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, L.; Sun, X.; Fan, F.; Ding, J.; Li, P.; Zhu, Y.; Xu, T.; Fang, Y. Improvement in the storage quality of fresh salmon (Salmo salar) using a powerful composite film of rice protein hydrolysates and chitosan. Food Control 2022, 142, 109211. [Google Scholar] [CrossRef]
- Wang, L.; Ding, J.; Fang, Y.; Pan, X.; Fan, F.; Li, P.; Hu, Q. Effect of ultrasonic power on properties of edible composite films based on rice protein hydrolysates and chitosan. Ultrason. Sonochemistry 2020, 65, 105049. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Fernandes, J.C.; Silva, S.I.; Pintado, M.E.; Malcata, F.X. Edible Films and Coatings from Whey Proteins: A Review on Formulation, and on Mechanical and Bioactive Properties. Crit. Rev. Food Sci. Nutr. 2012, 52, 533–552. [Google Scholar] [CrossRef]
- Lara, B.R.B.; Dias, M.V.; Guimarães Junior, M.; de Andrade, P.S.; de Souza Nascimento, B.; Ferreira, L.F.; Yoshida, M.I. Water sorption thermodynamic behavior of whey protein isolate/ polyvinyl alcohol blends for food packaging. Food Hydrocoll. 2020, 103, 105710. [Google Scholar] [CrossRef]
- Farsanipour, A.; Khodanazary, A.; Hosseini, S.M. Effect of chitosan-whey protein isolated coatings incorporated with tarragon Artemisia dracunculus essential oil on the quality of Scomberoides commersonnianus fillets at refrigerated condition. Int. J. Biol. Macromol. 2020, 155, 766–771. [Google Scholar] [CrossRef]
- Muley, A.B.; Singhal, R.S. Extension of postharvest shelf life of strawberries (Fragaria ananassa) using a coating of chitosan-whey protein isolate conjugate. Food Chem. 2020, 329, 127213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Z.; Li, Y.; Yang, Y.; Ju, X.; He, R. The preparation and physiochemical characterization of rapeseed protein hydrolysate-chitosan composite films. Food Chem. 2019, 272, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Tkaczewska, J. Peptides and protein hydrolysates as food preservatives and bioactive components of edible films and coatings—A review. Trends Food Sci. Technol. 2020, 106, 298–311. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Water Vapor Permeability and Solubility of Films from Hydrolyzed Whey Protein. J. Food Sci. 2000, 65, 700–703. [Google Scholar] [CrossRef]
- Al-Hatim, R.R.; Al-Rikabi, A.K.; Ghadban, A.K. The Physico-Chemical Properties of Bovine and Buffalo Whey Proteins Milk by Using Ultrafiltration Membrane Technology. Basrah J. Agric. Sci. 2020, 33, 122–134. [Google Scholar] [CrossRef]
- Perea, A.; Ugalde, U.; Rodriguez, I.; Serra, J.L. Preparation and characterization of whey protein hydrolysates: Applications in industrial whey bioconversion processes. Enzym. Microb. Technol. 1993, 15, 418–423. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Alias, A.K.; Mahmud, S.; Robal, M. Antimicrobial, rheological, and physicochemical properties of sago starch films filled with nanorod-rich zinc oxide. J. Food Eng. 2012, 113, 511–519. [Google Scholar] [CrossRef]
- Taheri, A.; Sabeena Farvin, K.H.; Jacobsen, C.; Baron, C.P. Antioxidant activities and functional properties of protein and peptide fractions isolated from salted herring brine. Food Chem. 2014, 142, 318–326. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Chang, W.-H.; Chen, C.-S.; Liao, J.-W.; Huang, C.-J.; Lu, F.-J.; Chia, Y.-C.; Hsu, H.-K.; Wu, J.-J.; Yang, H.-L. Antioxidant activities of Toona sinensis leaves extracts using different antioxidant models. Food Chem. Toxicol. 2008, 46, 105–114. [Google Scholar] [CrossRef]
- Bassan, J.C.; Goulart, A.J.; Nasser, A.L.M.; Bezerra, T.M.S.; Garrido, S.S.; Rustiguel, C.B.; Guimarães, L.H.S.; Monti, R. Buffalo Cheese Whey Proteins, Identification of a 24 kDa Protein and Characterization of Their Hydrolysates: In Vitro Gastrointestinal Digestion. PLoS ONE 2015, 10, e0139550. [Google Scholar] [CrossRef]
- Wali, A.; Yanhua, G.; Ishimov, U.; Yili, A.; Aisa, H.A.; Salikhov, S. Isolation and Identification of Three Novel Antioxidant Peptides from the Bactrian Camel Milk Hydrolysates. Int. J. Pept. Res. Ther. 2020, 26, 641–650. [Google Scholar] [CrossRef]
- Gounga, M.E.; Xu, S.-Y.; Wang, Z. Whey protein isolate-based edible films as affected by protein concentration, glycerol ratio and pullulan addition in film formation. J. Food Eng. 2007, 83, 521–530. [Google Scholar] [CrossRef]
- Vachon, C.; Yu, H.L.; Yefsah, R.; Alain, R.; St-Gelais, D.; Lacroix, M. Mechanical and Structural Properties of Milk Protein Edible Films Cross-Linked by Heating and γ-Irradiation. J. Agric. Food Chem. 2000, 48, 3202–3209. [Google Scholar] [CrossRef]
- Abugoch, L.E.; Tapia, C.; Villamán, M.C.; Yazdani-Pedram, M.; Díaz-Dosque, M. Characterization of quinoa protein–chitosan blend edible films. Food Hydrocoll. 2011, 25, 879–886. [Google Scholar] [CrossRef]
- de Jesus, G.L.; Baldasso, C.; Marcílio, N.R.; Tessaro, I.C. Demineralized whey–gelatin composite films: Effects of composition on film formation, mechanical, and physical properties. J. Appl. Polym. Sci. 2020, 137, 49282. [Google Scholar] [CrossRef]
- Park, S.-I.; Zhao, Y. Incorporation of a High Concentration of Mineral or Vitamin into Chitosan-Based Films. J. Agric. Food Chem. 2004, 52, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Maslekar, N.; Zetterlund, P.B.; Kumar, P.V.; Agarwal, V. Addition and Correction to “Mechanistic Aspects of the Functionalization of Graphene Oxide with Ethylene Diamine: Implications for Energy Storage Applications”. ACS Appl. Nano Mater. 2021, 4, 8637–8640. [Google Scholar] [CrossRef]
- Cao, L.; Liu, W.; Wang, L. Developing a green and edible film from Cassia gum: The effects of glycerol and sorbitol. J. Clean. Prod. 2018, 175, 276–282. [Google Scholar] [CrossRef]
- Kchaou, H.; Benbettaieb, N.; Jridi, M.; Nasri, M.; Debeaufort, F. Influence of Maillard reaction and temperature on functional, structure and bioactive properties of fish gelatin films. Food Hydrocoll. 2019, 97, 105196. [Google Scholar] [CrossRef]
- Zhou, W.; He, Y.; Liu, F.; Liao, L.; Huang, X.; Li, R.; Zou, Y.; Zhou, L.; Zou, L.; Liu, Y.; et al. Carboxymethyl chitosan-pullulan edible films enriched with galangal essential oil: Characterization and application in mango preservation. Carbohydr. Polym. 2021, 256, 117579. [Google Scholar] [CrossRef]
- Xue, F.; Gu, Y.; Wang, Y.; Li, C.; Adhikari, B. Encapsulation of essential oil in emulsion based edible films prepared by soy protein isolate-gum acacia conjugates. Food Hydrocoll. 2019, 96, 178–189. [Google Scholar] [CrossRef]
- Mann, B.; Kumari, A.; Kumar, R.; Sharma, R.; Prajapati, K.; Mahboob, S.; Athira, S. Antioxidant activity of whey protein hydrolysates in milk beverage system. J. Food Sci. Technol. 2015, 52, 3235–3241. [Google Scholar] [CrossRef]
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef]
- Fematt-Flores, G.E.; Aguiló-Aguayo, I.; Marcos, B.; Camargo-Olivas, B.A.; Sánchez-Vega, R.; Soto-Caballero, M.C.; Salas-Salazar, N.A.; Flores-Córdova, M.A.; Rodríguez-Roque, M.J. Milk Protein-Based Edible Films: Influence on Mechanical, Hydrodynamic, Optical and Antioxidant Properties. Coatings 2022, 12, 196. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Miralles, B.; Amigo, L.; Ramos, M.; Recio, I. Identification of antioxidant and ACE-inhibitory peptides in fermented milk. J. Sci. Food Agric. 2005, 85, 1041–1048. [Google Scholar] [CrossRef]
- Bierzunska, P.; Cais-Sokolińska, D.; Rudzinska, M.; Gramza-Michalowska, A. Evaluation of antioxidant activity of whey protein to improve cholesterol oxidation stability in fresh white cheese from buttermilk. J. Food Nutr. Res. 2017, 56, 101–108. [Google Scholar]
- Sohaib, M.; Anjum, F.M.; Sahar, A.; Arshad, M.S.; Rahman, U.U.; Imran, A.; Hussain, S. Antioxidant proteins and peptides to enhance the oxidative stability of meat and meat products: A comprehensive review. Int. J. Food Prop. 2017, 20, 2581–2593. [Google Scholar] [CrossRef]
- Ferreira, C.O.; Nunes, C.A.; Delgadillo, I.; Lopes-da-Silva, J.A. Characterization of chitosan–whey protein films at acid pH. Food Res. Int. 2009, 42, 807–813. [Google Scholar] [CrossRef]
- Agarwal, V.; Panicker, A.G.; Indrakumar, S.; Chatterjee, K. Comparative study of keratin extraction from human hair. Int. J. Biol. Macromol. 2019, 133, 382–390. [Google Scholar] [CrossRef]
- Semwal, A.; Singh, B.; Archana, D.; Verma, A.; Dutta, P. Macromolecular Chitosan/Ciprofloxacin Pro-Drugs: Synthesis, Physico-chemical and Biological Assess-ment for Drug Delivery Systems. J. Polym. Mater. 2012, 29, 1–13. [Google Scholar]
- Kumar, S.; Koh, J. Physiochemical, Optical and Biological Activity of Chitosan-Chromone Derivative for Biomedical Applications. Int. J. Mol. Sci. 2012, 13, 6102–6116. [Google Scholar] [CrossRef]
- Fernandes, R.; Borges, S.; Botrel, D.; Oliveira, C. Physical and chemical properties of encapsulated rosemary essential oil by spray drying using whey protein–inulin blends as carriers. Int. J. Food Sci. Technol. 2014, 49, 1522–1529. [Google Scholar] [CrossRef]
- Peng, J.; Wang, X.; Lou, T. Preparation of chitosan/gelatin composite foam with ternary solvents of dioxane/acetic acid/water and its water absorption capacity. Polym. Bull. 2020, 77, 5227–5244. [Google Scholar] [CrossRef]
- Phung Hai, T.A.; Sugimoto, R. Fluorescence control of chitin and chitosan fabricated via surface functionalization using direct oxidative polymerization. RSC Adv. 2018, 8, 7005–7013. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).