Abstract
A compact pH measuring electrochemical sensor module was developed for Smart Multi-Well Plates (SMWP) applicable for highly parallelized cell culture analysis using incorporated Organ-on-Chip devices. A specific electronic architecture was designed and manufactured containing an extended gate field effect transistor as the transducer device. Electrochemical electrodes were functionalized using pH sensitive metal-oxides and applied as the gate material. The composition and the related pH sensitivity of differently deposited materials were characterized and the suitability of ALD-deposited, non-stoichiometric titanium oxide (TiOx) for sensitive pH measurement was verified showing excellent responses close to the ideal Nernstian slope (59 mV/pH).
1. Introduction
Besides different marker molecules—such as relevant amino acids, carbohydrates, and drugs such as antibiotics—pH is another important parameter used to characterize the metabolic parameters of cell cultures and tissues in Organ-on-Chip applications. The good performance of the optical analysis method for pH measurement in Phenol red indicator-dye-containing media was proven, although a generally used, integrated electrochemical method could be more beneficial for various culture media.
Miniaturized and robust pH detectors based on potentiometric, conductometric methods or ion-selective field effect transistors (ISFET) and extended gate field effect transistors (EGFET) have been demonstrated utilizing various ion-sensitive metal-oxide (MOx) materials such as RuO2, IrO2, TiO2, SnO2, Ta2O5, WO3, ZnO, etc. In the case of the ISFET and EGFET pH sensors, the source–drain current of the transistor is controlled by the electrostatic field generated by the ion-sensitive material covered gate electrode.
The pH sensitivity of the gate materials can be explained by the surface charging of the metal-oxides, mainly due to the specific adsorption of H+/OH− ions and protonation and deprotonation reactions evolving at surface sites. The MOx sensing layer can be fabricated by screen printing, vacuum sputtering, and sol–gel or electrodeposition methods and characterized mainly by potentiometry, cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS) to define their sensing performance [1].
In this work, titanium-oxide layers with different compositions were deposited using alternative techniques, analyzed, and compared as potential sensitive materials of an EGFET-based pH sensor.
2. Materials and Methods
Electrochemical electrodes including integrated Pt reference/counter electrodes and comb-like metal-oxide working electrodes with Pt or Au contacts were manufactured on BorofloatTM glass substrate (Figure 1c). The Pt and Au metal layers were deposited by vacuum sputtering and vacuum evaporation, respectively. Titanium-oxide layers were deposited by vacuum sputtering and atomic layer deposition (ALD) at 300 °C and 100 °C so as to be compatible with the applied heat-sensitive materials. The metal wires and MOx layers were patterned by lift-off lithography. The different MOx layers were analyzed using X-ray Photoelectron Spectroscopy (XPS) to determine their specific composition. The MOx-functionalized electrochemical electrodes were connected as an extended gate in the proposed EGFET-based circuit represented in Figure 1a,b and the performance of the system was tested as demonstrated in Figure 1d.
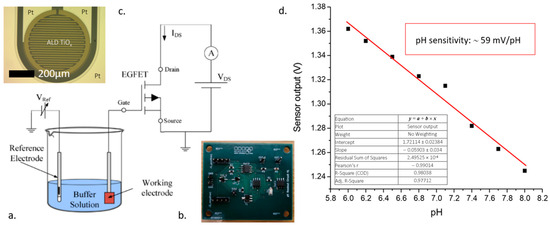
Figure 1.
Schematic measurement setup representing the electronic architecture of EGFET-based pH sensor (a), the manufactured read-out electronics (b), and the MOx-functionalized working electrode (c). The pH sensitivity of the non-stoichiometric TiOx layer was close to the Nernstian response (d).
3. Discussion
The pH sensitivity of the EGFET-based circuit containing different MOx materials was characterized using pH calibration solutions between 6 and 8 pH values. The differences between the pH sensitivities of various titanium-oxides can be explained by the material structures and compositions. The TiO2 layers deposited by sputtering and ALD at 300 °C showed moderated pH sensitivity according to their perfectly stoichiometric, inert structure. The TiOx layer deposited by ALD at 100 °C proved to be perfectly applicable for pH sensing applications, having a close-to-Nernstian 59 mV/pH response (Figure 1d). The surface analytical methods also confirmed that the low-temperature, ALD-deposited titanium-oxide layer is non-stoichiometric and has a Ti:O 1:1.65 atomic ratio.
Author Contributions
In this work, Z.S. was responsible for the design of EGFET-based read-out electronics; Z.S. and L.B. implemented the experimental characterization; S.S., O.H., A.S., C.D. and Z.B. contributed to the development of the ALD process and the analysis of the layers; and P.F. designed the electrode system and the system architecture. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the ECSEL JU and the National Research, Development and Innovation Fund (NKFIA) via the More4Medical H2020-ECSEL-2019-IA-876190 (www.moore4medical.eu, accessed on 10 April 2024) grant.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in this abstract.
Conflicts of Interest
The authors declare no conflicts of interest.
Reference
- Manjakkal, L.; Szwagierczak, D.; Dahiya, R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog. Mater. Sci. 2020, 109, 100635. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).