Abstract
(3-Cyano-5,6,7,8-tetrahydroquinolin-2(1H)-ylidene)malononitriles were prepared for the first time by the reaction of arylmethylidene malononitrile dimers with N-(1-cyclohexen-1-yl) morpholine.
1. Introduction
Arylmethylidene derivatives of malononitrile dimer (AMDM) 1 are highly reactive Michael acceptors and are convenient and widely used synthetic reagents for constructing various heterocyclic systems [1]. A literature survey reveals the methods for the preparation of (pyridin-2(1H)-ylidene)malononitriles and (isoquinolin-3(2H)-ylidene) malononitriles [2,3]. However, there are no data on the preparation of quinolines by the reaction of AMDMs with enamines. It is well known that quinoline derivatives have a wide spectrum of biological activity and are therefore of great interest for pharmaceutical chemistry.
2. Results and Discussion
The starting AMDMs 1 were prepared by the reaction of a malononitrile dimer with aromatic aldehydes under basic catalysis using piperidinium acetate. We succeeded to prepare (3-cyano-5,6,7,8-tetrahydroquinolin-2(1H)-ylidene)malononitriles 2 by the condensation of AMDMs 1 with N-(1-cyclohexen-1-yl) morpholine under prolonged heating (Scheme 1). Compounds were obtained in the form of off-white or yellow powders in 30%–60% yields.
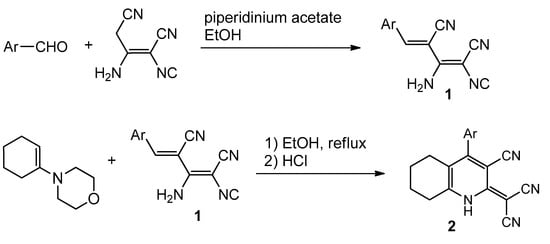
Scheme 1.
Ar = 4-BrC6H4; 3-thienyl; 4-CH3OC6H4; 4-OH-3-MeOC6H3; 2,4-Cl2C6H3.
We suggest that AMDMs reacted with N-(1-cyclohexen-1-yl)morpholine to form a Michael type adduct, followed by intramolecular cyclization through the attack of the amino group at position C-1 of the cyclohexene ring. The oxidation of the partially saturated pyridine ring with air oxygen occurs during the reaction. Subsequent acidification with hydrochloric acid leads to the elimination of morpholine molecule, and (3-cyano-5,6,7,8-tetrahydroquinolin-2(1H)-ylidene)-malononitriles are formed (Scheme 2).
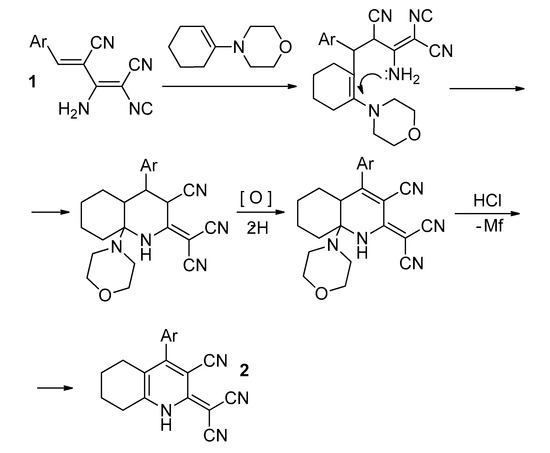
Scheme 2.
A possible mechanism of the formation of 2.
3. Experimental
[4-Aryl-3-Cyano-5,6,7,8-Tetrahydroquinolin-2(1H)-ylidene]malononitriles (2)
2-Amino-4-arylbuta-1,3-diene-1,1,3-tricarbonitrile (0.5 g) was dissolved in hot absolute EtOH (15 mL). To the solution formed, an excess (0.35–0.4 mL) of freshly distilled 4-(1-cyclohexen-1-yl) morpholine was added. A mixture was heated under reflux for 6–8 h (TLC thin layer chromatography control, eluent – EtOAc or acetone, “Sorbfil A” plates). Then, aq. HCl was added to adjust the pH to 2. The solid product was filtered off and recrystallized from the appropriate solvents.
Funding
Authors are grateful for financial support by the Russian Ministry of Education and Science (Project 0795-2020-0010).
References
- Dotsenko, V.V.; Krivokolysko, S.G.; Semenova, A.M. Heterocyclization reactions using malononitrile dimer (2-aminopropene-1,1,3-tricarbonitrile). Chem. Heterocycl. Compd. 2018, 54, 989–1019. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Ismiev, A.I.; Khrustaleva, A.N.; Frolov, K.A.; Krivokolysko, S.G.; Chigorina, E.A.; Snizhko, A.P.; Gromenko, V.M.; Bushmarinov, I.S.; Askerov, R.K.; et al. Synthesis, structure, and reactions of (4-aryl-3-cyano-6-oxopiperidin-2-ylidene) malononitriles. Chem. Heterocycl. Compd. 2016, 52, 473–483. [Google Scholar] [CrossRef]
- Hammouda, M.; El-Ahl, A.S.; El-Toukhee, Y.M.; Metwally, M.A. Reactions of ketonic Mannich bases with malononitrile and malononitrile dimer. J. Chem. Res. 2002, 2002, 89–94. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).