Abstract
The perspective catalysts usable for the fabrication of thermocatalytic gas sensors were studied. The analysis of CO oxidation kinetics by Pd decorated Al2O3, ZSM-5, SnO2, CeO2/ZrO2 and some other carriers of catalysts showed that the application of these catalysts leads to the ambiguity of sensor response (light-off effect). It was demonstrated that a catalyst based on CeO2/ZrO2 carrier could be used for the fabrication of sensors characterized by the univocal correspondence between CO concentration and sensor response. The developed model of the CO oxidation on all Pd catalysts with inert carrier enabled the description of the CO oxidation using a single value of activation energy.
Published: 4 December 2018
1. Introduction
The detection of CO concentrations in oxygen-containing atmosphere using thermocatalytic (calorimetric) gas sensors is possible for relatively high concentrations of this gas [1]. However, the problem, which restricts the application of such sensors, is the ambiguity of the sensor response. This means, that the same sensor response is observed at different CO concentrations. Under certain conditions, the same value of sensor response can correspond to 3 different gas concentrations! In this research, we tried to analyze kinetics of the CO oxidation on Pd containing catalysts and to find materials free of this disadvantage for development pellistor multisensory platform for the measurement СH4; NH3; CO and C4H10 gases based on silicon MEMS technology [2].
2. Motivation and Results
The scheme of the experimental setup is presented in Figure 1. The catalysts studied in this research were: Pd on inert carrier А12O3 and ZSM-5 Pd on catalytically active carrier SnO2 and mixed CeO2/ZrO2. The main result of the experiment consists in the conclusion that the so called “light off” of the catalyst used for the oxidation of CO in thermocatalytic gas sensors and in three way catalysts is an isothermal process.
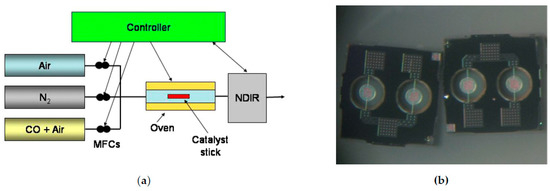
Figure 1.
(a) The scheme of the experimental setup. The concentration of CO on the inlet and the outlet of the reactor were controlled by Rosemount NDIR instrument, the inlet concentration being measured using a bypass tube. The measurements of temperature downstream the catalytic samples showed that the catalytic combustion of CO does not increase gas temperature by more than 3 °C; (b) Optical view of the 1 × 1mm2 pellistor chips for multisensory platform. The heaters in the centre of the membranes are is 120 µm diameter [2].
Two types of the experiments were carried out and gave self-consistent results. First group of experiments is measurement of the CO oxidation rate at constant temperature (Figure 2a). The second group of experiments was the measurement of the CO outlet concentration as a function of reactor inlet concentration (Figure 2b).
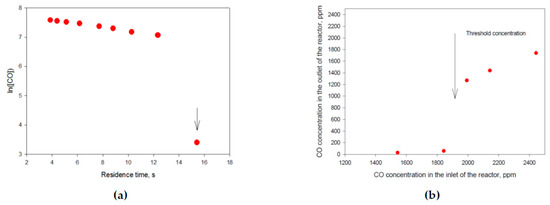
Figure 2.
(a) First order kinetics of CO oxidation on catalyst 2 wt.% Pd on SnO2 (55 m2/g). CO concentration on reactor inlet is 2310 ppm, O2 concentration is 16% in N2, T = 108 °C. Measured is CO concentration in the reactor outlet; (b) CO oxidation on a catalysts 4 wt.% Pd on Al2O3 (176 m2/g) O2 concentration is 15% in N2, T = 106 °C. A decrease in inlet CO concentration bellows a threshold value leads to stepwise increase in reaction rate and to decrease in outlet concentration.
Using model of process is due to the avalanche cleaning of the Pd surface from poisoning CO and different the rate substitution COads on O2ads This model was applied to the analysis of the isothermal light-off curves of the noble metal containing catalysts and used for the simulation of the results and for the calculation of kinetic parameters of the oxidation process. After all experiments was found only one catalyst based on CeO2/ZrO2 carrier characterized by the univocal correspondence between CO concentration and sensor response (Figure 3).
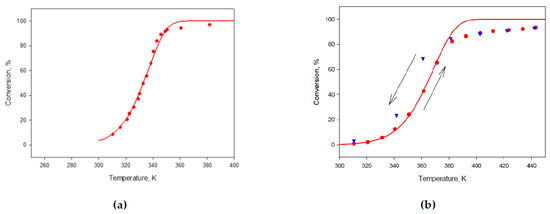
Figure 3.
(a) The temperature dependence conversion in the oxidation of CO on the catalyst. CeO2/ZrO2 + 4 wt.% Pd. The initial concentration of CO is 2270 ppm, the O2 concentration is 15% in N2; (b) The temperature dependence conversion in the oxidation of CO on the catalyst CeO2/ZrO2 + 4 wt.% Pd. The initial concentration of CO is 8250 ppm, the O2 concentration is 2% in N2.
Acknowledgments
This work was supported by the Ministry Education and Science Russian Federation (Grant No. 14.587.21.0053 from 21.02.2018, unique identifier RFMEFI58718X0053) in frame of joint Russian-Hungarian project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Samotaev, N.N.; Vasiliev, A.A.; Podlepetsky, B.I.; Sokolov, A.V.; Pisliakov, A.V. The mechanism of the formation of selective response of semiconductor gas sensor in mixture of CH4/H2/CO with air. Sens. Actuators B Chem. 2007, 127, 242–247. [Google Scholar] [CrossRef]
- Bíró, F.; Pap, A.E.; Bársony, I.; Dücső, C. Micro-pellistor with integrated porous alumina catalyst support. Procedia Eng. 2014, 87, 200–203. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).