A New Type of LSPR Sensor Featuring Immobilized Liposome or Phospholipid Single Layer †
Abstract
:1. Introduction
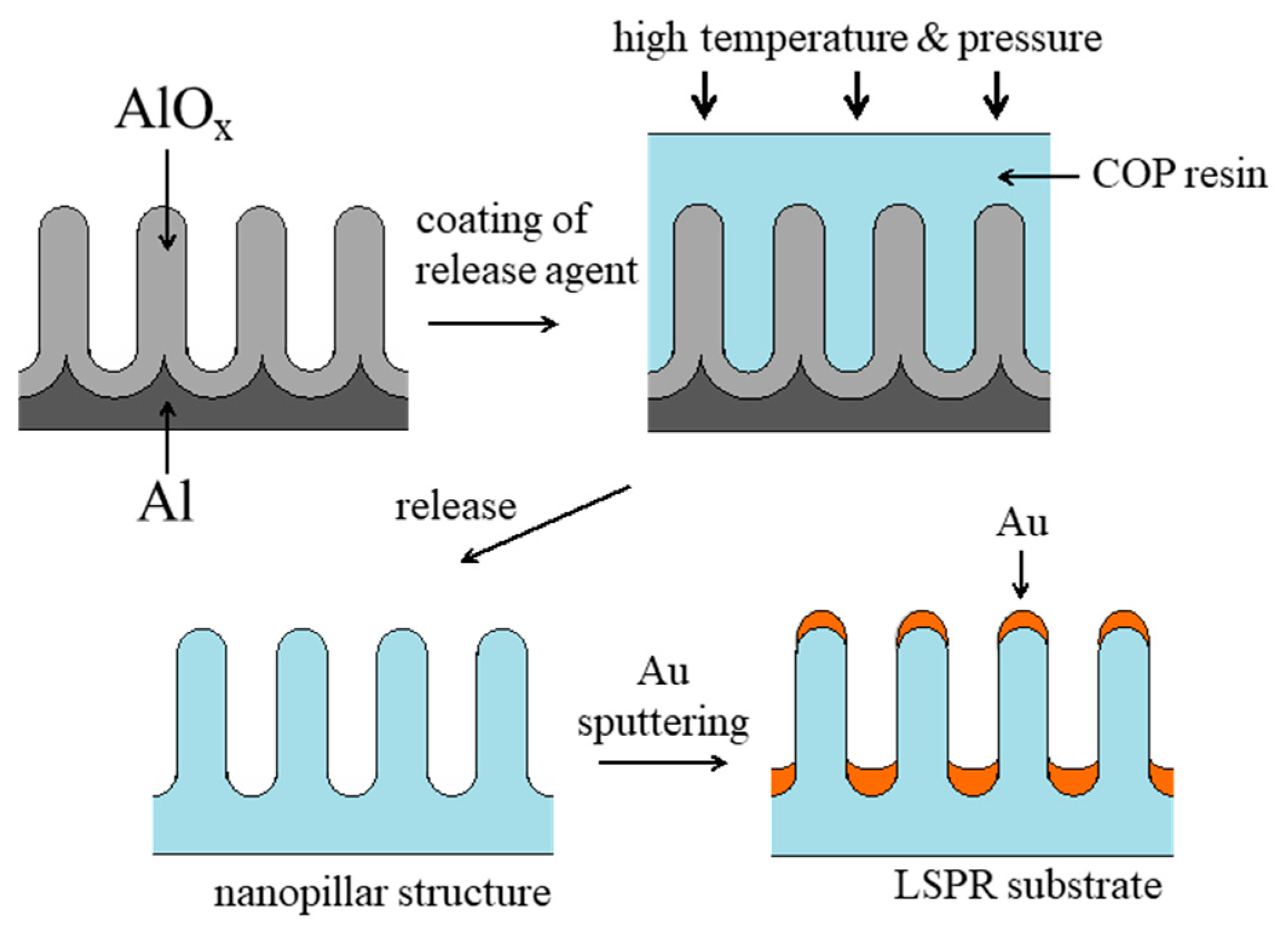
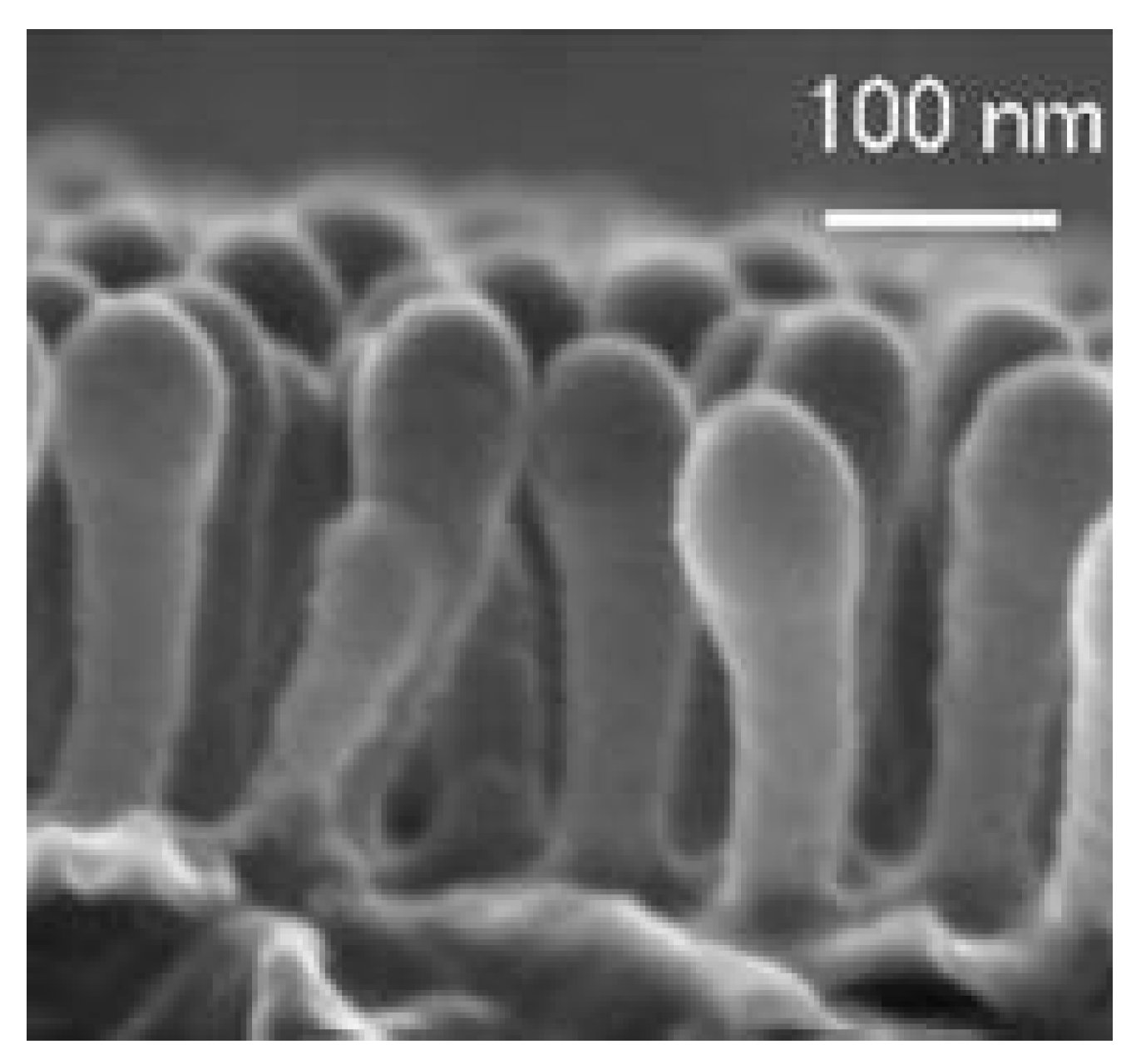
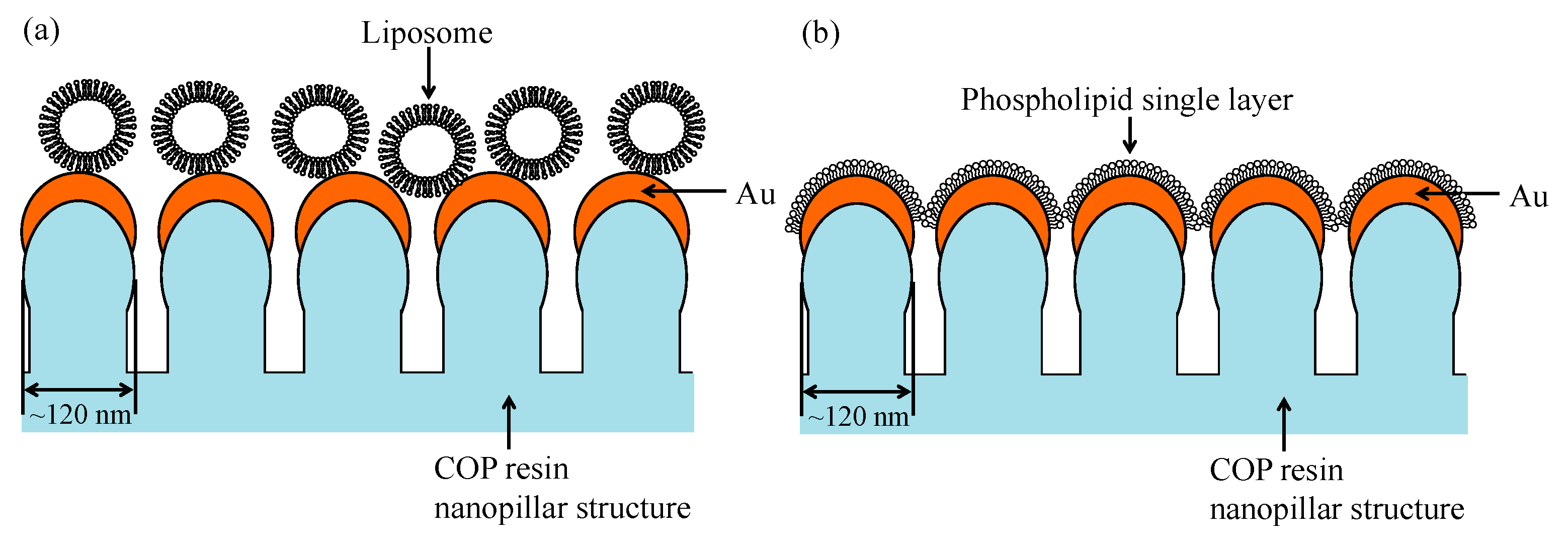
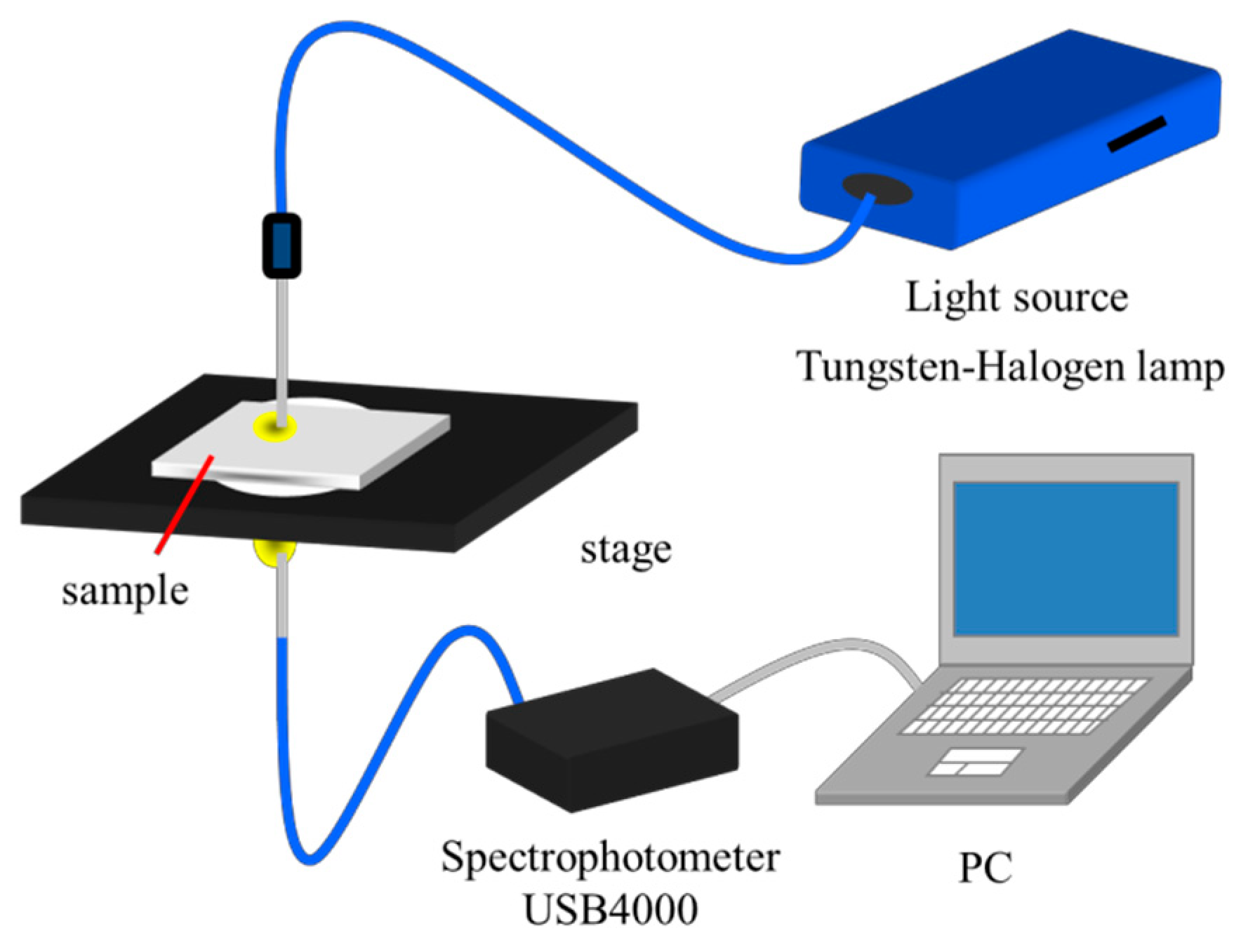
2. Materials and Methods
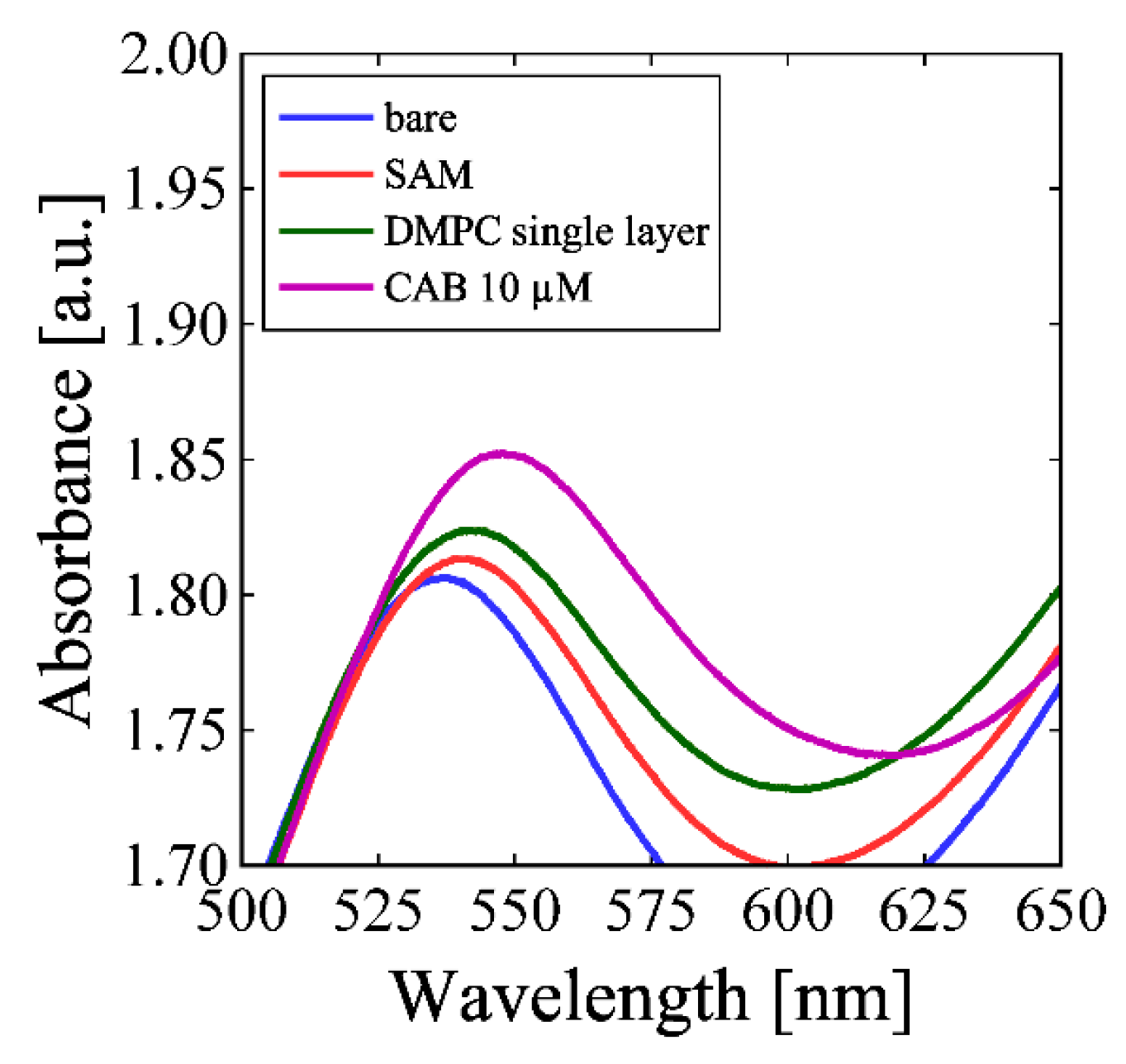
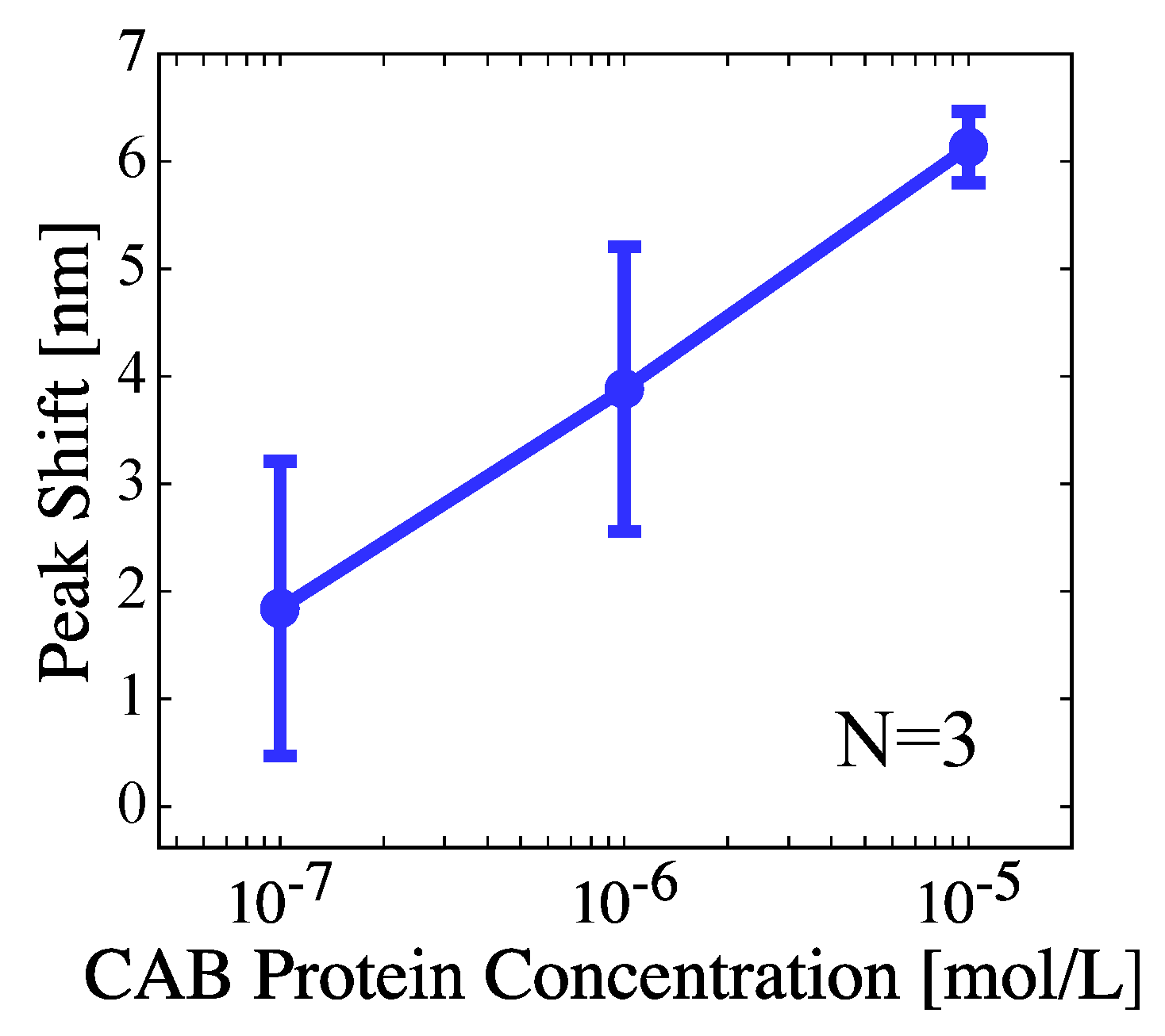
3. Results and Discussion
4. Conclusions
References
- Hiep, H.M.; Endo, T.; Saito, M.; Chikae, M.; Kim, D.K.; Yamamura, S.; Takamura, Y.; Tamiya, E. Label-Free Detection of Melittin Binding to a Membrane Using Electrochemical-Localized Surface Plasmon Resonance. Anal. Chem. 2008, 80, 1859–1864. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Kitamura, A.; Murahashi, M.; Yamanaka, K.; Hoa le, Q.; Yamaguchi, Y.; Tamiya, E. Novel Gold-Capped Nanopillars Imprinted on a Polymer Film for Highly Sensitive Plasmonic Biosensing. Anal. Chem. 2012, 84, 5494–5500. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawasaki, M.; Yoshikawa, H.; Saito, M.; Jiang, S.; Akeyama, T.; Tamiya, E.; Noda, M. A New Type of LSPR Sensor Featuring Immobilized Liposome or Phospholipid Single Layer. Proceedings 2018, 2, 791. https://doi.org/10.3390/proceedings2130791
Kawasaki M, Yoshikawa H, Saito M, Jiang S, Akeyama T, Tamiya E, Noda M. A New Type of LSPR Sensor Featuring Immobilized Liposome or Phospholipid Single Layer. Proceedings. 2018; 2(13):791. https://doi.org/10.3390/proceedings2130791
Chicago/Turabian StyleKawasaki, Masahiro, Hiroyuki Yoshikawa, Masato Saito, Shu Jiang, Takehiro Akeyama, Eiichi Tamiya, and Minoru Noda. 2018. "A New Type of LSPR Sensor Featuring Immobilized Liposome or Phospholipid Single Layer" Proceedings 2, no. 13: 791. https://doi.org/10.3390/proceedings2130791
APA StyleKawasaki, M., Yoshikawa, H., Saito, M., Jiang, S., Akeyama, T., Tamiya, E., & Noda, M. (2018). A New Type of LSPR Sensor Featuring Immobilized Liposome or Phospholipid Single Layer. Proceedings, 2(13), 791. https://doi.org/10.3390/proceedings2130791




