Abstract
Sound has a potential impact on animal welfare and production, but the impacts of soundscapes on aquaculture species in different aquaculture production systems have been rarely studied. This study investigated the impact of varying aquaculture soundscapes on largemouth bass (Micropterus salmoides). Three soundscapes were administered to replicated tanks: Recirculating Aquaculture System (RAS:107.7 dB re 1 Pa RMS), In-Pond Raceway System (IPRS:115.1 dB re 1 Pa RMS), and Ambient (70.4 dB re 1 Pa RMS) as the control. The initial weight of fish in the three groups was 3.59 ± 0.30 g. Following a 50-day experimental period, the average weight of the Ambient group (14.08 ± 0.13 g) was significantly greater than that of the IPRS group (12.79 ± 0.08 g) (p < 0.05). Examination of physiological samples revealed that the soundscape negatively impacted the fish’s immunological, anti-oxidation, and digestive enzymes. Furthermore, the external noise also influenced the locomotive patterns of the fish aggregations. Fish polarity and cohesion were significantly more discrete (p < 0.05) in both the RAS (47.79 ± 2.34° and 98.52 ± 3.22 mm) and IPRS groups (48.04 ± 0.70° and 87.70 ± 7.31 mm) compared to the Ambient group (42.76 ± 1.42° and 85.73 ± 1.57 mm). These results highlight the significant impacts of the aquaculture soundscape on the development, physiological activities, and behavioral traits of largemouth bass. Future research should focus on determining and optimizing the impact of different equipment noise to ensure optimal welfare and production performance in aquaculture systems.
Key Contribution:
The manuscript systematically investigates the impacts of soundscape on the growth, physiology, and behavior of Micropterus salmoides, filling a knowledge gap in this field. It reveals the underlying mechanisms of soundscape on farmed fish and provides valuable insights for noise control in practical production processes.
1. Introduction
Aquaculture has been expanding rapidly worldwide to meet the increasing demand for freshwater and seafood and to compensate for declining wild fish stocks [1,2]. This growth has brought numerous environmental challenges, including water pollution, habitat destruction, and noise pollution [3,4,5,6]. Among these issues, noise pollution has emerged as a growing concern due to its potential impact on the health, growth, and behavior of aquatic organisms, as well as the broader implications for aquatic ecosystems [7,8]. In particular, recirculating aquaculture systems (RAS) and in-pond raceway systems (IPRS) have gained popularity in recent years due to their resource efficiency and reduced environmental impact [9,10]. However, these systems often generate higher noise levels compared to natural or traditional aquaculture environments [11,12], raising concerns about the effects of noise pollution on the cultured species and the surrounding ecosystem.
The characteristics of soundscape in RAS and IPRS systems lie in their continuous and high-intensity noise. This noise primarily originates from the operation of equipment such as water pumps, air blowers, and bubbles generated by aeration systems [13,14]. Different aquaculture systems may exhibit variations in sound levels, spectra, and temporal patterns, which could potentially have varying effects on the growth performance, physiological and behavioral traits of the cultured species [15,16,17]. Understanding these effects is crucial for developing effective soundscape management strategies to mitigate the impact on cultured species and protect the surrounding aquatic ecosystems.
The acoustic frequency of mechanical disturbances in distinct aquaculture modalities generally falls below 2000 Hz, with sound pressure levels ranging from 100 to 150 dB re/μ Pa (RMS). Research by Davidson et al. (2007) indicates that low-frequency tonal sounds generated by nearby blowers and pumps (29 Hz and 59 Hz, respectively) permeate aquaculture tanks, contributing to the highest range of the sound spectrum [18]. Mean broadband sound pressure levels differ among intensive aquaculture systems, ranging from 100 dB re 1 pa in earthen ponds with inactive aerators to 130 dB in variously sized round fiberglass tanks [16]. The same study reported that sound pressure levels typically peaked at low frequencies, up to 135 dB re 1 pa at 25–1000 Hz and diminishing to 115 dB re 1 pa at 1–2 kHz. This auditory range is encompassed within the hearing spectrum of the majority of bony fish [19,20,21,22,23]. It is presumed that aquaculture systems possess higher underwater sound pressure levels compared to most natural habitats [24]. As a result, riverine species, such as largemouth bass, when cultivated in intensive recirculation systems, encounter more complex acoustic environments than those experienced in their native habitats. Distancing from noise sources is the most effective strategy to evade detrimental effects [25,26]. Contrary to fish in natural environments, those in intensive aquaculture settings have restricted mobility and are unable to efficiently avoid noise sources.
Certain investigations have examined the influence of sound generated by aquaculture systems on fish. Filiciotto et al. (2017) discovered that both offshore soundscapes (ship noise) and terrestrial soundscapes (concrete ponds) significantly impact the oxidative state and immune stress indices of juvenile snappers [12]. Furthermore, research has demonstrated that RAS noise adversely impacts the development of rainbow trout and largemouth bass [11,13,14]. In addition, anthropogenic underwater noise in nature, such as sounds from offshore piling, shipping, scientific research, and exploration operations [27,28,29], has also been shown to stress aquatic organisms, leading to environmental degradation, reduced reproductive success, altered hunting behavior, increased alertness, and reduced ability to perceive acoustic signals in the environment [30,31].
Largemouth bass (Micropterus salmoides) represents a vital species in aquaculture owing to its rapid growth cycle, high adaptability, and nutritional value [32]. While pond culture remains the dominant production method, there has been rapid development and widespread application of intensive aquaculture systems with advancing technologies. For example, to meet market demand for mature bass out of season, the species is cultivated as larvae in RAS. IPRSs are utilized for bass culture in ponds to enhance muscle nutritional quality. In this study, we posited that the RAS soundscape (107.7 dB re 1 Pa RMS) and IPRS soundscape (115.1 dB re 1 Pa RMS) would exert detrimental effects on largemouth bass, with the magnitude of these effects varying across different aquaculture systems. We aimed to substantiate this hypothesis by (1) evaluating the impact of soundscapes on the growth performance and physiology activities of largemouth bass within diverse aquaculture models and (2) quantifying the influence of soundscapes on the population polarity and cohesion of largemouth bass.
2. Materials and Methods
2.1. Fish
This investigation used 300 largemouth bass with an average body length and weight of 6.78 ± 0.16 cm and 3.59 ± 0.30 g, respectively, obtained from Jianfeng Agricultural Co., Ltd., Hangzhou, China. Prior to the experiment’s initiation, the fish underwent a two-week acclimatization period within a RAS system, where the sound level was maintained at 70.4 dB re 1 Pa RMS. The dissolved oxygen (DO), nitrite nitrogen (NO2−-N), and ammonia nitrogen (NH4+-N) were maintained around 6.5–7.0, 0.5, and 0.3 mg/L, respectively. NH4+-N was measured using Nessler’s reagent UV spectrophotometric method, and NO2−-N was measured using the N-1-Naphthylethylenediamine dihydrochloride colorimetric method. Dissolved oxygen (DO) was measured using an AR8606 dissolved oxygen meter manufactured by Smart Sensor, Suzhou, China.
2.2. Experimental System Setup
The experiment was conducted at the experimental base of Zhejiang University, Jianfeng Agricultural Development Co., Ltd. (Hangzhou, China). The experimental system encompassed a RAS, an underwater sound management system, and a video data acquisition system (Figure 1). The RAS provided a stable and reliable environment for the fish, while the underwater sound management system supplied the soundscape for the experimental conditions within the aquaculture tank. The video data acquisition system recorded the activity data of the fish throughout the experiment. The RAS consisted of a biological filter unit and an aquaculture pond. The aquaculture pond was a circular PVC structure with a height of 0.8 m and an interior diameter of 1 m. The biological filter unit consisted of a PVC water tank (1 m × 0.5 m × 0.8 m). It also had germicidal UV lights, aeration, oxygenation, and nitrification biofilms to remove NH4+-N and NO2−-N. The water circulation in the aquaculture ponds and biofilter units was driven by a whisper-quiet water pump, with water flow regulated by a flow control valve. The underwater sound management system consisted of a sound playback device and a sound analysis unit. Three players, three US-2150 power amplifiers, and nine US-0150 underwater speakers (80–2000 Hz) positioned at the base of the aquaculture tank constituted the sound playback unit (LingYan Electronic Technology Co., Ltd., Shanghai, China). The sound analysis equipment comprised an 8103 hydrophone, an AVANT MI-7008 data acquisition analyzer, and an AVANT MI-2004 power amplifier (Brüel & Kjaer Co., Ltd., Nærum, Denmark). The video data acquisition system included video cameras, LED lights, hard disk recorders, displays, and POE switches. For the purpose of recording the behavioral information of the experimental fish, cameras were installed on a stainless steel frame above the aquaculture pond. Noise reduction measures were implemented to mitigate system operation-related noise interference and external noise: (1) establish the experimental setup in a quiet laboratory; (2) erect a stainless steel framework around the aquaculture tank and envelop it with noise-absorbing shade fabric; (3) position a PVC shock-absorbing pallet at the base of the aquaculture tank; (4) utilize liquid oxygen for water oxygenation instead of air pumps; (5) employ a fixed biological bed for the biological filter rather than a movable one; (6) cover PVC pipes in the experimental system with sound-absorbing materials.
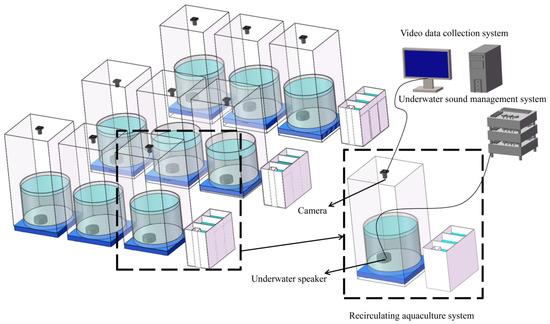
Figure 1.
Experimental system.
2.3. Experimental Design
The study comprised three groups: the RAS group, the IPRS group, and the Ambient group (Figure 2). The RAS soundscape was acquired from the recirculating aquaculture system of Jinchengfu Fisheries Technology Co., Ltd. (Suzhou, China, 107.7 dB re 1 Pa RMS). The IPRS soundscape was gathered from the intensive pond of Jianfeng Agricultural Development Co., Ltd. (Hangzhou, China, 115.1 dB re 1 Pa RMS). The Ambient group functioned as the control group (70.4 dB re 1 Pa RMS). For each group, three replicate aquaculture tanks containing 30 fish each were established, with the experiment lasting 50 days. Water quality and environmental conditions during the experiment remained consistent with those during the acclimation period. The recirculating system maintained a water flow rate of 300 L/h, and a daily water exchange of 5% of the total volume was conducted. Additionally, fecal matter at the bottom of the aquaculture tank was removed on a daily basis. The fish were provided with an adequate amount of feed twice daily, and any leftover feed was removed, dried, and weighed to determine the total feed consumption.
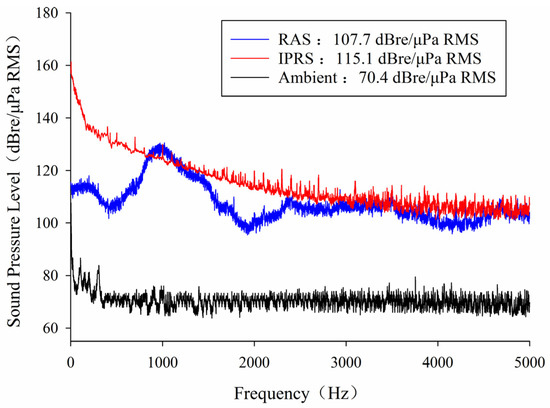
Figure 2.
The frequency spectrum of the RAS, IPRS, and Ambient groups.
2.4. Data Collection and Analysis
2.4.1. Growth
To determine the initial body length and weight, 20 fish were randomly sampled before the experiment. During the experiment, the fish were weighed every 10 days. At the end of the experiment, a growth assessment was performed on the fish in each tank, while the current number of fish in each group was also recorded.
where BW1 and BW2 represent the initial and final body weights of each fish (in grams) and T1 and T2 represent the time corresponding to those weights, Ni and Nf represent the initial and final number of fish, respectively.
FCR, Feed Conversion Ratio = Total feed intake (g)/Weight gain (g);
SGR, Specific growth rate (%/d) = 100 × (lnBW2 − lnBW1)/(T2 − T1);
PER, Protein Efficiency Ratio = Weight gain (g)/Protein intake (g);
HIS, Hepatosomatic Index (%) = (Liver weight/Body weight) × 100;
SR, Survival Rate (%) = 100 × Nf/Ni;
CF, Condition Factor (%) = (Weight/Length^3) × 100
2.4.2. Physiological Performance
Nine fish were randomly selected from each tank, anesthetized with 120 mg/L tricaine mesylate (MS-222) for five minutes during sampling to obtain blood from the fish’s tail vein and remove liver and intestinal samples for analysis. Glucose (Glu, catalog number A154-1-1, OD 505 nm, mmol/L) and immunoglobulin M (IgM, Cat.No. H109-1-1, OD 450 nm, μg/mL) in the blood, malondialdehyde (MDA, Cat.No. A003-4-1, 532 nm, nmol/mgprot) and lactate dehydrogenase (LDH, Cat.No. A020-1, OD 450 nm, U/gprot) in the liver, and lipase (LPS, Cat.No. A054-2-1, OD 570 nm, U/gprot) activity in the intestine were determined using kits from Jiancheng Bioengineering Institute (Nanjing, China) and measured using a microplate reader (Multiskan, Thermo Scientific, Waltham, MA, USA). Lysozyme (LZM, Cat.No. A050-1-1, OD 530 nm, U/mgprot). Amylase (AMS, Cat.No. C016-1-1, OD 660 nm, U/mgprot), protease (PEP, Cat.No. A080-1-1, OD 595 nm, U/mgprot), and cellulase (CE, Cat.No. A138, OD 550 nm, U/gprot) in the intestine, catalase (CAT, Cat.No. A007-2-1, OD 405 nm, U/mgprot), hexokinase (HK, Cat.No. A007-3, OD 340 nm, U/gprot), pyruvate kinase (PK, Cat.No. A076-1-1, OD 340 nm, U/gprot), and succinate dehydrogenase (SDH, Cat.No. A022-1-1, OD 600 nm, U/mgprot) in the liver, and polyphenol oxidase (PPO, Cat.No. A136-1-1, OD 420 nm, U/mL) activity in the blood were determined using assay kits from Nanjing Jiancheng Bioengineering Institute and measured using a spectrophotometer (Cary 60 UV-VIS, Agilent Technology, Beijing, China).
2.4.3. Behavioral Traits
A deep learning method known as the Mask Region Convolutional Neural Network (Mask RCNN) model [11] was used to study the largemouth bass’ collective swimming behavior. (Figure 3). Mask RCNN exemplifies a cutting-edge method for object instance segmentation that generates segmentation masks for individual fish by devising a model for the mask area within a convolutional neural network, thereby facilitating the identification and localization of the experimental fish in each frame [33].

Figure 3.
Measurement of angle and distance between the focal fish and its two nearest neighbor fish.
The Mask RCNN model, tailored for largemouth bass, was trained, utilizing a total of 1575 fish school images derived from nine aquaculture ponds, collected at 10-day intervals as the training dataset. Assessments of fish group swimming patterns were conducted by evaluating the angle and distance between the test fish and its nearest two companions [34,35]. During image data processing, a line connecting the head and tail of the focal fish was employed to represent its swimming direction. This line also facilitated the computation of the angle between the swimming direction of the focal fish and the swimming directions of its two closest neighbors. The line joining the line’s center and the centers of the two nearest neighbors were used to determine the shortest distance. By averaging 30 included angles and distances between 30 experimental fish and neighboring fish in each image, it was possible to determine the average angle and distance that represent the fish’s swimming capabilities. In each aquaculture tank, 120 photographs were randomly selected from a 5-min video daily at 03:00, 09:00, 15:00, 18:00, and 23:00. For the purpose of analyzing fish swimming behavior, 600 photos from each tank were chosen daily, amounting to a total of 194,400 photographs.
2.4.4. Data Analysis
Data are depicted as mean ± SD. The normality of the data distribution was assessed using the Kolmogorov–Smirnov test. Variations in growth, physiological, and behavioral parameters among treatments were then examined through one-way ANOVA, followed by Duncan’s test, with significance established at p < 0.05 in all instances. Statistical evaluations were conducted employing IBM SPSS Statistics version 22.0 (IBM) and SigmaPlot Version 14.0.
3. Results
3.1. Growth
At the beginning of the experiment, the fish in different soundscape groups had an initial weight of 3.59 ± 0.30 g. Throughout the experiment, the survival rate for each group remained at 100%. Aquaculture soundscapes led to a decrease in fish weight, with the average weight of fish in the IPRS group significantly lower than that of the Ambient and RAS groups (p < 0.05). Figure 4 presents the fish weights measured every 10 days during the experiment. On day 20, the mean weight of the RAS group started to be notably less than the Ambient group. On day 30, there were significant differences in average weights between the Ambient, RAS, and IPRS groups (p < 0.05), with the highest weight in the Ambient group at 8.97 ± 0.04 g and the lowest in the IPRS group at 8.09 ± 0.05 g. At the end of the experiment, there was no significant difference in weight between the Ambient and RAS groups, but the IPRS group had a significantly lower weight of 12.79 ± 0.08 g. Growth performance and feed utilization are shown in Table 1. Affected by noise, the feed consumption of the IPRS group significantly decreased, while the RAS group consumed less than the Ambient group, but the difference was not significant. The IPRS group had the lowest WG (g) and SGR (p < 0.05), but the differences in FCR among the groups were not significant. Similarly, no substantial disparities were observed in PER and HSI among the groups (p > 0.05).
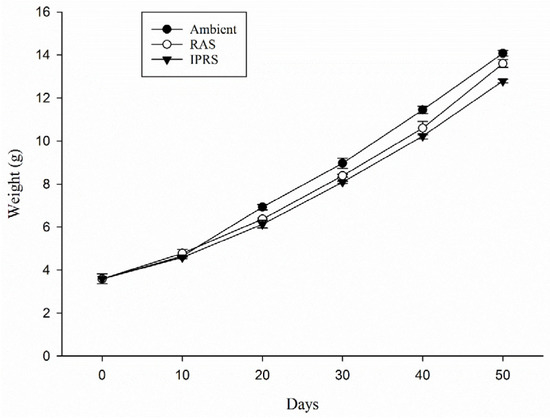
Figure 4.
Percentage difference in mean weights between treatments.

Table 1.
Growth evaluation and feed utilization performance of largemouth bass among the three treatments. Means bearing different superscript letters in the same row are significantly different (p < 0.05).
3.2. Physiological Performance
The results showed that at the end of the experiment, the Ambient group exhibited superior immune and antioxidant indicators (Table 2), with the serum glucose (Glu) in the Ambient group notably lower compared to the IPRS group. There were significant differences in malondialdehyde (MDA) activity among the three treatment groups (p < 0.05), with the lowest in the Ambient group at 0.92 ± 0.02 mg/mL. In terms of catalase (CAT), immunoglobulin M (IgM), lysozyme (LZM), and peroxidase (POX) activity, the Ambient group outperformed the RAS and IPRS groups, but the differences were not significant. Regarding digestive performance, the RAS group had higher CE activity, and the Ambient group had higher AMS activity, but neither showed significant differences (p > 0.05). The IPRS group had higher LPS activity, but its PEP activity was notably lower than that of the Ambient and RAS groups, at 3.67 ± 0.12 U/mgprot (Table 2). No significant differences in metabolic performance were observed among the three groups.

Table 2.
Physiological performance of anti-oxidation, immune, digestive enzymes, and metabolic enzymes indices among the three treatments (entries in the same row with different superscripts differed significantly).
3.3. Behavior
Affected by different aquaculture soundscapes, the swimming behavior of largemouth bass exhibited significant differences (Table 3). In terms of the angle between the focal fish and its closest neighbor, the Ambient group had an average angle of 42.76 ± 1.42° over 50 days, significantly smaller than the 48.04 ± 0.70° of the IPRS group. During the first 10 days of the experiment, the average angle in the Ambient group was the smallest, but the differences compared to the other two groups were not significant. The IPRS group had the largest angle at 50.47 ± 0.38°, significantly higher than the RAS group (p < 0.05). In contrast to the fish in the Ambient group swimming as a circle, the IPRS and RAS groups exhibited more disordered swimming angles. Regarding swimming distance, the average swimming distance in the RAS group during days 20–30 was significantly higher than that of the Ambient and IPRS groups (p < 0.05), at 108.22 ± 2.30 mm. In the subsequent culture period, the average swimming distance in the RAS group was also larger but not significantly different compared to the other two groups. Throughout the entire experiment, the average swimming distance of the Ambient group was the smallest, at 85.74 ± 1.57 mm, significantly smaller than the 98.52 ± 3.22 mm of the RAS group (p < 0.05).

Table 3.
Fish school swimming behavior performance in average angle and average distance between the nearest two fishes for the three treatments. Means bearing different superscript letters in the same row are significantly different (p < 0.05).
4. Discussion
This study investigated the impacts of soundscape pollution on the growth performance, physiological and behavioral traits of largemouth bass in IPRS and RAS aquaculture systems. The results revealed significant differences in growth, physiological responses, and behavior among the three experimental groups (Ambient, RAS, and IPRS).
4.1. Fish Growth
Several scholars have posited that rhythmic music may enhance fish growth and development [36,37,38,39], while other studies have emphasized the negative impacts of underwater noise on fish growth. The present study revealed that the soundscapes of RAS and IPRS impacted the production attributes of largemouth bass. In the RAS group, a significant difference in average weight compared to the Ambient group was observed on day 20 and day 30. However, as the experiment progressed, the gap between the RAS group and the control group gradually diminished, and the difference was no longer significant. This outcome concurs with prior research by Wysocki et al. (2007) and Davidson et al. (2009), which observed negative short-term consequences of RAS noise on rainbow trout growth [13,14]; however, there were no considerable differences during extended trials. Fish’s hearing is affected by noise, resulting in Temporary Threshold Shift (TTS) and Permanent Threshold Shift (PTS). The recovery period for a TTS depends on the noise type, the sensitivity of different fish species to noise, and the degree of threshold displacement; when noise does not subside, fish may experience a reduced sensitivity to noise following a recovery period, subsequently diminishing the impact of noise on their growth [40]. After a month of acclimation, largemouth bass became less sensitive to the RAS soundscape. In contrast, the IPRS group showed a significantly lower weight than the control group on day 30, which persisted until the end of the experiment. Although this study did not implement a strict experimental design to assess long-term growth performance under IPRS soundscapes, unlike the RAS group, the IPRS soundscape had higher low-frequency sound pressure levels and feed consumption was significantly lower than the Ambient group. Previous studies have shown that freshwater stream fish exposed to noise experience elevated auditory thresholds at 300 Hz and 400 Hz [41]. Furthermore, noise can also impact the auditory sensitivity of cyprinid fish, with their most sensitive range being 0.8–2.0 KHz [42]. However, further research is needed to determine the auditory sensitivity and responses of largemouth bass to different sound frequencies and intensities. Therefore, this could be due to the IPRS soundscape suppressing the appetite of largemouth bass or because the noise masked essential auditory cues, disrupting the fish’s ability to make informed decisions and, ultimately, leading to feeding failure [43,44,45]. In this study, the reduced feed intake observed in the IPRS group directly resulted in a significantly lower weight gain for largemouth bass compared to the RAS and Ambient groups.
4.2. Physiological Performance
In this study, fish exposed to the IPRS and RAS soundscapes exhibited higher MDA activity and Glu content in their serum, suggesting that higher sound pressure levels may cause oxidative stress responses in largemouth bass and disrupt their glucose metabolism [46,47]. The Ambient group performed better in indicators such as CAT, IgM, and LZM, implying that they may have a stronger ability to defend against pathogen invasions and prevent microbial infections [48,49]. IgM and LZM provide a defense when infection risk increases and POX can prevent microbial infections and protect fish when injured [50,51,52]. Through the analysis of largemouth bass’ digestive ability, we found that the PEP activity in the IPRS group was significantly lower. PEP is associated with various physiological processes, including learning and memory, emotion regulation, and pain transmission. Digestive protease activity is crucial for the effective absorption and utilization of ingested protein [53]. Although we did not find significant differences in FCR and PER between the IPRS group and the other two groups, the impact of low-frequency noise on the long-term growth performance and digestive performance of fish is still worth noting. SDH and LDH play a key role in the energy metabolism process within organisms, representing information about energy metabolism levels, energy supply status, stress responses and adaptability, and disease and physiological states. HK and PK play critical roles in glucose metabolism [54]. The liver enhances glucose utilization by increasing HK and PK activity, forming ATP to counteract the interference of the external environment on normal metabolism [55,56]. In this study, there were no significant differences in metabolic indicators among the three experimental groups, suggesting that the increase in sound pressure level did not affect the metabolism of largemouth bass.
4.3. Schooling Ability
The angle and separation between the closest members of a fish school can act as markers of how the school adjusts its grouping tactics to enhance collective collaboration and minimize energy use [35,57]. However, noise can distract their attention from essential tasks and alter their behavior. Higher low-frequency sound pressure resulted in higher average swimming angles of fish schools, reflecting lower swimming polarity within the group and a less apparent swimming trend in the same direction. Fish shoal polarity is an indicator that measures the degree to which shoal members swim in the same direction, reflecting the shoal’s cooperative behavior and information transmission. When influenced by environmental factors, shoals with higher polarity can make group decisions more quickly [34]. The results of this study suggest that the lower polarity of fish schools caused by higher sound pressure levels may be detrimental to maintaining stability and reducing energy loss during swimming. This outcome may be due to low-frequency underwater noise hindering communication signals between fish shoals [58,59,60], affecting group behavior and, consequently, reducing individual ecological advantages. In addition, the results of this study showed that higher sound pressure levels resulted in less cohesion of fish schools and more dispersed distribution in the culture pool. Lower cohesion can affect the defense strategies and social interactions within fish shoals, which may be influenced by environmental factors such as water flow, temperature, and shelter. Environmental noise can also disrupt acoustic information, reducing the cohesiveness of fish shoals [61,62,63]. Group behavior can improve feeding efficiency [64,65,66], increase defensive or offensive capabilities to reduce predation risk [67,68,69,70], conserve energy consumption [71,72,73], and enhance reproductive efficiency [74]. In intensive aquaculture characterized by constant water flow, effective collective cooperation can significantly reduce energy consumption, accelerate growth rates and, ultimately, bring economic benefits [75].
This study has practical implications for the aquaculture industry. Proper soundscape management in RAS and IPRS systems can potentially improve fish health, increase production efficiency, and mitigate the impacts on the surrounding ecosystem. Future research should focus on developing strategies to minimize soundscape pollution and exploring the long-term effects of noise pollution on fish populations and aquaculture systems. However, there are limitations to our study. The experimental period was relatively short, and further research is needed to investigate the long-term impacts of soundscape pollution on largemouth bass. Moreover, the study only focused on two aquaculture systems, and the generalizability of the results to other systems remains to be explored. In conclusion, our findings highlight the importance of understanding the impacts of soundscape pollution on the growth, physiology, and behavior of largemouth bass in aquaculture systems. Further research is needed to optimize soundscape management and promote sustainable aquaculture practices.
5. Conclusions
In conclusion, the study investigated the impacts of different aquaculture soundscapes on the growth, physiology, and behavior of largemouth bass. The results demonstrated that the fish reared in the IPRS soundscape exhibited lower growth performance compared to the Ambient and RAS groups. The physiological analyses revealed negative impacts on the fish’s immunological, anti-oxidation, and digestive enzyme activities in the IPRS group. Moreover, the swimming behavior of the fish was influenced by the soundscapes, with the IPRS group showing lower swimming polarity and cohesion. To optimize fish welfare and production performance, it is essential to implement effective soundscape management strategies in aquaculture systems. Further research should focus on evaluating the long-term effects of noise pollution, exploring its impacts on other fish species and aquaculture systems, and developing targeted measures to minimize noise pollution.
Author Contributions
Methodology, Y.Z. and J.Z.; software, S.H. and Y.Z.; formal analysis, H.Z. and Y.Z.; investigation, Z.Y.; resources, Y.Z.; data curation, Y.Z.; writing—original draft preparation, Y.Z.; writing—review and editing, A.S. and S.Z.; visualization, Y.Z.; supervision, S.Z., Z.Y. and W.X.; project administration, S.Z.; funding acquisition, Z.Y. and S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key R&D Program of China (Grant No. 2022YFD2001700), the Key Program of Science and Technology of Zhejiang Province (Grant No. 2023C02050), and the National Modern Agriculture Industrial Technology System Special Project—the National Technology System for Conventional Freshwater Fish Industries (Grant No. CARS-45-24).
Institutional Review Board Statement
The maintenance, handling, and experiments conducted on fish during this study were carried out in strict accordance with the guidelines of the Experimental Animal Welfare Ethics Committee of Zhejiang University (no. ZJU20190079).
Data Availability Statement
The data presented in this study are available on demand from the first author at (21713051@zju.edu.cn).
Acknowledgments
The authors sincerely thank Jianfeng Agricultural Development Co., Ltd. for site support and Peng Nantian and Zhang Yanfeng for technical support. We also thank the editors and reviewers of this paper for their work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmed, N.; Thompson, S.; Glaser, M. Global aquaculture productivity, environmental sustainability, and climate change adaptability. Environ. Manag. 2019, 63, 159–172. [Google Scholar] [CrossRef]
- Delgado, C.L.; Wada, N.; Rosegrant, M.W.; Meijer, S.; Ahmed, M. Outlook for Fish to 2020: Meeting Global Demand; Food Policy Report; International Food Policy Research Institute: Washington, DC, USA, 2003; Volume 15. [Google Scholar]
- Beveridge, M.C.M.; Phillips, M.J.; Macintosh, D.J. Aquaculture and the environment: The supply of and demand for environmental goods and services by Asian aquaculture and the implications for sustainability. Aquac. Res. 1997, 28, 797–807. [Google Scholar] [CrossRef]
- Islam, M.; Yasmin, R. Impact of aquaculture and contemporary environmental issues in Bangladesh. Int. J. Fish. Aquat. Stud. 2017, 5, 100–107. [Google Scholar]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, R.A.; Fuentes, M.; Montes, R.M.; Soto, D.; León-Muñoz, J. Environmental issues in Chilean salmon farming: A review. Rev. Aquac. 2019, 11, 375–402. [Google Scholar] [CrossRef]
- Hawkins, A.D.; Pembroke, A.E.; Popper, A.N. Information gaps in understanding the effects of noise on fishes and invertebrates. Rev. Fish Biol. Fish. 2015, 25, 39–64. [Google Scholar] [CrossRef]
- Hatch, L.; Wahle, C.; Gedamke, J.; Harrison, J.; Laws, B.; Moore, S.; Stadler, J.; Van Parijs, S. Can you hear me here? Managing acoustic habitat in US waters. Endanger. Species Res. 2016, 30, 171–186. [Google Scholar] [CrossRef]
- Fantini-Hoag, L.; Hanson, T.; Chappell, J. Production trials of in-pond raceway system growing stocker and foodsize hybrid Catfish plus Nile tilapia. Aquaculture 2022, 561, 738582. [Google Scholar] [CrossRef]
- Badiola, M.; Mendiola, D.; Bostock, J. Recirculating Aquaculture Systems (RAS) analysis: Main issues on management and future challenges. Aquac. Eng. 2012, 51, 26–35. [Google Scholar] [CrossRef]
- Hang, S.; Zhao, J.; Ji, B.; Li, H.; Zhang, Y.; Peng, Z.; Zhou, F.; Ding, X.; Ye, Z. Impact of underwater noise on the growth, physiology and behavior of Micropterus salmoides in industrial recirculating aquaculture systems. Environ. Pollut. 2021, 291, 118152. [Google Scholar] [CrossRef]
- Filiciotto, F.; Cecchini, S.; Buscaino, G.; Maccarrone, V.; Piccione, G.; Fazio, F. Impact of aquatic acoustic noise on oxidative status and some immune parameters in gilthead sea bream Sparus aurata (Linnaeus, 1758) juveniles. Aquac. Res. 2017, 48, 1895–1903. [Google Scholar] [CrossRef]
- Wysocki, L.E.; Davidson, J.W., III; Smith, M.E.; Frankel, A.S.; Ellison, W.T.; Mazik, P.M.; Popper, A.N.; Bebak, J. Effects of aquaculture production noise on hearing, growth, and disease resistance of rainbow trout Oncorhynchus mykiss. Aquaculture 2007, 272, 687–697. [Google Scholar] [CrossRef]
- Davidson, J.; Bebak, J.; Mazik, P. The effects of aquaculture production noise on the growth, condition factor, feed conversion, and survival of rainbow trout, Oncorhynchus mykiss. Aquaculture 2009, 288, 337–343. [Google Scholar] [CrossRef]
- Anderson, P.A.; Berzins, I.K.; Fogarty, F.; Hamlin, H.J.; Guillette, L.J., Jr. Sound, stress, and seahorses: The consequences of a noisy environment to animal health. Aquaculture 2011, 311, 129–138. [Google Scholar] [CrossRef]
- Bart, A.N.; Clark, J.; Young, J.; Zohar, Y. Underwater ambient noise measurements in aquaculture systems: A survey. Aquac. Eng. 2001, 25, 99–110. [Google Scholar] [CrossRef]
- Slater, M.; Fricke, E.; Weiss, M.; Rebelein, A.; Bögner, M.; Preece, M.; Radford, C. The impact of aquaculture soundscapes on whiteleg shrimp Litopenaeus vannamei and Atlantic salmon Salmo salar. Aquac. Environ. Interact. 2020, 12, 167–177. [Google Scholar] [CrossRef]
- Davidson, J.; Frankel, A.S.; Ellison, W.T.; Summerfelt, S.; Popper, A.N.; Mazik, P.; Bebak, J. Minimizing noise in fiberglass aquaculture tanks: Noise reduction potential of various retrofits. Aquac. Eng. 2007, 37, 125–131. [Google Scholar] [CrossRef]
- Amoser, S.; Ladich, F. Are hearing sensitivities of freshwater fish adapted to the ambient noise in their habitats? J. Exp. Biol. 2005, 208, 3533–3542. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, A.D.; Johnstone, A.D.F. The hearing of the Atlantic salmon, Salmo salar. J. Fish Biol. 1978, 13, 655–673. [Google Scholar] [CrossRef]
- Popper, A.N. Pure-tone auditory thresholds for the carp, Cyprinis carpio. J. Acoust. Soc. Am. 1972, 52, 1714–1717. [Google Scholar] [CrossRef]
- Radford, C.; Slater, M. Soundscapes in aquaculture systems. Aquac. Environ. Interact. 2019, 11, 53–62. [Google Scholar] [CrossRef]
- Wolff, D.L. Das Hörvermögen des Flußbarsches (Perca fluviatilis L.). Biol. Zent. Bl. 1967, 86, 449–460. [Google Scholar]
- Lugli, M.; Fine, M.L. Acoustic communication in two freshwater gobies: Ambient noise and short-range propagation in shallow streams. J. Acoust. Soc. Am. 2003, 114, 512–521. [Google Scholar] [CrossRef]
- Barber, J.R.; Crooks, K.R.; Fristrup, K.M. The costs of chronic noise exposure for terrestrial organisms. Trends Ecol. Evol. 2010, 25, 180–189. [Google Scholar] [CrossRef]
- Slabbekoorn, H.; Bouton, N.; van Opzeeland, I.; Coers, A.; ten Cate, C.; Popper, A.N. A noisy spring: The impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 2010, 25, 419–427. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, J.; Xu, W.; Zhan, W.; Zou, H.; Lin, J. Transcriptomic and Behavioral Studies of Small Yellow Croaker (Larimichthys polyactis) in Response to Noise Exposure. Animals 2022, 12, 2061. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.M.; Maruska, K.P. Noise during mouthbrooding impairs maternal care behaviors and juvenile development and alters brain transcriptomes in the African cichlid fish Astatotilapia burtoni. Genes Brain Behav. 2021, 20, e12692. [Google Scholar] [CrossRef]
- Passos, M.F.; Beirão, M.V.; Midamegbe, A.; Duarte RH, L.; Young, R.J.; de Azevedo, C.S. Impacts of noise pollution on the agonistic interactions of the saffron finch (Sicalis flaveola Linnaeus, 1766). Behav. Process. 2020, 180, 104222. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, H.; Chen, J.; Song, J.; Xu, K.; Lin, J.; Zhang, S. Potential effects of underwater noise from wind turbines on the marbled rockfish (Sebasticus marmoratus). J. Appl. Ichthyol. 2021, 37, 514–522. [Google Scholar] [CrossRef]
- Popper, A.N.; Hawkins, A.D. An overview of fish bioacoustics and the impacts of anthropogenic sounds on fishes. J. Fish Biol. 2019, 94, 692–713. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, J.; Nie, Z.; Gao, J.; Sun, Y.; Shao, N.; Li, Q.; Hu, J.; Xu, P.; Xu, G. Effects of stocking density on growth, serum parameters, antioxidant status, liver and intestine histology and gene expression of largemouth bass (Micropterus salmoides) farmed in the in-pond raceway system. Aquac. Res. 2020, 51, 5228–5240. [Google Scholar] [CrossRef]
- He, K.; Gkioxari, G.; Dollâr, P.; Girshick, R. R-CNN Mask 2020. IEEE Trans. Pattern Anal. Mach. Intell. 2020, 42, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, I.; Bradshaw, H.; Ha, T.T.; Halloy, J.; Godoy-Diana, R.; Thiria, B. Simple phalanx pattern leads to energy saving in cohesive fish schooling. Proc. Natl. Acad. Sci. USA 2017, 114, 9599–9604. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.I.; Lukeman, R.; Lizier, J.T.; Ward, A.J. Speed-mediated properties of schooling. R. Soc. Open Sci. 2019, 6, 181482. [Google Scholar] [CrossRef] [PubMed]
- Papoutsoglou, S.E.; Karakatsouli, N.; Louizos, E.; Chadio, S.; Kalogiannis, D.; Dalla, C.; Polissidis, A.; Papadopoulou-Daifoti, Z. Effect of Mozart’s music (Romanze-Andante of “Eine Kleine Nacht Musik”, sol major, K525) stimulus on common carp (Cyprinus carpio L.) physiology under different light conditions. Aquac. Eng. 2007, 36, 61–72. [Google Scholar] [CrossRef]
- Papoutsoglou, S.E.; Karakatsouli, N.; Papoutsoglou, E.S.; Vasilikos, G. Common carp (Cyprinus carpio) response to two pieces of music (“Eine Kleine Nachtmusik” and “Romanza”) combined with light intensity, using recirculating water system. Fish Physiol. Biochem. 2010, 36, 539–554. [Google Scholar] [CrossRef]
- Papoutsoglou, S.E.; Karakatsouli, N.; Skouradakis, C.; Papoutsoglou, E.S.; Batzina, A.; Leondaritis, G.; Sakellaridis, N. Effect of musical stimuli and white noise on rainbow trout (Oncorhynchus mykiss) growth and physiology in recirculating water conditions. Aquac. Eng. 2013, 55, 16–22. [Google Scholar] [CrossRef]
- Filiciotto, F.; Giacalone, V.M.; Fazio, F.; Buffa, G.; Piccione, G.; Maccarrone, V.; Di Stefano, V.; Mazzola, S.; Buscaino, G. Effect of acoustic environment on gilthead sea bream (Sparus aurata): Sea and onshore aquaculture background noise. Aquaculture 2013, 414, 36–45. [Google Scholar] [CrossRef]
- Dooling, R.J.; Leek, M.R.; Popper, A.N. Effects of noise on fishes: What we can learn from humans and birds. Integr. Zool. 2015, 10, 29–37. [Google Scholar] [CrossRef]
- Crovo, J.A.; Mendonça, M.T.; Holt, D.E.; Johnston, C.E. Stress and auditory responses of the otophysan fish, Cyprinella venusta, to road traffic noise. PLoS ONE 2015, 10, e0137290. [Google Scholar] [CrossRef]
- Scholik, A.R.; Yan, H.Y. Effects of underwater noise on auditory sensitivity of a cyprinid fish. Hear. Res. 2001, 152, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Voellmy, I.K.; Purser, J.; Flynn, D.; Kennedy, P.; Simpson, S.D.; Radford, A.N. Acoustic noise reduces foraging success in two sympatric fish species via different mechanisms. Anim. Behav. 2014, 89, 191–198. [Google Scholar] [CrossRef]
- Purser, J.; Radford, A.N. Acoustic noise induces attention shifts and reduces foraging performance in three-spined sticklebacks (Gasterosteus aculeatus). PLoS ONE 2011, 6, e17478. [Google Scholar] [CrossRef] [PubMed]
- Bracciali, C.; Campobello, D.; Giacoma, C.; Sara, G. Effects of nautical traffic and noise on foraging patterns of Mediterranean damselfish (Chromis chromis). PLoS ONE 2012, 7, e40582. [Google Scholar] [CrossRef]
- Xiong, D.; Fang, T.; Yu, L.; Sima, X.; Zhu, W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: Acute toxicity, oxidative stress and oxidative damage. Sci. Total Environ. 2011, 409, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Kim, S.; Ahn, J.H.; Youn, P.; Kang, J.S.; Park, K.; Yi, J.; Ryu, D.-Y. Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat. Toxicol. 2010, 100, 151–159. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, H.; Wang, X.; Wu, J.; Xue, Y. Effects of chronic exposure of 2, 4-dichlorophenol on the antioxidant system in liver of freshwater fish Carassius auratus. Chemosphere 2004, 55, 167–174. [Google Scholar] [CrossRef]
- Farombi, E.O.; Adelowo, O.A.; Ajimoko, Y.R. Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun River. Int. J. Environ. Res. Public Health 2007, 4, 158–165. [Google Scholar] [CrossRef]
- Rombout, J.H.; Abelli, L.; Picchietti, S.; Scapigliati, G.; Kiron, V. Teleost intestinal immunology. Fish Shellfish Immunol. 2011, 31, 616–626. [Google Scholar] [CrossRef]
- Salinas, I.; Zhang, Y.A.; Sunyer, J.O. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 2011, 35, 1346–1365. [Google Scholar] [CrossRef]
- Wang, L.G.; Li, E.C.; Qin, J.G.; Du, Z.Y.; Yu, N.; Kong, Y.Q.; Feng, D.X.; Chen, L.Q. Effect of oxidized fish oil and α-tocopherol on growth, antioxidation status, serum immune enzyme activity and resistance to Aeromonas hydrophila challenge of Chinese mitten crab Eriocheir sinensis. Aquac. Nutr. 2015, 21, 414–424. [Google Scholar] [CrossRef]
- Picos-Garcıa, C.; Garcıa-Carreno, F.L.; Serviere-Zaragoza, E. Digestive proteases in juvenile Mexican green abalone, Haliotis fulgens. Aquaculture 2000, 181, 157–170. [Google Scholar] [CrossRef]
- Allert, S.; Ernest, I.; Poliszczak, A.; Opperdoes, F.R.; Michels, P.A. Molecular cloning and analysis of two tandemly linked genes for pyruvate kinase of Trypanosoma brucei. Eur. J. Biochem. 1991, 200, 19–27. [Google Scholar] [CrossRef]
- Laiz-Carrión, R.; Sangiao-Alvarellos, S.; Guzmán, J.M.; Martín del Río, M.P.; Míguez, J.M.; Soengas, J.L.; Mancera, J.M. Energy metabolism in fish tissues related to osmoregulation and cortisol action. Fish Physiol. Biochem. 2002, 27, 179–188. [Google Scholar] [CrossRef]
- Marqueze, A.; Kucharski, L.C.; Da Silva, R.S.M. Effects of anoxia and post-anoxia recovery on carbohydrate metabolism in the jaw muscle of the crab Chasmagnathus granulatus maintained on carbohydrate-rich or high-protein diets. J. Exp. Mar. Biol. Ecol. 2006, 332, 198–205. [Google Scholar] [CrossRef]
- Chen, S.Y.; Fei YH, J.; Chen, Y.C.; Chi, K.J.; Yang, J.T. The swimming patterns and energy-saving mechanism revealed from three fish in a school. Ocean Eng. 2016, 122, 22–31. [Google Scholar] [CrossRef]
- Wang, Z.-T.; Akamatsu, T.; Nowacek, D.P.; Yuan, J.; Zhou, L.; Lei, P.-Y.; Li, J.; Duan, P.-X.; Wang, K.-X.; Wang, D. Soundscape of an Indo-Pacific humpback dolphin (Sousa chinensis) hotspot before windfarm construction in the Pearl River Estuary, China: Do dolphin engage in noise avoidance and passive eavesdropping behavior? Mar. Pollut. Bull. 2019, 140, 509–522. [Google Scholar] [CrossRef]
- Sabet, S.S.; Van Dooren, D.; Slabbekoorn, H. Son et lumiere: Sound and light effects on spatial distribution and swimming behavior in captive zebrafish. Environ. Pollut. 2016, 212, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.G.; Przeslawski, R.; Duncan, A.; Gunning, M.; Bruce, B. A critical review of the potential impacts of marine seismic surveys on fish & invertebrates. Mar. Pollut. Bull. 2017, 114, 9–24. [Google Scholar]
- Weilgart, L.S. The impacts of anthropogenic ocean noise on cetaceans and implications for management. Can. J. Zool. 2007, 85, 1091–1116. [Google Scholar] [CrossRef]
- Simpson, S.D.; Radford, A.N.; Nedelec, S.L.; Ferrari, M.C.; Chivers, D.P.; McCormick, M.I.; Meekan, M.G. Anthropogenic noise increases fish mortality by predation. Nat. Commun. 2016, 7, 10544. [Google Scholar] [CrossRef] [PubMed]
- Popper, A.N.; Hastings, M.C. The effects of anthropogenic sources of sound on fishes. J. Fish Biol. 2009, 75, 455–489. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.W.; Mangel, M. The evolutionary advantages of group foraging. Theor. Popul. Biol. 1986, 30, 45–75. [Google Scholar] [CrossRef]
- Creel, S.; Creel, N.M. Communal hunting and pack size in African wild dogs, Lycaon pictus. Anim. Behav. 1995, 50, 1325–1339. [Google Scholar] [CrossRef]
- Pitcher, T.J.; Magurran, A.E.; Winfield, I.J. Fish in larger shoals find food faster. Behav. Ecol. Sociobiol. 1982, 10, 149–151. [Google Scholar] [CrossRef]
- Nedelec, S.L.; Mills, S.C.; Lecchini, D.; Nedelec, B.; Simpson, S.D.; Radford, A.N. Repeated exposure to noise increases tolerance in a coral reef fish. Environ. Pollut. 2016, 216, 428–436. [Google Scholar] [CrossRef]
- Fels, D.; Ap Rhisiart, A.; Vollrath, F. The selfish crouton. Behaviour 1995, 132, 49–55. [Google Scholar] [CrossRef]
- Ranta, E.; Lindstroem, K. Behaviour of fish-ecological consequences. Ann. Zool. Fenn. 1990, 27, 50. [Google Scholar]
- Treherne, J.E.; Foster, W.A. Group transmission of predator avoidance behaviour in a marine insect: The Trafalgar effect. Anim. Behav. 1981, 29, 911–917. [Google Scholar] [CrossRef]
- Killen, S.S.; Marras, S.; Steffensen, J.F.; McKenzie, D.J. Aerobic capacity influences the spatial position of individuals within fish schools. Proc. R. Soc. B Biol. Sci. 2012, 279, 357–364. [Google Scholar] [CrossRef]
- Marras, S.; Killen, S.S.; Lindström, J.; McKenzie, D.J.; Steffensen, J.F.; Domenici, P. Fish swimming in schools save energy regardless of their spatial position. Behav. Ecol. Sociobiol. 2015, 69, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, L.; Baktoft, H.; Jepsen, N.; Aarestrup, K.; Berg, S.; Skov, C. Effect of boat noise and angling on lake fish behaviour. J. Fish Biol. 2014, 84, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Westneat, D.F.; Walters, A.; McCarthy, T.M.; Hatch, M.I.; Hein, W.K. Alternative mechanisms of nonindependent mate choice. Anim. Behav. 2000, 59, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Neo, Y.Y.; Hubert, J.; Bolle, L.; Winter, H.V.; Ten Cate, C.; Slabbekoorn, H. Sound exposure changes European seabass behaviour in a large outdoor floating pen: Effects of temporal structure and a ramp-up procedure. Environ. Pollut. 2016, 214, 26–34. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).