Abstract
Typhoons disturb the upper ocean, weaken the physical stratification, and induce temporal and spatial changes in primary production, which rapidly alter the distribution and diversity of fishery resources. This study analyzed the response of oceanic conditions and fishery resources on the sea area of the typhoon pathway in the East/Japan Sea (Type A: typhoon passed from southwest to northeast; Type B: typhoon dissipated in the southwest; Type C: typhoon passed from southeast to northeast; and Type D: typhoons passed from southwest to northwest). For Types A and B, the sea surface temperature (SST) decreased in all areas, and Chl-a showed the largest fluctuations in the southwest. For Type C, the SST variation was reduced in the eastern part, stratification was strengthened, and Chl-a did not differ significantly in each area. For Type D, SST and Chl-a showed significant variations in the western part. The biomass of fishery resources increased along the typhoon path for each type, and the diversity increased for Types A and D but decreased for Type B; however, the diversity and catch of fishery resources increased in the northeast for Type C. This study contributes to understanding the impact of typhoon pathway changes on the marine environment and ecosystem.
Key Contribution:
The East/Japan Sea (EJS) is divided into north and south along the subpolar front, and it is again divided into east and west environments according to the characteristics of ocean current circulation. As a result, when a typhoon moves from southwest to northeast, or it moves northward along the western coast in the EJS, changes in the marine environment and fishery are relatively large compared to the other pathways.
1. Introduction
The Northwest Pacific Ocean is strongly stratified by solar radiation, which limits material circulation between the upper and lower layers and reduces biological productivity in the summer [1]. Typhoons that occur in tropical seas pass through the Northwest Pacific between the middle of summer and the beginning of autumn, where they act as a major force driving a chain of physical, chemical, and biological environmental changes by disturbing the upper ocean [2,3,4,5,6]. The strong wind stress accompanying a typhoon decreases sea surface temperatures (SSTs) and weakens stratification structures through vertical mixing, Ekman transport upwelling, and turbulent mixing-induced entrainment [7,8,9,10]. Sea surface cooling (SSC), in which a decreased SST is present, occurs in the path of a typhoon in the range of 1–6 °C [11] and continues for several days due to the inertial motion after the typhoon dissipates [12,13,14,15,16]. The stratification structure is weakened as typhoons stimulate primary production through the upward mixing of deep nutrients toward the surface [17,18,19,20,21]. Changes in the physical oceanic environment have resulted in changes in rapid primary productivity and food webs, which cause changes in the distribution, species diversity, and catchment of fishery resources [22,23,24,25,26].
Post-typhoon changes in the marine environment and biological productivity depend on the pathway and intensity of the typhoon. Typhoon path changes continue the southward or northward winds, causing upwelling or downwelling in coastal areas. If the coastal region facing the land is located on the right side of the typhoon radius during a typhoon, the northward wind continues to generate upwelling, while if it is located on the left side, the coast is affected by the southward wind [27,28,29]. Upwelling has a beneficial effect on organisms by providing nutrients to the surface layer, whereas downwelling causes oligotrophic seawater to migrate downward to the bottom layer and results in reduced biological productivity [27]; therefore, changes in the marine environment caused by the typhoon path can be transmitted to fishery resources.
The East/Japan Sea (EJS) is the Northwest Pacific marginal sea that has moderate temperate climate characteristics at mid-latitudes and seasonal environmental changes. Typhoons enter the EJS between July and October and affect it through various pathways. The EJS has formed a subpolar front (SPF) at approximately 40° N with a strong water temperature gradient according to a cold current running southward along the Russian coast and a warm current running northward through the Korean Strait [30,31]. The current circulation of the EJS around the SPF is divided into counterclockwise and clockwise circulations in the northern and southern parts, respectively. In the northern part of the EJS, the Liman Cold Current and the North Korean Cold Current are separated around Vladivostok due to the northwest monsoon [32,33]. However, the East Korea Warm Current meanders along the coast of the EJS from the Korea Strait and forms an eddy in the southern part of the EJS [32,34,35]. These current interactions cause the EJS to have different physical and environmental, chemical (nutrients), and biological (primary production) characteristics [36,37,38,39,40]. Senju and Watanabe (1999) observed environmental changes in the EJS while typhoons passed along the northern coast of Japan [41], and Hong and Yoon (2003) proved that the post-typhoon SSC of the EJS was caused by coastal upwelling [12]. Additionally, Ekman transport, upwelling, and vertical mixing due to the passage of the typhoon change the temperature of middle, bottom, and surface waters [27,28,42,43], which affects the development and extinction of cold water along the coast of the EJS [29].
Previous research has explained the oceanic condition change processes by which typhoons with strong physical forces pass through the EJS and have also focused on the short-term changes in Chl-a and the landing of pelagic fish [22,24,25,26]. The typhoon strength and moving path were both found to be influencing factors; therefore, understanding the effects of typhoon path variations in the EJS on different marine environmental characteristics in each area and its impact on the distribution and diversity of marine life resources caused by the post-typhoon chain responses in marine ecosystems is crucial to understanding typhoon-induced changes in the EJS. The EJS has shown strong stratification during typhoon seasons, which has reduced biological productivity. Recent climate change has caused the future marine environment to be expected to further strengthen stratification and the primary productivity of the EJS to continually decrease over the past 10 years [36]. Additionally, the frequency of typhoons will increase, and the intensity of typhoons passing through the EJS will continue to increase as the regional SST increases [44,45].
Typhoons weaken the strong stratification, and upwelling and mixing relax the high water temperature phenomenon that occurs in the summer, thereby supplying nutrients to the surface and increasing primary productivity. The marine ecosystems along the coast and open ocean of the EJS in the summer have been expected to improve and change the distribution and catch of fish in the short term. Furthermore, changes in the typhoon route flowing into the EJS have various effects on its environment in areas with different physical and biological characteristics, but the post-typhoon response of fishery resources has been assumed to be unique. In this study, the response characteristics of the marine environment and fishery resources in each area according to the typhoon path in the EJS were investigated. To analyze this, among the typhoons affected by the Korean Peninsula in the last 10 years (2011–2020), the typhoon routes flowing into the EJS were organized by type and divided into four areas according to the characteristics of the oceanic conditions. Regional SST and Chl-a characteristics before and after the typhoon were compared by area using satellite data to analyze changes in the EJS environment and the diversity of fishery resources due to typhoon type. Additionally, the response (composition, catch, and distribution) patterns of the major fishery resources in the EJS by area were analyzed using catch data of fish by area.
2. Data and Methods
2.1. Study Area
In this study, the EJS was divided into four major regions based on its physical, biological, and marine environment characteristics [30,32,33,35]; Figure 1. The EJS was first divided into northern and southern parts based on the SPF, and these areas were subdivided into eastern and western parts.
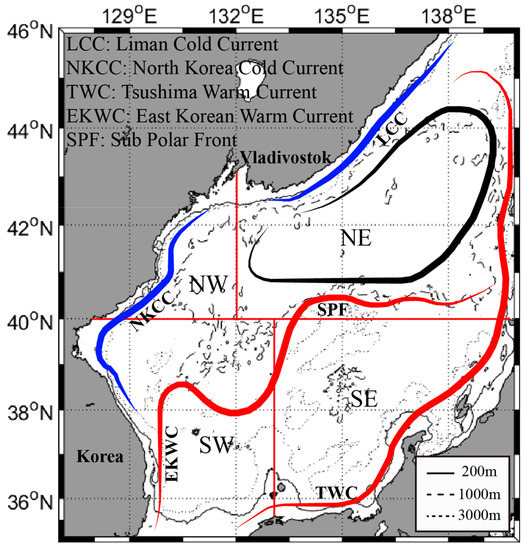
Figure 1.
Map showing study area divided based on subpolar front and surface current circulation in the East/Japan Sea (NW: Northwestern, NE: Northeastern, SW: Southwestern, SE: Southeastern); The red, blue, and black lines represent warm current, cold current, and counterclockwise circulation by northwestern monsoon, respectively.
2.2. Path of the Typhoon
The best track provided by the Typhoon Center of the Korea Meteorological Administration was used in this study. Typhoon movement trajectories were indicated for typhoons affected by the Korean Peninsula in the last 10 years (2011–2020) to identify typhoons that passed through the EJS or dissipated in the EJS (Figure A1). The trajectory of the typhoons was classified into four types according to their movement into the EJS (Figure 2). In Type A, the typhoon passed from the southwest area (SW) to the northeast area (NE) in the EJS, and in Type B, the typhoon disappeared within the EJS after it entered the southwest area (SW) of the EJS. In Type C, the typhoon moved north along the eastern area of the EJS from the southeast to the northeast area, and in Type D, the typhoon moved north along the eastern coast (western area) of the EJS from the southwest to the northwest (NW). In this study, typhoons with an intensity of 4 or higher according to the typhoon movement path (Table A1) were selected, and the physical and biological reaction characteristics of each area in the EJS according to the typhoon path and response of fishery resources were analyzed.
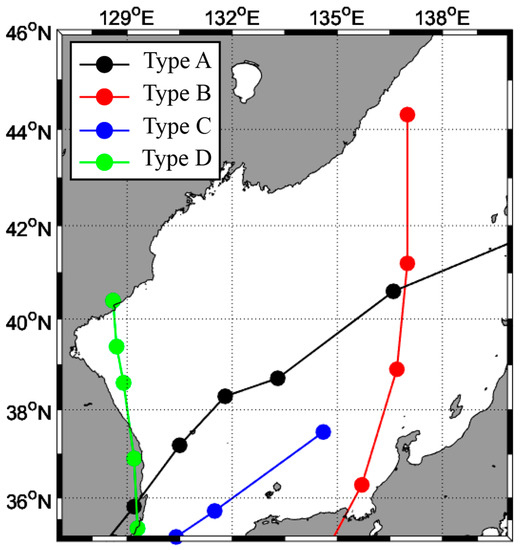
Figure 2.
Best tracks of the selected typhoon pathways that affected the East/Japan Sea.
2.3. Marine Environments of the EJS
2.3.1. Sea Surface Temperature (SST)
To analyze the change in the upper ocean temperature in the EJS after the typhoon, SST was calculated from the daily SST obtained from the daily Operational Sea Surface Temperature and Sea Ice Analysis (GHRSST OSTIA) data, which provided global SST at a spatial resolution of 0.05° [46]. Changes in SST were compared between 15 d before and after the typhoon.
2.3.2. Chlorophyll a (Chl-a)
To observe the Chl-a variations according to the typhoon passage, the Chl-a concentration was used for 15 d before and after the passing of the typhoon. Chl-a data were extracted from daily satellite ocean color images for 2011–2020 obtained from Moderate Resolution Imaging Spectrometer (MODIS) Aqua L3 products (http://oceancolor.gsfc.nasa.gov/ (accessed on 31 December 2021)) with a spatial resolution of 4 km.
2.3.3. Ekman Pumping Velocity (EPV)
The Ekman pumping velocity (EPV) was calculated from the European Center for Medium-Range Weather Forecasts (ECMWF) ERA5 reanalyzed wind data using the following equation to analyze the vertical flow (e.g., upwelling and downwelling) that occurred when the typhoon entered the EJS [47,48]. Wind data were provided with a spatial resolution of 0.25° and at hourly intervals [49]. Changes in EPV were compared between 15 d before and after the typhoon.
where is the wind stress, is the density of the water (1025.0 kg/m3), and is the Coriolis parameter. If the EPV was a positive number, an upward flow (upwelling) appeared in the sea area, and a negative value indicated that a downward flow (downwelling) occurred.
2.3.4. Mixed Layer Depth (MLD)
To analyze the changes in the mixed layer depths (MLDs) after passing the typhoon, the MLD was calculated from the daily MLD data for 2011–2020 obtained from the results of the Estimating the Circulation and Climate of the Ocean (ECCO2) data, which was calculated from satellite and in situ data with a spatial resolution of 0.25° [50]. Changes in the MLD were compared between 15 d before and after the typhoon.
2.4. Fishery Resources
To understand the response (i.e., the structure of fish assemblages, species composition and quantitative fluctuations, and diversity) of fishery resources according to short-term environmental changes after the typhoon, The fisheries catch data used in this study are the daily catch by area (0.5°) provided by the National Federation of Fisheries Cooperatives (NFFC, Republic of Korea). The data period is 2011–2020. These data are the total catch data not classified by type of fishery and fishing gear. In addition, since data on fishing efforts, such as the number of fishing ships and data classified into fishery and fishing gear types, are only available after 2018 (2019–2020), total catch data were used in this study. Although it is a short period, the changing trend is similar due to comparing the changing pattern of the catch per unit effort and the total catch over the past two years (Figure A6 and Figure A7). The species diversity index () [51] was calculated using the following equation and compared for 15 d before and after the typhoon passed:
where is the total number of species, Pi is the proportion of total catch of species i, and ln is the natural logarithm.
3. Results
3.1. Change in Oceanic Condition: SST, Chl-a, EPV, MLD
3.1.1. Type A
KONGREY occurred on 25 September 2018, and after entering the EJS on October 6, 2018, it was transformed into a temperate cyclone in the Sapporo Sea near Japan on 7 October 2018 (Figure 2, Table A1). The change in SST after the typhoon passed was from −1.9 to −2.4 °C, with the highest in the NW and the lowest in the SE and NE (Figure 3g–i, Table 1). The change in Chl-a after passing the typhoon was approximately 0.11–0.21 mg/m3, with the highest in the SW and the lowest in the SE and NE (Figure 3j–l, Table 1). Upward flow occurred in the SW and NE of the EJS near the typhoon path, and downward flow occurred in the SE and NW after the typhoon (Figure 3a–c, Table 1). The EPV was strongest in the SW and weakest in the NW. The MLD was 6.5–8.4 m deeper than before the typhoon, and the range of MLD variations was the largest in the SE and the smallest in the NE (Figure 3d–f, Table 1).
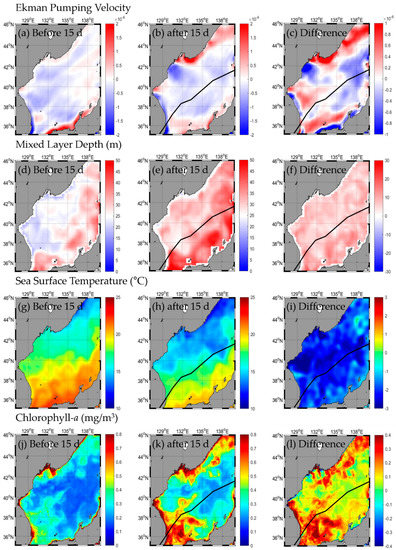
Figure 3.
Horizontal distribution of oceanic conditions (Ekman pumping velocity (a–c), mixed layer depth (d–f), sea surface temperature (g–i), chlorophyll a (j–l)) before (a,d,g,j) and after (b,e,h,k) the typhoon, and the difference (c,f,i,l) between 15 d before and after the typhoon KONGREY.

Table 1.
Changes in oceanic conditions (EPV; Ekman pumping velocity, MLD; mixed layer depth, SST; sea surface temperature, and Chl-a; Chlorophyll a) by sea area before and after the typhoon and the difference between 15 d before and after the typhoon.
3.1.2. Type B
DANAS occurred on 4 October 2013, and was transformed into a temperate cyclone in Dokdo after entering the EJS on 9 October 2013 (Figure 2, Table A1). The change in SST after the typhoon passed was from −1.7 to −2.5 °C, with the highest in the NW and the lowest in the SW and SE (Figure 4g–i, Table 1). The change in Chl-a after the typhoon passed was from 0.07 to 0.22 mg/m3, with the highest in the SW and in the lowest in the NW and NE (Figure 4j–l, Table 1). Upward flow occurred in the SE and NE of the EJS, and downward flow occurred in the SW and NW after the typhoon (Figure 4a–c, Table 1). The EPV was strongest in the SE and weakest in the NW. Furthermore, the MLD was 6.9–8.5 m deeper than before the typhoon, and the range of the MLD variations was the largest in the SW and the smallest in the SE (Figure 4d–f, Table 1).
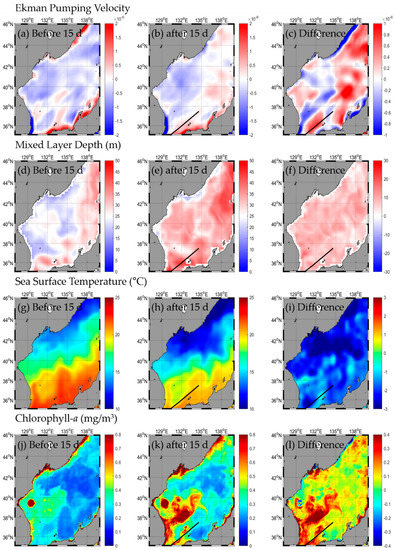
Figure 4.
Horizontal distribution of oceanic conditions (Ekman pumping velocity (a–c), mixed layer depth (d–f), sea surface temperature (g–i), chlorophyll a (j–l)) before (a,d,g,j) and after (b,e,h,k) the typhoon, and the difference (c,f,i,l) between 15 d before and after the typhoon DANAS.
3.1.3. Type C
HALONG occurred on 29 July 2014, after it moved northerly through the interior of Japan on 10 August 2014, and it was transformed into a temperate cyclone on 11 August 2014 (Figure 2, Table A1). The change in SST after passing the typhoon was −0.4 to −1.2 °C, with the highest in the eastern part (SE and NE) and the lowest in the western part (SW and NW; Figure 5g–i, Table 1). The change in Chl-a after the typhoon passed was from −0.01 to 0.01 mg/m3, but Chl-a was similar to before the typhoon. Upward flow occurred throughout the EJS (Figure 5j–l, Table 1). The EPV was the strongest in the SE and weakest in the NW (Figure 5a–c, Table 1). The MLD was 1.9–5.0 m deeper than before the typhoon, and the range of the MLD variations was the largest in the NE and the smallest in the SW (Figure 5d–f, Table 1).
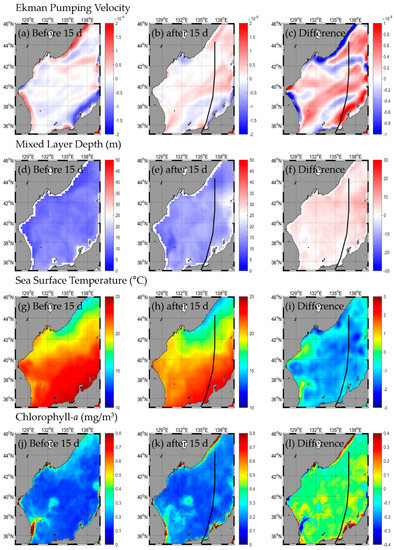
Figure 5.
Horizontal distribution of oceanic conditions (Ekman pumping velocity (a–c), mixed layer depth (d–f), sea surface temperature (g–i), chlorophyll a (j–l)) before (a,d,g,j) and after (b,e,h,k) the typhoon, and the difference (c,f,i,l) between 15 d before and after the typhoon HALONG.
3.1.4. Type D
HAISHEN occurred on 1 September 2020, entered the EJS, and was then transformed into a temperate cyclone on 7 September 2020 (Figure 2, Table A1). The change in SST after the typhoon passed was from −1.2 to −3.9 °C, with the highest in the western part (SW and NW) and the lowest in the eastern part (SE and NE; Figure 6g–i, Table 1). The change in Chl-a after the typhoon passed was from 0.08 to 0.61 mg/m3, with the highest in the NW and the lowest in the eastern part (Figure 6j–l, Table 1). Upward flow occurred in the northern part of the EJS, and downward flow occurred in the southern part after the typhoon (Figure 6a–c, Table 1). The EPV was strongest in the NE and weakest in the SE. The MLD was 5.6–12.7 m deeper than before the typhoon, and the range of its variations was largest in the SW and smallest in the NE (Figure 6d–f, Table 1).
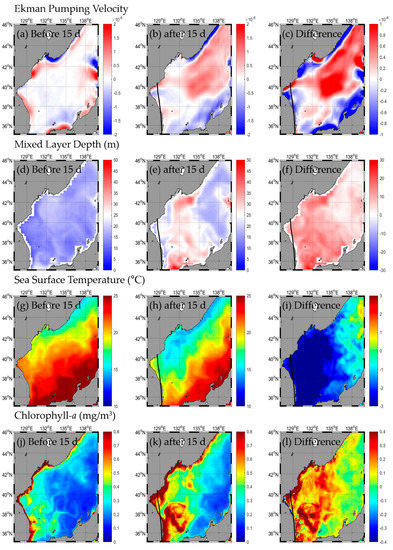
Figure 6.
Horizontal distribution of oceanic conditions (Ekman pumping velocity (a–c), mixed layer depth (d–f), sea surface temperature (g–i), chlorophyll a (j–l)) before (a,d,g,j) and after (b,e,h,k) the typhoon, and the difference (c,f,i,l) between 15 d before and after the typhoon HAISHEN.
3.2. Responses in Fishery Resources
3.2.1. Type A
A total of 45 species weighing 151,200.4 kg were caught in the EJS before the KONGREY typhoon passed, with extremes of 45 species in the SW, 2 species in the NE, 85,263.1 kg in the SW, and 24,213.5 kg in the SE, respectively (Table 2 and Table A2). A total of 52 species and 270,389.2 kg were caught after the typhoon, with extremes of 52 species in the SW, 2 species in the NE, 195,422.6 kg in the SW, and 37,354.4 kg in the SE. Therefore, after the typhoon, the number of species and the total catch increased (Table 2 and Table A2). Both the number of species and catch increased the most in the SW (Figure 7a–f, Table 2). In the NE, the number of species did not change, and the total catch decreased (Figure 7a–f, Table 2). The species diversity in the EJS decreased slightly from 1.8 before the typhoon to 1.7 after the typhoon (Figure 7g–i, Table 2). The diversity of each area decreased from extremes of 0.1 and 2.2 in the NE and SW, respectively, before the typhoon to those of 0.1 and 1.8 in the NE and SW, respectively, after the typhoon (Figure 7g–i, Table 2). Moreover, this diversity increased in the SE and NE (Figure 7g–i, Table 2); however, the number of species, total catch, and diversity increased along the typhoon path, with this trend being more evident in the SE, which is the right side in the southern part, and in the NE, which is the left side in the northern part, based on the typhoon path (Figure 7a–f). Additionally, responses of fishery resources (the number of species, total catch, and diversity) were compared during the non-typhoon period (Figure A2a–i) and the typhoon KONGREY period (Figure 3a–i). The responses of fishery increase along the typhoon pathway during the typhoon period was not seen during the non-typhoon period. In particular, diversity tends to decrease during non-typhoon periods in the NE.

Table 2.
Changes in fishery responses (number of species, total catch, and diversity) by sea area before and after the typhoon and the difference between 15 d before and after the typhoon.
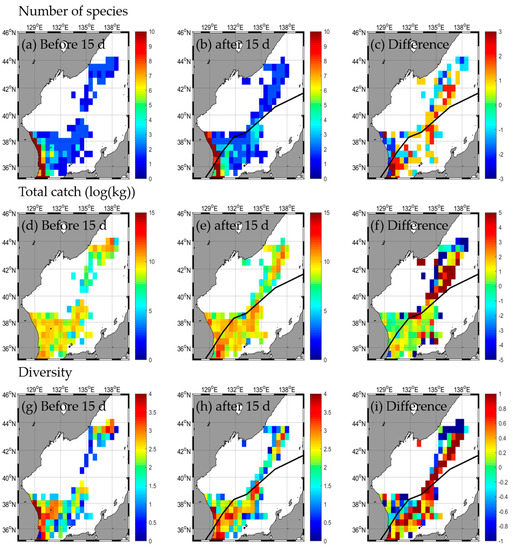
Figure 7.
Horizontal distribution of fishery responses (number of species (a–c), total catch (d–f), and diversity (g–i)) before (a,d,g) and after (b,e,h) the typhoon and the difference (c,f,i) between 15 d before and after typhoon KONGREY.
3.2.2. Type B
A total of 31 species and 215,391.7 kg were caught in the EJS before the DANAS typhoon passed, with extremes of 31 species in the SW, 2 species in the NE, 185,131.2 kg in the SW, and 8274.0 kg in the SE caught (Table 2 and Table A2). A total of 31 species and 219,588.9 kg were caught after the typhoon, with extremes of 30 species in the SW, 2 species in the NE, 217,913.9 kg in the SW, and 1675.0 kg in the SE (Table 2 and Table A2). The number of species and total catch did not change after the typhoon (Figure 8a–f, Table 2). In the SW area, the total catch increased the most, while the number of species and total catch decreased in the NE area (Figure 8a–f, Table 2). The species diversity in the EJS decreased from 1.8 before the typhoon to 1.7 after the typhoon (Figure 8g–i, Table 2). The diversity by each area decreased from extremes of 1.2 in the SW and 0.1 in the NE before the typhoon to those of 1.3 in the SW and 0 in the NE after the typhoon (Figure 8g–i, Table 2). There was a tendency in the SW for the diversity to increase and in the SE and NE for it to decrease. Overall, the number of species, total catch, and diversity increased (Figure 8a–i, Table 2), which was noticeable in the SE along the typhoon path (Figure 8a–i). In particular, this decrease was the largest in the NE, but the distribution pattern increased in the SW (Figure 8a–i, Table 2). After the typhoon, the fishing ground tends to reduce from the NE to the SW (Figure 8a–i), but unlike the typhoon DANAS period, the fishing ground tends to expand to the NW and shift northward (Figure A3a–i). In addition, during the typhoon period, responses of fishery decrease along the typhoon path. However, the number of species, total catch, and diversity tend to increase during the non-typhoon period compared to the typhoon period.
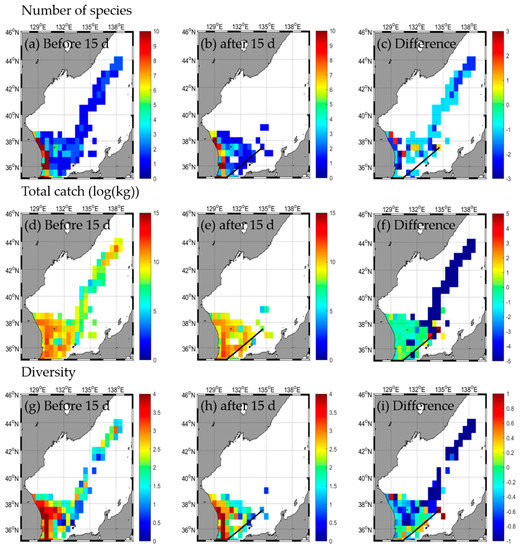
Figure 8.
Horizontal distribution of fishery responses (number of species (a–c), total catch (d–f), and diversity (g–i)) before (a,d,g) and after (b,e,h) the typhoon and the difference (c,f,i) between 15 d before and after typhoon DANAS.
3.2.3. Type C
A total of 31 species and 51,129.4 kg were caught in the EJS before the HALONG typhoon passed, with extremes of 31 species in the SW, 1 species in the NE, 32,992.0 kg in the SW, and 821.4 kg in the SE caught (Table 2 and Table A2). After the typhoon, 32 species and 93,161.3 kg were caught, with extremes of 30 species in the SW, 2 species in the NE, 25,744.3 kg in the SW, and 920.3 kg in the SE caught (Table 2 and Table A2). Changes in the number of species were not evident after the typhoon, but the total catch increased (Figure 9a–f, Table 2). The total catch tended to decrease in the SW but increased the most in the NE (Figure 9d–f, Table 2). The species diversity in the EJS decreased from 1.4 before the typhoon to 0.9 after the typhoon (Figure 9g–i, Table 2); however, regional diversity decreased from extremes of 1.8 in the SW and 0 in the NE before the typhoon to those of 2.0 in the SW and 0 in the NE after the typhoon (Figure 9g–i, Table 2). There was a tendency to increase throughout the EJS, but the range of change was smaller than that of other types (Figure 9g–i, Table 2). Additionally, responses of fishery resources were compared during the non-typhoon period (Figure A4a–i) and the typhoon HALONG period (Figure 5a–i). The responses of fishery increase along the typhoon pathway during the typhoon period was not seen during the non-typhoon period.
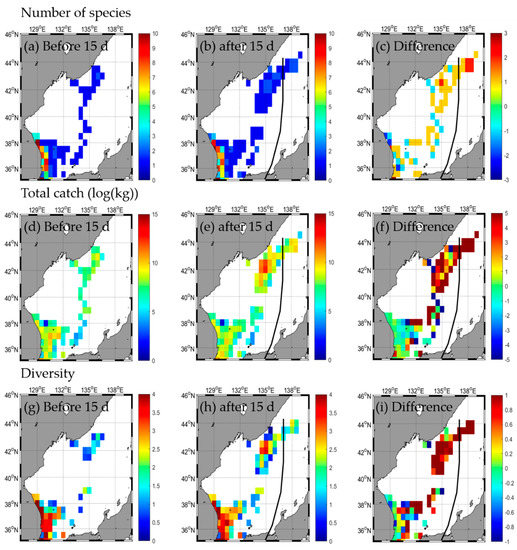
Figure 9.
Horizontal distribution of fishery responses (number of species (a–c), total catch (d–f), and diversity (g–i)) before (a,d,g) and after (b,e,h) the typhoon and the difference (c,f,i) between 15 d before and after the typhoon HALONG.
3.2.4. Type D
A total of 33 species and 49,900.0 kg were caught in the EJS before the HAISHEN typhoon passed, with extremes of 33 species in the SW, 1 species in the SE, 48,443 kg in the SW, and 1456.7 kg in the SE caught (Table 2 and Table A2). After the typhoon, 35 species and 146,598.1 kg were caught, with extremes of 35 species in the SW, 4 species in the SE, 117,438.9 kg in the SW, and 29,159.1 kg in the SE (Table 2 and Table A2). Changes in the number of species were not evident after the typhoon (Figure 10a–c, Table 2), but the total catch increased (Figure 10d–f, Table 2). The total catch had the largest increase in the SW, and the change in the number of species was the largest in the SE (Figure 10a–f, Table 2). The species diversity in the EJS decreased from 2.2 before the typhoon to 1.9 after the typhoon (Figure 10g–i, Table 2). The diversity of each area decreased from the extremes being 2.2 in the SW and 0 in the NE before the typhoon to those being 2.0 in the SW and 0.4 in the SE after the typhoon (Figure 10g–i, Table 2). There was a tendency to decrease in the SW, but an increase in the SE. The distribution of the number of species increased, but the total catch and diversity decreased along the typhoon path (Figure 10a–i). During the non-typhoon period, unlike the typhoon HAISHEN period, the fishing ground tends to expand from the SW to NE and shift northward (Figure A5a–i). In addition, the number of species, total catch, and diversity tend to decrease during the non-typhoon period compared to the typhoon period.
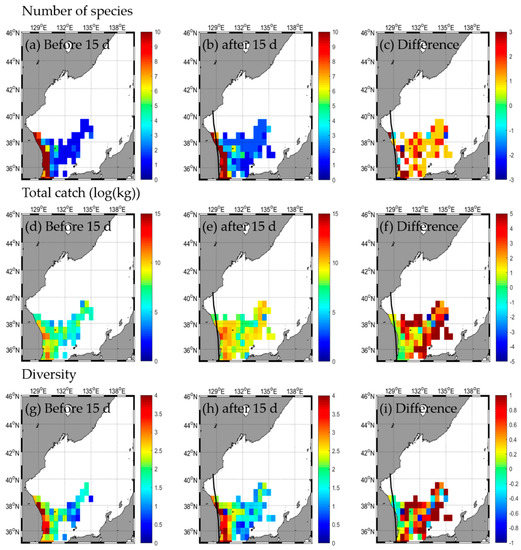
Figure 10.
Horizontal distribution of fishery responses (number of species (a–c), total catch (d–f), and diversity (g–i)) before (a,d,g) and after (b,e,h) the typhoon and the difference (c,f,i) between 15 d before and after the typhoon HAISHEN.
4. Discussion
The upper ocean changes due to typhoons passing through were affected by the pathways of the typhoons and the oceanic conditions (water temperature, stratification, etc.). These changes caused rapid ecosystem changes (distribution, species composition, food web, etc.). In this study, we classified the typhoon movement path entering the EJS by type and analyzed the response of the marine environment and fishery resources after the typhoon by type.
SSC and Chl-a blooming occurred after the typhoon, the mixed layer depth was deeper, and the vertical circulation was stronger than before the typhoon. These changes in the upper ocean result from solar radiation being blocked from the atmosphere when the typhoon approaches the EJS, which creates a strong counterclockwise wind that facilitates mixing with upwelling. Chl-a variations were similar after the typhoon in Types A and B, but SST in all areas decreased in Type A, whereas the change in SST was the largest in the northern part in Type B. In the case of Type C, SST was significantly reduced in the eastern part, and the change in Chl-a was similar to that before the typhoon in each area. In Type D, SST and Chl-a changes were the largest in the western part. Environmental changes due to the passage of the typhoons differed depending on the structure of the water mass and stratification by area [14,52]. In the northern part of the EJS, based on the polar front, the mixed layer was deeply distributed, and stratification formed shallower because the difference in water temperature between the surface and bottom waters was relatively lower. In particular, the eastern part of Vladivostok had a stronger vertical mixing and weaker stratification than the western part because the counterclockwise cycle formed due to the influence of the northeast monsoon [32,33]. In contrast, the East Korea Warm and North Korea Cold Currents integrate to form a vertical water temperature gradient in the southern part of the EJS. The mixed layer in the western part was shallower than that in the eastern part because this warm water layer was formed deeper in the western part [53,54,55]. The SSC caused by the typhoon was the strongest in the northern part, where the stratification was relatively weak and cold water was distributed at the bottom, while it was the lowest in the SW, where the stratification was strong, and the warm water layer was thick. This change is related to the active vertical mixing caused by the typhoon in the northern part, where the thickness of the warm water layer is shallow, and the density gradient by depth is relatively small. However, in the SW, where the vertical density gradient is more extensive, and the warm water layer is thickly distributed, the change range of SSC was lower than that in the northern part, and mixing was formed in the warm water layer after the typhoon. Chl-a was highly distributed in the northern part, which is relatively well mixed compared to the southern part. In such an environment, the change in EJS primary productivity after the typhoon is likely the result of a substantial supply of bottom nutrients in the SW mixed into the deep layer due to the typhoon.
The intense wind stress accompanying the typhoon caused vertical mixing with upwelling around the typhoon’s path. This change in the upper oceanic condition was maintained for several days after the typhoon, as the inertial movement repeated the vertical circulation. The changes in EPV and MLD were strongest in the surrounding area along the typhoon pathway. Changes in the typhoon pathways and the upper oceanic conditions affect the wind direction on the coast of the EJS and cause environmental changes. Mixing with upwelling was formed in Types A, B, and D, meaning that the typhoon was fueled by a northward wind maintained along the coast of the EJS. Low-temperature and high-nutrient bottom waters are supplied to the surface, causing SSC and Chl-a blooming to occur at the coast of the EJS. However, in Type C, the typhoon caused downwelling by the southward wind, and the high temperatures of the open sea were transferred to the coastal region, causing the range of cooling to be lower, and the change in Chl-a did not differ from its pre-typhoon levels. The SSC and Chl-a changes lasted 6–12 d after the typhoon. Unlike in the North Pacific Ocean, upwelling due to a low-pressure vortex only occurred stronger during the typhoon, and highly turbulent diffusion had a more significant effect than upwelling, which affects the environmental structure of the EJS [12,56]. In this study, the vertical circulation effect caused by the passage of the typhoon was strongest near the typhoon path when the typhoon entered the EJS, causing changes in the upper oceanic conditions.
These changes in the marine environment after a typhoon may directly or indirectly affect the structure of the marine ecosystem and the distribution of fishery resources [22,24,40]. The distribution of ectothermic animals, such as fish, requires water temperature conditions that can allow them to adapt to survival or environmental conditions and are favorable for feeding activities. In particular, changes in the water temperature of a habitat are among the most important factors affecting their distribution [57,58]. The change in oceanic conditions within a short period after a typhoon influences the behavior of fishery resources.
Overall, biomass and species diversity changes were affected by the intensity of the environmental changes caused by the passage of typhoons. After typhoons, diversity tended to decrease in areas with higher fluctuations in SST and Chl-a, and the number of species and the total catch decreased. In contrast, diversity increased in areas where fluctuations in SST and Chl-a were relatively low, and the number of species and total catch increased. According to the optimal environmental window hypothesis [59], changes in marine ecosystem structure are closely related to wind strength, and moderate disturbances in marine ecosystems provide an ecologically beneficial environment for increasing primary production using nutrients supplied to the surface and feeding ecology of juveniles. In the SW, where the disturbance caused by the typhoon was relatively weak, SSC and Chl-a blooming due to upwelling were strongly observed. Accordingly, an environment favorable for small fish to engage in feeding activities was formed, and it was determined that the number of species and total catch increased. However, in the northern part, where vertical mixing is strong due to the passage of typhoons, primary production was relatively lower, and catches were lower due to the spatial dispersion of fishery resources.
Changes in diversity are sensitive to changes in bycatch species and dominant species within the fish community [60,61]. Changes in the distribution of water temperature, prey organisms, and predators caused by typhoons are major causes of changes in the diversity of fishery resources in the short term. In particular, after typhoons, except for Type C, the water temperature in the upper layer became colder, and the temperature in the lower layer warmed, showing a tendency for the warm water layer to deepen. Due to this difference, catches of warm-current fish species (squid, anchovy, etc.) increased in the SW, and cold-current fish species, such as herring, moved to the deep layer, and the catch seemed to decrease.
These results are similar in Tachibana Bay [24]. After passing through the typhoon, the heat was transferred to a deeper layer. Accordingly, the area or depth of different water temperatures was moved according to the habitat characteristics of Engraulis japonicus, Trachurus japonicus, and Sarda orientalis [24,62]. It is suggested that vertical mixing through the water column in the EJS dispersed the thermal energy of the upper layer to the deep layer, expanding the habitat of pelagic fish. Therefore, the change in water temperature after the typhoon caused a change in the depth distribution of warm and cold water species habitats, and it is thought that biomass changes occurred accordingly. In particular, the squid tended to move south faster in Type B, where surface cooling occurred more strongly in the northern part than in Type A, where cooling occurred in all areas after the typhoon. These results were similar to the formation of squid fishing grounds in areas where Chl-a increased to avoid cold water areas formed after typhoons in the East China Sea [22,25].
Furthermore, the fishing ground of the red snow crab was formed in the SW, where pelagic fish were concentrated as Chl-a increased after the typhoon. This study could not clearly explain the connection between the change in the environment of the upper layer and the change in the distribution of red snow crabs after the typhoon; however, the increase in biological productivity in the upper layer owing to the influence of typhoons can also affect the deep-sea ecosystem. Organic matter is one of the food sources supplied to deep-sea ecosystems, as it preys on primary production at the surface and sinks into the bottom through the food web during the phytoplankton bloom [63,64] and can affect the distribution of demersal fish [64,65]. The sedimentation rate of organic particles sinking from the upper layer of the ocean to the bottom layer is approximately 70–200 m/day, and the sedimentation rate may be faster due to disturbances caused by typhoons [66,67,68]. Additionally, the disturbance of the upper ocean due to the typhoon affects the mortality rate of small fish such as juveniles [2,69,70,71] and changes in primary production and organic matter formed by the decomposition of the corpse, which can sink to the bottom layer and affect the short-term change in the distribution of demersal fish. The change in water temperature due to typhoons is transmitted to the upper and lower layers. Further research is needed to understand the link between deep-sea ecosystems and pelagic ecosystems, such as the response of deep-sea fishery resources after typhoons.
Recently, as SST has risen due to climate change and the North Pacific high pressure has expanded and strengthened to the northwest, the frequency of typhoons moving from south to north in the EJS is expected to increase [44,72,73]. Moreover, short-term atmospheric external forces such as typhoons are a major factor affecting the marine ecosystem environment through air–sea interactions, and predicting the distribution of fishery resources and the change in species diversity using the impact of typhoons through on-site observation and a physical–biological coupled model for fishery environment prediction and sustainable fishery is necessary.
5. Conclusions
Typhoons are accompanied by strong winds that change the vertical structure of the water column in the EJS through upwelling and mixing. Changes in the physical environment owing to the passage of typhoons activate the circulation of materials between the upper and lower layers. As a result, changes in Chl-a, species diversity, and biomass of fishery resources occur; however, the response characteristics of each area in the EJS differ depending on the typhoon pathway. In the EJS, when a typhoon moves from southwest to northeast, or it moves northward along the western coast, changes in the marine environment and fishery resources were relatively great compared to the other pathways due to the increase in residence time and right-side influence area of the typhoon. In the future, ocean warming is expected to intensify the stratification, and climate change will affect the intensity and path of typhoons and change their timing and frequency. For the sustainable use of fishery resources and effective fishing activities, we need to understand how typhoons change the shape of fisheries, and this study can provide important information for understanding this process.
Author Contributions
Conceptualization, Y.W.J. and C.I.L.; methodology, Y.W.J. and C.I.L.; validation, Y.W.J. and C.I.L.; formal analysis, Y.W.J.; investigation, Y.W.J.; resources, Y.W.J. and C.I.L.; data curation, Y.W.J.; writing—original draft preparation, Y.W.J. and C.I.L.; writing—review and editing, B.S.K. and H.K.J.; visualization, Y.W.J.; supervision, C.I.L.; project administration, C.I.L.; funding acquisition, C.I.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korea Institute of Marine Science & Technology Promotion (KIMST), funded by the Ministry of Oceans and Fisheries (20220214, 20220558).
Institutional Review Board Statement
This paper did not conduct experiments directly on animals, but used statistical data on catches provided by government agencies. Therefore, we think animal ethics information is not required for this paper.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was supported by the Ministry of Oceans and Fisheries, Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
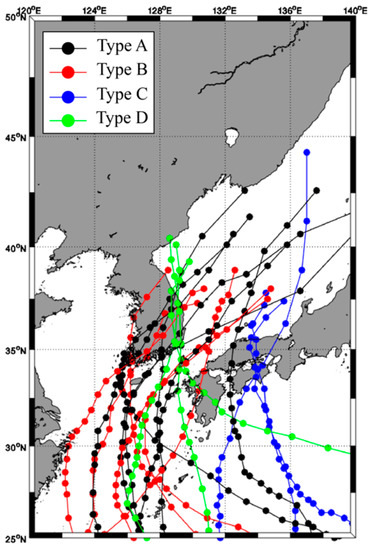
Figure A1.
Best track of the typhoon pathways affecting the East/Japan Sea.
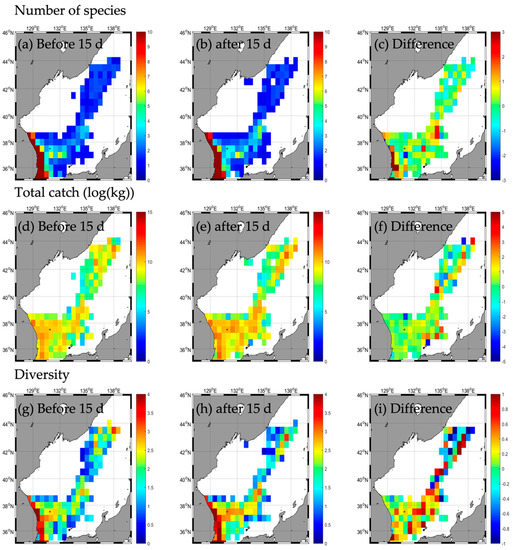
Figure A2.
Horizontal distribution of long-term mean (2016–2020) fishing conditions (number of species (a–c), total catch (d–f), and diversity (g–i) during the non–typhoon period similar to Typhoon KONGREY.
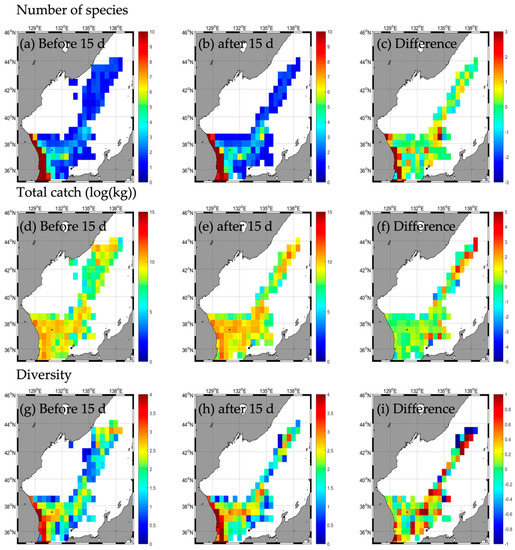
Figure A3.
Horizontal distribution of long-term mean (2016–2020) fishing conditions (number of species (a–c), total catch (d–f), and diversity (g–i) during the non–typhoon period similar to Typhoon DANAS.
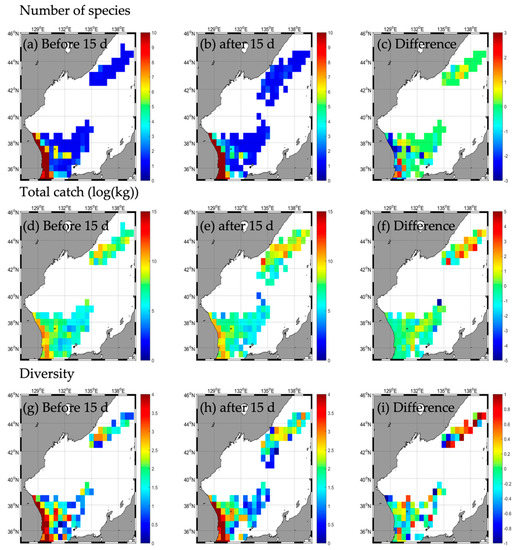
Figure A4.
Horizontal distribution of long-term mean (2016–2020) fishing conditions (number of species (a–c), total catch (d–f), and diversity (g–i) during the non–typhoon period similar to Typhoon HALONG.
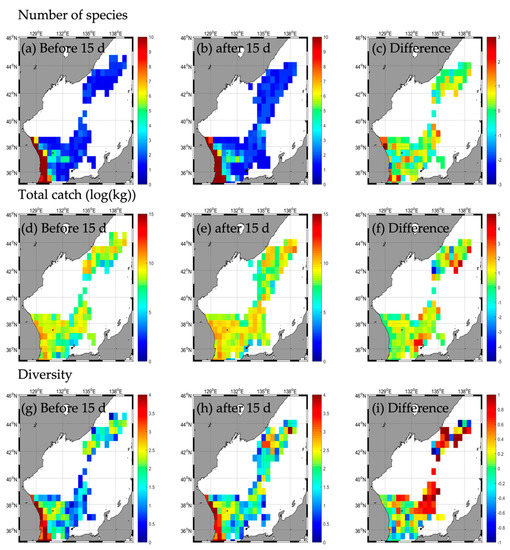
Figure A5.
Horizontal distribution of long-term mean (2016–2020) fishing conditions (number of species (a–c), total catch (d–f), and diversity (g–i) during the non–typhoon period similar to Typhoon HAISHEN.
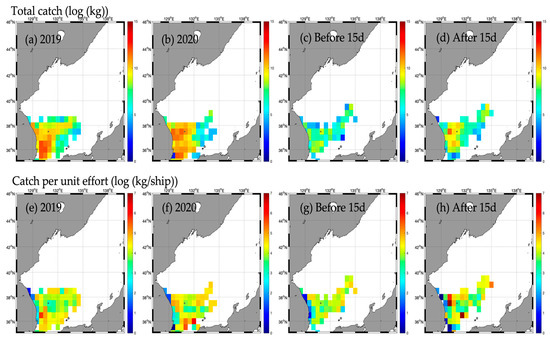
Figure A6.
Horizontal distribution of total catch (upper) and catch per unit effort (CPUE, lower) for major pelagic species (common squid) for 2019 (a,e), 2020 (b,f), and before (c,g) and after (d,h) the Typhoon HAISHEN.
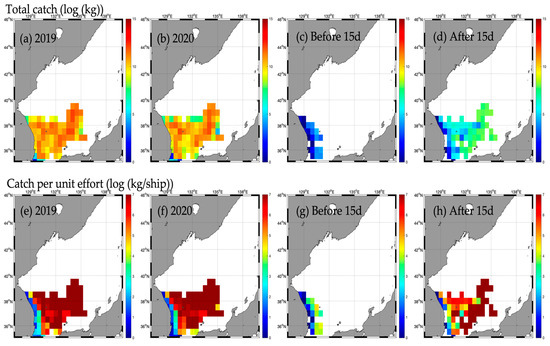
Figure A7.
Horizontal distribution of total catch (upper) and catch per unit effort (CPUE, lower) for major pelagic species (red snow crab) in 2019 (a,e), 2020 (b,f), and before (c,g) and after (d,h) the Typhoon HAISHEN.

Table A1.
Information on the target typhoon affecting the East/Japan Sea.
Table A1.
Information on the target typhoon affecting the East/Japan Sea.
| Type | Number | Name | Grade | Size | Occurrence | Enter | Dissipate |
|---|---|---|---|---|---|---|---|
| A | 1825 | KONGREY | 5 | M | September 2018 | 6 October 2018 | 7 October 2018 |
| B | 1324 | DANAS | 4 | M | 4 October 2013 | 9 October 2013 | 9 October 2013 |
| C | 1411 | HALONG | 5 | M | 29 July 2014 | 10 August 2014 | 15 August 2014 |
| D | 2010 | HAISHEN | 4 | L | 1 September 2020 | 7 September 2020 | 7 September 2020 |
M: medium, and L: large.

Table A2.
Changes in the landing (kg) of fish by sea area before, after, and difference between 15 d before and after the typhoon.
Table A2.
Changes in the landing (kg) of fish by sea area before, after, and difference between 15 d before and after the typhoon.
| Type A | Type B | Type C | Type D | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SW | SE | NW | NE | Total | SW | SE | NW | NE | Total | SW | SE | NW | NE | Total | SW | SE | NW | NE | Total | |||||||||||||||||||||
| b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | b | a | |
| Aptocyclus ventricosus | 38 | 194 | - | - | - | - | - | - | 38 | 194 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 17 | - | - | - | - | - | - | - | 17 |
| Arctoscopus japonicus | 452 | 3059 | - | - | - | - | - | - | 452 | 3059 | 383 | 467 | - | - | - | - | - | - | 383 | 467 | 213 | 80 | - | - | - | - | - | - | 213 | 80 | 3212 | 1440 | - | - | - | - | - | - | 3212 | 1440 |
| Batoidea | 33 | 20 | 200 | 125 | - | - | - | - | 233 | 145 | 50 | - | 100 | 300 | - | - | - | - | 150 | 300 | 30 | - | 100 | 100 | - | - | - | - | 130 | 100 | 59 | 55 | - | 238 | - | - | - | - | 59 | 293 |
| Chelidonichthys spinosus | 19 | 33 | - | - | - | - | - | - | 19 | 33 | - | 15 | - | - | - | - | - | - | - | 15 | - | - | - | - | - | - | - | - | - | - | 94 | 126 | - | - | - | - | - | - | 94 | 126 |
| Chionoecetes japonicus | 30,002 | 50,289 | 20,809 | 30,143 | - | - | - | - | 50,812 | 80,432 | 107,088 | 116,400 | 4000 | - | - | - | - | - | 111,088 | 116,400 | 545 | 741 | - | - | - | - | - | - | 545 | 741 | 1248 | 20,924 | - | 26,383 | - | - | - | - | 1248 | 47,306 |
| Clupea pallasii | 5956 | 2752 | - | - | - | - | - | - | 5956 | 2752 | 16 | 600 | - | - | - | - | - | - | 16 | 600 | 158 | 101 | - | - | - | - | - | - | 158 | 101 | 4154 | 5663 | - | - | - | - | - | - | 4154 | 5663 |
| Cololabis saira | - | 30 | - | - | - | - | - | - | - | 30 | 12 | - | - | - | - | - | - | - | 12 | - | - | 5 | - | - | - | - | - | - | - | 5 | - | - | - | - | - | - | - | - | - | - |
| Conger myriaster | 1217 | 1752 | - | - | - | - | - | - | 1217 | 1752 | 952 | 1502 | - | - | - | - | - | - | 952 | 1502 | 1579 | 1567 | - | - | - | - | - | - | 1579 | 1567 | 887 | 1642 | - | - | - | - | - | - | 887 | 1642 |
| Cottidae | 23 | 12 | - | - | - | - | - | - | 23 | 12 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 149 | 109 | - | - | - | - | - | - | 149 | 109 |
| Doederleinia berycoides | 590 | 514 | - | - | - | - | - | - | 590 | 514 | 200 | 175 | - | - | - | - | - | - | 200 | 175 | 548 | 183 | - | - | - | - | - | - | 548 | 183 | 467 | 571 | - | - | - | - | - | - | 467 | 571 |
| Engraulis japonicus | 6430 | 8404 | - | - | - | - | - | - | 6430 | 8404 | 8883 | 6740 | - | - | - | - | - | - | 8883 | 6740 | 2621 | 6233 | - | - | - | - | - | - | 2621 | 6233 | 8640 | 14,400 | - | - | - | - | - | - | 8640 | 14,400 |
| Gadus macrocephalus | 4580 | 3284 | - | - | - | - | - | - | 4580 | 3284 | 694 | 443 | - | - | - | - | - | - | 694 | 443 | 235 | 177 | - | - | - | - | - | - | 235 | 177 | 1114 | 2562 | - | - | - | - | - | - | 1114 | 2562 |
| Gymnothorax kidako | 295 | 324 | - | - | - | - | - | - | 295 | 324 | 154 | 110 | - | - | - | - | - | - | 154 | 110 | 26 | 126 | - | - | - | - | - | - | 26 | 126 | 87 | 225 | - | - | - | - | - | - | 87 | 225 |
| Hemitripterus villosus | 51 | 101 | - | - | - | - | - | - | 51 | 101 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 59 | 85 | - | - | - | - | - | - | 59 | 85 |
| Hexagrammos agrammus | 12 | 25 | - | - | - | - | - | - | 12 | 25 | 16 | 14 | - | - | - | - | - | - | 16 | 14 | 11 | 6 | - | - | - | - | - | - | 11 | 6 | 91 | 301 | - | - | - | - | - | - | 91 | 301 |
| Hexagrammos otakii | 34 | 7 | - | - | - | - | - | - | 34 | 7 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Konosirus punctatus | 198 | 343 | - | - | - | - | - | - | 198 | 343 | 2237 | 3632 | - | - | - | - | - | - | 2237 | 3632 | 3780 | 1758 | - | - | - | - | - | - | 3780 | 1758 | - | - | - | - | - | - | - | - | - | - |
| Labridae | 10 | 3 | - | - | - | - | - | - | 10 | 3 | 9 | - | - | - | - | - | - | - | 9 | - | - | - | - | - | - | - | - | - | - | - | 30 | - | - | - | - | - | - | - | 30 | - |
| Lateolabrax japonicus | 27 | 53 | - | - | - | - | - | - | 27 | 53 | - | - | - | - | - | - | - | - | - | - | 23 | 23 | - | - | - | - | - | - | 23 | 23 | - | - | - | - | - | - | - | - | - | - |
| Liparis tessellatus | 52 | 78 | - | - | - | - | - | - | 52 | 78 | 187 | 58 | - | - | - | - | - | - | 187 | 58 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Lophiomus setigerus | 4419 | 4118 | - | - | - | - | - | - | 4419 | 4118 | 804 | 446 | - | - | - | - | - | - | 804 | 446 | 2364 | 925 | - | - | - | - | - | - | 2364 | 925 | 2082 | 3400 | - | - | - | - | - | - | 2082 | 3400 |
| Miichthys miiuy | - | 7 | - | - | - | - | - | - | - | 7 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Mugil cephalus | 52 | 73 | - | - | - | - | - | - | 52 | 73 | - | - | - | - | - | - | - | - | - | - | 29 | - | - | - | - | - | - | - | 29 | - | 16 | 28 | - | - | - | - | - | - | 16 | 28 |
| Okamejei kenojei | - | 6 | - | - | - | - | - | - | - | 6 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 18 | - | - | - | - | - | - | - | 18 |
| Oncorhynchus keta | 113 | 209 | - | - | - | - | - | - | 113 | 209 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Pampus argenteus | 5 | 71 | - | - | - | - | - | - | 5 | 71 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Paralichthys olivaceus | 310 | 353 | - | - | - | - | - | - | 310 | 353 | 54 | 92 | - | - | - | - | - | - | 54 | 92 | 94 | 100 | - | - | - | - | - | - | 94 | 100 | 30 | 71 | - | - | - | - | - | - | 30 | 71 |
| Pennahia argentata | - | 19 | - | - | - | - | - | - | - | 19 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Pleurogrammus azonus | 52 | 150 | - | - | - | - | - | - | 52 | 150 | 81 | 24 | - | - | - | - | - | - | 81 | 24 | 50 | 3 | - | - | - | - | - | - | 50 | 3 | - | 20 | - | - | - | - | - | - | - | 20 |
| Pleuronectiformes | 10,184 | 21,674 | - | 75 | - | - | - | - | 10,184 | 21,749 | 8012 | 7852 | 75 | - | - | - | - | - | 8087 | 7852 | 5855 | 6953 | 50 | 83 | - | - | - | - | 5905 | 7036 | 15,743 | 29,055 | - | 50 | - | - | - | - | 15,743 | 29,105 |
| Pleuronichthys cornutus | 41 | 49 | - | - | - | - | - | - | 41 | 49 | 51 | 124 | - | - | - | - | - | - | 51 | 124 | 96 | 53 | - | - | - | - | - | - | 96 | 53 | - | - | - | - | - | - | - | - | - | - |
| Sardinops sagax | - | 8520 | - | - | - | - | - | - | - | 8520 | - | - | - | - | - | - | - | - | - | - | - | 30 | - | - | - | - | - | - | - | 30 | - | - | - | - | - | - | - | - | - | - |
| Saurida undosquamis | - | - | - | - | - | - | - | - | - | - | - | 150 | - | - | - | - | - | - | - | 150 | 20 | - | - | - | - | - | - | - | 20 | - | 130 | - | - | - | - | - | - | - | 130 | - |
| Scomber japonicus | 360 | 101 | - | - | - | - | - | - | 360 | 101 | 125 | - | - | - | - | - | - | - | 125 | - | - | - | - | - | - | - | - | - | - | - | 121 | 179 | - | - | - | - | - | - | 121 | 179 |
| Scomberomorus niphonius | 616 | 1307 | - | - | - | - | - | - | 616 | 1307 | 281 | 115 | - | - | - | - | - | - | 281 | 115 | 58 | 62 | - | - | - | - | - | - | 58 | 62 | 301 | 656 | - | - | - | - | - | - | 301 | 656 |
| Scombrops boops | 1 | 10 | - | - | - | - | - | - | 1 | 10 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4 | - | - | - | - | - | - | - | 4 |
| Scorpaenidae | 79 | 71 | - | - | - | - | - | - | 79 | 71 | 70 | 50 | - | - | - | - | - | - | 70 | 50 | 20 | 44 | - | - | - | - | - | - | 20 | 44 | 37 | 119 | - | - | - | - | - | - | 37 | 119 |
| Sebastiscus marmoratus | 19 | 21 | - | - | - | - | - | - | 19 | 21 | - | - | - | - | - | - | - | - | - | - | 40 | - | - | - | - | - | - | - | 40 | - | - | - | - | - | - | - | - | - | - | - |
| Seriola dumerili | - | - | - | - | - | - | - | - | - | - | 50 | 50 | - | - | - | - | - | - | 50 | 50 | 37 | 75 | - | - | - | - | - | - | 37 | 75 | - | - | - | - | - | - | - | - | - | - |
| Seriola lalandi | 35 | 31 | - | - | - | - | - | - | 35 | 31 | - | - | - | - | - | - | - | - | - | - | 28 | 30 | - | - | - | - | - | - | 28 | 30 | - | - | - | - | - | - | - | - | - | - |
| Seriola quinqueradiata | 4399 | 1474 | - | - | - | - | - | - | 4399 | 1474 | 228 | 30 | - | - | - | - | - | - | 228 | 30 | 43 | 50 | - | - | - | - | - | - | 43 | 50 | 208 | 309 | - | - | - | - | - | - | 208 | 309 |
| Sillago sihama | - | 8 | - | - | - | - | - | - | - | 8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Sparidae | 113 | 161 | 10 | - | - | - | - | - | 123 | 161 | 31 | 93 | - | - | - | - | - | - | 31 | 93 | 49 | 21 | - | - | - | - | - | - | 49 | 21 | 7 | 58 | - | - | - | - | - | - | 7 | 58 |
| Stephanolepis cirrhifer | 66 | 227 | - | - | - | - | - | - | 66 | 227 | 112 | 29 | - | - | - | - | - | - | 112 | 29 | 8 | 22 | - | - | - | - | - | - | 8 | 22 | 43 | 416 | - | - | - | - | - | - | 43 | 416 |
| Stichaeus grigorjewi | 6 | 9 | - | - | - | - | - | - | 6 | 9 | - | 70 | - | - | - | - | - | - | - | 70 | - | - | - | - | - | - | - | - | - | - | - | 3 | - | - | - | - | - | - | - | 3 |
| Tanakas snailfish | 231 | 185 | - | - | - | - | - | - | 231 | 185 | 82 | 336 | - | - | - | - | - | - | 82 | 336 | 47 | 30 | - | - | - | - | - | - | 47 | 30 | 110 | 438 | - | - | - | - | - | - | 110 | 438 |
| Tetraodontidae | 184 | 414 | - | 3775 | - | - | 453 | 455 | 637 | 4644 | 105 | 1204 | - | 1375 | - | - | 491 | - | 596 | 2579 | 15 | - | - | - | - | - | - | 115 | 15 | 115 | 271 | 90 | - | - | - | - | - | - | 271 | 90 |
| Gadus chalcogramma | 1 | 5 | - | - | - | - | - | - | 1 | 5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Thunnini | - | 60 | - | - | - | - | - | - | - | 60 | - | - | - | - | - | - | - | - | - | - | - | 100 | - | - | - | - | - | - | - | 100 | 1620 | 170 | - | - | - | - | - | - | 1620 | 170 |
| Todarodes pacificus | 12,759 | 82,825 | 3194 | 3237 | - | - | 41,271 | 37,157 | 57,225 | 123,219 | 52,003 | 71,964 | 4099 | - | - | - | 21,496 | - | 77,598 | 71,964 | 14,268 | 5870 | 671 | 737 | - | - | 17,316 | 66,382 | 32,255 | 72,989 | 4987 | 30,694 | 1457 | 2489 | - | - | - | - | 6444 | 33,183 |
| Trachurus japonicus | 124 | 428 | - | - | - | - | - | - | 124 | 428 | 375 | 3500 | - | - | - | - | - | - | 375 | 3500 | - | 10 | - | - | - | - | - | - | - | 10 | 128 | 460 | - | - | - | - | - | - | 128 | 460 |
| Tribolodon hakonensis | 5 | - | - | - | - | - | - | - | 5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 20 | 20 | - | - | - | - | - | - | 20 | 20 |
| Trichiurus lepturus | 49 | 282 | - | - | - | - | - | - | 49 | 282 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Zeus faber | - | 55 | - | - | - | - | - | - | - | 55 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 3 | - | - | - | - | - | - | - | 3 | - |
| Others | 1022 | 1227 | - | - | - | - | - | - | 1022 | 1227 | 1788 | 1630 | - | - | - | - | - | - | 1788 | 1630 | 104 | 367 | - | - | - | - | - | - | 104 | 367 | 2296 | 3112 | - | - | - | - | - | - | 2296 | 3112 |
| Total | 85,264 | 195,427 | 24,213 | 37,355 | - | - | 41,724 | 37,612 | 151,203 | 270,394 | 185,133 | 217,915 | 8274 | 1675 | - | - | 21,987 | - | 215,394 | 219,590 | 32,994 | 25,745 | 821 | 920 | - | - | 17,316 | 66,497 | 51,131 | 93,162 | 48,444 | 117,440 | 1457 | 29,160 | - | - | - | - | 49,901 | 146,599 |
b: 15 d before the typhoon and a: 15 d after the typhoon.

Table A3.
Fishery catch data classified fisher and fishing gear types from National Federation of Fisheries Cooperatives (NFFC).
Table A3.
Fishery catch data classified fisher and fishing gear types from National Federation of Fisheries Cooperatives (NFFC).
| Fish Species | Habitat | Fishing Gear |
|---|---|---|
| Flatfish | Demersal | Gill net |
| Red snow crab | Demersal | Gill net, trap fishing |
| Cod | Demersal | Gill net |
| Monkfish | Demersal | Gill net |
| Pollock | Demersal | Gill net |
| Squid | Pelagic | Gill net |
| Herring | Pelagic | Gill net, jigging |
| Mackerel | Pelagic | Gill net |
| Pacific saury | Pelagic | Gill net |
| Japanese Spanish markerel | Pelagic | Gill net |
References
- Ferland, J.; Gosselin, M.; Starr, M. Environmental control of summer primary production in the Hudson Bay system: The role of stratification. J. Mar. Syst. 2011, 88, 385–400. [Google Scholar] [CrossRef]
- Hsieh, H.Y.; Lo, W.T.; Chen, H.H.; Meng, P.J. Larval fish assemblages and hydrographic characteristics in the coastal waters of southwestern Taiwan during non-and post-typhoon summers. Zool. Stud. 2016, 55, e18. [Google Scholar] [PubMed]
- Menkes, C.E.; Lengaigne, M.; Lévy, M.; Éthé, C.; Bopp, L.; Aumont, O.; Jullien, S. Global impact of tropical cyclones on primary production. Glob. Biogeochem. Cycles 2016, 30, 767–786. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Kuwahara, V.S.; Yoshiki, T.; Nakajima, R.; Miyaguchi, H.; Kumekawa, N.; Toda, T. Phytoplankton community response and succession in relation to typhoon passages in the coastal waters of Japan. J. Plankton Res. 2014, 36, 424–438. [Google Scholar] [CrossRef]
- Zhang, H.; He, H.; Zhang, W.Z.; Tian, D. Upper ocean response to tropical cyclones: A review. Geosci. Lett. 2021, 8, 1. [Google Scholar] [CrossRef]
- Zhao, H.; Tang, D.; Wang, Y. Comparison of phytoplankton blooms triggered by two typhoons with different intensities and translation speeds in the South China Sea. Mar. Ecol. Prog. Ser. 2008, 365, 57–65. [Google Scholar] [CrossRef]
- Hong, C.H.; Masuda, A. Temperature Variations in the Mixed Layer with the Passage of Typhoons Using One-Dimensional Numerical Model. Korean J. Fish. Aquat. Sci. 2018, 51, 107–112. [Google Scholar]
- Jeong, Y.Y.; Moon, I.J.; Kim, S.H. A study on upper ocean response to typhoon Ewiniar (0603) and its impact. Atmosphere 2013, 23, 205–220. [Google Scholar] [CrossRef]
- Moon, I.J.; Kwon, S.J. Impact of upper-ocean thermal structure on the intensity of Korean peninsula landfall typhoons. Prog. Oceanogr. 2012, 105, 61–66. [Google Scholar] [CrossRef]
- Price, J.F. Metrics of hurricane-ocean interaction: Vertically-integrated or vertically-averaged ocean temperature? Ocean. Sci. 2009, 5, 351–368. [Google Scholar] [CrossRef]
- Price, J.F. Upper ocean response to a hurricane. J. Phys. Oceanogr. 1981, 11, 153–175. [Google Scholar] [CrossRef]
- Hong, C.H.; Yoon, J.H. A three dimensional numerical simulation of Typhoon Holly in the northwestern Pacific Ocean. J. Geophys. Res. Ocean. 2003, 108, 3282. [Google Scholar] [CrossRef]
- Lee, J.H.; Moon, J.H.; Kim, T. Typhoon-triggered phytoplankton bloom and associated upper-ocean conditions in the northwestern Pacific: Evidence from satellite remote sensing, Argo profile, and an ocean circulation model. J. Mar. Sci. Eng. 2020, 8, 788. [Google Scholar] [CrossRef]
- Lin, I.I.; Wu, C.C.; Pun, I.F.; Ko, D.S. Upper-ocean thermal structure and the western North Pacific category 5 typhoons. Part I: Ocean features and the category 5 typhoons’ intensification. Mon. Weather. Rev. 2008, 136, 3288–3306. [Google Scholar]
- Nam, S.H.; Yun, J.Y.; Kim, K. Observations on the coastal ocean response to typhoon MAEMI at the East Sea Real-time Ocean Buoy. Sea J. Korean Soc. Oceanogr. 2004, 9, 111–118. [Google Scholar]
- Shay, L.K.; Black, P.G.; Mariano, A.J.; Hawkins, J.D.; Elsberry, R.L. Upper ocean response to Hurricane Gilbert. J. Geophys. Res. Ocean. 1992, 97, 20227–20248. [Google Scholar] [CrossRef]
- Lee, C.I.; Park, M.O. Time series changes in sea-surface temperature, chlorophyll a, nutrients, and sea-wind in the East/Japan Sea on the left-and right-hand sides of Typhoon Shanshan’s track. Ocean. Sci. J. 2010, 45, 253–265. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, D.; Tang, S.; Morozov, E.; Liang, W.; Sui, Y. A case study of Chlorophyll a response to tropical cyclone Wind Pump considering Kuroshio invasion and air-sea heat exchange. Sci. Total Environ. 2020, 741, 140290. [Google Scholar] [CrossRef]
- Son, S.; Platt, T.; Bouman, H.; Lee, D.; Sathyendranath, S. Satellite observation of chlorophyll and nutrients increase induced by Typhoon Megi in the Japan/East Sea. Geophys. Res. Lett. 2006, 33, L05607. [Google Scholar] [CrossRef]
- Zhao, H.; Shao, J.; Han, G.; Yang, D.; Lv, J. Influence of typhoon matsa on phytoplankton chlorophyll-a off East China. PLoS ONE 2015, 10, e0137863. [Google Scholar] [CrossRef]
- Zheng, G.M.; Tang, D. Offshore and nearshore chlorophyll increases induced by typhoon winds and subsequent terrestrial rainwater runoff. Mar. Ecol. Prog. Ser. 2007, 333, 61–74. [Google Scholar] [CrossRef]
- Chang, Y.; Chan, J.W.; Huang, Y.C.A.; Lin, W.Q.; Lee, M.A.; Lee, K.T.; Kuo, Y.C. Typhoon-enhanced upwelling and its influence on fishing activities in the southern East China Sea. Int. J. Remote Sens. 2014, 35, 6561–6572. [Google Scholar] [CrossRef]
- Houde, E.D.; Bichy, J.; Jung, S. Effects of hurricane Isabel on fish populations and communities in Chesapeake Bay. Hurrican Isabel Perspect. Proc. A Conf. 2005, 5, 193–199. [Google Scholar]
- Yagi, M. Short-term change in fish assemblages after the passage of a typhoon in a temperate, coastal bay. Acta Ichthyol. Piscat. 2021, 51, 175–183. [Google Scholar] [CrossRef]
- Yu, J.; Tang, D.; Chen, G.; Li, Y.; Huang, Z.; Wang, S. The positive effects of typhoons on the fish CPUE in the South China Sea. Cont. Shelf Res. 2014, 84, 1–12. [Google Scholar] [CrossRef]
- Yu, J.; Tang, D.; Li, Y.; Huang, Z.; Chen, G. Increase in fish abundance during two typhoons in the South China Sea. Adv. Space Res. 2013, 51, 1734–1749. [Google Scholar] [CrossRef]
- Kim, S.W.; Lim, J.W.; Lee, Y.; Yamada, K. Response of water temperature in Korean Waters caused by the passage of Typhoons. J. Korean Soc. Mar. Environ. Saf. 2016, 22, 508–520. [Google Scholar] [CrossRef]
- Park, M.H.; Lee, J.S.; Suh, Y.S.; Kim, H.D.; Bae, H.K. Characteristics of Variation of Sea Surface Temperature in the East Sea with the Passage of Typhoons. J. Environ. Sci. Int. 2015, 24, 1657–1671. [Google Scholar] [CrossRef]
- Suh, Y.S.; Kim, D.S.; Kim, B.K.; Lee, D.I.; Kim, Y.S.; Kim, I.K. Temporal and spatial variation of SST related to the path of typhoons around the Korean waters in summer. J. Environ. Sci. 2002, 11, 627–636. [Google Scholar]
- Park, K.A.; Chung, J.Y.; Kim, K. Sea surface temperature fronts in the East (Japan) Sea and temporal variations. Geophys. Res. Lett. 2004, 31, L07304. [Google Scholar] [CrossRef]
- Park, K.A.; Ullman, D.S.; Kim, K.; Chung, J.Y.; Kim, K.R. Spatial and temporal variability of satellite-observed subpolar front in the East/Japan Sea. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2007, 54, 453–470. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, S.Y.; Choi, B.J.; Byun, D.S. Estimation of mean surface current and current variability in the East Sea using surface drifter data from 1991 to 2017. Sea J. Korean Soc. Oceanogr. 2019, 24, 208–225. [Google Scholar]
- Park, K.; Park, J.E.; Choi, B.J.; Byun, D.S.; Lee, E.I. An oceanic current map of the East Sea for science textbooks based on scientific knowledge acquired from oceanic measurements. Sea J. Korean Soc. Oceanogr. 2013, 18, 234–265. [Google Scholar]
- Hyun, J.H.; Kim, D.; Shin, C.W.; Noh, J.H.; Yang, E.J.; Mok, J.S.; Yoo, S. Enhanced phytoplankton and bacterioplankton production coupled to coastal upwelling and an anticyclonic eddy in the Ulleung Basin, East Sea. Aquat. Microb. Ecol. 2009, 54, 45–54. [Google Scholar] [CrossRef]
- Shin, H.R.; Shin, C.W.; Kim, C.; Byun, C.K.; Hwang, S.C. Movement and structural variation of warm eddy WE92 for three years in the Western East/Japan Sea. Deep Sea Res. II 2005, 52, 1742–1762. [Google Scholar] [CrossRef]
- Joo, H.; Son, S.; Park, J.W.; Kang, J.J.; Jeong, J.Y.; Lee, C.I.; Lee, S.H. Long-term pattern of primary productivity in the East/Japan Sea based on ocean color data derived from MODIS-aqua. Remote Sens. 2015, 8, 25. [Google Scholar] [CrossRef]
- Kim, D.; Yang, E.J.; Kim, K.H.; Shin, C.W.; Park, J.; Yoo, S.; Hyun, J.H. Impact of an anticyclonic eddy on the summer nutrient and chlorophyll a distributions in the Ulleung Basin, East Sea (Japan Sea). ICES J. Mar. Sci. 2012, 69, 23–29. [Google Scholar] [CrossRef]
- Kim, S.W.; Saitoh, S.I.; Ishizaka, J.; Isoda, Y.; Kishino, M. Temporal and spatial variability of phytoplankton pigment concentrations in the Japan Sea derived from CZCS images. J. Oceanogr. 2000, 56, 527–538. [Google Scholar] [CrossRef]
- Lim, J.H.; Son, S.; Park, J.W.; Kwak, J.H.; Kang, C.K.; Son, Y.B.; Kwon, J.N.; Lee, S.H. Enhanced biological activity by an anticyclonic warm eddy during early spring in the East Sea (Japan Sea) detected by the geostationary ocean color satellite. Ocean. Sci. J. 2012, 47, 377–385. [Google Scholar] [CrossRef]
- Shibano, R.; Yamanaka, Y.; Okada, N.; Chuda, T.; Suzuki, S.I.; Niino, H.; Toratani, M. Responses of marine ecosystem to typhoon passages in the western subtropical North Pacific. Geophys. Res. Lett. 2011, 38, L18608. [Google Scholar] [CrossRef]
- Senjyu, T.; Watanabe, T. A sudden temperature decrease along the Sanin coast induced by a typhoon. Umi Sora 1999, 75, 1–8. [Google Scholar]
- Chang, Y.; Liao, H.T.; Lee, M.A.; Chan, J.W.; Shieh, W.J.; Lee, K.T.; Lan, Y.C. Multisatellite observation on upwelling after the passage of Typhoon HaiTang in the southern East China Sea. Geophys. Res. Lett. 2008, 35, L03612. [Google Scholar] [CrossRef]
- Hahm, D.; Rhee, T.S.; Kim, H.C.; Jang, C.J.; Kim, Y.S.; Park, J.H. An observation of primary production enhanced by coastal upwelling in the southwest East/Japan Sea. J. Mar. Syst. 2019, 195, 30–37. [Google Scholar] [CrossRef]
- Mei, W.; Xie, S.P.; Primeau, F.; McWilliams, J.C.; Pasquero, C. Northwestern Pacific typhoon intensity controlled by changes in ocean temperatures. Sci. Adv. 2015, 1, e1500014. [Google Scholar] [CrossRef]
- Seol, D.I. Global warming and trends of typhoon variation. J. Navig. Port Res. 2010, 34, 453–458. [Google Scholar] [CrossRef]
- Donlon, C.J.; Martin, M.; Stark, J.; Roberts-Jones, J.; Fiedler, E.; Wimmer, W. The operational sea surface temperature and sea ice analysis (OSTIA) system. Remote Sens. Environ. 2012, 116, 140–158. [Google Scholar] [CrossRef]
- Pickett, M.H.; Paduan, J.D. Ekman transport and pumping in the California Current based on the US Navy’s high-resolution atmospheric model (COAMPS). J. Geophys. Res. Ocean. 2003, 108, 3327. [Google Scholar] [CrossRef]
- Smith, R.L. Upwelling. In Oceanography and Marine Biology An Annual Review; Taylor & Francis: Abingdon, UK, 1968; Volume 6, pp. 11–46. [Google Scholar]
- Hersbach, H.; Bell, B.; Berrisford, P.; Hirahara, S.; Horányi, A.; Muñoz-Sabater, J.; Thépaut, J.N. The ERA5 global reanalysis. Q. J. R. Meteorol. Soc. 2020, 146, 1999–2049. [Google Scholar] [CrossRef]
- Menemenlis, D.; Campin, J.; Heimbach, P.; Hill, C.; Lee, T.; Nguyen, A.; Schodlock, M.; Zhang, H. ECCO2: High resolution global ocean and sea ice data synthesis. Mercator Ocean. Q. Newsl. 2008, 31, 13–21. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949; pp. 1–117. [Google Scholar]
- Tang, D.; Ye, H.J.; Sui, Y.; Afanasyev, Y.D.; Wang, S. Typhoon Impacts on Subsurface Marine Ecosystems. Adv. Nat. Technol. Hazards Res. 2014, 40, 219–240. [Google Scholar]
- Furey, H.H.; Bower, A.S. Synoptic temperature structure of the East China and southeastern Japan/East Seas. Deep-Sea Res. II Top. Stud. Oceanogr. 2005, 52, 1421–1442. [Google Scholar] [CrossRef]
- Lim, S.; Jang, C.J.; Park, J. Climatology of the mixed layer depth in the East/Japan Sea. J. Mar. Syst. 2012, 96, 1–14. [Google Scholar] [CrossRef]
- Yun, J.Y.; Magaard, L.; Kim, K.; Shin, C.W.; Kim, C.; Byun, S.K. Spatial and temporal variability of the North Korean Cold Water leading to the near-bottom cold water intrusion in Korea Strait. Prog. Oceanogr. 2004, 60, 99–131. [Google Scholar] [CrossRef]
- Hong, C.H.; Sohn, I.S. Sea surface cooling in the East Sea with the passage of typhoons. Korean J. Fish. Aquat. Sci. 2004, 37, 137–147. [Google Scholar]
- Kaufman, L.S. Effects of hurricane Allen on reef fish assemblages near Discovery Bay, Jamaica. Coral Reefs 1983, 2, 43–47. [Google Scholar] [CrossRef]
- Kim, S.; Kang, S. The status and research direction for fishery resources in the East Sea/Sea of Japan. J. Korean Soc. Fish. Res. 1998, 1, 44–58. [Google Scholar]
- Cury, P.; Roy, C. Optimal environmental window and pelagic fish recruitment success in upwelling areas. Can. J. Fish. Aquat. Sci. 1989, 46, 670–680. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology; Harper and Row Publishers: New York, NY, USA, 1989; p. 654. [Google Scholar]
- Peet, R.K. The measurement of species diversity. Annu. Rev. Ecol. Syst. 1974, 5, 285–307. [Google Scholar] [CrossRef]
- Pough, F.H.; Janis, C.M.; Heiser, J.B. Vertebrate Life, 8th ed.; Benjamin Cummings: San Francisco, CA, USA, 2009; p. 752. [Google Scholar]
- Kojima, S.; Ohta, S. Patterns of bottom environments and macrobenthos communities along the depth gradient in the bathyal zone off Sanriku, Northwestern Pacific. J. Oceanogr. 1989, 45, 95–105. [Google Scholar] [CrossRef]
- Smith Jr, K.L.; Kaufmann, R.S.; Baldwin, R.J.; Carlucci, A.F. Pelagic-benthic coupling in the abyssal eastern North Pacific: An 8-year time-series study of food supply and demand. Limnol. Oceanogr. 2001, 46, 543–556. [Google Scholar] [CrossRef]
- Gooday, A.J.; Pfannkuche, O.; Lambshead, P.J.D. An apparent lack of response by metazoan meiofauna to phytodetritus deposition in bathyal north-eastern Atlantic. J. Mar. Biol. Soc. UK 1996, 76, 297–310. [Google Scholar] [CrossRef]
- Berelson, W.M. Particle settling rates increase with depth in the ocean. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2001, 49, 237–251. [Google Scholar] [CrossRef]
- Honda, M.C.; Sasai, Y.; Siswanto, E.; Kuwano-Yoshida, A.; Aiki, H.; Cronin, M.F. Impact of cyclonic eddies and typhoons on biogeochemistry in the oligotrophic ocean based on biogeochemical/physical/meteorological time-series at station KEO. Prog. Earth Planet. Sci. 2018, 5, 42. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, J.; Rho, T.; Lee, T.; Kang, D.J.; Chang, K.I.; Kim, K.R. Biogeochemical properties of sinking particles in the southwestern part of the East Sea (Japan Sea). J. Mar. Syst. 2017, 167, 33–42. [Google Scholar] [CrossRef]
- Lassig, B.R. The effects of a cyclonic storm on coral reef fish assemblages. Environ. Biol. Fishes 1983, 9, 55–63. [Google Scholar] [CrossRef]
- Mackenzie, B.R. Turbulence, larval fish ecology and fisheries recruitment: A review of field studies. Oceanol. Acta 2000, 23, 357–375. [Google Scholar] [CrossRef]
- Odeh, M.; Noreika, J.F.; Haro, A.; Maynard, A.; Castro-Santos, T.; Cada, G.F. Evaluation of the Effects of Turbulence on the Behavior of Migratory Fish; Final Report 2002; Bonneville Power Administration: Portland, OR, USA, 2002; pp. 1–46.
- Chan, J.C.; Liu, K.S. Global warming and western North Pacific typhoon activity from an observational perspective. J. Clim. 2004, 17, 4590–4602. [Google Scholar] [CrossRef]
- Haghroosta, T.; Ismail, W.R. Typhoon activity and some important parameters in the South China Sea. Weather. Clim. Extrem. 2017, 17, 29–35. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).