Abstract
The use of biological objects in monitoring the state of the environment and the changes caused by the impact of environmental pollution on marine and fresh waters is a promising tool due to a lower cost in comparison to traditional monitoring and the ability to receive immediate information about the ecosystem status. In this review, we summarize the biological information about shellfish biomonitors and the results of studies focused on the development and use of the bioindicator species in early warning systems in Russia. Since the mid-1980s, Russian specialists have developed online biomonitoring systems; as in the rest of world, there are two main approaches that are currently applied to study the physiological status of potential biosensor shellfish species and to monitor freshwater and marine systems: valvometry (registration of gaping activity in bivalve mollusks) and photoplethysmography (registration of cardiac activity in mollusks and crustaceans). Valve movement responses to stressors such as abnormal conditions and pollutants include the closure of shell valves for a long period, decrease in the average distance between valves, rapid shell opening, and higher closing frequency. Cardiac activity reactions of shellfish to stress factors include rapid increases in heart rate and stress index, higher variability in heart rate than under normal conditions, and longer periods required for heart rate recovery after stress. The most common bioindicators used to monitor environmental disturbances in marine ecosystems are blue mussels, Iceland scallops, and red king crabs in cold-water habitats and Black Sea mussels in warmer waters as well as freshwater mussels and crayfish in fresh waters.
1. Introduction
The assessment of harmful impacts on the aquatic environment and their effective control and minimization is a key factor in water quality management [,,]. Monitoring efforts started mainly in the 1960s and focused on physical and chemical parameters [,]. Millions of tons of natural and synthetic chemicals have been produced, used, and released into the aquatic environment worldwide without knowledge of their possible environmental impact on recipient ecosystems [,]. Although most of these chemical substances did not have an immediate effect on the structure and functioning of the aquatic systems, the cumulative effects of the total load of pollutants have certainly contributed to environmental shifts [,,].
The realization that anthropogenic chemicals can damage both wildlife and human health has led to the development and implementation of various procedures for testing threat-level concentrations of chemicals, including (a) laboratory toxicity tests that have been modified from conventional medical toxicology and (b) sensitive analytical methods for detecting specific chemicals in water and sediments [,]. Most of these studies have been conducted under controlled laboratory conditions []. These traditional approaches, however, have a number of serious limitations and cannot provide comprehensive real-time data relating to toxic events in aquatic systems []. For example, toxicity testing and environmental chemistry do not often provide insights into whether tested organisms are healthy, able to grow and reproduce, or perform their ecological roles []. The chemical approach cannot account for antagonistic, additive, or synergistic effects that might occur, and chemical data may be lacking for some constituents, particularly reaction products and trace metabolites, i.e., the chemical detection of a compound in water does not always imply that information is obtained about the biologically active form of this compound []. As the goal of aquatic biological monitoring is to provide reliable and proper information on the possible effects of chemicals present in the water due to human activities [], in order to enable the protection of the aquatic ecosystems, and particularly to give scientific guidance for legislation and enforcement, new approaches using bioindicators or biomonitors (i.e., the biological objects used in monitoring the state of the environment and the changes caused by the impact of environmental pollution on marine and freshwater ecosystems []) have been developed in the past decades [,].
The in situ use of aquatic organisms as natural monitors provides additional and factual data concerning the present status of and past trends in the environment []. Instead of direct measurements, the development of biomonitoring methods has resulted in an increased focus on the use of biological effect measures, or biomarkers [,]. Biomarkers are defined as biological responses that signal exposure to anthropogenic chemicals and the adverse effects of these pollutants on health. Biomarkers encompass a huge variety of molecular, cellular, physiological, and behavioral endpoints such as measuring the function of subcellular organelles involved in the detoxification of contaminants, genetic damage, induction of metabolic enzymes, immune function and disease resistance, endocrine disruption, locomotor behavior, as well as circulatory and respiratory physiology []. The most important biochemical biomarkers include metallothioneins, stress proteins, glutathione transferases, lipid peroxidation, haem, and porphyrins [], while the physiological biomarkers are heart rate, cardiac output, renal and hepatic blood flows, respiration rate, organ sizes, basal metabolism, and rate of cell turnover []. Behavioral biomarkers include movement, velocity, path changing, and space utilization. When affected, these functions may alter the life span, feeding habits, and even phylogenetic positions of aquatic organisms []. In most cases, biomarker analyses are less expensive and can be easier to perform than traditional chemical analyses [,]. Furthermore, biomarker responses may persist long after transient exposure to a pollutant that then degrades and is no longer detectable [].
Taking into account an increase in the global pollution load, there is a need for the development of early warning systems [,,,]. The latter (also referred to as online biomonitors, continuous biotests, or biotest automates) use the reactions of selected organisms, especially physiological and behavioral reactions to physical or chemical stress as a sensitive alarm system to detect exposure to contaminants within short exposure times and provide information about possible permit violations and the need for secondary treatment [,,].
In recent decades, many attempts have been made to develop bioassay models that will enable more or less continuous surveillance of water quality with respect to the possible presence of contaminants [,,,]. Most of the model systems consist of flow-through tanks in which aquatic organisms are exposed on a frequent or continuous basis to the water or wastewater being tested [,,,,]. A behavioral or physiological parameter of the organism is monitored by a recording device with the capability of responding to abnormal conditions indicated by the test organism [,].
Continuous biological monitoring systems for river and ocean monitoring have been developed since the 1990s, including novel online sensors ranging from conventional sensors to non-invasive sensor techniques using laser technology and optical sensors [,,]. Russian specialists have also been involved in this process, and there has been significant progress in this field in the past decades. The aim of our study was to provide an overview of Russian studies focused on the use of shellfish as biosensors in the real-time monitoring of aquatic ecosystems. In this paper, the following topics were considered: (a) early warning systems used for biomonitoring, (b) biological aspects of organisms used as biosensors, and (c) important results obtained by Russian specialists.
2. Methods and Solutions
2.1. Valvometry
Gaping activity (opening and closing of the valves) in bivalve mollusks reflects their biological processes and responses to environmental fluctuations []. The bivalve shell defends mollusks against adverse conditions and natural predators [,,,], and therefore its closure efficiently isolates bivalves from external threats or water contaminants []. The gaping activity of many species is closely related to physiological processes such as breathing, nutrition, and waste elimination, which respond to environmental conditions following rhythmic cycles [,]. Bivalves are known to alter their normal gaping behavior in response to the presence of stressors and fluctuations in environmental factors [,,,]. At rest, the valves are open, valve activity is low, and there are transient partial closures, whereas stress conditions lead to detectable valve activity. This feature can be employed for aquatic monitoring and assessments [,].
A system for continuous biomonitoring was developed by the Murmansk Marine Biological Institute in the mid-1980s. The system, based on a classical actograph [], has evolved from a mechanical recorder to analogous and, finally, digital recording complexes [,,,,,].
The latest version of this system includes measuring devices connected to a personal computer (Figure 1).
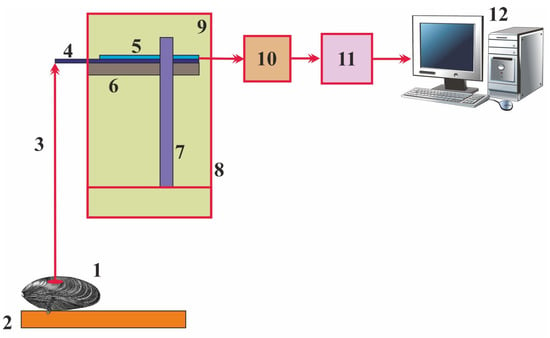
Figure 1.
Scheme of the gaping activity registration system developed by the Murmansk Marine Biological Institute []. 1—mussel, 2—platform, 3—flexible or rigid link, 4—thin flexible resilient plate, 5—tensoresistor, 6—horizontal base, 7—vertical rack, 8—protective case, 9—sensor, 10—amplifier, 11—analog-to-digital converter, 12—personal computer.
This complex has the following components: one or more sensors, an amplifier, an analog-to-digital converter (ADC), and a computer []. Each sensor is a tensoresistor attached to a horizontal base inside a protective enclosure and has the end of a flexible elastic sensor plate protruding from the enclosure so that this plate can be connected to the top valve of the tested mollusk. The output of each sensor is connected to the input of the amplifier. The latter consists of a zero-balancing device, an analog signal amplifier, and a signal shift device. Each sensor is connected to the bridge circuit of the zero-balancing device. The output of the amplifier is connected to the input of the ADC, whose output is connected to the input of an interfacing device. The latter transmits a digital signal to the input of the computer; then, the signal is processed with special software. Closing of the valves induces a signal that is transmitted through a flexible (or rigid) cable to the sensor plate whose bending leads to changes in the electromagnetic current in the tensoresistor. The signal is then transmitted to the amplifier, where it is amplified, filtered, and corrected. The signal is then quantized with a sampling frequency of 10 Hz and digitized in the ADC. A USB interface is used to transmit the signal to the computer for further data processing and visualization []. New sensors for this system are currently being developed and tested. These are traditional or modified serial strain and vibration sensors based on piezoelectric, ferro-electric ceramic, tensoresistive, and magnetoresistive materials. It is assumed that piezoceramic sensors based on a lead zirconate–lead titanate binary solid-solution might be a good alternative for traditional sensors [,].
Observations under laboratory conditions using this system detected the following bivalve behavioral acts in marine mussels [,]: (a) phase of activity = wide-open valves with rare closing; (b) resting phase = closed (usually not completely) valves with rare, short openings, (c) valve closing = single valve opening/closing, each cycle lasts for 5–10 min, (d) valve trembling = high-frequency and low-amplitude valve movements, (e) adduction = frequent valve closing, 2 to 10 closures per hour, (f) fast rhythm = periodical adduction against the background of the phase of activity or resting phase, (g) short rhythm = gradual changes in the mean opening amplitude, periodic alteration in the activity and resting phases, (h) open short rhythm = the predominance of the phase of activity with rare valve closing or trembling, and (k) closed short rhythm = the predominance of the resting phase with a decreased phase of activity (Figure 2a–k).
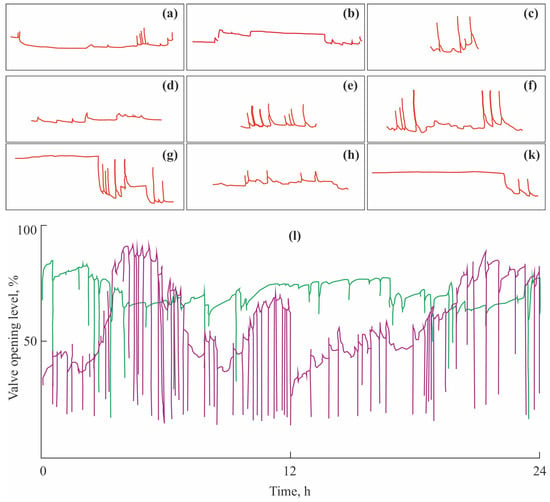
Figure 2.
Actograms showing typical behavioral patterns in blue mussels Mytilus edulis from the Barents Sea: (a) phase of activity, (b) resting phase, (c) valve closing, (d) valve trembling, (e) adduction, (f) fast rhythm, (g) short rhythm, (h) open short rhythm, (k) closed short rhythm, and (l) typical records of valve activity under normal (green) and stress conditions (violet). Modified from [,].
This method results in actograms (graphs representing measured parameters as functions of time; Figure 2l) and three parameters to be used for monitoring the mollusk behavior []: (a) valve-opening level (VOL, %), (b) valve closing frequency (VCF, closures per day), and (c) valve opening amplitude (VOA, %).
The most informative characteristic is the VOL, which is expressed in percent from the maximum according to the following formula []:
where VCL is the valve-closure level detected directly from actograms and max VOA is the maximum VOA registered during the study period.
In 2003, a submersible complex for continuous biomonitoring based on the behavior of the Black Sea mussel Mytilus galloprovincialis was developed by scientists from T.I. Vyazemsky Karadag Scientific Station, Feodosia. It was an analog of Musselmonitor [,]. In the next four years, this complex was tested and calibrated to develop an early warning system [,]. It was then slightly modified and improved in collaboration with specialists from the Marine Hydrophysical Institute, Sevastopol [,,]. This complex consists of a cylindrically shaped water-tight casing that contains rechargeable batteries and electronic components for the actual measurement and an external module for data communication and data evaluation. A bipolar Hall sensor SS496A1 and a magnet are attached to the valves of a mollusk to record the distance (range 1 to 12 mm, SE = 0.015 mm) between the valves []. These reactions are automatically transferred to a receiving device and are processed in specially designed software. The underwater complex is also equipped with a temperature sensor, a hydrostatic pressure sensor, and a light sensor (Figure 3).
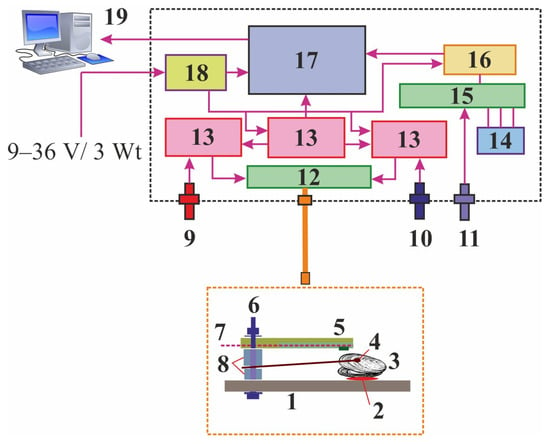
Figure 3.
Scheme of the gaping activity registration system developed in T.I. Vyazemsky Karadag Scientific Station and Marine Hydrophysical Institute [,,,]. 1—frame substrate, 2—water-resistant adhesive sealant, 3—mussel, 4—flexible plastic batten with neodymium magnet, 5—analogous Hall sensor, 6—stainless fixture, 7—three-core cable for power and analog signal transmission, 8—polyamide inserts, 9—light sensor, 10—hydrostatic pressure sensor, 11—temperature sensor, 12—32-channel switch, 13—conversion and power circuit, 14—etalons, 15—4-channel switch, 16—conversion circuit, 17—microcontroller block, communication interface, and 36-channel analog-to-digital converter, 18—power circuit, and 19—personal computer.
A block of measurement channels includes precision amplifiers, voltage regulators, and a 24-bit ADC. This block is switched to all sensors and in-circuit etalons. The external module of the system consists of two similar electronic boards with a real-time clock, non-volatile flash memory, and a digital temperature sensor controlled by a microcontroller. Power for the electronic boards is provided by a rechargeable battery pack or by a 12 V external power source. The electronic components of the underwater module are powered by 12 V/0.5 A via a link cable from the power supply or external batteries. The latter are charged using a 12 V charger from a normal network of 220 V. The maximum depth for this complex is 20 m, measuring frequency is 1 s−1, power consumption is 3 Watts for both the external and underwater modules, height of the underwater module is 90 cm, maximum diameter is 31 cm, and weight is 30 kg [].
A total of 16 mollusks can be monitored for their behavior, and a synchronous reaction of at least 70% of animals indicates an alarm signal. Valve gaping and movements are registered in real time and displayed as graphs showing changes in the valve-opening level (VOL) expressed in mm or %. VOL is 0% for fully closed and 100% for fully opened mollusks. A latent period for this method is as low as a few seconds or a few minutes after the detection of abnormal conditions. The time of autonomous operation is 23 d. This system can be operated on a 24/7 basis for a few years [].
2.2. Photoplethysmography
Photoplethysmography is an inexpensive and simple way of recording cardiac and ventilatory activity in shellfish organisms. The procedure involves the use of photosensitive detectors attached to the hard shell of an experimental animal. Low-intensity light from light sources passes through the dorsal part of the shell into the pericardium. During the heart’s cycle of action, varying amounts of light are reflected by the ventricle. Some of this light falls on the photosensor, which converts the energy into a proportional voltage change to operate an oscillograph []. This approach is widely used to monitor the physiological responses of aquatic animals to various stimuli [,,,,,,,,].
In 1999, an original fiber-optical method for recording the cardiac activity of shellfish was developed in the Laboratory of Experimental Ecology of Aquatic Systems of the St. Petersburg Research Center for Ecological Safety of the Russian Academy of Sciences [,,,]. This system includes a laser fiber-optical photoplethysmograph (LFOP), an optical cable, a sensor, an analog-to-digital converter (ADC), and a personal computer (Figure 4).
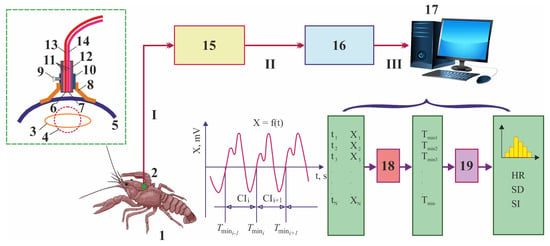
Figure 4.
Scheme of the cardiac activity registration system developed in Saint-Petersburg Scientific Research Center for Ecological Safety [,,]. 1—animal, 2—sensor, 3—heart (diastole), 4—heart (systole), 5—shell, 6—output end, 7—input end, 8—petal of the attached element, 9—screw, 10—cylinder of the attached element, 11—fixation element for fibers, 12—case, 13—transmitting optical fiber, 14—receiving optical fiber, 15—laser optical fiber photoplethysmograph, 16—analog-to-digital converter, 17—personal computer, 18—digital filter, and 19—distribution analysis. I—optic signal, II—analog signal, and III—digital signal. Data processing of cardiac intervals (CI) results in graphs, mean heart rate (HR), standard deviation of HR (SD), and stress index (SI).
In 2003–2004, specialized software was designed to provide real-time estimations of the physiological stress level in benthic invertebrates in the presence of pollutants []. In this system, only animals with hard exoskeletons (shells in the case of mollusks or carapaces in the case of crustaceans), to which a small sensor (of weight < 2 g) is attached via non-toxic water-resistant glue, can be used as biomonitors []. The infra-red light beam initially created in LFOP is transmitted to an animal by a thin optical cable through a small sensor attached to the shell, thus illuminating the heart area with scattered light. The sensor of physiological activity consists of a case with an element serving to attach to the body of the experimental organism, a source of optical radiation, and a receiver of optical radiation []. The optical signal modulated by the heart of the tested animal containing information about its cardiac activity is transmitted back to LFOP. After amplification and filtering procedures in this device, the analog signal is then transmitted to a digital storing oscilloscope, where it is converted to digital form by a 14-bit 16-channel ADC and then sent to a personal computer via a USB port for further processing. A resulting photoplethysmogram is then analyzed by mathematical and statistical methods in an original software program, VarPulse, which automatically reads data from the ADC, determines the duration of each cardiac interval in real time, and then calculates a set of heart rhythm variability characteristics such as mean cardiac interval (M, s), mean heart rate (HR, beats min−1), change in mean HR (dHR, %), standard deviation (SD) of cardiac intervals, and stress index (SI), according to the following formulas []:
where Mi is the duration of the ith cardiac interval, N is the sample size, HRt1 is the heart rate at time t1, and HRt2 is the heart rate at time t2.
Another important index is the recovery time of HR (Trec), i.e., the period between the end of exposure and the moment when HR reaches the normal values registered before the exposure [,]. It is judged that a good health status of an animal takes place when Trec is lower than 50–60 min [].
The obtained data can also be archived. The software allows for the simultaneous registering and analysis of 7–8 individuals. This method is considered non-invasive because the attached sensor does not affect the normal activity and physiology of the tested animals, including both mollusks and crustaceans. In 2005, a system for industrial biological water quality monitoring based on this method was created for real-time monitoring of toxicity level changes at the Neva River water intakes of St. Petersburg drinking Water Supply Stations using crayfish as biosensors [,].
The same approach has been developed and used by specialists from the Karelian Research Centre, Russian Academy of Sciences, Petrozavodsk, where remote monitoring of the cardiac muscle volume (plethysmogram) is based on infrared radiation of the heart region and reflected light recording [,]. Karelian scientists used a specially developed amplifier with a system of filters and a portable digital oscillograph (Fluke 125) that transmitted a signal to a personal computer. This signal is recorded as consecutive heart contraction waves and processed in FlukeView 3.0 software (Fluke Corporation, Washington, DC, USA).
3. Biological Aspects of Test Organisms
Various organisms have been tested as bioindicators; among these, fish and benthic invertebrates are the most common []. Potential bioindicators should meet the following requirements []: (a) high ecological relevance, (b) susceptibility to stressors in the wild and under laboratory conditions, (c) wide geographic distribution, (d) easy to culture and maintain in the laboratory, (e) high reproductive rate, and (f) ability to yield reproducible data under controlled laboratory conditions.
Russian scientists use the following organisms in physiological studies and real-time biomonitoring of both marine and fresh waters: blue mussels (Mytilus edulis) [,,,,,], Black sea mussels (Mytilus galloprovincialis) [,,,,], Iceland scallops (Chlamys islandica) [], freshwater mussels (Anodonta anatina and Anodonta cygnea) [,,], painter’s mussels (Unio pictorum) [,,], narrow-clawed crayfish (Pontastacus leptodactylus) [], and red king crabs (Paralithodes camtschaticus) [,].
3.1. Blue Mussels
Mytilus edulis is a bivalve mollusk with a smooth inequilateral shell, which features concentric growth lines emanating from the hinge. Fibrous brown byssal threads extend from the closed shell and are used for attachment to substrates []. Shell length varies from 2 to 20 cm and total weight from 1.4 to 6.5 g []. Mytilus edulis is found at coastal sites of the northern Atlantic Ocean, including North America, Europe, and the northern Palearctic. This species is found from the White and Barents Seas in Russia to southern France, throughout the British Isles, with abundant populations in Wash, Morecambe Bay, Conway Bay, and southwest England, north Wales, and west Scotland. In the western Atlantic, Mytilus edulis is distributed from the southern Canadian Maritime provinces to North Carolina [].
Mytilus edulis is a eurythermal species that tolerates freezing conditions for several months. It is well acclimated to a temperature range of 5–20 °C, with an upper sustained thermal tolerance limit of about 29 °C for adult individuals []. Blue mussels do not thrive in waters with salinities of less than 15 psu, but can withstand substantial environmental fluctuations []. The species occurs predominantly at 5–50 m depth []. Usually, Mytilus edulis occupies subtidal and intertidal beds on rocky shores.
Spawning in Mytilus edulis occurs from April to September, depending on water temperature, currents, and other environmental factors []. In most populations, resting gonads begin to develop from October to November, with gametogenesis occurring throughout winter so that gonads mature in early spring []. Partial spawning in spring is followed by rapid gametogenesis, with gonads maturing by early summer, resulting in a less intensive secondary spawning in late August or September. An individual female can produce from 5 to 8 million eggs []. Mature males and females (age 1–2 y) release the gametes into seawater for egg fertilization []. After the egg is fertilized, it turns into a ciliated trochophore larva. The trochophore larva then becomes a veliger, which persists for 1–1.5 months. Larval development may last 20 days under optimal conditions, but larval growth and metamorphosis between spring and early summer at 10 °C usually require 1 month [,]. A juvenile mollusk finds a primary settlement location on openings in the substrata, or amongst bryozoans or other filamentous structures away from mature mussels to decrease competition []. After 2–3 weeks there, the juvenile doubles in size and detaches to drift again and find a permanent substrate for attachment. Young specimens attach to the sea floor with a byssus thread or, if such an open substrate is unstable, may attach to another mussel, thus creating a mussel bed []. In the Barents Sea, the lifespan ranges from 12 to 14 years [,]. Mytilus edulis is a sessile species with a diet consisting of microalgae (dinoflagellates, small diatoms, and flagellates), zoospores, protozoans, and detritus filtered from the surrounding water [,]. The main predators of Mytilus edulis are sea urchins, starfish, gastropods, benthivorous fish, and birds [].
3.2. Black Sea Mussels
Mytilus galloprovincialis occurs in the Mediterranean and Black Seas, in Portugal, north to France, the British Isles, and Norway. Recently, this species has also been found in northern Norway and Svalbard []. Mytilus galloprovincialis was introduced into the northern Pacific from Europe by human activity in the early 20th century []. Invasive populations are also present in Japan, North Korea, Russian Far East, southern Africa, New Zealand, Australia, and South America [,]. The shell of Mytilus galloprovincialis tends to be higher and flatter than in Mytilus edulis. In Black Sea mussels, the anterior end of the shell is distinctly beaked or incurved, whereas that of blue mussels has a more snub-nosed appearance. The anterior edge of the shell of Mytilus galloprovincialis merges smoothly into the dorsal edge, giving rise to a rounded convex profile, while that of Mytilus edulis is angular [].
The optimal temperature range for Black Sea mussels is 14–20 °C, while the optimal salinity range is 12–25 psu [,,].
An individual female can produce 2–6 million eggs []. In the Black Sea, spawning of Mytilus galloprovincialis is biannual and depends on water temperature and the structure of phytoplankton communities. In mussels living at coastal sites on hard bottoms, the first spawning event is registered in spring at 8.5 °C, with a peak in March, and lasts for 1.5–3 months []. The second mass spawning occurs in autumn, with a peak in September at 15.5–18 °C, and lasts for 1.5 months [,]. Mussels living in mud habitats at 45–50 depth spawn in May–June and October–November at 7–8 °C []. Spawning is extended in time, and mussel larvae occur in the plankton all year round [,]. Mussels became mature at a shell size of 20 mm. Juveniles settled in spring become mature in autumn of the same year, while mussels from the autumn generation become mature in the spring of the next year []. The maximum growth rate (0.33–0.48 mm d−1) is registered in summer under favorable food conditions, while the minimum growth rate (0.10–0.23 mm d−1) is found in winter and spring []. Mytilus galloprovincialis can reach a shell size of 120 mm []. The maximum age is 26 years for deep-water mussels and 13 years for shallow-water ones []. Size at ages 0.5, 1, 1.5, 2, 2.5, 3, and 3.5 years is 24.4, 35.8, 51.1, 61.5, 66.7, 71.7, and 75.6 mm, respectively [,].
Large specimens can ingest particles of up to 0.6 × 2.7 μm, with an ingestion rate of 40–70 mg d−1 []. The diet of Black Sea mussels comprises 62 species of microalgae, of which 35 are diatoms and 23 are dinoflagellates (size 15–92 μm). The most common species are: Prorocentrum micans, P. compressum, P. balticum, Licmophora ehrenbergii, L. flabellate, and Scrippsiella acuminate [,]. Other less important food items are infusorians, planarians, nematodes, chaetognaths, and larvae of copepods and cladocerans. Detritus is the main food source in winter, while phytoplankton is the main source in spring and summer [].
3.3. Iceland Scallops
The Iceland scallop Chlamys islandica (Müller, 1776) is a sub-arctic species distributed from the Arctic seas to southern Massachusetts, USA. It occurs in Greenland, all around Iceland except the south coast, in Norwegian waters at coastal sites and fjords, and in the deeper waters of Bear Island, Spitsbergen, and Jan Mayen. The eastern distribution extends to the Barents, White, and Kara Seas [,].
In the Barents Sea, this species is widely distributed on hard coarse sediments consisting of sand, gravel, shells, and occasionally clay and fine sand at depths up to 500 m []. It is well acclimated to a temperature range of 0–7.2 °C and prefers a salinity range of 33.2–34.8 psu [].
Iceland scallop is a dioecious species with pinkish hue gonads in adult females and white gonads in adult males. In the Barents Sea, scallops become sexually mature at 3–6 years of age []. Scallop spawning occurs from March/April to June/July. The main driving factors of spawning are phytoplankton concentrations and local oceanography. The mean fecundity is 5.2 million eggs []. Scallop larvae occur in the plankton for about 2 months before settlement, during which time they are passive drifters. The most preferable substrate for larval settlement is dead hydroid perisarc of Tubularia, although larvae also settle on live hydrozoans, red algae, and artificial substrates. Iceland scallop is capable of limited swimming [,].
In the Barents Sea, Chlamys islandica demonstrates its greatest growth efficiency during the first 5 years of life. Usually, the lifespan of Chlamys islandica does not exceed 20–23 years []. In the Barents Sea, Iceland scallops can reach 151 mm in shell height and 500 g in weight [].
Chlamys islandica is a suspension feeder. Its main food items at shallow-water sites are various microalgae species, while in deep waters the scallops generally feed on detritus []. The natural predators of Chlamys islandica include starfish, cod, and red king crab [].
3.4. Freshwater Mussels Anodonta
Like marine mussels, freshwater mussels are mollusks that produce a bivalved shell with two valves by an elastic-like ligament along the dorsal hinge. The life cycle of freshwater mussels is different from that of marine mussels. During breeding, males release sperm into the water, and females must inhale it for fertilization to occur. Embryos develop into larvae called glochidia, which are released by the female. For further development, glochidia must encounter and attach to a host fish []. In Russia, host fishes are stickleback (Gasterosteus aculeatus), perch (Perca fluviatilis), pike (Esox lucius), tench (Tinca tinca), eel (Anguilla anguilla), and brown trout (Salmo trutta) []. Glochidia form cysts around themselves and remain on the host for several weeks. The primary selective advantage of such a strategy is that the glochidia are dispersed through the system, which may be particularly advantageous for upstream dispersal and in lentic systems where passive dispersal is poor because of limited water currents []. When ready, the juvenile mussels will release from the fish, fall to the bottom, burrow into the sediment, and begin their free-living existence []. During this time, they grow fast to protect against predators and the crushing and erosive force of rocks and water. Once mature, they spend most of their lives partially buried, with their posterior end sticking above the surface of the sediment. Freshwater mussels are confined to permanent water bodies, including creeks, rivers, ponds, and lakes []. The most preferable depth is 0.5–2 m [].
Anodonta spp. occur in Europe and Asia below 65° N latitude down to Portugal, Sicily, and Turkey and extending as far east as Siberia (Lake Baikal) [].
There are two species of the genus Anodonta in Russia: the duck mussel Anodonta anatina and the swan mussel Anodonta cygnea []. These species have no pseudocardinal and lateral teeth. The shell is thin, light, and fragile. In Anodonta anatina, the shell is very variable, usually greenish yellow, brown, or black, very solid, and often twice as heavy as in Anodonta cygnea []. Young animals are usually lighter and shinier than adults, the frontal part of the inside lower margin is thickened, the ligament is shorter and broader than in Anodonta cygnea, dorsal and ventral, the margins are less parallel, the ventral margin is often curved, and the dorsal margin can be straighter. Size varies from 80 × 50 × 25 mm to 150 × 60 × 35 mm (length × height × bulge) in Anodonta anatina and from 80 × 42 × 26 mm to 150 × 63 × 46 mm in Anodonta cygnea [,].
Anodonta are more tolerant to low oxygen content than most species and can thrive in small nutrient-rich water bodies. Anodonta are particularly vulnerable to water-level fluctuations. Dry periods usually expose animals, and predators (birds and mammals) can eat the mussels that remain exposed []. Anadonta anatina occurs on muddy or sandy substrates and tolerates slightly faster water currents than Anodonta cygnea []. Glochidia (0.35 × 0.35 mm) become mature in autumn and are released in spring []. The size and age of maturity depend on water temperatures: animals from warmer waters reach maturity after 2 years; in cold waters they need up to 5 years, and their shells remain smaller. In warm and eutrophic waters, Anodonta live only up to 5 years, but in cool and oligotrophic smaller running waters, they live up to 20 years [,]. These species are filter-feeders and can filter 15–45 L d−1 []. The main food items are phyto- and zooplankton and detritus [,].
3.5. Painter’s Mussels
The painter’s mussel Unio pictorum is a firewater mollusk belonging to the family Unionidae. It is distributed in Northern and North-Western Europe. In Scandinavia, this species is found up to 60° N. It occurs in the UK, excluding Ireland, and in central Europe and Russia, including far-eastern water bodies []. This species lives in rivers and old river arms and sometimes in lakes, reservoirs, and canals, mainly in lowlands inside the sandy or silty substrates, usually in deeper zones, up to 6 m deep. Painter’s mussels are capable of moving with as low a speed as 1–1.5 m h−1 [].
The shell of Unio pictorum is variable in color and shape, often greenish yellow or brownish without radial streaks []. In contrast to Anodonta, Unio pictorum has a more elongated and thick shell and possesses teeth on the hinge. In adults, shell length varies from 60 to 80 mm, with a height up to 100 mm and bulge up to 28 mm [].
This species attains a growth rate of 20 mm within the first year, after which annual growth increments fall considerably. In general, the shell grows at least 5 mm per year during the first 10 years of life []. In Russia, the maximum lifespan of Unio pictorum is 16 years []. Sexual maturity is reached after 2–4 years of growth []. Spawning occurs from April to June. Embryo development lasts for 20–40 d, and glochidia are released from May to August. The glochidia parasitize on perch, stickleback, ruffe (Gymnocephalus cernuus), white bream (Blicca bjoerkna), and roach (Rutilus rutilus) [].
Unio pictorum ingests particles of detritus or planktonic animals and microalgae, with a filtration rate of 3.2–4.6 L h−1 [].
3.6. Crayfish
The narrow-clawed crayfish Pontastacus leptodactylus is a widely distributed, commercially important species, with its native range in the Pontocaspian river basins. Its most abundant populations occur in Eastern Europe and the Middle East. Due to human-mediated translocation, it has spread to numerous European countries. Currently, this species is present across much of the continent, except for the Southwestern Balkans, Iberian Peninsula, Ireland, Scandinavia, and Estonia []. In Russian waters, Pontastacus leptodactylus is expanding its range to northwestern regions [].
Narrow-clawed crayfish have a wide habitat preference and are found in shallow and deep lakes, rivers, and streams, and on a wide range of bottom substrates, except those that are heavily silted []. This species can tolerate a wide range of temperature (4–32 °C) and salinity (4–14 psu) fluctuations, as well as temporary declines in oxygen concentration (down to 2 mg L−1) [].
Narrow-clawed crayfish can grow up to 30 cm in length [] and 500 g in wet weight, but usually, their body length is 10–17 mm and weight is 200–300 g, with females being smaller than males [,].
The age at maturity of Pontastacus leptodactylus is 3 years for males and 4 years for females. Pontastacus leptodactylus is a highly fecund species, with the number of eggs ranging from 75 to 325 [] and up to 800 in old females []. Mating occurs in autumn, when water temperature is about 5 °C. At mating, the male deposits the spermatophores on the ventral side of the female. Egg hatching occurs in December or January, when the released eggs are attached to the hairy pleopods under the tail. The incubation period lasts for 6 months until the following May or June. After hatching, the larvae cling together under the female for 10–12 d. Once they have first molted, the larvae start to disperse away from the female, seeking shelter in the immediate environment. Crayfish molt eight and five times in the 1st and 2nd years of life, respectively. In subsequent years, crayfish molt two times per year, while females molt one time per year after their fourth year []. Shell hardening after molting requires 8–10 d.
This species exhibits nocturnal foraging activity and has a diverse diet. The most important food items (90%) are macroalgae and aquatic plants. Animal food includes oligochaetes, mollusks, amphipods, insect larvae, tadpoles, frogs, and fish. Frog and fish carrion are also consumed by Pontastacus leptodactylus [].
3.7. Red King Crabs
The red king crab Paralithodes camtschaticus is a large commercially important crustacean belonging to the family Lithodidae [,,]. Its native distribution area is the North Pacific, including the Primorye, Ayan-Shantar region, South and North Kuril Islands, and West Kamchatka in Russia, and Norton Sound, Bristol Bay, the Aleutian Islands, the western Gulf of Alaska, and Southeast Alaska in the USA [,]. A new population also exists in the Barents Sea (Russian and Norwegian waters), after the successful introduction conducted by Russian scientists in the 1960s [,,,,].
In Russian waters, red king crabs occupy both shallow waters (juvenile crabs) and deeper waters (up to 335 m depth) (large males), at a temperature range from −0.8 to +8.5 °C [,,]. In April/May, they form mating aggregations. During August–September, red king crabs segregate by sex, with males and females forming aggregations at temperatures of 4–65 °C and 5–75 °C, respectively []. Females mature at carapace widths (CW) of 90–140 mm, and most mature at 110–130 mm CW [,,]. Males mature at 65–80 mm CW; however, they successfully couple only at 140–150 mm CW. Males and females couple in spring, with a peak in April, and eggs are fertilized externally []. Most females in Russian waters extrude the eggs at sites less than 90 m deep []. Reproductive events are initiated by adult males moving to shallow areas in December, followed by females in March/April. Shortly after their arrival on the spawning grounds, the females hatch their old eggs and start the process of molting and spawning new eggs. Absolute individual fecundity varies from 70,000 to 700,000 eggs [].
Mass hatching of larvae occurs in March/April at depths of 50–240 m and bottom temperatures of 0.5–1.95 °C []. As larvae develop, they feed on zooplankton in the pelagic layer for two months. During this period they molt four times. From the middle to the end of June, larvae settle at the bottom in the coastal areas []. Once settled on the substrate, the larvae turn into juvenile crabs with a CW of 2 mm [].
Starting from the age of 4, all juveniles molt once or twice a year. Adult females molt annually before mating []. Males with a CW less than 100 mm molt at least annually, and those larger than 110 mm CW molt less often [,]. Males larger than 150 and 190 mm CW molt once every three and four years, respectively []. The size of juvenile crabs at age 0–5 y has been estimated to be 1.2, 9.9, 30.0, 53.3, 71.8, and 83.8 mm carapace length per year, respectively []. The maximum reported size for the Barents Sea red king crab is 270 mm CW [].
The main food items of the Barents Sea red king crabs are bivalve mollusks Astarte sp., gastropods Naticidae g. spp., polychaetes Spiochaetopterus tipicus, and echinoderms Ophiura and Strongylocentrotus [,]. In areas with intensive multispecies fishing, they predominantly feed on fish offal. The main predators of red king crabs are cod, wolffish, and skates [].
4. Mollusks as Biomonitors
4.1. Blue Mussels
The first observations of valve movement activity were conducted in 1986 and 1987 in Dalnezelenetskaya Bay and Yarnyshnaya Bay, i.e., in typical coastal areas of the Barents Sea with no pollution [,]. Blue mussels (size 40–50 mm) were collected by hand in the upper and lower littoral and were placed into 4–5 L containers supplied with running seawater (rate 700–1200 mL min−1). These conditions were very close to those in the field [,]. Valve opening levels (VOLs) of the blue mussels tested demonstrated high sensitivity to environmental factors; significant effects were detected for the following changes: 0.1–0.05 °C for water temperature, 0.2 psu for salinity, 0.1 mg L−1 for suspended matter, and 300 cells L−1 for phytoplankton. There was a seasonal pattern in the mussel behavior: in spring and summer, an increase in temperature led to a smooth but expressed decrease in VOL. This result was associated with decreased salinity, which was the main limiting factor for blue mussels. In autumn, the seston concentration was found to be the driving factor of VOL. The detection limit for suspended matter, i.e., the lowest concentration causing changes in VOL, was 0.5–0.6 mg L−1. In winter, there was a direct positive relationship between water temperature and VOL [,].
Another seasonal study was undertaken in Kola Bay from November 2004 to December 2005. There was a clear trend towards higher levels in all parameters studied during the spring–summer season when compared to the autumn–winter season (Figure 5).
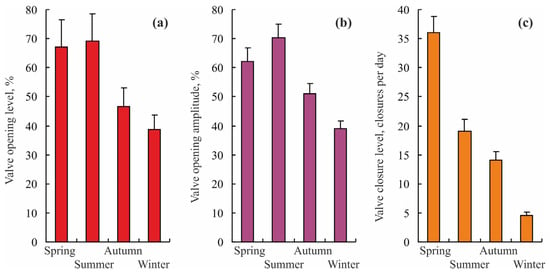
Figure 5.
Seasonal changes in valve activity of blue mussels Mytilus edulis from Kola Bay, Barents Sea (modified from []). (a) valve opening level, (b) valve opening amplitude, and (c) valve closing frequency.
This pattern was associated with a decrease in concentrations of phytoplankton and organic matter, i.e., less favorable conditions for blue mussels. Resting periods in this species followed vertical gradients in the concentrations of biogenic matter appearing first at 1.5 m depth in August and then registering in the surface layer in September [].
Recordings of gaping and cardiac activity were made simultaneously over a 10 d period from December 2006 to January 2007 under laboratory conditions to reveal possible relationships between VOL and HR using both valvometry and photoplethysmography. Daily HR varied from 0 to 20 beats min−1, averaging 12.6 beats min−1 at 10 °C []. This level was higher than that of blue mussels from the White Sea at −0.1 °C (5.3 beats min−1) []. Correlations between VOL and HR were unstable, changing from 0.7 to −0.5 []. In the first days, when the mussels were not acclimated to rearing conditions, this correlation was positive, while after acclimation, when the duration of resting phases increased, this correlation became negative. The most expressed correlation was found between VOL and variability in HR in the periods when VOL demonstrated substantial fluctuations []. The author concluded that the feeding conditions were the most important factors driving the cardiac activity in blue mussels under natural conditions. The same conclusion was made for the White Sea blue mussels monitored for their cardiac activity with a photoplethysmograph [,].
Under a Joint project of Murmansk Marine Biological Institute, French Nationals Center of Marine Research, and TOTAL Exploration and Production, a long-term monitoring study based on high-frequency non-invasive (HFNI) valvometery was undertaken for 1 year in Dalnezelenetskaya Bay []. The HFNI valvometer is a high frequency (10 Hertz), non-invasive biosensor employed to monitor the gaping behavior of bivalves. This device is equipped with a pair of electrodes with 1–1.5 m flexible cables glued onto each half of the mussel shell. The electrodes, designed to minimize disturbance to the bivalve’s behavior, are made up of two resin-coated electromagnets (56 mg each) []. The use of this technique provided new insights into the biological rhythms and shell opening status of the Barents Sea blue mussels. The authors reported that valve-closing activity was dependent on the tide and light regimes, with largely open valves and few valve movements during high tides and more frequent crossing during daytime [].
Cardiac activity in blue mussels from the White Sea was first studied by Bahmet et al. []. The authors reported that in sub-littoral individuals, HR was higher than in littoral ones (range 0–0.32 Hz, mean 0.239 ± 0.026 Hz vs. 0–0.25 Hz, and 0.179 ± 0.029 Hz). A decrease in salinity from 25 to 15 psu and an increase from 25 to 35 psu caused a decrease in HR by 80% [,]. Later studies confirmed these findings: HR levels in sub-littoral and littoral mussels were 18.6 ± 1.1 and 13.1 ± 0.5 beats min−1, respectively []. In contrast, recovery times (Trec) after salinity stress were 31 ± 2 and 38 ± 1.3 min, respectively []. However, in some individuals, this parameter reached 2 h []
Biotesting experiments performed to reveal the effects of 0.001% drilling fluid (0.01 g L−1) on VOL of blue mussels indicated that the duration of the resting phase demonstrated a 400% increase (from 2 to 8 h per day) when the mollusks were exposed to these pollutants for 14 days [,]. An extract of drilling mud at a 10% concentration caused a higher VOL (99%) and higher VOA []. These results were the first to demonstrate that the Barents Sea blue mussels have promising potential as biosensors to pollutants []. Similar findings were reported by Hamilton et al. [], who studied the effects of whole drilling mud (concentrations 50–600 mg L−1) on the shell movements of the bay scallop, Argopecten irradians, and found more intensive gaping activity after the exposure.
According to analyses of plethysmograms, Bakhmet et al. [,] reported that exposure to diesel fuel at concentrations of 8.0 and 38.0 mg L−1 caused a 30% increase in HR of blue mussels from Chupa Bay, Gulf of Kandalaksha, White Sea. On the second day, HR decreased, but rose over the control values again on the fourth day. After that, the cardiac activity remained higher than that in the control until the end of the experiment on the sixth day. A decreasing pattern in HR under the effect of lower concentrations of this pollutant (0.4 and 1.9 mg L−1) was observed on the fourth day (Figure 6a).
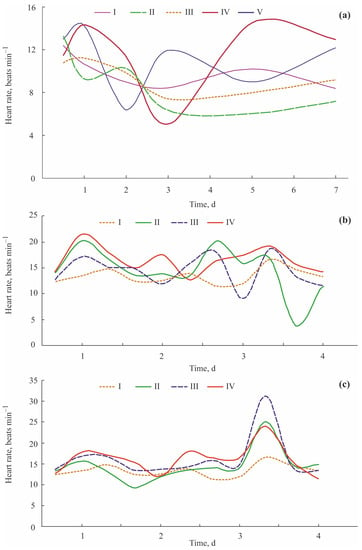
Figure 6.
Heart rate variations in blue mussels Mytilus edulis from the White Sea (modified from [,]): (a) exposure to diesel fuel (I—control, 0 μg L−1; II—0.35 mg L−1; III—1.88 mg L−1; IV—8.41 mg L−1, V—38.8 mg L−1), (b) exposure to Cu (I—control, 0 μg L−1; II—5 μg L−1; III—50 μg L−1; IV—250 μg L−1), (c) exposure to Cd (I—control, 0 μg L−1; II—10 μg L−1; III—100 μg L−1; IV—500 μg L−1).
Similar results were found for the effects of crude oil on the cardiac acridity of blue mussels at different salinity levels []. Further studies tested the effects of heavy metals on the cardiac activity of blue mussels [,,]. Exposure to Cu led to an increase in HR by 65, 23, and 46% at 5, 50, and 250 μg L−1, respectively (Figure 6b). In the case of Cd, HR increased by 11, 17, and 31% at 10, 100, and 500 μg L−1, respectively. Trec was 16 h for both metals, with some post-stress effects for 3 d after the end of the experiments (Figure 6c). Contrasting results were found by Curtis et al. [], who registered copper-induced bradycardia in blue mussels after 2 d of exposure to 12.5 μM of CuCl2. When exposed to Ni at a concentration of 500 μg L−1, the White Sea blue mussels decreased their HR by 32%, from 22.2 to 15 beats min−1 over 20 min. Individual variability in HR showed a 3–10-fold increase, indicating expressed stress reactions [].
In terms of early warning detection of pollutants, behavior is a more relevant tool for bioindication, because shifts in VOL and VLC are registered earlier (immediately after exposure) than significant changes in HR (30–60 min after exposure) [,]. For example, under laboratory conditions, the first significant changes in VOL were registered when concentrations of Cu and Cd were 5–10 times higher than their maximum allowable concentrations (MAC, Cu = 1 mg L−1, Cd = 0.001 mg L−1), whereas HR demonstrated significant changes when the MACs were oversubscribed by more than 50–100 times [].
4.2. Black Sea Mussels
A submersible complex on the basis of Hall sensors was used to study typical behavior patterns in Mytilus galloprovicialis. Valve gaping activity was registered in a group of 16 mollusks in an aquatic area of Kazachia Bay, Black Sea, under laboratory conditions [,,]. The authors found a clear 24 h rhythm in valve activity, with the maximal valve opening (6–8 mm, 6.38 ± 0.61 mm or 90–100%) at nighttime and minimal (4–6 mm, 4.67 ± 0.64 mm, 60–70%) during daytime [,,,]. The highest VOL was 10–12 mm. This feature seems to be species-specific because the same rhythm was noted for Mytilus galloprovicialis from other geographical locations as well []. Trusevich et al. [] detected two movement patterns in the daily rhythm of Mytilus galloprovicialis: (a) short rapid valve closures (amplitude 4–8 mm or 1–2 mm) maintaining excretion and metabolism and (b) slow, very short valve movements maintaining filtration and respiration. Ultradian rhythms in cardiac activity of Mytilus galloprovicialis lasted for 10–30 min were registered using analysis of their plethysmograms [].
Usually, VFL of Black Sea mussels fluctuate from 4–5 to 10 and more closures per min. The mollusks demonstrate immediate valve closure (2–3 s) as a typical response to various stimuli, including tapping the surface of the lab aquarium, sharp sounds, vibration, turning off lights, touching the shell, and changes in water current. The valves remain closed for a few seconds or a few minutes. Frequent repetition of these stimuli leads to less expressed reactions [,,]. Long-term observations detected no seasonal changes in the valve activity despite a smooth drop in water temperature from 22–23 °C in summer to 7–9 °C in winter, suggesting the appearance of feeding activity in this species during the colder season. Furthermore, rapid summer increases in water temperature to 26–28 °C or decreases to 9–17 °C (due to upwelling) led to stress reactions (a decrease in VOA) []. Trec, however, was as short as 3–5 h []. Feeding conditions can also affect the gaping activity. Observations indicated that the period of full valve closure in mussels reared in aerated seawater over a 13 d period increased from 13 to 32% [] and coupled with peaks in gaping activity when the water was renewed and food availability was improved [].
In 2007, cardiac activity of the Black Sea mussels was studied using the photoplethysmography method []. Daily HR varied from 0 to 20, averaging 15.6 beats min−1. This parameter varied from 8–18 (mean 14.8) beats min−1 at night to 6–19 (mean 13.7) beats min−1 in the morning hours, and to 9–20 (mean 16.2) beats min−1 in the afternoon hours. There were fluctuations in correlations between VOL and HR from 0.5 to −0.7. Slow heartbeat events (3 beats min−1) coincided with the minimum VOL []. Further studies revealed that mussels exposed to seawater with lower salinity (a decrease from 18 to 12 psu for 20 min) showed increased gaping activity 3 h after the exposure and were completely closed 15 after the exposure. This process was accompanied by an increase in HR from 20 to 28 beats min−1. The initial tachycardia occurred for 30 min after the end of this experiment [].
Reactions of 16 tested mussels to ammonia NH4OH at a concentration of 10 mg L−1 (=10 MAC) and hydroquinone at a concentration of 1 g L−1 were tested under laboratory conditions by valvometry. Acute stress led to a substantial drop in their valve activity, with a recovery period from a few minutes to a few days (Figure 7).
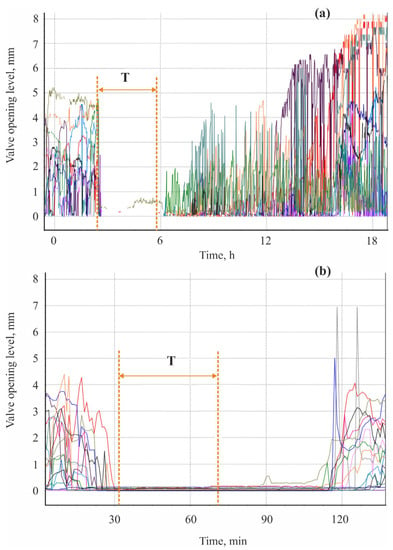
Figure 7.
Valve activity of Black Sea mussels Mytilus galloprovicialis from Kazachya Bay, Black Sea (adopted from []): (a) ammonia NH4OH (10 mg L−1), (b) hydroquinone 1 g L−1. T—period of exposure. Different color lines correspond to different mussel individuals.
Chronic stress caused rapid degradation of normal daily rhythm and an increase in the period when the mollusks were fully closed, from 4–6 h per day to 12–18 per day []. Hydroquinone was found to cause an increase in HR from 16.5 to 21 beats min−1 for 20 min followed by a sharp drop to 4.8–6 beats min−1. Trec for the cardiac activity and valve-opening activity in this experiment were 3 h 15 min and 12 h, respectively [].
According to valve movement responses, Trusevich et al. [] established that Black Sea mussels are sensitive to the following concentrations of ammonia NH4OH, copper CuSO4 * 5H2O, and sodium lauryl sulfate: 5, 0.1, and 3 mg L−1, respectively. For the latter detergent, this level was corrected to 1.7 mg L−1 thanks to research on cardiac activity []. Cooper ions were found to decrease HR by 50% in the mussels with Trec = 3 h []. According to Ait Fdil et al. [], a 1 h exposure of Mytilus galloprovincialis sampled on the Atlantic coast of Morocco to heavy metals induced a decrease in the time of normal opening and the appearance of sequences of stress behavior, including enhanced valve adductions and complete closure at high concentrations. They concluded that Hg was the most toxic to the valve activity, with a threshold effective concentration of 10 μg L−1, followed by Cu (20 μg L−1), Zn (100 μg L−1), and Cd (2000 μg L−1).
Using valvometry, Russian scientists studied how the Black Sea mussels respond to diesel oil. The mollusks with 55–65 mm shell lengths were reared in aquariums with running seawater [,]. The mussels were exposed for 2 h to a water emulsion of diesel oil at different concentrations. After 2–3 min, there were synchronous 30, 40, and 50% decreases in VOF at diesel oil concentrations of 25, 50, and 500 mg L−1, respectively. At the end of the experiment, VOL decreased to 20–25% at 25 mg L−1 and to 10% at 50 mg L−1, whereas VOL immediately decreased to 10% at 500 mg L−1. After a rapid decrease in VOA, lasting for a few minutes, this parameter was unstable, with periodical valve closures to 2–3% for 3–5 min. Mussels showed rapid recovery in VOA when the exposure was completed, and full recovery to normal rhythm was observed 2–4 h after the end of the experiment [,]. For comparison, the influence of 10 µL L−1 of diesel oil dispersed by the same concentration of the oil slick dispersant SD-25 caused a 50% decrease in HR of Mytilus galloprovicialis from the Adriatic Sea (from 18.7 to 9.1 beats min−1), with a Trec of 3 h 12 min []. Similar responses (decreased VCF and VOA) were reported for the brown mussel Perna perna exposed to 5 and 20% diesel water-accommodated fractions [,], and for the Asiatic clam Corbicula fluminea exposed to 28.2% crude oil from the North Sea []. In contrast, the clam Venus verrucosa collected on the north-eastern coast of Malta demonstrated a higher degree of valve activity after exposure to water-accommodated fractions of crude oil at a concentration of 610 µL L−1 []. Impaired valve activity in Mytilus galloprovicialis can be induced by other toxicants. For example, Cypermethrin, a widely used pyrethroid pesticide, caused a reduction in the time of normal valve opening in a concentration-dependent manner, with the lowest effect concentration at 100 µL L−1 [].
Analysis of cardiac activity and responses of Black Sea mussels to a drop in salinity from 18 to 9 psu was conducted for individuals collected at coastal sites with different levels of pollution using the photoplethysmographic technique. HR and Trec varied from 24.4 ± 12.3 to 33.3 ± 7.5 beats min−1 and from 31.7 ± 4.2 to 76.8 ± 9.1 min, respectively, being significantly higher at the site where higher levels of Cu and Pb were registered in the hepatopancreas and gills of the mussels tested []. At sites with higher levels of nitrates, nitrites, and silicates, Trec in Mytilus galloprovicialis after a salinity test was significantly higher than that at reference sites []. Similar results were found for the same species from Boka Kotorska Bay (South Adriatic Sea) [].
4.3. Iceland Scallops
Recording of gaping activity in Iceland scallops was first conducted during June–August 1987. Scallops were collected on Goose Bank at 70–80 m depth and then transferred to the laboratory in Dalnezelenetskaya Bay, where their behavior patterns were registered using valvometry. There were stable fluctuations in VOL (range 50–80%, mean 65%) with periodical attenuation of short rhythms. VCL varied from 18 to 26 closures per day, averaging 23 closures per day []. During a coastal MMBI expedition in June 2011 in Grøn-fjord (a glacier fjord of West Svalbard []), Iceland scallops were collected by divers and then placed in aquariums with aerated seawater (temperature 2 °C, salinity 34 psu) [,]. Under constant conditions and without feeding, the scallops demonstrated stable VOL (60 ± 23%) and VCF (12 ± 3 closures per day). The former parameter was close to that of Tridonta borealis (62 ± 10%) but lower than in Mya truncata (75 ± 18%), while there were zero VCL in Tridonta borealis and Mya truncata. The latter two species were concluded to be inappropriate biosensors due to their less expressed reactions to stimuli []. The same conclusion was made for the horse mussel Modiolus modiolus, which demonstrated a low-level VOA and less expressed fluctuations in VOL (10–40%, mean 25%) []. The lower VOL and VCF in Iceland scallops from Svalbard waters in comparison to their conspecifics reared in the coastal zone of the Barents Sea are associated with more favorable and less stable conditions in terms of higher food availability and water temperature [,].
Using the HFNI method described above, the joint Russian–French team studied growth patterns in Iceland scallops reared in a monitoring system installed at 16 m depth in Dalnezelenetskaya Bay. As the HFNI valvometry requires gluing of electrodes to each valve and measures the distance between the valves, this technique allows measuring growth increments in mollusks over time. The authors found a continuous growth from March to September, with a 0.4–0.5 μm daily increase in the valve distance. A dramatic decrease was found after the third week of May, indicating a shift in the physiological status of scallops associated with spawning events or changes in water quality [].
An experimental study was conducted by MMBI scientists to reveal the effects of diesel oil on Iceland scallops collected in Dalnezelenetskaya Bay and then reared in small aquariums with running seawater under conditions close to natural at 3–4 °C. The lower valve was glued to the substrate, while the upper valve was connected to a tenso-sensor of a valvometer []. After a 3 d acclimation period, the mollusks were exposed to diesel oil at concentrations of 1, 5, 10, and 15 mg L−1 for 1–3 h. The lowest concentration had no significant effects on the behavior patterns, while an increase in diesel oil concentrations from 1 to 5 mg L−1 and from 10 to 15 mg L−1 led to a 3- to 6-fold increase in VCF and a 3- to 10-fold decrease in VOL during the first 20–30 min (Figure 8).
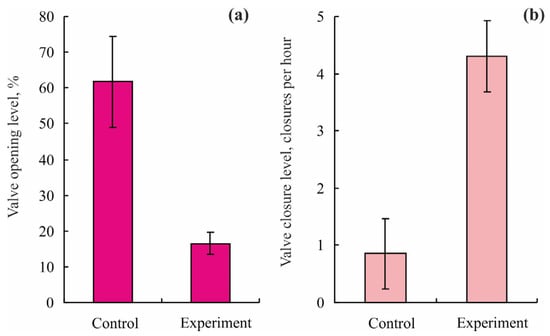
Figure 8.
Valve activity levels of Iceland scallops Chlamys islandica from the Barents Sea (adopted from []): (a) valve opening level after the exposure to diesel oil (5 mg L−1), (b) valve closing frequency after the exposure to diesel oil (15 mg L−1).
There was a complete recovery in respiratory and gaping activity 6 h after the treatment. The study demonstrated that concentrations of diesel oil higher than 5 mg L−1 are detectable by this method [].
4.4. Freshwater Mussels Anodonta
Laboratory research conducted by Sharov and Kholodkevich [] showed that HR in Anodonta anatina from the coastal zone of the Gulf of Finland at rest fluctuated from 8 ± 2 beats min−1 at 16 °C to 22 ± 5 beats min−1 at 26 °C. Ultradian and circadian rhythms were not expressed in this species. After a 24 d rearing period in aquariums, the freshwater mussels demonstrated a decrease in HR by 24%, reflecting adaptation to artificial conditions. Air exposure for 1 h caused a decrease in HR, but Trec was as low as 1–5 min. Interestingly, the mollusks did not completely close their valves during this test. There was a decrease in HR when the shell length (SL) increased (HR = −0.23SL + 39, R2 = 0.84 at 23 °C), and in large individuals (SL > 10 cm) at rest, bradycardia was registered for a few minutes in contrast to smaller mollusks []. A rapid (during 1–2 min) salinity increase (from 0 to 3 psu) led to increased HR, whereas a decrease in HR was always registered at salinity of 5–8 psu. Lower water temperatures (<7°) compensated for physiological reactions to salt stress, and HR was stable at 0 and 3–8 psu []. Mollusks at ages 3–6 had the same adaptive potential to unfavorable salinity conditions []. According to salinity test results (exposure to 6 mg L−1 NaCl for 1 h), Trec for Anodonta anatina collected at different sites varied from 35 ± 11 to 357 ± 33 following the level of pollution [,,].
The physiological status of Anodonta cygnea from locations with different pollution loads (Rybinsk Reservoir, Borok and the Yagorba River, Cherepovets, Russia) was studied by Kholodkevich et al. [,] using photoplethysmography. After a short-term salinity increase from 0 to 6 psu, HR increased from 12 to 14–16 beats min−1, and Trec for the specimens from a polluted location (320 ± 17 min) was significantly higher than that for the mollusks from a site with good ecological health (38 ± 6 min). A 1 h exposure of Anodonta cygnea to salinity of 3 psu caused an increase in HR from 12.3 to 18.2 beats min−1. Trec was 90 ± 18 min [].
4.5. Painter’s Mussels
According to laboratory observations, HR in Unio pictorum at rest varied from 12 ± 3 beats min−1 at 16 °C to 31 ± 7 beats min−1 at 26 °C. There was a positive relationship between log-transformed water temperature and HR []. For comparison, in the zebra mussel Dreissena polymorpha, HR without stress factors was 15 ± 1 beats min−1 at 20 °C. Like other freshwater mussels, Unio pictorum demonstrated no circadian or ultradian rhythms in cardiac activity []. Air exposure for 1 h led to a drop in HR and to closure of the valves, but this stress was not critical, and Trec was 1–5 min. Further laboratory research indicated that, according to HR responses, the critical thermal maximum for Unio pictorum is 35 °C []. Kuznetsova et al. [,] studied cardiac activity in Unio pictorum and found that mean HR was 18.6 ± 2.8 beats min−1 and variability in the cardiac rhythm was as low as 10%. Trec after salinity stress (exposure to 6–8 mg L−1 NaCl) was 45 ± 15 min.
A study focused on the testing threat-level concentrations of chemicals, including ammonia, copper CuSO4 * 5H2O, lead Pb(CH3COO)2, cadmium 3CdSO4 * 8H2O, and sodium lauryl sulfate, on the valve activity of Unio pictorum from the Chernaya River, Sevastopol, was conducted using the submersible complex developed by the Marine Hydrophysical Institute []. The authors observed an increase in VOL and a decrease in VOA down to full valve closure. Post-stress reactions were found to occur for a long time, depending on the pollutant type (Table 1).

Table 1.
Responses of Painter’s mussels Unio pictorum to different toxicants (adopted from []).
The most expressed changes in the gaping behavior of mussels were found for Cu. The negative effects of pollutants intensified substantially in non-flowing water [] but decreased in polluted natural freshwater rich in humic acids []. For example, toxic effects for Painter’s mussels exposed for 2 h to Pb, Ni, Cd, and Cr were registered at concentrations of 0.6, 2, 0.02, and 1 mg L−1, respectively [], thus indicating that the presence of humic acids in water should be considered when calculating the sensitivity of this biomonitoring system. A decrease in valve gaping activity was reported by Jou et al. [] for the freshwater clam Corbicula fluminea exposed to 19.5 μg L−1 Cu based on a response time of 30 min. Further laboratory studies established that Unio pictorum demonstrates high sensitivity to seawater, acetic acid, and liquid washing powders [].
Another study examined the effects of diesel oil on the behavior of Painter’s mussels reared in 120 L aquariums supplied with running fresh water from the Black River, Sevastopol, at a flow rate of 4 L min−1 []. The bivalves demonstrated high sensitivity to this pollutant, and detectable reactions occurred at 0.005 mL L−1. The most expressed responses were found for the highest tested level (0.5 mL L−1): the mollusks rapidly increased VOF to 10–20 closures per h and decreased VOA by 40%. Some specimens remained completely closed for the whole exposure period and 8−10 h after the pollutant removal [].
Diclofenac at a concentration of 0.1 µg L−1 caused an increase in HR of Unio pictorum from 17.9 to 20.1 beats min−1 at 25 °C and from 24.9 to 29.5 beats min−1 at 30 °C. This toxicant, at a concentration of 1 µg L−1, increased HR to 19.15 and 27.6 beats min−1, respectively. For the lower concentration, Trec was 39.5 and 119 min at 25 °C and 30 °C, respectively, while the higher dose resulted in Trec of 207.8 and 95 min, respectively []. HR in Unio pictorum was also found to be affected by cyanobacteria: a decrease from 14.3 to 12.9 beats min−1 was registered as a response to exposure to 3.5 ± 0.5 mL L−1. Correspondingly, Trec increased from 82 to 193 min []. The effects of toxic microalgae at a concentration of 3500 cells mL−1 were also evident for the oyster Crassostrea gigas. This mollusk demonstrated an increased daily valve-opening duration and micro-closure activity but decreased VOA [].
According to long-term observations, the following criteria were developed to assess the quality status of an ecosystem based on Trec of Unio pictorum (northern populations) after a salinity test: (a) high (Trec < 50 min), (b) good (Trec = 50–70 min), (c) satisfactory (Trec = 70–100 min), (d) bad (Trec = 100–200 min), and (e) very bad (Trec > 200 min) [,].
5. Crustaceans as Biomonitors
5.1. Crayfish
Narrow-clawed crayfish were used as biosensor organisms in the early warning system at St. Petersburg drinking Water Supply Stations based on the photoplethysmograhy method []. The circadian rhythms of this species represent periodic alterations in HR, with an increase from 35–38 beats min−1 at daytime to 85–87 bats min−1 at nighttime, reflecting the life-history traits of this species associated with nocturnal feeding activity []. A similar trend in cardiac activity was found for the red claw crayfish Cherax quadricarinatus: during daytime, its HR varied from 50 to 56 beats min−1, while at nighttime, it varied from 121 to 127 beats min−1. The light switch caused an increase in HR of Pontastacus leptodactylus to 126–148 beats min−1 [,].
Pontastacus leptodactylus demonstrated a rapid (1–2 min) two-fold increase in HR from 55 to 120 beats min−1 for 4–5 min as a response to handling. A 2 h air exposure caused an increase in the duration of heart rhythm from 0.80 to 0.99 s and an increase in the mean amplitude from 14.4 to 15.0% []. Salt stress reactions in narrow-clawed crayfish were studied by Kozák et al. []. They found that at 100, 400, 800, and 1600 mg L−1 NaCl, only a few specimens demonstrated changes in HR. More expressed responses (an increase in HR from 42–75 to 54–94 beats min−1 and an increase in stress index, SI, from 21–297 to 92–2257 units) were registered for 3200, 6400, 12,800, and 25,600 mg L−1 NaCl. Both HR and SI returned to normal parameters within a few minutes or hours after NaCl addition []. Although an increase in SI after the salt exposure occurred somewhat later (after 2–4 min) than the immediate increase in HR, SI was concluded to be a more appropriate indicator of the physiological status, due to its much higher variation [].
Kozák et al. [] studied the effects of nitrites on the cardiac activity of narrow-clawed crayfish and found that significant changes in SI and HR occurred at concentrations of 7.5 and 30 mg L−1, respectively. Fluctuations in pH significantly affected HR in narrow-clawed crayfish. Exposure to a low pH (3.4) resulted in an increased HR (115.7 ± 43.8 beats min−1) in comparison to conditions at a normal pH of 6.8 (39.1 ± 3.2 beats min−1) [].
In an early warning system, a rapid salinity increase from 0 to 6.5 psu led to a 30% decrease in HR, and such a response is considered an “alarm” signal []. In the case of low toxicant concentrations, when a short-term (1–5 min) “sensory response” occurs, the alarm signal is detected if the difference between actual HR and HR at rest exceeds three standard deviations at rest [].
In another experiment, the authors added ammonium (450 mg L−1 NO3−) and ammonium phosphate (35 mg L−1 PO4−) in the water and observed a dramatic increase in SI to 7000 units 2–3 min after the exposure. The stress period was 5–7 min. A two-fold increase in HR and an increase in SI from 0 to 10,000 units were registered as typical reactions of the crayfish to hydroquinone at a concentration of 1 g L−1 [,]. Crayfish demonstrated first reactions 3–4 min after the exposure (Figure 9).
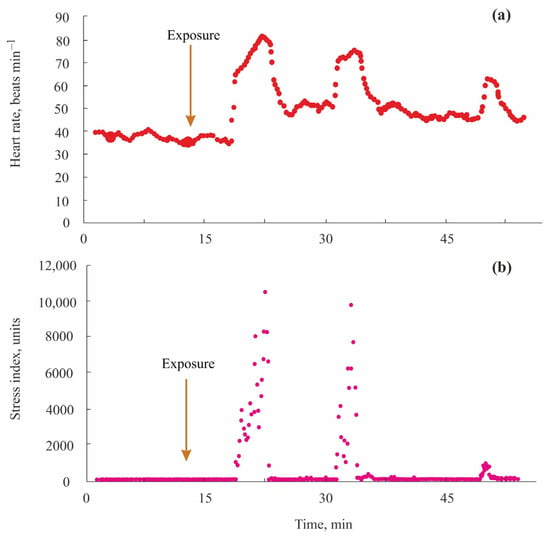
Figure 9.
Heart rate (a) and stress index (b) in narrow-clawed crayfish Pontastacus leptodactylus exposed to hydroquinone (1 g L−1) (adopted from []).
In general, at rest, HR of Pontastacus leptodactylus is about 20–40 beats min−1, while under stress conditions, it increases to 100–120 beats min−1 [].
5.2. Red King Crabs
Photoplethysmography was used to study the physiological responses of commercial male red king crabs (carapace width > 150 mm) captured in their non-native area, the Barents Sea. The crabs were transferred to the laboratory of the Russian Federal Research Institute of Fisheries and Oceanography, Moscow, and reared in individual 200 L aquariums supplied with artificial seawater at a salinity of 32 psu and temperature of 5 °C [,]. After an acclimation period that lasted for 10–14 d, the crabs were tested for stress reactions. Food consumption, handling, temperature fluctuations, and air exposure had a significant impact on cardiac activity (Table 2).

Table 2.
Cardiac activity of red king crabs Paralithodes camtschaticus under different conditions (adopted from []).
HR of red king crabs at rest (18–25 bets min−1 at 5 °C) was lower than those in the green crab Carcinus maenas (30–90 beats min−1 at 16 °C) [] and in the pebble crab Gaetice depressus (75–189 beats min−1 at 27 °C) [], probably due to species-specific adaptations [].
There was an increase both in HR by 10 beats min−1 and in SI by 500 units during feeding, and the increased levels occurred more than 1 h after the feeding finished (Figure 10a).
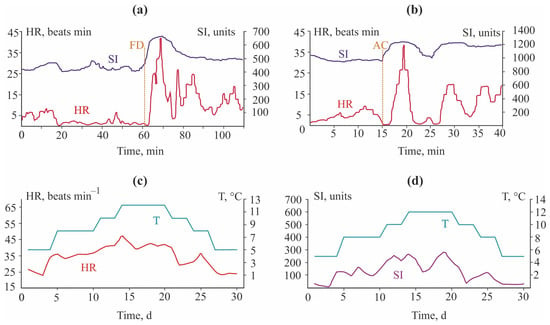
Figure 10.
Heart rate (HR) and stress index (SI) of red king crab Paralithodes camtschaticus from the Barents Sea (modified from []: (a) during food consumption (FD—food added), (b) during mechanical stress (AC—aquarium cleaning), and (c,d) during temperature fluctuations.
A similar result was found for the Dungeness crab Cancer magister, which showed a higher HR when feeding (84 vs. 74 beats min−1) []. Mechanical stimuli associated with cleaning procedures in the rearing aquariums caused increases in HR and SI of Paralithodes camtschaticus (Figure 10b) and prolonged stress reactions for a few hours. HR in red king crabs followed changes in temperature, demonstrating higher levels when the water temperature increased. Stress reactions to increased temperatures, however, were not expressed because SI remained relatively stable (Figure 10c). Similar responses were detected in the lobster Homarus americanus, whose HR increased from 20 beats min−1 at 2 °C to 40–45 beats min−1 at 10 °C and to 50 beats min−1 at 12 °C [], and in Carcinus maenas, whose HR increased from 62 to 100 beats min−1 between 10.2 and 25 °C [].
Air exposure led to an increase in HR of red king crabs from 30 to 40 beats min−1. Three hours later, HR decreased to 20–25 beats min−1. During the following 20 h, HR smoothly decreased to 15 beats min−1. When the crab was placed into seawater after the end of this 24 h experiment, its HR remained low for 30 min but then increased to 40 beats min−1. SI was 3500 units. During the next day, these parameters varied from 42 to 47 beats min−1 and from 200 to 2000 units, respectively []. A longer experiment indicated that some red king crabs can endure a 47 h aerial exposure period, but this period was fatal for other crabs [].
As the red king crab is a cold-water species, its cardiac activity can be used as an indicator of stress conditions at high latitudes.
6. Conclusions
More than three decades of Russian research has focused on the development of biomonitoring techniques and searching for reliable shellfish biosensor species, in accordance with world trends, resulting in two relatively simple and low-cost approaches: valvometry (i.e., monitoring of valve movement activity of bivalve mollusks using actographs and specialized sensors) and photoplethysmography (i.e., registration of cardiac activity using photosensors). Physiological assessment of gaping activity in bivalve mollusks and cardiac activity in mollusks and crustaceans is non-invasive and provides a complete understanding of environmental impacts on the organisms serving as water quality monitors in both freshwater and marine environments. In Russia, the most appropriate bioindicators in terms of their ecological relevance, availability, susceptibility to stressors, ease of maintenance, and ability to provide reproducible results are blue mussels, Black sea mussels, Iceland scallops, freshwater mussels, crayfish, and red king crabs. Although in the fluctuating environmental conditions that occur in nature, it is difficult to attribute cause and effect; it is even less certain how exposure–response relationships that have been demonstrated in the laboratory are modified under natural conditions. Long-term biotesting studies have shown that stress factors such as salinity and temperature fluctuations, handling, changes in light regimes, and pollutants including ammonia, detergents, heavy metals, and oil products lead to significant changes in the gaping and cardiac activity of biosensor organisms, indicating that their responses may be used as alarm signals. Early warning systems based on technological solutions of Russian scientists are currently being used in practice at the Neva River water intakes of St. Petersburg drinking Water Supply Stations and in research laboratories.
Author Contributions
Conceptualization, A.G.D.; methodology, A.G.D.; validation, A.G.D. and V.G.D.; investigation, A.G.D. and V.G.D.; visualization V.G.D.; writing—original draft, A.G.D.; writing—review and editing, A.G.D. and V.G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Ministry of Science and Higher Education of the Russian Federation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to the anonymous reviewers for valuable comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oertel, N.; Salánki, J. Biomonitoring and bioindicators in aquatic ecosystems. In Modern Trends in Applied Aquatic Ecology; Ambasht, R.S., Ambasht, N.K., Eds.; Springer: Boston, MA, USA, 2003; pp. 219–246. [Google Scholar]
- Feio, M.J.; Hughes, R.M.; Callisto, M.; Nichols, S.J.; Odume, O.N.; Quintella, B.R.; Kuemmerlen, M.; Aguiar, F.C.; Almeida, S.F.P.; Alonso-EguíaLis, P.; et al. The biological assessment and rehabilitation of the World’s rivers: An overview. Water 2021, 13, 371. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, G.L.; Zamparas, M.G.; Kapsalis, V.C. Investigating the human impacts and the environmental consequences of microplastics disposal into water resources. Sustainability 2022, 14, 828. [Google Scholar]
- Zolkefli, N.; Sharuddin, S.S.; Yusoff, M.Z.M.; Hassan, M.A.; Maeda, T.; Ramli, N.A. Review of current and emerging approaches for water pollution monitoring. Water 2020, 12, 3417. [Google Scholar] [CrossRef]
- Chu, X.; Wu, D.; Wang, H.; Zheng, F.; Huang, C.; Hu, L. Spatial distribution characteristics and risk assessment of nutrient elements and heavy metals in the Ganjiang River basin. Water 2021, 13, 3367. [Google Scholar] [CrossRef]
- Zhang, F.; Lin, L.; Li, W.; Fang, D.; Lv, Z.; Li, M.; Ma, G.; Wang, Y.; Wang, L.; He, L. Long-term study of monitoring history and change trends in surface water quality in China. Water 2022, 14, 2134. [Google Scholar]
- Cabral, H.; Fonseca, V.; Sousa, T.; Costa Leal, M. Synergistic effects of climate change and marine pollution: An overlooked interaction in coastal and estuarine areas. Int. J. Environ. Res. Public Health 2019, 16, 2737. [Google Scholar]
- Miloloža, M.; Kučić Grgić, D.; Bolanča, T.; Ukić, Š.; Cvetnić, M.; Ocelić Bulatović, V.; Dionysiou, D.D.; Kušić, H. Ecotoxicological assessment of microplastics in freshwater sources—A review. Water 2021, 13, 56. [Google Scholar] [CrossRef]
- Depledge, M.H.; Galloway, T.S. Healthy animals, healthy ecosystems. Front. Ecol. Environ. 2005, 3, 251–258. [Google Scholar]
- Connon, R.E.; Geist, J.; Werner, I. Effect-Based tools for monitoring and predicting the ecotoxicological effects of chemicals in the aquatic environment. Sensors 2012, 12, 12741–12771. [Google Scholar] [CrossRef]
- Kuklina, I.; Kouba, A.; Kozák, P. Real-time monitoring of water quality using fish and crayfish as bio-indicators: A review. Environ. Monit. Assess. 2013, 185, 5043–5053. [Google Scholar]
- Agrawal, A.; Gopal, K. Biomonitoring of Water and Waste Water; Springer: New Dehli, India, 2013. [Google Scholar]
- Savoca, D.; Pace, A. Bioaccumulation, biodistribution, toxicology and biomonitoring of organofluorine compounds in aquatic organisms. Int. J. Mol. Sci. 2021, 22, 6276. [Google Scholar] [CrossRef] [PubMed]
- Depledge, M.H.; Aagard, A.; Györkös, P. Assessment of trace metal toxicity using molecular, physiological and behavioural biomarkers. Mar. Pollut. Bull. 1995, 31, 19–27. [Google Scholar]
- Bae, M.-J.; Park, Y.-S. Biological early warning system based on the responses of aquatic organisms to disturbances: A review. Sci. Total Environ. 2014, 466–467, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Ravera, O. Monitoring of the aquatic environment by species accumulator of pollutants: A review. J. Limnol. 2001, 60 (Suppl. 1), 63–78. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Giordano, M.E. Pollution Biomarkers in the Framework of Marine Biodiversity Conservation: State of Art and Perspectives. Water 2021, 13, 1847. [Google Scholar]
- Borcier, E.; Charrier, G.; Couteau, J.; Maillet, G.; Le Grand, F.; Bideau, A.; Waeles, M.; Le Floch, S.; Amara, R.; Pichereau, V.; et al. An integrated biomarker approach using flounder to improve chemical risk assessments in the heavily polluted seine estuary. J. Xenobiot. 2020, 10, 14–35. [Google Scholar] [CrossRef]
- Kataoka, C.; Kashiwada, S. Ecological risks due to immunotoxicological effects on aquatic organisms. Int. J. Mol. Sci. 2021, 22, 8305. [Google Scholar]
- Gerhardt, A.; Ingram, M.K.; Kang, J. In situ on-line toxicity biomonitoring in water: Recent developments. Environ. Toxicol. Chem. 2006, 25, 2263–2271. [Google Scholar]
- Di Giacinto, F.; Berti, M.; Carbone, L.; Caprioli, R.; Colaiuda, V.; Lombardi, A.; Tomassetti, B.; Tuccella, P.; De Iuliis, G.; Pietroleonardo, A.; et al. Biological early warning systems: The experience in the Gran Sasso-Sirente aquifer. Water 2021, 13, 1529. [Google Scholar] [CrossRef]
- Tang, J.Y.; Glenn, E.; Thoen, H.; Escher, B.I. In vitro bioassay for reactive toxicity towards proteins implemented for water quality monitoring. J. Environ. Monitor. 2012, 14, 1073–1081. [Google Scholar]
- Sivakumar, P.; Srinivasan, G.; Janardhanam, M.; Sivakumar, R.; Niranjani Marcus, P.; Balasubramaniam, S.; Singaram, G.; Harikrishnan, T. A Rapid bioassay test for assessing environmental contamination using the marine sedentary polychaete Hydroides elegans. Water 2022, 14, 1713. [Google Scholar]
- Haron, F.K.; Shah, M.D.; Yong, Y.S.; Tan, J.K.; Lal, M.T.M.; Venmathi Maran, B.A. Antiparasitic Potential of methanol extract of brown alga Sargassum polycystum (Phaeophyceae) and its LC-MS/MS metabolite profiling. Diversity 2022, 14, 796. [Google Scholar] [CrossRef]
- Lemos, M.F.L.; Duarte, B.; Fonseca, V.F.; Novais, S.C. Effects on biomarkers in stress ecology studies. Well, So What? What Now? Biology 2022, 11, 1777. [Google Scholar]
- Ates, M.; Arslan, Z.; Demir, V.; Daniels, J.; Farah, I.O. Accumulation and toxicity of CuO and ZnO nanoparticles through waterborne and dietary exposure of goldfish (Carassius auratus). Environ. Toxicol. 2015, 30, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Leese, J.M.; McMahon, J.; Colosi, J.C. Effects of wastewater treatment plant effluent in a receiving stream on reproductive behavior of fathead minnows (Pimephales promelas). Fishes 2021, 6, 14. [Google Scholar]
- Rabbane, M.G.; Kabir, M.A.; Habibullah-Al-Mamun, M.; Mustafa, M.G. Toxic effects of arsenic in commercially important fish Rohu Carp, Labeo rohita of Bangladesh. Fishes 2022, 7, 217. [Google Scholar]
- De Araújo, E.R.L.; Torres, M.F.; Da Costa, B.M.P.A.; Hamoy, M.; Sampaio, L.A.; Barbas, L.A.L. Electroencephalographic response in juvenile tambaqui, Colossoma macropomum, exposed to short-term anaesthetic baths with geraniol and citronellol. Biology 2023, 12, 90. [Google Scholar]
- Yap, C.K.; Pang, B.H.; Cheng, W.H.; Kumar, K.; Avtar, R.; Okamura, H.; Horie, Y.; Sharifinia, M.; Keshavarzifard, M.; Ong, M.C.; et al. Heavy metal exposures on freshwater snail Pomacea insularum: Understanding its biomonitoring potentials. Appl. Sci. 2023, 13, 1042. [Google Scholar] [CrossRef]
- Kramer, K.J.M.; Foekema, E.M. The “Musselmonitor®” as biological early warning system. In Biomonitors and Biomarkers as Indicators of Environmental Change 2: A Handbook; Butterworth, F.M., Gunatilaka, A., Gonsebatt, M.E., Eds.; Springer: New York, NY, USA, 2001; pp. 59–87. [Google Scholar]
- Kramer, K.G.M.; Jenner, H.A.; de Zwart, D. The valve movement response of mussels: A tool in biological monitoring. Hydrobiologia 1989, 188, 433–443. [Google Scholar] [CrossRef]
- Sluyts, H.; van Hoof, F.; Cornet, A.; Paulussen, J. A dynamic new alarm system for use in biological early warning systems. Environ. Toxicol. Chem. 1995, 15, 1317–1323. [Google Scholar]
- Scully, P. Optical techniques for water quality monitoring. In Monitoring of Water Quality; Colin, F., Quevauviller, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 15–35. [Google Scholar]
- Ballesta-Artero, I.; Witbaard, R.; Carroll, M.L.; van der Meer, J. Environmental factors regulating gaping activity of the bivalve Arctica islandica in northern Norway. Mar. Biol. 2017, 164, 116. [Google Scholar] [CrossRef] [PubMed]
- Nedeau, E.J.; Smith, A.K.; Stone, J.; Sarina, J. Freshwater Mussels of the Pacific Northwest; The Xerces Society: Portland, OR, USA, 2009. [Google Scholar]
- Johnson, E.H. Experimental tests of bivalve shell shape reveal potential tradeoffs between mechanical and behavioral defenses. Sci. Rep. 2020, 10, 19425. [Google Scholar] [CrossRef] [PubMed]
- Mikac, B.; Tarullo, A.; Colangelo, M.A.; Abbiati, M.; Costantini, F. Shell infestation of the farmed Pacific oyster Magallana gigas by the endolith bivalve Rocellaria dubia. Diversity 2021, 13, 526. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Biological aspects, fisheries, and aquaculture of yesso scallops in Russian waters of the Sea of Japan. Diversity 2022, 14, 399. [Google Scholar]
- Sow, M.; Durrieu, G.; Briollais, L.; Ciret, P.; Massabuau, J.-C. Water quality assessment by means of HFNI valvometry and high-frequency data modeling. Environ. Monit. Assess. 2011, 182, 155–170. [Google Scholar]
- Tran, D.; Nadau, A.; Durrieu, G.; Ciret, P.; Parisot, J.-P.; Massabuau, J.-C. Field chronobiology of a molluscan bivalve: How the moon and sun cycles interact to drive oyster activity rhythms. Chronobiol. Int. 2011, 28, 307–317. [Google Scholar] [CrossRef]
- Borcherding, J. Ten years of practical experience with the Dreissena-Monitor, a biological early warning system for continuous water quality monitoring. Hydrobiologia 2006, 556, 417–426. [Google Scholar] [CrossRef]
- Gnyubkin, V.F. An early warning system for aquatic environment state monitoring based on an analysis of mussel valve movement. Russ. J. Mar. Biol. 2009, 35, 431–436. [Google Scholar]
- Guarini, J.-M.; Hinz, S.; Coston-Guarini, J. Designing the next generation of condition tracking and early warning systems for shellfish aquaculture. J. Mar. Sci. Eng. 2021, 9, 1084. [Google Scholar] [CrossRef]
- Dodgson, R.W. Report on mussels purification. In Ministry of Agriculture and Fisheries: Investigations; Her Majesty’s Stationary Office: London, UK, 1928; Series II; Volume 10. [Google Scholar]
- Gudimov, A.V. Behavioral Reactions of Mussels under Fluctuations of Environmental Factors of the Eastern Murmansk Coast. Ph.D. Thesis, Murmansk Marine Biological Institute, Murmansk, Russia, 2004. (In Russian). [Google Scholar]
- Gudimov, A.V. Method of Operational Bioindication. Patent RF. RU 2,395,082, C1. G01N 33/18. (2006.01), 20 July 2010. Bull. 20. (In Russian). [Google Scholar]
- Gudimov, A.V. System for Rapid Biological Monitoring and Indication. Patent RF. 2,437,093, Int. Cl. G01N 33/18 (2006.01), 20 December 2011. Bull. 35. (In Russian). [Google Scholar]
- Gudimov, A.V. Autonomous System of Operational Biological Monitoring and Indications (Variants). Patent RF. RU 101,838, U1. G01N 33/18 (2006.01), 27 January 2011. (In Russian). [Google Scholar]
- Gudimov, A.V.; Burdygin, A.I.; Nesterov, V.P.; Mitrofanov, V.F. Equipment System for Continuous Detection and Measurement of Motor Activity of Bivalve Mollusks. Patent RF. RU 2,452,949, C1. G01N 33/18. (2006.01), 10 June 2012. Bull. 16. (In Russian). [Google Scholar]
- Gudimov, A.V.; Komarova, E.P. Environmental control: From inertia of the standard biomonitoring to speed of the online one. IOP Conf. Ser. Earth Environ. Sci. 2021, 625, 12013. [Google Scholar] [CrossRef]
- Komarova, E.P.; Gudimov, A.V.; Burdygin, A.I. Sensors selection in the system online biomonioring of aquatic media. Vestn. Lugansk Vladimir Dahl Nat. Univ. 2019, 7, 202–205. (In Russian) [Google Scholar]
- Komarova, E.P.; Gudimov, A.V.; Burdygin, A.I.; Yurasov, Y.I. Sensors for online biomonitoring of the water quality. In The Future of the Arctic Begins Here: Materials of the All-Russian Scientific and Practical Conference with International Participation; Dyachenko, N.G., Ed.; Apatity Branch of MAGU: Apatity, Russia, 25–26 April 2019; pp. 173–179. (In Russian) [Google Scholar]
- Gudimov, A.V. Elementary behavioral acts of valve movements in mussels (Mytilus edulis L.). Dokl. Biol. Sci. 2003, 391, 346–348. [Google Scholar] [PubMed]
- Massabuau, J.C.; Gudimov, A.; Blanc, P. Environmental monitoring of Arctic waters with unmanned bivalve biosensor technology: One year of background data acquisition in the Barents Sea. In Proceedings of the SPE Russian Petroleum Technology Conference, Moscow, Russia, 26–28 October 2015. SPE-176681-MS. [Google Scholar]
- Kramer, K.J.M. Continuous monitoring of waters by biological early warning systems. In Rapid Chemical and Biological Techniques for Water Monitoring; Gonzalez, C., Greenwood, R., Quevauviller, P.P., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 197–219. [Google Scholar]
- Trusevich, V.V.; Stolbov, A.A.; Mishurov, V.Z.; Shejanov, V.A. Locomotor activity of valves as an instrument for biological monitoring of water environment. Mar. Ecol. 2006, 71, 64–67. (In Russian) [Google Scholar]
- Trusevich, V.V.; Gaiskii, P.V.; Kuz’min, K.A. Automatic biomonitoring of aqueous media based on the response of bivalves. Mar. Hydrophys. J. 2010, 3, 75–83. (In Russian) [Google Scholar]
- Gaisky, P.V.; Trusevich, V.V.; Zaburdaev, V.I. Automatic bioelectronic complex designed for early detection of toxic pollution of fresh and marine waters. Mar. Hydrophys. J. 2014, 3, 44–53. (In Russian) [Google Scholar]
- Gajskij, P.V. Device for Measuring Motor Activity of Clam Shells. Patent RF. RU 2,625,673, A01K 61/00 (2006.01), G01N 33/18 (2006.01), 18 July 2017. Bull. 20. (In Russian). [Google Scholar]
- Gaisky, V.A.; Gaisky, P.V. Bioelectronic automatic aquaculture monitoring station. Tr. VNIRO 2021, 184, 159–168. (In Russian) [Google Scholar] [CrossRef]
- Trusevich, V.V.; Gaysky, P.V.; Mishurov, V.Z.; Kuzmin, K.A. Automated monitoring of the aquatic environment based on behavioral reactions of the Black Sea mussels. Assessment of sensitivity to some toxicants. In Environmental Control Systems—2016, Proceedings of the International Scientific and Technical Conference, Sevastopol, Russia, 27–27 October 2016; Polonsky, A.B., Voskresenskaya, E.N., Gaisky, V.A., Grekov, N.A., Krasnodubets, L.A., Gaisky, P.V., Eds.; Federal State Budgetary Scientific Institution “Institute of Natural and Technical Systems”: Sevastopol, Russia, 2016; p. 57. (In Russian) [Google Scholar]
- Depledge, M.H. Photoplethysmograph—A non-invasive technique for monitoring heart beat and ventilation rate in decapod crustaceans. Comp. Biochem. Physiol. A 1984, 77, 369–371. [Google Scholar]
- Aagaard, A.; Andersen, B.B.; Depledge, M.H. Simultaneous monitoring of physiological and behavioural activity in marine organisms using non-invasive, computer-aided techniques. Mar. Ecol. Prog. Ser. 1991, 73, 277–282. [Google Scholar] [CrossRef]
- Aagaard, A.; Styrishave, B.; Warman, C.G.; Depledge, M.H. The use of cardiac monitoring in the assessment of mercury toxicity in the subtropical pebble crab (Gaetice deoressus). Sci. Mar. 2000, 64, 381–386. [Google Scholar] [CrossRef]
- Frederich, M.; Pörtner, H.O. Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. Am. J. Physiol. Regulat. Integr. Compar. Physiol. 2000, 279, R1531–R1538. [Google Scholar]
- Seo, E.; Sazi, T.; Togawa, M.; Nagata, O.; Murakami, M.; Kojima, S.; Seo, Y. A portable infrared photoplethysmograph: Heartbeat of Mytilus galloprovincialis analyzed by MRI and application to Bathymodiolus septemdierum. Biol. Open 2016, 5, 1752–1757. [Google Scholar] [PubMed]
- Kushinsky, D.; Morozova, E.O.; Marder, E. In vivo effects of temperature on the heart andpyloric rhythms in the crab Cancer borealis. J. Exp. Biol. 2019, 222, jeb199190. [Google Scholar] [PubMed]
- Zarykhta, V.V.; Zhang, Z.; Kholodkevich, S.V.; Kuznetsova, T.V.; Sharov, A.N.; Zhang, Y.; Sun, K.; Lv, M.; Feng, Y. Comprehensive assessments of ecological states of Songhua River using chemical analysis and bivalves as bioindicators. Environ. Sci. Pollut. Res. 2019, 26, 33341–33350. [Google Scholar]
- Maus, B.; Gutsfeld, S.; Bock, C.; Pörtner, H.-O. Non-invasive MRI studies of ventilatory and cardiovascular performance in edible crabs Cancer pagurus during warming under elevated CO2 levels. Front. Physiol. 2021, 11, 596529. [Google Scholar] [CrossRef]
- Maus, B.; Gutsfeld, S.; Pörtner, H.O.; Bock, C. Non-invasive quantification of cardiac stroke volume in the edible crab Cancer Pagurus. Front. Zool. 2019, 16, 46. [Google Scholar]
- Martinović, R.; Joksimović, D.; García-March, J.R.; Vicente, N.; Gačić, Z. Evaluation of physiological state of pen shell Pinna nobilis (Linnaeus, 1758) by a non-invasive heart rate recording under short-term hyposalinity test. Micromachines 2022, 13, 1549. [Google Scholar]
- Fedotov, V.P.; Kholodkevich, S.V.; Strochilo, A.G. Study of contractile activity of the crayfish heart with the aid of a new non-invasive technique. J. Evol. Biochem. Physiol. 2000, 36, 288–293. [Google Scholar]
- Kholodkevich, S.V.; Donchenko, V.K.; Ivanov, A.V.; Kornienko, E.L.; Kurakin, A.S.; Fedotov, V.P. A Sensor of Physiological Activity of Invertebrate with Hard Skeleton and a System Based on the Sensor for Biological Monitoring of Environment. Patent RF (Useful Model) RU 52,190, U1. Int. Cl. G01N 33/18 (2006.01), 10 March 2006. (In Russian). [Google Scholar]
- Kholodkevich, S.V.; Fedotov, V.P.; Ivanov, A.V.; Kuznetsova, T.V.; Kurakin, A.S.; Kornienko, E.L. Fiber-optical remote biosensor systems for permanent biological monitoring of the surface waters quality and bottom sediments in the real time. ICES CM CM 2007, 1, 15. [Google Scholar]
- Kholodkevich, S.V.; Ivanov, A.V.; Kurakin, A.S.; Kornienko, E.L.; Fedotov, V.P. Real time biomonitoring of surface water toxicity level at water supply stations. J. Environ. Bioindic. 2008, 3, 23–34. [Google Scholar]
- Kurakin, A.S.; Kholodkevich, S.V.; Ivanov, A.V. Software and algorithmic aspects of the real-time bioelectronic systems for heart rhythm analysis in exoskeleton invertebrates applied to the surface water quality assessment. Environ. Control Syst. 2017, 10, 38–47. (In Russian) [Google Scholar]
- Kholodkevich, S.V.; Ivanov, A.V.; Trusevich, V.V.; Kuznetsova, T.V. New physiological biomarkers for express indication of aquatic ecosystems state on the base of adaptive capacities assessment of bivalves using standard test-stimuli. In Ecosystem Protection in a Sustainable World: A Challenge for Science and Regulation, Proceedings of the SETAC Europe 21th Annual Meeting, Milan, Italy, 15–19 May 2011; pp. 168–169. Available online: https://www.setac.org/store/ (accessed on 12 September 2022).
- Kholodkevich, S.V.; Kuznetsova, T.V.; Sharov, A.N.; Kurakin, A.S.; Lips, U.; Kolesova, N.; Lehtonen, K.K. Applicability of a bioelectronic cardiac monitoring system for the determination of biological effects of pollution in bioindicator species in the Gulf of Finland. J. Mar. Syst. 2017, 171, 8151–8158. [Google Scholar]
- Kuznetsova, T.V.; Kholodkevich, S.V.; Kurakin, A.S. Experience on ecological status assessment based on adaptive potential diagnostics in selected invertebrates of the Baltic Sea sub–regions. Fundamental. Prikladn. Gidrofiz. 2018, 11, 75–85. [Google Scholar]
- Sladkova, S.V.; Lyubimtsev, V.A.; Kholodkevich, S.V. A study of the impact of biologically treated wastewater discharged into the Neva Bay on the functional state of crustaceans. In Anthropogenic Impact on Aquatic Organisms and Ecosystems, Proceedings of VII All-Russian Conference on Aquatic Ecotoxicology Dedicated to the Memory of Doctor of Biological Sciences, Professor B.A. Flerov. Modern Methods of Research and Assessment of Water Quality, the State of Aquaticorganisms and Ecosystems under Anthropogenic Stress: Materials of the School-Seminar for Young Scientists, Graduate Students and Students, Borok, Russia, 16–19 September 2020; Filigran: Yaroslavl, Russia, 2020; pp. 180–182. (In Russian) [Google Scholar]
- Ipatov, A.A.; Bakhmet, I.N.; Yekimov, D.A.; Kuldin, N.A. Automatic early warning device for environmental risks at waterbodies and its trials. Proc. Karelian Sci. Cent. Russ. Acad. Sci. 2015, 12, 80–86. (In Russian) [Google Scholar]
- Bakhmet, I.; Aristov, D.; Marchenko, J.; Nikolaev, K. Handling the heat: Changes in the heart rate of two congeneric blue mussel species and their hybrids in response to water temperature. J. Sea Res. 2022, 185, 102218. [Google Scholar] [CrossRef]
- Resh, V.H. Which group is best? Attributes of different biological assemblages used in freshwater biomonitoring programs. Environ. Monit. Assess. 2008, 138, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.S.; Salierno, J.D.; Brewer, S.K. Fish models in behavioral toxicology: Automated techniques, updates and perspectives. In Methods in Aquatic Toxicology; Ostrander, G.K., Ed.; Lewis Publishers: Boca Raton, FL, USA, 2005; pp. 559–590. [Google Scholar]
- Gudimov, A.V. Discharge of drill cuttings in the Arctic seas and responses of bottom fauna: Bivalve Mytilus edulis L. IOP Conf. Ser. Earth Environ. Sci. 2021, 937, 22041. [Google Scholar]
- Gudimov, A.V.; Malavenda, S.S. Responses of bottom invertebrates to pollution in the Arctic: Bioassays with blue mussel Mylilus edulis L. BIO Web Conf. EDP Sci. 2022, 52, 71. [Google Scholar] [CrossRef]
- Bakhmet, I.N. Estimation of oil product’s effects on the mussel cardiac rhythm. Probl. Ecol. Monit. Ecosyst. Model. 2009, 22, 267–277. (In Russian) [Google Scholar]
- Bakhmet, I.N. Bioindication role of filterers: Response of mussels Mytilus edulis L. to heavy metals. Probl. Ecol. Monit. Ecosyst. Model. 2010, 23, 268–275. (In Russian) [Google Scholar]
- Bakhmet, I.; Fokina, N.; Ruokolainen, T. Changes of heart rate and lipid composition in Mytilus edulis and Modiolus modiolus caused by crude oil pollution and low salinity effects. J. Xenobiot. 2021, 11, 46–60. [Google Scholar] [CrossRef]
- Trusevich, V.V.; Gaysky, P.V.; Kuzmin, K.A.; Mishurov, V.Z. Biomarkers of behavioural reactions of black sea mussel for the automated biomonitoring of ecological state of water environment. Environ. Control Syst. 2015, 1, 13–18. (In Russian) [Google Scholar]
- Trusevich, V.V.; Vyshkvarkova, E.V.; Zhuravsky, V.Y. Shellfish reactions to pollution of the aquatic environment by extracts of drilling sludge and diesel fuel. In Environmental Control Systems—2020, Proceedings of the International Scientific and Technical Conference, Sevastopol, Russia, 9–12 November 2020; Bardin, M.Y., Voskresenskaya, E.N., Vyshkvarkova, E.V., Gaisky, V.A., Gaisky, P.V., Grekov, N.A., Grekov, A.N., Eds.; IP Kulikov: Sevastopol, Russia, 2020; p. 83. (In Russian) [Google Scholar]
- Kholodkevich, S.V.; Kuznetsova, T.V.; Kurakin, A.S.; Soldatov, A.A.; Gostukhina, O.L.; Golovina, I.V.; Andreenko, T.I.; Kirin, M.P. New methodological approach to express assessment of ecological state for the coastal sea waters. Izv. TINRO 2018, 194, 215–238. (In Russian) [Google Scholar]
- Gudimov, A.V. Bioassay of diesel oil for the online bio-indicating. In Pollution of Marine Environment: Ecological Monitoring, Bioassay, Standardization, Proceedings of the Russian Scientific Conference with International Participation Devoted to 125th Anniversary of prof. V.A. Vodyanitsky, Sevastopol, Russia, 28 May 2018–1 June 2018; Rudneva, I.I., Ed.; Colorit: Sevastopol, Russia, 2018; pp. 78–82. (In Russian) [Google Scholar]
- Kholodkevich, S.; Sharov, A.; Nikolić, M.; Joksimović, A. Bioindication of aquatic ecosystems on the base of the assessment of functional state of freshwater bivalve mollusks biomarkers. In Proceedings of the 4th Mediterranean Conference on Embedded Computing, MECO, IEEE, Budva, Montenegro, 14–18 June 2015; MECO: Budva, Montenegro, 2015; pp. 345–348. Available online: https://ieeexplore.ieee.org/document/7181939 (accessed on 12 September 2022).
- Kholodkevich, S.V.; Sharov, A.N.; Kuznetsova, T.V. Perspectives and problems of application of bioelectronic systems for monitoring of environmental safety state in the Gulf of Finland aquatoria. Reg. Ecol. 2015, 2, 16–26. (In Russian) [Google Scholar]
- Kholodkevich, S.V.; Chuiko, G.M.; Sharov, A.N.; Kuznetsova, T.V.; Pesnya, D.S. Indicators of cardiac activity and oxidative stress in the mollusk Anodonta cygnea under short-term salt test load as biomarkers for assessing the state of the organism and the quality of the environment. Inland Water Biol. 2021, 14, 739–746. [Google Scholar]
- Trusevich, V.V.; Kuzmin, K.A.; Mishurov, V.J. Biomonitoring of the surface water quality with use of fresh-water bivalvia moluscs. Environ. Control Syst. 2017, 7, 83–93. (In Russian) [Google Scholar]
- Trusevich, V.V.; Mishurov, V.Z.; Kuzmin, K.A. Assessment of the sensitivity of pearl oysters (Unio pictorum) used in automated control systems biomonitoring of the aquatic environment, to oil pollution. In Environmental Control Systems—2021, Proceedings of the International Scientific and Practic Conference, Sevastopol, Russia, 9–12 November 2021; Bardin, M.Y., Voskresenskaya, E.N., Gaisky, V.A., Grekov, N.A., Grekov, A.N., Kebkal, K.G., Krasnodubets, L.A., Eds.; IP Kulikov: Sevastopol, Russia, 2021; p. 41. (In Russian) [Google Scholar]
- Berezina, N.A.; Sharov, A.N.; Chernova, E.N.; Malysheva, O.A. Effects of diclofenac on the reproductive health, respiratory rate, cardiac activity, and heat tolerance of aquatic animals. Environ. Toxicol. Chem. 2022, 41, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Kovatcheva, N.P.; Kholodkevitch, S.V.; Vasilyev, R.M.; Ivanov, A.V.; Zagorsky, I.A.; Kornienko, E.L. Real time assessment of red king crabs functional status by measuring of its cardioactivity. Probl. Fish. 2008, 9, 513–517. (In Russian) [Google Scholar]
- Kovacheva, N.P.; Kholodkevich, S.V.; Vasilyev, R.M.; Zagorsky, I.A.; Ivanov, A.V.; Kornienko, E.L. Red king crab functional state assessment by non-invasive real-time control of their cardiac activity in aquaculture. Izv. TINRO 2009, 157, 197–205. (In Russian) [Google Scholar]
- Gosling, E. Bivalve Molluscs Biology, Ecology and Culture; Fishing News Books: Oxford, UK, 2003. [Google Scholar]
- Mallet, A.L.; Carver, C.E. Comparative growth and survival patterns of Mytilus trossulus and Mytilus edulis in Atlantic Canada. Can. J. Fish. Aquat. Sci. 1995, 52, 1873–1880. [Google Scholar]
- Seed, R.; Suchanek, T.H. Population and community ecology of Mytilus. In The Mussel Mytilus: Ecology, Physiology, Genetics and Culture; Gosling, E.M., Ed.; Elsevier: Amsterdam, The Netherlands, 1992; pp. 87–169. [Google Scholar]
- Gudimov, A.V. Research of mussels in the Barents Sea: From theory to practice. In Formation of the Foundations of a Modern Strategy of Environmental Management in the Euro-Arctic Region; Vinogradov, A.N., Ed.; Kola Scientific Center of the Russian Academy of Sciences: Apatity, Russia, 2005; pp. 304–315. (In Russian) [Google Scholar]
- Matveeva, T.A. The biology of Mytilus edulis L. in the Eastern Murman. Tr. Murm. Mar. Biol. Stn. USSR 1948, 1, 215–241. (In Russian) [Google Scholar]
- Thompson, R.J. Fecundity and reproductive effort in the blue mussel (Mytilus edulis), the sea urchin (Strongylocentrotus droebachiensis), and the snow crab (Chionoecetes opilio) from populations in Nova Scotia and Newfoundland. J. Fish. Res. Bd. Can. 1979, 36, 955–964. [Google Scholar] [CrossRef]
- Gudimov, A.V. Blue mussels Mytilus edulis L. In Harvesting and Perspective for Uses Algae and Invertebrates of the Barents and White Seas; Matishov, G.G., Ed.; KSC RAS Press: Apatity, Russia, 1998; pp. 529–580. (In Russian) [Google Scholar]
- Lande, E. Growth, spawning and mortality of the mussel (Mytilus edulis L.) in Prestvaagen, Trondheimsfjorden. Det Kgl. Nor. Vidensk. Selsk. Mus. Misc. 1973, 11, 1–26. [Google Scholar]
- Kuznetsov, V.V.; Matveeva, T.A. Materials on the biological characteristics of marine invertebrates in the Eastern Murman. Tr. Murm. Mar. Biol. Stn. USSR 1948, 1, 242–260. (In Russian) [Google Scholar]
- Mathiesen, S.S.; Thyrring, J.; Hemmer-Hansen, J.; Berge, J.; Sukhotin, A.; Leopold, P.; Bekaert, M.; Sejr, M.K.; Nielsen, E.E. Genetic diversity and connectivity within Mytilus spp. in the subarctic and Arctic. Evol. Appl. 2017, 10, 39–55. [Google Scholar] [CrossRef]
- Wonham, M.J. Mini-review: Distribution of the Mediterranean mussel Mytilus galloprovincialis (Bivalvia: Mytilidae) and hybrids in the Northeast Pacific. J. Shellfish Res. 2004, 23, 535–543. [Google Scholar]
- Grant, W.S.; Cherry, M.I. Mytilus galloprovincialis Lmk. in Southern Africa. J. Exp. Mar. Biol. Ecol. 1985, 90, 179–191. [Google Scholar] [CrossRef]
- Lutaenko, K.A.; Kolpakov, E.V. The extension of the distributional range of an invasive mussel, Mytilus galloprovincialis (Bivalvia: Mytilidae) in the Sea of Japan. Bull. Russ. Far East Malacol. Soc. 2016, 20, 57–76. (In Russian) [Google Scholar]
- Gosling, E.M. The systematic status of Mytilus galloprovincialis in Western Europe: A review. Malacologia 1984, 55, 551–568. [Google Scholar]
- Ivanov, V.N.; Kholodov, V.I.; Senicheva, M.I.; Pirkova, A.V.; Bulatov, K.V. Biology of Cultivated Mussels; Naukova Dumka: Kiev, Russia, 1989. (In Russian) [Google Scholar]
- Zaika, V.E.; Valovaya, N.A.; Povchun, A.C.; Revkov, N.K. Mytilids of the Black Sea; Naukova Dumka: Kiev, Russia, 1989. (In Russian) [Google Scholar]
- Chertok, A.I.; Belous, K.A.; Sytnik, N.A. Current state of natural mussel populations in the Black Sea. In Education, Science and youth—2018, Proceedings of the Scientific and Practical Conferences of the KSMTU, Kerch, Russia, 2–13 April 2018; Masyutkin, E.P., Ed.; Kerch State Marine Technological University: Kerch, Russia, 2018; pp. 67–77. (In Russian) [Google Scholar]
- Chelyadina, N.S.; Popov, M.A. Mortality of the mussel Mytilus galloprovincialis (Lamark, 1819) depending on sex. Tomsk State Univ. J. Biol. 2021, 55, 166–176. [Google Scholar]
- Pirkova, A.V. Reproduction, embryogenesis, larval development and growth of Mytilus galloprovincialis. In Mariculture of Mussel on the Black Sea; Ivanov, V.N., Ed.; EKOSI-Gidrofizika: Sevastopol, Ukraine, 2007; pp. 138–167. (In Russian) [Google Scholar]
- Sytnik, N.A.; Zolotnitsky, A.P. State of natural populations and dynamics of the number of mussel larvae in the Kerch Strait and the Black Sea Pre-strait. Vestn. Kerch State Mar. Technol. Univ. 2020, 1, 53–68. (In Russian) [Google Scholar]
- Gorbunova, T.L.; Basharova, M.P.; Matova, N.I. Morphometric characteristics of Black Sea mussels Mytilus galloprovincialis Lam. as biomarkers of the anthropogenic impact on the Black Sea coastal biocenoses in tourist destinations. Amur. Zool. J. 2022, 14, 516–530. (In Russian) [Google Scholar]
- Pospelova, N.V.; Bulycheva, D.S.; Ryabushko, L.I. Microalgae in the nutrition spectrum of cultivated mussels (Crimea, Black Sea). In Proceedings of the V International Scientific and Practical Conference “Marine Research and Education (MARESEDU-2016)”, Moscow, Russia, 18–21 October 2016; Feoria: Moscow, Russia, 2016; pp. 434–438. (In Russian). [Google Scholar]
- Priymak, A.S.; Pospelova, N.V. On the feeding of the mussel Mytilus galloprovincialis Lamarck, 1819. In Study of Aquatic and Terrestrial Ecosystems: History and Contemporary State, Proceedings of the 2nd International Academic Conference, Sevastopol, Russia, 5–9 September 2022; IBSS: Sevastopol, Russia, 2022; pp. 134–135. (In Russian) [Google Scholar]
- Garcia, E.G. The fishery for Iceland scallop (Chlamys islandica) in the Northeast Atlantic. Adv. Mar. Biol. 2006, 51, 1–55. [Google Scholar] [PubMed]
- Duncan, P.F.; Brand, A.R.; Strand, Ø.; Foucher, E. The European scallop fisheries for Pecten maximus, Aequipecten opercularis, Chlamys islandica, and Mimachlamys varia. In Scallops Biology, Ecology, Aquaculture, and Fisheries, 3rd ed.; Shumway, S.E., Parsons, G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 781–858. [Google Scholar]
- Denisenko, S.G. Ecology and Resources of the Iceland Scallop in the Barents Sea; KSC RAS Press: Apatity, Russia, 1989. (In Russian) [Google Scholar]
- Denisenko, S.G. Temperature conditions and spawning periods of the Iceland scallop in the Eastern Murmansk coast. In Studies of the Biology, Morphology and Physiology of Hydrobionts; Matishov, G.G., Ed.; MMBI Press: Apatity, Russia, 1983; pp. 90–94. (In Russian) [Google Scholar]
- Zolotarev, P.N. Biology and Fishery of the Icelandic Scallop Chlamys Islandica in the Barents and White Seas; PINRO Press: Murmansk, Russia, 2016. (In Russian) [Google Scholar]
- Gruffydd, L.D. Swimming in Chlamys islandica in relation to current speed and an investigation of hydrodynamic lift in this and other scallops. Norw. J. Zool. 1976, 24, 365–378. [Google Scholar]
- Zolotarev, P.N. Shell morphometry of the Icelandic scallop (Chlamys islandica, Pectinidae, Bivalvia) from the Barents and White Seas. Zool. Zh. 2010, 89, 1200–1204. (In Russian) [Google Scholar]
- Bogolubov, A.S. Computer Digital Atlas-Guide of Freshwater Invertebrates of Russia. 2018. Available online: http://ecosystema.ru/04materials/guides/10water.htm (accessed on 12 September 2022). (In Russian).
- Aldridge, D.C.; McIvor, A.L. Gill evacuation and release of glochidia by Unio pictorum and Unio tumidus (Bivalvia: Unionidae) under thermal and hypoxic stress. J. Mollusc. Stud. 2003, 69, 55–59. [Google Scholar]
- Vaughn, C.C.; Nichols, S.J.; Spooner, D.E. Community and foodweb ecology of freshwater mussels. J. N. Am. Benthol. Soc. 2008, 27, 409–423. [Google Scholar] [CrossRef]
- Graf, D.L.; Cummings, K.S. Review of the systematics and global diversity of freshwater mussel species (Bivalvia: Unionoida). J. Mollusc. Stud. 2007, 73, 291–314. [Google Scholar] [CrossRef]
- Tomilova, A.A. Morphological Variability and Phylogeography of the Duck Mussel Anodonta Anatina in Russia and Adjacent Territories. Ph.D. Thesis, FIC KIA RAS, Arkhangelsk, Russia, 2021. (In Russian). [Google Scholar]
- Schultes, F.W. Species Summary for Anodonta anatine. 2013. Available online: http://www.animalbase.uni-goettingen.de/zooweb/servlet/AnimalBase/home/species?id=2118 (accessed on 12 September 2022).
- Hinzmann, M.; Lopes-Lima, M.; Teixeira, A.; Varandas, S.; Sousa, R.; Lopes, A.; Froufe, E.; Machado, J. Sexual strategy and reproductive cycle of Anodonta anatina (L., 1758): Notes on hermaphroditism. J. Exp. Zool. A 2013, 309, 378–390. [Google Scholar]
- Strayer, D.L. Freshwater Mussel Ecology. A Multifactor Approach to Distribution and Abundance; Freshwater Ecological Series; University of California Press: Berkely, CA, USA, 2008. [Google Scholar]
- Lima, P.; Carvalho, F.; Vasconcelos, V.; Machado, J. Studies on growth in the early adult of the freshwater mussel, Anodonta cygnea. Invertebr. Reprod. Dev. 2004, 45, 117–125. [Google Scholar] [CrossRef]
- Aldridge, D.C. The morphology, growth and reproduction of Unionidae (Bivalvia) in a fenland waterway. J. Mollusc. Stud. 1999, 65, 47–60. [Google Scholar]
- Rizhinashvili, A.L. Determination of the Maximum lifespan of bivalves as exemplified by Unio-like mussels (Bivalvia, Unionidae). Dokl. Biol. Sci. 2009, 424, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Kryger, J.; Riisgård, H.U. Filtration rate capacities in 6 species of European freshwater bivalves. Oecologia 1988, 77, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Holdich, D.M.; Pöckl, M. Invasive crustaceans in European inland waters. In Biological Invaders in Inland Waters: Profiles, Distribution and Threats; Invading Nature—Springer Series in Invasion Ecology; Gherardi, F., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 29–75. [Google Scholar]
- Berezina, N.A.; Terentiev, P.M.; Sharov, A.N.; Maximov, A.A. New records and disappearance from old sites of narrow-clawed crayfish Pontastacus leptodactylus in northwestern Russia. BioInvas. Rec. 2021, 10, 894–903. [Google Scholar] [CrossRef]
- Skurdal, J.; Taugbøl, T. Astacus. In Biology of Freshwater Crayfish; Holdich, D.M., Ed.; Blackwell Science: Oxford, UK, 2002; pp. 467–510. [Google Scholar]
- Füreder, L. Crayfish in Europe. Biogeography, ecology and conservation. In Freshwater Crayfish: A Global Overview; Faulkes, Z., Kawai, T., Scholtz, G., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 594–697. [Google Scholar]
- Souty-Grosset, C.; Holdich, D.M.; Noël, P.Y.; Reynolds, J.D.; Haffner, P. Atlas of Crayfish in Europe; Museum National D’Histoire Naturelle: Paris, France, 2006. [Google Scholar]
- Alexandrov, B.M. About crayfish of Karelia. Proc. Karelian Branch GosNIORKH 1966, 4, 188–209. (In Russian) [Google Scholar]
- Kuzmin, S.A.; Gudimova, E.N. Introduction of the Kamchatka (Red King) Crab in the Barents Sea: Peculiarities of Biology, Perspectives of Fishery; KSC RAS Press: Apatity, Russia, 2002. (In Russian) [Google Scholar]
- Dvoretsky, A.G.; Bichkaeva, F.A.; Baranova, N.F.; Dvoretsky, V.G. Fatty acid composition of the Barents Sea red king crab (Paralithodes camtschaticus) leg meat. J. Food Compos. Anal. 2021, 98, 103826. [Google Scholar]
- Dvoretsky, A.G.; Bichkaeva, F.A.; Baranova, N.F.; Dvoretsky, V.G. Fatty acids in the circulatory system of an invasive king crab from the Barents Sea. J. Food Compos. Anal. 2022, 110, 104528. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Red king crab (Paralithodes camtschaticus) fisheries in Russian waters: Historical review and present status. Rev. Fish Biol. Fish. 2018, 28, 331–353. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. New echinoderm-crab epibiotic associations from the coastal Barents Sea. Animals 2021, 11, 917. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Distribution of amphipods Ischyrocerus on the red king crab, Paralithodes camtschaticus: Possible interactions with the host in the Barents Sea. Estuar. Coast. Shelf Sci. 2009, 82, 390–396. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Some aspects of the biology of the amphipods Ischyrocerus anguipes associated with the red king crab, Paralithodes camtschaticus, in the Barents Sea. Polar Biol. 2009, 32, 463–469. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Commercial fish and shellfish in the Barents Sea: Have introduced crab species affected the population trajectories of commercial fish? Rev. Fish Biol. Fish. 2015, 25, 297–322. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Epibiotic communities of common crab species in the coastal Barents Sea: Biodiversity and infestation patterns. Diversity 2022, 14, 6. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Renewal of the recreational red king crab fishery in Russian waters of the Barents Sea: Potential benefits and costs. Mar. Policy 2022, 136, 104916. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Inter-annual dynamics of the Barents Sea red king crab (Paralithodes camtschaticus) stock indices in relation to environmental factors. Polar Sci. 2016, 10, 541–552. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Effects of environmental factors on the abundance, biomass, and individual weight of juvenile red king crabs in the Barents Sea. Front. Mar. Sci. 2020, 7, 726. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Epibionts of an introduced king crab in the Barents Sea: A second five-year study. Diversity 2023, 15, 29. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Population dynamics of the invasive lithodid crab, Paralithodes camtschaticus, in a typical bay of the Barents Sea. ICES J. Mar. Sci. 2013, 70, 1255–1262. [Google Scholar] [CrossRef]
- Matyushkin, V.B. Peculiarities of reproduction of the red king crab in fjord waters of the western Murman. In The Red King Crab in the Barents Sea; Berenboim, B.I., Ed.; PINRO Press: Murmansk, Russia, 2003; pp. 88–100. (In Russian) [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Size at maturity of female red king crab, Paralithodes camtschaticus, from the costal zone of Kola Peninsula (southern Barents Sea). Cah. Biol. Mar. 2015, 56, 49–54. [Google Scholar]
- Dvoretsky, A.G.; Tipisova, E.V.; Elfimova, A.E.; Alikina, V.A.; Dvoretsky, V.G. Sex hormones in hemolymph of red king crabs from the Barents Sea. Animals 2021, 11, 2149. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Tipisova, E.V.; Alikina, V.A.; Elfimova, A.E.; Dvoretsky, V.G. Thyroid hormones in hemolymph of red king crabs from the Barents Sea. Animals 2022, 12, 379. [Google Scholar] [CrossRef]
- Bakanev, S.V. Fecundity and some other reproductive parameters of red king crab in the Barents Sea. In The Red King Crab in the Barents Sea; Berenboim, B.I., Ed.; PINRO Press: Murmansk, Russia, 2003; pp. 78–88. (In Russian) [Google Scholar]
- Bakanev, S.V. Larvae of red king crab in the coastal areas and large bays of Murman. In The Red King Crab in the Barents Sea; Berenboim, B.I., Ed.; PINRO Press: Murmansk, Russia, 2003; pp. 122–133. (In Russian) [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Ecology and distribution of red king crab larvae in the Barents Sea: A review. Water 2022, 14, 2328. [Google Scholar]
- Dvoretsky, A.G.; Bichkaeva, F.A.; Baranova, N.F.; Dvoretsky, V.G. Fatty acid profiles in the gonads of red king crab (Paralithodes camtschaticus) from the Barents Sea. Animals 2023, 13, 336. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Hemolymph molting hormone concentrations in red king crabs from the Barents Sea. Polar Biol. 2010, 33, 1293–1298. [Google Scholar]
- Pinchukov, M.A.; Berenboim, B.I. Molting and growth of red king crab in the Barents Sea. In The Red King Crab in the Barents Sea; Berenboim, B.I., Ed.; PINRO Press: Murmansk, Russia, 2003; pp. 100–106. (In Russian) [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Size-at-age of juvenile red king crab (Paralithodes camtschaticus) in the coastal Barents Sea. Cah. Biol. Mar. 2014, 55, 43–48. [Google Scholar]
- Pavlova, L.V.; Dvoretsky, A.G. Prey selectivity in juvenile red king crabs from the coastal Barents Sea. Diversity 2022, 14, 568. [Google Scholar] [CrossRef]
- Pavlova, L.V.; Zuyev, Y.A.; Dvoretsky, A.G. Shallow-water benthic communities on soft bottoms of a sub-arctic fjord (southern Barents Sea, Russia) along a gradient of ecological factors. Diversity 2023, 15, 84. [Google Scholar] [CrossRef]
- Evseeva, O.Y.; Ishkulova, T.G.; Dvoretsky, A.G. Environmental drivers of an intertidal bryozoan community in the Barents Sea: A case study. Animals 2022, 12, 552. [Google Scholar]
- Gudimov, A.V. Behavior of blue mussels (Mytilus edulis L.) under the fluctuating environmental conditions. Dokl. Biol. Sci. 2006, 409, 314–316. [Google Scholar] [CrossRef]
- Gudimov, A.V. Continuous biomonitoring of behavioral responses of mussels: The first experience in the Kola Bay (Barents Sea). Dokl. Biol. Sci. 2011, 439, 218–220. [Google Scholar] [CrossRef]
- Bakhmet, I.N.; Zdorovenov, P.E. Variability in cardiac activity of the bivalves Mytilus edulis and Modiolus modiolus. Russ. J. Mar. Biol. 2010, 36, 223–225. [Google Scholar] [CrossRef]
- Gudimov, A.V. Cardiac activity and behavior of blue mussels from the Barents Sea under controlled conditions. Dokl. Biol. Sci. 2009, 427, 335–338. [Google Scholar] [PubMed]
- Bakhmet, I.N. Cardiac activity and oxygen consumption of blue mussels (Mytilus edulis) from the White Sea in relation to body mass, ambient temperature and food availability. Polar Biol. 2017, 40, 1959–1964. [Google Scholar]
- Bakhmet, I.N.; Sazhin, A.; Maximovich, N.; Ekimov, D. In situ long-term monitoring of cardiac activity of two bivalve species from the White Sea, the blue mussel Mytilus edulis and horse mussel Modiolus Modiolus. J. Mar. Biol. Assoc. UK 2019, 99, 833–840. [Google Scholar] [CrossRef]
- Andrade, H.; Massabuau, J.-C.; Cochrane, S.; Ciret, P.; Tran, D.; Sow, M.; Camus, L. High frequency non-invasive (HFNI) bio-sensors as a potential tool for marine monitoring and assessments. Front. Mar. Sci. 2016, 3, 187. [Google Scholar] [CrossRef]
- Bahmet, I.N.; Berger, V.J.; Halaman, V.V. Heart rate in the blue mussel Mytilus edulis (Bivalvia) under salinity change. Russ. J. Mar. Biol. 2005, 31, 314–317. [Google Scholar] [CrossRef]
- Bakhmet, I.N. Characteristic property of blue mussel Mytilus edulis L. adaptation to pollutants. Proc. Petrozavodsk. State Univ. 2013, 8, 17–19. (In Russian) [Google Scholar]
- Sharov, A.N.; Kholodkevich, S.V. Heart activity in the littoral and sublittoral White Sea blue mussels Mytilus edulis. In Marine Biological Research: Achievements and Perspectives, Proceedings of All-Russian Scientific-Practical Conference with International Participation Dedicated to the 145th Anniversary of Sevastopol Biological Station, Sevastopol, Russia, 19–24 September 2016; Gaevskaya, A.V., Ed.; EKOSI-Gidrofizika: Sevastopol, Russia, 2016; Volume 3, pp. 346–349. (In Russian) [Google Scholar]
- Chuiko, G.M.; Kholodkevich, S.V.; Sharov, A.N.; Kuznetsova, T.V.; Kurakin, A.S. The reaction of the cellular system of antioxidant protection, cardioactivity and motor activity of the White Sea mussel (Mytilus edulis Linnaeus, 1758) to a short-term decrease in water salinity. In Study of Aquatic and Terrestrial Ecosystems: History and Contemporary State, Proceedings of the International Scientific Conference Dedicated to the 150th Anniversary of the Sevastopol Biological Station—A. O. Kovalevsky Institute of Biology of the Southern Seas and to the 45th Anniversary of Research Vessel “Professor Vodyanitsky”, Sevastopol, Russia, 13–18 September 2021; Egorov, V.N., Gorbunov, R.V., Eds.; IBSS RAS: Sevastopol, Russia, 2021; pp. 448–449. (In Russian) [Google Scholar]
- Hamilton, P.V.; Winter, M.A.; Pegg, R.K. Effects of whole drilling mud and selected components on the shell movements of the bay scallop, Argopecten irradians. Gulf Mex. Sci. 1981, 5, 13–20. [Google Scholar] [CrossRef]
- Bakhmet, I.N.; Fokina, N.N.; Nefedova, Z.A.; Ruokolainen, T.R.; Nemova, N.N. Blue mussels Mytilus edulis L. in the White Sea as bioindicators under diluted oil impact. Proc. Karelian Sci. Cent. Russ. Acad. Sci. 2012, 2, 38–46. (In Russian) [Google Scholar]
- Bakhmet, I.N.; Kantserova, N.P.; Lysenko, L.A.; Nemova, N.N. Effect of copper and cadmium ions on heart function and calpain activity in blue mussel Mytilus edulis. J. Environ. Sci. Health A 2012, 47, 1528–1535. [Google Scholar]
- Curtis, T.M.; Williamson, R.; Depledge, M.H. Simultaneous, long-term monitoring of valve and cardiac activity in the blue mussel Mytilus edulis exposed to copper. Mar. Biol. 2000, 136, 837–846. [Google Scholar]
- Bakhmet, I.N.; Ekimov, D.A. Effect of nikel ions on cardiac activity in the blue mussel Mytilus edulis Linnaeus, 1758. Proc. Karelian Sci. Cent. Russ. Acad. Sci. 2020, 11, 64–69. (In Russian) [Google Scholar]
- Gudimov, A.V. Ecological biomonitoring of aquatic ecosystems: On the way to the newest technologies. In Marine Ecosystems and Communities in the Conditions of Current Climatic Changes; Matishov, G.G., Ed.; Renome: St. Petersburg, Russia, 2014; pp. 326–344. (In Russian) [Google Scholar]
- Gudimov, A.V. Methods of online biosensor monitoring and online bioindication: Responses to toxicants on mussel behavior and cardiac activity. In Proceedings of the VII International Conference “Marine Research and Education”, Moscow, Russia, 19–22 November 2018; OOO PolyPRESS: Tver, Russia, 2019; pp. 385–392. (In Russian). [Google Scholar]
- Trusevich, V.V.; Kuz’min, K.A.; Mishurov, V.Z.; Zhuravsky, V.Y.; Vyshkvarkova, E.V. Features of behavioral responses of the Mediterranean mussel in its natural habitat of the Black Sea. Inland Water Biol. 2021, 14, 10–19. [Google Scholar] [CrossRef]
- Zhuravsky, V.Y.; Voskresenskaya, E.N.; Trusevich, V.V.; Lubkov, A.S. Data analysis for automation of aquatic biomonitoring in the Black Sea region. Environ. Control Syst. 2019, 4, 66–71. (In Russian) [Google Scholar]
- Gnyubkin, V.F. The circadian rhythms of valve movements in the mussel Mytilus galloprovincialis. Russ. J. Mar. Biol. 2010, 36, 419–428. [Google Scholar]
- Vyshkvarkova, E.V.; Trusevich, V.V.; Kuzmin, K.A.; Mishurov, V.Z.; Zhuravsky, V.Y. Features of behavioral reactions of the Black Sea mussel. In Comprehensive Research of the World Ocean, Proceedings of the V All-Russian Scientific Conference of Young Scientists, Kaliningrad, Russia, 18–22 May 2020; Mevedev, I.P., Ed.; Atlantic Branch of the Federal State Budgetary Institution of Science “P.P. Shirshov Institute of Oceanology of the Russian Academy of Sciences”: Kaliningrad, Russia, 2020; pp. 241–242. (In Russian) [Google Scholar]
- Comeau, L.A.; Babarro, J.M.; Longa, A.; Padin, X.A. Valve-gaping behavior of raft-cultivated mussels in the Ría de Arousa, Spain. Aquac. Rep. 2018, 9, 68–73. [Google Scholar]
- Kholodkevich, S.V.; Ivanov, A.V.; Kuznetsova, T.V.; Kurakin, A.S.; Kornienko, E.L.; Khalatov, A.N.; Pan’Kov, S.L. Ultradian rhythms in cardiac activity of Bivalvia. Dokl. Biol. Sci. 2009, 426, 216–218. [Google Scholar] [CrossRef]
- Kazankova, I.I.; Gaisky, P.V.; Kazantsev, S.V. Individual variability of biorhythmic elements of Mytilus galloprovincialis mussels under experimental conditions. In Terrestrial and Marine Ecosystems of the Black Sea Region and Their Protection, Proceedings of the Scientific and Practical School-Conference, Novorossiysk, Russia, 23–27 April 2018; Bykhalova, O.N., Korobushkin, D.I., Marin, I.N., Maslova, V.N., Skuratovskaya, E.N., Eds.; Institute of Natural and Technical Systems: Sevastopol, Russia, 2018; pp. 53–54. (In Russian) [Google Scholar]
- Kazankova, I.I.; Gaisky, P.V.; Kazantsev, S.V. Variability of the level of opening of the flaps of adult mussels Mytilus galloprovincialis lam. in laboratory conditions. In Environmental Control Systems—2018, Proceedings of the International Scientific and Technical Conference, Sevastopol, Russia, 5–9 November 2018; Bardin, M.Y., Voskresenskaya, E.N., Vyshkvarkova, E.V., Gaisky, V.A., Gaisky, P.V., Grekov, N.A., Grekov, A.N., Eds.; Kolorit: Sevastopol, Russia, 2018; p. 114. (In Russian) [Google Scholar]
- Gudimov, A.V. Marine mussels of the Karadag (Black Sea): Population decay, ecology, and physiological adaptations. Dokl. Biol. Sci. 2008, 422, 330–332. [Google Scholar]
- Ait Fdil, M.; Mouabad, A.; Outzourhit, A.; Benhra, A.; Maarouf, A.; Pihan, J.C. Valve movement response of the mussel Mytilus galloprovincialis to metals (Cu, Hg, Cd and Zn) and phosphate industry effluents from Moroccan Atlantic coast. Ecotoxicology 2006, 15, 477–486. [Google Scholar] [CrossRef]
- Vyshkvarkova, E.V.; Trusevich, V.V.; Grekov, A.N.; Kuzmin, K.A.; Mishurov, V.Z.; Zhuravsky, V.Y. Biomarkers of behavioral reactions of mollusks in automated biomonitoring systems in conditions of pollution of the aquatic environment with petroleum hydrocarbons and drilling sludge. In Comprehensive Research of the World Ocean, Proceedings of the VI All-Russian Scientific Conference of Young Scientists, Moscow, Russia, 18–24 April 2021; Alekseev, D.A., Ed.; IO RAN: Moscow, Russia, 2021; pp. 242–243. (In Russian) [Google Scholar]
- Martinovic, R.; Gacic, Z.; Kljajic, Z. The influence of oil, dispersed oil and the oil dispersant sd-25, on the heart rate of the Mediterranean Mussel (Mytilus galloprovincialis L.). In Sustainable Development of Sea-Corridors and Coastal Waters; Stylios, C., Floqi, T., Marinski, J., Damiani, L., Eds.; Springer: Cham, Switzerland, 2015; pp. 21–27. [Google Scholar]
- Guterres, B.D.V.; Guerreiro, A.D.S.; Botelho, S.S.D.C.; Sandrini, J.Z. Perna perna mussels network as pollution biosensors of oil spills and derivatives. IFAC Pap. 2020, 53, 16727–16732. [Google Scholar]
- Da Silveira Guerreiro, A.; de Vargas Guterres, B.; Costa, P.G.; Bianchini, A.; da Costa Botelho, S.S.; Sandrini, J.Z. Combined physiological and behavioral approaches as tools to evaluate environmental risk assessment of the water accommodated-fraction of diesel oil. Aquat. Toxicol. 2022, 249, 106230. [Google Scholar]
- Miserazzi, A.; Sow, M.; Gelber, C.; Charifi, M.; Ciret, P.; Dalens, J.M.; Weber, C.; Le Floch, S.; Lacroix, C.; Blanc, P.; et al. Asiatic clam Corbicula fluminea exhibits distinguishable behavioural responses to crude oil under semi-natural multiple stress conditions. Aquat. Toxicol. 2020, 219, 105381. [Google Scholar] [CrossRef] [PubMed]
- Axiak, V.; George, J.J. Behavioral responses of a marine bivalve (Venus verrucosa) to pollution by petroleum hydrocarbons. Water Air Soil Pollut. 1987, 35, 395–410. [Google Scholar]
- Ait Ayad, M.; Ait Fdil, M.; Mouabad, A. Effects of Cypermethrin (pyrethroid insecticide) on the valve activity behavior, byssal thread formation, and survival in air of the marine mussel Mytilus galloprovincialis. Arch. Environ. Contam. Toxicol. 2011, 60, 462–470. [Google Scholar] [PubMed]
- Sigacheva, T.B.; Chesnokova, I.I.; Gostyukhina, O.L.; Kholodkevich, S.V.; Kuznetsova, T.V.; Andreenko, T.I.; Kovrigina, N.P.; Gavruseva, T.V.; Kirin, M.P.; Kurakin, A.S. Assessment of recreational potential of Sevastopol bays using bioindication methods. South Russ. Ecol. Dev. 2021, 16, 151–167. (In Russian) [Google Scholar]
- Nikolic, M.; Kuznetsova, T.; Kholodkevich, S.; Gvozdenovic, S.; Mandic, M.; Joksimovic, D.; Teodorovic, I. Cardiac activity in the Mediterranean mussel (Mytilus galloprovincialis Lamarck, 1819) as a biomarker for assessing sea water quality in Boka Kotorska Bay, South Adriatic Sea. Mediterr. Mar. Sci. 2019, 20, 680–687. [Google Scholar]
- Gudimov, A.V. The first records of behavioral responses of the bivalves, Icelandic scallop Chlamys islandica and horse mussel Modiolus modiolus. Vestn. KamchatGTU 2012, 20, 50–55. (In Russian) [Google Scholar]
- Evseeva, O.Y.; Dvoretsky, A.G. Shallow-water bryozoan communities in a glacier fjord of West Svalbard, Norway: Species composition and effects of environmental factors. Biology 2023, 12, 185. [Google Scholar]
- Gudimov, A.V. The behavior of Svalbard bivalves in controlled conditions. Vestn. KamchatGTU 2012, 22, 77–82. (In Russian) [Google Scholar]
- Gudimov, A.V. Behavioral research of the bivalve Chlamys islandica in the Grønfjord (West Spitsbergen). In Complex Investigations of Spitsbergen and Offshore Nature, Proceedings of the XIV Scientific Conference with International Participation, Murmansk, Russia, 30 October 2018–2 November 2018; Makarevich, P.R., Ed.; FRC Kola Science Centre RAS: Apatity, Russia, 2018; pp. 27–28. (In Russian) [Google Scholar]
- Sharov, A.; Kholodkevich, S. Some features of using heart rate monitoring of freshwater bivalve molluscs with a fiber-optical method for ecotoxicological research. Principy Èkologii 2015, 4, 21–28. (In Russian) [Google Scholar] [CrossRef]
- Smirnov, I.S.; Sharov, A.N.; Kholodkevich, S.V. Cardioactivity features of bivalve mollusks of the Gulf of Finland. Reg. Ecol. 2017, 2, 35–39. (In Russian) [Google Scholar]
- Kholodkevich, S.V.; Sharov, A.N.; Kuznetsova, T.V.; Chuiko, G.M.; Gapeeva, M.V.; Lozhkina, R.A. Quality assessment of freshwater ecosystems by the functional state of bivalved mollusks. Water Res. 2019, 46, 249–257. [Google Scholar] [CrossRef]
- Kholodkevich, S.V.; Motruk, M.K.; Lyubimtsev, V.A.; Susloparova, O.N. Comparative bioelectronic diagnostics of the ecological state of contaminated water areas (on the example of some ducts of the Volga River Delta). Pharm. Formulas 2021, 3, 84–91. (In Russian) [Google Scholar]
- Kholodkevich, S.V.; Kuznetsova, T.V.; Lyubimtsev, V.A.; Susloparova, O.N. Assessment of the ecosystem health of coastal waters of the eastern Gulf of Finland (case study of recreational waters quality of the Kurortny district of St. Petersburg) based on the study of the functional state of the mollusks living in them. In Proceedings of the XXI International Environmental Forum «Baltic Sea Day», St. Petersburg, Russia, 23–24 March 2021; OOO Svoe Izdatelstvo: St. Petersburg, Russia, 2021; pp. 30–32. [Google Scholar]
- Verbitsky, V.B.; Sharov, A.N.; Kholodkevich, S.V. Determination of the bivalve Unio pictorum critical temperature maximum by cardioactivity. Trans. IBIW 2020, 89, 50–57. [Google Scholar]
- Kuznetsova, T.V.; Kholodkevich, S.V.; Manvelova, A.B.; Frumin, G.T. Some problems and approaches for their solution in searching reference sites and reference values in assessing the ecological state of aquatoria in the Eastern Gulf of Finland. Reg. Ecol. 2019, 3, 102–114. (In Russian) [Google Scholar]
- Kuznetsova, T.V.; Kholodkevich, S.V.; Manvelova, A.B. Searching for reference sites and reference functional indicators for a comparative assessment of the ecological state of water areas on the basis of heart rate of bivalve mollusks of the family Unionidae (Mollusca, Bivalvia) and crustaceans (Crustacea, Decapoda). In Anthropogenic Impact on Aquatic Organisms and Ecosystems, Proceedings of the VII All-Russian Conference on Aquatic Ecotoxicology Dedicated to the Memory of Doctor of Biological Sciences, Professor, B.A. Flerov. Modern Methods of Research and Assessment of Water Quality, the State of Aquaticorganisms and Ecosystems under Anthropogenic Stress: Materials of the School-Seminar for Young Scientists, Graduate Students and Students, Borok, Russia, 16–19 September 2020; Filigran: Yaroslavl, Russia, 2020; pp. 112–115. (In Russian) [Google Scholar]
- Trusevich, V.V.; Mishurov, V.J.; Kuzmin, K.A. System of the continuous quality control of water and the warning of threats of ecological danger automated in real time on water intakes of city water supply. In Environmental, Industrial and Energy Security—2018, Proceedings of the International Scientific and Practical Conference, Sevastopol, Russia, 24–27 September 2018; Lukina, L.I., Ed.; Sevastopol State University: Sevastopol, Russia, 2018; pp. 1177–1181. (In Russian) [Google Scholar]
- Jou, L.J.; Chen, B.C.; Chen, W.Y.; Liao, C.M. Sensory determinants of valve rhythm dynamics provide in situ biodetection of copper in aquatic environments. Environ. Sci. Pollut. Res. 2016, 23, 5374–5389. [Google Scholar]
- Gaisky, P.V.; Stepanova, O.A. Behavioral responses of freshwater bivalve mussel Unio pictorum to a number of common abiotic chemical contaminants. Monitor. Syst. Environ. 2020, 2, 87–96. (In Russian) [Google Scholar]
- Berezina, N.A.; Verbitsky, V.B.; Sharov, A.N.; Chernova, E.N.; Meteleva, N.Y.; Malysheva, O.A. Biomarkers in bivalve mollusks and amphipods for assessment of effects linked to cyanobacteria and elodea: Mesocosm study. Ecotoxicol. Environ. Saf. 2020, 203, 110994. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Haberkorn, H.; Soudant, P.; Ciret, P.; Massabuau, J.C. Behavioral responses of Crassostrea gigas exposed to the harmful algae Alexandrium minutum. Aquaculture 2010, 298, 338–345. [Google Scholar] [CrossRef]
- Kholodkevich, S.V.; Kuznetsova, T.V.; Kirin, M.P.; Smirnov, I.S.; Rudakova, O.A.; Lyubimtsev, V.A.; Manvelova, A.B.; Susloparova, O.N.; Perelygin, V.V.; Sakharova, O.A. Bioindication of the ecological state (health) of coastal waters based on the use of automated bioelectronic systems. Pharm. Formulas 2020, 2, 64–73. [Google Scholar]
- Kholodkevich, S.V.; Rudakova, O.A.; Kuznetsova, T.V.; Manvelova, A.B.; Susloparova, O.N. Ranking of the ecosystem state of water areas on the basis of operational testing of the functional state of bivalve mollusks inhabiting them (on the example of recreational water areas of the Kurortny District of St. Petersburg). In Anthropogenic Impact on Aquatic Organisms and Ecosystems, Proceedings of the VII All-Russian Conference on Aquatic Ecotoxicology Dedicated to the Memory of Doctor of Biological Sciences, Professor B.A. Flerov. Modern Methods of Research and Assessment of Water Quality, the State of Aquaticorganisms and Ecosystems under Anthropogenic Stress: Materials of the School-Seminar for Young Scientists, Graduate Students and Students, Borok, Russia, 16–19 September 2020; Filigran: Yaroslavl, Russia, 2020; pp. 216–219. (In Russian) [Google Scholar]
- Kuznetsova, T.V.; Sladkova, G.V.; Kholodkevich, S.V. Evaluation of functional state of crayfish Pontastacus leptodactylus in normal and toxic environment by characteristics of their cardiac activity and hemolymph biochemical parameters. J. Evol. Biochem. Physiol. 2010, 46, 241–250. [Google Scholar]
- Sladkova, S.; Kholodkevich, S.; Safronova, D.; Borisov, R. Cardiac activity of crayfish Cherax quadricarinatus (von Martens 1868) in different physiological states. Principy Èkologii 2017, 6, 40–53. (In Russian) [Google Scholar]
- Sladkova, S.V.; Lyubimtsev, V.A.; Kholodkevich, S.V. Study of cardioactivity of Astacus leptodactylus Crayfish and Cherax quadricarinatus in a wide temperature range. In Actual Problems of Studying Crustaceans, Proceedings of the Scientific and Practical Conference, Borok, Russia, 23–25 May 2022; Institute of Natural and Technical Systems: Sevastopol, Russia, 2022; p. 59. (In Russian) [Google Scholar]
- Kozák, P.; Policar, T.; Fedotov, V.P.; Kuznetsova, T.V.; Buric, M.; Kholodkevich, S.V. Effect of chloride content in water on heart rate in narrow-clawed crayfish (Astacus leptodactylus). Knowl. Manag. Aquat. Ecosyst. 2009, 394–395, 8. [Google Scholar]
- Kuznetsova, T.V. Change of salinity of medium as a function loading in estimating functional state of the crayfish Astacus leptodactylus. J. Evol. Biochem. Physiol. 2013, 49, 498–502. [Google Scholar]
- Kozák, P.; Policar, T.; Fedotov, V.P.; Kuznetsova, T.V.; Buric, M.; Kouba, A.; Kuklina, I.; Kholodkevich, S.V. Stress reaction in crayfish: Chlorides help to withstand stress in high nitrite concentration conditions—Preliminary study. Knowl. Manag. Aquat. Ecosyst. 2011, 401, 5. [Google Scholar] [CrossRef]
- Udalova, G.P.; Kholodkevich, S.V.; Fedotov, V.P.; Kornienko, E.L. Changes in heart rate and circadian cardiac rhythm as physiological biomarkers for estimation of functional state of crayfish Pontastacus leptodactylus Esch. upon acidification of the environment. Inland Water Biol. 2012, 5, 119–127. [Google Scholar]
- Sladkova, S.V.; Lyubimtsev, V.A.; Kholodkevich, S.V. On the use of river crayfish as bioindicators in bioelectronic water quality monitoring systems at wastewater discharge sites in the Gulf of Finland. In Biodiagnostics of the State of Natural and Natural-Technogenic Systems, Proceedings of the XIV All-Russian Scientific and Practical Conference with International Participation, Kirov, Russia, 5–8 December 2016; Degteva, S.V., Litvinets, S.G., Ashikhmina, T.Y., Domracheva, L.I., Kondakova, L.V., Shirokikh, I.G., Dabakh, E.V., Eds.; LLC Raduga-PRESS: Kirov, Russia, 2016; Volume 2, pp. 245–249. (In Russian) [Google Scholar]
- Lyubimtsev, V.A.; Kholodkevich, S.V.; Druzhinin, I.I. Measuring systems designed for working with living organisms as biosensors. Features of their metrological maintenance. J. Phys. Conf. Ser. 2019, 1379, 12077. [Google Scholar]
- Fedotov, V.P.; Zhuravlev, V.L.; Khalatov, A.N.; Kholodkevich, S.V. Comparative analysis of heart activity of the crayfish Pontastacus leptodactylus by methods of plethysmography and electrocardiography. J. Evol. Biochem. Physiol. 2009, 45, 527–529. [Google Scholar] [CrossRef]
- McGaw, I.J. Does feeding limit cardiovascular modulation in the Dungeness crab Cancer magister during hypoxia? J. Exp. Biol. 2005, 208, 83–91. [Google Scholar]
- Worden, M.K.; Clark, C.M.; Conaway, M.; Qadri, S.A. Temperature dependence of cardiac performance in the lobster Homarus americanus. J. Exp. Biol. 2006, 209, 1024–1034. [Google Scholar]
- Giomi, F.; Pörtner, H.-O. A role for haemolymph oxygen capacity in heat tolerance of eurythermal crabs. Front. Physiol. 2013, 4, 110. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).