Abstract
Understanding the relationship between environmental temperature and the biological traits of organisms is fundamental to inferring the potential impacts of climate change. In the case of marine poikilotherm species, seawater temperature is one of the main driving forces of biological processes, with consequences at higher levels of organization such as population and ecosystem. In this study, we analysed differences in maximum carapace width (CWmax), size at maturation, relative size at maturation, and duration of the reproductive season for the green crab (Carcinus maenas) along a temperature gradient. An extensive review of bibliographic data was performed on studies published between 1962 and 2020, gathering C. maenas data from 55 different populations, spread over 20 degrees of latitude and 14.2 °C of sea surface temperature (SST). In addition, green crab data were collected at five different lagoons and estuaries along the continental Portuguese coast. The relationship between average SST and CWmax, age of maturation, reduction of size at maturation, and duration of egg bearing was analysed to understand the role of SST in driving variation in these C. maenas characteristics across a latitudinal gradient. There was a significant relationship between SST and CWmax for males and SST and CW of females at maturation, respectively. The results extrapolate for each local projected temperature increase caused by climate change and suggest an effect on the morphometric and reproductive traits of C. maenas across regions. These changes comprise an overall reduction in C. maenas body size, an enlargement of the reproductive season, a shortening in the duration of larval developmental time, and a decrease in the relative size of crabs at maturation. Secondary consequences on the fecundity and connectivity of populations are discussed.
Keywords:
European green crab; carapace width; sexual maturation; sea surface temperature; global warming; climate change Key Contribution:
This manuscript provides a comprehensive analysis of the relationship between environmental temperature and latitude, and different reproductive and morphometric traits of the green crab Carcinus maenas. This study identified different life history traits of the species that will be both negatively and positively affected by temperature increase.
1. Introduction
As a consequence of global warming, the Earth’s surface temperature is expected to rise between 1.7 (Representative Concentration Pathway 4.5 (RCP 4.5)) and 3.2 °C (RCP 8.5) by the end of the XXI century [1]. The implications on the ecology of marine species under these scenarios are difficult to infer due to different trade-offs and constraints governing life history traits. For instance, changes in body size due to climate warming could imply changes in the fecundity and phenology of organisms [2] affecting species population dynamics and ecosystem trophic relationships via cascading effects.
The influence of temperature on biological processes in ectotherms and on biological traits (intraspecific phenotypes) has been observed to vary across temperature gradients, often represented by latitude. This variability encompasses aspects such as body size (e.g., Bergmann’s rule), growth, and reproduction [3,4,5]. Existing biological variation across latitude can be used to identify how species traits respond to different temperature conditions and can thus be used to predict how species will respond to changes in those conditions due to climate warming.
The European green crab Carcinus maenas (Linnaeus 1758) is a brachyuran decapod native to the coasts and estuaries of the Northeast Atlantic, from Mauritania to Norway, including Iceland [6,7,8]. Over the past centuries, this species has spread its geographic distribution and settled in five major regions of the globe (Northeast, Northwest and Southwest Pacific Ocean, and Northwest and Southeast Atlantic Ocean) [9,10]. Carcinus maenas is also included in the top 100 of the most invasive species by the IUCN [11]. An integral component of its success as an invasive species is the high phenotypic plasticity and its wide range of tolerance to salinity and temperature [12,13]. Across its entire geographic distribution, C. maenas holds significant ecological importance, playing a pivotal role as a key epibenthic species. It is deeply involved in various estuarine ecological processes through trophic interactions, holding a central position in the marine food chain by linking primary producers, invertebrates and top predators [14,15]. Similarly, its expansion throughout its non-native range has been related to a drastic reduction of native benthic species in North America, such as the bivalve Mya arenaria [16] and the American oyster (Crassostrea virginica) [17].
The effect of temperature on the growth and adult size of C. maenas has been observed by field observations [18,19], and laboratorial experiments to analyse the effect of temperature on larval development [3,5,20]. In most studies evaluating the effect of temperature, it has been observed that at lower temperatures, individuals reach larger carapace widths [19,21].
Understanding the effect of temperature on C. maenas is fundamental to assessing the potential consequences of climate warming in this species. The aim of this study is to complement prior studies [19], expanding the geographic range under analysis, examining reproductive traits in addition to morphometric traits, and developing projections under future climate conditions. More specifically, in this research, we study the relationship between the environmental temperature (sea surface temperature, SST) and morphological and reproductive characteristics of C. maenas. To determine that, we combine existing data on C. maenas traits across a wide range of its geographic distribution and new field data gathered in Portugal at five lagoons and estuaries. These data allowed us to estimate the potential consequences of local SST increases on the morphometric and reproductive traits of C. maenas.
2. Material and Methods
Data on different biological traits of Carcinus maenas were obtained from two sources: (1) metadata that included a literature review of C. maenas studies with associated geographic information, and (2) field sampling carried out at five locations along the Portuguese coast (Figure 1). Regardless of the data source, the biological traits compiled included maximum carapace width (CWmax, in mm) for both sexes, minimum size at maturation for females (CWmat, in mm), and the duration period of egg-bearing females (EggTime, in months). The biological data from the literature review encompassed a broad amount of information covering a large spatial extent.
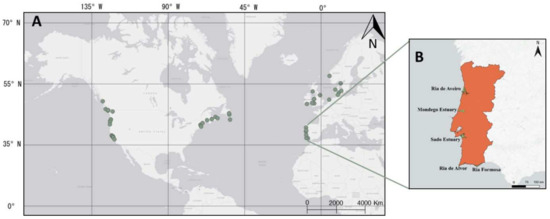
Figure 1.
Geographical location of the different biological traits data on Carcinus maenas populations obtained throughout literature (A) and throughout field work in five study areas on the Portuguese Coast: Ria de Aveiro, Mondego estuary, Sado estuary, Ria de Alvor and Ria Formosa (B).
2.1. Literature Review Data
A comprehensive bibliographic review was carried out considering studies published between 1962 and 2020 (Table 1 and Table 2). The scientometric analysis was conducted using the Web of Science scientific database (http://webofknowledge.com, between January 2019 and January 2021 accessed on 7 April 2022). The search and choice of keywords and Boolean operators followed a research methodology where three fields were used to define the search steps, all connected by the AND option in the connection boxes from one to the other. The first search field was defined to insert the words related to carapace width: “Carcinus maenas AND Carapace Width” OR “Carcinus maenas AND CW” OR “Carcinus maenas AND Size” OR “Carcinus maenas AND Carapace” OR “Green crab AND Carapace Width” OR “Green crab AND Size”. The second search field comprised keywords related to sexual maturation, with the following terms used: “Carcinus maenas AND Sexual maturation” OR “Carcinus maenas AND Reproduction”. The third search field comprised keywords related to egg-bearing females, with the following terms used: “Carcinus maenas AND Egg” OR “Carcinus maenas AND Ovigerous” OR “Carcinus maenas AND Larval release”. Furthermore, a main search was performed only with the word “Carcinus maenas”. The data information includes both native populations (Table 1) and non-native populations (Table 2) along a wide latitudinal gradient, spreading over 20 degrees in the northern hemisphere (Figure 1). The information was obtained from values included in the text, or data provided in tables and figures, followed by a quality assessment of the data. Manuscripts with poor or sparse data were not used in our review, with the exclusion criteria being the number of individuals analysed (minimum: 50 individuals) and the sampling periods (minimum: 2 samplings). The search considered 326 reviewed articles and was reduced to 46 articles that provided data on 55 different populations.

Table 1.
Source studies on Carcinus maenas native populations found in the bibliographic search, listed from north to south. NA = data not available; SST = sea surface temperature (°C); RCP = representative concentration pathway.

Table 2.
Source studies on Carcinus maenas non-native populations found in the bibliographic search, listed from north to south. NA = data not available; SST = sea surface temperature (°C); RCP = representative concentration pathway.
2.2. Field Work Data
Field samplings of C. maenas were carried out along Portuguese estuaries and lagoons to obtain in situ biological data: Ria de Aveiro (40°38′ N 8°41′ W), Mondego Estuary (40°08′ N, 8°50′ W), Sado Estuary (38°25′ N 8°48′ W), Ria de Alvor (37°08′ N 8°37′ W), and Ria Formosa (37°03′ N 7°43′ W) (Figure 1). In the Mondego estuary, the population of C. maenas was sampled monthly from June 2003 to December 2018 during the night, at high water of spring tides, using a 2 m beam trawl with one tickler chain and 5 mm mesh size in the cod end (see more details in Monteiro et al. 2021) [13]. For the remaining systems, C. maenas populations were sampled monthly from June 2018 to December 2020 using fishing traps deployed for 24 h.
2.3. Environmental Data
The annual mean sea surface temperature (SST) at each location was obtained from the Copernicus dataset (https://cds.climate.copernicus.eu/cdsapp#!/dataset/sis-biodiversity-cmip5-global?tab=form, accessed on 13 September 2022) choosing the variable “Sea Surface Temperature” (Monthly mean) from the Model “IPSL-CM5A-LR”, the Ensemble member “r1i1p1” and the Experiment “RCP 4.5 and RCP 8.5”. With this dataset it was possible to obtain the present mean annual SST (mean SST between 1960–2020) and the future mean annual SST (mean SST expected between 2080–2100) for each location (Table 1 and Table 2). The pathways RCP 4.5 and RCP 8.5 describe different climate change scenarios, all of which are considered possible depending on the volume of greenhouse gases (GHG) emitted in future years. RCP 4.5 is described by the IPCC as an intermediate scenario, and RCP 8.5 is generally taken as the worst-case climate change scenario.
2.4. Data Analysis
Analysis within regions was avoided due to a limited number of samples per region (less than 20) and narrow ranges of sea surface temperature (SST) between locations within each region (See Supplemental Information, Figures S1 and S2). As expected, a statistically significant negative linear relationship between latitude and SST data (indicating lower temperatures at higher latitudes) (p < 0.001, Figure 2) was observed across sampling sites. Consequently, SST was considered as the explanatory variable for all tested relationships.

Figure 2.
Relationship between latitude and sea surface temperature (SST) (°C).
The relationship between C. maenas morphometric and reproductive traits (response variables) and SST (explanatory variable) was assessed using linear regression. The traits considered were: (1) maximum carapace width (CW) for females (CWmax females) and males (CWmax males); (2) the difference between maximum CW of the sexes (CWMaxMales—CWMaxFemales); (3) CW at sexual maturation (CWmat) for females; (4) the relative size at maturation (CWmat/CWmax), defined as the ratio between the size at maturation and the maximal carapace width of females; (5) the duration of the period, in months, when egg-bearing females are present (EggTime). Model validation was performed by visual inspection of residuals and by fulfilling the linearity, homoscedasticity, independence and normality assumptions and a probability level α < 0.05 was used in all analyses in order to reject the null hypothesis. All statistical analyses were performed in IBM SPSS software 29.0 (Statistical Package for the Social Sciences).
The future predicted CWmax for each sex, the CWmat for females and EggTime, were recalculated using the estimated linear model (based on present data) by substituting the projected sea surface temperature (SST) values for the end of the 21st century under scenarios RCP 4.5 and RCP 8.5. Changes in fecundity and pelagic larval duration (PLD) for the present scenario and both RCP scenarios were calculated using equations developed for C. maenas by [51,52], respectively.
The fecundity and PLD regressions mentioned above encompass a range of SST values observed within the SST range collected in this study across all populations. While the equations derived from these studies were initially designed for specific regions, the tested values for estimating fecundity and PLD, as included in the studies by the latter authors, fall within the range of SST collected across all populations in our study. As a result, the estimations made here serve as robust proxies for the anticipated effects of SST in the RCP 4.5 and 8.5 scenarios.
To evaluate the direction and extent of climate impacts, we computed both the absolute and relative (%) changes in morphometric and reproductive C. maenas traits values for each RCP scenario. This involved calculating the disparity between projected future alterations and the current scenario. In order to categorize populations based on their overall biological traits, we employed the K-means similarity classification method from data mining analysis. This technique aims to divide n-populations of biological traits into k-clusters, where each observation (morphometric and reproductive trait) is assigned to the cluster with the closest mean. Populations exhibiting comparable changes in biological traits were grouped together within the same cluster. The sum of squared Euclidian distance was used to determine the distances between k-mean clusters and provide an index of oddity of the cluster.
To determine the optimal number of clusters that best elucidates data classification, we conducted an ANOVA. We tested various numbers of K-clusters (ranging from 1 to 6), and ANOVA p-values for K-means revealed that 5 clusters provided the optimal fit, effectively indicating the number of clusters that most accurately described the data. The data on each response variable was then averaged for each K-mean cluster for both RCP 4.5 and RCP 8.5 analyses, using the population individual values observed from each location (Supplementary Information).
3. Results
The biological trait data of Carcinus maenas were collected from 60 distinct locations, including 55 sourced from a literature review and 5 obtained through field samplings. These locations spanned more than 140 degrees of longitude and 20 degrees of latitude in the northern hemisphere (see Figure 1). Among these, 24 populations were categorized as native (Table 1), while 36 were classified as non-native (Table 2) locations.
The observed mean sea surface temperatures (SST) exhibited considerable variation across the different populations, ranging from 4.8 °C at North Harbour, Canada (48° N), to 19 °C at Ria Formosa Lagoon, Portugal (37° N). Similarly, the biological trait values encompassed a wide range of phenotypic diversity within the species. For females, the maximum carapace width (CWmax) spanned from 54 mm CW (at 37° N [16]) to 93 mm (at 49° N [38]), while for males, the range extended between 67 mm CW (at 37° N [16]) and 114 mm CW (at 49° [38]).
The relationship between SST and CWmax was statistically significant exclusively for males (p = 0.014), while the relationship for females showed no statistical significance (p = 0.324; refer to Figure 3A,B). Notably, males exhibited a higher CWmax compared to females. Moreover, the relationship between SST and the difference in maximum CW between males and females was also statistically significant (p = 0.023), indicating that in warmer locations, the discrepancy in CWmax between males and females is reduced (Figure 3C).

Figure 3.
Relationship between morphometrics Carcinus maenas traits and sea surface temperature (SST). (A) Female maximum carapace width mm (Female CWmax); (B) Male maximum carapace width mm (Male CWmax); (C) difference between Male CWmax and Female CWmax. In each figure it is possible to observe the linear regression and the 95% confidence intervals.
Considering the reproductive-related traits, it was observed that the relationship between SST and CWmat of females (the data was unavailable for males) was negative and statistically significant (p = 0.023; Figure 4A). On the contrary, the negative relationship between SST and the relative size of females at maturation (CWmat/CWmax) was not statistically significant (p = 0.116; Figure 4B). Finally, the relationship between SST and duration of the period when females carry their eggs in a year was positive and statistically significant (p < 0.001; Figure 4C), with egg-bearing females being found during 3 months in colder locations (≅6 °C) and up to 8 months in warmer locations (≅18 °C).

Figure 4.
Relationship between reproductive Carcinus maenas traits and sea surface temperature (SST). (A) Female carapace width at maturation (mm) (Female CWmat); (B) females’ relative size at maturation (Carapace width at maturation/maximum carapace width); (C) time of egg-bearing females (months) (EggTime). In each figure it is possible to observe the linear regression and the 95% confidence intervals.
With the rise in temperature predicted by the end of the XXI century, 2080–2090 (Table 1 and Table 2), changes in the morphometric and reproductive traits of C. maenas become predictable (Figure 5 and Table 3, Supplementary Information Tables S1 and S2). For C. maenas males, a reduction of approximately 0.9 to 6.0 mm in CWmax (corresponding to a decrease of around 1.20% to 6.63%) is foreseen across various locations under RCP 4.5 and RCP 8.5 scenarios. For females, a minor decrease in the CWmax of about 0.36 and 2.38 is expected, corresponding to 0.51% and 1.30% for RCP 4.5 and RCP 8.5, respectively. Moreover, the size at maturation (CWmat) for females is expected to decrease by 0.47 to 3.04 mm, constituting a reduction of roughly 1.71% to 8.84% for RCP 4.5 and RCP 8.5, respectively. Conversely, an increase in the duration of the egg-bearing period by 0.22 to 1.45 months is estimated, corresponding to 3.59% and 56.80% for RCP 4.5 and RCP 8.5, respectively. Due to the anticipated reduction in CW, a decrease in fecundity of between 2570 and 16710 eggs is also projected, corresponding to a decrease of approximately 0.84% and 4.87% under RCP 4.5 and RCP 8.5, respectively. Lastly, the negative relationship between SST and PLD suggests a potential decrease in PLD by 2 to 36 days, representing a decrease of 5.71% to 32.35% depending on the location.
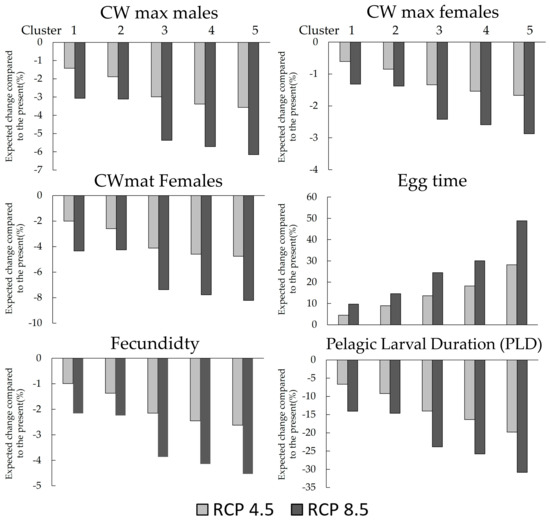
Figure 5.
Expected changes (%) in the morphometric and reproductive traits of Carcinus maenas caused by the expected rise of sea surface temperature (SST) of 1.7 and 3.2 °C in comparation with the actual value. CWmax—Carapace width maximum; CWmat—Carapace width at maturation; EggTime—Duration of egg-bearing females; Fecundity—Eggs carried by a female; PLD—Pelagic larvae duration.

Table 3.
Expected changes in the morphometric and reproductive traits of Carcinus maenas caused by the expected rise of sea surface temperature (SST). The different climate consequence clusters were obtained through Figure 5. N—Number of populations of each consequence; CWmax—Carapace width maximum (mm); CWmat—Carapace width at maturation (mm); EggTime—Duration of egg-bearing females (months); Fecundity—Average number of eggs carried by a female; PLD—Pelagic larvae duration (days); RCP = Representative concentration pathway. Note that fecundity is closely related to CWmax females, so this relationship should be corrected by this negative relationship.
The k-means tests organize k-clusters in a latitudinal way based on morphometric and reproductive analyses (Figure 5 and Figure 6A,B, Table 3). The results denote a tropicalization effect due to SST on the biological traits; here, lower latitudes near temperate areas are less affected than north areas. Across the k-clusters, it is possible to observe that populations from locations with presently low SST will be more affected than populations from warmer locations (Figure 6A,B), which is observed in both RCP scenarios. In addition, we expect a greater effect from the increase of SST (in both RCP scenarios) on the biological trait of C. maenas populations from the east coast of North America, and a lower effect on the European populations (Figure 6A,B; for detailed information, see Supplementary Information Tables S1 and S2). Since the cluster 1 group populations have expected lower changes in the biological traits, regardless of the scenario, and cluster 5 has the highest expected changes in the biological traits, cluster 1 was defined as “low consequences”, cluster 2 as “low-medium consequences”, cluster 3 as “medium consequences”, cluster 4 as “medium-high consequences”, and, finally, cluster 5 as “high consequences” (Table 3).
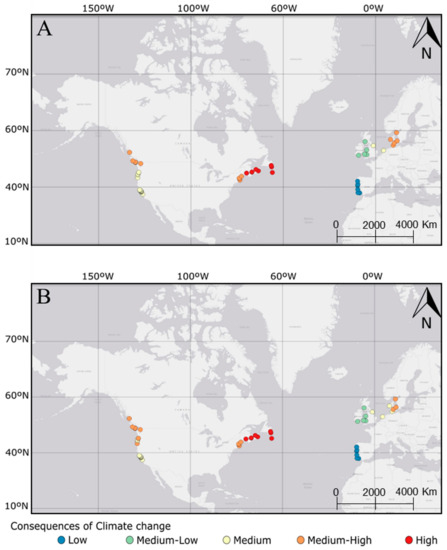
Figure 6.
Consequence of SST increase in each Carcinus maenas population according to different scenarios of climate change, RCP (representative concentration pathway) 4.5 (A) and RCP 8.5 (B). In a gradient of colour, the populations in blue presented lower consequences and, in red, higher consequences.
4. Discussion
This study provides a comprehensive analysis of the relationship between different Carcinus maenas biological traits and the average environmental temperature over its range of occurrence, continuing the work by Kelley et al. [19]. In comparison with this previous study, we expand the geographic range under analysis, incorporate reproductive parameters and predict changes in the morphometric and reproductive traits of C. maenas caused by global warming. The final data set evaluated covered 20° of latitude and 140° of longitude (representing an SST range between 4.8 °C and 19 °C). With this data set we provide a comprehensive analysis of the relationship between SST and different morphometric and reproductive traits of C. maenas. By doing so, we can project the potential consequences of the anticipated SST increase until 2100, thus allowing us to increase the knowledge about the consequences of SST increase, with data taken in the field and not with laboratory experiments that always present some constraints or variables that are not possible to control.
4.1. Morphological Trait
Significant differences in the maximum carapace width (CWmax) of C. maenas related to SST along the spatial range analysed were found only for males (Figure 3). Larger male crabs were found at locations with the lower SST (114 mm at 52° N; Gillespie et al. 2015) [38], while the smallest maximum CW was recorded at the higher SST locations (67 mm at the southern Portuguese coast). At all the locations examined, the observed CWmax was larger in males than in females, which is probably driven by male competition for access to females and resources [10,53]. This finding is also supported by Hartnoll (2006) [54], who suggests that females do not execute intraspecific competition effectively, resulting in more energy diverted into reproduction regardless of the resources/temperature of the environment. We also found that the sexual difference in CWmax is higher in populations where crabs are larger (colder locations) (Figure 3C). This finding is a consequence of the relationships found between SST and male CWmax, which was not found for females. However, it should be noted that the size of the largest crabs captured in non-native areas has been decreasing over the last few decades, likely due to the beginning of crab fishing in these regions. As fisheries target larger individuals, they exert selective pressure that favours smaller crabs, leading to a reduction in the maximum recorded size and potentially causing maturation at smaller sizes [55].
Kelley et al. (2015) [19] observed a negative correlation between SST and CW on a small regional scale along the U.S. North Pacific coast. These authors’ results were consistent with Bergmann’s rule, as well as the temperature–size rule (TSR) established for ectotherms [21], which generally establishes that the size of individuals in a given species is negatively correlated with temperature. However, the maximum carapace width (CWmax) in Kelley et al. (2015) was not significantly correlated with SST in females. Complementary to Kelley et al. (2015), this study revealed that such an effect occurs at short and long/global spatial scales. The CWmax of male C. maenas was negatively correlated with SST, whereas, for females, this relationship did not reach statistical significance (Figure 3A). These means presented findings that do not meet the premises of the latter “thermic” rules for females. In Kelley et al. (2015), despite the lack of correlation between female CWmax and SST, a negative correlation existed to all other morphometric–SST relationships tested (i.e., mean size and mean maximum size). In our study, predominantly conducted with metadata, the relationships between both mean and mean maximum size with SST could not be analysed. Different ecological explanations are found in the literature for the TSR: while some refer to the different velocity of developmental and growth rates [56], other theories point towards environmental pressures optimizing fitness, having that body size is a final result of food availability and quality, environmental temperature, and predator pressures [57]. At warmer temperatures, the individual’s metabolism requirements usually outpace energy acquisition rates, resulting in smaller-sized individuals compared to those at colder temperatures [58].
The temperature–size rule (TSR), mentioned before, is a form of phenotypic plasticity that can be described from laboratory analysis. However, it is not possible to conclude whether the patterns reported in our study are driven by plasticity or by genetic variation among populations (most likely it is both), and we cannot rule out other unexplored hypotheses. One of the hypotheses is the effect of the large intraspecific genetic variation that C. maenas populations exhibit throughout their geographical distribution [59]. This intraspecific genetic variation may also be one of the explanations for the variation in CWmax across the geographic distribution, due to a temperature-mediated modification in the expression of genes governing growth, which can result in a plastic response of size [19,60]. Furthermore, crustacean maximum size is known to be influenced by other environmental patterns, such as salinity and available oxygen [61]. In the present study, these patterns were not evaluated. Carcinus maenas is an euryhaline intertidal species, adapted to live in a wide range of salinity and oxygen conditions [10] and thus inhabiting different systems (lagoons, estuaries, etc.), with local conditions affecting populations biological traits [10,62]. Oxygen solubility increases as both salinity and temperature decrease [63], and, in a previous study [61], it was observed that the maximum size of crustacean species is influenced by oxygen availability. In systems with high temperature or salinity, the available oxygen is lower, leading to an increase in metabolic rates, resulting in higher tissue maintenance costs and less energy allocated to growth. In addition, the morphological characteristics of C. maenas are also affected by the presence of competitors/predators [Enemy hypothesis] [53]. For instance, the species distribution and reproductive/feeding behaviour of C. maenas can be affected by interspecific competition with other crab species [10,63].
4.2. Reproductive Trait
We have found that the size at maturation in females varies negatively with SST. The relationship between CWmat and temperature has also been observed in other species of crabs [64] and marine invertebrates [65]. This finding, in combination with other studies relating a relationship between maximum body size and size at maturation in crustaceans [66,67], could imply that female C. maenas are not properly sampled, probably due to behavioural processes. Nevertheless, warm-water crustaceans are known to reach maturity at a smaller size than their cold-water conspecifics since the moult frequency of crustaceans declines in colder water, resulting in longer intermoult periods [68]. These longer periods favour energy conservation and, thus, larger somatic growth between each intermoult period compared to individuals in warmer waters [67,68,69]. Our observations support this theory, as in populations where SST was lower, the size of females at sexual maturation was higher. The onset of sexual maturation implies a trade-off between the amount of energy allocated to reproduction and growth, which organisms in colder environments pay at larger relative sizes, ensuring that they have enough internal energetic resources [54,70].
Interestingly, we found that not only CWmat and CWmax decrease with SST, but the ratio CWmat/CWmax (the relative size at maturation) does as well. This implies that populations from warmer locations can reproduce at smaller sizes at the cost of reaching smaller maximum sizes. This finding could be directly related to fecundity, given the close relationship between female size and the number of eggs [8,62]. Temperature also has an influence on egg size [71,72]. Presently, this relationship could not be analyzed due to the limited number of published research papers with measurements of C. maenas eggs. However, it is expected that at lower temperatures, egg development time would be slower, but egg size and hatching rates would be higher [71]. Moreover, water temperature can exert a strong influence on the spawning period, with a positive correlation between SST and the months when egg-bearing females can be found. This variation ranges from 3 months in colder locations to up to 8 months in warmer locations. The spawning period of aquatic species, including fish and crabs, is influenced by SST [66,67,73], as the optimal temperature range for spawning is extended in warmer conditions. This extended spawning period is made possible by the consistent maintenance of suitable temperatures [73], resulting in a continuous spawning period or even the occurrence of two reproductive periods in females in temperate water [62].
4.3. Warming Impacts
Understanding the ecological impacts of climate change is a crucial challenge of the 21st century. Considering the total reproductive potential of C. maenas during its lifetime, an increase of SST could, overall, imply different trade-offs with different directions (Figure 5, and Table 3). The duration of the period when egg-bearing females can be found increases with temperature. On the other hand, body size, size at maturity, larval duration, fecundity, and life span were all negatively related to SST, pointing in the opposite direction for the relationship between temperature and reproductive potential. It is worth noting that decreasing body size due to SST could affect fecundity and life span [10,62]. The duration of the egg-bearing period in females is expected to increase with the increase in SST, and the periods of reproduction will change from once to twice a year due to climate change, as happens in warmer water populations [74]. Owing to the reduction of CW, we could expect a decrease in female fecundity for both climate change scenarios. However, the final outcome between this decrease in potential fecundity and the enlargement of the reproductive period is difficult to infer in terms of the expected effect on population recruitment/dynamic. Moreover, a negative relationship between SST and the duration of the pelagic larval phase is also expected, which could negatively affect the connectivity between populations or the mortality rate at the earlier life stages [2,74,75]. With the information obtained in this study, it is difficult to predict if the morphometric and reproductive changes observed imply an increase or decrease of populations, given the antagonistic effects on fecundity, pelagic larval duration, and reproductive period, because the net effects cannot be determined.
The climate change effects in C. maenas are expected to occur in all distribution areas, but populations in warmer locations will be less impacted than those in colder locations, because in warmer locations, C. maenas is already exposed to upper limit conditions close to their biological thermic preference (see more in Young and Elliot 2020) [10]. Even though some European and North American populations can be found at similar latitudes, we predict that the ones from the North American coast will suffer harsher consequences of the expected increase in SST, which may help to mitigate the presence of this species in these invaded areas. Furthermore, since our model is linear, regions where there is less predicted impact of warming temperatures have lower predicted consequences for the morphological and reproductive patterns of crabs, and this reflects the known fact that temperature increases will be greater near the poles and will be greater in some regions than other regions inhabited by C. maenas populations.
As observed in our study, Daufresne et al. (2009) found evidence that reduced body size is the third universal ecological response to global warming in aquatic systems, alongside shifts in species ranges toward higher altitudes and latitudinal and seasonal changes in life cycle events. These highlight the impact of global warming on shifting biological traits of crabs, namely body size and reproductive patterns, and other marine aquatic ectotherm species. Such an effect has also been recorded for other crabs [76] and fish species [77], where a decrease in body size has occurred as a result of global warming, with this effect exerting more influence in males than in females.
4.4. Final Considerations
Understand the consequences of global warming in C. maenas is extremely important, and this study gives an overview of some potential consequences of climate change on this species. Overall, our findings show that the reproductive period of the green crab will increase, but it is difficult to predict if this will lead to a higher reproductive potential, since that will require observing decreasing body size and phenology (PLD, time to maturity, life span, etc.). This species’ features, distribution, and adaptation to increased marine warming can be the reason for C. maenas’ success in colonizing new areas as an invasive species. Our results should be interpreted with caution; however, the overall results indicate a potential reduction in C. maenas body size, affecting its fecundity, an enlargement of the reproductive season, a shortening in the duration of larval developmental time, and a decrease of the relative size of crabs at maturation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes8100485/s1, Figure S1: Relationship between Carcinus maenas traits and sea surface temperature (SST) for each population; Figure S2: Results of the statistical analysis, carried out through separate analysis by the different populations (ANCOVAS). Table S1: Expected changes in the morphometric and reproductive traits of Carcinus maenas caused by the expected rise of SST, in each local; Table S2: Expected changes (%) in the morphometric and reproductive traits of Carcinus maenas caused by the expected rise of SST of 1.7 and 3.2 °C in comparation with the actual value, for each local.
Author Contributions
J.N.M.: Concepturalization, Methodology, Investigation, Formal analysis, Writing—original draft. J.B.-P.: Methodology, Formal analysis, Writing—review & editing. M.P.: Investigation, Writing—review & editing. M.A.P.: Supervision, Writing—review & editing, Funding acquisition. F.M.: Investigation, Supervision, Writing—review & editing, Funding acquisition. F.L.: Methodology, Supervision, Formal analysis, Writing—review & editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the projects: “CRUSTAPANHA—Estudo da ecologia, biologia e dinâmica populacional dos pequenos caranguejos com interesse comercial existentes ao longo da costa Portuguesa (Operação 16-01-04-FMP-0005—CRUSTAPANHA)” that is funded by Programa Operacional Mar 2020, Portugal 2020 and Fundo Europeu dos Assuntos Marítimos e das Pescas (FEAMP); “CLIMA-PESCA (MAR-01.03.02-FEAMP-0052)—Vulnerability of fishing sector to climate change: adaptation measures” that is funded by operational program MAR2020-FEAMP; CLIMFISH—A framework for assess Committee vulnerability of coastal fisheries to climate change in Portuguese coast founded by Portugal 2020 and FCT SAICT-45-2017-02; ALG-01-0145-FEDER-028518; PTDC/ASP-PES/28518/2017; “MYTAG—Integrating natural and artificial tags to reconstruct fish migrations and ontogenetic niche shifts (PTDC/MAR-EST/2098/2014)”, under the Project 9471—Reforçar a Investigação, o Desenvolvimento Tecnológico e a Inovação (Projeto 9471-RIDTI) and subsidized by the European Regional Development Fund (FEDER, POCI-01-0145-FEDER-016787); and “RENATURE—Valorization of the Natural Endogenous Resources of the Centro Region (CENTRO-01-0145-FEDER-000007)”, funded by the Comissão de Coordenação da Região Centro (CCDR-C) and subsidized by the European Regional Development Fund (FEDER). This study also received Portuguese national funds from the Foundation for Science and Technology (FCT) through projects UIDB/04326/2020, UIDP/04326/2020 and LA/P/0101/2020. JNM was funded by FCT PhD fellowship SFRH/BD/06336/2021. JBP was funded by EU H2020 (FutureMARES, contract no. 869300). MP received an FCT PhD fellowship SFRH/BD/11426/2022. FM was funded by FCT, in the scope of the Decree-Law 57/2016. FL received Portuguese national founds from FCT contract program DL57/2016/CP1361/CT0008 and FCT 2022.04803.CEECIND.
Data Availability Statement
Relevant information is included in the article. Raw data supporting the conclusions are available from the author, J.N.M., upon request.
Acknowledgments
Special thanks to the Direção Geral dos Recursos Marinhos (DGRM), which provided the crab landing data, enabling this study to collect Portuguese crab data. Special thanks to all the fishers who, through their assistance, made it possible to collect the samplings.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC. Global Warming of 1.5 °C; An IPCC Special Report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; 616p. [Google Scholar] [CrossRef]
- Bueno, J.; López-Urrutia, Á. The Offspring-Development-Time/Offspring-Number Trade-Off. Am. Nat. 2012, 179, E196–E203. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, M. Combined effects of temperature and salinity on the zoeal development of the green crab, Carcinus maenas (Linnaeus, 1758) (Decapoda: Portunidae). Sci. Mar. 1993, 57, 1–8. [Google Scholar]
- Rodríguez-Félix, D.; Cisneros-Mata, M.A.; Aragón-Noriega, E.A. Variability of size at maturity of the warrior swimming crab, Callinectes bellicosus (Stimpson, 1859) (Brachyura, Portunidae), along a latitudinal gradient in the Gulf of California. Crustaceana 2015, 88, 979–989. [Google Scholar] [CrossRef]
- Spitzner, F.; Giménez, L.; Meth, R.; Harzsch, S.; Torres, G. Unmasking intraspecific variation in offspring responses to multiple environmental drivers. Mar. Biol. 2019, 166, 112. [Google Scholar] [CrossRef]
- Crothers, J.H. The biology of the shore crab Carcinus maenas (L.). I. The background anatomy, growth and life history. Field Stud. 1967, 2, 407–434. [Google Scholar]
- Carlton, J.T.; Cohen, A.N. Episodic global dispersal in shallow water marine organisms: The case history of the European shore crabs Carcinus maenas and C. aestuarii. J. Biogeogr. 2003, 30, 1809–1820. [Google Scholar] [CrossRef]
- Rewitz, K.; Styrishave, B.; Depledge, M.H.; Andersen, O. Spatial and Temporal Distribution of Shore Crabs Carcinus maenas in a Small Tidal Estuary (Looe Estuary, Cornwall, England). J. Crustac. Biol. 2004, 24, 178–187. [Google Scholar] [CrossRef]
- Thresher, R.E.; Werner, M.; Høeg, J.T.; Svane, I.; Glenner, H.; Murphy, N.E.; Wittwer, C. Developing the options for managing marine pests: Specificity trials on the parasitic castrator, Sacculina carcini, against the European crab, Carcinus maenas, and related species. J. Exp. Mar. Biol. Ecol. 2000, 254, 37–51. [Google Scholar] [CrossRef]
- Young, A.M.; Elliott, J.A. Life History and Population Dynamics of Green Crabs (Carcinus maenas). Fishes 2020, 5, 4. [Google Scholar] [CrossRef]
- Leignel, V.; Stillman, J.H.; Baringou, S.; Thabet, R.; Metais, I. Overview on the European green crab Carcinus spp. (Portunidae Decapoda) one of the most famous marine invaders and ecotoxicological models. Environ. Sci. Pollut. Res. 2014, 21, 9129–9144. [Google Scholar] [CrossRef]
- Kelley, A.L.; de Rivera, C.E.; Buckley, B.A. Cold tolerance of the invasive Carcinus maenas in the east Pacific: Molecular mechanisms and implications for range expansion in a changing climate. Biol. Invasions 2013, 15, 2299–2309. [Google Scholar] [CrossRef]
- Monteiro, J.N.; Pinto, M.; Crespo, D.; Pardal, M.A.; Martinho, F. Effects of climate variability on an estuarine green crab Carcinus maenas population. Mar. Environ. Res. 2021, 169, 105404. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, E. Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. J. Sea Res. 2008, 59, 30–43. [Google Scholar] [CrossRef]
- Vermeiren, P.; Sheaves, M. Predicting habitat associations of five intertidal crab species among estuaries. Estuar. Coast. Shelf Sci. 2014, 149, 133–142. [Google Scholar] [CrossRef]
- Cohen, A.N.; Carlton, J.T.; Fountain, M.C. Introduction, dispersal and potential impacts of the green crab Carcinus maenas in San Francisco Bay, California. Mar. Biol. 1995, 122, 225–238. [Google Scholar] [CrossRef]
- Pickering, T.R.; Poirier, L.A.; Barrett, T.J.; McKenna, S.; Davidson, J.; Quijón, P.A. Non-indigenous predators threaten ecosystem engineers: Interactive effects of green crab and oyster size on American oyster mortality. Mar. Environ. Res. 2017, 127, 24–31. [Google Scholar] [CrossRef]
- Freitas, V.; Cardoso, J.F.M.F.; Lika, K.; Peck, M.A.; Campos, J.; Kooijman, S.A.L.M.; van der Veer, H.W. Temperature tolerance and energetics: A dynamic energy budget-based comparison of North Atlantic marine species. Philos. Trans. R. Soc. Land B 2010, 365, 3553–3565. [Google Scholar] [CrossRef]
- Kelley, A.L.; de Rivera, C.E.; Grosholz, E.D.; Ruiz, G.M.; Yamada, S.B.; Gillespie, G. Thermogeographic variation in body size of Carcinus maenas, the European green crab. Mar. Biol. 2015, 162, 1625–1635. [Google Scholar] [CrossRef]
- Torres, G.; Giménez, L. Temperature modulates compensatory responses to food limitation at metamorphosis in a marine invertebrate. Funct. Ecol. 2020, 34, 1564–1576. [Google Scholar] [CrossRef]
- Atkinson, D. Temperature and Organism Size—A Biological Law for Ectotherms? Adv. Ecol. Res. 1994, 25, 1–58. [Google Scholar]
- Yamada, S.B.; Dumbauld, B.R.; Kalin, A.; Hunt, C.E.; Figlar-Barnes, R.; Randall, A. Growth and persistence of a recent invader Carcinus maenas in estuaries of the northeastern Pacific. Biol. Invasions 2005, 7, 309–321. [Google Scholar] [CrossRef]
- Mouritsen, K.N.; Geyti, S.N.S.; Lützen, J.; Høeg, J.T.; Glenner, H. Population dynamics and development of the rhizocephalan Sacculina carcini, parasitic on the shore crab Carcinus maenas. Dis. Aquat. Org. 2018, 131, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Lützen, J. Growth, reproduction, and life span in Sacculina carcini Thompson (Cirripedia: Rhizocephala) in the Isefjord, Denmark. Sarsia 1984, 69, 91–105. [Google Scholar] [CrossRef]
- Sneddon, L.U.; Huntingford, F.A.; Taylor, A.C. Weapon size versus body size as a predictor of winning in fights between shore crabs, Carcinus maenas (L.). Behav. Ecol. Sociobiol. 1997, 41, 237–242. [Google Scholar] [CrossRef]
- Aagaard, A.; Warman, C.G.; Depledge, M.H. Tidal and seasonal changes in the temporal and spatial distribution of foraging Carcinus maenas in the weakly tidal littoral zone of Kerteminde Fjord, Denmark. Mar. Ecol. Prog. Ser. 1995, 122, 165–172. [Google Scholar] [CrossRef]
- Dries, M.; Adelung, D. Die Schlei, ein Modell für die Verbreitung der Strandkrabbe Carcinus maenas. Helgol. Mar. Res. 1982, 35, 65–77. [Google Scholar] [CrossRef]
- McVean, A. The incidence of autotomy in Carcinus maenas (L.). J. Exp. Mar. Biol. Ecol. 1976, 24, 177–187. [Google Scholar] [CrossRef]
- Hunter, E.; Naylor, E. Intertidal migration by the sore crab Carcinus maenas. Mar. Ecol. Prog. Ser. 1993, 101, 131–138. [Google Scholar] [CrossRef]
- McGaw, I.J.; Edgell, T.C.; Kaiser, M.J. Population demographics of native and newly invasive populations of the green crab Carcinus maenas. Mar. Ecol. Prog. Ser. 2011, 430, 235–240. [Google Scholar] [CrossRef][Green Version]
- Reid, D.G.; Abello, P.; Warman, C.G.; Naylor, E. Size-related mating success in the shore crab Carcinus maenas (Crustacea: Brachyura). J. Zool. 1994, 232, 397–401. [Google Scholar] [CrossRef]
- Broekhuysen, G.J. On Development, Growth and Distribution of Carcinides maenas (L.). Arch. Neerl. Zool. 1936, 2, 257–399. [Google Scholar] [CrossRef]
- Naylor, E. Seasonal Changes in a Population of Carcinus maenas (L.) in the Littoral Zone. J. Anim. Ecol. 1962, 31, 601–610. [Google Scholar] [CrossRef]
- Lyons, L.J.; O’riordan, R.M.; Cross, T.F.; Culloty, S.C. Reproductive biology of the shore crab Carcinus maenas (Decapoda, Portunidae): A macroscopic and histological view. Invertebr. Reprod. Dev. 2012, 56, 144–156. [Google Scholar] [CrossRef]
- Souza, A.T.; Ilarri, M.I.; Campos, J.; Marques, J.C.; Martins, I. Differences in the neighborhood: Structural variations in the carapace of shore crabs Carcinus maenas (Decapoda: Portunidae). Estuar. Coast. Shelf Sci. 2011, 95, 424–430. [Google Scholar] [CrossRef]
- Baeta, A.; Cabral, H.N.; Neto, J.M.; Marques, J.C.; Pardal, M.A. Biology, population dynamics and secondary production of the green crab Carcinus maenas (L.) in a temperate estuary. Estuar. Coast. Shelf Sci. 2005, 65, 43–52. [Google Scholar] [CrossRef]
- Bessa, F.; Baeta, A.; Martinho, F.; Marques, S.; Pardal, M.A. Seasonal and temporal variations in population dynamics of the Carcinus maenas (L.): The effect of an extreme drought event in a southern European estuary. J. Mar. Biol. Assoc. UK 2010, 90, 867–876. [Google Scholar] [CrossRef]
- Gillespie, G.E.; Norgard, T.C.; Anderson, E.D.; Haggarty, D.R.; Phillips, A.C. Distribution and Biological Characteristics of European Green Crab, Carcinus maenas, in British Columbia, 2006–2013; Canadian technical report of fisheries and aquatic sciences; Fisheries and Oceans Canada: Nanaimo, BC, Canada, 2015; p. 3120.
- Gillespie, G.E.; Phillips, A.C.; Paltzat, D.L.; Therriault, T.W. Status of the European Green Crab, Carcinus maenas, in British Columbia—2006; Canadian technical report of fisheries and aquatic sciences; Fisheries and Oceans Canada: Nanaimo, BC, Canada, 2007; p. 39.
- Yamada, S.B.; Gillespie, G.E. Will the European green crab (Carcinus maenas) persist in the Pacific Northwest? ICES J. Mar. Sci. 2008, 65, 725–729. [Google Scholar] [CrossRef]
- Best, K.; McKenzie, C.; Couturier, C. Reproductive biology of an invasive population of European green crab, Carcinus maenas, in Placentia Bay, Newfoundland. Manag. Biol. Invasions 2017, 8, 247–255. [Google Scholar] [CrossRef]
- Bergshoeff, J.A.; McKenzie, C.H.; Favaro, B. Improving the efficiency of the Fukui trap as a capture tool for the invasive European green crab (Carcinus maenas) in Newfoundland, Canada. PeerJ 2019, 7, e6308. [Google Scholar] [CrossRef]
- Audet, D.; Miron, G.; Moriyasu, M. Biological Characteristics of a Newly Established Green Crab (Carcinus maenas) Population in the Southern Gulf of St. Lawrence, Canada. J. Shellfish Res. 2008, 27, 427–441. [Google Scholar] [CrossRef]
- Tremblay, M.J.; Thompson, A.; Paul, K. Recent Trends in the Abundance of the Invasive Green Crab (Carcinus maenas) in Bras d’Or Lakes and Eastern Nova Scotia Based on Trap Surveys; Fisheries and Ocean Canada, Bedford Institute of Oceanography: Dartmouth, NS, Canada, 2006; p. 32.
- MacDonald, A.J.; Kienzle, H.M.; Drolet, D.; Hamilton, D.J. Distribution and Habitat Use of the Invasive Carcinus maenas L. (European Green Crab) and the Native Cancer irroratus (Say) (Rock Crab) in Intertidal Zones in the upper Bay of Fundy, Canada. Northeast. Nat. 2018, 25, 161–180. [Google Scholar] [CrossRef]
- Quinn, B.K. Dramatic decline and limited recovery of a green crab (Carcinus maenas) population in the Minas Basin, Canada after the summer of 2013. PeerJ 2018, 6, e5566. [Google Scholar] [CrossRef] [PubMed]
- Rossong, M.A.; Quijón, P.A.; Snelgrove, P.V.R.; Barrett, T.J.; McKenzie, C.H.; Locke, A. Regional differences in foraging behaviour of invasive green crab (Carcinus maenas) populations in Atlantic Canada. Biol. Invasions 2012, 14, 659–669. [Google Scholar] [CrossRef]
- Berrill, M. The Life Cycle of the Green Crab Carcinus maenas at the Northern End of Its Range. J. Crustac. Biol. 1982, 2, 31–39. [Google Scholar] [CrossRef]
- Fulton, B.A.; Warner, R.; Fairchild, E.A. The green crab Carcinus maenas in two New Hampshire estuaries. Part 1: Spatial and temporal distribution, sex ratio, average size, and mass. J. Crustac. Biol. 2013, 33, 25–35. [Google Scholar] [CrossRef]
- Young, A.M.; Elliott, J.A.; Incatasciato, J.M.; Taylor, M.L. Seasonal catch, size, color, and assessment of trapping variables for the European green crab Carcinus maenas (Linnaeus, 1758) (Brachyura: Portunoidea: Carcinidae), a nonindigenous species in Massachusetts, USA. J. Crustac. Biol. 2017, 37, 556–570. [Google Scholar] [CrossRef]
- Griffen, B.D. Linking individual diet variation and fecundity in an omnivorous marine consumer. Oecologia 2013, 174, 121–130. [Google Scholar] [CrossRef]
- Derivera, C.E.; Hitchcock, N.G.; Teck, S.J.; Steves, B.P.; Hines, A.H.; Ruiz, G.M. Larval development rate predicts range expansion of an introduced crab. Mar. Biol. 2006, 150, 1275–1288. [Google Scholar] [CrossRef]
- Klassen, G.; Locke, A. A Biological Synopsis of the European Green Crab, Carcinus maenas; Canadian technical report of fisheries and aquatic sciences; Fisheries and Oceans Canada: Moncton, NB, Canada, 2007; Volume 2818, pp. 1–75.
- Hartnoll, R.G. Reproductive Investment in Brachyura. Hydrobiologia 2006, 557, 31–40. [Google Scholar] [CrossRef]
- Daufresne, M.; Lengfellner, K.; Sommer, U. Global warming benefits the small in aquatic ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12788–12793. [Google Scholar] [CrossRef]
- Forster, J.; Hirst, A.G. The temperature-size rule emerges from ontogenetic differences between growth and development rates. Funct. Ecol. 2012, 26, 483–492. [Google Scholar] [CrossRef]
- Manyak-Davis, A.; Bell, T.M.; Sotka, E.E. The Relative Importance of Predation Risk and Water Temperature in Maintaining Bergmann’s Rule in a Marine Ectotherm. Am. Nat. 2013, 182, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, D.J.; Levinton, J.S. Latitudinal Differentiation in Copepod Growth: An Adaptation to Temperature. Ecology 1985, 66, 1397–1407. [Google Scholar] [CrossRef]
- Lehnert, S.J.; DiBacco, C.; Jeffery, N.W.; Blakeslee, A.M.H.; Isaksson, J.; Roman, J.; Wringe, B.F.; Stanley, R.R.E.; Matheson, K.; McKenzie, C.H.; et al. Temporal dynamics of genetic clines of invasive European green crab (Carcinus maenas) in eastern North America. Evol. Appl. 2018, 11, 1656–1670. [Google Scholar] [CrossRef]
- Johnston, I.A.; Bennett, A.F. Animals and Temperature: Phenotypic and Evolutionary Adaptation; Society for Experimental Biology Seminar Series; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar] [CrossRef]
- Chapelle, G.; Peck, L.S. Polar gigantism dictated by oxygen availability. Nature 1999, 398, 114–115. [Google Scholar] [CrossRef]
- Monteiro, J.N.; Ovelheiro, A.; Ventaneira, A.M.; Vieira, V.; Teodósio, M.A.; Leitão, F. Variability in Carcinus maenas Fecundity along Lagoons and Estuaries of the Portuguese Coast. Estuaries Coasts 2022, 45, 1716–1727. [Google Scholar] [CrossRef]
- Debelius, B.; Gómez-Parra, A.; Forja, J.M. Oxygen solubility in evaporated seawater as a function of temperature and salinity. Hydrobiologia 2009, 632, 157–165. [Google Scholar] [CrossRef]
- Azra, M.N.; Aaqillah-Amr, M.A.; Ikhwanuddin, M.; Ma, H.; Waiho, K.; Ostrensky, A.; Tavares, C.P.d.S.; Abol-Munafi, A.B. Effects of climate-induced water temperature changes on the life history of brachyuran crabs. Rev. Aquac. 2019, 12, 1211–1216. [Google Scholar] [CrossRef]
- Hosono, T. Effect of temperature on growth and maturation pattern of Caprella mutica (Crustacea, Amphipoda): Does the temperature–size rule function in caprellids? Mar. Biol. 2011, 158, 363–370. [Google Scholar] [CrossRef]
- Hartnoll, R.G. Growth in Crustacea—Twenty years on. In Advances in Decapod Crustacean Research, Proceedings of the 7th Colloquium Crustacea Decapoda Mediterranea, Lisbon, Portugal, 6–9 September 1999; Springer: Dordrecht, The Netherlands, 2001; pp. 111–122. [Google Scholar] [CrossRef]
- Groner, M.L.; Shields, J.D.; Landers, D.F.; Swenarton, J.; Hoenig, J.M. Rising Temperatures, Molting Phenology, and Epizootic Shell Disease in the American Lobster. Am. Nat. 2018, 192, 163–177. [Google Scholar] [CrossRef]
- Johnson, D.S.; Crowley, C.; Longmire, K.; Nelson, J.; Williams, B.; Wittyngham, S. The fiddler crab, Minuca pugnax, follows Bergmann’s rule. Ecol. Evol. 2019, 9, 14489–14497. [Google Scholar] [CrossRef]
- Cunningham, S.R.; Darnell, M.Z. Temperature-Dependent Growth and Molting in Early Juvenile Blue Crabs Callinectes sapidus. J. Shellfish Res. 2015, 34, 505–510. [Google Scholar] [CrossRef]
- Aguilar-Alberola, J.A.; Mesquita-Joanes, F. Breaking the temperature-size rule: Thermal effects on growth, development and fecundity of a crustacean from temporary waters. J. Therm. Biol. 2014, 42, 15–24. [Google Scholar] [CrossRef]
- Steele, D.H.; Steele, V.J. The biology of Gammarus (Crustacea, Amphipoda) in the northwestern Atlantic. XI. Comparison and discussion. Can. J. Zool. 1975, 53, 1116–1126. [Google Scholar] [CrossRef]
- Shakuntala, K.; Reddy, S.R. Crustacean egg size as an indicator of egg fat/protein reserves. Int. J. Invertebr. Reprod. 1982, 4, 381–384. [Google Scholar] [CrossRef]
- Brown, N.P.; Shields, R.J.; Bromage, N.R. The influence of water temperature on spawning patterns and egg quality in the Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture 2006, 261, 993–1002. [Google Scholar] [CrossRef]
- Baptista, V.; Costa, E.F.S.; Carere, C.; Morais, P.; Cruz, J.; Cerveira, I.; Castanho, S.; Ribeiro, L.; Pousão-Ferreira, P.; Leitão, F.; et al. Does consistent individual variability in pelagic fish larval behaviour affect recruitment in nursery habitats? Behav. Ecol. Sociobiol. 2020, 74, 67. [Google Scholar] [CrossRef]
- Pinto, M.; Monteiro, J.N.; Crespo, D.; Costa, F.; Rosa, J.; Primo, A.L.; Pardal, M.A.; Martinho, F. Influence of oceanic and climate conditions on the early life history of European seabass Dicentrarchus labrax. Mar. Environ. Res. 2021, 169, 105362. [Google Scholar] [CrossRef]
- De Grande, F.R.; Granado, P.; Costa, T.M. Size-at-age or structure shift: Which hypothesis explains smaller body size of the fiddler crab Leptuca uruguayensis in northern populations? Estuar. Coast. Shelf Sci. 2021, 254, 107358. [Google Scholar] [CrossRef]
- Todd, C.D.; Hughes, S.L.; Marshall, C.T.; MacLean, J.C.; Lonergan, M.E.; Biuw, E.M. Detrimental effects of recent ocean surface warming on growth condition of Atlantic salmon. Glob. Chang. Biol. 2008, 14, 958–970. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).